TO THE EDITOR: Acute flaccid myelitis (AFM) is a poliomyelitis-like paralyzing illness in children. Since an outbreak of AFM in the United States in 2014, evidence has emerged suggesting that enterovirus D68 (EV-D68) causes AFM.1 The virus has been detected in respiratory specimens obtained from patients with AFM, but it has rarely been detected in the cerebrospinal fluid. There have been few autopsy studies of the disorder, and its pathogenesis has been inferred mainly from in vivo or in vitro models of EV-D68 infection or historical comparisons with poliovirus infection.
We identified a previously published case of a 5-year-old boy who died of an AFM-like illness in 2008. EV-D68 was detected in the cerebrospinal fluid, and preserved formalin-fixed and paraffin-embedded autopsy tissue was available for assessment.2 In a reconsideration of this case, we detected EV-D68 RNA and protein in anterior horn motor neurons and their axons in a section of the cervical spinal cord that we examined with the use of in situ hybridization and immunohistochemical methods (as described in the Supplementary Appendix, available with the full text of this letter at NEJM.org, and shown in Fig. 1 and Figs. S1 and S2 in the Supplementary Appendix). The precise axial level of the cervical spinal cord section was not certain because of the time that had passed between obtaining the autopsy material and performing this pathological examination. Another section of the thoracic spinal cord could not be completely examined but had immunohistochemical evidence of EV-D68. Asymmetric arm weakness was present at the onset of the syndrome, 2 days before the patient began to have difficulty walking.
Figure 1. The Presence of EV-D68 RNA and Capsid Protein in the Anterior Horn Neurons of the Spinal Cord.
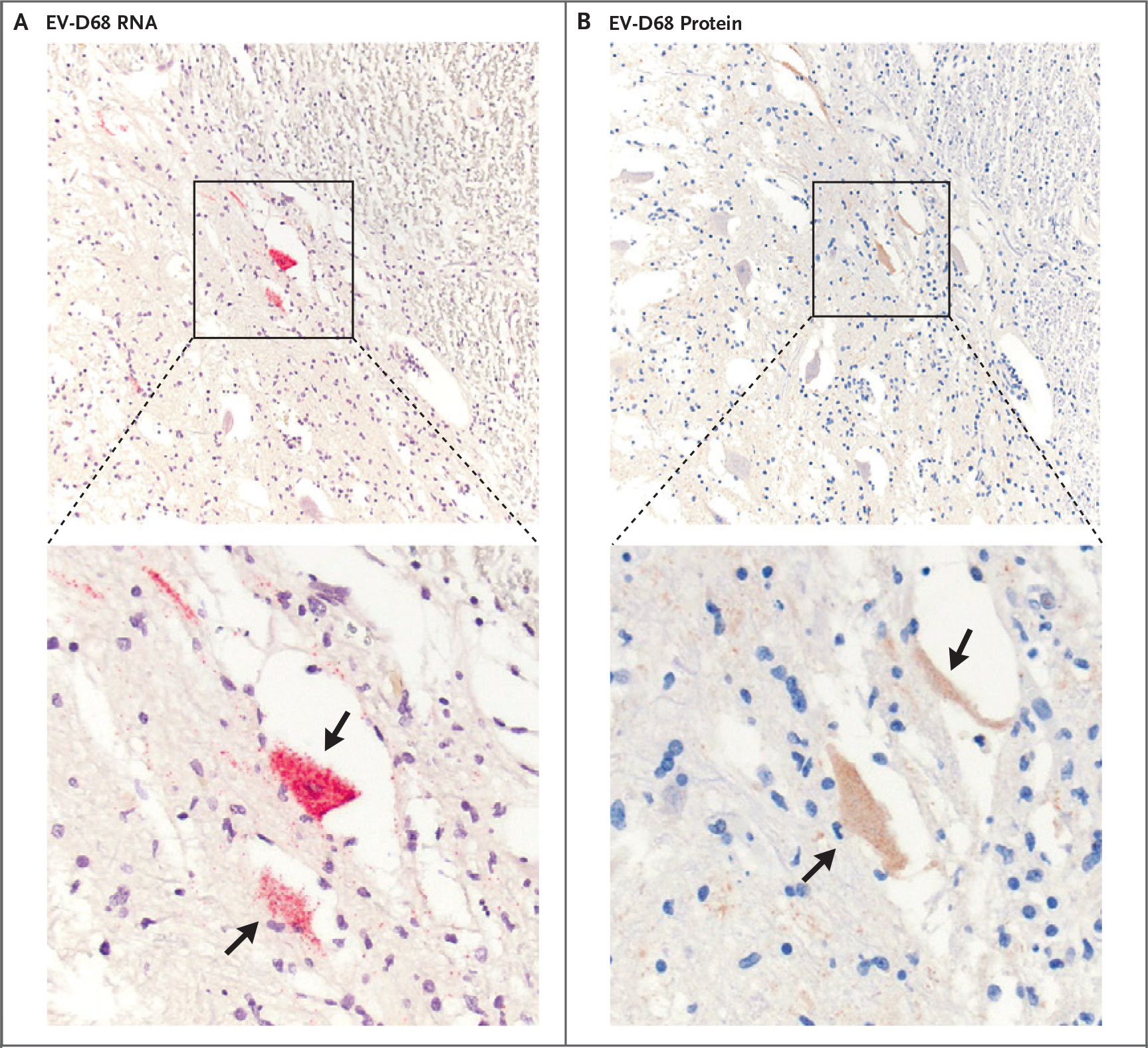
Enterovirus D68 (EV-D68)–specific genomic RNA is demonstrated by means of in situ hybridization (Panel A) and viral capsid protein by means of immunohistochemical methods (Panel B) in the same neuronal structures (arrows) in serial sections of spinal cord. Both neuronal cell bodies and axons stain for RNA and protein. The lower panels show enlargement of the boxed areas in the upper panels.
