Abstract
High seed vigour ensures good quality seed and higher productivity. Early seedling growth parameters indicate seed vigour in rice. Seed vigour via physiological growth parameters is a complex trait controlled by many quantitative trait loci. A panel was prepared representing a population of 274 rice landraces by including genotypes from all the phenotypic groups of sixseedling stage physiological parameters including germination % for association mapping. Wide variations for the six studiedtraits were observed in the population. The population was classified into 3 genetic groups. Fixation indices indicated the presence of linkage disequilibrium in the population. The population was classified into subpopulations and each subpopulation showed correspondence with the 6 physiological traits. A total of 5 reported QTLs viz., qGP8.1 for germination % (GP); qSVII2.1, qSVII6.1 and qSVII6.2 for seed vigour index II (SVII), and qRSR11.1 for root-shoot ratio (RSR) were validated in this mapping population. In addition, 13 QTLs regulating the physiological parameters such as qSVI 11.1 for seed vigour index I; qSVI11.1 and qSVI12.1 for seed vigour index II; qRRG10.1, qRRG8.1, qRRG8.2, qRRG6.1 and qRRG4.1 for rate of root growth (RRG); qRSR2.1, qRSR3.1 and qRSR5.1 for root-shoot ratio (RSR) while qGP6.2 and qGP6.3 for germination %were identified. Additionally, co-localization or co-inheritance of QTLs, qGP8.1 and qSVI8.1 for GP and SVI-1; qGP6.2 and qRRG6.1 for GP and RRG, and qSVI11.1 and qRSR11.1 for SVI and RSR were detected. The QTLs identified in this study will be useful for improvement of seed vigour trait in rice.
Introduction
Rice is the most important cereal crop for human consumption as more than half the global population uses this as staple food and hence there is a need to increase the production [1]. Food requirement for the increasing human population, theglobal demand is expected to reach almost double by 2050 which is a big challenge under the adverse effects of the climate change [2,3]. A good quality seed not only ensures high yield but also considered as a mega factor for enhancing crop productivity [4]. The genetic potential of a plant variety is fully manifested by use of quality seed which is considered as the basic and most vital input for crop production. Seed vigour is one of the most important determinant of seed quality, directly influences the crop productivity by delivering the genetic and yield potentials of the seed while ensuring uniformity in seed germination, seedling growth, establishment of seedling in the field and withstanding unfavourable environmental condition [5,6]. In addition, high seed vigour is also equally important for direct seeding as it enhances early crop establishment [7,8] and produces vigorous seedling that can compete with weeds [9,10]. Improving the seed vigour of rice remains a breeding challenge [11] as it is not only essential to enhance the yield but also can improve crop resilience against adverse effects of climate change and biotic impediments to crop yields [12].
Seed vigour via physiological growth parameters is a complex trait controlled by many quantitative trait loci (QTLs). To investigate the inheritance of such complex trait, the QTL analysis has successfully proven as a powerful tool in the last decades. The detected QTLs for rice seed vigour were associated with many physiological traits viz., root length, shoot length, dry weight of seedling, germination rate, radicle length, root activity, coleoptile length, mesocotyl length, germination potential, germination index and time for 50% germination [13–25]. The QTLs reported for seed vigour in rice were associated with many physiological traits viz., root length, shoot length, dry weight of seedling, germination rate, radicle length, root activity, coleoptile length, mesocotyl length, germination potential, germination index and time for 50% germination [13–25]. Many QTLs controlling germination % in rice were reported by earlier researchers [17–19,26–32]. Good seedling root features playimportant role in water and nutrient absorption from the soil and support to the plant. However, information on the vigour related seedling root mapping studies are limited [15,17,23,33–35]. The rapid growth of seedlings after germination is an important feature of good seedling vigour. Also a limited number of mapping information on shoot length and its related parameters are available [25–38]. However, the QTLs reported for the physiological traits described above are not always consistent for the mapped position.
Majority of the genes/QTLs reported for seed vigour in rice were based on bi-parental segregating populations. Association mapping based on linkage disequilibrium (LD) is a very successful approach of complex traits mapping. In addition, the QTL identifiedin this approachshowbetter resolution by utilizing the available natural variation [3,39,40]. The microsatellite markers are widely used to assess genetic diversity and genetic structure in rice as they are hyper variable, multi-allelic in nature,robust, co-dominant, chromosome specific and greatly facilitate linkage map construction [41–45]. Population genetic structure is useful in detecting a perfect marker-phenotype association. The population structure (Q) with relative kinship (K) analyses is used to check and correct the panel population composition for linkage disequilibrium (LD) mapping analyses [46,47]. Association analysis based on both the models of General linear model (GLM) and Mixed linear model (MLM)is considered appropriate for mapping complex traits that have shown to perform better than other models analysis. However, very limited information is available on genetic analysis of seedling stage physiological parameters related to seed vigour in a variable rice natural population.
In the present investigation, association mapping of seedling growth parameters including germination %with SSR markers was performed in a representative shortlisted population by including genotypes from all the phenotypic classesfor the early seedling growth parameters namely seed vigour index I, seed vigour index II, relative root growth, root shoot ratio, rate of plumule elongationand germination per cent from a total of 274rice landraces. The study will reveal the population genetic structure, diversity and candidate genes/QTLs controlling the 6seedling growth parameter associated with seed vigour in rice.
Material and methods
Seed material
The freshly harvested seeds of 274 landraces collected from five states viz., Assam, MP, Kerala, Odisha and Manipur of India were used for association mapping of seedling stage physiological parameters with molecular markers. The germplasm lines of Odisha state were from the Jeypore tract, the secondary center of origin of rice and known for availability of rich diversity of rice. All the germplasm lines were collected from Gene bank, ICAR-NRRI, Cuttack and grown during wet season, 2018 (S1 Table). The harvested seeds were used for estimation of 6seedling growth parameters after a storage period of three months to overcome the seed dormancy. A panel population was developed and raised during the wet seasons of 2019 and 2020. Thepanel population comprising 120landraceswas used for mapping of the 6growth parameters including germination % (Table 1).
Table 1. Mean estimates of SVI-1, SVI-II, RRG, RPE, RSR, GP and genetic structure ancestry value at K = 3 for the panel population containing 120 landraces.
| Sl.No. | Accession No./Name of the germplasm line | SVI-I | SVI-II | RRG | RPE | RSR | GP | Inferred ancestry value at K = 3 | Genetic structure group | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | |||||||||
| 1 | Jhagrikartik | 202.12 | 0.057 | 0.970 | 0.53 | 2.148 | 20.00 | 0.008 | 0.99 | 0.002 | SP2 |
| 2 | Dadghani | 357.40 | 0.137 | 0.110 | 0.15 | 0.933 | 50.00 | 0.005 | 0.992 | 0.003 | SP2 |
| 3 | Shayam | 676.52 | 0.127 | 0.810 | 0.24 | 1.460 | 78.00 | 0.001 | 0.002 | 0.996 | SP3 |
| 4 | Basumati | 481.60 | 0.100 | 0.290 | 0.29 | 0.888 | 80.00 | 0.005 | 0.113 | 0.883 | SP3 |
| 5 | Bharati | 131.64 | 0.070 | 0.127 | 0.19 | 0.778 | 14.00 | 0.001 | 0.997 | 0.002 | SP2 |
| 6 | Joha | 103.60 | 0.100 | 0.220 | 0.20 | 1.151 | 20.00 | 0.002 | 0.997 | 0.002 | SP2 |
| 7 | Adira-1 | 380.12 | 0.216 | 0.230 | 0.40 | 0.962 | 28.00 | 0.019 | 0.619 | 0.362 | A |
| 8 | Adira-2 | 1273.20 | 0.592 | 0.753 | 0.43 | 1.074 | 84.00 | 0.002 | 0.997 | 0.001 | SP2 |
| 9 | Adira-3 | 602.68 | 0.293 | 0.533 | 0.21 | 1.133 | 50.00 | 0.003 | 0.593 | 0.403 | A |
| 10 | PK6 | 400.24 | 0.178 | 0.463 | 0.15 | 1.782 | 46.00 | 0.004 | 0.982 | 0.014 | SP2 |
| 11 | Vachaw | 523.72 | 0.316 | 0.463 | 0.19 | 1.390 | 74.00 | 0.002 | 0.961 | 0.037 | SP2 |
