Abstract
This study investigated the druggability, pharmacokinetics and ethyl acetate extract of Teucrium polium (EA T. polium) and the protective effect against carbon tetrachloride (CCl4) induced liver cirrhosis in rats. The total antioxidant capacity (TAC) and scavenging activity of the extract were examined. The in vivo protective study was based on the use of an animal model of CCl4-induced liver cirrhosis. Four groups of rats have been used: Group I: control rats; Group II: received CCl4 in olive oil (0.5 mL/kg); Group III: received the EA T. polium (25 mg/kg) of pretreatment for seven days by gavage then CCl4 in olive oil by gavage for 15 days. Group IV: received the EA of T. polium for seven days (25 mg/kg). EA T. polium was found to possess significant antioxidant capacity. CCl4 caused a hepatotoxicity associated increase in both levels of AST and ALT, which were reduced back to normal values following EA T. polium pretreatment. Hepatotoxicity associated structural modifications of liver tissues and increase in thiobarbituric acid reactive substances (TBARS), conjugated dienes (CD) and carbonyl proteins (CP), associated decreases in several assessed antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT). The in vivo findings on the protective effect of T. polium were supported by its druggability, its pharmacokinetic properties and molecular docking assays. These results confirm the modulatory antioxidant and hepatoprotective potential of T. polium in this experimental liver cirrhosis model. T. polium phytochemicals are good candidates for further pharmaceutical explorations and drug design.
Keywords: liver steatosis, Teucrium polium, CCl4, antioxidant, oxidative injury, pharmacokinetics, histopathology, in silico
1. Introduction
Oxidative stress plays a crucial role in the progression of liver diseases by toxic xenobiotics [1]. Many drugs derived from plants have been applied for the treatment of such pathologies. Among these, T. polium is a plant which belongs to the Labiatae family. Both the structural diversity of natural products and the better understanding of their nutraceutical/biological activities have led to increased interest in their applications in drug discovery and development [2,3,4,5]. Teucrium species are commonly used as alimentary plants, and some of them are currently used in herbal teas and infusions of leaves. This genus is very important in food industries because many species are endowed with antioxidant, antimicrobial and antifungal activities which enhance their use as biological preservatives [6,7,8]. It is one of the richest sources of neoclerodane diterpenes, which possess a key structure in several biological activities. Recent records indicate that more than 220 of their secondary metabolites have been described, and many of them showed considerable medicinal properties and/or ecological interest [7,8,9]. It is also commonly used for antibacterial, antispasmodic, antiulcer, anorexic, and antipyretic agents and has a hepato-protective activity against CCl4 induced hepato-oxidative stress in rats [10,11]. It has been reported that extracts of T. polium are rich in phenolic compounds, which allow them to possess hepato-protection via the inhibition of lipoperoxidation and enhancing the activity of antioxidant enzymes [12]. Recently, using high resolution-liquid chromatography mass spectroscopy (HR-LCMS), it was reported that T. polium dominant compounds include several small peptides associated (1) 13R-hydroxy-9E,11Z-octadecadienoic acid, (2) Rhoifolin, (3) Sericetin diacetate, (4) Selinidin, (5) Valtratum, (6) Triptonide, (7) Koparin 2′-Methyl Ether, (8) Dihydrosamidin, (9) 4, 16alpha, 17beta-Estriol 3-(beta-Dglucuronide), (10) Cepharanthine, (11) Carapin-8 (9)-Ene, (12) 1-dodecanoyl-sn-glycerol [7,8]. In previous works by our team, the protective effect of the aqueous extract of T. polium against CCl4-induced toxicity in rats was assessed. Using ethyl acetate (EA), many more components would be extracted due to the increased polarity and the biological effects might be more efficient. In fact, several studies reported that the use of the plant whole extracts are usually more efficient than the separated compounds.
The current work is devoted to study the protective effect of EA T. polium against hepatotoxicity and oxidative stress induced by carbon tetrachloride in rats. Furthermore, the druggability and pharmacokinetic properties have been evaluated for the major T. polium phytochemicals based on their absorption, distribution, metabolism, excretion and toxicity (ADMET) attributes. Likewise, the molecular interactions of these phytochemicals with two key receptors; cytochrome P450 (CYP) and the stimulator of interferon genes receptors (STING), which are commonly targeted in the treatment of liver diseases, have been explored.
2. Materials and Methods
2.1. Preparation of EA T. polium
The aerial parts of the T. polium specimens were washed with tap water, dried and then crushed with a mortar. An amount of 100 g of the powder was first subjected to a three-day maceration step in 80% ethanol. The obtained hydroalcoholic filtrate underwent evaporation followed by solubilization in water. Later on, the obtained aqueous phase was subjected to a fractionation step using hexane, butanol and ethyl acetate. The fraction obtained was dried with the lyophilizer and then weighed to calculate the yield. Finally, the resulting fractions were evaporated using a vacuum to get the following weights: 0.79 g for hexane, 1.25 g for butanol and 3.2 g for ethyl acetate. The extracts were stored in the dark at 4 °C until they were used for further analyses.
2.2. Total Antioxidant Capacities (TAC) Assay
TAC of EA T. polium was measured following the method described by Prieto et al. [13] using the phosphomolybdenum and compared to the ascorbic acid as a standard. The method included the reduction of Mo (VI) to Mo (V) by the assessed specimen, at acidic pH, via the formation of phosphate/Mo (V): a green complex. TAC levels were expressed as mg of ascorbic acid equivalents (AAEs)/gram of dry extract.
2.3. Scavenging Activity of ABTS Radical Cation
The trolox equivalent antioxidant capacity (TEAC) method, which is based on the reduction of the 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical cation by the specimen’s antioxidants was used to assess the as previously reported by Laporta et al. [14]. Briefly, the method was assessed spectrophotometrically and the absorbance was measured at 734 nm. Methanol was used as the blank and various concentrations of Trolox (0–20 µM) have been used for the calibration curve. Results were expressed as mM concentration of Trolox/g of dry extract.
2.4. In Vivo Antioxidant Properties
2.4.1. Animals Experiments
All the experimental procedure including the animal housing, the way they have been treated, sacrificed… met the international standards (Council of European Communities 2010). This study followed the guidelines for the care and use of laboratory animals at Sciences Faculty of Sfax, University of Sfax (Tunisia), which refers to the National Academies Press (NAP) Guide for the Care and Use of Laboratory Animals (https://www.nap.edu/read/12910/chapter/1 (accessed on 15 October 2021)), and was approved by the local Ethical Committee (12/ES/15-2021).
Male Wistar rats weighing of about 180–200 g were purchased from the Central Pharmacy of Tunisia (SIPHAT, Sfax, Tunisia). Standard housing conditions were used during the experimental procedure: 22 ± 3 °C, 12 h/12 h and 40 ± 2%, for average temperature, light/dark periods and humidity, respectively. The rats were given standard food (SICO, Sfax, Tunisia) and drinking water ad libitum.
2.4.2. Experimental Protocols
For the purposes of this study, 24 rats were divided in four groups of six rats each. Free access was given to both food and water. Dose, duration and route of treatment were selected according to previous published reports in rats and ethno-pharmacological studies [7,8,12].
Group I received drinking water for 15 days and constituted the control group.
Group II received 0.5 mL/kg of CCl4 in olive oil by gavage for 15 days and constituted the CCl4 group.
