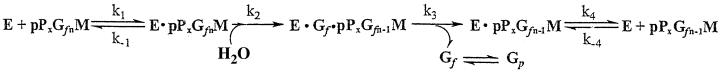Abstract
Extracellular Penicillium fellutanum exo-β-d-galactofuranosidase, with a mass of 70 kDa, was purified to apparent homogeneity. The enzyme was used to investigate the influence of phosphodiesters of the peptidophosphogalactomannans pP2GMii and pP25GMii (containing 2 and 25 phosphodiester residues, respectively, per mol of polymer) on the kinetic parameters of galactofuranosyl hydrolysis of these two polymers, of 1-O-methyl-β-d-galactofuranoside, and of two galactofuranooligosaccharides. The enzyme did not hydrolyze phosphorylated galactose residues of pP2GMii or pP25GMii. The kcat/Km value for pP25GMii is 1.7 × 103 M−1 s−1, that for 1-O-methyl-β-d-galactofuranoside is 1.1 × 104 M−1 s−1, that for pP2GMii is 1.7 × 10 4 M−1 s−1, and those for 5-O-β-d-galactofuranooligosaccharides with degrees of polymerization of 3.4 and 5.5 are 1.7 × 105 and 4.1 × 105 M−1 s−1, respectively. Variability in the kcat/Km values is due primarily to differences in Km values; the k−1/k1 ratio likely provides the most influence on Km. kcat increases as the degree of polymerization of galactofuranosyl residues increases. Most of the galactofuranosyl and phosphocholine residues were removed by day 8 in vivo from pPxGMii added to day 3 cultures initiated in medium containing 2 mM phosphate but not from those initially containing 20 mM phosphate. The filtrates from day 9 cultures initiated in 2 mM inorganic phosphate in modified Raulin-Thom medium contained 0.2 mM inorganic phosphate and 2.2 U of galactofuranosidase ml−1h−1. No galactofuranosidase activity but 15 mM inorganic phosphate was found in filtrates from day 9 cultures initiated in 20 mM phosphate. In vivo the rate of galactofuranosyl hydrolysis of pPxGMii and of related polymers is proportional to the kcat/Km value of each polymer. The kinetic data show that the kcat/Km value increases as the number of phosphodiesters of pPxGMii decreases, also resulting in an increase in the activity of exo-β-d-galactofuranosidase.
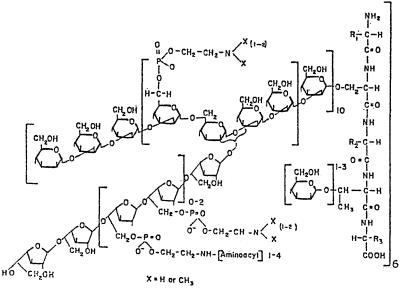
β-d-Galactofuranosyl residues occur in several genera and species of fungi (5–7, 10, 12, 18, 20, 22–24, 27, 34). Complex cell walls (9) of Penicillium fellutanum (formerly P. charlesii) and extracellular phosphorylated glycopeptides (peptidophosphogalactomannan pPxGM, with x phosphodiester residues per mol of polymer) have been isolated and partially characterized (8, 14, 16, 34, 40–42). pPxGM fractionates into four related species, pPxGMi, pPxGMii, pPxGMiii, and pPxGMiv, based on the affinity toward DEAE-cellulose · borate (36). pPxGMii (Fig. 1) is the major species and constitutes 80% or more of the total pPxGMs.
FIG. 1.
Structural features of peptidophosphogalactomannan. These features are based on methylation analyses and 13C and 31P NMR spectroscopy (9, 16, 40, 41). The diagram shows one phosphogalactomannan repeating unit attached to a 3-kDa peptide unit. The 5-O-β-galactofuranosyl-containing chain is attached to a mannotetraosyl residue through the C-3 position of a mannopyranosyl residue. Phosphocholine phosphodiester is the major phosphodiester. It is attached to C-6 of a mannopyranosyl residue. Phospho-1′-O-[N-peptidyl-(2′-aminoethanol)] residues are associated primarily, if not exclusively, with the galactofuran chains. (Reprinted from reference 32 with permission from the publisher.)
Two types of phosphodiesters occur in extracellular pPxGM. d-Mannopyranosyl-6-O-phosphocholine is a major phosphodiester species (40, 41) that occurs as part of the mannan. d-Galactofuranosyl-6-O-phospho-1′-O-[N-peptidyl-(2′-aminoethanol)] is the second type of phosphodiester found (8, 9). Removal of phosphocholine phosphodiester residues from pPxGMii converts it into pPxGMiii (32), a species that binds tightly to DEAE-cellulose · borate (36). Based on 31P nuclear magnetic resonance (NMR) spectroscopy (36), phosphocholine phosphodiester represents 90% or more of the phosphodiesters in pPxGMi. pPxGMi is a minor species of pPxGM.
The physiological roles of pPxGMii as a reserve source of carbon-, nitrogen-, and phosphate-containing precursors required during the process of osmotic protection or sulfate storage have been reported (29–32). During our investigations, indirect evidence was obtained which suggested that a nonspecific R-O-phosphocholine phosphodiester:phosphocholine hydrolase (15, 37) and exo-β-d-galactofuranosidase (29) produced by P. fellutanum may work in concert during the depolymerization of pPxGMs. An understanding of the mechanism of depolymerization of pPxGMii species and related extracellular species and how this depolymerization is regulated may provide insight into the physiology of cell wall and membrane turnover, stress management, cell growth, and other cellular processes. Enzyme-catalyzed depolymerization of cell walls and extracellular polymers and reutilization of their products as nutrients seem to be critical for the survival of P. fellutanum under various environmental conditions and conditions of exogenous nutrient depletion (9, 29–32).
Early work showed that increasing the pH of culture filtrates of modified Raulin-Thom medium from 2 to 4 with (NH4)2HPO4, (NH4)2CO3, Na2CO3, or NaOH resulted in the appearance of exo-β-d-galactofuranosidase activity in day 8 cultures (33) and that pPxGMii contained approximately 10 phosphodiesters. In contrast, P. fellutanum cultured for 4 to 5 days in a medium enriched with trace elements and containing citrate as the secondary source of carbon (36) produced pPxGMii that contained up to 60 phosphodiesters (8, 9); no exo-β-d-galactofuranosidase was detected in the medium. Reduction of the initial phosphate concentration in the medium from 20 to 2 mM resulted in a 35-fold increase in the activity of R-O-phosphocholine phosphodiester:phosphocholine hydrolase with p-nitrophenyl-phosphocholine as a substrate (37). The phosphodiester content of pPxGMii species obtained from day 10 cultures which initially contained 20 or 2 mM phosphate was 20 or 1 residues, respectively (36). It was also observed (39) that exo-β-d-galactofuranosidase obtained from Raulin-Thom medium did not bind to an affinity support obtained by reacting pP30GMii isolated from a medium containing citrate with cyanogen bromide-activated Sepharose 4B (35). These data lead us to question whether phosphodiesters of pPxGMii influence the exo-β-d-galactofuranosidase-catalyzed depolymerization of galactofuranosyl-containing galactan chains.