| 12 | Kozhivalan | 571.12 | 0.355 | 0.093 | 0.31 | 1.076 | 66.00 | 0.002 | 0.997 | 0.001 | SP2 |
| 13 | Marathondi | 264.20 | 0.112 | 0.360 | 0.37 | 0.652 | 26.00 | 0.034 | 0.516 | 0.45 | A |
| 14 | Ezhoml-2 | 526.84 | 0.215 | 0.243 | 0.05 | 1.079 | 74.00 | 0.001 | 0.998 | 0.001 | SP2 |
| 15 | Jyothi | 190.40 | 0.112 | 0.423 | 0.19 | 1.088 | 28.00 | 0.001 | 0.998 | 0.001 | SP2 |
| 16 | Kantakopura | 885.20 | 0.525 | 0.447 | 0.72 | 0.891 | 70.00 | 0.001 | 0.997 | 0.002 | SP2 |
| 17 | Kantakaamala | 713.20 | 0.419 | 0.640 | 0.67 | 0.987 | 68.00 | 0.109 | 0.675 | 0.216 | A |
| 18 | Kapanthi | 1349.76 | 0.684 | 0.677 | 0.54 | 1.366 | 76.00 | 0.341 | 0.304 | 0.355 | A |
| 19 | Karpurkanti | 661.12 | 0.151 | 0.403 | 0.55 | 0.842 | 70.00 | 0.001 | 0.001 | 0.998 | SP3 |
| 20 | Kathidhan | 523.20 | 0.327 | 0.420 | 0.63 | 1.118 | 38.00 | 0.097 | 0.898 | 0.005 | SP2 |
| 21 | Kundadhan | 213.92 | 0.076 | 0.157 | 0.38 | 0.907 | 20.00 | 0.005 | 0.994 | 0.001 | SP2 |
| 22 | Champaeisiali | 1011.72 | 0.479 | 1.113 | 0.80 | 1.403 | 58.00 | 0.003 | 0.993 | 0.004 | SP2 |
| 23 | Latamahu | 318.48 | 0.182 | 0.830 | 0.81 | 1.074 | 28.00 | 0.003 | 0.996 | 0.002 | SP2 |
| 24 | Latachaunri | 633.20 | 0.303 | 0.417 | 0.50 | 1.336 | 64.00 | 0.005 | 0.993 | 0.002 | SP2 |
| 25 | AC5993 (Mikirahu) | 88.16 | 0.248 | 0.260 | 0.35 | 1.320 | 16.00 | 0.003 | 0.995 | 0.003 | SP2 |
| 26 | AC6221(Ana pachidhan) | 483.56 | 0.310 | 0.617 | 0.16 | 1.309 | 70.00 | 0.007 | 0.991 | 0.002 | SP2 |
| 27 | AC6183 (Kanai muluk) | 451.44 | 0.198 | 1.617 | 0.31 | 2.185 | 36.00 | 0.072 | 0.925 | 0.003 | SP2 |
| 28 | AC6170 (Bengali joha) | 532.16 | 0.179 | 0.447 | 0.20 | 1.170 | 84.00 | 0.003 | 0.995 | 0.002 | SP2 |
| 29 | AC6023 (Tili bora) | 151.84 | 0.160 | 1.070 | 0.38 | 1.380 | 16.00 | 0.02 | 0.978 | 0.002 | SP2 |
| 30 | AC6172 (ManoharSali) | 684.80 | 0.490 | 0.600 | 0.21 | 1.184 | 80.00 | 0.051 | 0.948 | 0.002 | SP2 |
| 31 | AC6027 (Chingforechokua) | 141.92 | 0.134 | 0.553 | 0.27 | 1.004 | 28.00 | 0.002 | 0.005 | 0.993 | SP3 |
| 32 | AC6007 (Manipur local) | 323.28 | 0.093 | 0.973 | 0.21 | 1.203 | 30.00 | 0.004 | 0.995 | 0.001 | SP2 |
| 33 | AC9006 (Aujari) | 717.36 | 0.583 | 0.736 | 0.16 | 0.896 | 45.00 | 0.02 | 0.971 | 0.009 | SP2 |
| 34 | AC9021 (Kabokphou) | 865.33 | 0.477 | 0.240 | 0.23 | 0.907 | 58.33 | 0.013 | 0.982 | 0.005 | SP2 |
| 35 | AC9028 (Mayangkhang-I) | 459.00 | 0.637 | 0.923 | 0.06 | 0.814 | 30.00 | 0.069 | 0.928 | 0.003 | SP2 |
| 36 | AC9030 (Moiranghouanganba) | 514.27 | 0.898 | 0.527 | 0.10 | 0.596 | 35.00 | 0.006 | 0.994 | 0.001 | SP2 |
| 37 | AC9035 (Taothali) | 397.80 | 0.515 | 0.917 | 0.14 | 0.585 | 25.00 | 0.004 | 0.983 | 0.014 | SP2 |
| 38 | AC9038 (Mayangkhang-II) | 891.87 | 1.020 | 0.903 | 0.20 | 0.804 | 53.33 | 0.001 | 0.998 | 0.001 | SP2 |
| 39 | AC9043 (Phoudum) | 799.87 | 1.123 | 0.280 | 0.13 | 0.744 | 50.00 | 0.002 | 0.997 | 0.001 | SP2 |
| 40 | AC9044 (Changli) | 690.00 | 0.740 | 1.247 | 0.09 | 0.919 | 40.00 | 0.003 | 0.993 | 0.005 | SP2 |
| 41 | AC20920 (Pondremunduria) | 771.67 | 0.504 | 1.133 | 0.26 | 0.884 | 60.00 | 0.003 | 0.993 | 0.004 | SP2 |
| 42 | AC20907 (Lalmunduria) | 318.60 | 0.168 | 0.267 | 0.41 | 0.854 | 30.00 | 0.002 | 0.997 | 0.001 | SP2 |
| 43 | AC20845 (Jhitikuji) | 629.84 | 0.453 | 0.510 | 0.14 | 0.829 | 42.00 | 0.003 | 0.996 | 0.001 | SP2 |
| 44 | AC20770 (Tikichudi) | 842.24 | 0.336 | 0.287 | 0.25 | 0.725 | 56.00 | 0.002 | 0.993 | 0.005 | SP2 |
| 45 | AC20627 (Chudi) | 492.52 | 0.212 | 0.227 | 0.37 | 0.696 | 42.00 | 0.002 | 0.997 | 0.001 | SP2 |
| 46 | AC20686 (Radhabati) | 169.16 | 0.048 | 0.147 | 0.20 | 0.863 | 16.00 | 0.002 | 0.997 | 0.001 | SP2 |
| 47 | AC20664 (Liktimachi) | 121.84 | 0.078 | 0.220 | 0.43 | 0.821 | 16.00 | 0.003 | 0.996 | 0.001 | SP2 |
| 48 | AC20614(Baranga) | 371.20 | 0.245 | 0.320 | 0.41 | 1.066 | 40.00 | 0.001 | 0.996 | 0.002 | SP2 |
| 49 | AC10608 (Ampang) | 320.56 | 0.175 | 0.440 | 0.12 | 1.093 | 38.00 | 0.006 | 0.994 | 0.001 | SP2 |
| 50 | AC10187 (Mimahambel) | 1060.20 | 0.412 | 1.917 | 0.22 | 1.598 | 80.00 | 0.081 | 0.917 | 0.002 | SP2 |
| 51 | AC10162 (Ahimachutki) | 256.24 | 0.071 | 1.067 | 0.35 | 1.272 | 28.00 | 0.023 | 0.96 | 0.017 | SP2 |
| 52 | AC7282 (Mimagisim) | 166.32 | 0.074 | 1.137 | 0.35 | 1.025 | 28.00 | 0.001 | 0.002 | 0.997 | SP3 |
| 53 | AC7269 (Champalidhan) | 113.00 | 0.194 | 0.570 | 0.66 | 1.124 | 18.00 | 0.002 | 0.997 | 0.001 | SP2 |
| 54 | AC7134 (Memabalbok) | 142.72 | 0.140 | 0.687 | 0.29 | 1.186 | 20.00 | 0.009 | 0.793 | 0.198 | A |
| 55 | AC7008 (Kartiksal) | 129.76 | 0.088 | 0.660 | 0.11 | 1.071 | 16.00 | 0.002 | 0.998 | 0.001 | SP2 |
| 56 | AC9093 (Turnaianganba) | 446.16 | 1.298 | 0.457 | 0.37 | 0.811 | 44.00 | 0.001 | 0.995 | 0.004 | SP2 |
| 57 | AC9090 (Chakhaosimpak) | 451.36 | 1.050 | 0.477 | 0.42 | 1.354 | 38.00 | 0.004 | 0.987 | 0.01 | SP2 |
| 58 | AC9076A(Phoaujaarangbele) | 173.76 | 0.180 | 0.543 | 0.58 | 1.669 | 12.00 | 0.006 | 0.993 | 0.001 | SP2 |
| 59 | AC9065 (Moirangphon) | 498.24 | 1.094 | 0.270 | 0.38 | 1.054 | 46.00 | 0.003 | 0.963 | 0.034 | SP2 |
| 60 | AC9063 (Chingphou) | 326.12 | 0.856 | 0.367 | 0.11 | 0.917 | 34.00 | 0.007 | 0.992 | 0.001 | SP2 |
| 61 | AC9058 (Langmanbu) | 332.96 | 0.962 | 0.237 | 0.39 | 0.816 | 38.00 | 0.001 | 0.998 | 0.001 | SP2 |
| 62 | AC9053A(Kakchengphou) | 105.28 | 0.080 | 0.140 | 0.27 | 0.756 | 16.00 | 0.153 | 0.839 | 0.008 | SP2 |
| 63 | AC9050 (Phongangangamphou) | 261.08 | 0.564 | 0.217 | 0.25 | 1.027 | 34.00 | 0.002 | 0.994 | 0.004 | SP2 |
| 64 | AC9005 (Phourrel) | 849.97 | 0.563 | 0.803 | 0.29 | 1.172 | 48.33 | 0.004 | 0.993 | 0.002 | SP2 |
| 65 | AC20389 (Kusumal) | 999.32 | 0.908 | 0.513 | 0.27 | 0.809 | 80.00 | 0.046 | 0.943 | 0.011 | SP2 |
| 66 | AC20371 (Kakudimanji) | 1356.84 | 1.318 | 0.347 | 0.19 | 0.763 | 94.00 | 0.007 | 0.992 | 0.001 | SP2 |
| 67 | AC20423 (Salati) | 475.64 | 0.204 | 1.090 | 0.21 | 1.071 | 32.00 | 0.003 | 0.996 | 0.001 | SP2 |
| 68 | AC20362 (Kaberi) | 1129.04 | 0.886 | 0.377 | 0.55 | 0.818 | 84.00 | 0.018 | 0.975 | 0.007 | SP2 |
| 69 | AC20328 (Batachudi) | 1255.68 | 1.161 | 0.540 | 0.34 | 0.796 | 86.00 | 0.009 | 0.979 | 0.012 | SP2 |
| 70 | AC20317 (Barda) | 569.16 | 0.442 | 0.347 | 0.33 | 0.999 | 68.00 | 0.003 | 0.973 | 0.024 | SP2 |
| 71 | AC20282 (Mahipaljeera) | 1149.28 | 0.789 | 0.883 | 0.42 | 0.805 | 70.00 | 0.108 | 0.886 | 0.006 | SP2 |
| 72 | AC20246 (BodiKaberi) | 660.96 | 0.452 | 0.403 | 0.58 | 0.685 | 48.00 | 0.024 | 0.89 | 0.086 | SP2 |
| 73 | AC20347 (Dudhamani) | 310.60 | 0.138 | 0.563 | 0.38 | 0.809 | 28.00 | 0.066 | 0.932 | 0.001 | SP2 |
| 74 | AC44603 (Sonamasuri) | 1490.00 | 1.120 | 1.250 | 0.50 | 1.678 | 100.00 | 0.985 | 0.014 | 0.001 | SP1 |
| 75 | AC44585 (Bilijaya) | 1396.67 | 2.190 | 1.083 | 0.80 | 0.906 | 100.00 | 0.989 | 0.003 | 0.008 | SP1 |
| 76 | AC44598 (Jira) | 835.11 | 0.608 | 1.300 | 0.45 | 1.332 | 66.67 | 0.988 | 0.007 | 0.006 | SP1 |
| 77 | AC44592 (Malbar) | 1900.00 | 1.630 | 1.133 | 0.40 | 1.286 | 100.00 | 0.991 | 0.001 | 0.008 | SP1 |
| 78 | AC44646 (Jaya Padma) | 1621.44 | 1.709 | 1.567 | 0.70 | 1.164 | 96.67 | 0.998 | 0.001 | 0.001 | SP1 |
| 79 | AC44604 (Lusai) | 1526.67 | 2.720 | 0.150 | 0.40 | 1.399 | 100.00 | 0.977 | 0.013 | 0.01 | SP1 |
| 80 | AC44597 (Bilipandya) | 1890.33 | 2.804 | 1.200 | 0.35 | 0.985 | 96.67 | 0.997 | 0.002 | 0.001 | SP1 |
| 81 | AC44638 (Kalame) | 1536.00 | 0.875 | 2.583 | 0.50 | 1.231 | 93.33 | 0.29 | 0.001 | 0.71 | A |
| 82 | AC44595 (Chitapa) | 1664.44 | 1.699 | 0.283 | 0.70 | 0.711 | 93.33 | 0.99 | 0.004 | 0.006 | SP1 |
| 83 | AC44588 (Badra) | 1420.00 | 1.940 | 0.250 | 0.50 | 0.841 | 100.00 | 0.997 | 0.002 | 0.001 | SP1 |
| 84 | AC44591 (Gouri) | 1443.33 | 1.050 | 0.333 | 0.50 | 0.904 | 100.00 | 0.998 | 0.002 | 0.001 | SP1 |
| 85 | AC44594 (Pandya) | 1986.67 | 2.430 | 1.350 | 0.70 | 0.789 | 100.00 | 0.99 | 0.008 | 0.002 | SP1 |
| 86 | AC43737 (karinellu) | 1498.33 | 0.920 | 3.833 | 0.60 | 0.854 | 70.00 | 0.996 | 0.002 | 0.002 | SP1 |
| 87 | AC43660 (Manavari) | 1215.50 | 0.625 | 3.422 | 0.40 | 0.727 | 45.00 | 0.997 | 0.002 | 0.001 | SP1 |
| 88 | AC3732 (Koompallai) | 1163.50 | 1.050 | 2.550 | 0.20 | 0.838 | 80.00 | 0.998 | 0.001 | 0.001 | SP1 |
| 89 | AC43661 (MDU-5) | 737.83 | 0.443 | 2.583 | 0.10 | 0.925 | 45.00 | 0.995 | 0.004 | 0.001 | SP1 |
| 90 | AC43738 (Belimuruduga) | 668.33 | 0.740 | 2.200 | 0.40 | 1.165 | 50.00 | 0.996 | 0.002 | 0.002 | SP1 |
| 91 | AC43669 (Tulasi) | 1809.67 | 0.950 | 3.533 | 0.35 | 0.982 | 70.00 | 0.994 | 0.004 | 0.002 | SP1 |
| 92 | AC43663 (TKM10) | 1041.33 | 0.890 | 1.200 | 0.40 | 0.587 | 70.00 | 0.997 | 0.001 | 0.002 | SP1 |
| 93 | AC43658 (Noorthipathu) | 758.00 | 0.590 | 3.000 | 0.80 | 0.584 | 50.00 | 0.998 | 0.001 | 0.001 | SP1 |
| 94 | AC43662 (PMK2) | 1287.83 | 0.985 | 2.633 | 0.70 | 0.754 | 80.00 | 0.979 | 0.002 | 0.018 | SP1 |
| 95 | AC43670 (Aditya) | 1117.41 | 0.567 | 2.583 | 0.65 | 1.130 | 55.56 | 0.827 | 0.002 | 0.171 | SP1 |
