Summary
Background and Aim
Bulevirtide (BLV) blocks the uptake of the hepatitis D virus (HDV) into hepatocytes via the sodium/bile acid cotransporter NTCP. BLV was conditionally approved by the EMA but real‐life data on BLV efficacy are limited.
Methods
Patients were treated with BLV monotherapy. Patients who did not achieve further decreases in HDV‐RNA after 24 weeks were offered PEG‐IFN as an add‐on therapy in a response‐guided manner.
Results
Twenty‐three patients (m: 10, f: 13; mean age: 47.9 years, cirrhosis: 16; median ALT: 71 IU/ml; median HDV‐RNA: 2.1 × 105copies/ml) started BLV monotherapy (2 mg/day: 22; 10 mg/day: 1). Twenty‐two completed ≥24 weeks of treatment (24–137 weeks): Ten (45%) were classified as BLV responders at week 24. BLV was stopped in two patients with >6 months HDV‐RNA undetectability, but both became HDV‐RNA positive again. One patient was transplanted at week 25. One patient terminated treatment because of side effects at week 60. Ten patients are still on BLV monotherapy. Adding PEG‐IFN in eight patients induced an HDV‐RNA decrease in all (1.29 ± 0.19 [SD] log within 12 weeks). HDV‐RNA decreased by >2log or became undetectable in 45%(10/22), 55%(11/20), 65% (13/20) and 69% (9/13); and ALT levels normalised in 64% (14/22), 85% (17/20), 90% (18/20) and in 92% (12/13) patients at weeks 24, 36, 48 and 60, respectively. Portal pressure decreased in 40% (2/5) of patients undergoing repeated measurement under BLV therapy.
Conclusion
Long‐term BLV monotherapy is safe and effectively decreases HDV‐RNA and ALT—even in patients with cirrhosis. The optimal duration of BLV treatment alone or in combination with PEG‐IFN remains to be established. An algorithm for a response‐guided BLV treatment approach is proposed.
The novel antiviral drug Bulevirtide (BLV) was studied in 23 patients (70% had cirrhosis) with hepatitis D virus infection. At week 24 (W24), 45% (10/22, one dropped out) were classified as virologic responders (2‐log decline or HDV‐RNA undetectablity) to BLV monotherapy. Interferon treatment was added in eight patients who achieved no further decline in HDV‐RNA after W24, inducing a synergistic antiviral effect. The proportion of patients who achieved virologic response and normalisation of ALT continuously increased from W24 to W60. No clinically relevant side effects were attributed to BLV treatment, despite marked increases in bile acid levels.

1. INTRODUCTION
Infection with hepatitis D virus (HDV) frequently causes progression to cirrhosis and hepatocellular carcinoma. 1 , 2 HDV relies on hepatitis B virus (HBV), specifically on HBV surface antigen (HBsAg) to form infectious HDV particles. Worldwide, about 0.16% (0.11–0.25) of the general population, totaling 12.0 (8.7–18.7) million people are estimated to be anti‐HDV positive. 3 Pegylated interferon alpha (PEG‐IFN) achieves sustained suppression of HDV replication only in 25% of patients. 4 Nucleos(t)ide analogs (NUCs) are ineffective against HDV. 5 Therefore, there is an urgent need for novel HDV therapies. The HBV entry inhibitor Bulevirtide (BLV) is a synthetic N‐acylated preS1 lipopeptide that blocks the sodium/bile acid cotransporter (NTCP/SLC10A1), 6 serving as receptor for the entry of HBV and HDV into hepatocytes. 7 , 8 The results of recent clinical studies on BLV monotherapy have only been published as abstracts 9 , 10 or as a preliminary report in six patients 11 and demonstrated a ≥2 log decline or undetectable HDV‐RNA levels in up to 50% of patients after 24 weeks when used as monotherapy and undetectable HDV‐RNA levels after 48 weeks of treatment in 60% of patients when used in combination with PEG‐IFN (two out of three, i.e. 40% of overall patients maintained undetectability after another 24 weeks). BLV was well tolerated—including in patients with compensated cirrhosis—with the only documented laboratory side effect being an increase in serum bile acids levels. The European Medical Agency (EMA) approved BLV for treatment of HDV 12 given the urgent medical need in an orphan disease. Unfortunately, the phase 2 study 10 with 90 patients assigned to six different treatment arms and the interim data of two ongoing phase 3 studies 13 , 14 do not provide sufficient guidance for the use of BLV treatment for HDV in clinical practice. Thus, important information on the most effective dose of BLV, the duration of treatment, the need for combination with PEG‐IFN or NUCs is lacking.
Here, we report the real‐life efficacy and safety of BLV monotherapy using doses of 2 to 10 mg per day in 23 HDV patients mostly with cirrhosis.
