Abstract
Objectives
To describe the real‐world effectiveness and safety of bosutinib in patients with chronic myeloid leukemia (CML).
Methods
This was a multi‐center, retrospective, non‐interventional chart review study conducted in 10 hospitals in the United Kingdom and the Netherlands.
Results
Eighty‐seven patients were included. Bosutinib was the third‐line tyrosine kinase inhibitor (TKI) in 33 (38%) and fourth‐line in 44 (51%) patients. Median treatment duration was 15.6 months. Among 84 patients in chronic phase (CP) at baseline, 26 (31%) switched to bosutinib due to resistance and 57 (68%) due to intolerance to prior TKIs. Cumulative complete cytogenetic and major molecular response rates in CP patients were 67% and 55%, respectively. After a median follow‐up of 21.5 months, nine (11%) patients in CP died; estimated overall survival rates at 1 and 2 years postbosutinib initiation were 95% and 91%, respectively. Overall, 33/87 (38%) patients discontinued bosutinib due to either lack of efficacy/disease progression (17%), adverse events (14%), death (2%), or other reasons (5%). Eighty‐two (94%) patients experienced ≥1 adverse event possibly related to bosutinib, most commonly diarrhea (52%).
Conclusions
Bosutinib used in routine clinical practice in heavily pretreated patients with CML is an effective treatment for patients in CP and is generally tolerable.
Keywords: bosutinib, chronic myeloid leukemia, retrospective studies, tyrosine kinase inhibitor
1. INTRODUCTION
The introduction of tyrosine kinase inhibitors (TKIs) targeting the BCR‐ABL1 tyrosine kinase transformed the treatment of Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML). 1 , 2 , 3 , 4 , 5 Imatinib was the first TKI introduced into clinical practice, followed by 4 second‐ and third‐generation TKIs, namely dasatinib, nilotinib, bosutinib, and ponatinib. 2 The success of TKI therapy for the treatment of CML has led to significant improvement in survival in these patients. 1 , 2 , 5
Despite this success, some patients experience resistance or intolerance and require a change of TKI. 5 , 6 Based on European LeukemiaNet 2020 criteria, treatment failure occurs in approximately 30%–35% of patients with first‐line imatinib. 5 , 7 , 8 In some cases, imatinib resistance is due to the development of mutations within the kinase domain of BCR‐ABL1. 5 , 9 However, not all mutations conferring resistance to imatinib also confer resistance to second‐generation TKIs, and, therefore, the presence of mutations can direct the choice of the subsequent TKI. 5 , 10 Side effects of TKIs occur with variable frequency and may require the patient to switch to an alternative TKI when management with supportive care and/or dose modifications is inadequate or in the case of certain serious side effects. 11
The choice of TKI should take into account a number of factors, including responses to previous lines of treatment, mutation analyses, comorbidities, prior adverse events (AEs), and patient preferences. However, data on TKI treatment sequencing from randomized controlled trials are limited, and, therefore, real‐world data can provide valuable information to assist treatment decisions.
The aim of this study was to characterize the use of bosutinib in patients with CML receiving treatment in a real‐world, clinical practice setting in the United Kingdom (UK) and the Netherlands from 2010 to 2016. Bosutinib was approved by the European Medicines Agency (EMA) in March 2013 for the treatment of adult patients in all phases of Ph+ CML previously treated with one or more TKIs and for whom imatinib, nilotinib, and dasatinib were not considered appropriate treatment options. 12 In the UK, most patients included in this study received bosutinib via a compassionate use program or the Cancer Drugs Fund since bosutinib was not approved by the National Institute for Health and Care Excellence for patients with Ph+ CML with prior therapy until August 2016. Bosutinib was subsequently approved by the EMA as first‐line therapy in 2018. 13
2. METHODS AND MATERIALS
2.1. Study design and setting
This retrospective, non‐interventional research study of patients with Ph+ CML treated with bosutinib in the real‐world clinical setting was conducted in 10 centers, eight in the UK and two in the Netherlands; all 10 were university hospitals. Data related to study outcomes were collected from patients' hospital medical records by members of the direct care team or an independent researcher between October 2015 and January 2017.
In the UK, the study was approved by the Health Research Authority National Research Ethics Service Committee (reference 15/NE/0263). In the Netherlands, the VU University Medical Center Medical Ethics Review Committee confirmed that official ethical approval was not required for this study (reference 2015.325).
2.2. Patients
Patients with a diagnosis of Ph+ CML were eligible for the study if aged ≥18 years at bosutinib initiation and received bosutinib via a compassionate use program prior to the EMA marketing authorization (March 27, 2013) 12 or as part of routine clinical practice after this date. Patients were excluded if they were prescribed bosutinib in an interventional clinical trial, were initiated on bosutinib <3 months prior to data collection, or declined consent for data collection (see Appendix S1).