Group III received a seven-day pretreatment with the EA T. polium (25 mg/kg) then received 0.5 mL/kg of CCl4 in olive oil for 15 days. Both EA T. polium and CCl4 were given by oral gavage. This group constituted the CCl4 + EA T. polium group.
Group IV received the EA of T. polium (25 mg/kg) by gavage for 15 days and constituted the EA of T. polium group.
2.4.3. Samples Collection and Preparation
At the end of the experimental period, all rats were sacrificed by decapitation. Serum samples were collected following blood centrifugation (4000 rpm, 15 min, 4 °C). The organ samples were also collected from each rat and used for biochemical and histological studies.
2.4.4. Serum Parameters
Standard commercial kits referenced as 80127 and 92027 (France) were used to measure the serum activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), respectively. The activity of these enzymes was expressed as IU/L.
2.4.5. Thiobarbituric Acid Reactive Substances (TBARS) Measurement
Measurement of TBARS as estimation of the lipid peroxidation was determined spectrophotometrically according to the procedure of Yagi [15]. The absorbance was read at 530 nm and TBARS levels were expressed as nmol/mg protein.
2.4.6. Determination of Carbonyl Protein Content
Levels of carbonyl protein were recorded using the method of Ardestani et al. [16]. Briefly, 1 mL of 2, 4-dini-trophenylhydrazine (DNPH, 10 mM) in 2M HCl was mixed with 1 mg of liver samples, then incubated at room temperature for 30 min. Cold TCA (1 mL of 10%, w/v) was added and the mixture was centrifuged (3000× g for 10 min). Ethanol/ethyl acetate (2 mL, 1:1, v/v) was used to wash the protein pellet, which was finally dissolved in of guanidine hydrochloride (1 mL, 6 M, pH 2.3). The absorbance was checked at 370 nm and the carbonyl contents were expressed as nmol/mg protein.
2.4.7. Assessment of Conjugated Dienes (CDs)
CDs were assessed as previously described by Slater (1984) [17] based on the use of a chloroform-methanol mixture (2:1) and centrifugation at 1000× g for 5 min. After evaporating the chloroform at 50 °C, the resulted lipid residue was dissolved in methanol (1.5 mL) and the absorbance was recorded at 233 nm.
2.4.8. Superoxide Dismutase (SOD) Assay
The SOD levels were measured as described by Asada et al. [18], which is based on the inhibition of nitro-blue tetrazolium (NBT). Briefly, 1 mL of the liver homogenate was mixed with phosphate buffer (50 mM, pH 7.8), methionine (39 mM), NBT (2.6 mM) and EDTA-Riboflavin (2.7 mM). Twenty minutes after starting the reaction by turning on the light, the changes in the absorbance were followed at 560 nm. One unit of SOD corresponded to the required amount to inhibit 50% of NBT photoreduction. SOD activity is reported as Units/mg of protein.
2.4.9. Glutathione Peroxidase (GPx) Assay
GPx activity was assessed spectrophotometrically as reported by Flohe and Gunzler [19]. The liver supernatant issued by centrifugation was mixed with phosphate buffer (0.1 M pH 7.8) and DTNB (0.4 mg/mL) at 0.2 and 0.7 mL, respectively. The absorbance was recorded at 420 nm. GPx activity was expressed as µmol GSH min−1 mg protein−1.
2.4.10. Catalase (CAT) Assay
CAT was assessed using the method previously described by Aebi [20]. The reaction started by adding H2O2 to the reaction mixture. The decrease in the absorbance, as decomposition of H2O2 was recorded for 1 min, at 420 nm. CAT activity was expressed as µmol H2O2/mg protein.
2.4.11. Protein Measurement
Protein measurement in liver samples was studied using the method of Lowry et al. (1951), in which bovine serum albumin was used as a standard.
2.5. In Silico Molecular Interactions and Pharmacokinetic Assays
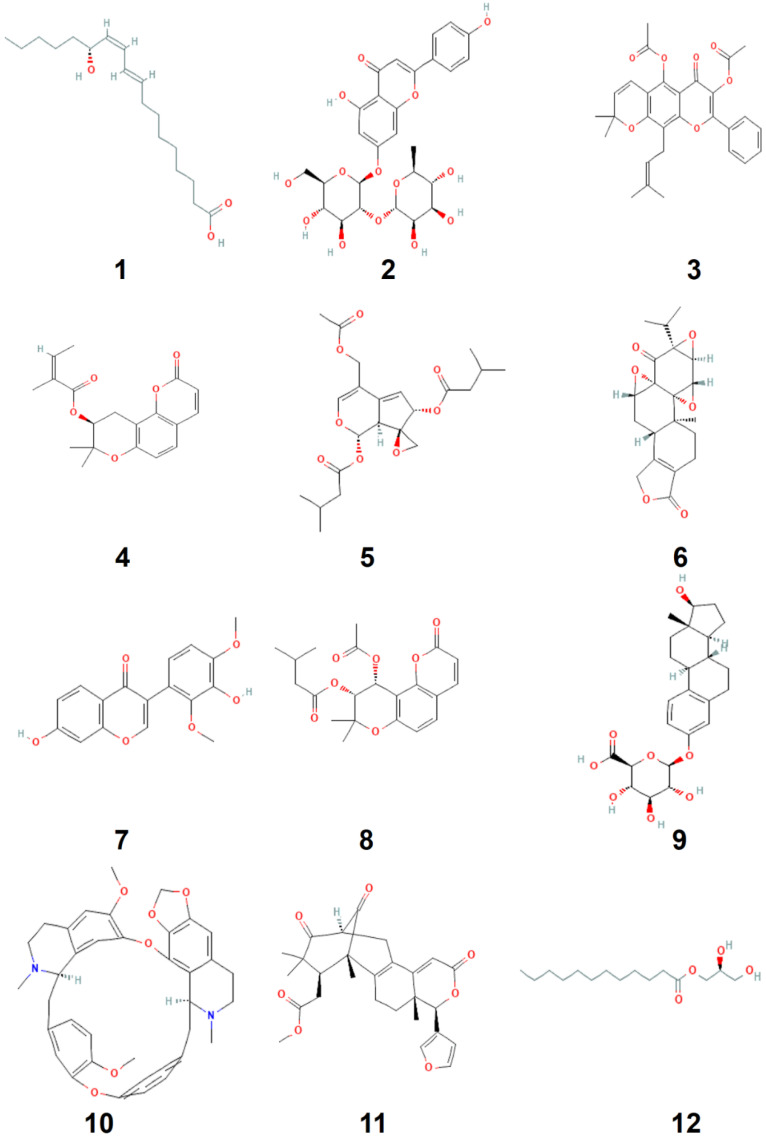
The physicochemical and pharmacokinetic properties of the T. polium phytochemicals, which we had recently determined [7,8], were assessed. ChemDraw Pro 12.0 was used to draw the chemical structures of these phytochemicals (Figure 1). The most pertinent physicochemical parameters were used to assess the draggability and pharmacokinetic properties based on ADMET. These parameters have been assessed in March 2022 using ADMET properties. The tridimensional (3D) structure of CYP and STING receptors (1OG2 and 6DXL respectively), (Doi: 10.2210/pdb1OG2/pdb and 10.2210/pdb6DXL/pdb respectively), and selinidin, (compound no. 4, Accession number: 668079) were obtained from the RCSB PDB and pubchem®, respectively. Both ligand and receptors were processed for minimization and saved in pdbqt format as previously described [21,22,23].