Infiltrates of CD8+ T cells and CD68+ macrophages were found in EV-D68–infected regions (Fig. S3). A comparison of quantified transcripts related to oncologic and immunologic processes in inflamed and control regions of spinal cord revealed up-regulated inflammatory gene transcripts in inflamed tissues on microscopic examination, particularly transcripts involved in antigen processing for presentation on major histocompatibility complex I molecules (Fig. S4).
These findings support the view that EV-D68 infection is a cause of AFM. The pathogenesis of AFM may involve a combination of the direct effects of viral infection of spinal cord motor neurons and damage resulting from local inflammation. This view is consistent with signal changes in the anterior horn of the spinal cord on magnetic resonance imaging in children with AFM and validates some features of murine models of EV-D68–associated AFM, which show infection of spinal cord neurons and perineural inflammation mainly with macrophages and T cells.3,4 These data, however, are in contrast with ex vivo and in vitro models of EV-D68 infection that have demonstrated astrocyte infection.5 Since the material in the current report captures only a single time point in a single infection, EV-D68 may have tropism for non-neuronal cells that was not detected. Other viruses may be found to be implicated in AFM.
Transcriptomic analysis of autopsy samples from patients with AFM may identify more specific immune-related treatment targets than the currently used glucocorticoids and intravenous immune globulin for treatment of the disorder.
Supplementary Material
Acknowledgments
Supported by a grant from the National Institute of Allergy and Infectious Diseases (K08 AI156125) and the Pediatric Infectious Diseases Society–St. Jude Children’s Hospital Fellowship Program in Basic Research to Dr. Vogt, with the fellowship funded by St. Jude Children’s Research Hospital and jointly sponsored by the Pediatric Infectious Diseases Society and St. Jude Children’s Research Hospital; a grant from the National Institute of Allergy and Infectious Diseases (U19 AI117905) to Dr. Crowe; a grant from the National Cancer Institute (5P30 CA68485-19) to the Vanderbilt Translational Pathology Shared Resource (TPSR) for the performance of histology experiments; and a Shared Instrumentation Grant (S10 OD023475-01A1) from the National Institutes of Health Office of the Director to the TPSR for the Leica Bond RX. The University of North Carolina Bioinformatics and Analytics Research Collaborative, which is supported by a University of North Carolina at Chapel Hill School of Medicine strategic fund, performed statistical analyses.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Matthew R. Vogt, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC
Peter F. Wright, Dartmouth Hitchcock Medical Center, Lebanon, NH
William F. Hickey, Dartmouth Hitchcock Medical Center, Lebanon, NH
Tristan De Buysscher, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC
Kelli L. Boyd, Vanderbilt University Medical Center, Nashville, TN
James E. Crowe, Jr., Vanderbilt University Medical Center, Nashville, TN
References
- 1.Messacar K, Asturias EJ, Hixon AM, et al. Enterovirus D68 and acute flaccid myelitis — evaluating the evidence for causality. Lancet Infect Dis 2018;18(8):e239–e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med 2011;135:793–6. [DOI] [PubMed] [Google Scholar]
- 3.Hixon AM, Yu G, Leser JS, et al. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog 2017;13(2):e1006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrey JD, Wang H, Hurst BL, et al. Causation of acute flaccid paralysis by myelitis and myositis in enterovirus-D68 infected mice deficient in interferon αβ/γ receptor deficient mice. Viruses 2018;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld AB, Warren AL, Racaniello VR. Neurotropism of enterovirus D68 isolates is independent of sialic acid and is not a recently acquired phenotype. mBio 2019;10(5):e02370–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