Here we report (i) a procedure for the purification of exo-β-d-galactofuranosidase, different from that reported previously (26); (ii) the influence of phosphodiester residues of extracellular pPxGMii on the kinetic properties of the purified enzyme, and (iii) the influence of phosphate concentration in the medium on the presence of β-d-galactofuranosyl and phosphocholine phosphodiester residues in pPxGMii in day 8 and day 9 cultures.
MATERIALS AND METHODS
Chemical and reagents.
All chemicals used, including l-[methyl-13C]methionine and 2H2O, were reagent grade and were purchased from Sigma Chemical Co., St. Louis, Mo., or Fisher Scientific Co., Pittsburgh, Pa. Commercial enzyme preparations were obtained from Sigma or Worthington Biochemical Co. Sodium (trimethylsilane)-1-propanesulfonate was obtained from Wilmad Glass Co., Buena, N.J.
Culture conditions and growth media.
P. fellutanum (formerly P. charlesii G. Smith; NRRL 1887) conidiospores were obtained from day 14 cultures. The conditions and procedures for obtaining the conidiospores are described elsewhere (1, 9, 35).
Analytical methods. (i)Determination of carbohydrate and phosphate.
Total carbohydrate was determined by a modified (1) phenol-sulfuric acid method (13) using 0.3 ml of sample, 20 μl of 80% phenol, and 1.0 ml of concentrated sulfuric acid. After the reaction, the A490 was compared with that of a solution of 0.9 mM galactose–0.3 mM mannose. Reducing sugars were determined by the Nelson procedure (28).
After a sample was reduced to ash (2), the quantity of phosphate remaining was determined at 820 nm (3). The reference was 0.4 μmol of KH2PO4. Inorganic phosphate was determined at 830 nm as the reduced phosphomolybdate complex (38) in filtrates of P. fellutanum cultured in Raulin-Thom medium modified to contain either 2 or 20 mM ammonium phosphate.
(ii) Determination of protein.
Protein was determined by the microbicinchoninic acid (micro-BCA) method of Pierce Biochemicals. Bovine serum albumin (10 to 200 μg/ml) served as the reference.
(iii) Determination of formaldehyde.
Oligosaccharides or pPxGMii (4 to 7 μmol of carbohydrate) in H2O were reacted with a fivefold molar excess of sodium metaperiodate for 18 h at 4°C in the dark (35). Excess periodate was destroyed with sodium arsenite. Formaldehyde generated by the oxidation was reacted with MacFadyn chromotropic acid reagent (25). The A570 was compared with that of the formaldehyde generated from 0.65 μmol of erythritol.
Enzyme assays. (i) Exo-β-d-galactofuranosidase activity.
Exo-β-d-galactofuranosidase activity was determined by the procedure of Rietschel-Berst et al. (35) using 1-O-methyl-β-d-galactofuranoside as a substrate. The galactose released during incubation at pH 4.0 and 40°C was determined as either micromoles of reducing sugar (28) or micromoles of galactose reacted in a coupled oxidation of galactose and o-cresol catalyzed by galactose oxidase and peroxidase, respectively, as described by Worthington Biochemical Co. One unit of exo-β-d-galactofuranosidase activity releases 1.0 μmol of galactose min−1 ml−1 at pH 4.0 and 40°C. Routine estimation of exo-β-d-galactofuranosidase activity was determined by monitoring the change in the optical rotation of the reaction mixture in either a decimeter JASCO DIP digital polarimeter or a Rudolph digital polarimeter with a 7-ml cell. An increase of +35 millidegrees in these 1-dm cells represents the hydrolysis of 1.0 μmol of substrate ml−1 and the accumulation of 1.0 μmol of d-galactose ml−1 using specific optical rotations in water of 1-O-methyl-β-d-galactofuranoside and d-galactose of −110 and +83.5 degrees, respectively. A concentration of 5 mM (observed change of −107 millidegrees) 1-O-methyl-β-d-galactofuranoside was used routinely, except in one experiment, in which 20 mg (61 μmol of galactofuranosyl residues) of pPxGMii was used as a substrate to measure enzyme activities in culture filtrates of day 9 modified Raulin-Thom medium that contained 0.2 or 15 mM inorganic phosphate.
(ii) Activities of glycohydrolases and phosphomono- and phosphodiesterases.
Synthetic p-nitrophenyl derivatives of carbohydrates and phosphomono- and phosphodiesters served as substrates in some experiments in which activities of glycohydrolase and acid phosphoesterases were measured. The p-nitrophenol formed over 2 h at pH 4.0 and 40°C was quantified as p-nitrophenolate (E410, = 18.3 mM−1 cm−1) after the addition of an equal volume of 0.2 N NaOH.
Preparation of 1-O-methyl-β-d-galactofuranoside, pPxGMii species, and 5-O-β-d-galactofuranooligosaccharides.
1-O-Methyl-β-d-galactofuranoside was prepared by the procedure of Augstead and Berner (4) and fractionated on powdered cellulose as described previously (35). Substrate preparations had specific optical rotations of −105° to −108°.
pPxGMii, pP2GMii, and pP25GMii were isolated from culture filtrates and purified on Whatman DE-52 cellulose as reported previously (9, 16, 34, 35). The samples were desalted and stored as lyophilized products until used.
Galactofuranooligosaccharides were prepared by treatment of pP2GMii with 10 mM HCl in a molar ratio of hexose to HCl of 1:50 at 100°C for 90 min. The reaction was neutralized, and low-molecular-weight substances were removed by dialysis through Spectrapor membranes (molecular weight cutoff, 3,500). These saccharides were concentrated and passed through a DE-52 column. The filtrate was concentrated and fractionated on 200-mesh Bio-Gel P4. Two 5-O-β-d-galactofuranooligosaccharide-containing fractions had degree of polymerizations (DP) of 3.4 and 5.5. The DP was determined from the ratio of total carbohydrate to formaldehyde.
Enzyme purification.
On day 17, solid phenylmethylsulfonyl fluoride (17 μg/ml) was added to cultures on Raulin-Thom medium (10, 35). The cultures were harvested on day 18 and filtered through Whatman no. 4 filter paper. Filtrates were dialyzed in Spectrapor membrane tubing with an MWCO of 14,000 against 50 mM sodium citrate (pH 5.0) at 4°C. The dialysates were concentrated approximately 10-fold in YM-30 membrane (MWCO, 30,000). Enzyme preparations and buffers were filter sterilized. The crude enzyme preparations were fractionated on a DE-52 column preequilibrated with 50 mM sodium citrate (pH 5.0).
Fractions 10 to 27 containing exo-β-d-galactofuranosidase activity had little or no pPxGMs. These fractions were combined, concentrated, and applied to DE-52 preequilibrated with 50 mM morpholinepropanesulfonic acid (MOPS) (pH 7.5). The gel was irrigated with a stepwise gradient of MOPS (fractions 1 to 20), MOPS–0.12 M NaCl (fractions 21 to 30), and MOPS–0.25 M NaCl. Fractions 31 to 40, which contained exo-β-d-galactofuranosidase activity, were combined, dialyzed against 12.5 mM sodium tartrate buffer (pH 3.0), and fractionated on CM-Sepharose preequilibrated with 12.5 mM sodium tartrate (pH 3.0). The gel was irrigated with the same buffer containing 0.12 M NaCl (fractions 21 to 45) and the same buffer containing 0.25 M NaCl (fractions 46 to 80). Fractions 55 to 70, which contained enzyme activity, were pooled and concentrated. Samples (200 μl) which contained 20 to 200 μg of protein in 10 mM sodium acetate–10 mM NaCl (pH 4.0) were filtered through a 0.22-μm-pore-size filter and fractionated twice on a Superose-12 fast protein liquid chromatography (FPLC) gel column of (Pharmacia; void volume, 7.5 ml).