2. PATIENTS AND METHODS
2.1. Study population
Since April 2018, the compassionate use program by MyrPharma (Leipzig/Germany) allowed us to treat eight patients according to the choice of the physician (PF, HZ). MyrPharma provided BLV until 8/2020, after EMA approval BLV for treatment of HDV on 31 July 2020. After market authorisation by EMA 2 mg/day BLV was reimbursed by medical insurance to HDV patients with cirrhosis and/or ALT >100 IU/mL. Demographic, clinical, laboratory and virological parameters were collected.
Patients were recruited from six hospitals (Medical University of Vienna: 14; Klinik Ottakring, Wien: 2; Kepler University Linz: 2; Paracelsus University Salzburg: 1; Medical University Innsbruck: 3, Krankenhaus Hall/Tirol: 1). Similar to previous studies most patients were immigrants (Mongolia: 6; Romania: 5; Moldavia: 1; Turkey: 5; Usbekistan: 1; Georgia: 1; Syria 1, Nigeria:1) and only one patient was an Austrian native.
2.2. HDV‐RNA quantification
In patients from Vienna, Linz and Salzburg HDV‐RNA was quantified by PCR 15 with a lower limit of quantification of 100 copies/ml. The Innsbruck and the Hall group used the RoboGene® assay (Roboscreen Diagnostics). Based on parallel determination of the samples of patient #1 by this assay and the RoboGene® assay showed a similar sensitivity 16 (see Figure S1) and allowed to convert the Robogen® results (IU/ml) to copies/mL by multiplication by 37.
2.3. Efficacy and safety parameters
We used the definitions of efficacy according to previous studies. 4 , 17 Virological response was defined by an on‐treatment decline of baseline HDV‐RNA levels by at least 2‐log or undetectability of HDV‐RNA. Biochemical response was defined as on‐treatment normalisation of ALT. These endpoints are also used in current phase 3 studies. 13 , 14 However, limitations of these response criteria have been recently discussed. 18
2.4. Elastography
Liver stiffness measurements were performed by transient elastography (Fibroscan®; Echosens), as previously described ( 19 ).
2.5. Measurement of portal pressure
The hepatic venous pressure gradient (HVPG) was assessed in accordance with a standardised protocol described in detail elsewhere ( 20 ). Of note, in patients on non‐selective betablockers (NSBB), NSBB therapy was interrupted 5 days before HVPG measurements. Clinically significant portal hypertension (CSPH) was defined as an HVPG ≥10 mmHg, and the achievement of a meaningful decrease of 10% was investigated in accordance with recommendations by the Baveno Consensus. 21
2.6. Response‐guided approach
The ultimate aim was to achieve complete virological suppression of HDV‐RNA replication. Based on our initial experience we adopted a response‐guided approach.
Patients who achieved virological response on BLV monotherapy at week 24 continued BLV monotherapy and were offered to terminate treatment if HDV‐RNA remained undetectable at least at three time points within 6 months. In patients without further HDV‐RNA decline after week 24–48 combination therapy with PEG‐IFN alfa‐2a (Pegasys®, Roche) was initiated irrespective of the response classification (see Figure 1B).
FIGURE 1.
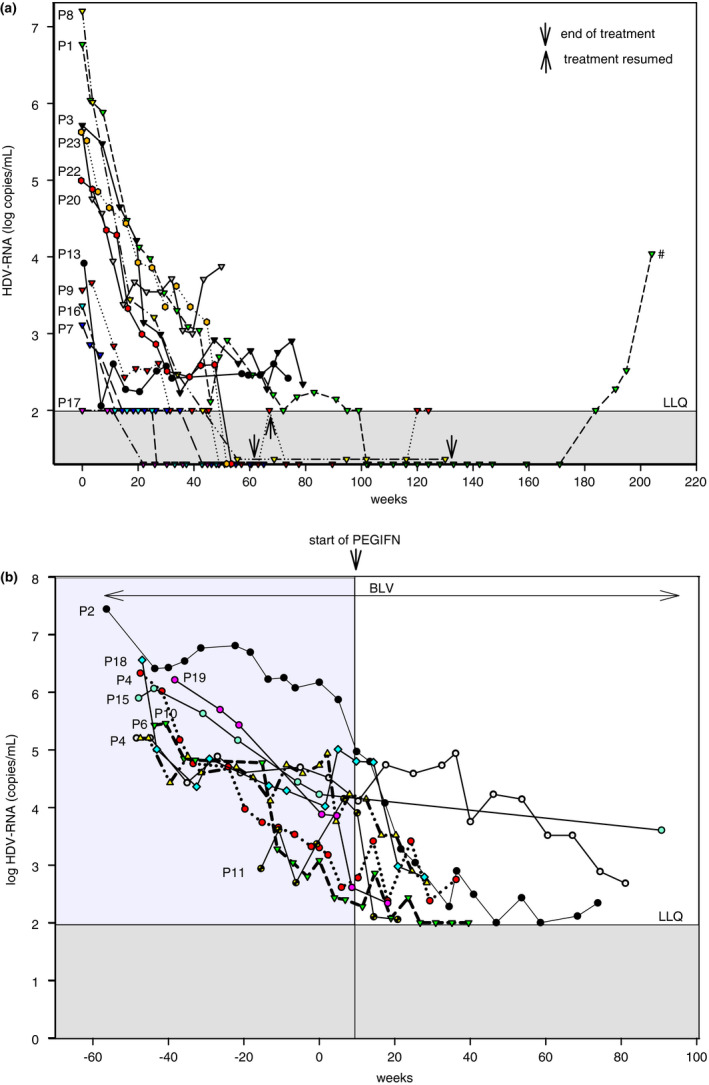
(A) HDV‐RNA in patients on BLV monotherapy responding to BLV. Data of treatment of patient P1 until week 24 of follow‐up were published. 16 # retreatment with 2 mg BLV/day is considered. (B) Changes in HDV‐RNA before and after the addition of PEG‐IFN
As safety parameters, routine laboratory parameters including blood cell counts and liver biochemistries were collected. In addition, HBsAg levels were quantified (Architect, Abbott) and bile acid levels were measured.