Figure 1.
Chemical structure of the T. polium identified compounds. (1) 13R-hydroxy-9E,11Z-octadecadienoic acid, (2) Rhoifolin, (3) Sericetin diacetate, (4) Selinidin, (5) Valtratum, (6) Triptonide, (7) Koparin 2′-Methyl Ether, (8) Dihydrosamidin, (9) 4, 16alpha, 17beta-Estriol 3-(beta-Dglucuronide), (10) Cepharanthine, (11) Carapin-8 (9)-Ene, (12) 1-dodecanoyl-sn-glycerol.
The binding assessment used in molecular docking and calculation of the binding energy was based on the CHARMm force field using MGL tools and Vina® software (version 4.2.6) as previously described [3,21,24]. The ability of the ligands (major component of T. polium: selinidin) to bind to androgen receptors CYP-LBD and STING-LBD was studied after the removal of heteroatoms (warfarin) and water molecules and adding Coleman charges. Both CYP and STING receptors are involved in xenobiotic biotransformation and the etiology of diverse liver pathology including hepatotoxicity, liver cancer and infectious diseases [25,26].
2.6. Histopathological Analysis
The liver was collected and fixed in 10% formalin and then processed for standard histological preparation. Several sections of 5 µm-thick were prepared and then stained with hematoxylin and eosin (H&E). The histological slides have been examined for histopathological changes under an optic microscope.
2.7. Statistical Analysis
All results were expressed as mean ± standard deviation. To check the significant differences between the different groups, a one-way ANOVA and Tukey’s post hoc test were carried out using the SPSS software package. Differences were considered significant whenever p < 0.05.
3. Results
Extraction with different solvents showed that EA T. polium has a better activity compared with those of two other solvents; hexane and butanol.
3.1. TAC of EA T. polium In Vitro
The TAC of EA T. polium was measured spectrophotometrically using the phosphomolybdenum method. The TAC of EA T. polium extract was 123.85 ± 60 mg AAEs/g of dry extract (Table 1). In this study, the ABTS radical scavenging assay was also used. The principle of the method is based on the ability of an antioxidant to stabilize the ABTS+ by trapping a proton with the antioxidant. A comparison was made with the ability of Trolox to capture ABTS+. Our results showed that T. polium has a significant antioxidant capacity equal to 0.62 mM equivalents Trolox/mg of extract (Table 1). A statistical analysis showed that both TAC and ABTS scavenging activity of EA T. polium were much better than hexane and butanol extract.
Table 1.
Antioxidant activity of EA T. polium by TAC and ABTS tests.
| Parameters/Fractions | Hexane | Butanol | Ethyl Acetate |
|---|---|---|---|
| TAC (EAAs/g extract) | 60.21 ± 33.02 | 92.11 ± 36.33 | 123.85 ± 60 * |
| ABTS (mM Equivalents Trolox/mg extract) | 0.11 ± 0.02 | 0.23 ± 0.06 * | 0.62 ± 0.14 ***### |
Values are mean ± SD of three distinct measurements. * p < 0.5 vs. Hexane. *** p < 0.001 vs. Hexane. ### p < 0.5 vs. Butanol.
3.2. Serum Biochemical Parameters
The activities of AST and ALT, in the different studied groups, are shown in Table 2. Both AST and ALT activities increased significantly in the CCl4 group once compared to control. Pretreatment with EA T. polium decreased the activity of the above enzymes with statistically significant levels. Animals given EA T. polium showed no disrupted levels of ALT and AST as compared to the control group.
Table 2.
Effects of CCl4, EA of T. polium (25 mg/kg) and their combination on serum ALT and AST activity (IU/L).
| Enzymes (IU/L) | Control | EA T. polium | CCl4 | CCl4 + EA T. polium |
|---|---|---|---|---|
| ALT | 1738 ± 44.3 ## | 1768 ± 73.74 # | 1913 ± 37.08 **## | 1805 ± 29.39 |
| AST | 137.7 ± 16.47 | 139.8 ± 5.48 | 575.5 ± 58.72 ## | 523.5 ± 52.42 ## |
Values are mean ± SD for six determinations. ** p < 0.01 vs. Control. # p < 0.5 vs. CCl4. ## p < 0.01 vs. CCl4.
3.3. Oxidative/Antioxidative Status
The administration of CCl4 significantly elevated the TBARS level, an indicator of lipid peroxidation, while the pretreatment with EA T. polium resulted in the reduction of the serum activity of liver marker enzymes when compared to CCl4 intoxicated rats. This hepatoprotective activity may be due to the anti-oxidant activity of EA T. polium. The effects of CCl4, EA of T.polium and their combination on serum conjugated dienes (CD) and carbonyl protein (CP) in the different groups are shown in Table 3. As compared to controls, the levels of these parameters were significantly increased in CCl4 treated rats. The pretreatment with EA T. polium lowered the level of these parameters. No difference was detected between the group pretreated with EA T. polium and control.
Table 3.
Thiobarbituric acid reactive substances (TBARS), carbonyl protein (CP) and conjugated diene (CD) levels of control and treated rats with CCl4, EA of T. polium (25 mg/kg) and their combinations (CCl4 + EA T. polium).
| Treatment/Parameters | Control | EA T. polium | CCl4 | CCl4 + EA T. polium |
|---|---|---|---|---|
| TBARS (µmol/mg protein) | 5.96 ± 3.09 | 5.90 ± 2.97 | 10.2 ± 3.53 ** | 8.2 ± 2.80 **## |
| Carbonyl protein (nmol/mg protein) | 0.69 ± 0.023 | 0.69 ± 0.007 | 1.38 ± 0.013 *** | 1.296 ± 0.004 |
| Conjugated dienes (nmol/mg protein) | 9 ± 0.02 | 9.45 ± 0.14 | 13.21 ± 0.04 ** | 11.11 ± 0.37 # |
Values are mean ± SD for six determinations. ** p ≤ 0.01 and *** p ≤ 0.001 vs. Control group. #p< 0.05 vs. CCl4 group. ## p< 0.01 vs. CCl4 group.
The lipid peroxidation was associated with a concomitant decline in the activity of antioxidant enzymes such as SOD, GPx and CAT (enzymes involved in the defense against oxidative stress) respectively in the CCl4 treated rats compared to the control rats. However, pretreatment with EA T. polium significantly restored the activity of antioxidant enzymes in CCl4 treated rats (Table 4). The administration of EA T. polium was also able to significantly restore the enzyme level compared to CCl4 alone.
Table 4.
Effects of CCl4, EA T. polium (25 mg/kg) and their combination (CCl4 + EA T. polium) on the SOD, GPx and CAT enzymatic activities in hepatic tissues of different studied groups.
| Parameter/Treatment | Control | EA T. polium | CCl4 | CCl4 + EA T. polium |
|---|---|---|---|---|
| SOD (Units/mg protein) | 1.62 ± 0.19 | 1.65 ± 0.27 | 0.44 ± 0.09 *** | 0.51 ± 0.01 |
| GPx (µmol GSH min−1 mg protein−1) | 0.91 ± 0.014 | 0.92 ± 2.5 | 0.49 ± 0.01 *** | 0.93 ± 0.01 # |
| CAT (µmol H2O2/mg protein) | 174.02 ± 44.3 | 176.32 ± 22.18 | 85.9 ± 6.25 *** | 149.4 ± 44.29 ## |
Values are mean ± SD for six rats in each group. *** p ≤ 0.001 vs. Control group. # p< 0.05, ## p< 0.01 vs. CCl4 group.
