Abstract
This study investigated the alteration of tumour necrosis factor (TNF-α) and interleukin-19 (IL-19) at different clinical disease stages, lymph node metastasis, and ductal carcinoma in women with breast cancer. Serum samples were collected from 90 individuals with an age range of 25–61 years. These individuals were divided into a control group (healthy people), consisting of 31 individuals, and a breast cancer patients (BCP) group, consisting of 59 individuals. The pathological data (tumour stage, lymph node metastasis, and ductal carcinoma) was obtained from the medical record of patients and confirmed by experienced histopathology. Enzyme-linked immunosorbent assay (ELISAs) technology was used to measure the serum concentrations of IL-19 and TNF-α. The results showed significant differences (P≤0.002) in the mean of BMI, interleukin-19, and TNF-α in BCP compared to controls, while the age factor did not play an important role. The stages of breast cancer caused clear differences in the levels of TNF-α and IL-19. According to the findings, BCPs had a greater level of TNF-α in lymph node metastases than healthy persons. The concentration of serum IL-19 in BCP with lymph node metastasis was significantly different in non-lymph node metastasis patients and healthy people. Additionally, BCP with ductal carcinoma showed significant differences in the mean levels of IL-19 and TNF-α in comparison with healthy people.
Keywords: breast cancer, BMI, interleukin-19, TNF-α, metastasis
INTRODUCTION
Breast cancer is the uncontrolled growth of breast cells that forms a malignant tumour, which could metastasize to other areas and tissues of the body [1]. Although this cancer primarily affects women, a few cases are reported in men [2, 3]. Breast cancer is one of the most frequent types of cancer globally, and it is the second leading cause of cancer-related deaths among women in the United States of America (40,610 deaths in 2017) [1]. Breast cancer is characterized by inflammation (such as immune cells) and some types of proteins (such as perforin), and pro-inflammatory cytokines [2]. It is noteworthy to mention that previous studies indicated that cytokines could motivate or inhibit the growth of breast cancer depending on two factors; the concentration of cytokines and the existence of modulating factors [3]. For example, Calcinotto et al. [4] reported that the presence of tumour necrosis factor (TNF-α) could promote the growth of breast cancer; this effect could be attributed to the ability of TNF-α to inhibit the immune response. At the same time, Calcinotto et al. [4] and Rao et al. [3] demonstrated that some types of cytokines, such as IL-6 and IL-18, could inhibit the progress of breast cancer by enhancing the anti-tumour ability of the immune system. Thus, the presence of TNF-α scarifying cells, such as macrophages, could promote the development of tumour cells [5]. Some researchers believe that the effect of TNF-α on the growth of breast cancer is not only due to its influence on the immune response but also its ability to induce the expression of angiogenic factors [6]. Although the mechanism of the influence of TNF-α on the development of breast cancer is not proven yet, a wide body of literature demonstrates that TNF-α plays a role in linking the inflammation and the growth of breast cancer [7].
Additionally, a high cancer risk has been found in individuals with elevated TNF-α levels [8, 9]. Other studies indicated that some interleukins, especially IL-19, act as mediators in breast cancer [8, 9], where high disease-specific survival (DSS) and metastasis-free survival (MFS) were noticed in patients with low levels of IL-19 compared with patients with high levels of IL-19 [10]. Moreover, Hsing et al. [10] indicated that IL-19 is also responsible for inducing fibronectin expression and cancer cell proliferation. It is noteworthy to highlight that anti-IL-19 monoclonal antibodies could be used to minimise the negative impacts of IL-19 [10]. In this context, the current study investigates the alteration of TNF-α and IL-19 at different clinical disease stages, lymph node metastasis, and ductal carcinoma in women with breast cancer.
Finally, it is noteworthy to mention that there are many potential reasons for the widespread cancer. For instance, some studies indicated that the consumption of polluted water with metals, nitrate, nitrite, by-products of chemical water disinfection, organics, phenols, or azo dyes could cause a wide range of cancers [11–14]. However, recent studies demonstrated that such pollutants could be removed efficiently from water using different treatment methods, such as adsorption, electrocoagulation and/or hybrid methods. In addition, air pollutants could also cause cancer [15]. Thus, it is recommended to use advanced technologies to monitor the concentrations of such pollutants in water and air.