2.7. Statistical analyses
Sigma Plot (version 13, Sysstat, Düsseldorf, Germany) was used for computing of the figures and for statistical analyses. Continuous variables are presented as median and range, while nominal parameters are shown as absolute numbers (or proportions) of patients with the respective attribute. The ethics committee of the Medical University of Vienna (EC Vote No. 1515/2020) approved the retrospective evaluation of the HDV treatment data.
3. RESULTS
3.1. Patient characteristics
Twenty‐three patients (m: 10/f:13; mean age: 47.9 years, cirrhosis: n = 16; median ALT: 71 IU/mL (range 21–341); HDV‐RNA: 2.1 × 105 [range: 1.0 × 102–2.1 × 107] copies/mL) received BLV (2 mg/day in n = 21; 10 mg/day in n = 2). Twenty‐one patients (84.6%) were on concomitant NUCs (ETV:3, TDF:16, TAF:2), 18 were previous PEG‐IFN non‐responders (Table 1). 17/23 patients had progressed to cirrhosis at inclusion. All but one cirrhotic patient showed Child‐Turcotte‐Pugh stage A cirrhosis, and none had a history of hepatic decompensation. Twenty‐two patients completed at least ≥24 weeks of BLV treatment (range: 24–137 weeks). One patient (#8) did not show up after week 8.
TABLE 1.
Study population
| Patients with cirrhosis (n = 16) | Patients without cirrhosis (n = 7) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2 | P4 | P6 | P7 | P9 | P10 | P11 | P12 | P13 | P14 | P17 | P18 | P19 | P20 | P21 | P23 | P1 | P3 | P5 | P8 | P15 | P16 | P22 | |
| Sex/Age | M/68 | F/46 | F/67 | M/52 | M/51 | F/37 | M/42 | F/45 | M/66 | F68 | F36 | M/59 | M/37 | F/50 | F/64 | M/53 | F/66 | F/42 | M/42 | F/29 | F36 | F39 | M/30 |
| Previous IFN | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| HDV‐RNA copies/mL | 2.8e6 | 2.1e6 | 1.6e5 | 7.1e2 | 3.7e3 | 2.7e5 | 3.1e4 | 1.2e4 | 5.8e3 | 8.2e5 | 1.0e2 | 1.0e6 | 1.6e6 | 4.3e5 | 2.1e7 | 2.1e7 | 5.9e6 | 5.2e5 | 9.5e4 | 4.3e5 | 1.1e6 | 2.3e3 | 2.1e7 |
| HBeAg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg | pos | neg | neg | neg |
| HBV‐DNA (IU/mL) | <20 | neg | <20 | neg | neg | 63 | <20 | neg | neg | neg | neg | neg | neg | 347 | neg | 0 | 21 | <20 | neg | <20 | neg | <20 | 6000 |
| HBsAg, quant | 3142 | 9377 | 420 | 1229 | 1663 | — | 13,649 | 181 | 45 | 2090 | 2266 | 4673 | 2183 | 1161 | — | — | 9780 | 11,825 | 1090 | 17,326 | 5890 | 3643 | — |
| VCTE‐LSM (kPa) | 26.3 | 17.2 | 10.2 | — | 35.8 | 14.6 | 22.7 | 18.6 | 7.5 | — | 24 | — | 48 | 19.4 | — | 31.6 | — | 9.1 | 7.4 | 5.5 | 7 | 9.5 | 6.8 |
| Bilirubin (mg/dl) | 1.38 | 0.52 | 0.35 | 0.49 | 0.95 | 1.2 | 1.37 | 1.12 | 1.35 | 1.54 | 0.65 | 1.24 | 0.8 | 1.6 | 0.3 | 1.6 | 0.36 | 0.47 | 0.52 | 0.5 | 0.28 | 0.65 | 0.64 |
| Albumin (g/dl) | 34.4 | 31.0 | 44.8 | 44.1 | 46.1 | 43.0 | 38.1 | 41.8 | 39.9 | 33.9 | 49.9 | 41.9 | 36.7 | 32.5 | 41.0 | 34.9 | 41.3 | 45.6 | 44.0 | 35.6 | 43.0 | 45.6 | 47.6 |
| INR | 1.3 | 1.4 | 1.0 | 1.0 | 1.2 | 1.2 | 1.2 | 1.0 | 1.4 | 1.7 | 1.2 | 1.2 | 2.4 | 1.3 | 1.0 | 1.4 | 1.0 | 1.0 | 1.0 | 1.2 | 1.1 | 1.3 | 1.0 |
| Platelets (G/L) | 77 | 98 | 131 | 166 | 84 | 129 | 109 | 29 | 90 | 63 | 85 | 86 | 78 | 53 | 126 | 140 | 216 | 251 | 162 | 288 | 257 | 206 | 166 |
| AST (IU/L) | 55 | 93 | 64 | 21 | 68 | 48 | 87 | 35 | 36 | 94 | 24 | 101 | 56 | 315 | 50 | 120 | 111 | 76 | 68 | 53 | 51 | 203 | 43 |