2.3. Outcomes and definitions
The primary outcome measure was the cumulative response rate with bosutinib, including complete cytogenetic response (CCyR) and molecular responses (major molecular response [MMR]/MR3, MR4, and MR4.5), assessed according to European LeukemiaNet (ELN) 2013 criteria 6 (see Appendix S1). Secondary outcome measures included patient demographic and clinical characteristics at initiation, bosutinib treatment (initial dosing, dose intensity, dose changes/interruptions, permanent discontinuation, reasons for changes/interruptions/discontinuation, treatment duration), disease progression, overall survival (OS), and bosutinib‐related AEs (see Appendix S1). Treatment failure was defined as failure to achieve ELN 2013 response levels. 6 Loss of achieved response was defined as loss of MMR, CCyR, or complete hematologic response. Disease progression was defined as a transformation from chronic phase (CP) to accelerated phase or blast crisis.
2.4. Statistical analysis
As a retrospective, non‐interventional, single‐cohort study, no formal power calculation was performed (see Appendix S1).
Data were analyzed using descriptive statistics. Categorical variables are presented as number (frequency). Quantitative variables are presented as mean (standard deviation) or median (range). Denominators are presented where data were missing. Multivariable logistic regression analyses were conducted to identify independent predictors of treatment response (see Appendix S1). Results are reported as odds ratios (OR) with 95% confidence intervals (CI); 95% CIs excluding 1 were considered predictive of outcome. OS was evaluated using Kaplan–Meier analyses and summarized as OS rates at 1 and 2 years (see Online Supplementary Methods).
Patient demographics, details of bosutinib treatment, and safety data are presented in all patients. Treatment response was evaluated in patients in CP at bosutinib initiation only. OS was evaluated in patients in CP at bosutinib initiation and in all patients.
Data were analyzed using Stata v14 (StataCorp).
3. RESULTS
3.1. Patient demographic and clinical characteristics
The study included 87 patients (median age 62.7 years; 54% male; median duration of disease 7.1 years; Table 1) who commenced bosutinib between January 2010 and January 2016. Eighty‐four (97%) patients were in CP at bosutinib initiation. One or more comorbidities were recorded in 60 (69%) patients; 22 (25%) had vascular disease, 12 (14%) had hypertension, 10 (11%) had chronic pulmonary disease, nine (10%) had diabetes, nine (10%) had renal disease, and five (6%) had gastrointestinal disease; no patient had a record of comorbid liver disease. BCR‐ABL1 mutations were documented for eight patients at baseline and included G250E, Q252H, Y253H, E255K, E279K, F359V, F455S, and E462K. Two patients had multiple mutations.
TABLE 1.
Patient demographic and clinical characteristics
| Characteristic | Overall patient population (n = 87) |
|---|---|
| Age at bosutinib initiation, median (range), years | 62.7 (24.8–90.1) |
| Male, n (%) | 47 (54) |
| Disease duration, median (range), years | 7.1 (0.2–35.7) |
| Number of comorbidities, n (%) | |
| 0 | 26 (30) |
| 1 | 19 (22) |
| 2 | 22 (25) |
| ≥3 | 19 (22) |
| Not known | 1 (1) |
| Selected comorbidities, n (%) | |
| Vascular disease a | 22 (25) |
| Hypertension | 12 (14) |
| Chronic pulmonary disease | 10 (11) |
| Diabetes mellitus | 9 (10) |
| Renal disease | 9 (10) |
| Gastrointestinal disease | 5 (6) |
| Connective tissue disease | 4 (5) |
| Chronic heart failure | 3 (3) |
| Malignant lymphoma | 3 (3) |
| Malignant solid tumor | 3 (3) |
| Liver disease | 0 (0) |
| Prior transplant, n (%) | |
| Allograft | 4 (5) |
| Autograft | 5 (6) |
| Number of prior TKIs, n (%) | |
| 1 | 4 (5) |
| 2 | 33 (38) |
| 3 | 44 (51) |
| 4 | 6 (7) |
| Patients receiving ≥1 CML‐related non‐TKI therapies, n (%) | 25 (29) |
| Best response to most recent CML‐related therapy prior to bosutinib, n (%) | |
| MR4.5 | 12 (14) |
| MR4 | 10 (11) |
| MMR | 16 (18) |
| CCyR | 10 (11) |
| PCyR | 7 (8) |
| Minor CyR | 5 (6) |
| CHR | 16 (18) |
| PHR | 2 (2) |
| No response | 4 (5) |
| Not known | 5 (6) |
| Reason for switching to bosutinib, n (%) | |
| TKI intolerance | 59 (68) |
| TKI resistance | 27 (31) |
| Other | 1 (1) |
| Phase at initiation, n (%) | |
| Chronic phase | 84 (97) |
| Accelerated phase | 1 (1) |
| Blast crisis | 2 (2) |
| BCR‐ABL mutation at initiation, n (%) | |
| ≥1 mutation | 8 (9) |
| No mutation | 64 (74) |
| Not known | 15 (17) |
Abbreviations: CCyR, complete cytogenetic response; CHR, complete hematologic response; CML, chronic myeloid leukemia; CyR, cytogenetic response; MMR, major molecular response; MR, molecular response; PCyR, partial cytogenetic response; PHR, partial hematologic response; TKI, tyrosine kinase inhibitor.
