Abstract
Despite the challenging conditions in the pre-Saharan areas of Algeria, such as weak plant cover and a harsh climate, beekeeping is being developed and spread. In the present work, honey samples collected from ten locations in the El Oued region were examined during the spring of 2021. A melissopalynological analysis was carried out, followed by a floristic investigation. The 10 honey samples were also investigated for their physicochemical properties and antioxidant and antibacterial activity against five strains: Escherichia coli, Staphylococcus aureus, Bacillus subtilus, Listeria innocua, and Micrococcus luteus. The floristic analysis found 65 species belonging to 33 botanical families, with a dominance of the Asteraceae family accounting for 18.461% of the total. The melissopalynological study revealed only one monofloral honey (Ziziphus lotus), whereas the nine others were multi-floral. The honey’s color changed from light to dark amber, and most tested honey was of high quality, fulfilling international criteria. The total phenol and flavonoid contents varied considerably amongst the various honey samples. Furthermore, LC-MS-MS phenolic profile analysis identified the presence of 20 chemicals, of which only three phenols were found in all honey types. Antioxidant capacity analyzed with FRAP test and antiradical activities against DPPH differed from one honey sample to another. Moreover, a significant correlation was recorded between the antioxidant activity, honey’s color, polyphenol, and flavonoid contents. The S. aureus strain was the most sensitive regarding honey antibacterial activity, while M. luteus and B. subtilis strains were only moderately sensitive.
Keywords: honey, melissopalynology, physicochemical analysis, LC-MS-MS, antioxidant test, antimicrobial activity
1. Introduction
Honey is a natural product with a complex chemical composition, and it is also the only and the most well-known sweetener that can be consumed raw by humans [1]. Bees collect nectar and pollen from plants and produce honey, which has been revered for centuries for its nutritional and therapeutic properties [2]. Honey has been resurrected as a therapy for burns, gastrointestinal diseases, asthma, infected wounds, and skin ulcers in human and animal medicine [3,4].
Honey contains several constituents of small amounts, such as minerals, free amino acids, proteins, vitamins, enzymes, organic acids, flavonoids, phenolic acids, and other organic acids in addition to other phytochemicals compounds [5]. The amount of these components is determined by several factors, including the honey’s geographical origin, floral source, meteorological circumstances, any treatments applied [6], and seasonality [7]. Honey’s composition can be affected by processing, handling, and storage [8]. The quality of honey also depends on floral resources and the treatment of the beekeepers [9].
Honey’s botanical and geographical origins have traditionally been determined by evaluating pollen quality and quantity and organoleptic and physicochemical testing. In addition, data derived from the sensory profile, bioactive components, and novel methods of investigation should be added to this information [10,11].
Water content, sugar reduction, sucrose, insoluble matter, ash, free acid, pH, electrical conductivity, specific rotation, and sensory and microbiological properties are the basis for the quality assessment of honey [12,13]. Honey’s components have a variety of beneficial biological actions, such as antioxidant, antifungal, antibacterial and antiviral, anti-browning effects, and antioxidants effects in natural foodstuffs [14,15]. Various studies have demonstrated that antioxidant activity highly correlates to total phenolic levels [16]. Moreover, darker honey has been reported to have a higher total phenolic content and thus more significant antioxidant activity [17]. Honey’s composition includes various components, including hydrogen peroxide and polyphenols, and is also strongly linked to antibacterial activity [18]. The latter is diverse and yet not fully understood. Several components of honey have been shown to have a critical role in honey’s antibacterial effects. Honey’s ability to fight different sorts of microorganisms is determined by various variables, including the kind and natural structure of the nectar and the environmental circumstances in which the bees were raised [19].
Despite severe environmental conditions and poor plant cover, beekeeping is being developed and promoted in pre-Saharan regions. The western and central parts of Algeria are the focus of studies on the kinds of honey of the Saharan region [20]. However, in our perspective, no work has addressed the research of kinds of honey from the south-eastern region of the country, particularly those bordering Tunisia. In the Algerian Sahara, the inhabitants frequently use Saharan honey as a remedy for many infections because of its medicinal attributes and higher efficacy than in North Africa [21]. This present study aims to investigate honey plants in a pre-Saharan area of Algeria and appraise their honey using physicochemical parameters, pollen and color analyses, phenolic compounds and their dosages, and an evaluation of their antioxidant activities. Honey’s antibacterial activity was also assessed against Gram-positive and Gram-negative strains.
2. Materials and Methods
2.1. Honey Samples Origin
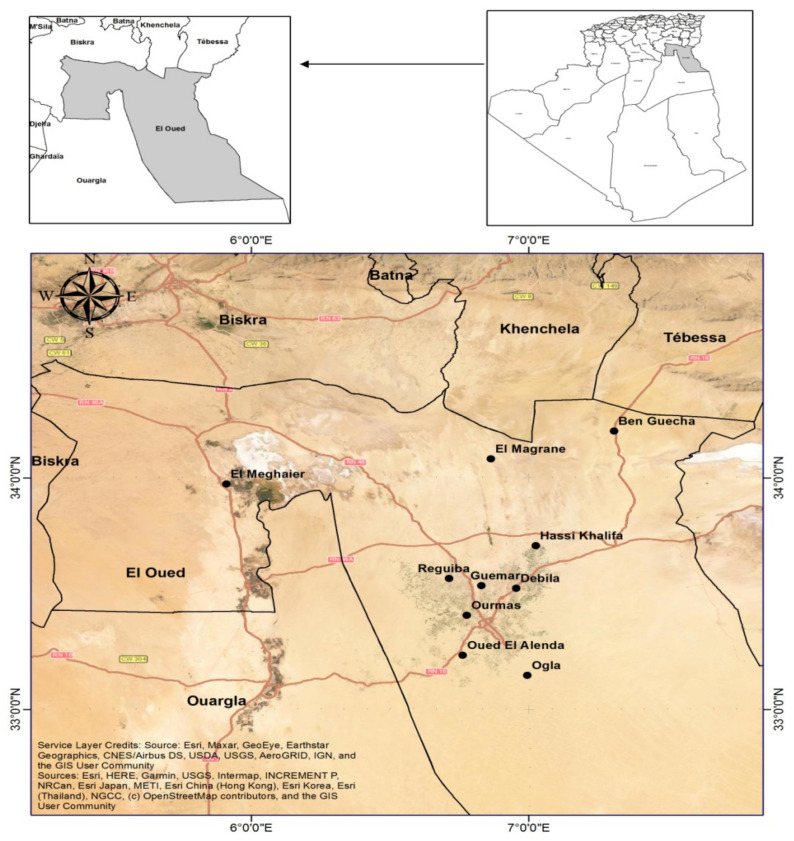
The current study was conducted on ten honey samples obtained from Apis mellifera intermission apiaries installed in various localities in the El-Oued region, a 77 km2 area located in the northeastern Sahara of Algeria (Low Sahara basin) (Figure 1 and Table 1), and characterized by a dry to hyper-arid climate. Ten sites, of which two are natural and eight cultivated, were selected according to their distance from each other and the beekeepers’ activity. Nine sites are located in the Oued Souf region, whereas the tenth site is situated in the Oued Righ area. The samples were collected at the end of May 2021, and all honey samples were kept at 4 °C until the analysis.
Figure 1.
Distribution of the study sites in El Oued region.
Table 1.
Geographical Origin of the honey samples.
| Samples’ Number | Study Sites | Longitude | Latitude | Altitude |
|---|---|---|---|---|
| H01 | Ben Guecha | 7°18′28.1″ E | 34°12′07.2″ N | 28 m |
| H02 | Oued El Alenda | 6°45′43.6″ E | 33°14′01.1″ N | 105 m |
| H03 | Guemar | 6°49′47.1″ E | 33°32′06.7″ N | 61 m |
| H04 | El Megrane | 6°51′49.6″ E | 34°04′56.0″ N | 70 m |
| H05 | Debila | 6°57′16.5″ E | 33°31′22.8″ N | 65 m |
| H06 | Reguiba | 6°42′44.4″ E | 33°33′55.6″ N | 59 m |
| H07 | Ourmas | 6°46′38.4″ E | 33°24′22.5″ N | 80 m |
| H08 | Hassi Khalifa | 7°01′31.8″ E | 33°42′24.6″ N | 31 m |
| H09 | El Meghaier | 5°54′33.4″ E | 33°58′23.6″ N | 02 m |
| H10 | Ogla | 6°59′42.7″ E | 33°08′50.1″ N | 89 m |
2.2. Vegetation Study
The flora investigation and plant cover were conducted in the spring using the quadrat technique in a 500 m2 area [22] by installing quadrat plots every 200 m in the bee-foraging radius. From November until May, flowering plants were collected weekly and haphazardly at each study site. According to our observations and beekeepers’ information, day-long (from 8 h to 10 h and from 12 h to15 h 30 h) melliferous plants foraged by honey bees were divided into three percentages cover categories: frequent (>60%), average (˂60% to >30%), and less often (<30%) [22]. The identification of plant flowering species was determined based on Ozenda [23] and the flora of North Africa [24].