SDS-PAGE.
Polyacrylamide gel electrophoresis (PAGE) of galactofuranosidase was performed on a mini-slab gel apparatus at 200 V (constant) using the Laemmli buffer system (21). Gels were cast on glass plates (7 by 10 cm). Separating and stacking gels contained 12 and 4% acrylamide, respectively. Samples in sodium dodecyl sulfate (SDS) reducing buffer (pH 6.8; 50 mM Tris-HCl in 10% glycerol–2% SDS–5% mercaptoethanol–0.002% bromophenol blue) were heated at 100°C for 5 min. The gels were stained with 0.1% Coomassie brilliant blue R-250 and then with Bio-Rad silver.
Nondenaturing gel electrophoresis.
Nondenaturing gel electrophoresis of exo-β-d-galactofuranosidase was performed with the apparatus described above. Buffer systems are described in Sigma technical bulletin MKR-137. Separating and stacking gels contained 7 to 10% and 4% acrylamide solutions, respectively. Samples were suspended in reducing buffer (pH 6.7; 50 mM Tris-HCl–glycerol–H2O–bromophenol blue [1:1:1:250, vol/vol/vol/wt]). The molecular weights were determined using Ferguson plots (19) as explained in detail in MKR-137. A retardation coefficient is obtained from a determination of the slope obtained upon plotting log10(Rf × 100) versus percent gel concentration for each protein. Plotting the log10 molecular weight of each reference protein versus the log10 negative slope establishes a linear plot for estimating the molecular weight of exo-β-d-galactofuranosidase.
IEF.
Isoelectric focusing (IEF) of exo-β-d-galactofuranosidase was performed with a PhastSystem (Pharmacia). PhastGel IEF medium precast in homogeneous (5% T, 3% C) polyacrylamide (pH 4.0 to 6.5) was used for determination of the isoelectric point (17).
RESULTS
Ion-exchange chromatography and gel permeation chromatography of exo-β-d-galactofuranosidase.
Enzyme partially purified from Raulin-Thom medium (35) contains acid phosphatases and phosphodiesterases that also bind to pPxGMii-Sepharose 4B (39). These phosphatases were removed during the purification of exo-β-d-galactofuranosidase as described in Materials and Methods. The glycohydrolase was purified approximately 100-fold (Table 1 and Fig. 2). Forty-five percent (25 U mg−1) of the enzyme activity in the extracellular fluid was recovered.
TABLE 1.
Purification of exo-β-d-galactofuranosidase
| Fraction | Protein (mg)a | Total activity (U) | Sp act (U/mg)b | Recovery
|
|
|---|---|---|---|---|---|
| Foldc | % | ||||
| Cruded | 77 | 19 | 0.25 | 100 | |
| DEAE Ie | 47 | 14 | 0.30 | 1.2 | 74 |
| DEAE IIf | 13 | 10 | 0.77 | 3.1 | 53 |
| CMg | 1.2 | 5.8 | 4.8 | 19 | 31 |
| FPLC IIh | 0.34 | 8.5 | 25 | 100 | 45 |
Protein was measured by the microbicinchoninic acid assay using bovine serum albumin as a reference.
Specific activity is defined as micromoles of product formed milligram of protein−1 minute−1 milliliter−1 from the substrate, 1-O-methyl-β-d-galactofuranoside.
Fold increase in specific activity.
Crude enzyme was obtained by filtering day 18 cultures. Filtrate (500 ml) was concentrated approximately 10-fold on a membrane with an MWCO of 30,000. Chromatographic procedures are described in Materials and Methods.
DEAE I, fractions 10 to 27, from the first DE-52 column chromatography.
DEAE II, fractions 31 to 40 from the second DE-52 column chromatography.
CM, fractions 55 to 70, from CM-Sepharose column chromatography.
FPLC II, fractions 31 to 37, from the second Superose-12 gel filtration column chromatography.
FIG. 2.
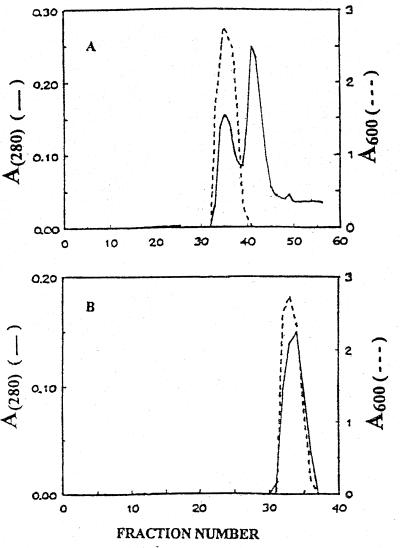
Fractionation of exo-β-d-galactofuranosidase by FPLC on Superose-12. Fractions 55 to 70 from the CM-Sepharose column were pooled, concentrated, and applied to a column of Superose-12 preequilibrated with 10 mM sodium acetate–10 mM NaCl (pH 4.0). The elution rate was 0.12 ml min−1 and the fraction size was 250 μl. Protein and exo-β-d-galactofuranosidase activities in 20-μl alternate fractions are represented by solid and broken lines, respectively. (A and B) First and second passes respectively, of enzyme through the FPLC column.
The increases in both total activity and specific activity that occurred in the second pass through the size exclusion column may have resulted from the removal of a protein in fractions above 40 that interacted with the galactofuranosidase.
Gel electrophoresis of exo-β-d-galactofuranosidase.
Electrophoresis of the purified enzyme in SDS resulted in a single band at 75 kDa. Nondenaturing PAGE of this purified enzyme followed by staining with Coomassie brilliant blue R-250 and Bio-Rad silver revealed bands at approximately 150 and 70 kDa (data not shown). IEF of a purified preparation on PhastGel IEF medium (pH 4.0 to 6.5) resulted in one band at a pI of 4.35. In a companion experiment, the lane of gel containing the enzyme was cut into 2-mm sections, and each section was assayed for exo-β-d-galactofuranosidase activity. Only one section contained significant activity; that activity coincided with the location of the protein (data not shown).
Carbohydrate content.
Mannose (2.7 μg) but no galactose was found in 16 μg of protein. Thus, the enzyme contains approximately 60 mannosyl residues per mol of enzyme, based on a molecular mass of 70 kDa for the glycoprotein.
Properties of exo-β-d-galactofuranosidase.
The purified enzyme had optimal activity from pH 4.0 to pH 4.5. When the enzyme was incubated at 24°C for 24 h, the pH for optimum stability was maximal at pH 4.0 to 5.0 (20 U/mg); incubation at pH 3.5 and at pH 6.0 resulted in activities of 17 and 10 U mg of protein−1, respectively. The optimum temperature for enzyme activity in 60-min assays was 40°C. Activities decreased 10 and 40% at 50 and 60°C, respectively.