| 96 | AC43675 (Kalimekri 77–5) | 653.33 | 0.480 | 2.383 | 0.20 | 0.759 | 50.00 | 0.99 | 0.002 | 0.007 | SP1 |
| 97 | AC43676 (Pratao) | 1131.17 | 0.945 | 2.183 | 0.20 | 0.567 | 65.00 | 0.959 | 0.008 | 0.033 | SP1 |
| 98 | Palinadhan-1 | 102.88 | 0.038 | 0.267 | 0.28 | 1.049 | 16.00 | 0.237 | 0.393 | 0.371 | A |
| 99 | Chatuimuchi | 608.80 | 0.329 | 0.663 | 0.22 | 1.167 | 84.00 | 0.001 | 0.001 | 0.998 | SP3 |
| 100 | Uttarbangalocal-9 | 56.92 | 0.048 | 0.200 | 0.14 | 1.432 | 10.00 | 0.094 | 0.904 | 0.001 | SP2 |
| 11 | Gochi | 171.96 | 0.120 | 0.250 | 0.09 | 0.884 | 26.00 | 0.065 | 0.927 | 0.008 | SP2 |
| 12 | Sugandha-2 | 540.80 | 0.208 | 0.457 | 0.24 | 1.399 | 84.00 | 0.001 | 0.002 | 0.997 | SP3 |
| 13 | Jhingesal | 370.44 | 0.169 | 0.610 | 0.37 | 1.128 | 34.00 | 0.002 | 0.997 | 0.001 | SP2 |
| 104 | Cheruvirippu | 411.04 | 0.149 | 0.300 | 0.28 | 1.126 | 56.00 | 0.004 | 0.994 | 0.001 | SP2 |
| 105 | Mahamaga | 250.08 | 0.066 | 0.443 | 0.19 | 0.974 | 42.00 | 0.047 | 0.951 | 0.002 | SP2 |
| 106 | Jaya | 442.00 | 0.300 | 0.527 | 0.24 | 1.303 | 64.00 | 0.009 | 0.99 | 0.001 | SP2 |
| 107 | D1 | 185.36 | 0.130 | 0.357 | 0.20 | 1.024 | 34.00 | 0.049 | 0.94 | 0.011 | SP2 |
| 108 | PK21 | 1151.00 | 0.400 | 0.787 | 0.27 | 0.835 | 100.00 | 0.016 | 0.983 | 0.001 | SP2 |
| 109 | Gandhakasala | 397.76 | 0.152 | 0.720 | 0.14 | 1.387 | 58.00 | 0.003 | 0.004 | 0.993 | SP3 |
| 110 | Sreyas | 235.36 | 0.127 | 0.667 | 0.19 | 1.158 | 32.00 | 0.002 | 0.995 | 0.002 | SP2 |
| 111 | Gondiachampeisiali | 1010.48 | 0.647 | 0.513 | 0.68 | 0.854 | 66.00 | 0.003 | 0.995 | 0.002 | SP2 |
| 112 | Chinamal | 637.40 | 0.411 | 0.583 | 0.41 | 1.248 | 46.00 | 0.002 | 0.983 | 0.015 | SP2 |
| 113 | Magra | 390.36 | 0.138 | 1.213 | 0.09 | 1.392 | 30.00 | 0.002 | 0.995 | 0.003 | SP2 |
| 114 | Landi | 672.60 | 0.184 | 0.747 | 0.65 | 1.168 | 46.00 | 0.002 | 0.997 | 0.002 | SP2 |
| 115 | Lalgundi | 334.88 | 0.054 | 0.243 | 0.68 | 0.802 | 36.00 | 0.004 | 0.989 | 0.006 | SP2 |
| 116 | Balisaralaktimachi | 579.96 | 0.244 | 1.193 | 0.51 | 1.264 | 40.00 | 0.003 | 0.993 | 0.003 | SP2 |
| 117 | Laxmibilash | 257.00 | 0.096 | 0.273 | 0.60 | 0.970 | 22.00 | 0.003 | 0.427 | 0.57 | A |
| 118 | Kaniar | 918.28 | 0.619 | 0.407 | 0.56 | 0.799 | 66.00 | 0.005 | 0.981 | 0.015 | SP2 |
| 119 | Kanakchampa | 277.68 | 0.204 | 0.733 | 0.33 | 0.942 | 24.00 | 0.004 | 0.982 | 0.015 | SP2 |
| 120 | Magura-s | 400.40 | 0.136 | 0.647 | 0.45 | 0.766 | 32.00 | 0.002 | 0.914 | 0.084 | SP2 |
| Mean | 672.85 | 0.54 | 0.85 | 0.36 | 1.04 | 52.20 | |||||
| CV% | 12.35 | 13.41 | 14.25 | 10.23 | 11.62 | 9.31 | |||||
| LSD 5% | 127.422 | 0.131 | 0.728 | 0.137 | 0.286 | 9.650 | |||||
Phenotyping forsix seedling stage physiological traits
Seed physiological characteristics such as seed vigour index I, seed vigour index II, rate of root growth, rate of plumule elongation, root shoot ratio andgermination per cent were estimated for the mapping study. Fifty seeds were germinated in three replicationsby adopting the top of paper method of Rao et al. [48] for panel development. Plastic trays were used for germination and raising the seedlings for phenotyping of the panel population. For estimating the sixgrowth parameters, observations observation on five seedlings were recorded from each replication and averaged to get the value of each replication. The germination % (GP) is the percentage of germinated seeds at 10th day was referred as the final germination percentage. Root length (RL) was measured on 10th day of germination and expressed in cm. The increase in plumule length per day was considered as rate of plumule elongation (RPE) and expressed in cm day-1. The increase in root length per day i.e rate of root grow (RRG) recorded on 7th day and 10th day of germination were considered and expressed in cm day-1. The seedlings used for recording root:shoot ratio (RSR) were oven dried at 70 °C for 48 hours after removing the cotyledon and seedling dry weight was expressed in gram per seedling as per the protocol of Kleyer et al. [49]. Seed vigour indices (SVI I and SVI II) were calculated using the formula suggested by Abdul-Baki and Anderson [50].
Analysis of variance (ANOVA) of each traits including the estimation of mean, range, and coefficient of variation (CV %) were estimated by using Cropstat software 7.0 [51]. Pearson’s correlation coefficients were analyzed to find out the relationship among the various physiological traits, based on the mean values of the 120 genotypes and presented in correlation matrix heatmap. The mean estimates of the 6 physiological parameters were classified into 4 groups as very high, high, medium and low value containing germplasm lines for this study.
Genomic DNA isolation, PCR analysis and selection of SSR markers
Genomic DNA of the germplasm lines was extracted from 15 days-old plant by adopting CTAB method [52]. The 136 SSR (simple sequence repeat) markers were taken from the data base available in the public domain (S1 Table). The isolated DNA was quantified by resolving the DNA fragments in gel electrophoresis. PCR analysis was done using the markers selected based on position covering all the chromosomes to illustrate the diversity and to identify the polymorphic loci among the 120 rice landraces (Table 1). Conditions of PCR reaction was set to initial denaturation step (2 min, 95°C), followed by 35 cycles of denaturation (30 s, 95°C) and annealing/extension (30 min, 55°C), extension (2 min, 72ºC), final extension (5 min, 72ºC) and store at 4ºC (infinity). The PCR products were electrophoresed using 2.5% agarose gel containing 0.80g ml-1 ethidium bromide. To determine the size of amplicons, 50 bp DNA ladder was used. The gel was run at 2.5V cm-1 for 4 hrs and photographed using a Gel Documentation System [53]. The procedures followed in earlier publications were adopted in this work [54–56].
Molecular data analysis
Data scoring was carried out from the presence or absence of amplified products obtained on the basis genotype-primer combination. A binary data matrix was used as discrete variables for the entry of our result data. Software, ‘Power Marker Ver3.25’ was used to analyze the parameters namely polymorphic information content (PIC), observed heterozygosity (H), number of alleles (N), major allele frequency (A) and gene diversity (GD) for each SSR locus [57]. A Bayesian model based clustering approach STRUCTURE 2.3.6 software was used to analysis genetic data and obtain population structure [58]. To derive the ideal number of groups (K), STRUCTURE software was run with K varying from 1 to 10, with 10 iterations for each K value. A high throughput parameter set of burn-in period of the 150,000 followed by 150,000 Markov Chain Monte Carlo (MCMC) replications was adapted during the running period. Highest value of ΔK was pick up from Evanno table used to detect the subpopulation groups from the panel of populations in the next step. The maximal value of L(K) was identified using the exact number of sub-populations. The model choice criterion to detect the most probable value of K was ΔK, an ad-hoc quantity related to the second-order change of the log probability of data with respect to the number of clusters inferred by STRUCTURE. Structure Harvester was used for estimation of the ΔK-value as function of K showing a clear peak as the optimal K-value [59]. The principal coordinate analysis of all the genotypes and unweighted neighbor joining unrooted tree for NEI coefficient dissimilarity index with bootstrap value of 1,000 were obtained by using DARwin5 software [60]. The presence of molecular variance across the whole population, within a population and between the sub-population structures (FIT, FIS, FST) was calculated by the deviation from Hardy-Weinberg expectation and estimated through Analysis of molecular variance (AMOVA) using GenAlEx 6.5 software [61]. All the detailed protocols of the above mentioned softwares were described in earlier publications [39,62].
Software, “TASSEL 5.0” was used to analyze the marker-trait association for mapping study of the seed vigor traits in rice. General linear model and Mixed linear model in TASSEL 5.0 were used to perform the genetic association between the phenotypic traits and molecular markers [63]. By considering the significant p-value and r2 value convincing associated markers were identified. The associations of markers were further confirmed by the Q-Q plot generated by the software. Linkage disequilibrium plot was obtained using LD measured r2, between pair of markers is plotted against the distance between the pair. Also, the accuracy of the marker-trait association by estimating the FDR adjusted p-values (q-values) using R software as described in the earlier publications [39,47].