2.3. Pollen Analysis
The melissopalynological qualitative study was reported by Louveaux et al. [25]. A sample of 10 g of honey was dissolved in 20 mL of acidic water 5% (5 g H2SO4 per one liter of distilled water), and the mixture was centrifuged at 3500 rpm for 10 min, after which the supernatant was discarded. The precipitate was soaked in 10 mL of distilled water and centrifuged again for 5 min. The deposit containing the pollen grains was spread on a slide. After drying, a drop of glycerin gelatin was added to it, where it was then covered with a cover slip for identification. To determine frequency classes, 500 pollen grains were counted per sample. The four following categories were used for frequency classes: predominant pollen (>45%) of pollen grains counted, secondary pollen (16–45%), important minor pollen (3–15%), and minor pollen (1–3%) [25,26]. The pollen grains were observed using an optical microscope, and pollen types were identified using a reference collection collected from the beehives’ area. We also used the pollen atlas established by Riccardeli D’Albore [27].
2.4. Physicochemical Analyses
The physicochemical characteristics of the honey samples were determined three times using the International Honey Commission methods (IHC) [28]. First, water content was determined by using a refractometric method. All honey samples were placed in firmly closed, sterile vials and were incubated in a water bath at 50 degrees Celsius for 30 min, then cooled at room temperature until 20 °C. Refractive index values were then measured, and a standard CHATAWAY table was used to calculate the associated percent humidity (g/100 g honey). Second, the pH of honey was measured using a pH meter whereby 10 g of honey at a temperature of 20 °C was dissolved in ultra-pure water under a magnetic agitator to avoid the precipitation of sugars and maintain the solution homogeneity. Third, the titrimetric approach was employed to determine free acidity. After dissolving 10 g of honey with 75 mL of pure water, the volume of 0.1 M NaOH was added to the resulting solution until reaching a pH of 8.5. The results were expressed in meq/kg. To determine electrical conductivity, a conductivity bridge was employed to analyze a 20% (w/v) distilled water–honey solution [28]. The electrical conductivity values were given in mS/cm. To determine the color of honey, solutions of 50% were heated to 50 degrees until the sugars were wholly dissolved. Absorbance was measured at 635 nm, and absorbance values were classified according to the Pfund scale [29].
2.5. Total Polyphenols and Flavonoids Content
The Folin–Ciocalteu procedure was employed to measure the overall phenol content of the sampled honey with a few modifications [30]. Each gram of each honey sample was diluted into 10 mL of distilled water, filtered through Whatman paper No.1, then mixed with 2.5 mL of 0.2 N Folin–Ciocalteu reagents. The mixture was left to stand for 5 min. Then, 2 mL of 20% aqueous sodium carbonate solution (Na2CO3) was added. The reaction was kept in the dark at room temperature for 2 h, and the absorbance was measured with a spectrophotometer at 760 nm. Total phenolic content was calculated using the linear regression equation of the plotted calibration curve of gallic acid using the linear regression equation of the plotted calibration curve gallic acid (dilutions from 50 to 250 mg GA/L methanol). According to [31], the flavonoid contents were determined with some modifications. Hence, 5 mL of honey solutions (0.01 g of honey/mL distilled water) were combined with the same volume of 2% aluminum chloride solution diluted in methanol and then incubated at room temperature for 30 min. The absorbance was read at 415 nm, and the total flavonoid content was reported as mg of quercetin equivalent (Q.E.) per 100 g of honey [32].
2.6. Extraction of Phenolic Compounds for LC-MS-MS Analysis
The extraction process was modified slightly from what was initially reported by Azar et al. [33] and Wahdan [34]. Thus, 10 g of a honey sample was diluted in 50 mL of pure water (20%), and the mixture was stirred for a while to ensure optimum homogenization. The pH of this solution was adjusted to 2 with HCl 0.1 Μ (mol/L). After filtering through absorbent cotton, the phenolic compounds in the honey solution were extracted using 50 mL of ethyl acetate for the first time, followed by 25 mL for the second and third times. At 40 °C, the ethyl acetate extract was evaporated in a rotary evaporator under a vacuum. The residue was placed in 5 mL of methanol and kept at 18 °C. Prior to chromatography, all samples were filtered via Millex-LCR (PTFE) filters with 0.45 m pore sizes.
2.7. Liquid Chromatography-Mass Spectrometry Analysis Conditions LC-MS-MS
UPLC-ESI-MS-MS Shimadzu 8040 Ultra-High sensitivity with UFMS technology was employed and equipped with binary bump Nexera XR LC-20AD. For optimization of polyphones standards, we used direct injection without column.
The ESI conditions were as follows: CID gas, 230 KPs; conversion dynode, −6.00 Kv; interface temperature, 350 °C; DL temperature, 250 °C; nebulizing gas flow, 3.00 L/min; heat block, 400 °C; drying gas flow, 15.00 L/min.
All standards were prepared in methanol with a 500 μg/L concentration. The ion trap mass spectrometer was used in both negative and positive ions with MRM mode (multiple reaction monitoring). The mobile phase was constituted of water, 0.1% formic acid, and 70% methanol. The flow rate was 0.3 mL/min, and the injection volume was 6 µL.
The samples were separated using an Ultra-force C18 column (I.D. 2.5 mm ×100 mm, 1.8 µm particle size; Restek), and the oven temperature was 25 °C. Isocratic elution was applied with 0.1% formic acid and methanol (30:70, v/v). The flow rate was 0.30 mL/min, and the injection volume was 10 mL.
2.8. Antioxidant Activity
2.8.1. DPPH Test
The DPPH radical scavenging capacity of the sampled honey was measured following the procedure described by Ferreira et al. [35], with modifications. Thus, 1 ml of honey solution (w/v) was added to 2.7 mL of a methanolic solution containing DPPH radical (0.024 mg/mL). The mixture was stirred with a vortex before being left in the dark for 60 min. The absorbance was then measured at 517 nm against a DPPH-free blank. The data were provided as a percentage of radical DPPH inhibition. The radical inhibition of DPPH was estimated using the following equation: Percentage of Inhibition % = 100 × [(blank absorbance − sample absorbance)/blank absorbance].
2.8.2. FRAP Test
According to Tuberoso et al. [36], the FRAP test was performed with minor modifications.
The ferric reduction antioxidant test (FRAP) uses a spectrophotometric test to lower ferric 2,4,6-tris(2-pyridyl)-1,3,5-triazine [Fe (III)-TPTZ] to the ferric complex at low pH. The FRAP solution is composed of three ingredients: 300 mM sodium acetate, TPTZ (10 mM) diluted in 40 mM HCl, and FeCl3 (20 mM). To perform the test, 500 µL of honey solution (0.1 g/1 mL) was combined with 750 µL of FRAP reagent. The absorbance was measured at 593 nm after homogenization and incubation for 5 min at 37 °C. The values are given in milligrams per 100 g of honey.
2.9. Antibacterial Activity
2.9.1. Bacterial Strains
The inhibitory effect of the different selected honey samples was tested on five human pathogenic bacterial strains: one is a Gram-negative strain (Escherichia coli ATCC 8737), and the others are Gram-positive (Micrococcus luteus ATCC 9314, Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCCN 6538, Listeria innocua CLIP 7491). Bacterial strains were kept at −80 °C in brain–heart agar broths containing glycerol until they were used. The strains were grown afterward in agar broths for 24 h at 37 °C in an incubator before being tested for antibacterial activity.
2.9.2. Minimal Inhibitory Concentration (MIC)
The minimum inhibitory concentration (MIC) of honey is defined as the lowest concentration that can prevent bacteria development [37]. The antibacterial activity of the investigated honey samples was examined using the technique performed by Baydar et al. [38]. The disc diffusion method was used to conduct this test, which involves soaking discs in each sample. A pure and young culture (18 h old) should be used to create bacterial suspensions with an optical density of 0.5 Mc Ferland (EQ105UFC/mL). A 1 mL volume of each bacterial strain was inoculated into Petri dishes and then filled with Muller–Hinton agar medium at a thickness of 4 mm, whereby it was then dried for 3 to 5 min at room temperature. Following that, three disks (8 mm in diameter) soaked with the same honey solution concentration were put on the Petri dishes and placed on the growing medium’s surface to achieve full contact with the agar. Next, sterilized control disks were impregnated in a 20 µL volume of distilled water. Petri dishes were then kept for pre diffusion at 4 °C for three hours and then incubated at 37 °C for 24 h. The inhibitory zone diameter was determined in millimeters by using a caliper. For each bacterial strain, the experiment was performed three times.
2.10. Statistical Analyses
All tests were carried out in triplicate. Results were reported as mean values with a standard deviation (SD). Microsoft Office Excel 2007 and Pearson’s correlation coefficient (R) using Prism Graph Pad.8 software were used to test correlations between the analytical parameters. ANOVA analyzed data with Tukey test and Matlab vers. 17 for Windows. The level of confidence was set at 95% (α = 0.05). Multivariate PCA analysis was performed using PAST—PAlaeontological STatistics, ver. 1.89 (free software, http://folk.uio.no/ohammer/past, accessed on 14 May 2022).