3.4. Druggability and Pharmacokinetic Properties of EA T. polium Main Compounds
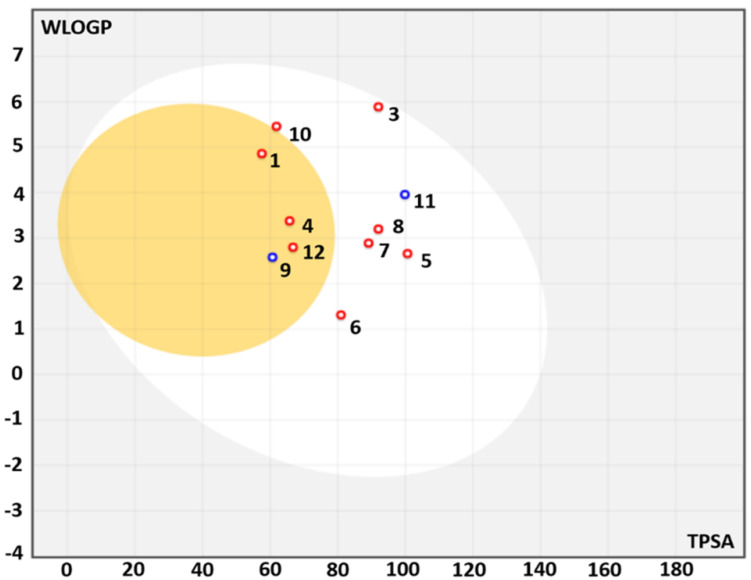
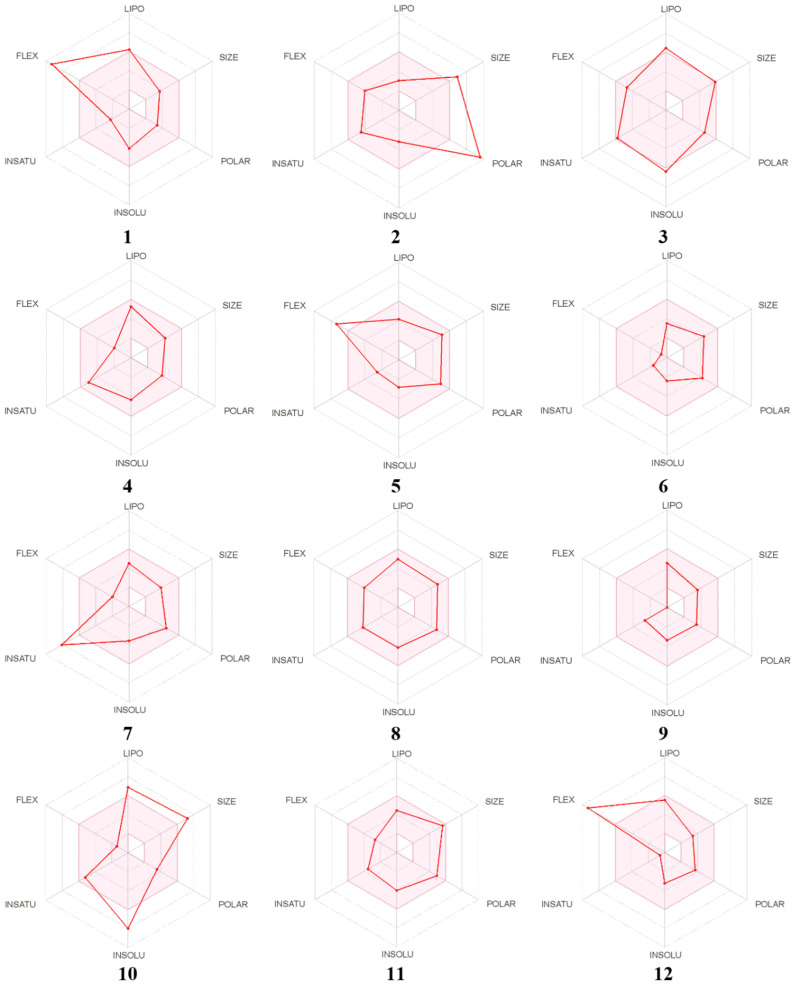
The previous T. polium identified compounds were studied to assess their druggability and pharmacokinetic properties [7,8]. Interestingly, the studied compounds displayed nil alert. Furthermore, regardless of the compound no. 2 (rhoifolin), which stands out of the boiled egg model (Figure 2), all the other phytochemical compounds had acceptable consensus Log Po/w, obeyed to the Lipinski’s rule and have good gastro-intestinal absorption, lipophilicity and bioavailability score (0.55–0.58). This permit these compounds to be placed in the pink zone of the bioavailability polygons (Figure 3). Moreover, all the assessed T. polium compounds are not suitable for transdermal delivery. All the T. polium identified phytochemicals displayed negative skin permeability ranging from −0.55 to −9.94 cm/s (Table 5), which indicates low to moderate skin permeability. These results might support the selected route of T. polium administration in our study (gavage) and their potential biological activities: antioxidant and hepato-protective effects. Selinidin, which possessed good pharmacokinetic properties and oral bioavailability, have been further assessed for potential hepato-protective effect using in silico analyses.
Figure 2.
Boiled-egg model of the T. polium studied compounds using ADMET properties. (1) 13R-hydroxy-9E,11Z-octadecadienoic acid, (2) Rhoifolin, (3) Sericetin diacetate, (4) Selinidin, (5) Valtratum, (6) Triptonide, (7) Koparin 2′-Methyl Ether, (8) Dihydrosa-midin, (9) 4, 16alpha, 17beta-Estriol 3-(beta-Dglucuronide), (10) Cepharanthine, (11) Carapin-8 (9)-Ene, (12) 1-dodecanoyl-sn-glycerol.
Figure 3.
Bioavailability polygons of T. polium identified compounds based on their physicochemical parameters using ADMET properties. (1) 13R-hydroxy-9E,11Z-octadecadienoic acid, (2) Rhoifolin, (3) Sericetin diacetate, (4) Selinidin, (5) Valtratum, (6) Triptonide, (7) Koparin 2′-Methyl Ether, (8) Dihydrosa-midin, (9) 4, 16alpha, 17beta-Estriol 3-(beta-Dglucuronide), (10) Cepharanthine, (11) Carapin-8 (9)-Ene, (12) 1-dodecanoyl-sn-glycerol.LIPO: Lipophilicity, SIZE: Molecular size, POLAR: Polarity, INSOLU: Insolubility, INSATU: Insaturation, FLEX: Flexibility, The pink area represents the most suitable physicochemical zone for oral bioavailability. Note that some compounds, including selinidin, stand in the pink area, which is the most suitable for oral bioavailability.
Table 5.