Results
Phenotyping of the population for seedling stage physiological traits in rice
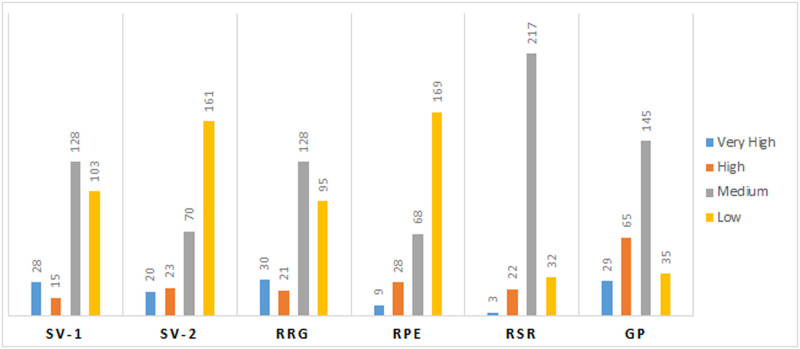
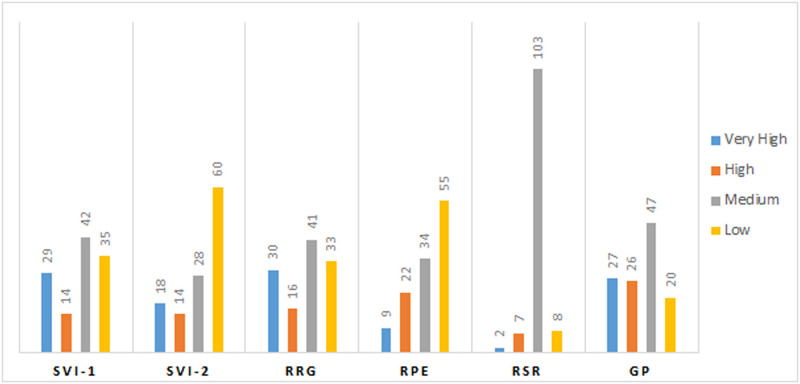
The mean values of 6 physiological traits viz., GP, SVI, SVII, RRG, RPE, and RGR related to seed vigour were estimated from274 landraces during the wet seasons of 2019 and 2020 (S1 Table). Significant differences were noticed among the germplasm lines for these 6 traits. The frequency distribution of the 274 germplasm lines were broadly classified into 4 groups each for the 6 physiological parameters (Fig 1). The distribution of germplasm lines into various groups were categorized into groups or subpopulations. A representative panel population containing 120 landraces was developed from the original population by shortlisting germplasm lines from all the phenotypic groups of each parameter (Table 1; Figs 2 and 3). The mean values of the 6 physiological traits estimated from the studied panel population also showed significant variation among the genotypes for each trait (Table 1). AC. 9038, AC. 9043, AC. 20282, AC. 20328, AC. 20362, AC. 20371, and AC. 20389 showed very high values for both seed vigour index I and seed vigour index II. High seed vigour index-II and germination per cent exhibited by the landraces Kapanthi, PK21, AC. 10187, AC. 20389, AC. 20371, AC. 20362 and AC. 20328. Rate of root growth and root shoot rate were high in the germplasm lines Champaisali, Jhagirikartik, Latamahu, Adira-2, AC. 6183, AC. 6023, AC. 6007, AC. 10187, AC. 10162, AC. 7282, AC. 9005 and AC. 20423. Landraces Champaisali, Jhagirikartik and Latamahu showed high value for the traits, RPE, RSR and RRG. Landraces showinghigh values for > 4 parameters identifiedfrom the landraces were Champeisali, AC. 10187, AC. 3663, AC. 44638, AC. 44604, AC. 44646, AC. 44598, AC. 20362, AC. 9038, Kapanthi and Adira-2 (Table 1).
Fig 1. Frequency distribution of germplasm lines for each of the seedling stage physiological parameters for SVI-1, SVI-2, RRG, RPE, RSR and GP estimated from 274rice landraces.
Fig 2. Frequency distribution of germplasm lines for each of the seedling stage physiological parameters for SVI-1, SVI-2, RRG, RPE, RSR and GP estimated from the panel population containing 120rice landraces.
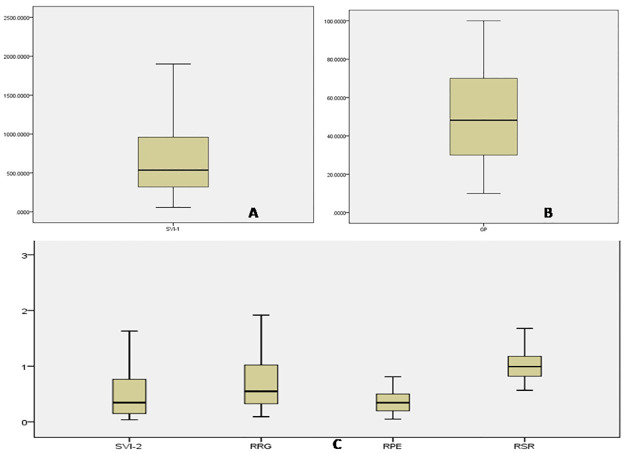
Fig 3. Box plots representing the phenotypic variation in the physiological parameters for (A) Seed vigour index-I (B) Germination %, (C) for Seed vigour index-II, RRG, RPE and RSR in a panel containing 120 rice landraces.
Genotype-by-trait biplot and correlation analyses
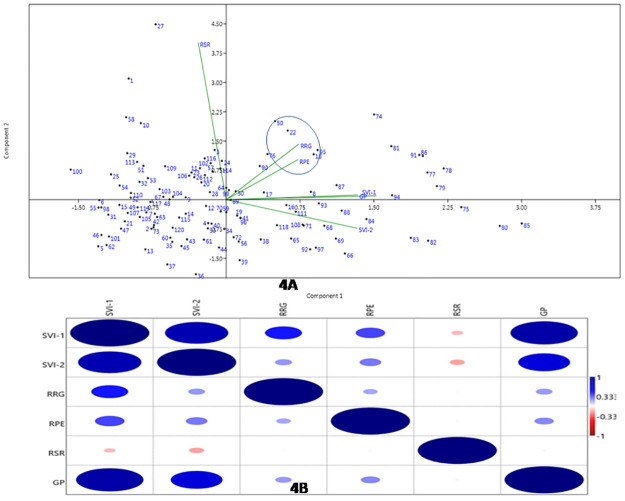
The scatter diagram was plotted by taking the first two principal components to generate genotype-by-trait biplot graph for the 6 physiological traits estimated from the 120 genotypes present in the panel (Fig 4A). The first and second principal components based on the correlation values showed 47.7.651 and 16.9of the total variability with eigen value of 2.86 and 1.02, respectively. RSR contributed maximum diversity among the 6 physiological parameters followed by SVII and SVI for the panel population based on the principal component analysis (Fig 4A). The scattering pattern of genotypes in the 4 quadrants indicated that genotypes containing high seedling stage growth parameters are placed in opposite direction of the quadrant 1 and II. Landraces with higher valuesof the physiological parameters have been encircled in the figure (Fig 4A). The top right (Ist quadrant) and bottom right (2nd quadrant) accommodated majority of the genotypes containing high estimates of the studied physiological parameters. The 3rd (bottom left) quadrant kept most of the moderate value containing landraces while the 4th quadrant (top left) accommodated majority of low value carrying germplasm lines (Fig 4A).
Fig 4. (A) Genotype-by-trait bi-plot diagram showing 120rice landraces in two PCs for 6 physiological traits (B) Heat map showing Pearson’s correlation coefficients for physiological traits. The dot numbers in the diagram depict the serial number of the germplasm line listed in Table 1.
Significant correlations are colored either in red (negative correlation at 0.01 level) or blue hues (positive correlation at 0.01 level).
The association among 6 physiological traits revealed a strong positive correlation (r≥0.7) of SV1 with SVI 2 and GP; SV2 with GP observed a moderate positive correlation (r:0.5–0.7). Weak positive correlation (r < 0.5) was observed for SVI with RRG and RPE; SV 2 with RRG, RPE and GP; RRG with RPE; GP with RRG and RPE. However, weak negative correlation was noticed for SVI II with RSR (Fig 4B).
Genetic diversity parameters analysis
The constituted panel containing 120landraces from the original population of 274 landraces exhibited wide variation for the 6 physiological traits. The landraces were genotyped using 120SSR markers. The gene diversity, loci used for genetic diversity and other diversity related parameters are presented in S3 Table. A total of 544 marker alleles were obtained with average value of 4 alleles per locus. The range of alleles per locus varied from 2 to 7 per marker showing the highest number of alleles by RM493in the studied panel for the 6 physiological parameters. The average value of the major allele frequency of the parameters linked to the polymorphic markers was observed to be 0.561 which varied from 0.279 (RM8044) to0.925 (RM6054) (S3 Table). The range for PIC value was estimated to be from 0.137 (RM6054) to 0.787 (RM493) with mean value of 0.496. The observed average heterozygosity (Ho) in the population was 0.114 which varied from 0.00 to 0.958. The gene diversity (He) in the panel ranged from 0.1415 (RM6054) to 0.8126 (RM493) showing a mean value of 0.5545.
Population genetic structure analysis
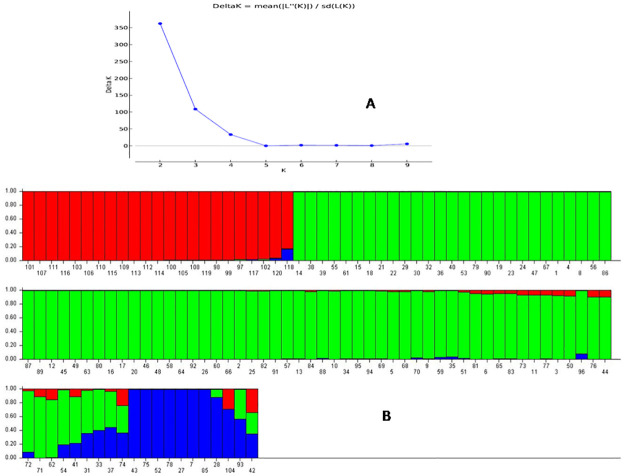
The genotypes in the panel exhibiting variation for the studied seedling stage physiological parameters were evaluated for genetic structure by adopting the probable sub-populations (K) and selecting higher delta K-value estimated by STRUCTURE 2.3.6 software. The delta K value is related to the rate of change in the log probability of data between successive K values. It categorized the genotypes into two sub-populations with a high ΔK peak value of 362.4at K = 2 among the assumed K (S1 Fig). The proportions of genotypes in the inferred clusters were 0.277 and 0.723 in subpopulation 1 and subpopulation 2, respectively. The two subpopulations showed correspondence with the studied physiological parameters but presence of many unrelated landraces observed in the clusters. Hence, the next highest peak at the ΔK peak value of 108.8 was considered and the population was categorized into 3 subpopulations. The proportions of genotypes in the inferred clusters were 0.208, 0.689 and 0.103 for the sub-population 1, 2 and 3, respectively. The fixation index (Fst) values were 0.339, 0.166 and 0.370 for the sub-population 1, 2 and 3, respectively. The expected average distances or heterozygosity between individual in the clusters were 0.382, 0.428, and 0.393 in the sub-population 1, 2, and 3, respectively. The genotypes with ≥80% inferred ancestry value were categorized for that subpopulation (Table 1; Fig 5).
Fig 5. A) Graph of ΔK value, to the rate of change in the log probability of data between successive K values; B) Population structure for the 120 panel population based on membership probability fractions of individual genotypes at K = 3.
The genotypes with the probability of ≥80% membership proportions were assigned as subgroups while others grouped as admixture group. The numbers in the diagram depict the serial number of the germplasm lines listed in Table 1.
The seedling stage physiologicalparameters showed a relatively fair correspondence at K = 3 with the structure subpopulations presentin the panel population. Majority of the landraces showing high to very high seed vigour indices and root growth parameterswere present in the subpopulation SP1. Landraces such as AC44638, AC43663, AC44646, AC44604 and AC44598 present in this subpopulation are potential donor lines for seed vigour indices, germination % and root growth parameters. Majority of the members with poor to moderatein these parameters are found in the subpopulation SP2. Less priority may be given to the members of this subpopulation for use in the improvement of the target traits. The subpopulation 3 accommodated majority of the landraces showing moderate in seed vigour indices and root growth parameters. There is existence of a 4th structure group for the admix landraceswherein majority of the moderate to high parameters carrying landraces are observed. The panel also showed a low alpha value (alpha = 0.0473) by the structure analysis at K = 3. Positively skewed leptokurtic distribution was observed for the mean alpha-value detected from the analysis. The distributions of Fst1, Fst2 and Fst3 subpopulations were symmetrically skewed showing a distinct variation in the distribution among the Fst values (S1 Fig).