3. Results and Discussion
3.1. Vegetation Features
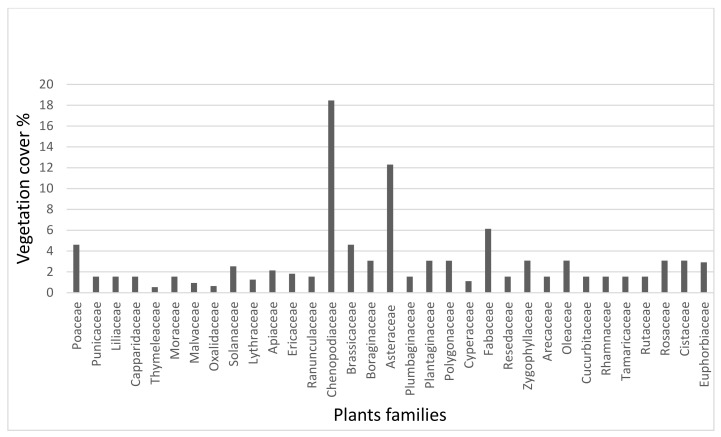
We found 65 species in total, divided into 33 botanical families, where most species belong to Chenopodiaceae (18.46%), followed by Asteraceae (12.307%), Fabaceae (6.135%), Brassicaceae, and Poaceae (4.615%). Boraginaceae, Cucurbitaceae, Plantaginaceae, Polygonaceae, Rosaceae, Solanaceae, and Zygophyllaceae were all under 3.076 percent, with only one species represented (Figure 2). These findings are similar to those published in the exact location [24,39]. The bees visited spontaneous plants, primarily flora, accounting for more than 70% of inventoried plants. Fruit trees, market gardening, fodder crops, and Phoenix culture make up the rest.
Figure 2.
Percentage cover of the prominent botanical families encountered in the study area.
3.2. Qualitative Pollen Analysis
Twenty-three families and thirty-three taxa were identified from the honey samples (Table 2 and Table 3). The taxa Arecaceae, Tamaricaceae, Poaceae, Plantaginaceae, Euphorbiaceae, Ericaceae, Chenopodiaceae, Asteraceae, and Diplotaxis have a large distribution (>50%) in the honey samples.
Table 2.
Frequency classes of pollen types in the studied honey samples.
| Family | Pollen Type | % | Pollen Class | Max % | |||
|---|---|---|---|---|---|---|---|
| Predominant (P) | Secondary (S) | Important Minor (I) | Minor (M) | ||||
| Apiaceae | Thapsia | 20 | --- | --- | --- | 2 | 1.98 |
| Arecaceae * | Phoenix dactylifera * | 90 | --- | --- | 5 | 2 | 5.23 |
| Asteraceae | Echinops | 50 | --- | --- | --- | 3 | 1.33 |
| Centaurea | 30 | --- | --- | 3 | 2 | 8.11 | |
| Scolymus | 40 | --- | --- | 1 | 2 | 3.65 | |
| Calendula | 30 | --- | --- | 2 | 3 | 10.58 | |
| Asteraceae | 80 | --- | 3 | 5 | --- | 30.74 | |
| Boraginaceae | Echium | 10 | --- | --- | --- | 1 | 1.95 |
| Boraginaceae | 30 | --- | --- | 1 | 2 | 6.94 | |
| Brassicaceae | Diplotaxis | 60 | --- | --- | 3 | --- | 15.42 |
| Brassicaceae | 30 | --- | 2 | 2 | 1 | 33.51 | |
| Chenopodiaceae * | Chenopodiaceae * | 70 | --- | --- | 3 | 3 | 3.80 |
| Cistaceae * | Cistus * | 30 | --- | --- | 2 | 1 | 4.35 |
| Ericaceae | Ericaceae | 60 | --- | --- | 1 | 5 | 13.39 |
| Euphorbiaceae | Euphorbia sp. | 60 | --- | --- | 5 | 3 | 15.5 |
| Fabaceae | Ononis | 10 | --- | --- | --- | 1 | 1.39 |
| Retama retam | 50 | --- | --- | 5 | --- | 16.54 | |
| Acacia | 10 | --- | --- | --- | 1 | 1.23 | |
| Fabaceae | 50 | --- | --- | 5 | --- | 16.54 | |
| Liliaceae | Liliaceae | 10 | --- | --- | 1 | --- | 8.76 |
| Malvaceae | Malva | 10 | --- | --- | 1 | --- | 3.12 |
| Oleaceae | Olea europea * | 80 | --- | --- | --- | 8 | 2.88 |
| Plantaginaceae * | Plantago * | 80 | --- | --- | 5 | 3 | 5.95 |
| Poaceae * | Poaceae * | 90 | --- | --- | 3 | 6 | 7.60 |
| Oxalidaceae | Oxalis | 10 | --- | --- | --- | 1 | 2.53 |
| Resedaceae | Reseda alba | 40 | --- | --- | 1 | 3 | 5.23 |
| Ranunculaceae | Ranunculaceae | 20 | --- | --- | --- | 2 | 1.20 |
| Rhamnaceae | Ziziphus lotus | 30 | 1 | --- | 2 | --- | 48.93 |
| Rosaceae | Rosaceae | 50 | --- | --- | 1 | 4 | 6.24 |
| Rutaceae | Citrus | 20 | --- | 2 | --- | --- | 20.9 |
| Tamaricaceae | Tamarix | 90 | --- | --- | 2 | 6 | 8.97 |
| Zygophylaceae | Peganum harmala | 30 | --- | --- | 2 | 1 | 3.65 |
*, nectarless species; Max, maximum recorded pollen frequency; %, percentage of the presence of each pollen type in honey samples; P, >45%; S, 16–45%; I, 3–15%; M, 1–3%.
Table 3.
Qualitative pollen analysis of honey samples.
| Honey Samples | Pollen Type Classes | |||
|---|---|---|---|---|
| >45% | 16–45% | 3–16% | <3% | |
| H01 | Brassicaceae | Scolymus, Calendula, Chenopodiaceae, Plantago, Phoenix dactylifera, Retama retam, Boraginaceae | Ericaceae, Reseda alba, Poaceae, Tamarix, Olea europea, Echinops | |
| H02 | Ziziphus lotus | Ericaceae, Cistus, Asteraceae, Plantago, Phoenix dactylifera | Boraginaceae, Chenopodiaceae, Peganum harmala, Tamarix, Poaceae, Thapsia, Euphorbia sp., Brassicaceae, Calendula, Echinops, Olea europea, Phoenix dactylifera, Poaceae, Rosaceae, Rhenonculaceae | |
| H03 | Asteraceae | Centaurea, Fabaceae, Poaceae, Plantago, Malva, Euphorbia sp., Asteracreae, Chenopodiaceae | Phoenix dactylifera, Oxalis, Brassicaceae, Euphorbia sp., Olea europea, Tamarix | |
| H04 | Retama retam, Cistus, Peganum harmala, Euphorbia sp., Diplotaxix, Asteraceae, Ziziphus lotus, Plantago, Poaceae, Phoenix dactylifera | Chenopodiaceae, Ericaceae, Tamarix, Thapsia, Echinops, Olea europea, Ranunculaceae, Rosaceae, Calendula | ||
| H05 | Brassicaceae, Citrus | Ziziphus lotus, Calendula, Phoenix dactylifera, Euphorbia sp., Peganum harmala, Tamarix | Thapsia, Ononis, Echinops, Echium, Cistus, Ericaceae, Plantago, Olea europea, Rosaceae | |
| H06 | Asteraceae | Calandula, Asteraceae, Rosaceae, Euphorbia sp., Plantago, Boraginaceae, Diplotaxix | Cistus, Renonculaceae, Thapsia, Echinops, Poaceae, Rosaceae, Centaurea, Brassicaceae | |
| H07 | Citrus | Asteraceae, Brassicaceae, Euphorbia sp., Fabaceae, Retama retam, Phoenix dactylifera | Chenopodiaceae, Poaceae, Reseda alba, Tamarix, Olea europea, Scolymus, Poaceae, Calendula | |
| H08 | Asteraceae | Centaurae, Brassicaceae, Plantago, other fabaceae, Liliaceae, Diplotaxix | Boraginaceae, Olea europea, Tamarix, Reseda alba, Euphorbia sp., Phoenix dactylifera, Ericaceae, Poaceae, Rosaceae | |
| H09 | Asteraceae | Brassicaceae, Fabaceae, Poaceae, Reseda alba, Rosaceae, Tamarix, Reatama retam | Centaurea, Ericaceae, Plantago, Olea europea, Thapsia, Acacia, Calendula | |
| H10 | Asteraceae, Centaurea, Retama retam, Fabaceae, citrus, Poaceae, Phoenix dactylifera, Chenopodiaceae | Scolymus, Euphorbia sp., Plantago, Tamarix, Olea europea | ||
Qualitative pollen analysis highlighted the predominant Ziziphus lotus pollen in one honey sample (H02) with a frequency of 48.93%. In general, honey is considered monofloral when the relative pollen frequency of one taxon exceeds 45% [26]. All other kinds of honey are multifloral, showing no pollen as predominant. Moreover, we noted the secondary presence of pollens from Brassicaceae, Asteraceae, and Citrus; these taxa constitute an essential source of nectar and pollen.