| ALT (IU/L) | 63 | 80 | 75 | 25 | 125 | 56 | 115 | 35 | 44 | 69 | 24 | 132 | 73 | 307 | 42 | 100 | 224 | 91 | 207 | 42 | 59 | 341 | 98 |
| GGT (IU/L) | 30 | 52 | 43 | 37 | 98 | 31 | 177 | 16 | 26 | 48 | 30 | 88 | 141 | 82 | 45 | 91 | 44 | 64 | 86 | 16 | 32 | 58 | 49 |
| Concomitant NAtherapy | ETV | ETV | TDF | TDF | ETV | TAF | TDF | No | TDF | TAF | TDF | TDF | TDF | TDF | TDF | TDF | TDF | TDF | TDF | No | TDF | TDF | TDF |
Abbreviations: ETV, entecavir; NUC, nucleos(t)ide analog; TAF, tenofovir alafenamide; TDF, tenofovir disoprixile fumarate; VCTE‐LSM, liver stiffness measurement by vibration controlled transient elastography.
3.2. Virological responders
At week 24, 10 (45%) patients were classified as virological responders to BLV monotherapy (Table 2). In two patients being HDV‐RNA undetectable for >24 weeks therapy was terminated. The first part of the clinical course of P1 was previously published. 16 , 22 Sixty weeks after therapy HDV‐RNA became detectable again, but ALT is still in the normal range. In a cirrhotic patient (P9) treatment with 2 mg/day BLV was terminated after 63 weeks. After 4 weeks, HDV‐RNA became again detectable and treatment was resumed. P14 listed for liver transplantation for hepatocellular carcinoma received BLV treatment until a donor organ became available at week 25 (after having achieved a 2.1‐log drop in HDV‐RNA at W24). After transplantation, he is on monotherapy with tenofovir. HDV‐RNA is still undetectable after 1 year.
TABLE 2.
Efficacy of Bulevirtide therapy
| Weeks BLV therapy | Dose (mg/day) | Week 24 | Week 36 | Week 48 | Week 60 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change HDV‐RNA (log) vs. baseline | ALT normal (Y/N) | CR (Y/N) | Change HDV‐RNA (log) vs. baseline | ALT normal (Y/N) | CR (Y/N) | Change HDV‐RNA (log) vs. baseline | ALT normal (Y/N) | CR (Y/N) | Change HDV‐RNA (log) vs. baseline | ALT normal (Y/N) | CR (Y/N) | |||
| P1 | 137 | 10 | −2.80 | N | N | −3.68 | Y | Y | −4.65 | Y | Y | TND | Y | Y |
| P2 a | 130 | 2‐10 | −0.68 | N | N | −0.72 | N | N | −1.16 | N | N | −1.20 | Y | N |
| P3 | 77 | 2 | −2.70 | N | N | −3.49 | Y | Y | −2.80 | Y | Y | −2.94 | Y | Y |
| P4 a | 64 | 2 | −2.60 | Y | Y | −2.59 | Y | Y | −3.00 | Y | Y | −2.90 | Y | Y |
| P5 | 8 | 2 | DO | — | — | |||||||||
| P6 a | 74 | 2 | −0.50 | Y | N | −0.46 | Y | N | −0.47 | Y | N | −2.31 | Y | Y |
| P7 a | 65 | 2 | −0.63 | Y | N | −0.85 | Y | N | −0.52 | Y | N | −0.82 | Y | N |
| P8 | 116 | 2 | −3.30 | Y | Y | TND | Y | N | TND | Y | Y | TND | Y | Y |
| P9 a | 63 + 52 | 2 | −1.14 | N | N | −1.56 | Y | N | TND | Y | Y | TND | Y | Y |
| P10 a | 83 | 2 | −2.15 | Y | Y | −2.39 | Y | Y | −3.00 | Y | Y | −3.00 | Y | Y |
| P11 a | 24 + 37 | 2 | −1.32 | N | N | DO, restartc | — | — | ||||||
| P12 a | 62 | 2 | −0.86 | Y | N | −0.86 | Y | N | TND | Y | Y | TND | Y | Y |
| P13 a | 62 | 2 | −1.33 | Y | N | Missing | Y | N | −1.37 | Y | N | −1.25 | Y | N |
| P14 a | 24 | 2 | −2.10 | Y | Y | LTX | — | — | ||||||
| P15 | 60 | 2 | −0.74 | Y | N | Missing | Y | N | −1.67 | Y | N | −2.30 | N | N |
| P16 | 48 | 2 | −1.36 | Y | N | TND | Y | Y | TND | Y | Y | |||