MATERIAL AND METHODS
The current study involved 90 individuals with ages ranging from 25–61 years; 59 people were diagnosed with breast cancer BCP (patients group), while the rest (31 people) were healthy (control group). The body mass index (BMI) of each participant has been calculated using the following equation [16]:
Where W and H represent the participant's weight (in kg) and height (in m), respectively, the pathological data (tumour stage, lymph node, metastasis, and ductal carcinoma) for each participant was obtained from the medical record of patients and confirmed by experienced histopathologist. In addition, serum concentrations of IL-19 and TNF-α were calculated by enzyme-linked immunosorbent assay (ELISAs).
RESULTS
The results showed that there are significant differences (P≤0.002) between the BCP and controls in terms of BMI, IL-19, and TNF-α, while the age of participants did not show any significance (Table 1). Additionally, the levels of both TNF-α and IL-19 significantly varied (P<0.001) with the stage of the disease (Table 2). The obtained results also indicated a significantly increased level of TNF-α in the lymph node metastasis compared to healthy people (Figure 1).
Table 1.
Comparison of age, BMI, TNF-α, and IL-19 according to the study groups.
| Variable | Mean±SD Patient groups (n=59) | Mean±SD Control groups (n=31) | Significance (P) |
|---|---|---|---|
| Age (year) | 37.39±8.39 | 7.70±34.74 | 0.12 |
| BMI (kg/m2) | 6.42±31.38 | 5.10±23.90 | 0.002* |
| Serum TNF-α (pg/mL) | 7.43±30.89 | 2.86±12.50 | 0.001* |
| Serum IL-19 (pg/ml) | 6.90±36.53 | 4.86±21.26 | 0.001* |
BMI – Body Mass Index; TNF-α – Tumor necrosis factor-α; IL-19 – Interleukin 19; * – P-value is significant ≤0.05 levels; SD – Standard deviation.
Table 2.
The differences between TNF-α and IL-19 at different stages of breast cancer.
| Parameters | No. | Mean | Std. Deviation | LSD (0.05) | |
|---|---|---|---|---|---|
| TNF-α (pg/mL) | Stage I | 22 | 34.79 | 8.68 | 0.001* |
| Stage II | 17 | 29.66 | 5.19 | ||
| Stage III | 20 | 29.33 | 6.21 | ||
| IL-19 (pg/mL) | Stage I | 22 | 36.17 | 7.52 | 0.008* |
| Stage II | 17 | 36.71 | 6.36 | ||
| Stage III | 20 | 39.51 | 11.99 | ||
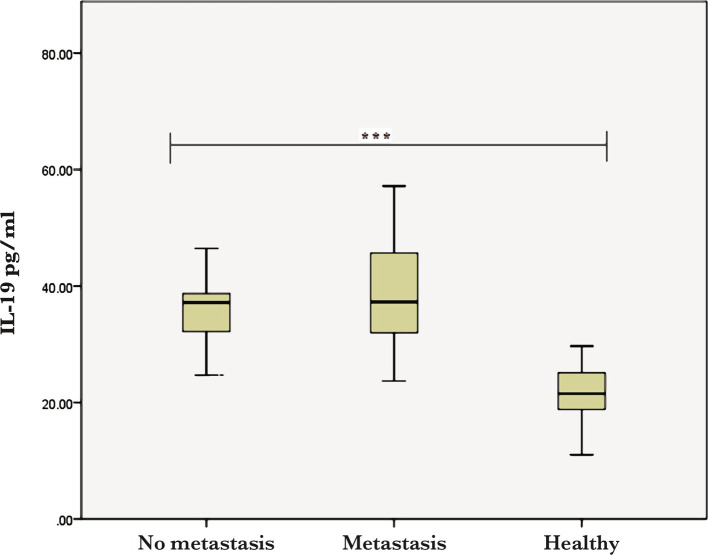
Figure 1.
Serum IL-19 levels in healthy women and BCP with lymph node metastasis (*** – p<0.0001).