The pollens of nectarless species such as Arecaceae, Poaceae, Chenopodiaceae, Cistaceae, Plantaginaceae, and Oleaceae were calculated. Although they do not provide nectar, they are essential for pollen in describing the geographical origin [26]. Our results are similar to those from the Steppe region in Algeria, which is also characterized by a hot and dry climate [40,41]. However, Myrtaceae, Apiaceae, and Ericaceae families are most frequently found in honey samples in the Algerian north [42]. Fabaceae, Asteraceae, Apiaceae, and Lamiaceae are the most representative plant groups, accounting for 36% of the total pollen types encountered in honey samples taken from the Kabylia region in Algeria [43].
3.3. Physicochemical Properties
Results of the physicochemical properties of the studied honey samples are reported in Table 4.
Table 4.
Physicochemical characteristics of the studied honey samples (different superscripts letters indicate a statistically significant difference between values).
| Honey Samples | pH | Free Acidity (meq/kg) | Water Content (%) | Electrical Conductivity (mS/cm) | Color (Pfund Index) |
|---|---|---|---|---|---|
| H01 | 4.102 ± 0.013 d | 12.230 ± 0.020 b,c | 17.800 ± 0 b,c,d | 0.213 ± 0.005 g | 47.462 ± 0.030 i |
| H02 | 3.944 ± 0.008 g | 25.300 ± 0.050 b,c | 18.600 ± 0.001 e | 0.254 ± 0.001 f,g | 109.856 ± 0.002 g |
| H03 | 4.203 ± 0.011 c | 15.500 ± 0.090 a | 16.600 ± 0001 b | 0.235 ± 0.020 a | 159.993 ± 0.005 b |
| H04 | 4.006 ± 0.017 e | 21.200 ± 0.060 g | 17.400 ± 0.006 bc | 0.240 ± 0.002 e,f,g | 80.144 ± 0.001 f |
| H05 | 3.991 ± 0.013 e | 27.800 ± 0.050 b | 18.600 ± 0.001 c,d,e | 0.275 ± 0.004 d,e | 70.860 ± 0.002 g |
| H06 | 4.016 ± 0.017 e | 20.820 ± 0.050 c | 19 ± 0.004 d,e | 0.268 ± 0.007 d,f,e | 129.911 ± 0.001 d |
| H07 | 4.313 ± 0.011 a | 18.600 ± 0.020 f | 18.600 ± 0.006 a | 0.562 ± 0.008 d | 154.422 ± 0.003 c |
| H08 | 4.373 ± 0.011 b | 16.980 ± 0.020 e | 14.200 ± 0.002 c,d,e | 0.290 ± 0.019 b | 171.135 ± 0.003 a |
| H09 | 3.884 ± 0.015 f | 27.300 ± 0.080 e,f | 19.800 ± 0.001 c,d,e | 0.948 ± 0.005 d,e,f | 70.488 ± 0.006 e |
| H10 | 4.086 ± 0.004 d | 20.000 ± 0.080 d | 17.800 ± 0.002 b,c,d | 0.520 ± 0.012 c | 67.888 ± 0.002 h |
| Mean ± SD | 4.088 ± 0.127 | 20.573 ± 3,57 | 17.48 ± 1.08 | 0.381 ± 0.177 | 106.216 ± 38.84 |
| F-value | 234.72 | 253.60 | 20.69 | 859.22 | 9458.41 |
According to the Tukey test, different letters in the same column indicate highly significant differences (p < 0.001) across samples.
Results highlight that the analyzed honey samples are of good quality and clearly within the Codex Alimentarus (2001)-acceptable criteria. Furthermore, these results align with those found in other Algerian honey [42,44,45].
The variance analysis showed a very significant difference (p = 0.000, p < 0.001) of all the physicochemical analyses tested according to the different types of honey.
The water content varied between 14.200 ± 0.002% to 19.800 ± 0.001%, with an average value of 17.48 ± 1.08%. After sugars, water is the second most prevalent component in honey [46]. The amount of water in the honey is related to several factors: the relative humidity of the harvested season, the level of maturity attained in the hive, processing procedures, and storage conditions. It also varies based on the parent plant’s water content and the nectar and honeydew [47].
The pH value is another crucial factor during honey extraction and preservation. The texture, consistency, and shelf life of honey are all affected by the potential of hydrogen [48].
The honey samples analyzed in this study were acidic (Table 3). pH levels ranged from 3.880 ± 0.015 to 4.373 ± 0.011, with a mean pH value of 4.088 ± 0.127. These values are close to those reported for honey samples from the Kabylia area (Algeria) [43], along with certain Tunisian honey [48], Spanish samples [1], and Malaysian honey [49].
The acidity of honey is due to the presence of organic acid and compounds, such as the content of lactones, esters, phosphates ions, sulfates ions, and chlorides ions [50]. The free-acidity values of all the honey samples ranged between 12.230 ± 0.020 and 27.800 ± 0.050 meq/kg, with an average value of 20.573 ± 3573 meq/kg.
The highest free-acidity values were found in the H05 honey sample (27.800 meq/kg), whereas the lowest free-acidity values were found in the H01 one (12.23 meq/kg). Our findings are consistent with those obtained for various Tunisian honey, which ranged from 7.11 to 27.70 meq/kg [48], whereas Otmani et al. [51] found that the free acidity of two honey samples collected in northern Algeria was higher (37 and 41 meq/kg). The acidity variations have been attributed to flowers’ origin and the harvesting season [52].
Electrical conductivity (E.C.) strongly correlates with organic acids, proteins, mineral or total ash concentrations, and salts. It is a characteristic that varies greatly depending on the honey’s floral origin [48,51]. Electrical conductivity values should be less than 0.8 mS cm−1 for floral honey, whereas values for honeydew should be greater than 0.8 mS/cm [53]. All of the results (except for the H09 sample, in which the E.C. value was greater than 0.8 mS/cm) were below the required maximum level of electrical conductivity for honey (<0.800 mS/cm).
Honey comes in various colors, ranging from pale yellow to amber and dark amber and from dark amber to black, with some rare shades of green and red in severe situations or even the color red [9].
According to Pfund’s index, the color values obtained vary from 47.462 ± 0.0302 to 171.135 ± 0.003 mm, with an average value of 106.216 ± 38.847 mm (Table 4); 10% of the honey samples examined, including only one sample, were extra-light amber in color, and 20% were a dark color. Moreover, 30% of samples were dark amber, and 40% (four samples) were light amber. Our results of Pfund’s index are similar to those of Ghorab et al. [43] (37 to 135 mm) and Frankel et al. [54] (31.12 to 166.68 mm).
Several authors have demonstrated that the number of phenols, minerals, and acids is much higher in dark honey than in light honey; thus, a robust antioxidant activity has been registered in dark honey [49,54].
3.4. Total Polyphenols and Flavonoids Results
Results of the polyphenol contents, flavonoids, and antioxidant capacities of the tested sample honey are presented in Table 5.
Table 5.
Phenolic content (mg GAE/100 g), total flavonoid (mg Q.E./100 g), FRAP (μM Fe (II)/Kg), and DPPH (mg/mL) IC50 values of the studied honey samples (different superscripts letters indicate a statistically significant difference between values).
| Honey Samples | Total Phenolic Content (mg GAE/100 g) | Total Flavonoids (mg QE/100 g) | DPPH, IC50 (mg/mL) | FRAP Assay (μM Fe(II)/Kg) |
|---|---|---|---|---|
| H01 | 44.186 ± 0.006 j | 36.111 ± 0.004 h | 10.390 ± 0.040 a | 44.186 ± 0.030 a |
| H02 | 75.609 ± 0.006 h | 48.777 ± 0.177 f | 23.950 ± 0.050 c | 75.609 ± 0.080 c |
| H03 | 508.536 ± 0.006 b | 215.606 ± 0.128 b | 4.070 ± 0.080 j | 508.536 ± 0.070 h |
| H04 | 289.635 ± 0.005 f | 20.444 ± 0.012 i | 10.490 ± 0.060 e | 289.634 ± 0.060 e |
| H05 | 215.630 ± 0.001 g | 101.666 ± 0.012 c | 8.140 ± 0.080 d | 215.630 ± 0.070 d |
| H06 | 318.097 ± 0.007 d | 74.888 ± 0.132 e | 6.395 ± 0.030 f | 381.097 ± 0.040 f |
| H07 | 507.731 ± 0.006 c | 338.558 ± 0.002 d | 5.504 ± 0.030 g | 459.552 ± 0.030 g |
| H08 | 459.552 ± 0.001 a | 94.777 ± 0.004 a | 3.117 ± 0.090 i | 570.731 ± 0.070 i |
| H09 | 353.252 ± 0.001 e | 74.444 ± 0.012 e | 11.901 ± 0.070 h | 353.252 ± 0.030 e |
| H10 | 63.617 ± 0.001 i | 45.555 ± 0.003 g | 6.677 ± 0.060 b | 63.617 ± 0.020 h |
| Mean ± SD | 296.184 ± 158.440 | 105.088 ± 68.800 | 9.063 ± 4.090 | 123.796 ± 45.290 |
| F-value | 163,983.77 | 28,334.9 | 39,962.48 | 10,479.04 |
The variance analysis showed a very significant difference (p = 0.000, p < 0.001) of all the antioxidants tested according to the different types of honey.