Draggability and pharmacokinetic properties of T. polium main compounds based on the ADMET properties. (1) 13R-hydroxy-9E,11Z-octadecadienoic acid, (2) Rhoifolin, (3) Sericetin diacetate, (4) Selinidin, (5) Valtratum, (6) Triptonide, (7) Koparin 2′-Methyl Ether, (8) Dihydrosa-midin, (9) 4, 16alpha, 17beta-Estriol 3-(beta-Dglucuronide), (10) Cepharanthine, (11) Carapin-8 (9)-Ene, (12) 1-dodecanoyl-sn-glycerol.
| Entry | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Druglikeness/Medicinal Chemistry | ||||||||||||
| Consensus Log Po/w | 4.52 | −0.81 | 5.19 | 3.40 | 2.78 | 1.93 | 2.27 | 3.19 | 2.43 | 5.36 | 3.27 | 3.22 |
| Lipinski’s Rule | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability Score | 0.85 | 0.17 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| PAINS (alerts) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Synthetic accessibility | 4.01 | 6.33 | 4.74 | 4.08 | 6.17 | 5.96 | 3.20 | 4.58 | 3.74 | 7.01 | 6.36 | 3.42 |
| Pharmacokinetics | ||||||||||||
| GI absorption | High | Low | High | High | High | High | High | High | High | High | High | High |
| BBB permeant | Yes | No | No | No | No | No | No | No | Yes | No | No | Yes |
| P-gp substrate | No | Yes | No | No | No | No | No | No | Yes | No | Yes | No |
| CYP1A2 inhibitor | Yes | No | No | No | No | No | Yes | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | Yes | No | No | No | No | Yes | No | No | No | No |
| CYP2C9 inhibitor | Yes | No | Yes | No | No | No | Yes | Yes | No | No | No | No |
| CYP2D6 inhibitor | Yes | No | No | No | Yes | No | Yes | No | Yes | No | No | Yes |
| CYP3A4 inhibitor | No | No | No | No | Yes | No | Yes | Yes | No | No | No | No |
| Log Kp (cm/s) | −4.31 | −9.94 | −5.2 | −8.87 | −7.63 | −8.02 | −0.55 | −6.38 | −6.32 | −5.36 | −7.53 | −5.02 |
3.5. In Silico Molecular Interactions
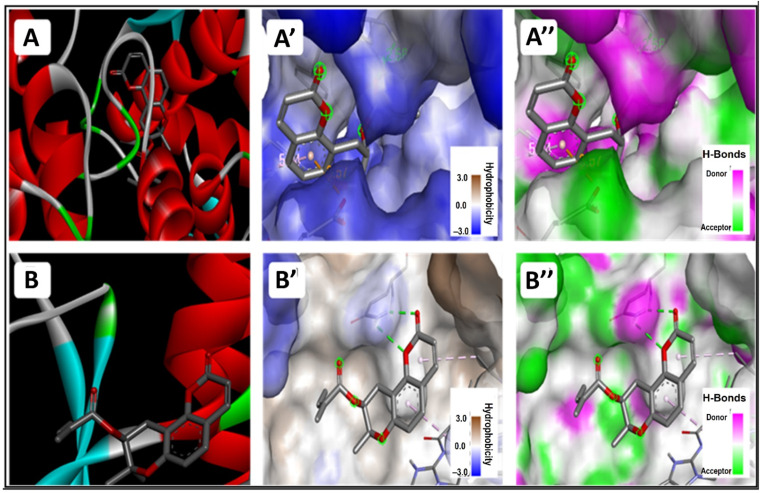
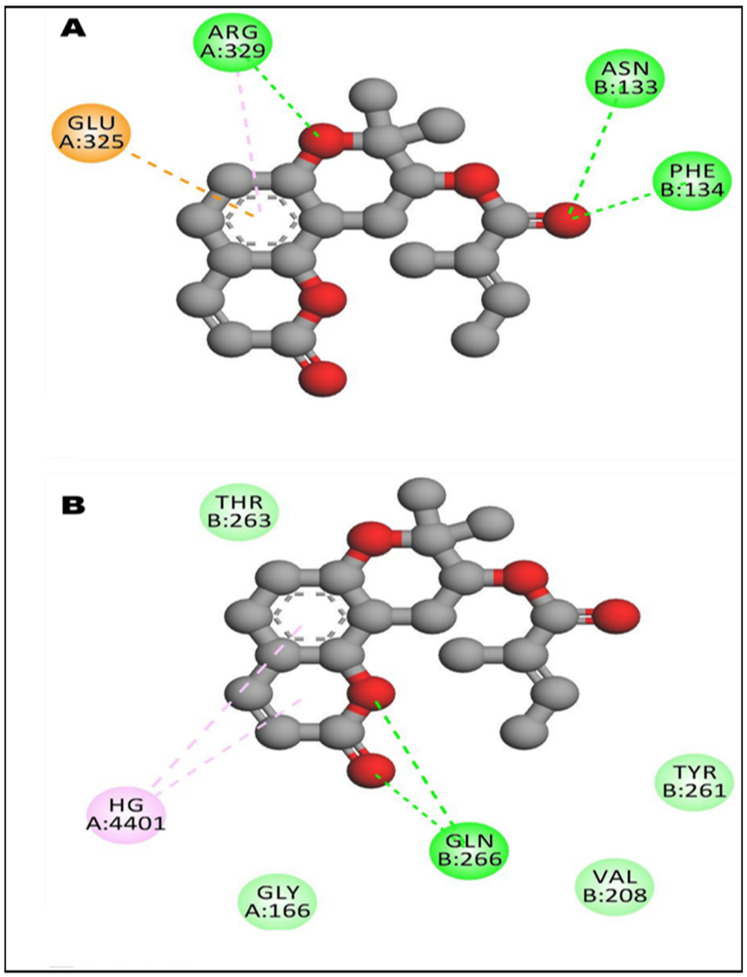
Selinidin established three H-bonds with CYP via both its heterocyclic ring (with Arg329) and its branched chain (with Asn133 and Phe134). With STING receptor, the Gln266 established a couple of H-bonds with the first heterocyclic ring (Figure 4 and Figure 5). Furthermore, selinidin was found to be strongly embedded particularly with the pocket region of the CYP receptor. It showed a network of conventional H-bonds, electrostatic and hydrophobic bonds.
Figure 4.
Photograph of the 3D structure of selinidin bounded to the ribbon structure of cytochrome P450 (top: A) and stimulator of interferon genes (STING, bottom: B) receptors. 3D interaction’s illustrations of the hydrophobicity (A’ and B’ respectively) and conventional hydrogen bond (A’’ and B’’ respectively).
Figure 5.
Illustration of the 2D diagrams of interactions with the pocket region of CYP (A) and STING (B) receptors, respectively.