| P17 a | 48 | 2 | TND | Y | Y | TND | Y | Y | TND | Y | Y | |||
| P18 a | 51 | 2 | −0.86 | Y | N | −1.75 | Y | N | −2.71 | Y | Y | |||
| P19 a | 65 | 2 | −1.29 | N | N | −1.69 | N | N | −1.32 | Y | N | −1.90 | Y | N |
| P20 a | 49 | 2 | −2.09 | Y | Y | −2.59 | Y | Y | −1.75 | Y | N | |||
| P21 a | 48 | 2 | −2.50 | Y | Y | −2.50 | Y | Y | −4.15 | Y | Y | |||
| P22 | 48 | 2 | −2.13 | N | N | −2.56 | N | N | −2.40 | N | N | |||
| P23 a | 52 | 2 | −1.76 | N | N | −2.00 | Y | Y | −2.46 | Y | Y | |||
| Total | VR b :10/22 (45%) | 14/22 (64%) | 7/22 (32%) | VR b :11/20 (55%) | 17/20 (85%) | 10/20 (50%) | VR b :13/20 (65%) | 18/20 (90%) | 12/20 (60%) | VR b :9/13 (69%) | 12/13 (92%) | 8/13 (62%) | ||
Note: Highlighted in yellow background: PEGIFN added prior to this date.
Abbreviations: CR, combined response; DO, drop out; TND, target not detected; LTX, liver transplantation.
Patient with cirrhosis.
VR, virological response: >2log decline or TND.
PEGIFN was added at week 20 of retherapy.
3.3. Addition of PEG‐IFN
PEG‐IFN (Pegasys®, Roche) was added in two virological responders (P4, P10) and in six non‐responders (P2, P6, P11, P15, P18, P19) (Figure 1B). Doses were 90 μg/week in 7, 180 μg/week in 1. P10 had a 2‐log drop of HDV‐RNA after 24 weeks of BLV, but HDV‐RNA re‐increased at week 44. P4 had a significant drop in viral load at week 24 of BLV, which did not further decrease; PEG‐IFN was thus added at week 39. P2 had cirrhosis with marked portal hypertension (HVPG 18 mm Hg, thrombo‐ and leucopenia). He started on 2 mg/day BLV and was increased after 24 weeks to 10 mg/day. Addition of PEG‐IFN (week 58) together with 25 mg Eltrombopag QD and 48 MU/week filgrastim led to a rapid decline of HDV‐RNA. Currently, he is at week 115 of treatment. P11 (cirrhosis Child B) showed a decline of 1.32 log copies/mL at week 24, but treatment was stopped because of severe alcohol abuse. Retreatment was started after 6 months of sobriety and PEG‐IFN was added after 20 weeks. In P15 treatment was terminated because of intolerability of the combination PEG‐IFN with BLV at week 60. Overall, 10 patients are still on BLV monotherapy.
3.4. Virological and biochemical response
All patients started BLV monotherapy. The median declines of HDV‐RNA at BLV monotherapy treatment at weeks 24, 36 and 48 were as follows: 1.57 log copies/mL (0.51–3.76; n = 22), 2.00 (0.46–4.15; n = 20) and 2.44 (0.26–4.65; n = 20) respectively (Table 2). ALT became normal in 14/22 (64%) patients at week 24, in 17/20 (85%) at week 36, in 18/20 (90%) at week 48 and in 12/13 (92%) at week 60. A decrease of HDV‐RNA >2log was achieved in 10/22 (45%) patients at week 24, in 11/20 (55%) at week 36, in 13/20 (65%) at week 48 and in 9/13 (69%) at week 60. Combined response, that is ALT normalisation and a > 2log decline of HDV‐RNA was observed in 7/22 (32%) patients at week 24, in 10/20 (50%) at week 36, in 12/20 (60%) at week 48 and 8/13 (62%) at week 60. Overall, a total of seven patients achieved HDV‐RNA undetectability: One at week 24, three at week 36, one at week 48, one at week 60 and one additional after a treatment period of 100 weeks. 16 , 22 No significant changes in HBsAg levels were observed during BLV therapy.