According to the Tukey test, different letters in the same column indicate highly significant differences (p < 0.001) across samples.
Total Phenolic Content
Polyphenols are essential components of honey found in tiny amounts and generated from the pollen of plants frequently visited by bees [55]. The total phenolic compounds in our honey samples varied from 44.186 ± 0.006 to 508.536 ± 0.006 mg/kg, whereas the average total phenolic level ranging from 41.800 mg GAE/100 g to 128.300 mg GAE/100 g observed in honey [56] was less than those obtained in our findings. Moreover, these samples contained more than those found in the Malaysian regions [57], with reported phenolic content values of honey samples in an interval between 110.394 mg GAE/100 g and 196.500 mg GAE/100 g. On the other hand, Dżugan et al. [56] found that the total phenolics in Polish honey varied from 205.41 to 1353.66 mg GAE/100 g.
Plant secretions are the primary source of phenolic chemicals, which are secondary metabolites; the difference in total phenolic content might be attributable to the geographical location of the various floral sources [58,59].
Flavonoids are phenolic chemicals with a low molecular weight that give honey its fragrance and antioxidant properties [49]. The total flavonoid content was found to be 105.088 ± 68.808 mg Q.E./100 g per sample of honey on average, with a low value of 20.444 ± 0.012 mg Q.E./100 g per sample of honey and a high value of 338.558 ± 0.002 mg Q.E./100 g per sample of honey (Table 5). These honey specimens have a more excellent content of flavonoids than Burkinafassou honey [58], Italian honey [59], and Algerian honey tested by Khalil et al. [60].
3.5. Phenolic Compounds LC-MS-MS Analysis
The chromatography results showed the presence of 20 different phenolic compounds across the board for all honey samples (Table 6).
Table 6.
LC-MS-MS-determined phenolic compounds of honey samples.
| Compound Name. | Charge +/− | Precursor m/z | Product m/z | H01 | H02 | H03 | H04 | H05 | H06 | H07 | H08 | H09 | H10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylsalicylic Acid | [MH]+ | 181.1 | 98.59 131 |
ND | ND | ND | ND | ND | ND | ND | ND | D | ND |
| Cinnamic Acid | [MH]+ | 149.1 | 77.2 | D | D | D | D | ND | D | ND | D | D | D |
| p-Coumaric Acid | [MH]+ | 165.1 | 59.1 | ND | ND | ND | ND | ND | ND | ND | ND | D | ND |
| Gallic Acid | [MH]− | 168.8 | 125.1 | ND | ND | ND | ND | ND | ND | ND | ND | D | ND |
| Caffeic Acid | [MH]− | 178.8 | 135.1 | D | D | D | D | D | D | D | ND | D | D |
| Chlorogenic Acid | [MH]+ | 355 | 73.15 | D | D | D | ND | D | D | D | D | D | D |
| Chrysin | [MH]+ | 255.1 | 223.3 207.25 |
D | D | D | D | D | D | D | D | D | D |
| 4-Hydroxycoumarin | [MH]− | 160.8 | 117.1 | D | D | D | ND | D | D | ND | ND | ND | D |
| Esculin | [MH]+ | 341.3 | 309.4 | D | D | D | D | D | D | D | D | D | D |
| Butylhydroxyanisole | [MH]+ | 181.1 | 99.15 81.05 |
ND | D | D | D | D | ND | D | D | D | ND |
| Kaempferol | [MH]+ | 287.1 | 255.25 | D | D | D | D | D | D | ND | D | D | D |
| Lawsone | [MH]+ | 175.1 | 134.2 | D | D | D | ND | D | D | D | D | D | D |
| Naringenin | [MH]+ | 273.1 | 191.1 232.2 |
D | D | D | D | D | D | D | D | D | ND |
| Quercetin | [MH]+ | 303.1 | 262.2 | ND | D | D | D | D | D | D | D | D | D |
| Resorcinol | [MH]+ | 111.1 | 79.15 | D | D | ND | D | D | ND | D | D | D | D |
| Rutin | [MH]+ | 611.2 | 73.2 | D | D | D | D | D | D | D | D | D | D |
| Vanillin | [MH]+ | 153.1 | 71.15 | ND | ND | ND | D | D | ND | D | D | D | D |
| Verbascoside | [MH]+ | 625.2 | 593.4 | D | D | D | D | D | D | D | D | D | D |
| Butylated hydroxytoluene | [MH]+ | 221 | 161.3 203.25 |
ND | ND | D | ND | ND | D | D | D | D | ND |
| Myricetin | [MNH4]+ | 336.2 | 46.15 | D | D | D | D | D | D | D | D | D | D |
D, detected; ND, not detected.
Myricetin, verbascoside, esculin, rutin, and chrysin were found in all honey samples. However, acetylsalicylic acid, p-coumaric acid, and gallic acid were detected only in honeydew honey (H09) and which is the richest in phenolic compounds. These results align with those obtained by Trautvetter et al. [61].
3.6. Antioxidant Activity
3.6.1. DPPH Test
DPPH radical scavenging activity showed a significant variance (Table 5). Antioxidant levels of the tested honey samples ranged from 3.117 ± 0.090 to 23.950 ± 0.050 mg/mL. The sample H08 honey had considerably greater antiradical ability (3.117 mg/mL) than the other honey samples. The lowest capacity was recorded in the sample H02 (23.950 mg/mL). The lowest IC50 value implies a remarkable ability to scavenge free radicals. Meda et al. [58] found a similar antioxidant activity that ranged from 1.630 to 29.130 mg/mL, which was lower than monofloral Turkish honey samples, with values ranging from 12.010 to 65.520 mg/mL [62].
The difference between the antioxidant capacity values of the tested honey samples may be due to their antioxidants’ nature and the quality and quantity of their phenol content [17].
3.6.2. FRAP Test
The FRAP test is based on the capacity to decrease ferrous iron Fe3+ to ferric iron Fe2+ by using antioxidants. Power reduction is one of the antioxidant processes [63]. According to the results recorded in Table 5, the values of antioxidant activity by the FRAP test of the honey samples vary from 44.186 ± 0.030 to 570.731 ± 0.070 (μM Fe(II)/Kg). This interval is similar to that obtained by Gül et Pehlivan [62].
3.6.3. Correlation Analysis
Correlation analysis between the studied honey sample parameters underlined highly significant differences. A higher negative correlation between phenolic content and DPPH (r = −0.9504, p < 0.0001), phenolic content and FRAP (r = −0.9568, p < 0.0001), and between phenolic content and color (r = −0.9666, p < 0.0001) were discovered. Our correlation coefficients are in perfect accordance with those obtained in the study of Boussaid et al. [48]. A negative correlation also was observed between DPPH and color (r = 0.8917, p = 0.0005), and these findings are similar to those mentioned by Baltrušaitytė et al. [64] (r = −0.716). The positive correlation between FRAP and DPPH values was statistically significant (r = 0.9648; p< 0.0001). However, compared to Perna et al. [59] (r = 0.61), this correlation coefficient was significantly greater.
3.6.4. Antibacterial Results
New treatment techniques are required due to pathogenic bacteria’s increasing antibiotic resistance and a shortage of therapeutic choices [65,66].
Honey’s natural components have a variety of antimicrobial properties against various bacteria. Honey’s antibacterial action is believed to be affected by the pasture where the bees were reared, climatic circumstances, and blossom nectar’s natural composition [19,66].