3.6. Histopathological Study
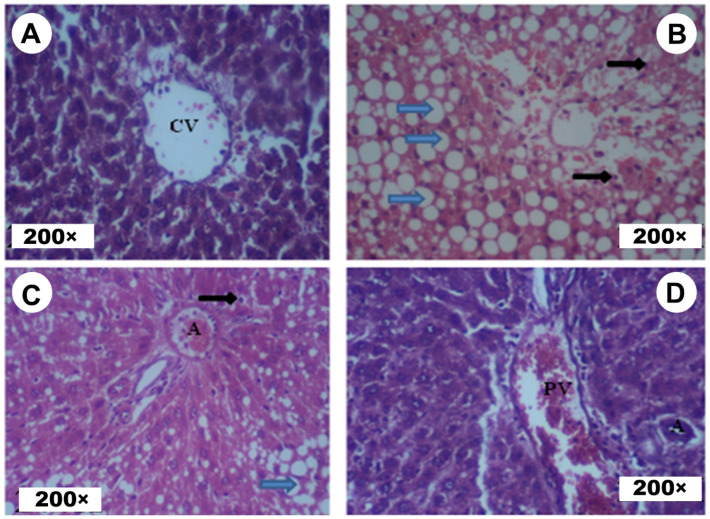
Histological study performed on the liver showed healthy liver parenchyma (normal liver cells, centrilobular and sinusoidal veins and normal hepatocyte trabeculae) in the control rats. Moreover, there was a severe alteration of the architecture with the presence of inflammatory aspects, leukocyte infiltration and cytoplasmic vacuolization of the hepatocytes and hepatic necrosis in CCl4 treated rats. These modifications were less pronounced in CCl4 + EA T. polium treated rats. This study shows that there was conservation of the usual liver structure in rats treated with EA T. polium in comparison to controls (Figure 6). These results parallel both biochemical and oxidative/antioxidative status findings.
Figure 6.
Histological study of the liver: (A) = Control group; (B) = CCl4 group; (C) = CCl4 + EA T. polium group and (D) = EA T. polium group. CV: Centrilobular Vein; Blue arrows: Hydropic Degenerations; Black arrows: Hepatic Necrosis; A: Arteriole; PV: Portal Vein.
4. Discussion
Antioxidants from medicinal herbs may play an important protective role against oxidative injury and maintain the healthy physiological function of cells. T. polium was reported to possess a potent scavenging activity against the reactive oxygen species [7,8,11,27]. Hence, the obtained results data revealed that the EA fraction of the studied plant possesses potent anti-oxidant properties. The results of our study supported those of Movahedi et al. [28] who demonstrated that the EA extract of T. polium possessed the highest antioxidant activity by DPPH and FRAP tests, and by total phenolic and flavonoid contents. Our results are also in agreement with previous research activities which revealed that T. polium possessed an antioxidant activity by DPPH method and the IC50 values were about 90 μg/mL [7,8,29]. These results parallel both the biochemical and oxidative/antioxidative findings.
AST and ALT enzymatic activities associated carbohydrate and amino acid metabolisms. The increased activity of these enzymes reflects liver injury and the inflammation of hepatic parenchyma [1]. Our results revealed that that of CCl4 induced significant increases in both AST and ALT activities, which corroborate the findings of previous studies that also reported the interest of phytotherapy in reducing the increases of these parameters comparatively with CCl4 treated rats [30].
Our results supported those of Rahmouni et al. [31], who showed that subchronic exposure to CCl4 increased TBARS levels and decreased antioxidant enzymes. Our studies also supported those of Sun et al. [32], who reported new insights into the acute liver pathogenesis via CCl4 and some potential novel strategies for its treatment. Similarly, Bellassoued et al. (2018) evaluated the protective effect of Mentha piperita L. (which also belongs to Lamiacee family), and reported potential benefits mainly due to menthol and its derivatives.
A recent phytochemical screening study using HR-LCMS revealed that the T. polium composition included several beneficial compounds and small peptides [7,8]. The phytochemical profiling of the plant was associated with promising bioactivities such as antioxidant, anticancer, antibacterial and antiviral effects [7,8]. In this study, these compounds were assessed for their draggability and pharmacokinetics based on their ADMET characteristics, as previously described [5,22,33]. The assessment of such parameters was reported to be important to avoid drug failure in the advanced steps of clinical applications [23,24]. The majority of the compounds obeyed the Lipinski’s rule and all of them were associated with zero alerts as they meet the Lipinski rule of five. Such results indicate the safe use of the T. polium phytochemicals and/or extracts as reported in previous studies, [7,8] and supported its ethno-pharmacological applications [8,31]. The partition coefficient between octanol and water, as an indicator of lipophilicity (Log Po/w), also indicated that all the assessed compounds, except no. 2, possessed acceptable lipophilicity, as Log Po/w varied between 1.93 and 5.36. Previous reports indicated that Log Po/w < 5 is usually associated with good lipophilicity [5,21,22,33]. Their bioavailability scores prove their potential biological activities and the possibility of oral administration. This was further confirmed by the bioavailability polygons (Figure 3). Regardless of compound No. 2, all T. polium studied phytochemicals possessed high gastro-intestinal absorption (GI). Compounds No. 1, 9 and 12 were categorized as blood-brain-barrier (BBB) permeant. The majority of T. polium identified compounds were predicted not to be a substrate of P-glycoprotein (P-gp), which indicates the absence of significant disruption of drug transport [21,22]. Compounds No. 2, 4, 6, 10 and 11 inhibited none of the targeted CYP isoforms CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. None of the remaining compounds was predicted to be a potent inhibitor of the CYP studied isoforms, thus, indicating the lack of disrupted metabolism and excretion [21,23]. Log Kp varied between –0.55 and –9.94, which indicated moderate to low skin permeability. Taken together, T. polium sounds containing suitable drug-like molecules with good synthetic scores that might have promising systemic effects, including alleviation of hepatic disorders.
CYP and STING receptors have been selected, as they are commonly associated with both liver pathogenesis and are targeted in drug design and development [34,35]. The molecular interactions of T. polium’s major compounds with these key receptors, which are targeted in liver therapy, were studied. Both CYP and STING are considered key areas of focus for liver diseases, and thus constituted excellent targets for liver medication [36,37].
The selinidin heterocyclic ring and branched chain were predicted to establish three H-bonds with CYP residues: Arg329, Asn133 and Phe134. With STING receptor, the Gln266 established a couple of H-bonds with the first selinidin heterocyclic ring. Moreover, selinidin was found to be strongly embedded, particularly with the pocket region of the CYP receptor. In fact, the distance to the closest residues was 1.811 Å for CYP and 2.461 Å for STING receptors. These results prove that a hepatoprotective effect of the studied extract is possible. The in silico and molecular docking results of the current study mirror the pharmacokinetic, biochemical and histological findings. Furthermore, selinidin associated with the other phytochemical compounds of T. polium may possess a synergistic effect. These molecular interactions may justify the in vivo findings in rats, specifically the hepato-protective potential of the T. polium extract via its rich phytochemical profile. It was previously suggested that the use of the cure plant extracts normally has better effects than the plant phytochemical components [3,24,38,39]. In this study, the whole extract was used, and further analyses of molecular dynamics may support our results.
Similar results were obtained by Sakr et al. in rats intoxicated by CCl4 [40]. In fact, the liver is a major metabolizing organ for which the biochemical markers are commonly disturbed by toxic products including CCl4 [1,41,42]. The current results parallel those of Sreelatha and Padma [43] and Seghatoleslam et al. [44], who reported liver damage associated severe pathological/degenerative features following subchronic exposure to CCl4. This study proved the conservation of the usual liver structure in rats treated with EA T. polium in comparison to controls.