Molecular variance (AMOVA) and LD decay plot analysis
The closely related plants in a population are clustered into isolated groups and form various subpopulations. Genetic variations between and within the sub-populations at K = 3 were detected through analysis of molecular variance (AMOVA) (Table 2). The genetic variations obtained between and within at K = 3 was computed to be 18% among the populations, 63% among individuals and 19% variation within individuals of the panel population. Deviation from Hardy-Weinberg’s prediction was calculated from Wright’s F statistics estimates. Different parameters like uniformity of individual within the subpopulation (FIS) and individual within the total population (FIT) were estimated for differentiation of population. The FIT and FIS values of total population and within population based on 136 marker loci were 0.769 and 0.811, whereas FST was 0.181 between the two subpopulations. Fst is estimated to measure the population differentiation or the subpopulations within the total population. The Fst values of each sub-population and their distribution pattern showed a clear differentiation between the 3 sub-populations from each other (S2 Fig).
Table 2. Analysis of molecular variance (AMOVA) of the sub-populations present in the panel population at K = 3 for 6 physiological parameters in 120 rice germplasm lines.
| Sources of variation | AMOVA for the four subpopulations at K = 3 | |||
|---|---|---|---|---|
| df. | Mean sum of squares | Variance components | Percentage variation | |
| Among populations | 3 | 368.485 | 7.569 | 18% |
| Among individuals (accessions) within population | 116 | 60.415 | 26.272 | 63% |
| Within individuals (accessions) | 120 | 7.871 | 7.871 | 19% |
| Total | 239 | 41.712 | 100% | |
| F-Statistics | Value | P-value | ||
| FST | 0.181 | 0.001 | ||
| FIS | 0.769 | 0.001 | ||
| FIT | 0.811 | 0.001 | ||
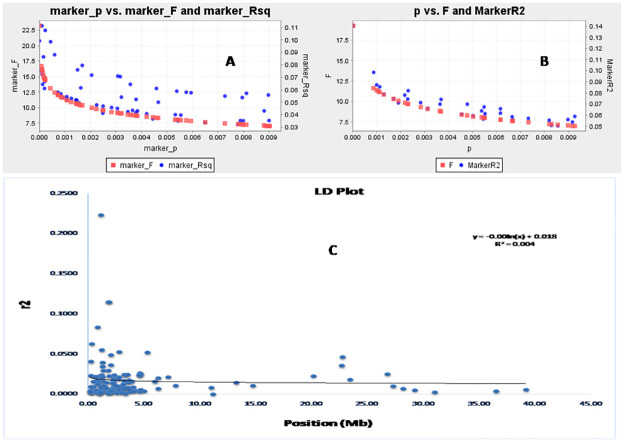
The association of markers alleles at different loci is successfully utilized for marker-trait association study. The LD decay rate is important factor for getting marker–trait association. The decay rate will facilitate the discovery of reliable markers associated with the physiological parameters and will facilitate the discovery of new genes or allelic variants controlling these traits. Syntenicr2 was used to plot the LD decay of the population against the physical distance in million base pair (Fig 6). Tightly linked markers have the highest r2 and average r2 rapidly decreases as linkage distance increases. There was a sharp decline in LD decay for the linked markers at 1–2 mega base pair and thereafter a very slow and gradual decay was noticed. Overall, it is clear that LD decay occur for the studied seedling stage physiological growth parameters. The genotypes with admixture type of ancestry values may be originated due to the LD decay of traits including the studied 6 traits. The trend is also seen in the marker ‘F’ versus marker ‘P’ and marker R2 (Fig 6) curve. The detected markers from this study indicated the strength of the markers for selection of the seedling stage physiological traits in rice.
Fig 6. The marker ‘P’ versus marker ‘F’ and marker R2 detected using (A) GLM approach (B) MLM approach and (C)The physical distance (Mb) between pairs of loci on chromosomes against linkage disequilibrium (LD) decay (r2) curve plotted in rice.
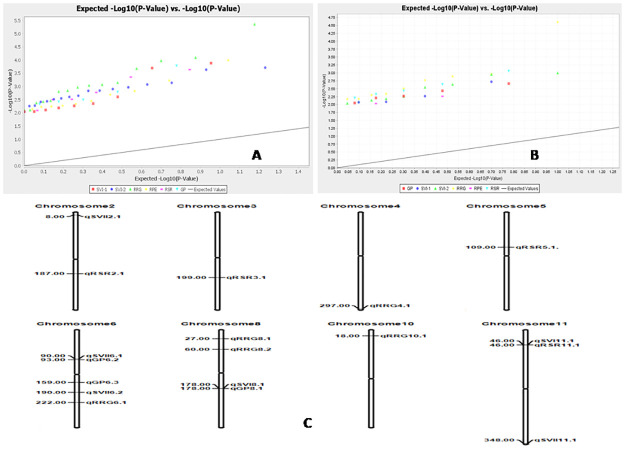
The decay started in million bp estimated by taking 95th percentile of the distribution of r2 for all unlinked loci.
Genetic relatedness among the landracesby principal coordinates and cluster analyses
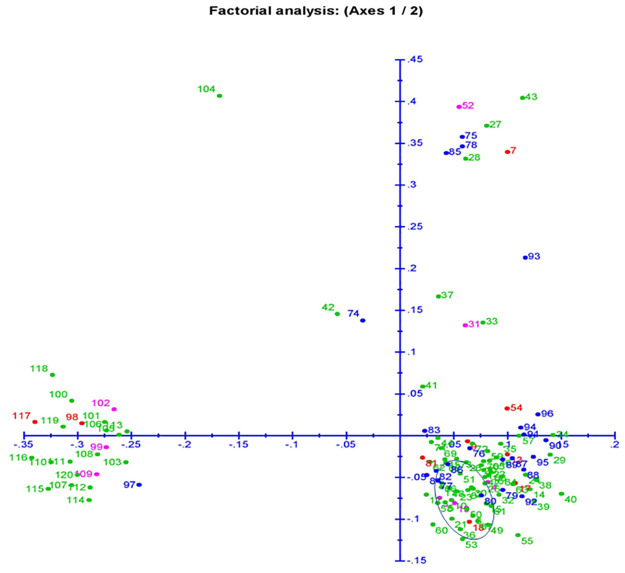
The two dimensions diagram for principal coordinate analysis (PCoA) is drawn based on 136 markers genotyping data which grouped the landracesbased on the genetic relatedness among them (Fig 7). The component 1 accounted for 11.7% inertia and component 2 for 7.49% of total inertia. The panel containing landraceswere placed in various spots on the 4 quadrants which formed twomajor and two minor groups (Fig 7). Majority of the landraces showing higher values for the 6 physiological parameters are present in the quadrant 2 (right bottom). Almost all landraces in the subpopulation 1 and subpopulation 2 were with higher in seedling stage physiological traits present in this quadrant. The genotypes belonging to the 3rd and 4th quadrant showed poor to moderate in the studied seedling stage growth parameters. Majority of the admix type landraces depicted in red color are present in the quadrant 2. The best landraces with higher seedling stage physiological parameters were present in the 2nd quadrantand encircled in the quadrant (Fig 7).
Fig 7. Principal coordinate analysis (PCoA) of 120landraces present in the panel population for 6 physiological traits using 136 molecular markers.
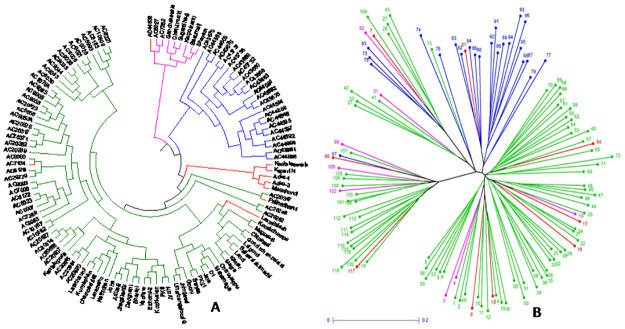
The dot numbers in the figure represent the serial number of the genotypes enlisted in Table 1. The numbers are coloured on the basis of sub-populations obtained from structure analysis (SP1: Blue; SP2: pink; SP3: green and Admix: red).
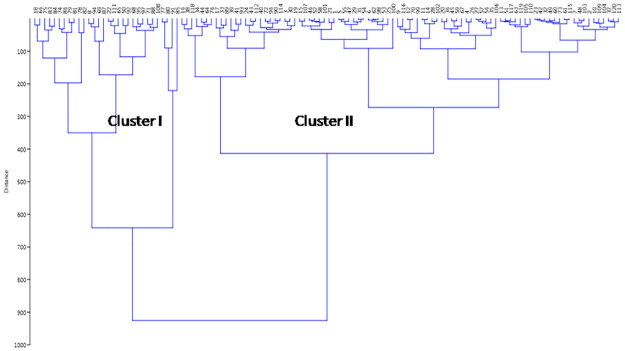
The dendrogram is broadly classified into cluster I and cluster II based on the mean values of studied growth parameters (Fig 8). Cluster II is the biggest cluster which is classified into two sub clusters. Also, the cluster I is divided into two sub-clusters. Each sub-cluster is finally grouped into sub-sub clusters based on the presence of the growth parameter traits in the landraces. Majority of the germplasm lines present in the cluster I showed high or very high estimates of growth parameters in the landraces. Cluster II which accommodated 90 landraces while cluster I showed 30 genotypes. The germplasm lines present in the cluster II of the dendrogram were poor to moderate carrying parameters in it. The sub-clusters of Cluster II are finally grouped into sub-sub clusters based on the value of the growth parameters in the germplasm lines.
Fig 8. Wards’s clustering based on the estimates of 6 physiological traits for clustering of 120 germplasm lines.
The UPGMA tree constructed based on the genotyping results using136 markers for the panel population that classified the germplasm lines into 4 groups including the admix type as in the case of PCoA. The colors of the 4 subpopulations are blue for SP1; green for SP2; pink for Sp3 and red for admix type (Fig 9A). Clusters SP1 was differentiated from SP2 by the presence of high estimates for the majority of the 6 studied physiological parameters. The landraces with poor and moderate in physiological parameters are in structure group 2. The germplasm lines with admix type of population are depicted in red color in the neighbour joining tree (Fig 9A). The phylogenetic tree is alsodrawn using unrooted tree. There is no common ancestor or node in this tree. The variations among the landraces are assessed from the distancefor each landrace depicted in the diagram (Fig 9B). The relationships among the landraces are determined using both the trees without considering the evolutionary time.
Fig 9. Tree constructed based on the genotyping results of 120 landraces using 136 SSR markers for depicting clustering pattern (A) UPGMA Unrooted tree (B) Neighbour-joining tree coloured on the basis of the sub-populations obtained from structure analysis at K = 3 (SP1: Blue; SP2: pink; SP3: green and Admix: red).
Association of marker alleles with the seedling stage physiological parameters in rice
Association of molecular markers with 6 physiological parameters was computed using Mixed Linear Model (MLM/ K+Q model) and Generalized Linear Model (GLM) by TASSEL 5 software. The marker-trait comparisons were subjected to filtration at less than 1% error i.e. 99% confidence (p<0.01). Five parameters showed significant associations with markers using both the models at p<0.01. A total of 39 and 32 significant marker-trait associations were detected by GLM and MLM, respectively at p<0.01 and markers R2 value >0.05. The marker R2 values computed by GLM approach was from 0.05001 to 0.1113 while the R2by MLM approach varied from 0.05042 to 0.14020 (S4 and S5 Tables). Significant marker-trait associations were detected for SVI-I with 2 markers; GPwith 3 markers; SVI-II and RSR with 4 markers;and RRG with 5 markers by both GML and MLM models at p<0.01 and considering markers R2 value at >0.05. Considering the marker r2 value of about 0.10 and above at p<0.01, marker RM223 exhibited associations with the parameter, RRG and RM405 with RSR analyzed by both the models (Table 3; S5 Table). The Q-Q plot also confirmed the association of these markers with the associated seed quality traits in rice (Fig 10).