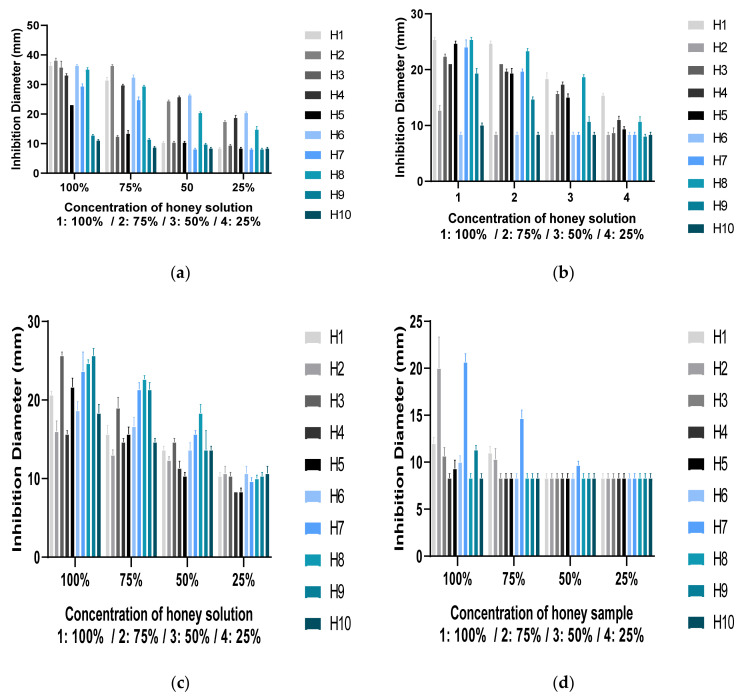
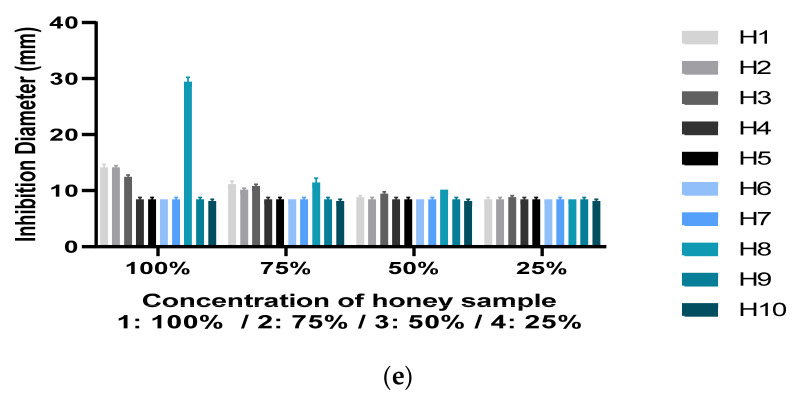
Figure 3 and Table 7 compare the antibacterial activity of different honey samples at four concentrations (100%, 75%, 50%, and 25% w/v) against Gram-positive and Gram-negative bacteria by well diffusion experiments.
Figure 3.
Antibacterial activity of honey samples against: (a) S. aureus (S2); (b) E. coli (S1); (c) L. innocua (S3); (d) B. subtilis (S4); (e) M. luteus (S5).
Table 7.
Inhibition % of different honey samples against bacterial strains.
| Honey Sample/Bacteria Strain | E. coli | S. aureus | L. innocua | B. subtilis | M. luteus |
|---|---|---|---|---|---|
| H01 | 5% | 50% | 25% | 75% | 75% |
| H02 | 75% | 5% | 25% | 75% | 75% |
| H03 | 2% | 25% | 25% | 100% | 75% |
| H04 | 25% | 5% | 50% | N I | N I |
| H05 | 25% | 50% | 50% | 100% | N I |
| H06 | N I | 5% | 25% | 100% | N I |
| H07 | 75% | 50% | 25% | 50% | N I |
| H08 | 25% | 5% | 25% | N I | 50% |
| H09 | 50% | 5% | 25% | 100% | N I |
| H10 | 100% | 75% | 25% | N I | N I |
N I, not inhibited. Zone Inhibition ≥ 10 mm.
Generally, all studied kinds of honey were efficient against Staphylococcus aureus and Listeria innocua. The H01, H02, and H03 honey samples inhibited all pathogens examined. Staphylococcus aureus was the most affected Gram-positive bacteria with the most considerable inhibitory zone impact.
Bacillus subtilis and Micrococcus luteus were not inhibited by H04 and H10 honey samples. Except for H06, all concentrations of the tested honey samples inhibited E. coli. Honey exhibited a more potent antibacterial effect on three Gram-positive pathogens than on Gram-negative bacteria. (Table 7). The H02 sample had the best zone of inhibition against S. aureus, with an inhibition diameter measured from 38.66 ± 0.88 to 17.33 ± 0.44 mm (100% to 25%) (Figure 3a). The best effect on the E. coli strain was observed in the H 01 sample, with an average diameter ranging from 25.33 ± 0.44 to 15.33 ± 0.44 mm (Figure 3b). Listeria innocua was more sensitive to H03 than the other honey samples, with a sensitivity interval of 25.66 ± 0.44 to 10.33 ± 0.44 mm (Figure 3c). Bacillus subtilis and Micrococcus luteus were the most resistant to the other pathogenic bacteria, whereby the best inhibition for B. subtilis was noticed in samples H02 and H07 (20.66 ± 0.88 to 10.33 ± 1.11 mm) (Figure 3d). For M. luteus, a diameter of 29.33 ± 0.88 to 10 ± 0.00 mm was obtained with the H08 sample effect (Figure 3e).
Table 6 presents the MIC values of the studied honey samples against all tested pathogenic bacterial strains. MIC values range from 5% to 100%. According to the minimum inhibitory concentration (MIC) findings, S. aureus was the most sensitive bacteria, while M. luteus and B. subtilis were resistant. (Table 7). Honey was also found to be efficient against Gram-positive (S. aureus, Bacillus subtilis, Bacillus cereus, Enterococcus faecalis, and Micrococcus luteus) and Gram-negative (Escherichia coli, P. aeruginosa, and Salmonella typhi) bacteria [67]. Additionally, according to many studies, S. aureus has a high susceptibility [68,69] compared to Gram-negative bacteria. In contrast, the Ukrainian honey samples were efficient against Listeria monocytogenes ATCC 7644 and could not inhibit Staphylococcus aureus CCM 4223 growth [18,70].
The variance analysis showed a very significant difference in the variation of inhibition diameter according to the different honey types, the strains and concentrations, and the interaction between factors, with probability values (p = 0.000; p < 0.001).
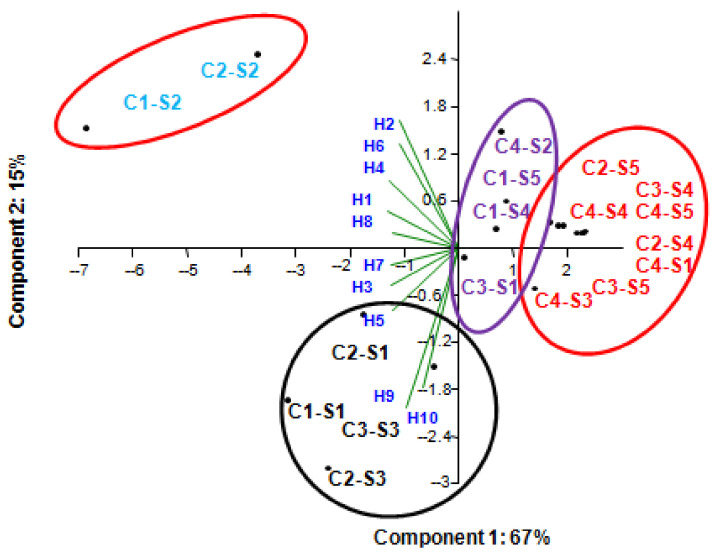
The principal component analysis (PCA) is satisfactory for the studied parameters (honey samples, bacterial strains, and concentration factor) since more than 80% of the variance is expressed on the first two axes (Figure 4). The PCA vertical axis vertical explained 67% of the total variance while the horizontal axis, a further 15% as well as the APC highlighted four groups: the first one, Group A, was constituted by the C1_2, and C2_S2 is correlated with the following honey samples: H02, H06, H04, H01, and H08.
Figure 4.
A plot of the two first components of the PCA of antibacterial activity. C1: 100%, C2: 75%, C3: 50%, C4: 25%, S1: E. coli, S2: S. aureus, S3: L. innocua, S4: B. subtilis, S5: M. luteus.
The second group correlated C1_S1, C2_S1, C2_S2, and C3_S3 with H03, H05, H09, and H10. At the same time, no correlation was recorded in the other two groups with the honey samples.
4. Conclusions
High-quality honey production is feasible given the floristic biodiversity in Algeria. This research has led to the identification of an extensive range of melliferous plants in the pre-Saharan zone, with most of them are spontaneous species. Overall, the results of the physicochemical analysis revealed that the Algerian honey samples from this region were of outstanding quality, conforming to international standards and having a composition mainly determined by their botanical origin. Furthermore, all honey samples demonstrated potent antioxidant and antibacterial capabilities. This could be due to the high polyphenol and flavonoid content. As a result, we propose that these plants should be preserved and protected, mainly because some of them are beneficial to human health and the ecosystem.
Acknowledgments
The authors express their deep appreciation for the region’s beekeepers. They also want to express their gratitude to the staff of Echahid Hamma Lakhdar University, Oued Souf, Algeria. Furthermore, the authors would like to thank the General Directorate of Scientific Research and Technological Development, who financially assisted in realizing the chemical analyses. Finally, the author M.H.A introduces her thanks to Taif University Researchers, supporting project number TURSP-2020/91, Taif University, Saudi Arabia.