Vascular disease includes ischemic heart disease, myocardial infarction, cerebrovascular disease, peripheral vascular disease, and vascular disease of unspecified type.
The majority of patients had received two (38%) or three (51%) lines of TKI therapy prior to bosutinib initiation; the median (range) time from initiation of first CML treatment until bosutinib initiation was 6.6 (0.2–29.2) years. A total of 79 (91%) patients had been treated with imatinib (median [range] treatment duration 33.3 [0.2–144.0] months; n = 77); 74 (85%) had been treated with nilotinib (median [range] treatment duration 8.3 [0.1–80.6] months); 67 (77%) had been treated with dasatinib (median [range] treatment duration 15.6 [<0.1–92.0] months; n = 66); and 6 (7%) patients had been treated with ponatinib (median [range] treatment duration 13.0 [2.5–25.9] months). All six patients receiving ponatinib had received ≥4 previous lines of CML‐related treatment; none were documented as having the BCR‐ABL1 T315I mutation, and one patient had multiple BCR‐ABL1 mutations documented after ponatinib was discontinued. Forty‐nine (56%) patients had a total duration of prior therapy >5 years (Figure S1). Nine (10%) patients received hematopoietic stem cell transplantation (HSCT; allograft: n = 4; autograft: n = 5) prior to bosutinib initiation. The best response to the treatment received immediately prior to bosutinib was CCyR, MMR, MR4, and MR4.5 in 10 (11%), 16 (18%), 10 (11%), and 12 (14%) patients, respectively (Table 1). A total of 59 (68%) patients switched to bosutinib due to intolerance to prior TKI therapy, and 27 (31%) switched due to resistance to prior TKI therapy. One (1%) patient switched for other reasons, and this patient was included in the intolerant group for the response analysis and for calculating dose intensity, as this is the most conservative approach. The TKI treatment pathways for patients receiving bosutinib were mapped according to the order of TKI therapy and reasons for discontinuing treatment (Figure S2).
3.2. Response to bosutinib
Among the 84 patients in CP at baseline, the cumulative CCyR rate was 67% (n = 56/84), and 40% (n = 14/35) of patients without baseline CCyR achieved CCyR (Figure 1). The cumulative MMR rate was 55% (n = 46/84), and 37% (n = 19/51) of patients without a baseline MMR achieved MMR (Figure 1). In the overall patient population, the cumulative CCyR and MMR rates were 66% (n = 57/87) and 53% (n = 46/87), respectively.
FIGURE 1.
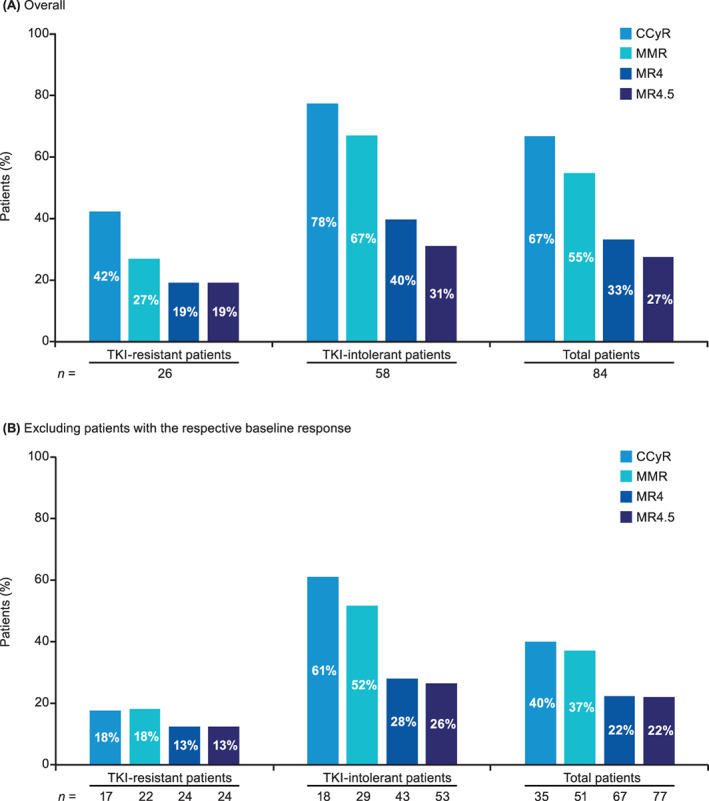
Cumulative cytogenetic and molecular response rates in patients in CP at baseline stratified by TKI resistance or intolerance (A) overall and (B) excluding patients with the respective baseline response. CCyR, complete cytogenetic response; CP, chronic phase; MMR, major molecular response; MR, molecular response; TKI, tyrosine kinase inhibitor
The response to bosutinib was also evaluated separately in patients in CP at baseline with intolerance (n = 58) or resistance (n = 26) to prior TKI therapy. The cumulative rates of CCyR in patients intolerant and resistant to prior TKI therapy were 78% (n = 45/58) and 42% (n = 11/26), respectively; 61% (n = 11/18) and 18% (n = 3/17) of patients without a baseline CCyR achieved CCyR (Figure 1). The cumulative MMR rates in patients intolerant and resistant to prior TKI therapy were 67% (n = 39/58) and 27% (n = 7/26), respectively; 52% (n = 15/29) and 18% (n = 4/22) of patients without a baseline MMR achieved MMR (Figure 1).