3.5. Paired comparison of liver stiffness and portal pressure
Paired data on liver stiffness by VCTE at baseline and at 48 weeks of treatment was available in 11 patients included in our cohort. Liver stiffness decreased in nine out of 11 patients. Notably, decreases in liver stiffness did not seem to correlate with virological response, and the only two patients who showed increases in liver stiffness were P11, in whom an increase in HVPG was also observed, most likely due to alcohol abuse, and P4, who was an excellent virological responder, as shown in Table 3.
TABLE 3.
Liver stiffness measurement (LSM values) before and during treatment
| Pat # | Cirrhosis | Pretreatment LSM (kPa) | BLV year 1 LSM (kPa) | Virological response at year 1 (∆log HDV‐RNA) |
|---|---|---|---|---|
| P 2 | Yes | 26.3 | 15.6 (−41%) | −1.16 |
| P 3 | No | 9.1 | 4.9 (−46%) | −2.80 |
| P 4 | Yes | 17.2 | 18.2 (+6%) | −3.00 |
| P 6 | Yes | 10.2 | 5.3 (−48%) | −0.47 |
| P 7 | Yes | 18.6 | 9.6 (−48%) | −0.52 |
| P 8 | No | 5.5 | 5.1 (−7%) | RNA undetectable |
| P 9 | Yes | 35.8 | 28.3 (−21%) | RNA undetectable |
| P 11 | Yes | 25.3 | 27.5 (+9%) | −2.08 a |
| P 13 | Yes | 10.2 | 8.9 (−13%) | −1.37 |
| P 19 | Yes | 48.0 | 26.8 (−44%) | −1.32 |
| P 22 | No | 6.8 | 5.5 (−19%) | −2.40 |
P11 had intermittent, significant alcohol abuse.
Green background: clinically relevant decrease in liver stiffness or HVPG; Yellow background: insignificant change in liver sitffness or HVPG; Red background: clinically increase in liver stiffness or HVPG.
In five patients with pre‐treatment portal hypertension (i.e. HVPG ≥6 mmHg; 4 of 5 with clinically significant portal hypertension, i.e. HVPG ≥10 mmHg), HVPG measurement was repeated after 1 or 2 years on BLV treatment (Table 4). Among four patients with pre‐treatment CSPH, a clinically meaningful (i.e. ≥10% 23 ) decrease in HVPG was observed in two patients, who also achieved virological response, while one patient had no change in HVPG, and, as previously mentioned, HVPG even increased in one patient who had a virological non‐response and consumed excessive amounts of alcohol. The patient with subclinical portal hypertension pre‐treatment and virological response did not progress to CSPH.
TABLE 4.
Hepatic venous pressure gradient (HVPG) before and during treatment
| Pat # | Pretreatment | BLV year 1 | BLV year 2 | Virological response at HVPG (∆log HDV‐RNA) | PEG‐IFN at 2nd HVPG |
|---|---|---|---|---|---|
| HVPG (mmHg) | HVPG (mmHg) | HVPG (mmHg) | |||
| P 2 | 19 | — | 15 (−21%) | −5.17 | Yes |
| P 4 | 17 | 13 (−24%) | — | −3.00 | Yes |
| P 9 | 6 | — | 7 (+17%) | RNA undetectable | No |
| P 11 | 18 | 21 (+17%) | −2.08 a | Yes | |
| P 13 | 10 | 10 (±0%) | −1.37 | No |
P11 had intermittent, significant alcohol abuse.
Green background: clinically relevant decrease in liver stiffness or HVPG; Yellow background: insignificant change in liver sitffness or HVPG; Red background: clinically increase in liver stiffness or HVPG.
3.6. Treatment‐induced side effects
Patient 11 developed hepatic decompensation (ascites, jaundice) shortly after the second HVPG measurement. He had a virological response to combined BLV and PEG‐IFN (90 μg/week, reduced to 45 μg/week) but a further increase in HVPG, most likely due to alcohol abuse. To date, this is the only patient who progressed to decompensated cirrhosis included in this study. All patients on PEG‐IFN had a decrease in leucocytes and platelets, but only one required haemopoetic growth factors. Serum bile acids increased during BLV in all patients treated at the Vienna General Hospital. The magnitude of increase varied considerably among the patients (maximum: +64‐fold, minimum: 2.2‐fold). Except for one patient none complained about pruritus. This patient developed severe pruritus which became unbearable when combination with PEG‐IFN was added and treatment was terminated at week 48 (P15). We previously reported severe pruritus in a patient (not part of this study) a few days after starting BLV 24 which was due to an allergic reaction.