Author Contributions
Data curation, L.A.B., S.M. and M.H.A.; Formal analysis, S.B.A., W.B. and F.A.A.; Funding acquisition, M.H.A. and S.M.A.K.; Investigation, S.B.A. and F.A.A.; Methodology, S.B.A., L.A.B., S.M., W.B. and S.M.A.K.; Resources, M.H.A., W.B. and T.B.H.; Software, T.B.H.; Validation, F.A.A.; Visualization, L.A.B. and T.B.H.; Writing—Original draft, L.A.B. and S.M.; Writing—Review & editing, S.M.A.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The APC funded by TURSP2020/91.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FTerrab A., Recamales A.F., Hernanz D., Heredia F.J. Characterisation of Spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chem. 2004;88:537–542. doi: 10.1016/j.foodchem.2004.01.068. [DOI] [Google Scholar]
- 2.Rana S., Mishra M., Yadav D., Subramani S.K., Katare C., Prasad G. Medicinal uses of honey: A review on its benefits to human health. Prog. Nutr. 2018;20:5–14. doi: 10.23751/pn.v20i1-S.6394. [DOI] [Google Scholar]
- 3.Molan P., Betts J. Clinical usage of honey as a wound dressing: An update. J. Wound Care. 2004;13:353–356. doi: 10.12968/jowc.2004.13.9.26708. [DOI] [PubMed] [Google Scholar]
- 4.Nigussie K., Subramanian P., Mebrahtu G. Physicochemical analysis of Tigray honey: An attempt to determine major quality markers of honey. Bull. Chem. Soc. Ethiop. 2012;26:127–133. doi: 10.4314/bcse.v26i1.14. [DOI] [Google Scholar]
- 5.Terrab A., Díez M.J., Heredia F.J. Palynological, physico-chemical and colour characterization of Moroccan honeys. II. Orange (Citrus sp.) honey: Characterization of Moroccan citrus honeys. Int. J. Food Sci. Technol. 2003;38:387–394. doi: 10.1046/j.1365-2621.2003.00714.x. [DOI] [Google Scholar]
- 6.Benfekih L.A., Bellache M., Aoudia B., Mahmoudi A. Impact of Insecticides on Pollinator Populations: Role of Phytosanitary Performance Indicators in Tomato Crops. AGR. 2018;3:5–13. doi: 10.7251/AGRENG1802005A. [DOI] [Google Scholar]
- 7.Clearwater M.J., Revell M., Noe S., Manley-Harris M. Influence of genotype, floral stage, and water stress on floral nectar yield and composition of mānuka (Leptospermum scoparium) Ann. Bot. 2018;121:501–512. doi: 10.1093/aob/mcx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertoncelj J., Dobersek U., Jamnik M., Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105:822–828. doi: 10.1016/j.foodchem.2007.01.060. [DOI] [Google Scholar]
- 9.El Sohaimy S.A., Masry S.H.D., Shehata M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015;60:279–287. doi: 10.1016/j.aoas.2015.10.015. [DOI] [Google Scholar]
- 10.Özcan M.M., Ölmez Ç. Some qualitative properties of different monofloral honeys. Food Chem. 2014;163:212–218. doi: 10.1016/j.foodchem.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 11.Can Z., Yildiz O., Sahin H., Turumtay E.A., Silici S., Kolayli S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015;180:133–141. doi: 10.1016/j.foodchem.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Gomes S., Dias L.G., Moreira L.L., Rodrigues P., Estevinho L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2010;48:544–548. doi: 10.1016/j.fct.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Belay A., Solomon W., Bultossa G., Adgaba N., Melaku S. Physicochemical properties of the Harenna forest honey, Bale, Ethiopia. Food Chem. 2013;141:3386–3392. doi: 10.1016/j.foodchem.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Stagos D., Soulitsiotis N., Tsadila C., Papaeconomou S., Arvanitis C., Ntontos A., Karkanta F., Adamou-Androulaki S., Petrotos K., Spandidos D., et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018;42:726–734. doi: 10.3892/ijmm.2018.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pauliuc D., Dranca F., Oroian M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods. 2020;9:306. doi: 10.3390/foods9030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasa M., Candiracci M., Accorsi A., Piacentini M.P., Albertini M.C., Piatti E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006;97:217–222. doi: 10.1016/j.foodchem.2005.03.039. [DOI] [Google Scholar]
- 17.Beretta G., Granata P., Ferrero M., Orioli M., Facino R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta. 2005;533:185–191. doi: 10.1016/j.aca.2004.11.010. [DOI] [Google Scholar]
- 18.Grecka K., Kuś P.M., Worobo R.W., Szweda P. Study of the Anti-Staphylococcal Potential of Honeys Produced in Northern Poland. Molecules. 2018;23:260. doi: 10.3390/molecules23020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aal A.M.A.-E., El-Hadidy M.R., El-Mashad N.B., El-Sebaie A.H. Antimicrobial effect of bee honey in comparison to antibiotics on organisms isolated from infected burns. Ann. Burn. Fire Disasters. 2007;20:83–88. [PMC free article] [PubMed] [Google Scholar]
- 20.Laallam H., Boughediri L., Bissati S., Menasria T., Mouzaoui M.S., Hadjadj S., Hammoudi R., Chenchouni H. Modeling the synergistic antibacterial effects of honey characteristics of different botanical origins from the Sahara Desert of Algeria. Front. Microbiol. 2015;6:1239. doi: 10.3389/fmicb.2015.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boukraâ L., Niar A. Sahara Honey Shows Higher Potency against Pseudomonas aeruginosa Compared to North Algerian Types of Honey. J. Med. Food. 2007;10:712–714. doi: 10.1089/jmf.2006.256. [DOI] [PubMed] [Google Scholar]
- 22.Braun-blanquet J. Plant Sociology. The Study of Plant Communities. 1st ed. McGraw-Hill Book Co., Inc.; New York, NY, USA: London, UK: 1932. [(accessed on 29 November 2021)]. Available online: https://www.cabdirect.org/cabdirect/abstract/19331600801. [Google Scholar]
- 23.Ozenda P. Flora of the Sahara. 2nd ed. CNRS; Paris, France: 1977. [(accessed on 20 April 2022)]. Available online: https://www.cabdirect.org/cabdirect/abstract/19790656031. [Google Scholar]
- 24.Chenchouni H. Diversité floristique d’un lac du bas-sahara algérien. Flora diversity of a lake at algerian low-sahara. Acta Bot. Malacit. 2012;37:33–44. doi: 10.24310/abm.v37i0.2664. [DOI] [Google Scholar]
- 25.Louveaux J., Maurizio A., Vorwohl G. Methods of Melissopalynology. Bee World. 1978;59:139–157. doi: 10.1080/0005772X.1978.11097714. [DOI] [Google Scholar]
- 26.Von Der Ohe W., Oddo L.P., Piana M.L., Morlot M., Martin P. Harmonized methods of melissopalynology. Apidologie. 2004;35((Suppl. 1)):S18–S25. doi: 10.1051/apido:2004050. [DOI] [Google Scholar]
- 27.Ricciardelli D’Albore G. Mediterranean Melissopalynology. Università Degli Studi di Perugia, Facoltà di Agraria, Istituto di Entomologia Agrarian; Perugia, Italy: 1998. [Google Scholar]
- 28.Bogdanov S., Martin P., Luellmann C. Harmonised Methods of the European Honey Commission. Apidologie (France). 1997. [(accessed on 30 November 2021)]. Available online: https://scholar.google.com/scholar_lookup?title=Harmonised+methods+of+the+European+Honey+Commission&author=Bogdanov%2C+S.+%28Eidg+Forschungsanatalt+fuer+Milchwirtshaft%2C+Bern+%28Suisse%29.+Bee+Department%29&publication_year=1997.