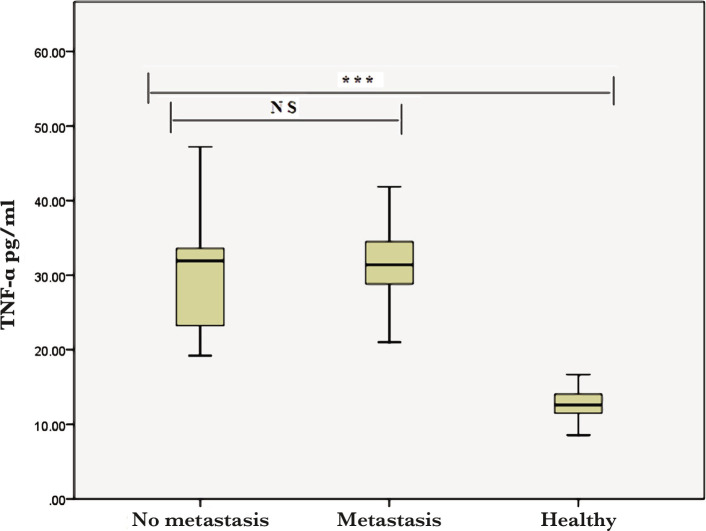
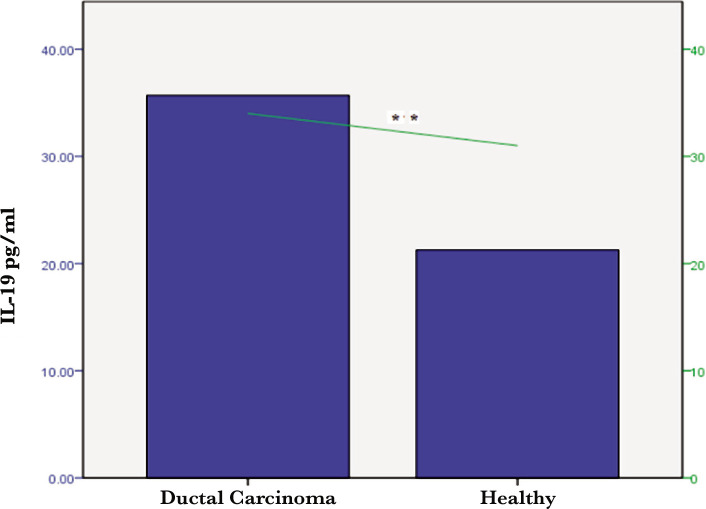
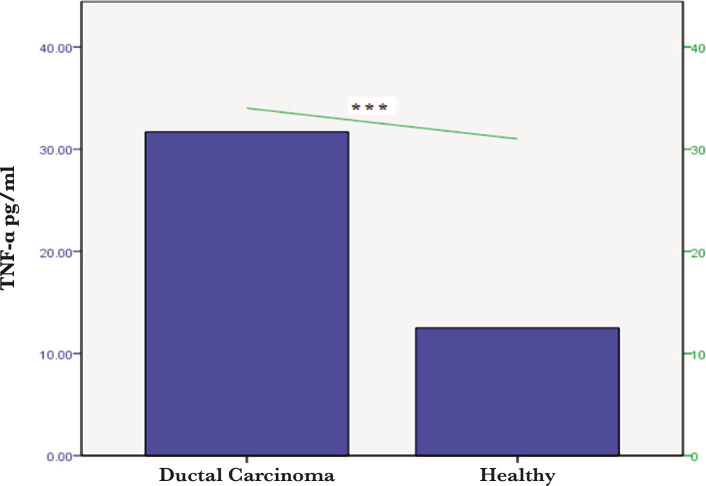
However, there was no significant difference between the BCP and controls in terms of TNF-α in the lymph node metastasis compared to no lymph node metastasis (Figure 2). Individuals with ductal carcinoma recorded significant differences (p<0.001) in the mean levels of the interleukin-19 and TNF-α compared with healthy groups (Figures 3 and 4).
Figure 2.
Serum TNF-α level in both healthy women and BCP with lymph node metastasis. NS – non-significant (*** – p<0.0001).
Figure 3.
Serum IL-19 levels in healthy women and patients with ductal carcinoma (** – p<0.01).
Figure 4.
Serum TNF-α levels in healthy women and patients with ductal carcinoma (*** – p<0.0001).