Multivariable logistic regression analyses were carried out to identify independent predictors of cumulative CCyR, MMR, and MR4. Patients with intolerance vs resistance to prior TKI therapy were more likely to have a CCyR (OR 19.64; 95% CI: 3.31–116.6) or MMR (OR 14.76; 95% CI: 2.99–72.80). No other variables were independently associated with response to treatment (Table S1).
The proportions of patients in CP at baseline achieving optimal responses at 3, 6, and 12 months following bosutinib initiation were 64% (n = 54/84), 64% (n = 54/84), and 48% (n = 40/84), respectively, based on the ELN 2013 criteria for second‐line TKI therapy 6 (Table S2). The best responses recorded during bosutinib treatment were CCyR, MMR, MR4, and MR4.5 in 12%, 21%, 6%, and 27% of patients, respectively (Figure 2).
FIGURE 2.
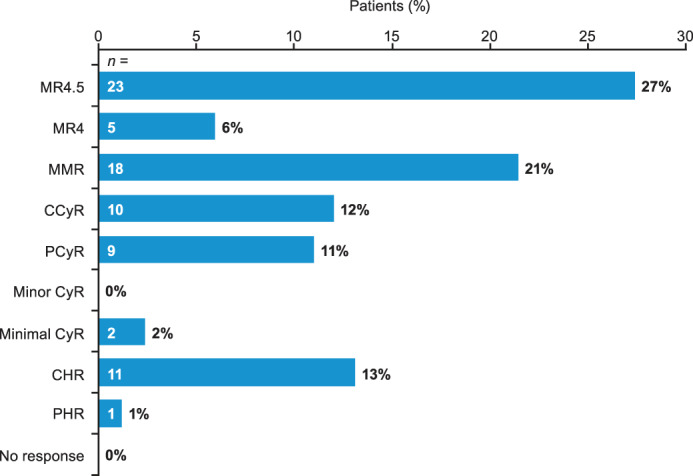
Best response to bosutinib in patients in CP at baseline. Five patients discontinued bosutinib before response to treatment was assessed. CCyR, complete cytogenetic response; CHR: complete hematologic response; CP: chronic phase; CyR: cytogenetic response; MMR: major molecular response; MR: molecular response; PCyR: partial cytogenetic response; PHR: partial hematologic response
3.3. Disease progression
Of the 84 patients in chronic phase at initiation, three (4%) progressed to blast crisis ≤12.4 months after bosutinib initiation and subsequently died (median time from initiation to death: 10.4 months). Of these, two patients progressed and died on bosutinib, and one progressed on bosutinib and died after starting follow‐on treatment.
3.4. Overall survival
After a median (range) follow‐up of 21.5 (0.9–66.0) months, nine (11%) patients in CP at baseline died: four from disease progression, one each from thromboembolic complication, stroke, myocardial infarction, and anal carcinoma, and one from unknown cause. Three patients died on bosutinib (including up to 30 days after stopping bosutinib) and six died >30 days after stopping bosutinib. Of the three patients who died on bosutinib, two died from disease progression and one died due to other causes. The median (range) time from bosutinib initiation to death was 8.5 (0.9–10.4) months in patients who died on bosutinib (n = 3) and 20.5 (3.0–56.3) months in patients who died after stopping bosutinib (n = 5; n = 1 missing date of death). The estimated cumulative OS rates at 1 and 2 years post‐bosutinib initiation were 95% and 91% (Figure 3), respectively, with 82% and 50% having adequate follow‐up (i.e., died prior to or were followed until the respective year). Only 21% of patients had adequate follow‐up at 3 years.
FIGURE 3.