- 29.White J.W., Beaty M.R., Eaton W.G., Hart B., Huser W., Killion E., Lamssies R.R., Lee T., Moen W.E., Nelson S.L., et al. Instrumental Color Classification of Honey: Collaborative Study. J. AOAC Int. 1984;67:1129–1131. doi: 10.1093/jaoac/67.6.1129. [DOI] [Google Scholar]
- 30.Pontis J.A., Da Costa L.A.M.A., Da Silva S.J.R., Flach A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. Technol. 2014;34:69–73. doi: 10.1590/S0101-20612014005000015. [DOI] [Google Scholar]
- 31.Djeridane A., Yousfi M., Nadjemi B., Maamri S., Djireb F., Stocker P. Phenolic extracts from various Algerian plants as strong inhibitors of porcine liver carboxylesterase. J. Enzym. Inhib. Med. Chem. 2006;21:719–726. doi: 10.1080/14756360600810399. [DOI] [PubMed] [Google Scholar]
- 32.Özkök A., D’Arcy B., Sorkun K. Total Phenolic Acid and Total Flavonoid Content of Turkish Pine Honeydew Honey. J. ApiProd. ApiMed. Sci. 2010;2:65–71. doi: 10.3896/IBRA.4.02.2.01. [DOI] [Google Scholar]
- 33.Azar M., Verette E., Brun S. Identification of Some Phenolic Compounds in Bilberry Juice Vaccinium myrtillus. J. Food Sci. 1987;52:1255–1257. doi: 10.1111/j.1365-2621.1987.tb14056.x. [DOI] [Google Scholar]
- 34.Wahdan H.A.L. Causes of the antimicrobial activity of honey. Infection. 1998;26:26–31. doi: 10.1007/BF02768748. [DOI] [PubMed] [Google Scholar]
- 35.Isabel C.F.R., Ferreira E.A., JCM B., Estevinho L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009;114:1438–1443. doi: 10.1016/j.foodchem.2008.11.028. [DOI] [Google Scholar]
- 36.Tuberoso C.I.G., Bifulco E., JerkoviĆ I., Caboni P., Cabras P., Floris I. Methyl Syringate: A Chemical Marker of Asphodel (Asphodelus microcarpus Salzm. et Viv.) Monofloral Honey. J. Agric. Food Chem. 2009;57:3895–3900. doi: 10.1021/jf803991j. [DOI] [PubMed] [Google Scholar]
- 37.Kuś P.M., Szweda P., Jerkovic I., Tuberoso C.I.G. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016;62:269–276. doi: 10.1111/lam.12541. [DOI] [PubMed] [Google Scholar]
- 38.Baydar N.G., Özkan G., Sagdic O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control. 2004;15:335–339. doi: 10.1016/S0956-7135(03)00083-5. [DOI] [Google Scholar]
- 39.Youcef H., Lamine B.M., Hocine B., Rabah M., Ali L., Mohamed B. Diversity of Halophyte Desert Vegetation of the Different Saline Habitats in the Valley of Oued Righ, Low Sahara Basin, Algeria. Res. J. Environ. Earth Sci. 2012;4:308–315. [Google Scholar]
- 40.Mekious S., Houman Z., Bruneau É., Masseaux C., Guillet A., Hance T. Caractérisation des miels produits dans la région steppique de Djelfa en Algérie. Biotechnol. Agron. Soc. Environ. 2015;19:221–231. [Google Scholar]
- 41.Zerrouk S., Escuredo O., Rodríguez-Flores M.S., Seijo M.C. Palynological characterisation of sedra honeys (Ziziphus lotus) produced in Algeria. Grana. 2021;60:69–80. doi: 10.1080/00173134.2020.1770853. [DOI] [Google Scholar]
- 42.Ouchemoukh S., Louaileche H., Schweitzer P. Physicochemical characteristics and pollen spectrum of some Algerian honeys. Food Control. 2007;18:52–58. doi: 10.1016/j.foodcont.2005.08.007. [DOI] [Google Scholar]
- 43.Ghorab A., Rodríguez-Flores M.S., Nakib R., Escuredo O., Haderbache L., Bekdouche F., Seijo M.C. Sensorial, Melissopalynological and Physico-Chemical Characteristics of Honey from Babors Kabylia’s Region (Algeria) Foods. 2021;10:225. doi: 10.3390/foods10020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makhloufi C., Kerkvliet J.D., D’Albore G.R., Choukri A., Samar R. Characterization of Algerian honeys by palynological and physico-chemical methods. Apidologie. 2010;41:509–521. doi: 10.1051/apido/2010002. [DOI] [Google Scholar]
- 45.Homrani M., Escuredo O., Rodríguez-Flores M.S., Fatiha D., Mohammed B., Homrani A., Seijo M.C. Botanical Origin, Pollen Profile, and Physicochemical Properties of Algerian Honey from Different Bioclimatic Areas. Foods. 2020;9:938. doi: 10.3390/foods9070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehryar L., Esmaiili M., Hassanzadeh A. Evaluation of Some Physicochemical and Rheological Properties of Iranian Honeys and the Effect of Temperature on its Viscosity. Environ. Sci. 2013;13:807–819. [Google Scholar]
- 47.Da Silva P.M., Gauche C., Gonzaga L.V., Costa A.C.O., Fett R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 48.Boussaid A., Chouaibi M., Rezig L., Hellal R., Donsì F., Ferrari G., Hamdi S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2018;11:265–274. doi: 10.1016/j.arabjc.2014.08.011. [DOI] [Google Scholar]
- 49.Moniruzzaman M., Sulaiman S.A., Khalil I., Gan S.H. Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: A comparison with manuka honey. Chem. Cent. J. 2013;7:138. doi: 10.1186/1752-153X-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majewska E., Drużyńska B., Wołosiak R. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol. 2019;28:1307–1314. doi: 10.1007/s10068-019-00598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otmani I., Abdennour C., Dridi A., Kahalerras L., Halima-Salem A. Characteristics of the bitter and sweet honey from Algeria Mediterranean coast. Vet. World. 2019;12:551–557. doi: 10.14202/vetworld.2019.551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Rodríguez G.O., de Ferrer B.S., Ferrer A., Rodríguez B. Characterization of honey produced in Venezuela. Food Chem. 2004;84:499–502. doi: 10.1016/S0308-8146(02)00517-4. [DOI] [Google Scholar]
- 53.Mondragón-Cortez P., Ulloa J.A., Rosas-Ulloa P., Rodríguez-Rodríguez R., Resendiz Vázquez J.A. Physicochemical characterization of honey from the West region of México. CyTA J. Food. 2013;11:7–13. doi: 10.1080/19476337.2012.673175. [DOI] [Google Scholar]
- 54.Frankel S., Robinson G.E., Berenbaum M.R. Antioxidant capacity and correlated characteristics of 14 unifloral honeys. J. Apic. Res. 1998;37:27–31. doi: 10.1080/00218839.1998.11100951. [DOI] [Google Scholar]
- 55.Anand S., Deighton M., Livanos G., Morrison P.D., Pang E.C., Mantri N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison with Important Commercial Honeys. Front. Microbiol. 2019;10:263. doi: 10.3389/fmicb.2019.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dżugan M., Tomczyk M., Sowa P., Grabek-Lejko D. Antioxidant Activity as Biomarker of Honey Variety. Molecules. 2018;23:2069. doi: 10.3390/molecules23082069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chua L.S., Rahaman N.L.A., Adnan N.A., Tan T.T.E. Antioxidant Activity of Three Honey Samples in relation with Their Biochemical Components. J. Anal. Methods Chem. 2013;2013:e313798. doi: 10.1155/2013/313798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 59.Perna A., Simonetti A., Intaglietta I., Sofo A., Gambacorta E. Metal content of southern Italy honey of different botanical origins and its correlation with polyphenol content and antioxidant activity: Honey: Metal and polyphenol contents. Int. J. Food Sci. Technol. 2012;47:1909–1917. doi: 10.1111/j.1365-2621.2012.03050.x. [DOI] [Google Scholar]
- 60.Khalil I., Moniruzzaman M., Boukraâ L., Benhanifia M., Islam A., Islam N., Sulaiman S.A., Gan S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules. 2012;17:11199–11215. doi: 10.3390/molecules170911199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trautvetter S., Koelling-Speer I., Speer K. Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS. Apidologie. 2009;40:140–150. doi: 10.1051/apido/2008072. [DOI] [Google Scholar]
- 62.Gül A., Pehlivan T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018;25:1056–1065. doi: 10.1016/j.sjbs.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karagözler A.A., Erdağ B., Emek Y., Uygun D.A. Antioxidant activity and proline content of leaf extracts from Dorystoechas hastata. Food Chem. 2008;111:400–407. doi: 10.1016/j.foodchem.2008.03.089. [DOI] [PubMed] [Google Scholar]
- 64.Baltrušaitytė V., Venskutonis P.R., Čeksterytė V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007;101:502–514. doi: 10.1016/j.foodchem.2006.02.007. [DOI] [Google Scholar]
- 65.Al-Masaudi S.B., Hussain M.B., Al-Maaqar S.M., Al Jaouni S., Harakeh S. In vitro antibacterial activity of honey against multidrug-resistant Shigella sonnei. Complement. Ther. Clin. Pract. 2020;41:101257. doi: 10.1016/j.ctcp.2020.101257. [DOI] [PubMed] [Google Scholar]
- 66.Sharaf M., Hamouda H., Shabana S., Khan S., Arif M., Rozan H.E., Abdalla M., Chi Z., Liu C. Design of lipid-based nanocarrier for drug delivery has a double therapy for six common pathogens eradication. Colloids Surf. A Physicochem. Eng. Asp. 2021;625:126662. doi: 10.1016/j.colsurfa.2021.126662. [DOI] [Google Scholar]
- 67.Mohapatra D.P., Thakur V., Brar S.K. Antibacterial Efficacy of Raw and Processed Honey. Biotechnol. Res. Int. 2011;2011:1–6. doi: 10.4061/2011/917505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bucekova M., Jardekova L., Juricova V., Bugarova V., Di Marco G., Gismondi A., Leonardi D., Farkasovska J., Godocikova J., Laho M., et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules. 2019;24:1573. doi: 10.3390/molecules24081573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bartkiene E., Lele V., Sakiene V., Zavistanaviciute P., Zokaityte E., Dauksiene A., Jagminas P., Klupsaite D., Bliznikas S., Ruzauskas M. Variations of the antimicrobial, antioxidant, sensory attributes and biogenic amines content in Lithuania-derived bee products. LWT. 2020;118:108793. doi: 10.1016/j.lwt.2019.108793. [DOI] [Google Scholar]
- 70.Cilia G., Fratini F., Marchi M., Sagona S., Turchi B., Adamchuk L., Felicioli A., Kačániová M. Antibacterial Activity of Honey Samples from Ukraine. Vet. Sci. 2020;7:181. doi: 10.3390/vetsci7040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available.