Table 3. Marker alleles association with seed vigour index, root parameters and germination per cent in ricelandraces present in the panel population detected by both the models of GLM and MLM analyses at p<0.01.
| SL.No | Traits | Marker | QTLs identified | Position (cM) | GLM | MLM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker_F | Marker_p | Marker_R2 | q -value | Marker_F | Marker_p | Marker_R2 | q- value | |||||
| 1 | SVI-1 | RM3701 | qSVI11.1 | 46 | 14.73858 | 2.02E-04 | 0.06101 | 0.0039 | 8.02083 | 0.00545 | 0.05653 | 0.008364 |
| 2 | SVI-1 | RM502 | qSVI8.1 | 178 | 15.66579 | 1.31E-04 | 0.06439 | 0.005657 | 7.21569 | 0.00829 | 0.05085 | 0.007171 |
| 3 | SVI-2 | RM13335 | qSVII2.1 | 8 | 10.66622 | 0.00143 | 0.05185 | 0.001377 | 7.02387 | 0.00917 | 0.05385 | 0.00926 |
| 4 | SVI-2 | RM103 | qSVII6.1 | 90 | 12.04771 | 7.29E-04 | 0.05793 | 0.001377 | 11.38112 | 0.00101 | 0.08726 | 0.008424 |
| 5 | SVI-2 | RM3 | qSVII6.2 | 190 | 10.93544 | 0.00126 | 0.05304 | 0.001377 | 11.14453 | 0.00113 | 0.08545 | 0.00926 |
| 6 | SVI-2 | RM441 | qSVII11.1 | 348 | 14.81491 | 1.95E-04 | 0.06973 | 0.0039 | 7.23687 | 0.0082 | 0.05549 | 0.00926 |
| 7 | RRG | RM222 | qRRG10.1 | 18 | 16.71582 | 8.04E-05 | 0.07491 | 0.003159 | 9.10484 | 0.00313 | 0.06625 | 0.007171 |
| 8 | RRG | RM337 | qRRG8.1 | 27 | 14.65199 | 2.10E-04 | 0.06669 | 0.0039 | 8.37216 | 0.00455 | 0.06092 | 0.007171 |
| 9 | RRG | RM223 | qRRG8.2 | 60 | 23.21457 | 4.42E-06 | 0.09917 | 0.001377 | 19.27957 | 2.51E-05 | 0.14029 | 0.00926 |
| 10 | RRG | RM494 | qRRG6.1 | 222 | 16.10333 | 1.07E-04 | 0.0725 | 0.001377 | 11.23597 | 0.00108 | 0.08176 | 0.008424 |
| 11 | RRG | RM16686 | qRRG4.1 | 297 | 11.58296 | 9.14E-04 | 0.05399 | 0.000287 | 10.85419 | 0.00131 | 0.07898 | 0.000853 |
| 12 | RSR | RM3701 | RSR11.1 | 46 | 9.14305 | 0.00307 | 0.07096 | 0.001377 | 8.27567 | 0.00478 | 0.06994 | 0.007171 |
| 13 | RSR | RM405 | qRSR5.1. | 109 | 14.45053 | 2.31E-04 | 0.10758 | 0.003301 | 11.65994 | 8.81E-04 | 0.09854 | 0.007171 |
| 14 | RSR | RM6641 | qRSR2.1 | 187 | 13.09154 | 4.41E-04 | 0.09849 | 0.0039 | 8.78196 | 0.00369 | 0.07421 | 0.008424 |
| 15 | RSR | RM168 | qRSR3.1 | 199 | 10.34346 | 0.00168 | 0.07951 | 0.005657 | 7.7835 | 0.00617 | 0.06578 | 0.008424 |
| 16 | GP | RM225 | qGP6.2 | 93 | 10.41636 | 0.00162 | 0.06153 | 0.001377 | 8.80688 | 0.00365 | 0.06994 | 0.007171 |
| 17 | GP | RM7179 | qGP6.3 | 159 | 9.05346 | 0.00322 | 0.05406 | 0.002389 | 9.82798 | 0.00218 | 0.07805 | 0.008364 |
| 18 | GP | RM502 | qGP8.1 | 178 | 15.17228 | 1.65E-04 | 0.08637 | 0.0039 | 7.06356 | 0.00898 | 0.0561 | 0.008424 |
Fig 10. Distribution of marker-trait association and quantile–quantile (Q-Q) plot generated from Generalized Linear Model analysis for six antioxidant traits at (A) p < 0.05, (B) at p < 0.01 and (C) the positionsof the QTLs on the chromosomes for RSG, RGR, AGR and MGR detected byassociation mapping inrice.
Two markers showed significant association with SVII detected by GLM and MLM models at p<0.01. Significant associations of markers RM3701 and RM502 with SVI were detected by both the models. Four markers namely RM13335, RM103, RM3 and RM441 located at 8, 90, 190 and 348 cM positions on chromosome 2, 6, 6 and 11, respectively were associated with the parameter, SVII. QTLs for germination % showed significant associations with RM225 on chromosome 6, RM7179 on chromosome 6 and RM502 on chromosome 8. The root-shoot parameter, RSR showed significant associations with markers, RM168, RM225, RM7179 and RM502. Markers viz., RM222, RM337, RM223, RM7179, RM494, RM16686 and RM243 showed significant association with the parameter, RRG. The parameter, RPE was detected to be significantly associated with 9 markers by GLM and by 2 markers in MLM analyses. But, no common marker was detected from the analysis by both the models. However, higher marker r2 and low p-values were shown by the marker RM25181, RM403 and RM309 analyzed by GLM model. Marker RM502 was strongly associated with parameters, SVI and GP. Marker RM7179 significantly associated with GP and RRG and hence both the traits are co-localized and controlled by segment near to 159 cM region on the chromosome 6. In addition, the traits controlled by SVI and RSR also associated with RM3701 (Table 3). The Q-Q plot also confirmed the associations of these markers with the estimated physiological parameters in rice (Fig 10).
Discussion
Seed vigour improvement in rice is an important breeding objective mainly for the direct seeded rice. Genetics of this trait is complex in nature and hence directly and indirectly associated traits including the physiological traits are considered for the association study. Reports on association of markers with physiological traits are very less available in rice. The landraces included in this study were significantly different from each other for the 6 studied seedling stage physiological traits (Table 1). High PCV % and GCV % were estimated for SVI, SVII, RRG, RPE, RSR and GP indicating the usefulness of the landraces for the improvement programs. Higher magnitude of correlation coefficients of the few parameters will be helpful in deciding the traits associated with the seed vigour improvement and hence better for selection of progenies. The existenceof higher molecular diversity parameters in the population and also higher phenotypic variations for these 6 physiological traits indicated clear cut differentiations and classes in the studied population (Figs 5 and 7–9). The landraces used in this association study were collections from the states where existence of rich rice genetic diversity was reported in earlier studies [62–66]. Landraces from Jayapur region of Odisha state, the secondary centre of origin were also used in this experiment. However, the available diversity in the panel was from the collections made from 5 states of India, only. Few landraces viz., Champeisali, AC. 10187, AC. 3663, AC. 44638, AC. 44604, AC. 44646, AC. 44598, AC. 20362, AC. 9038, Kapanthi and Adira-2 showed presence of multiple seedling stage traits identified from the population (Table 1). Thus, inclusion of donor lines from this population will be effective for improvement of seed vigour. Use of diverse lines in many breeding and mapping studies for improvement traits are reported by many researchers in rice [1,67–77]. The panel population is broadly classified into cluster I and cluster II based on the mean values of studied growth parameters. Majority of the germplasm lines present in the cluster I showed presence of high or very high estimates of growth parameters in the landraces. The germplasm lines present in cluster II of the dendrogram were poor to moderate for the 6 studied seedling growth parameters in them. Three genetic structure groups carrying different Fst values were observed to be underlinkage disequilibrium for the studied traits in the population. Presence of many admix type landraces and low alpha value in the population provided clue for evolution of the traits from single source which formed different admix genotypes during the evolution process. Relatedness among the members of a structure subpopulation for the studied traits was proved from this study. The correspondence among the members of a structure group with seed vigour related traits were published by earlier workers [72,78]. Additionally, publications on phenotype of various traits and structure group relatedness have been published by many researchers [39,53,69,72–80].
Five traits for seedling stage physiological parameters were detected to be significantly associated with 18 SSR markers analyzed by both GLM and MLM approaches (Table 3). The markers associatedwith the parameters in this study were detected using both the models at p<0.01, low ‘p’ and higher marker r2 values are considered to be very robust and useful for seed vigour improvement program. Therefore the detected molecular markers namely RM3701 and RM502 with SVI; RM13335, RM103, RM3 and RM441 for SVI-II; RM225, RM7179 and RM502 for GP; RM168, RM225, RM7179 and RM502 for RSR; RM222, RM337, RM223, RM7179, RM494, RM16686 and RM243 with RRG are useful in molecular breeding for improvement of seed vigour trait in rice (Table 3). The Q-Q plot also confirmed the associations of these markers with various seed vigour influencing traits in rice (Fig 10). Molecular markers for seed vigour improvement were reported from many mapping studies [25,72,73,78,81].
Many QTLs controlling germination % in rice were reported by earlier researchers [17–23,26,28,30,31]. In our investigation, the markers RM225, RM7179 and RM502 were significantly associated with germination % and the QTLs were located near 92 cM and 159cM on the chromosome 6 and at 177 cM on chromosome 8, respectively. The two QTLs detected in our investigation were different from the genes reported by the above workers. These two QTLs detected for the trait in this mapping study were not reported in earlier studies and designated as qGP6.2 and qGP6.3. The QTL detected by Jin et al. [31] on chromosome 8 is nearer to the QTL detected by us. Therefore, the QTL reported from earlier study, qGP8.1 may be the detected QTL in our investigation. Anandan et al. [78] detected association of seed vigour index with marker, RM341 on chromosome 2. Diwan et al. [82] reported a QTL for seed vigour index on chromosome 2 within the marker interval RM174-RM145. In our investigation, markers RM13335 located on chromosome 2 showed significant associations with SVII by both the models. This QTL detected by us may be in the marker interval reported by Dewan et al. [82]. The QTL may be designed as qSVII2.1 and validated usingthis mapping population. In addition, Diwan et al. [82] reported two QTLs on chromosome 6 present in the marker interval of RM3-RM162 and RM136-RM3 which controlled the seed vigour. We also detected two QTLs on chromosome 6, one associated with RM3 and other one near to it which are within the above two marker intervals. These QTLs may be designated as qSVII6.1 and qSVII6.2which are validated in this mapping study. However, the QTL for the trait detected by us on chromosome8 near to 178 cM and chromosome 11 near to 46cM position for SV1 along with 348 cM position for SVII on chromosome 11 were not reported in earlier studies. Location ofvigour index reported in mapping studies of Liu et al.[22] and Zhang et al. [23] on the chromosome 11 were at different positions. These 3 QTLs are designated as qSVI8.1, qSVI 11.1 and qSVII 11.1.
The trait relative root growth showed significant association with five markers namely RM222, RM337, RM223 RM494 and RM16686 present on the chromosomes 10, 8, 8, 6, and 4, respectively. According to the earlier mapping study, the QTLs for root length were reported on chromosome 8 [17] and chromosome 6 [15,33,34,35] but at different locations. The QTLs detected in this investigation for regulating RRG were designated as qRRG10.1, qRRG8.1, qRRG8.2, qRRG6.1 and qRRG4.1. The trait, root shoot ratio showed significant association with four markers viz., RM6641, RM168, RM405 and RM3701 present on chromosome 2, 3, 5 and 11, respectively. As per earlier publications of Xu et al., Li et al. and Zhao et al. [25,36,37] the QTLs for the trait werealso reported but at different locations from the results of the present investigation. The detected QTLs were designated as qRSR2.1, qRSR3.1 and qRSR5.1. However, the root length QTL reported by Sabar et al. [38] was located in the genomic region within the marker interval of RM202-RM229. In the present study, marker RM3701 is present within this interval and showed strong association with this trait. As the region reported by Sabar et al. [38] was for root related trait and the present mapping result is for root-shoot ratio, the reported QTL may be same one with the present detected QTL, qRRL11.1.