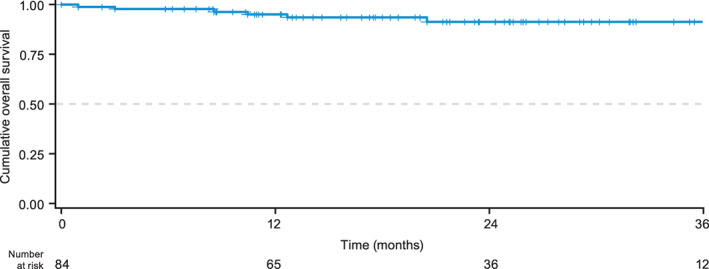
Overall survival from bosutinib initiation in patients in CP at baseline. Date of death was not available for one patient. CP, chronic phase
During a median (range) follow‐up of 20.5 (0.3–66.0) months, 11 (13%) patients in the overall patient population died. The estimated cumulative survival rates at 1 and 2 years post‐bosutinib initiation were 93% and 89%, respectively (Figure S3). Of the three patients in an advanced phase at bosutinib initiation, two died less than 12 months post‐initiation; one patient in blast crisis was still alive and on bosutinib at 15 months post‐initiation.
3.5. Bosutinib treatment and discontinuation
At data collection, 62% of patients in the overall population were still receiving bosutinib, and the median (range) treatment duration was 15.6 (0.3–66.0) months. Overall, 45% of patients had one or more dose decreases and 45% had temporary discontinuations; 37% of patients had dose decreases due to AEs and 40% had temporary discontinuations due to AEs (Table 2). The median (range) duration of temporary discontinuations was 15 (3–269) days.
TABLE 2.
Bosutinib treatment and discontinuation in patients stratified according to the reason for bosutinib initiation
| Overall patient population (n = 87) | Reason for bosutinib initiation a | ||
|---|---|---|---|
| Intolerance to prior TKI (n = 59) | Resistance to prior TKI (n = 27) | ||
| Dose at initiation, n (%) | |||
| 100 mg/day | 14 (16) | 14 (24) | 0 (0) |
| 200 mg/day | 14 (16) | 12 (20) | 2 (7) |
| 300 mg/day | 25 (29) | 14 (24) | 11 (41) |
| 400 mg/day | 11 (13) | 5 (8) | 6 (22) |
| 500 mg/day b | 23 (26) | 14 (24) | 8 (30) |
| Dose increase, n (%) | 57 (66) | 38 (64) | 19 (70) |
| Dose decrease, n (%) | 39 (45) | 29 (49) | 10 (37) |
| Dose decrease due to AEs, n (%) | 32 (37) | 24 (41) | 8 (30) |
| Temporary discontinuation, n (%) | 39 (45) | 27 (46) | 12 (44) |
| Temporary discontinuation due to AEs, n (%) | 35 (40) | 25 (42) | 10 (37) |
| Dose intensity, median (range), mg/day |
324.5 (31.8–568.3) |
300.0 (31.8–568.3) |
360.9 (115.4–500.0) |
| Patients remaining on bosutinib at end of observation, n (%) | 54 (62) | 37 (63) | 16 (59) |
| Reasons for discontinuation, n (%) | |||
| AE | 12 (14) | 10 (17) | 2 (7) |
| Treatment failure | 10 (11) | 4 (7) | 6 (22) |
| Loss of response | 4 (5) | 2 (3) | 2 (7) |
| Patient request | 2 (2) | 2 (3) | 0 (0) |
| Death | 2 (2) | 1 (2) | 1 (4) |
| Disease progression | 1 (1) | 1 (2) | 0 (0) |
| Other | 2 (2) | 2 (3) | 0 (0) |
Abbreviations: AE, adverse event; TKI, tyrosine kinase inhibitor.
One patient who initiated bosutinib for other reasons was excluded from the subgroup analyses.
Recommended bosutinib starting dose. 13
The most common dose of bosutinib at initiation was 300 mg/day, and median (range) bosutinib dose intensity was 324.5 (31.8–568.3) mg/day (Table 2). In 59 patients intolerant to prior TKI therapy, 24% started on 100 mg/day and 20% on 200 mg/day, compared with 0% and 7%, respectively, for the 27 patients resistant to prior TKI therapy. Conversely, 32% of patients intolerant to prior TKI therapy and 52% of patients resistant to prior TKI therapy started on a bosutinib dose of ≥400 mg/day. Median (range) bosutinib dose intensity in patients intolerant to prior TKI therapy was 300.0 (31.8–568.3) mg/day compared with 360.9 (115.4–500.0) mg/day in patients resistant to prior TKI therapy (Table 2).
Overall, 33 (38%) patients permanently discontinued bosutinib. The most commonly recorded reasons were lack of efficacy/disease progression (n = 15 [17%]) and AEs (n = 12 [14%]; Table 2). Twenty‐two (37%) patients intolerant to prior TKI therapy discontinued bosutinib, most commonly because of AEs (n = 10 [17%]) and lack of efficacy/disease progression (n = 7 [12%]). Eleven (41%) patients resistant to prior TKI therapy discontinued bosutinib, most commonly due to lack of efficacy/disease progression (n = 8 [30%]) and AEs (n = 2 [7%]). One patient discontinued bosutinib due to bone pain and dizziness, consistent with documented intolerances to previous TKIs; no other patient who discontinued bosutinib due to intolerance had evidence of cross‐intolerance between bosutinib and previous TKI therapy.