DISCUSSION
It was found that the expression of the cell TNF-α in inflammatory breast carcinoma is related to the grade of the tumour. Additionally, it was found that the TNF-α expression is a key parameter in the metastatic behaviour of breast cancer. Furthermore, the results showed that the level of TNF-α was high in BCP with progressed tumour phenotypes in comparison with those with less progressed tumour phenotypes. Recently, some researchers believe that TNF-α plays a role in carcinogenesis by activating the nuclear factor kappa-light-chain-enhancer (NF-κB); the latter plays an important role in expressing cancer-related genes [17]. In addition, it was reported that TNF-α motivates the activation of the inducible nitric oxide synthase (iNOS), which is involved in different cellular changes resulting in malignancy [18]. Therefore, the results from the current investigation agree with previous studies [18, 19]. These findings could be explained by the fact that inflammation helps develop and progress cancer [9, 20].
In terms of IL-19, it was found that its expression is influenced by several factors, such as mitotic figures and the stage of the disease. Additionally, it was found that IL-19 directly helps proliferate and migrate breast cancer and indirectly promotes the progression of the tumour by providing the required microenvironment. A wide body of clinical and experimental works confirms that IL-19 is a key agent in breast cancer. It is involved in different processes, such as cell proliferation and migration, which accelerate tumour growth [21]. These results confirm that IL-19 is an indicator for breast cancer, which is in high agreement with the results of Chen et al. [21].
In terms of aging, the current study indicated no correlation between the age of participants and breast cancer. However, some previous studies indicated that other key factors, such as obesity and age, could motivate the tumour's genesis by providing the required inflammatory environment [22, 23]. Previous studies indicated three important facts. Firstly, the patients with breast cancer show apparent inflammatory responses [24]. Secondly, a direct correlation was found between the concentration of IL-19 and TNF-α and tumour grade in breast cancer patients [9]. Thirdly, IL-19 is an indicator for breast cancer as it helps to provide the required micro-environment for the progression of tumours [17, 25]. Finally, obesity contributes to tumorigenesis as it helps to develop an inflammatory environment [17, 26]. Thus, the literature recommends treating cancer by treating the inflammatory micro-environment [17, 27].
CONCLUSIONS
Our results indicated that high levels of IL-19 and TNF-α, linked to clinical disease stage and lymph node metastasis, have an autocrine effect on breast cancer cells and produce a milieu conducive to tumour growth. Additionally, it could be concluded from the obtained results that inhibiting IL-19 could be helpful in the treatment of breast cancer.
For future studies, it is recommended to carry out the same research in other countries to check whether these results are applicable elsewhere too.
ACKNOWLEDGMENTS
Conflict of interest
The author declares no conflict of interest
Ethical approval
The study was approved by the Institutional Review Board of Kirkuk University, Iraq (No. 603, August, 2021).
Consent to participate
Written informed consent was obtained from the participants.
Authorship
AKM contributed to conceptualizing, the methodology, data collection, data analysis, data curation, editing, and writing the original draft.
References
- 1.Mills RC., 3rd Breast Cancer Survivors, Common Markers of Inflammation, and Exercise: A Narrative Review. Breast Cancer (Auckl) 2017;11:1178223417743976. doi: 10.1177/1178223417743976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012 Mar 15;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 3.Rao VS, Dyer CE, Jameel JK, Drew PJ, Greenman J. Potential prognostic and therapeutic roles for cytokines in breast cancer (Review) Oncol Rep. 2006 Jan;15(1):179–85. doi: 10.3892/or.15.1.179. [DOI] [PubMed] [Google Scholar]
- 4.Calcinotto A, Grioni M, Jachetti E, Curnis F, et al. Targeting TNF-α to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol. 2012 Mar 15;188(6):2687–94. doi: 10.4049/jimmunol.1101877. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Deng J, Rychahou PG, Qiu S, et al. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009 May 5;15(5):416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushita S, Nitanda T, Furukawa T, Sumizawa T, et al. The effect of a thymidine phosphorylase inhibitor on angiogenesis and apoptosis in tumors. Cancer research. 1999;59(8):1911–1916. [PubMed] [Google Scholar]