5. Conclusions
Our study revealed that T. polium displayed an efficient antioxidant activity using both in vitro and in vivo tests such as ABTS scavenging, TAC, TBARS, SOD... EA T. polium was proven to be effective in preventing CCl4 induced hepatic injury in rats by restoring both the levels of liver biochemical markers (AST and ALT) and oxidative/antioxidative status, which resulted in correction of liver histological morphology. Druggability, pharmacokinetics and molecular docking assays support the in vitro and in vivo approaches. In fact, the in silico results suggest the satisfactory draggability and the promising properties of the T. polium phytochemicals, particularly selinidin. The therapeutic potential may include CYP and STING receptors as assessed by docking and molecular interactions. Further investigations regarding the molecular dynamics of T. polium components with several receptors are needed for a better understanding of the pathway and the action mechanism to encourage the use of the plant extract against liver injury. In fact, its beneficial effects are promising and encouraging for its use in pharmaceutical processes.
Acknowledgments
The authors want to acknowledge the funder (Scientific Research Deanship at University of Ha’il—Saudi Arabia through project number “RD-21 042”) for the financial support.
Author Contributions
Conceptualization, F.R., R.B. and T.R.; Methodology, F.R., H.B.-N. and R.B.; Experimental analysis, F.R. and R.B.; Validation, F.B., A.J.S., S.E., M.S. (Mongi Saoudi) and M.S. (Mohd Saeed); Formal analysis, R.B. and M.S. (Mejdi Snoussi); Investigation, H.B.-N., A.J.S. and R.B., Resources, R.B. and T.R., Software, F.R. and R.B.; Writing–original draft preparation, F.R. and R.B.; Writing–review and editing, F.R., R.B. and T.R.; Supervision, R.B. and T.R.; Project administration, R.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data of the current study are included in this article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This research has been funded by Scientific Research Deanship at University of Ha’il (UoH)—Saudi Arabia through project number “RD-21 042”.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Badraoui R., Ben-Nasr H., Bardakçi F., Rebai T. Pathophysiological impacts of exposure to an endocrine disruptor (tetradifon) on α–amylase and lipase activities associated metabolic disorders. Pestic Biochem. Physiol. 2020;167:104606. doi: 10.1016/j.pestbp.2020.104606. [DOI] [PubMed] [Google Scholar]
- 2.Mzid M., Badraoui R., Ben Khedir S., Sahnoun Z., Rebai T. Protective effect of ethanolic extract of Urtica urens L. against the toxicity of imidacloprid on bone remodeling in rats and antioxidant activities. Biomed. Pharmacother. 2017;9:1022–1041. doi: 10.1016/j.biopha.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Akacha A., Badraoui R., Rebai T., Zourgui L. Effect of Opuntia ficus indica extract on Methotrexate-induced testicular injury: A biochemical, docking and histological study. J. Biomol. Str. Dyn. 2022;40:4341–4351. doi: 10.1080/07391102.2020.1856187. [DOI] [PubMed] [Google Scholar]
- 4.Saoudi M., Badraoui R., Rahmouni F., Jamoussi K., El Feki A. Antioxidant and protective effects of Artemisia campestris essential oil against chlorpyrifos-induced kidney and liver injuries in rats. Front. Physiol. 2021;12:194. doi: 10.3389/fphys.2021.618582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zammel N., Jedli O., Rebai T., Hamadou W.S., Elkahoui S., Jamal A., Alam J.M., Adnan M., Siddiqui A.J., Alreshidi M.M., et al. Kidney injury and oxidative damage alleviation by Zingiber officinale: Pharmacokinetics and protective approach in a combined murine model of osteoporosis. 3 Biotech. 2022;12:1–16. doi: 10.1007/s13205-022-03170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahramikia S., Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae) Phytother. Res. 2012;26:1581–1593. doi: 10.1002/ptr.4617. [DOI] [PubMed] [Google Scholar]
- 7.Alreshidi M., Noumi E., Bouslama L., Ceylan O., Veettil V.N., Adnan M., Danciu C., Elkahoui S., Badraoui R., Al-Motair K.A., et al. Phytochemical Screening, Antibacterial, Antifungal, Antiviral, Cytotoxic, and Anti-Quorum-Sensing Properties of Teucrium polium L. Aerial Parts Methan Extract. Plants. 2020;9:1418. doi: 10.3390/plants9111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noumi E., Snoussi M., Anouar E.H., Alreshidi M., Veettil V.N., Elkahoui S., Adnan M., Patel M., Kadri A., Aouadi L., et al. HR-LCMS-Based Metabolite Profiling, Antioxidant, and Anticancer Properties of Teucrium polium L. Methanolic Extract: Computational and In Vitro Study. Antioxidants. 2020;9:1089. doi: 10.3390/antiox9111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosselli S., Maggio A., Bellone G. Antibacterial and anticoagulant activities of coumarins isolated from the flowers of Magydaris tomentosa. Planta Med. 2007;73:116. doi: 10.1055/s-2006-951772. [DOI] [PubMed] [Google Scholar]
- 10.Patil K., Prasad R., Skouby K. A survey of worldwide spectrum occupancy measurement campaigns for cognitive radio; Proceedings of the International Conference on Devices and Communication (ICDeCom); Mesra, India. 24–25 February 2011. [Google Scholar]
- 11.Rahmouni F., Saoudi M., Amri N., El-Feki A., Rebai T., Badraoui R. Protective effect of Teucrium polium on carbon tetrachloride induced genotoxicity and oxidative stress in rats. Arch. Physiol. Biochem. 2018;124:1–9. doi: 10.1080/13813455.2017.1347795. [DOI] [PubMed] [Google Scholar]
- 12.Rahmouni F., Saoudi M., Rebai T. Therapeutics studies and biological properties of Teucrium polium (Laminaceae) BioFactors. 2021;47:952–963. doi: 10.1002/biof.1782. [DOI] [PubMed] [Google Scholar]
- 13.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 14.Laporta O., Perez-Fons L., Mallavia R. Isolation, characterization and antioxidant capacity assessment of the bioactive compound derived from Hypoxis rooperi corm extract (African potato) Food Chem. 2007;101:1425–1437. doi: 10.1016/j.foodchem.2006.03.051. [DOI] [Google Scholar]
- 15.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 16.Ardestani A., Yazdanparast R. Inhibitory effects of ethyl acetate extract of Teucrium polium on in vitro protein glycoxidation. Food Chem. Toxicol. 2007;45:2402–2411. doi: 10.1016/j.fct.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Slater T.F. Overview of methods used for detecting lipid peroxidation. Meth. Enzymol. 1984;105:283–293. doi: 10.1016/s0076-6879(84)05036-9. [DOI] [PubMed] [Google Scholar]
- 18.Asada K., Kiso K., Yoshikawa K. Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J. Biol. Chem. 1974;249:2175–2181. doi: 10.1016/S0021-9258(19)42815-9. [DOI] [PubMed] [Google Scholar]
- 19.Flohe L., Gunzler W.A. Methods in Enzymology. Volume 105. Academic Press; Cambridge, MA, USA: 1984. Assays of glutathione peroxidase; pp. 114–120. [DOI] [PubMed] [Google Scholar]