Conclusion
Estimation of six seedling stage physiological parameters of a population containing 274 landraces showed wide genetic variations among the genotypes for the traits. Presence of linkage disequilibrium (LD) was detected in the panel population based on the fixation indices of the subpopulations. Moderate to high values of gene diversity, polymorphic information content (PIC) and other diversity parameters were estimated from the population by genotyping with 136 SSR markers. The population was classified into 3 genetic groups. The population was classified into subpopulations and each subpopulation showed relatedness for the 6 seedling stage physiological traits among the members in the subgroup. A total of 5 reported QTLs viz., qGP8.1 for germination %; qSVII2.1, qSVII6.1 and qSVII6.2 for seed vigour index II, and qRSR11.1 for root-shoot ratio were validated in this mapping population and will be useful for marker-assisted breeding. In addition, 13 QTLs regulating the physiological parameters such as qSVI 11.1 for seed vigour index I; qSVII 11.1, and qSVI12.1 for seed vigour index II; qRRG10.1, qRRG8.1, qRRG8.2, qRRG6.1 and qRRG4.1 for rate of root growth; qRSR2.1, qRSR3.1 and qRSR5.1 for root-shoot ratio while qGP6.2 and qGP6.3 for germination % were identified using both the models of GLM and MLM analysis. Additionally, co-localization or co-inheritanceof QTLs, qGP8.1 and qSVI8.1 for GP and SVI-1, qGP6.2 and qRRG6.1 for GP and RRG and SVI and qSVI11.1 and qRSR11.1 for SVI and RSR were detected. The QTLs identified in this study will be useful for improvement of seed vigour trait in rice.
Supporting information
The genotypes with the probability of ≥80% membership proportions were assigned as subgroups while others grouped as admixture group. The numbers in the diagram depict the serial number of the germplasm lines listed in Table 1.
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors acknowledge the support of Director, ICAR-National Rice Research Institute, Cuttack for providing all the necessary facilities including the funding for conducting the experiment.
Declarations
Ethics approval and consent to participate. The authors declare that this study complies with the current laws of the country in which the experiments were performed.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
No funding received.
References
- 1.Pandit E, Pawar S, Barik SR, Mohanty SP, Meher J, Pradhan SK. Marker-Assisted Backcross Breeding for Improvement of Submergence Tolerance and Grain Yield in the Popular Rice Variety ‘Maudamani’.Agronomy. 2021; 11(7): 1263. doi: 10.3390/agronomy11071263 [DOI] [Google Scholar]
- 2.Hunter MC, Smith RG, Schipanski ME, Atwood LW, Mortensen DA. Agriculture in 2050: Recalibrating targets for sustainable Intensification. BioScience.2017; 67: 386–391. doi: 10.1093/biosci/bix010 [DOI] [Google Scholar]
- 3.Pradhan SK, Pandit E, Pawar S, Bharati B, Chatopadhyay K, Singh S, et al. Association mapping reveals multiple QTLs for grain protein content in rice useful for biofortification. Mol Genet Genom. 2019a; 294: 963–983. doi: 10.1007/s00438-019-01556-w [DOI] [PubMed] [Google Scholar]
- 4.Chauhan JS, Singh LA, Prasad RS, Pal S. Quality seed: A mega factor in enhancing crop productivity in (ED: Singh, LA) Recent advances in crop physiology. Daya publishing house. Astral International PVT Ltd. New Delhi 2015; 2: 357–366. [Google Scholar]
- 5.Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, et al. Seed germination and vigour. Annual Review Plant Biology. 2012;63: 507–533. [DOI] [PubMed] [Google Scholar]
- 6.Ventura L, Dona M, Macovei A, Carbonera D, Buttafava A, Mondoni A, et al. Understanding the molecular pathways associated with seed vigor.Plant Physiol Biochem. 2012; 60: 196–206. doi: 10.1016/j.plaphy.2012.07.031 [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi M, Winn T. Rice seed vigor and seedling establishment in anaerobic soil. Crop Sci.1996; 36:680–686. [Google Scholar]
- 8.Mahender A, Anandan A, Pradhan SK. Early seedling vigour, an imperative trait for direct seeded rice: an overview on physio-morphological parameters and molecular markers. Planta.2015;241:1027–1050. doi: 10.1007/s00425-015-2273-9 [DOI] [PubMed] [Google Scholar]
- 9.Dingkuhn M, Johnson De, Audeberta Y. Relationship between upland rice canopy characteristics and weed competitiveness. Field Crop Res.1999; 61:71–95. [Google Scholar]
- 10.Rao AN, Johnson DE, Sivaprasad B, Ladha JK, Mortimer AM. Weed management in direct-seeded rice. Adv Agron. 2007;93:153–255. [Google Scholar]
- 11.Finch-Savage WE, Bassel GW. Seed vigour and crop establishment: extending performance beyond adaptation. J Exp Bot.2016; 67:567–591. doi: 10.1093/jxb/erv490 [DOI] [PubMed] [Google Scholar]
- 12.Daniel OI. Biology of Seed Vigor in the Light of omics Tools. In Jimenez- Lopez JC(Ed) Advances in Seed Biology 2017; 6. doi: 10.5772/intechopen.71258 Available from:https://www.intechopen.com/books/advances-in-seed-biology/biology-of-seed-vigor-in-the light-of-omics-tools. [DOI] [Google Scholar]
- 13.Regan KL, Siddique KHM, Turner NC, Whan BR. Potential for increasing early vigour and total biomass in spring wheat. II. Characteristics associated with early vigour. Australian J of Agril Res. 1992;43:541–553. doi: 10.1071/ar9920541 [DOI] [Google Scholar]
- 14.Redona ED, Mackill DJ. Molecular mapping of quantitative trait loci in japonica rice. Genome. 1996;39: 395–403. doi: 10.1139/g96-050 [DOI] [PubMed] [Google Scholar]
- 15.Cui KH, Peng SB, Xing YZ, Xu CG, Yu SB, Zhang Q. Molecular dissection of seedling vigor and associated physiological traits in rice. Theo Appl Genet.2002;105:745–753. doi: 10.1007/s00122-002-0908-2 [DOI] [PubMed] [Google Scholar]
- 16.Miura K, Lin S, Yano M, Nagamine T. Mapping quantitative trait loci controllingseed longevity in rice (Oryzasativa L.). Theor Appl Genet. 2002; 104:981–986. doi: 10.1007/s00122-002-0872-x [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZH,Qu XS, Wan S, Chen LH, Zhu YG. Comparison of QTL controlling seedling vigor under different temperature conditions using recombinant inbred lines in rice (Oryza sativa).Ann Bot. 2005;95(3):423–429. doi: 10.1093/aob/mci039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujino K, Sekiguchi H, Matsuda Y, Sugimoto K, Ono K, Yano M. Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc. of Natl Acad Sci.2008; 105: 12623–12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Wan J, Bao Y, Wang F, Zhang H. Quantitative trait loci analysis for rice seed vigor during the germination stage. J. Zhejiang Univ. Sci. B. Biomed and Biotechnol.2010; 11(12): 958–964. doi: 10.1631/jzus.B1000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang X,Thi TGT, Dong G, Wang H, Edzesi WM, Hong D. Genetic diversity and association mapping of seed vigour in rice (Oryza sativa L.). Planta.2014; 239: 1309–1319. doi: 10.1007/s00425-014-2060-z [DOI] [PubMed] [Google Scholar]
- 21.Xie L, Tan Z, Zhou Y, Xu R, Feng L, Xing Y, et al. Identification and fine mapping of quantitative trait loci for seed vigor in germination and seedling establishment in rice. J Integr Plant Biol. 2010; 56:749–759. [DOI] [PubMed] [Google Scholar]
- 22.Liu LF, Lai YY, Cheng JP, Wang L, Du WL, Wang ZF, et al. Dynamic quantitative trait locus analysis of seed vigor at three maturity stages in rice. PLoS ONE.2014;9: e115732. doi: 10.1371/journal.pone.0115732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Zhong K, Shahid MQ, Tong H. Association analysis in rice: from application to utilization. Front in Plant Sci. 2017; 7:1202. doi: 10.3389/fpls.2016.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Zhao W, Jiang C, Wang X, Xiong H, Todorovska EG, et al. Genetic Architecture and Candidate Genes for Deep-Sowing Tolerance in Rice Revealed by Non-syn GWAS. Front. Plant Sci.2018; 9:332. doi: 10.3389/fpls.2018.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Jiang C, Rehman RMA, Zhang HL, Li J, Li ZC. Genetic analysis of roots and shoots in rice seedling by association mapping. GenesGenom.2019; 41: 95–105. doi: 10.1007/s13258-018-0741-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Liu X, Xie K, Wang Y, Liu F, Lin Q, et al. qLTG-9, a stable quantitative trait locus for low-temperature germination in rice (Oryza sativa L.). Theor Appl Genet. 2013;126: 2313–2322. doi: 10.1007/s00122-013-2137-2 [DOI] [PubMed] [Google Scholar]
- 27.Liu LF, Lai YY, Cheng JP, Wang L, Du WL, Wang ZF, et al. Dynamic quantitative trait locus analysis of seed vigor at three maturity stages in rice.PLoS ONE. 2014;9: e115732. doi: 10.1371/journal.pone.0115732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang J, Xing F, Wang C, Zeng X. Identification and Analysis of Rice Yield-Related Candidate Genes by Walking on the Functional Net work original research article. Front Plant Sci. 2018;20. doi: 10.3389/fpls.2018.01685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Zheng H, Wu W. et al. QTL Mapping and Candidate Gene Analysis for Alkali Tolerance in Japonica Rice at the bud Stage Based on Linkage Mapping and Genome-Wide Association Study. Rice.2020; 48. doi: 10.1186/s12284-020-00412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Vieira FG, Crawford JE, Chu C, Nielsen R. Asian wild rice is a hybrid swarm with extensive gene flow and feralization from domesticated rice.Genome Res.2017; 27(6):1029–1038. doi: 10.1101/gr.204800.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin J. QTL Mapping of Seed Vigor of Backcross Inbred Lines Derived From Oryza longistaminata Under Artificial Aging. Frontiers in Plant Sci.2018; 9: doi: 10.3389/fpls.2018.01909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X, Feng X, Sun D, Liu F, Bao Y, He Y. Rapid and Nondestructive Measurement of Rice Seed Vitality of Different Years Using Near-Infrared Hyperspectral Imaging. Molecules. 2019; 24:2227. doi: 10.3390/molecules24122227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitomi Y, Nakao E, Kawai S, Kanno N, Ando T, Fukuoka S, et al. Fine Mapping of QUICK ROOTING 1 and 2, Quantitative Trait Loci Increasing Root Length in Rice. G3 Bethesda.2018; 8(2):727–735. doi: 10.1534/g3.117.300147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuda S, Sasaki K, Kazama Y, Kisara C, Takeda S, Hanzawa E, et al. Mapping of quantitative trait loci related to primary rice root growth as a response to inoculation with Azospirillum sp. strain B510. Communicative & Integrative Biol. 2018; 11(3): 1–6, doi: 10.1080/19420889.2018.1502586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Zheng H, Wu W. et al. QTL Mapping and Candidate Gene Analysis for Alkali Tolerance in Japonica Rice at the bud Stage Based on Linkage Mapping and Genome-Wide Association Study. Rice. 2020; 13: 48. doi: 10.1186/s12284-020-00412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu CG, Li XQ, Xue Y, Huang YW, Gao J, Xing YZ. Comparison of quantitative trait loci controlling seedling characteristics at two seedling stages using rice recombinant inbred lines. Theor Appl Genet.2004;109: 640–647. doi: 10.1007/s00122-004-1671-3 [DOI] [PubMed] [Google Scholar]