A total of 20 (61%) patients who discontinued bosutinib went on to receive further medical treatment. Treatments after bosutinib included imatinib (n = 5), nilotinib (n = 2), dasatinib (n = 8), ponatinib (n = 3), and bosutinib after ponatinib (n = 1; Table S3). Of the 20 patients who went on to receive further medical treatment, eight discontinued bosutinib due to resistance, seven of whom received an alternative TKI (imatinib, nilotinib, dasatinib, or ponatinib), and nine discontinued bosutinib due to intolerance, eight of whom received an alternative TKI (imatinib, dasatinib, or ponatinib). Three patients who received further medical treatment discontinued bosutinib due to reasons other than resistance or intolerance. Four patients who did not receive follow‐on medical treatment after bosutinib went on to receive HSCT, noting that data on follow‐on medical treatment and HSCT were collected separately in the case report form.
3.6. Bosutinib‐related adverse events
Overall, 82 (94%) patients reported a total of 297 any‐grade AEs, and 18 (21%) patients experienced a total of 22 grade 3–4 AEs. The most commonly reported AE was diarrhea, reported in 45 (52%) patients; only three (3%) patients had grade 3–4 diarrhea (Figure 4). Any‐grade increased alanine aminotransferase (ALT) was reported in 15 (17%) patients; three (3%) patients had grade 3–4 increased ALT. The only other grade 3–4 AE reported in more than one patient was thrombocytopenia (n = 4 [5%]; Figure 4). Seven patients had cardiac AEs, which included atrial fibrillation (n = 3; grade 3/4, n = 1), cardiac failure (n = 3; grade 3/4, n = 1), and hypertension (n = 1; grade 3/4, n = 0).
FIGURE 4.
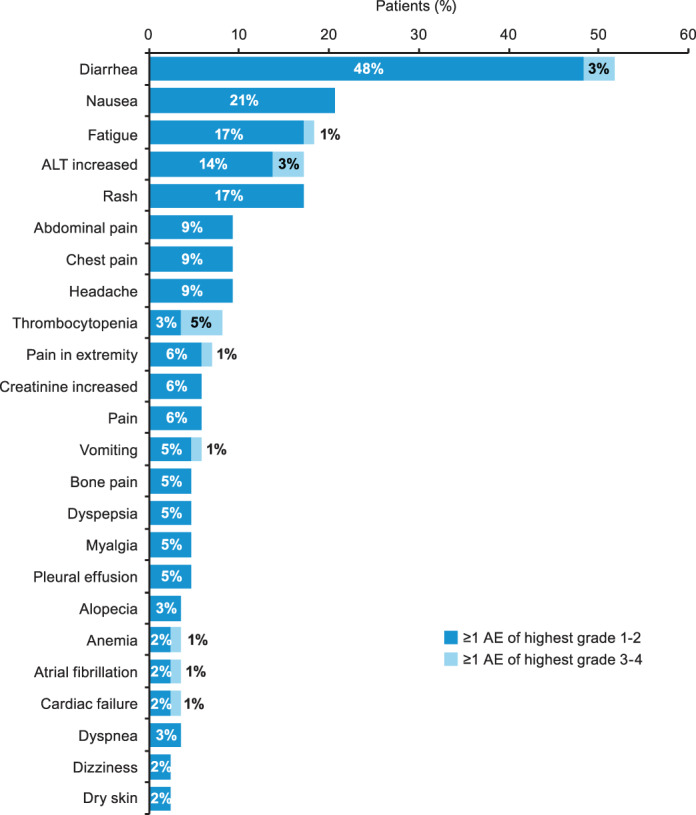
AEs reported in ≥2 (2%) patients categorized according to highest grade experienced. AE, adverse event; ALT, alanine aminotransferase
Of the 22 grade 3–4 AEs, nine led to dose reductions and three to temporary discontinuation. Three grade 3–4 AEs (increased ALT, thrombocytopenia, and vomiting) led to permanent discontinuation of bosutinib (Table S4). AEs leading to dose reduction in ≥5% of patients were diarrhea (8%), nausea (7%), fatigue (5%), increased ALT (5%), and thrombocytopenia (5%). AEs leading to dose interruption or permanent discontinuation in ≥5% of patients were increased ALT (13%), diarrhea (9%), rash (7%), and chest pain (6%). AEs leading to dose reductions or discontinuations by bosutinib dose are shown in Table S5.