In experiments containing 0.0076 U of enzyme and 1-O-methyl-β-d-galactofuranoside as a control, none of the following potential substrates was hydrolyzed: 1-O-(p-nitrophenyl)-β-d-galactopyranoside, 1-O-(p-nitrophenyl)-α-d-galactopyranoside, 1-O-(p-nitrophenyl)-β-N-acetyl-d-glucopyranosylamine, p-nitrophenyl-phosphocholine, p-nitrophenyl-phosphate, or bis-(p-nitrophenyl)-phosphate. The enzyme catalyzed the hydrolysis of 1-O-(p-nitrophenyl)-β-d-galactofuranoside, with the release of p-nitrophenol. These data suggest that R-O-phosphocholine phosphodiester:phosphocholine hydrolase and other phosphoesterase activities that bind pPxGM-Sepharose 4B (39) have been removed.
Time course and extent of pP2GMii and pP25GMii degalactosylation.
Galactose (15 μmol) was released from pP2GMii (4.7 mg; ∼3.0 mM nonreducing terminal galactofuranosyl residues) with 4 μg of exo-β-d-galactofuranosidase ml−1 at 24°C at a rate of 0.075 μmol h−1 over 200 h, as monitored by optical rotation. Galactose (5.7 μmol) was released from pP25GMii (3.2 mg; ∼1.8 mM nonreducing terminal galactofuranosyl residues) with 4 μg of enzyme ml−1 at 24°C over 120 h. No galactose was released from pP25GMii after 96 h. Thus, 87 and 52% of the galactose in pP2GMii and pP25GMii, respectively, was released by treatment with the enzyme. This treatment decreased the average chain length of the galactofuran chains attached to pP2GMii and pP25GMii from a DP of 5.4 to 1.3 and from a DP of 6.0 to 3.7, respectively (Table 2). The number of galactofuran chains per mol of pP2GMii decreased from 32 to 17, and that of pP25GMii decreased from 23 to 18. There was a negligible loss of phosphodiesters from the polymers during treatment with the enzyme.
TABLE 2.
Influence of treatment of pP2GMii and pP25GMii with exo-β-d-galactofuranosidase (exo-β-Galfase) on the DP of galactofuran chainsa
| Phosphate and carbohydrate components of pPGMx analyzed | Molar ratio for component pairs of the following polymers after the indicated treatment:
|
|||
|---|---|---|---|---|
| pP25GMii
|
pP2GMii
|
|||
| None | Exo-β-Galfasea | None | Exo-β-Galfase | |
| Galactose/total carbohydrate | 0.55 | 0.37 | 0.60 | 0.16 |
| Galactose/nrtGalactose | 6.0 | 3.7 | 5.4 | 1.3 |
| Total carbohydrate/PO4 | 10 | 9.2 | 166 | 109 |
| Galactofuran chains/mol | 23 | 18 | 32 | 17 |
Reaction mixtures with 4 to 8 μmol of galactofuranosyl residues from pP2GMii (50 kDa) or pP25GMii (47 kDa) in 1.5 ml of 66 mM acetate buffer (pH 4.0) containing 6 μg of purified enzyme were incubated in a 1-dm cuvette in a JASCO DIP polarimeter at room temperature for 200 or 130 h, respectively. Galactose was removed through a Spectrapor membrane. The retentate was analyzed for total phenol-sulfuric acid-positive carbohydrate, galactose, nonreducing terminal galactofuranosyl residues (nrtGalactose), and phosphate. Similar untreated samples of polymers served as controls.
Similar treatment of (1 → 5)-β-d-galactofuranooligosaccharides with DP of 3.4 and 5.5 or 1-O-methyl-β-d-galactofuranoside with exo-β-d-galactofuranosidase resulted in greater than 99% hydrolysis in less than 96 h. Galactose was the only saccharide produced by the enzyme-catalyzed hydrolysis of pP25GMii.
Based on paper chromatography with butanol-pyridine-H2O, (6:4:3), the only saccharide product that eluted from a column of Bio-Gel P2 was coincident with reference galactose. Partially degraded pP25GMii and protein eluted in the voided volume.
Kinetic properties of exo-β-d-galactofuranosidase.
The initial velocities of exo-β-d-galactofuranosidase-catalyzed hydrolysis of nonreducing terminal galactofuranosyl residues of pP2GMii and pP25GMii were determined from 0.25 mM to 1.05 and 1.4 mM, respectively. Concentrations of 0.1 to 0.75 mM galactofuranooligosaccharides with DP of 3.4 and 5.5 were used. The concentration range for 1-O-β-methyl-d-galactofuranoside was 2.4 to 11 mM.
The rate of hydrolysis of nonreducing terminal galactofuranosyl residues of pP25GMii increased linearly to 1.4 mM (∼3 mg/ml). The linearity shows that the range of substrate concentrations is far below the apparent Km. A calculated kcat/Km value of 1.7 × 103 M−1 s−1 was estimated from the reciprocal of the slope (Table 3). Concentrations of pP25GMii of greater than 1.6 mM in nonreducing terminal residues were too viscous to obtain valid data. The kcat/Km values for 1-O-methyl-β-galactofuranoside, pP2GMii, and 5-O-β-d-galactofuranosides with DP of 3.4 and 5.5 are 1.1 × 103, 1.7 × 104, 1.7 × 105, and 4.1 × 105 M−1 s−1, respectively. These data show the influence of phosphodiesters on the DP in the region of first-order rate of hydrolysis.
TABLE 3.
Kinetic constants for exo-β-d-galactofuranosidase-catalyzed hydrolysis of substratesa
| Substrate | Km (mM) | kcat (s−1) | kcat/Km (M−1 s−1) |
|---|---|---|---|
| pP2GMii | 0.80 | 14 | 1.7 × 104 |
| pP25GMii | >1.5 | ND | 1.7 × 103b |
| β-d-Galf(5,6) | 0.10 | 41 | 4.1 × 105 |
| β-d-Galf(3,4) | 0.25 | 43 | 1.7 × 105 |
| 1-O-Methyl-β-Galf | 2.6 | 29 | 1.1 × 104 |
Abbreviations: β-Galf(5,6), mixture of penta- and hexa-β-(1→5)-d-galactofuranooligosaccharides with a DP of 5.5; β-d-Galf(3,4), mixture of tri- and tetra-β-d-(1,5)-galactofuranooligosaccharides with a DP of 3.4; 1-O-Methyl-β-Galf, 1-O-methyl-β-d-galactofuranoside. ND, not determined. The concentration of nonreducing terminal galactofuranosyl residues in pPxGMii was calculated based on the percentage of galactofuranosyl in the total carbohydrate of the polymer and the ratio of total galactose to nonreducing terminal galactofuranosyl residues. The enzyme activities were referenced to 25 U mg−1 with 1-O-methyl-β-d-galactofuranoside as a substrate. Apparent Km and kcat were determined from Cornish-Bowden–Wharton plots (11). The kcat was calculated based on an enzyme molecular mass of 70 kDa.
The kcat/Km for the exo-βd-galactofuranosidase-catalyzed depolymerization of pP25GMii was estimated from the slope of the first order region of the curve for the initial velocity versus the nonreducing terminal galactofuranosyl-pP25GMii concentration.
kcat and Km values were obtained for 1-O-β-methyl-d-galactofuranoside, pP2GMii, and the galactofuranooligosaccharides. kcat and Km values for galactofuranooligosaccharides with DP of 3.4 and 5.5 were 43 s−1 and 0.25 mM and 41 s−1 and 0.10 mM, respectively; those for 1-O-methyl-β-d-galactofuranoside and pP2GMii were 29 s−1 and 2.6 mM and 14 s−1 and 0.80 mM, respectively. Removal of phosphodiesters and increasing the DP decrease the apparent Km and increase the kcat, resulting in higher kcat/Km values.