- 37.Li J, Xie Y, Dai A, Liu L, Li Z. Root and shoot traits responses to phosphorus deficiency and QTL analysis at seedling stage using introgression lines of rice. J Genet Genomics.2009; 36: 173–183. doi: 10.1016/S1673-8527(08)60104-6 [DOI] [PubMed] [Google Scholar]
- 38.Sabar M, Shabir G, Shah SM, Aslam K, Naveed SA, Arif M. Identification and mapping of QTLs associated with drought tolerance traitsin rice by a cross between Super Basmati and IR55419-04.Breeding Science.2019; 69: 169–178. doi: 10.1270/jsbbs.18068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandit E, Tasleem S, Barik SR, Mohanty DP, Nayak DK, Mohanty SP, et al. Genome-wide association mapping reveals multiple QTLs governing tolerance response for seedling stage chilling stress in indicarice. Front Plant Sci. 2017; 8:552. doi: 10.3389/fpls.2017.00552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pawar S, Pandit E, Mohanty IC, Saha D, Pradhan SK. Population genetic structure and association mapping for iron toxicity tolerance in rice. Plos One. 2021. doi: 10.1371/journal.pone.0246232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barik SR, Pandit E, Mohanty SP, Nayak DK, Pradhan SK, Mohapatra T. Parental polymorphism survey and phenotyping of recombinant inbred lines for reproductive stage drought tolerance parameters in rice. Oryza.2016;53: 374–384. [Google Scholar]
- 42.Barik SR, Pandit E, Pradhan SK, Mohanty SP, Mohapatra T. Genetic Mapping of morphophysiological traits involved during reproductive stage drought tolerance in rice. Plos One. 2019; 14(12): e0214979. doi: 10.1371/journal.pone.0214979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pradhan SK, Nayak DK, Pandit E, Behera L, Anandan A, Mukherjee AK, et al. Incorporation of bacterial blight resistance genes in to low land rice cultivar through marker-assisted backcross breeding. Phytopathology. 2016a. doi: 10.1094/PHYTO-09-15-0226-R [DOI] [PubMed] [Google Scholar]
- 44.Das S, Pandit E, Guru M, Nayak DK, Tasleem S, Barik SR, et al. Genetic diversity, population structure, marker validation and kinship analysis for seedling stage cold tolerance in indica rice. Oryza.2018; 55(3): 396–405. [Google Scholar]
- 45.Barik SR, Pandit E, Mohanty SP, Nayak DK, Pradhan SK. Genetic mapping of physiological traits associated with terminal stage drought tolerance in rice. BMC Genetics. 2020;21:76. doi: 10.1186/s12863-020-00883-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu J, Pressoir G, Briggs WH, Vroh Bi I, Yamasaki M, Doebley JF, et al. A unified mixed model method for association mapping that accounts for multiple levels of relatedness. Nat Genet. 2006; 38: 203–208. doi: 10.1038/ng1702 [DOI] [PubMed] [Google Scholar]
- 47.Pradhan SK, Pandit E, Pawar S, Naveenkumar R, Barik SR, Mohanty SP, et al. Linkage disequilibrium mapping for grain Fe and Zn enhancing QTLs useful for nutrient dense rice breeding. BMC Plant Bio. 2020; 20:57 doi: 10.1186/s12870-020-2262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowell D and Larinde M. 2006. Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity International, Rome, Italy. [Google Scholar]
- 49.Kleyer M., Bekker R. M., Knevel I. C., Bakker J. P., Thompson K., Sonnenschein M., et al. (2008).The LEDA Traitbase: A database oflife-history traits of the Northwest European flora. Journal of Ecology. 96, 1266–1274. doi: 10.1111/j.1365-2745.2008.01430.x [DOI] [Google Scholar]
- 50.Abdul-Baki AA, Anderson JO. Vigour determination of soybean seed by multiplecriteria.Crop Science.1973; 13: 630 – 633. doi: 10.2135/cropsci1973.0011183X00130006013x [DOI] [Google Scholar]
- 51.IRRI, 2009. CropStat 7.2 for Windows. Crop Research Informatics Laboratory, International Rice Research Institute, Los Banos, Philippines.
- 52.Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980; 8(19):4321–5. doi: 10.1093/nar/8.19.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gel documentation system. Syngene Gel Doc, A Division of Synoptics Group, Cambridge, CB4 1TF, United kingdom.
- 54.Pradhan SK, Nayak DK, Mohanty S, Behera L, Barik SR, Pandit E, et al. Pyramiding of three bacterial blight resistance genes for broad-spectrum resistance in deep water rice variety, Jalmagna. Rice. 2015; doi: 10.1186/s12284-015-0051-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pradhan SK, Pandit E, Pawar S, Baksh SY, Mukherjee AK, Mohanty SP. Development of flash-flood tolerant and durable bacterial blight resistant versions of mega rice variety ‘Swarna’ through marker-assisted backcross breeding. Scientific reports,2019b; 9:12810 doi: 10.1038/s41598-019-49176-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohapatra S, Bastia AK, Meher J, Sanghamitra P, Pradhan SK. Development of submergence tolerant, bacterial blight resistant and high yielding near isogenic lines of popular variety,‘Swarna’through marker-assisted breeding approach. Frontiers in Plant Sci.2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu K, Muse SV. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21(9):2128–9. doi: 10.1093/bioinformatics/bti282 [DOI] [PubMed] [Google Scholar]
- 58.Evanno G., Regnaut S.; Goudet J. Detecting the number of clusters of individuals using the software structure: a simulation study. Molecular Ecology. 2005; 14: 2611–2620 doi: 10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- 59.Earl DA, Vonholdt BM. Structure Harvester: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Res. 2012; 4: 359–361. [Google Scholar]
- 60.Perrier X, Flori A, Bonnot F. Data analysis methods. In: Hamon P, Seguin M, Perrier X, Glaszmann JC, editors. Genetic Diversity of Cultivated Tropical Plants. Montpellier, France: Science Publis.2003; pp. 43–76. [Google Scholar]
- 61.Peakall R.O.D. and Smouse P.E. GENALEX 6.5: genetic analysis in Excel. Population genetic software for teaching and research. Molecular ecology notes, 2006; 6(1), pp.288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pandit E, Panda RK, Sahoo A, Pani DR, Pradhan SK. Genetic Relationship and Structure Analysis of Root Growth Angle for Improvement of Drought Avoidance in Early and Mid-Early Maturing Rice Genotypes. Rice Sci.2020; 27(2): 124–132. [Google Scholar]
- 63.Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diversesamples. Bioinformatics. 2007; 23(19): 2633–5 doi: 10.1093/bioinformatics/btm308 . [DOI] [PubMed] [Google Scholar]
- 64.Patra BC, Dhu SR. Agro-morphological diversity scenario in upland rice germplasm of Jeypore tract. Genet Resour Crop Evol. 2003; 50(8): 825–828. doi: 10.1023/A:1025963411919 [DOI] [Google Scholar]
- 65.Latha M, Abdul Nizar M, Abraham Z, Joseph John K, Asokan Nair R., Mani S, et al. (2013) Rice landraces of Kerala State of India: A documentation International Journal of Biodiversity and Conservation 5(4): 250–263. doi: 10.5897/IJBC12.138 [DOI] [Google Scholar]
- 66.Vanlalsanga SSP, Singh YT. Rice of Northeast India harbor rich genetic diversity as measured by SSR markers and Zn/Fe content. BMC Genet.2019; 20: 79 doi: 10.1186/s12863-019-0780-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh S, Pradhan SK, Singh AK, Singh ON. Marker validation in recombinant inbred lines and random varieties of rice for drought tolerance. Australian Journal of Crop Sci. 2012; 6 (4):606–612. [Google Scholar]
- 68.Pandit E, Sahoo A, Panda RK, Mohanty DP, Pani DR, Anandan A, et al. Survey of rice cultivars and landraces of upland ecology for phosphorous uptake 1(pup1) QTL using linked and gene specific molecular markers. Oryza. 2016; 53(1): 1–9. [Google Scholar]
- 69.Mohapatra S, Pandit E, Barik SR, Patra BC, Pradhan SK. Genetic diversity and population structure in early duration rice genotypes. Oryza.2017; 54(2): 158–168. doi: 10.5958/2249-5266.2017.00021.2 [DOI] [Google Scholar]
- 70.Panda RK, Pandit E, Dash SK, Kar MK, Pradhan SK. Comparison of morpho-physiological traits and root architecture of tolerant and susceptible rice genotypes under both phosphorus and water stressed and normal condition. Oryza. 2017; 54(1): 21–28. [Google Scholar]
- 71.Pandit E, Panda RK, Pani DR, Chandra R, Singh S, Pradhan SK. Molecular marker and phenotypic analyses for low phosphorus stress tolerance in cultivars and landraces of upland rice under irrigated and drought situations.Indian J Genet.2018; 78(1): 59–68. [Google Scholar]
- 72.Pawar S, Pandit E, Arjun P, Wagh M, Bal D, Panda S, et al. Genetic variation and association of molecular markers for Fe toxicity tolerance in rice. Oryza. 2017; 54(4): 356–366. doi: 10.5958/2249-5266.2017.00066.2 [DOI] [Google Scholar]
- 73.Pradhan SK, Pandit E, Nayak DK, Behera L, Mohapatra T. Genes, pathways and transcription factors involved in seedling stage chilling stress tolerance in indica rice through RNA-SeqanalysisBMC Plant Biology.2019c;19:352. doi: 10.1186/s12870-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pradhan SK, Barik SR, Nayak DK, Pradhan A, Pandit E, Nayak P, et al. Genetics, MolecularMechanisms and Deployment of Bacterial Blight Resistance Genes in Rice. Critical Reviews in Plant Sciences. 2020b; 39: 360–385. doi: 10.1080/07352689.2020.1801559 [DOI] [Google Scholar]
- 75.Pradhan SK and Mani SC. Genetic diversity in basmati rice. Oryza 2005; 42 (2), 150. [Google Scholar]
- 76.Bose LK, Pradhan SK, Mohanty A, Nagaraju M. Genetic variability and association of yield attributing characters with grain yield in deepwater rice. Korean Journal of Crop Science 2005;50 (4):262–264. [Google Scholar]
- 77.Shukla V, Singh S, Singh H, Pradhan SK. Multivariate analysis in tropical japonica" New plant type" rice (Oryza sativa L.). Oryza 2006;43 (3), 203–207. [Google Scholar]
- 78.Anandan A, Anumalla M, Pradhan SK, Ali J. Population structure, diversity and trait association analysis in rice (Oryza sativa L.) germplasm for early seedling vigour (ESV) using trait linked SSR markers. PLoS One 2016; 11: 406. doi: 10.1371/journal.pone.0152406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanghamitra P, Nanda N, Barik S, Sahoo S, Pandit E, Bastia R, et al. Genetic structure and molecular markers-trait association for physiological traits related to seed vigour in rice. Plant Gene 2021; 28, doi: 10.1016/j.plgene.2021.100338 [DOI] [Google Scholar]
- 80.Pradhan SK, Barik SR, Sahoo A, Mohapatra S, Nayak DK, Mahender A, et al. Population structure, genetic diversity and molecular marker-trait association analysis for high temperature stress tolerance in rice. PLoS ONE.2016b; 11: 123. doi: 10.1371/journal.pone.0160027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abe A, Kosugi S, Yoshida K, Natsume S, Takagi H, Kanzaki H, et al. Genome sequencing reveals agronomically important loci in rice using MutMap. Nature Biotech.2012; 30: 174–178. doi: 10.1038/nbt.2095 [DOI] [PubMed] [Google Scholar]
- 82.Diwan J, Channbyregowda M, Shenoy V, Salimath P, Bhat R. Molecular mapping of early vigour related QTLs in rice. Res Jour of Biol.2013; 1: 24–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The genotypes with the probability of ≥80% membership proportions were assigned as subgroups while others grouped as admixture group. The numbers in the diagram depict the serial number of the germplasm lines listed in Table 1.
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.