4. DISCUSSION
The results of this retrospective, observational study are broadly consistent with those of the phase I/II study of bosutinib (Study 200) and a retrospective study of fourth‐line bosutinib in Spain. 14 , 15 , 16 , 17 , 18 In this study, the majority of patients were treated in the fourth‐ or later‐line setting, and most patients received bosutinib via a compassionate use program or the Cancer Drugs Fund. A total of 67%, 55%, and 27% of patients maintained or achieved CCyR, MMR, and MR4.5, respectively, after initiation of bosutinib. The best responses to bosutinib were CCyR, MMR, and MR4.5 in 12%, 21%, and 27% of patients in CP, respectively, compared with 11%, 18%, and 14% for the treatment immediately prior to bosutinib, suggesting that the majority of patients maintained baseline responses or achieved better responses on bosutinib than they had achieved with the previous therapy. Cytogenetic and molecular responses (maintained and achieved) were observed in both patients with resistance and intolerance to prior TKI therapy. However, patients with a more resistant phenotype showed lower response rates overall and were less likely to achieve a response (MMR/CCyR) compared with patients with intolerance to prior TKI therapy. Despite the high burden of comorbidities in our patient population, comorbidities were not found to adversely affect treatment response. Only 4% of patients in the present study who were in CP at baseline progressed (all progressed to blast crisis), and the Kaplan–Meier estimates of cumulative survival at 1 and 2 years were consistent with results from Study 200. 14 , 16 , 17
The recommended dose of bosutinib is 500 mg once daily 13 ; however, the median dose intensity was 324.5 mg/day in the overall patient population. In addition to reflecting dose adjustments due to AEs, the lower median dose intensity in our study also reflects a lower starting dose, with most patients initiated on <500 mg/day. The majority of patients switching to bosutinib for intolerance to prior TKI therapy were initiated on doses ≤300 mg/day, whereas the majority of patients with resistance to prior TKIs were initiated on a higher dose (either 400 or 500 mg/day).
The overall proportion of patients reporting AEs was consistent with results of bosutinib clinical trials and observational studies, although the incidence of diarrhea was lower. 13 , 15 , 19 , 20 This may result from underreporting of diarrhea or could reflect physician and patient awareness, resulting in use of lower doses of bosutinib at initiation and prompt treatment of any episodes of diarrhea. In addition, the incidence of AEs may have been generally reduced in the current study as compared with clinical trials because many patients started with a lower dose than the currently recommended 500 mg/day. Only 14% of the overall patient population discontinued bosutinib treatment due to AEs, despite the fact that 68% of patients started bosutinib because of intolerance to previous TKI therapy, suggesting limited cross‐intolerance between bosutinib and other TKIs. These results lend support to the results of previous studies suggesting that, although AEs are common, they are generally tolerable, and cross‐intolerance between bosutinib and other TKIs is relatively uncommon. 14 , 15
The limitations of this study largely relate to the retrospective nature of the data collection and the accuracy and completeness of the available clinical records. Furthermore, the differences in the requirement for consent between centers and availability of data on deceased patients may have introduced selection bias leading to under‐ or over‐estimation of study outcomes. Differences in the healthcare systems between the UK and Netherlands may also have introduced selection bias or resulted in differences in patient care and/or interpretation of the data between sites.
In conclusion, these results confirm the effectiveness of bosutinib when used in unselected patients treated in a real‐world setting at centers across the UK and the Netherlands, the majority of whom had received at least two previous TKIs. Most patients maintained a response, with some patients further achieving a deeper response with bosutinib, particularly those intolerant to previous TKI therapy. Although nearly all patients experienced AEs, these were mostly low grade despite most patients having pre‐existing comorbidities. There was a little evidence of cross‐intolerance between bosutinib and other TKIs as has previously been described between other TKIs, supporting the potential benefits of changing to bosutinib in patients with intolerance. Bosutinib was able to improve or maintain response rates in a patient population that was older, had a higher comorbidity burden, and was more heavily pretreated than the patients with CML who are usually included in clinical trials. Taken together, these results lend support for bosutinib as an effective treatment for CML in routine clinical practice, particularly for those patients with comorbidities and previous intolerance.