Influence of phosphate concentration in the culture medium on the activity of exo-β-d-galactofuranosidase.
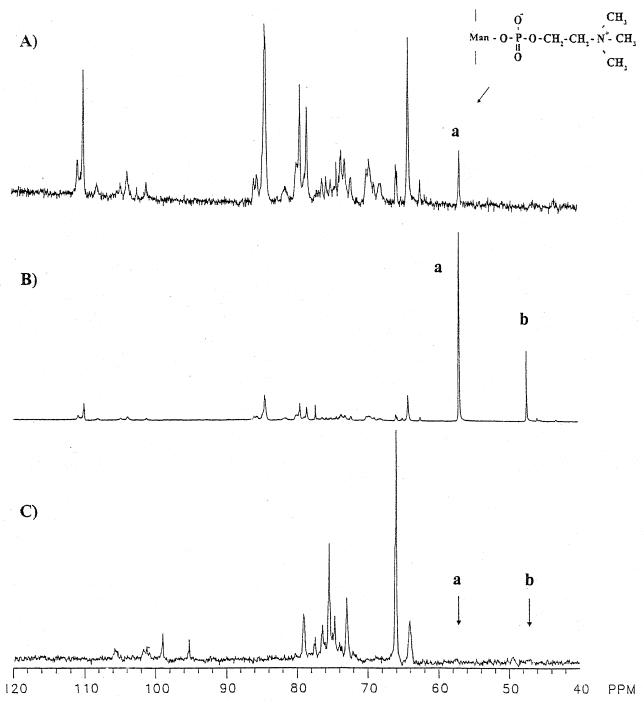
The kinetic data suggest that the exo-β-d-galactofuranosidase-catalyzed hydrolysis of galactofuranosyl residues of phosphodiesters of pP2GMii and especially those of pP25GMii decreases with increasing phosphodiester content. An experiment was performed to determine if P. fellutanum cultures on medium initially containing either 2 or 20 mM phosphate removed the galactofuranosyl as well as the phosphocholine residues from added pPxGMii. pPxGMii from day 8 cultures in medium initially containing 20 mM phosphate served as the first control (Fig. 3A). Natural-abundance 13C NMR signals at 110.6 and 109.6 ppm are those of the C-1 atom of nonreducing terminal and internal galactofuranosyl residues, respectively; the signal at 84.0 ppm is that of C-2 and C-4 and that at 80.1 ppm is that of C-5 of 5-O-β-d-galactofuranosyl residues (42). The signal at 56.83 ppm is that of methyl groups of the phosphocholine phosphodiesters of pPxGMii (29, 30, 40). All other signals are from the mannopyranosyl residues.
FIG. 3.
Effect of phosphate concentration on the release of galactofuranosyl residues from pPxGMii. Proton-decoupled 13C NMR spectra of extracellular pPxGMii isolated from day 8 culture filtrates are shown. (A) Spectrum of pPxGMii obtained from a culture originally in 20 mM phosphate medium [methyl- 13C]phosphocholine-containing pPxGMii (200 mg per 200 ml of medium) was added on day 3 to separate cultures originally in 20 mM and 2 mM phosphate. (B and C) Spectra of pPxGMii from cultures in 20 mM phosphate and 2 mM phosphates, respectively. Spectra of pPxGMii were recorded with 13,476, 18,557, and 13,649 acquisitions for panels A, B, and C, respectively. Ninety-degree radiofrequency pulses of 25 μs were applied at 4-s intervals. The signal designated “a” at 56.75 ppm is of the methyl carbons of phosphocholine attached to C-6 of mannopyranosyl residues in pPxGMii. The signals at 110.6, 109.6, 84.0, and 80.1 are those of the nonreducing terminal C-1 and internal C-1, C-2 and C-4, and C-5 atoms of 5-O-β-d-galactofuranosyl residues, respectively, of pPxGMii. The signals are as assigned by Unkefer et al. (40, 42). (Reprinted from reference 29 with permission from the publisher.)
A culture containing 200 mg of [methyl-13C]phosphocholine-enriched pPxGMii was added to day 3 culture medium of P. fellutanum initially containing 20 mM phosphate. The 13C NMR spectrum (Fig. 3B) of pPxGMii isolated from this culture after day 8 had major signals at 56.8 and 47.1 ppm resulting from the 13C-enriched methyl groups of phosphocholine phosphodiesters and its 2-N,N′-dimethyl-aminoethanol analog (29, 40). Signals at 109.6, 84.0, and 80.1 ppm indicate that pPxGMii from cultures initially containing 20 mM phosphate also had unenriched [13C]galactofuranosyl residues (43). The 13C NMR spectrum (Fig. 3C) of pPxGMii from a companion culture also containing [methyl-13C]phosphocholine-enriched pPxGMii but modified initially to contain only 2 mM phosphate did not have β-d-galactofuranosyl signals at 109.6, 110.6, 84.0, and 80.1 ppm or a signal at 56.83 ppm for the methyl group of phosphocholine. These data show that galactofuranosyl and choline or phosphocholine residues were removed from added pP25GMii between days 3 and 8.
Although it has been shown (29, 32) that the activity of nonspecific R-O-phosphocholine phosphodiester:phosphocholine hydrolase is low in cultures containing 20 mM phosphate and that this activity peaks at days 6 to 8 in cultures containing 2 mM phosphate, the phosphate concentration in the culture filtrate during the interval from day 3 to day 9 was not known. The relative concentrations of inorganic phosphate in cultures of Raulin-Thom medium initially containing 2 and 20 mM inorganic phosphate are shown in Table 4. The concentration of inorganic phosphate decreased from 2 to 0.42 mM in day 2 cultures and decreased more slowly over the next 12 days. In contrast, the decrease in phosphate concentration in 20 mM phosphate cultures was much slower; the concentration was approximately 15 mM on day 14.
TABLE 4.
Changes in concentration of inorganic phosphate and activity of exo-β-d-galactofuranosidase as a function of culture age and initial phosphate concentrationa
| Culture age (days) | Inorganic phosphate concn (mM) in the presence of an initial phosphate concn of:
|
Exo-β-d-galactofuranosidase activity (U h−1 ml−1) in the presence of an initial phosphate concn of:
|
||
|---|---|---|---|---|
| 2.0 mM | 20 mM | 2.0 mM | 20 mM | |
| 2 | 0.42 | 19.8 | ND | ND |
| 3 | 0.40 | 19.2 | ND | ND |
| 5 | 0.38 | 17 | ND | ND |
| 7 | 0.33 | 16 | ND | ND |
| 9 | 0.20 | 15 | ND | 2.2 |
| 14 | 0.13 | 15 | ND | 1.0 |
| 17 | <0.05 | 9.6 | ND | ND |
Approximately 3 ml of culture filtrate was collected through sintered glass and centrifuged for 4 min at 4,000 × g to pack any particulate material. Sample volumes ranging from 20 to 300 μl of culture filtrate were reacted with ammonium molybdate (38), and the A820 was compared with that of reference concentrations ranging from 0.05 to 1.0 μmol of KH2PO4. One milliliter of culture filtrate was added to 60 ml of 50 mM sodium acetate (pH 4.3) in H2O containing 20 mg of pPxGM. This reaction mixture was placed in a Rudolph polarimeter cell, and an optical rotation of 0.74 millidegrees was obtained. The reaction mixture was maintained at 25°C, and the observed optical rotation was measured again after 4 hr. ND, none detected.