4. DISCUSSION
BLV, an inhibitor of the main hepatic bile acid uptake system NTCP, has been approved by the EMA for treatment of HDV infection based on its anti‐viral efficacy and good safety profile in phase 2 9 , 10 and ongoing phase 3 trials. 13 , 14 However, some important issues regarding the use of BLV in clinical practice were not addressed sufficiently:
First, there is a lack of an accepted surrogate for virological efficacy of BLV therapy. In ongoing studies, the combination of a >2log decline in viral load and normalisation of ALT (combined response) is used as primary endpoint. 25 In the present study 45% (10/22) had a > 2log drop at week 24 and 65% (13/20) at week 48 respectively. In the interim analysis of the French BLV Early Access Program the combined response rates at weeks 24 and 48 were 52.3% and 68.2% respectively. 26 As recently outlined, 18 the use of this endpoint implies that many patients still have detectable HDV‐RNA and the impact of incomplete viral suppression on the further evolution of chronic hepatitis D remains unknown. Furthermore, this approach is problematic in patients with a low baseline viral load ≤104 log copies/mL and low/normal ALT levels. These criteria applied to three and four patients in our study. ALT is an uncertain marker of liver disease and patients with advanced chronic liver disease may have ALT in the normal range. 27 , 28
In the absence of a validated virological/biochemical surrogate endpoint that confers a clinical benefit, we performed paired HVPG measurements, as a 10%‐decrease (i.e. HVPG response) in HVPG translated into a decreased risk of hepatic decompensation in patients achieving HCV cure 23 as well as favourable outcomes in studies investigating medical therapies for portal hypertension. 29 Among three patients with virological response, two patients showed HVPG response, while the third patient had subclinical portal hypertension at baseline and did not progress to CSPH. The patient with a suboptimal virological response and pre‐treatment CSPH showed no change in HVPG. The final patient (P11) who interrupted treatment as a consequence of excessive alcohol consumption had a virological response after combining BLV with low‐dose PEG‐IFN but showed an increase in HVPG and progressed to decompensated cirrhosis. Decompensation was possibly triggered by PEG‐IFN. Accordingly, we observed changes in HVPG that may confer a meaningful benefit to those achieving virological response. In line with observations in patients achieving HCV cure, 23 , 30 LSM was unable to capture the dynamics of portal hypertension on an individual patient level. Generally, it seems that elastography might be similarly inaccurate in diagnosing/longitudinally observing cirrhosis in HDV patients than in HCV patients upon SVR 31 , 32 or patients with stable cirrhosis due to Wilson disease. 33
Based on the association of virological and HVPG response, we propose that the efficacy HDV‐directed therapies should be measured by its ability to achieve sustained suppression of HDV replication. At present, it remains unknown whether this can be achieved by BLV monotherapy. Combination therapy with another anti‐viral agent may be necessary.
Second, the optimal dose of BLV is uncertain; the market authorisation has only been obtained for the 2 mg/d dose, while higher BLV doses may be more effective. 13 In the present study, 20 of 21 patients who received the initial dose of 2 mg/d had some virological response (any log decline) at week 24. One patient (P2) did not sufficiently respond even after increasing the dose to 10 mg/day.
Third, the optimal treatment duration remains unknown. So far, only five patients became HDV‐RNA undetectable for at least 6 months, in two treatment was terminated 63 to 130 weeks, and both relapsed; suggesting that treatment for more than 2 years may be required to reach complete viral suppression.
Fourth, the impact of (add‐on) interferon therapy is controversial for treatment of chronic hepatitis D. 5 Interferon may have an important role during anti‐viral therapy of HDV. A long‐term follow‐up showed that high doses of interferon alpha‐2a given for many years significantly improved the long‐term outcome and survival of a small cohort of patients with chronic hepatitis D. 34 , 35 In a meta‐analysis, PEG‐IFN had only a limited effectiveness in HDV treatment, but at least one‐third of patients achieved viral clearance and normalised ALT levels. 36 In our own experience, two patients (not part of this study) with chronic hepatitis D had a seroconversion HBsAg to anti‐HBs with loss of HDV‐RNA after >2 years on PEG‐IFN monotherapy (data not shown).
Adding PEG‐IFN to BLV therapy may increase response rates (Figure 1B). Following entry into hepatocytes (which is inhibited by BLV), replicative intermediates of HDV‐RNA are sensed by the pattern recognition receptor MDA5 (melanoma differentiation antigen 5) resulting in interferon (IFN)‐β/λ induction. This IFN response strongly suppresses the cell division‐mediated spread of HDV genomes, however, it only marginally affects HDV‐RNA replication in already infected, resting hepatocytes. 37 Strong synergy against HDV was confirmed using 10 mg BLV in combination with PEG‐IFN. 38 With all three new treatment options against HDV (BLV, lonafarnib and nucleic acid polymers) the best response was achieved in combination with PEG‐IFN. 4 , 39 NUCs had no impact on the response and it remains unclear whether they are needed during BLV therapy. Since most of our patients received NUCs, we cannot comment on this issue.
Serum bile acids levels increased with a wide variation among individual patients during therapy as expected due to the inhibition of NTCP. 6 Determination of serum bile acids may be helpful to monitor adherence to therapy.