CONFLICT OF INTEREST
S.C. reports personal fees from Pfizer. J.J.W.M.J. reports research funding from Novartis and Bristol Myers Squibb; speaker fees from Incyte and Pfizer; advisory board honoraria from AbbVie, Incyte, Novartis, and Pfizer; and is the President of the Apps for Care and Science Foundation that develops the HematologyApp and that has received funding from Abbvie, Amgen, Astellas, Celgene/Bristol Myers Squibb, Daiichi‐Sankyo, Incyte, Janssen, Jazz, Novartis, Sanofi Genzyme, Takeda, Roche, and Servier. J.B. reports Pfizer advisory board and speaker fees and personal fees from Pfizer during the conduct of the study. G.S. reports advisory board attendance and honoraria from Ariad/Incyte, Bristol Myers Squibb, Novartis, and Pfizer. N.B. reports grants from Bristol Myers Squibb, Incyte, Novartis, Pfizer, and ZonMw. M.R. reports speaker fees from Incyte, Novartis, and Pfizer. M.S. reports grants from Pfizer Ltd, UK, during the conduct of the study (site fees for data collection) and personal fees from ARIAD (speaker fees). R.E.C. reports research funding from Bristol Myers Squibb, Novartis, and Pfizer, and honoraria from AbbVie, Bristol Myers Squibb, Jazz Pharmaceuticals, Novartis, and Pfizer. S.M‐S. and A.M.C. are employees of PH Associates Ltd, doing business as OPEN Health, who were commissioned by Pfizer to provide support for study design, implementation, data analysis and interpretation, and development of this manuscript. D.M. reports honoraria and speakers bureau fees from Novartis, Pfizer, Bristol Myers Squibb and Incyte. J.F.A. reports advisory board membership for Ariad/Incyte, Novartis, and Pfizer; speaker at satellite symposia for Ariad/Incyte, Bristol Myers Squibb, Novartis, and Pfizer; and research funding from Ariad/Incyte and Pfizer.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank all the participating patients and their families, study research nurses, study coordinators, and operations staff. The authors would also like to thank Dr. Andres Virchis and Dr. Hugues De Lavallade for their contributions to this study. Pfizer was involved in study design, interpretation of data, and review and approval of this manuscript. pH Ltd, doing business as OPEN Health, was commissioned by Pfizer Ltd, UK, to provide support for study design, implementation, data analysis and interpretation, and development of this manuscript. J.F.A. is a NIHR Senior Investigator and together with D.M. and S.C. would like to acknowledge the support provided by the Imperial College NIHR‐BRC. S.C., D.M., and J.F.A. acknowledge the support of the NIHR Imperial Biomedical Research Center. Medical writing support was provided by Emily Balevich, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Claudiani S, Janssen JJWM, Byrne J, et al. A retrospective observational research study to describe the real‐world use of bosutinib in patients with chronic myeloid leukemia in the United Kingdom and the Netherlands. Eur J Haematol. 2022;109(1):90‐99. doi: 10.1111/ejh.13775
Funding informationThis study was sponsored by Pfizer.
Clinicaltrials.gov identifier: NCT02546375.
DATA AVAILABILITY STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions. Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
REFERENCES
- 1. Hoglund M, Sandin F, Simonsson B. Epidemiology of chronic myeloid leukaemia: an update. Ann Hematol. 2015;94(Suppl 2):S241‐S247. [DOI] [PubMed] [Google Scholar]
- 2. Apperley JF. Chronic myeloid leukaemia. Lancet. 2015;385:1447‐1459. [DOI] [PubMed] [Google Scholar]
- 3. Chereda B, Melo JV. Natural course and biology of CML. Ann Hematol. 2015;94(Suppl 2):S107‐S121. [DOI] [PubMed] [Google Scholar]
- 4. Baccarani M, Castagnetti F, Gugliotta G, Rosti G. A review of the European LeukemiaNet recommendations for the management of CML. Ann Hematol. 2015;94(Suppl 2):S141‐S147. [DOI] [PubMed] [Google Scholar]
- 5. Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123:1353‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3‐year follow‐up from a randomized phase 3 trial (DASISION). Blood. 2014;123:494‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soverini S, Branford S, Nicolini FE, et al. Implications of BCR‐ABL1 kinase domain‐mediated resistance in chronic myeloid leukemia. Leuk Res. 2014;38:10‐20. [DOI] [PubMed] [Google Scholar]
- 10. Ernst T, Hochhaus A. Chronic myeloid leukemia: clinical impact of BCR‐ABL1 mutations and other lesions associated with disease progression. Semin Oncol. 2012;39:58‐66. [DOI] [PubMed] [Google Scholar]
- 11. Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Medicines Agency . BOSULIF® (Bosutinib) European Public Assessment Report. EMA; 2013. [Google Scholar]
- 13. European Medicines Agency . BOSULIF® (Bosutinib) Summary of Product Characteristics. EMA; 2019. [Google Scholar]
- 14. Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI‐606) in chronic phase Philadelphia chromosome‐positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567‐4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. García‐Gutiérrez V, Martinez‐Trillos A, Lopez Lorenzo JL, et al. Bosutinib shows low cross intolerance, in chronic myeloid leukemia patients treated in fourth line. Results of the Spanish compassionate use program. Am J Hematol. 2015;90:429‐433. [DOI] [PubMed] [Google Scholar]
- 16. Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119:3403‐3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cortes JE, Khoury HJ, Kantarjian HM, et al. Long‐term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. García‐Gutiérrez V, Milojkovic D, Hernandez‐Boluda JC, et al. Safety and efficacy of bosutinib in fourth‐line therapy of chronic myeloid leukemia patients. Ann Hematol. 2019;98:321‐330. [DOI] [PubMed] [Google Scholar]
- 19. Gambacorti‐Passerini C, Brummendorf TH, Kim DW, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: minimum 24‐month follow‐up. Am J Hematol. 2014;89:732‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanaizi Z, Unkrig C, Enzmann H, et al. The European medicines agency review of bosutinib for the treatment of adult patients with chronic myelogenous leukemia: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2014;19:421‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions. Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.