Table 4 also shows the activity of exo-β-d-galactofuranosidase in culture filtrates on days 3, 5, 7, and 9. pPxGMii (1 mg ml of culture−1) was added on day 3 to separate flasks of Raulin-Thom medium initially containing 2 or 20 mM inorganic phosphate. Each flask was pretreated for 24 h prior to sample removal with 0.1 mg of phenylmethylsulfonyl fluoride ml of culture−1. No activity was detected in day 3, 5, or 7 cultures. However, 2.2 U of exo-β-d-galactofuranosidase activity was found in culture filtrates of day 9 medium containing 0.2 mM inorganic phosphate but not in companion culture filtrates of medium containing 15 mM phosphate.
DISCUSSION
The question of whether the phosphodiesters of pPxGMii species have a role in pPxGMii depolymerization could be answered only by determining the kinetic properties of purified exo-β-d-galactofuranosidase on two major pPxGMii species and oligosaccharides derived from pPxGMii species. Preliminary studies showed that exo-β-d-galactofuranosidase did not bind to pPxGMii-Sepharose 4B when pPxGMii was obtained from cultures maintained on a defined standard growth medium initially containing 20 mM phosphate (39). Furthermore, culture filtrates from this medium, analyzed daily to day 30, contained no significant exo-β-d-galactofuranosidase activity. At the outset of this work, it became evident that affinity-purified (39) exo-β-d-galactofuranosidase also contained acid phosphomonoesterase and R-O-phosphocholine:phosphocholine hydrolase activities. A procedure for purification of the enzyme was undertaken with the ultimate objective of determining the kinetic properties of purified exo-β-d-galactofuranosidase in reaction with each of the following substrates: 1-O-β-methyl-d-galactofuranoside, 5-O-β-d-galactofuranooligosaccharides, pP2GMii, and pP25GMii.
Table 3 shows that the apparent Km values for the substrates ranged from 0.1 mM for 5-O-β-d-galactofuranooligosaccharide with a DP of 5.5 to 2.6 mM for 1-O-β-methyl-d-galactofuranoside. The apparent Km for pP25GMii was too high to measure. However, its kcat/Km value, which measures the rate of conversion of substrate to the E · pP25GMii complex at concentrations of substrate where the reaction is first order with respect to the substrate, was nearly sevenfold lower than that of 1-O-β-methyl-d-galactofuranoside. This result and the fact that velocity increased linearly over the range of pP25GMii concentrations up to 1.5 mM suggest that the rate of pP25GMii binding to the enzyme (k1) is much lower than the k1 values of other substrates binding to the enzyme, especially 5-O-β-d-galactofuranooligosaccharides. Furthermore, if the rate of binding is decreased, then it is likely that the stability of the E · pP25GMii binary complex will be decreased in such a manner as to increase k−1. The kcat of these substrates varied from 43 s−1 to 14 s−1. The Km and kcat/Km values for pP25GMii are consistent with the inability of the enzyme to bind to pP10GMii-Sepharose 4B (39).
The multiple charges and N-trimethyl groups in pP25GMii, compared with those in pP2GMii, may inhibit the binding of galactofuranosyl residues in the proper orientation and may also decrease the stability of the E · pP25GMii complex compared with that of the E · pP2GMii complex. Thus, a decreased rate of binding of the E · pP25GMii complex and the formation of a less stable E · pP25GMii complex that has an increased k−1 are also consistent with the Km values of pP25GMii and pP2GMii and with those of 5-O-β-d-galactofuranooligosaccharides.
The kcat values ranged from 43 s−1 for galactofuranooligosaccharide with a DP of 5.5 to 14 s−1 for pP2GMii. The kcat for pP25GMii could not be calculated; however, if kcat for pP25GMii was also 14 s−1, then Km for that substrate would be 8.2 mM. From the data it is apparent that multiple phosphodiesters attached to the saccharides of pPxGMii species serve to modulate the activity of exo-β-d-galactofuranosidase.
Based on these data, the least complex representation of pPxGMii binding to the enzyme, catalysis, and release of products is shown in Fig. 4. This model describes the release of one galactofuranose (Gf) residue in a reaction sequence describing the initial-velocity steady-state condition in which the polymer dissociates after each nth round of hydrolysis. Note that the second and third steps are shown as being not significantly reversible. Under these conditions and because the values for k−2 and k−3 are both ∼0, the terms expressing Km can be shown in equation 1 and those for kcat and kcat/Km can be shown in equations 2 and 3:
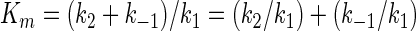 |
1 |
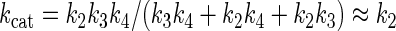 |
2 |
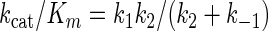 |
3 |
The hydrolytic step (k2) in 55.5 M H2O drives product formation, and the equilibrium between bound furanose, Gf, and unbound pyranose, Gp, forms leans more than 20-fold toward pyranose forms. The rate of release of Gf (k3) may be approximately constant and much more rapid than either k2 or k4. Furthermore, k2 probably has the greatest influence on determining the value of kcat based on the observations that the kcat values of 1-O-β-methyl-d-galactofuranoside and the two galactofuranooligosaccharides are about the same and that the kcat value of pP2GMii is only threefold lower than the maximum rate. We conclude that the two phosphodiesters in pP2GMii do not play large role in dictating the magnitude of kcat for hydrolysis.
FIG. 4.
Proposed sequence of the exo-β-d-galactofuranosidase-catalyzed reaction. The sequence is based on the kinetic parameters of the catalyzed depolymerization of pPxGfnMii. Abbreviations: E, exo-β-d-galactofuranosidase; pPxGfnM, peptidophosphogalactomannan with x phosphopdiesters and n glactofuranosyl (Gf) residues; M, mannan to which the galactofuranosyl chains are attached; k1, bimolecular rate constant for binding of the substrate to the enzyme; E · pPxGfnM, enzyme-substrate complex; k2, bimolecular rate constant for hydrolysis of a Gf residue; k3, unimolecular rate constant for Gf release from the ternary complex, E · Gf · pPxGf(n − 1)M; k4, unimolecular rate constant for E · Gf(n − 1)M enzyme-product binary complex disassociation.
At a low concentration of enzyme relative to substrate, as exists in culture filtrates, the values of k1 and k−1 become major determinants of the apparent Km, especially when k1 is only about 2 orders of magnitude higher than k−1. Furthermore, the kcat/Km ratio at a very low concentration of pPxGMii is approximated by the reciprocal of the slope of the line generated by a plot of 1/[pPxGMii] versus 1/V0, that is, k1k2/(k2 + k−1), if k2 is much lower than k3 and k 4. However, the k−1/k2 ratio has the potential to be of major importance if k−1 increases with increasing numbers of phosphodiester residues and k1 decreases.