- 7.Hwang JR, Jo K, Lee Y, Sung BJ, et al. Upregulation of CD9 in ovarian cancer is related to the induction of TNF-α gene expression and constitutive NF-κB activation. Carcinogenesis. 2012 Jan;33(1):77–83. doi: 10.1093/carcin/bgr257. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 2003;5(1):31–6. doi: 10.1186/bcr554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheen-Chen SM, Chen WJ, Eng HL, Chou FF. Serum concentration of tumor necrosis factor in patients with breast cancer. Breast Cancer Res Treat. 1997 May;43(3):211–5. doi: 10.1023/a:1005736712307. [DOI] [PubMed] [Google Scholar]
- 10.Hsing CH, Cheng HC, Hsu YH, Chan CH, et al. Upregulated IL-19 in breast cancer promotes tumor progression and affects clinical outcome. Clin Cancer Res. 2012 Feb 1;18(3):713–25. doi: 10.1158/1078-0432.CCR-11-1532. [DOI] [PubMed] [Google Scholar]
- 11.Hashim KS, Shaw A, AlKhaddar R, Kot P, Al-Shamma'a A. Water purification from metal ions in the presence of organic matter using electromagnetic radiation-assisted treatment. Journal of Cleaner Production. 2021;280(2):1–17. doi: 10.1016/j.jclepro.2020.124427. [DOI] [Google Scholar]
- 12.Hashim KS, Shaw A, Al Khaddar R, Pedrola MO, Phipps D. Energy efficient electrocoagulation using a new flow column reactor to remove nitrate from drinking water-Experimental, statistical, and economic approach. J Environ Manage. 2017 Jul 1;196:224–233. doi: 10.1016/j.jenvman.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Karaghool HAK, Hashim K, Kot P, Muradov M. Preliminary Studies of Methylene Blue Remotion from Aqueous Solutions by Ocimum basilicum. Environments. 2022;9(2):1–7. doi: 10.3390/environments9020017. [DOI] [Google Scholar]
- 14.Al-Hashimi O, Hashim K, Loffill E, Marolt Čebašek T, et al. A Comprehensive Review for Groundwater Contamination and Remediation: Occurrence, Migration and Adsorption Modelling. Molecules. 2021 Sep 29;26(19):5913. doi: 10.3390/molecules26195913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grmasha RA, Al-Sareji OJ, Salman JM, Hashim KS, Jasim IA. Polycyclic Aromatic Hydrocarbons (PAHs) in Urban Street Dust Within Three Land-Uses of Babylon Governorate, Iraq: Distribution, Sources, and Health Risk Assessment. Journal of King Saud University-Engineering Sciences. 2020;33:1–18. [Google Scholar]
- 16.Mandal R, Biswas S, Chaterjee K. BMI and Body fat percentage difference between caesarean and non-caesarean school girls. 2017;2(1):339–341. [Google Scholar]
- 17.Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Bustos M, et al. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta. 2011 Jun;1807(6):664–78. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006 May 25;441(7092):431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 19.Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4(2):65–9. doi: 10.1186/bcr425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015 Apr 13;27(4):462–72. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YY, Li CF, Yeh CH, Chang MS, Hsing CH. Interleukin-19 in Breast Cancer. Clinical and Developmental Immunology. 2013:1–9. doi: 10.1155/2013/294320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001 May;8(3):131–6. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002 Feb;23(1):90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 24.Standish LJ, Sweet ES, Novack J, Wenner CA, et al. Breast cancer and the immune system. J Soc Integr Oncol. 2008 Fall;6(4):158–68. [PMC free article] [PubMed] [Google Scholar]
- 25.Gao W, Wen H, Liang L, Dong X, et al. IL20RA signaling enhances stemness and promotes the formation of an immunosuppressive microenvironment in breast cancer. Theranostics. 2021 Jan 1;11(6):2564–2580. doi: 10.7150/thno.45280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saud Hussein A, Ibraheem Salih N, Hashim Saadoon I. Effect of Microbiota in the Development of Breast Cancer. Arch Razi Inst. 2021 Oct 31;76(4):761–768. doi: 10.22092/ari.2021.355961.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda A, Hasegawa E, Nakao S, Ishikawa K, et al. Vitreous levels of interleukin-35 as a prognostic factor in B-cell vitreoretinal lymphoma. Sci Rep. 2020 Sep 24;10(1):15715. doi: 10.1038/s41598-020-72962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]