- 20.Aebi H. Catalase in vitro. Methods Enzymol. 1984;8105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 21.Badraoui R., Saeed M., Bouali M., Hamadou W.S., Elkahoui S., Alam M.J., Siddigui A.J., Adnan M., Saoudi M., Rebai T., et al. Expression profiling of selected immune genes and trabecular microarchitecture in breast cancer skeletal metastases model: Effect of α–tocopherol acetate supplementation. Calcif. Tissue Int. 2022;110:475–488. doi: 10.1007/s00223-021-00931-3. [DOI] [PubMed] [Google Scholar]
- 22.Hchicha K., Korb M., Badraoui R., Naïli R. A novel sulfate-bridged binuclear copper (II) complex: Structure, optical, ADMET and in vivo approach in a murine model of bone metastasis. N. J. Chem. 2021;45:13775–13784. doi: 10.1039/D1NJ02388H. [DOI] [Google Scholar]
- 23.Mhadhbi N., Issaoui N., Hamadou W.S., Alam J.M., Elhadi A.S., Adnan M., Naϊli H., Badraoui R. Physico-Chemical Properties, Pharmacokinetics, Molecular Docking and In-Vitro Pharmacological Study of a Cobalt (II) Complex Based on 2-Aminopyridine. ChemistrySelect. 2022;7:e202103592. doi: 10.1002/slct.202103592. [DOI] [Google Scholar]
- 24.Zammel N., Saeed M., Bouali N., Elkahoui S., Alam J.M., Rebai T., Kausar M.A., Siddigui A.J., Adnan M., Badraoui R. Antioxidant and anti-inflammatory effects of Zingiber officinale roscoe and Allium subhirsutum: In silico, biochemical and histological study. Foods. 2021;10:1383. doi: 10.3390/foods10061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams P.A., Cosme J., Ward A., Angove H.C. Crystal Structure of Human Cytochrome P450 2C9 with Bound Warfarin. Nature. 2003;424:464. doi: 10.1038/nature01862. [DOI] [PubMed] [Google Scholar]
- 26.Ramanjulu J.M., Pesiridis G.S., Yang J., Concha N., Singhaus R. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564:439–443. doi: 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoudi S., Khali M., Mahmoudi N. Etude de l’extraction des composés phénoliques de différentes parties de la fleur d’artichaut (Cynara scolymus L.) Nat. Technol. 2013;9:35. [Google Scholar]
- 28.Movahedi A., Basir R., Rahmat A., Charaffedine M., Othman F. Remarkable anticancer activity of Teucrium polium on hepatocellular carcinogenic rats. Evid. Based Complem. Alter. Med. 2014;2014:726724. doi: 10.1155/2014/726724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang H., Hammack C., Ogden S.C. Zika virus infects human cortical neural progenitors and attenuates their growth. Cellul. Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellassoued K., Hsouna A.B., Athmouni K. Protective effects of Mentha piperita L. leaf essential oil against CCl4 induced hepatic oxidative damage and renal failure in rats. Lipids Health Dis. 2018;17:9. doi: 10.1186/s12944-017-0645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahmouni F., Hamdaoui L., Badraoui R., Rebai T. Protective effects of Teucrium polium aqueous extract and ascorbic acid on hematological and some biochemical parameters against carbon tetrachloride (CCl4) induced toxicity in rats. Biomed. Pharmacother. 2017;91:43–48. doi: 10.1016/j.biopha.2017.04.071. [DOI] [PubMed] [Google Scholar]
- 32.Sun H.J., Chen J., Zhang H., Ni B., van Velkinburgh J.C., Liu Y., Wu Y.-Z., Yang X. Von Willebrand factor protects against acute CCl4-induced hepatotoxicity through phospho-p38 MAPK signaling pathway inhibition. Immun. Res. 2017;65:1046–1058. doi: 10.1007/s12026-017-8946-7. [DOI] [PubMed] [Google Scholar]
- 33.Badraoui R., Adnan M., Bardakci F., Alreshidi M.M. Chloroquine and hydroxychloroquine interact differently with ACE2 domains reported to bind with the coronavirus spike protein: Mediation by ACE2 polymorphism. Molecules. 2021;26:673. doi: 10.3390/molecules26030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu D., Tian Y., Xia Q., Ke B. The cGAS-STING Pathway: Novel Perspectives in Liver Diseases. Front. Immunol. 2021;12:682736. doi: 10.3389/fimmu.2021.682736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villeneuve J.-P., Pichette V. Cytochrome P450 and Liver Diseases. Curr. Drug. Metab. 2004;5:273–282. doi: 10.2174/1389200043335531. [DOI] [PubMed] [Google Scholar]
- 36.Sun L., Zhang Y., Wen S., Li Q., Chen R., Lai X., Zhang Z., Zhou Z., Xie Y., Zheng X., et al. Extract of Jasminum grandiflorum L. alleviates CCl4-induced liver injury by decreasing inflammation, oxidative stress and hepatic CYP2E1 expression in mice. Biomed. Pharmacother. 2022;152:113255. doi: 10.1016/j.biopha.2022.113255. [DOI] [PubMed] [Google Scholar]
- 37.Zhong W., Rao Z., Xu J., Sun Y., Hu H., Wang P., Xia Y., Pan X., Tang W., Chen Z., et al. Defective mitophagy in aged macrophages promotes mitochondrial DNA cytosolic leakage to activate STING signaling during liver sterile inflammation. Aging Cell. 2020;21:e13622. doi: 10.1111/acel.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badraoui R., Rebai T., Elkahoui S., Alreshidi M., Veettil V.N., Noumi E., Al-Motair K.A., Aouadi K., Kadri A., De Feo V., et al. Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study. Antioxidants. 2020;9:1003. doi: 10.3390/antiox9101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guesmi F., Saidi I., Soussi R., Hfaiedh N., Landoulsi A. Antioxidant potential of four species of natural product and therapeutic strategies for cancer through suppression of viability in the human multiple myeloma cell line U266. Biomed. Environ. Sci. 2019;32:22–33. doi: 10.3967/bes2019.003. [DOI] [PubMed] [Google Scholar]
- 40.Sakr L., Dutau H. Massive hemoptysis: An update on the role of bronchoscopy in diagnosis and management. Respir. J. 2010;80:38–58. doi: 10.1159/000274492. [DOI] [PubMed] [Google Scholar]
- 41.Amri N., Rahmouni F., Chokri M.A., Rebai T., Badraoui R. Histological and biochemical biomarkers analysis reveal strong toxicological impacts of pollution in hybrid sparrow (Passer domesticus × Passer hispaniolensis) in southern Tunisia. Environ. Sci. Pollusion Res. 2017;24:17845–17852. doi: 10.1007/s11356-017-9352-3. [DOI] [PubMed] [Google Scholar]
- 42.Rahmouni F., Badraoui R., Amri N., Elleuch A., ElFeki A. Hepatotoxicity and nephrotoxicity in rats induced by carbon tetrachloride and the protective effects of Teucrium polium and vitamin C. Toxicol. Mech. Methods. 2019;29:313–321. doi: 10.1080/15376516.2018.1519864. [DOI] [PubMed] [Google Scholar]
- 43.Sreelatha S., Padma P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009;64:303. doi: 10.1007/s11130-009-0141-0. [DOI] [PubMed] [Google Scholar]
- 44.Seghatoleslam A., Khoshdel Z., Ghafouri R., Fakher S., Molaei M., Namavari M., Zal F. Composition and Anti-Toxicity Effects of Cichorium intybus Distillate on Serum Antioxidant Status in Carbon Tetrachloride-Treated Rats. Arch. Razi. Inst. 2021;76:107–117. doi: 10.22092/ari.2019.127303.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data of the current study are included in this article.