At physiological concentrations of pPxGMii (∼50 μM nonreducing terminal galactofuranosyl residues), the average rate of hydrolysis of pP2GMii nonreducing terminal galactofuranosyl residues in day 18 medium containing 1.5 μg of enzyme ml−1 is approximately 66 μM h−1. Thus, that for pP25GMii would be 6.6 μM h−1. Therefore, pP2GMii is approximately a 10-fold better substrate than pP25GMii at physiological extracellular concentrations of both potential substrates (Table 3).
Extended treatment of pP2GMii that contained only about two phosphodiester residues with exo-β-d-galactofuranosidase reduced the average galactan chain DP to 1.3 residues and reduced the number of galactan chains from 32 to 17. After similar treatment of pP25GMii, the DP was not reduced below 3.7 by further incubation, and there were only five fewer galactan chains. We conclude that the phosphodiester residues in pPxGMii serve to limit the rate of release of galactofuranosyl residues. As the charged phosphodiester residues are removed, the number of galactofuranosyl residues in the galactofuran chains serves to regulate their rate of release.
The presence of extracellular exo-β-d-galactofuranosidase activity is influenced indirectly by the concentration of phosphate in the medium. Extracellular pPxGMii added to day 3 cultures initially containing 2 mM phosphate lost its galactofuranosyl residues as well as its choline or phosphocholine residues by day 8. A burst of R-O-phosphocholine:phosphodiester phosphocholine hydrolase activity between days 4 and 8 has been noted previously (15, 31). In contrast, pPxGMii isolated from control cultures initially containing 20 mM phosphate retained galactofuranosyl and phosphocholine phosphodiester residues. Negligible R-O-phosphocholine:phosphocholine hydrolase or exo-β-d-galactofuranosidase activities are present in P. fellutanum culture filtrates obtained between days 3 and 16 from cultures initially containing 20 mM phosphate (36). Extracellular exo-β-d-galactofuranosidase activity usually appears in the medium soon after day 16. The addition of pPxGMii to day 3 low-phosphate medium resulted in the release of sufficient R-O-phosphocholine:phosphodiester phosphocholine hydrolase and exo-β-d-galactofuranosidase activities to remove essentially all of the phosphocholine and galactofuranosyl residues from extracellular pPxGMii by day 8 (Fig. 3C).
In separate experiments, the concentration of inorganic phosphate was measured in filtrates of P. fellutanum cultures initially containing 2 or 20 mM inorganic phosphate. The data establish that there is a rapid depletion of phosphate to 0.4 mM by day 2 and 0.2 mM by day 9 in cultures initially containing 2 mM inorganic phosphate (Table 4). The culture that initially contained 20 mM phosphate absorbed only about one-half of the phosphate over 17 days.
Approximately 2.2 U of exo-β-d-galactofuranosidase was found in day 9 culture filtrates containing approximately 0.2 mM phosphate after the addition of 200 mg of pPxGMii on day 3 and 25 mg of phenylmethylsulfonyl fluoride on day 8 (Table 4). No galactofuranosidase activity was detected in cultures initially containing 20 mM phosphate. These data are consistent with those obtained upon examining pPxGMii by NMR spectroscopy (Fig. 3C).
Examination of the results of subjecting pP25GMii and pP2GMii to digestion for 130 and 200 h, respectively, with exo-β-d-galactofuranosidase showed that both the rate and the extent of galactofuranosyl hydrolysis of pP25GMii were diminished compared with those of pP2GMii as a substrate. Nevertheless, both the DP of pP25GMii galactan chains and the average number of galactofuranosyl residues per chain were decreased significantly, even though slowly. These data are also consistent with the previous finding of as many as 40 galactan chains per molecule of pP40GMii in day 4 to 5 cultures (9, 15) but only 10 galactan chains in day 10 culture filtrates that had low exo-β-d-galactofuranosidase activity (35).
We conclude that the phosphodiesters of extracellular pPxGMii modify the kinetic parameters of exo-β-d-galactofuranosidase activity and thus modulate the rate of galactofuranosyl hydrolysis until these phosphodiesters are removed by extracellular phosphodiesterases. This notion suggests a relationship in which the early depletion of phosphate from the medium results in the release of extracellular R-O-phosphocholine:phosphocholine hydrolase activity, which in turn initiates the removal of phosphocholine residues from pP60GMii. As the number of phosphocholine residues in pPxGMii decreases, the pPxGMii species become increasingly better substrates for exo-β-d-galactofuranosidase because the kcat/Km value increases with decreasing number of phosphodiesters.
ACKNOWLEDGMENTS
We thank James F. Preston, Department of Microbiology and Cell Science, University of Florida, and Sandra J. Bonetti, Department of Chemistry, University of Southern Colorado, for reviewing the manuscript and for useful comments and Penelope A. Naranjo, Los Alamos National Laboratory, for assistance with some of the experiments.
This research was supported by the Florida Agricultural Experiment Station.
Footnotes
Florida Agricultural Experiment Station Journal Series no. R06884.
REFERENCES
- 1.Abbas C A, Groves S, Gander J E. Isolation, purification, and properties of Penicillium charlesii alkaline protease. J Bacteriol. 1989;171:5630–5637. doi: 10.1128/jb.171.10.5630-5637.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames B N. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- 3.Ames B N, Durbin D T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960;235:769–775. [PubMed] [Google Scholar]
- 4.Augstead I, Berner E. Chromatographic separation of anomeric glycosidases. II. New crystalline methylfuranosides of galactose, arabinose and xylose. Acta Chem Scand. 1954;8:251–256. [Google Scholar]
- 5.Azuma I, Kimura H, Hirao F, Tsubura E, Yamamura Y, Misaki A. Biochemical and immunological studies on Aspergillus. III. Chemical and immunological properties of glycopeptides obtained from Aspergillus fumigatus. Jpn J Microbiol. 1971;15:237–246. [PubMed] [Google Scholar]
- 6.Bardalaye C P, Nordin J H. Chemical structure of the galactomannan from the cell wall of Aspergillus niger. J Biol Chem. 1977;252:2584–2591. [PubMed] [Google Scholar]
- 7.Barker S A, Basarab O, Cruickshank C N D. Galactomannan peptides of Trichophyton mentagrophytes. Carbohydr Res. 1967;3:325–332. [Google Scholar]
- 8.Bonetti S J, Gander J E, Tuekam B A. The N-peptidylethanolamine phosphodiesters:phosphate bridging structures in the galactan chains of Penicillium glycopeptides. FASEB J. 1993;7:A1260. [Google Scholar]
- 9.Bonetti S J, Groves S E, Black B, Gander J E. Heat shock treatment releases Penicillium phosphoglycopeptide. Phytochemistry. 1998;47:567–575. doi: 10.1016/s0031-9422(97)00522-0. [DOI] [PubMed] [Google Scholar]
- 10.Clutterbuck P W, Haworth W N, Raistrick H, Smith G, Stacey M. Studies on the biochemistry of microorganisms. XXXVI. Metabolic products of Penicillium charlesii G. Smith Biochem J. 1934;28:94–110. doi: 10.1042/bj0280094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornish-Bowden A, Wharton C W. Enzyme kinetics. Oxford, United Kingdom: IRL Press; 1990. pp. 12–14. [Google Scholar]
- 12.Dow J M, Callou J A. Partial characterization of glycopeptides from culture filtrates of Fulvia fulva (Cooke) Ciferi syn. Cladosporium flavum, the tomato leaf mould pathogen. J Gen Microbiol. 1979;113:57–66. [Google Scholar]
- 13.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 14.Gander J E, Beachy J C, Unkefer C J, Tonn S J. Toward understanding the structure, biosynthesis and function of a membrane bound fungal glycopeptide. Structural studies. Am Chem Soc Symp Ser. 1980;126:49–79. [Google Scholar]
- 15.Gander J E, Bonetti S J, Brouillette J R, Abbas C E. Depolymerization of structural polymers: use of phosphodiesterases and glycohydrolases. Biomass Bioenergy. 1993;5:223–239. [Google Scholar]
- 16.Gander J E, Jentoft N H, Drewes L R, Rick P D. The 5-O-β-d-galactofuranosyl-containing exocellular glycopeptide of Penicillium charlesii. Characterization of the phosphogalactomannan. J Biol Chem. 1974;249:2063–2072. [PubMed] [Google Scholar]
- 17.Garfin D E. Isoelectric focusing. Methods Enzymol. 1990;182:459–477. doi: 10.1016/0076-6879(90)82037-3. [DOI] [PubMed] [Google Scholar]
- 18.Groisman J F, de Lederkremer R M. d-Galactofuranose in the N-linked sugar chain of a glycopeptide from Ascorbolus furfuraceus. Eur J Biochem. 1987;165:327–332. doi: 10.1111/j.1432-1033.1987.tb11445.x. [DOI] [PubMed] [Google Scholar]
- 19.Hendrick J L, Smith A J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968;126:155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- 20.James P G, Cherniak R. Galactoxylomannans of Cryptococcus neoformans. Infect Immun. 1992;60:1084–1088. doi: 10.1128/iai.60.3.1084-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Leal J A, Guerrero C, Gomez-Miranda B, Prieto A, Bernabe M. Chemical and structural similarities in wall polysaccharides of some Penicillium, Eupenicillium and Aspergillus species. Microbiol Lett. 1992;90:165–168. doi: 10.1016/0378-1097(92)90622-u. [DOI] [PubMed] [Google Scholar]
- 23.Livingston R S, Scheffer R P. Isolation and characterization of host-selective toxin from Helminthosporium sacchari. J Biol Chem. 1981;256:1705–1710. [PubMed] [Google Scholar]
- 24.Lloyd K O. Molecular organization of a covalent peptido-phospho-polysaccharide complex from the yeast form of Cladosporium wernecki. Biochemistry. 1972;11:3384–3390. doi: 10.1021/bi00771a008. [DOI] [PubMed] [Google Scholar]
- 25.MacFadyn D A. Estimation of formaldehyde with chromotropic acid. J Biol Chem. 1945;158:107–133. [Google Scholar]
- 26.Milletti L C, Marino C, Marino K, de Lederkremer R M, Colli W, Alves M J. Immobilized 4-aminophenyl 1-thio-beta-d-galactofuranoside as a matrix for affinity purification of an exo-beta-d-galactofuranosidase. Carbohydr Res. 1999;320:176–182. doi: 10.1016/s0008-6215(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima T, Yoshida M, Nakamuro M, Huiro N, Matsuda K. Structure of the cell wall proteogalactomannan from Neurospora crassa. II. Structural analysis of the polysaccharide part. J Biochem. 1984;96:1013–1020. doi: 10.1093/oxfordjournals.jbchem.a134917. [DOI] [PubMed] [Google Scholar]
- 28.Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- 29.Park Y-I, Buszko M L, Gander J E. Utilization of phosphocholine from extracellular complex polysaccharide as a source of cytoplasmic choline derivatives in Penicillium fellutanum. J Bacteriol. 1997;179:1186–1192. doi: 10.1128/jb.179.4.1186-1192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park Y-I, Gander J E. Choline derivatives involved in osmotolerance of Penicillium fellutanum. Appl Environ Microbiol. 1998;64:273–278. doi: 10.1128/aem.64.1.273-278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park Y-I, Buszko M L, Gander J E. Glycine betaine: reserve form of choline in Penicillium fellutanum in low-sulfate medium. Appl Environ Microbiol. 1999;65:1340–1342. doi: 10.1128/aem.65.3.1340-1342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park Y-I, Bonetti S J, Gander J E. Influence of osmotic and nutritional stress on physiology of Penicillium fellutanum in removal of phosphocholine and modification of phospho-1-O-[N-peptidyl-(2-aminoethanol)] phosphodiesters of peptidophosphogalactomannan species. Appl Environ Microbiol. 2000;66:832–835. doi: 10.1128/aem.66.2.832-835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pletcher C H, Lohmar P D, Gander J E. Factors affecting the accumulation of exocellular exo-β-d-galactofuranosidase and other enzymes from Penicillium charlesii. Exp Mycol. 1981;5:133–139. [Google Scholar]
- 34.Preston J F, Lapis E, Gander J E. Isolation and partial characterization of the exocellular polysaccharides of Penicillium charlesii. III. Heterogeneity in size and composition of high molecular weight exocellular polysaccharides. Arch Biochem Biophys. 1969;134:324–334. doi: 10.1016/0003-9861(69)90291-4. [DOI] [PubMed] [Google Scholar]
- 35.Rietschel-Berst M, Jentoft N H, Rick P D, Pletcher C H, Fang F, Gander J E. Extracellular exo-β-d-galactofuranosidase from Penicillium charlesii. Isolation, purification and properties. J Biol Chem. 1977;252:3219–3226. [PubMed] [Google Scholar]
- 36.Salt S D, Gander J E. Variations in phosphoryl substituents in extracellular peptidophosphogalactomannans from Penicillium charlesii G. Smith Exp Mycol. 1985;9:9–19. [Google Scholar]
- 37.Salt S D, Gander J E. Peptidophosphogalactomannans from Penicillium charlesii. Effects of culture pH and phosphate limitation. Exp Mycol. 1988;12:243–257. [Google Scholar]
- 38.Sumner J B. A method for the colorimetric determination of phosphorus. Science. 1944;100:413–414. doi: 10.1126/science.100.2601.413. [DOI] [PubMed] [Google Scholar]
- 39.Thompson M A, Gander J E. Purification of extracellular exo-β-d-galactofuranosidase: a glycoprotein from Penicillium charlesii. Fed Proc. 1984;43:1553. [Google Scholar]
- 40.Unkefer C J, Jackson C L, Gander J E. The 5-O-β-d-galactofuranosyl-containing peptidophosphogalactomannan of Penicillium charlesii. Identification of phosphocholine attached to C-6 of mannopyranosyl residues of the mannan region. J Biol Chem. 1982;257:2491–2497. [PubMed] [Google Scholar]
- 41.Unkefer C J, Gander J E. The 5-O-β-d-galactofuranosyl-containing peptidophosphogalactomannan of Penicillium charlesii. Characterization of the mannan by 13C NMR spectroscopy. J Biol Chem. 1990;265:685–689. [PubMed] [Google Scholar]
- 42.Unkefer C J, Gander J E. The 5-O-β-d-galactofuranosyl-containing peptidophosphogalactomannan from Penicillium charlesii. Carbon 13 nuclear magnetic resonance studies. J Biol Chem. 1979;254:12131–12135. [PubMed] [Google Scholar]