Although we observed an association between virological and HVPG response, a key question is whether HDV‐RNA suppression without HDV eradication is associated with a clinical benefit. In this study, only 7/22 (32%) patients had undetectable HDV‐RNA upon many weeks of treatment. In two of them, treatment was terminated but HDV‐RNA became detectable again. Studies are ongoing to investigate the impact of treatment on liver fibrosis and direct endpoints such as morbidity and mortality. Beyond the anti‐viral response BLV may exert hepatoprotective effects since NTCP‐inhibition leads to reduced hepatocellular bile acid load. Altered bile acid transport could be an important modulator of liver fibrosis, thus preventing disease progression. 40 Thus, NTCP‐inhibition may be beneficial in selected forms of cholestasis 41 and may have anti‐inflammatory effects. 42
Limitations of this study include the lack of a pre‐defined treatment protocol and missing long‐term outcome data after cessation of BLV therapy, since most patients are still on treatment. The proposed treatment algorithm (Figure 2) is based on our “learning‐by‐treatment” approach and has to be validated in a prospective study. Basically, we followed our experience with response‐guided therapy for chronic hepatitis C. 43 In chronic hepatitis D treated with BLV we observed three patterns: Some patients had an almost linear decline in viral load (responders). In some patients, however, this robust decline levelled off and patients plateaued at a lower level or even slightly increased. There was no definite time point when the change in viral kinetics happened. When the plateau became evident, we added PEG‐IFN, if not contraindicated. The efficacy criteria for stopping BLV treatment are not well‐defined. We terminated treatment only in two patients under close clinical monitoring according to our proposed efficacy criteria and both patients relapsed, however, with one relapse having been observed after >1 year of BLV cessation. This is likely due to persisting HBsAg levels that allow for the formation of new HDV particles. This finding is novel and clinically relevant, since no long‐term follow‐up data have been reported from previous studies using treatment with BLV. 9 , 10 , 26 On the other hand, spontaneous HDV elimination occurred in 20% of patients with chronic hepatitis D without clearance of HBV. 44 This observation opens new questions by which mechanism HDV can be eliminated.
FIGURE 2.
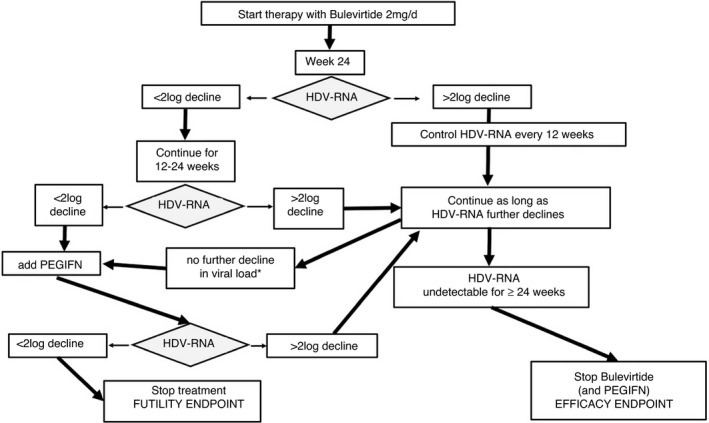
Proposed algorithm for anti‐viral treatment of chronic hepatitis D. *Further decline is defined by a continuous decrease of HDV‐RNA levels (see text)
In summary, the novel NTCP inhibitor BLV is well tolerated and exerts promising virologic as well as biochemical response rates and ameliorates portal hypertension in patients with advanced chronic hepatitis D. Unfortunately, not a single patient had a “sustained” virological response, and only 7/22 (32%) reached undetectable HDV‐RNA on BLV treatment. An individualised approach and prolonged treatment duration could increase the chance of HDV suppression. Future studies should address the long‐term efficacy and safety of BLV in HDV patients and its combination with PEG‐IFN in selected patients.
AUTHOR CONTRIBUTIONS
Mathias Jachs: Data curation (supporting); writing – original draft (supporting); writing – review and editing (supporting). Caroline Schwarz: Data curation (equal). Marlene Panzer: Data curation (equal). Teresa Binter: Data curation (equal); writing – original draft (supporting). Stephan Aberle: Data curation (equal); investigation (equal). Lukas Hartl: Data curation (equal). Kristina Dax: Data curation (equal). Elmar Aigner: Data curation (equal). Albert F. Stattermayer: Validation (equal). Petra Steindl Munda: Data curation (equal). Ivo Graziadei: Data curation (equal). Heidemarie Holzmann: Data curation (equal); investigation (equal). Michael Trauner: Resources (equal); supervision (equal). Heinz Zoller: Data curation (equal). Michael Gschwantler: Data curation (equal). Mattias Mandorfer: Supervision (supporting); writing – original draft (supporting); writing – review and editing (supporting). Thomas Reiberger: Conceptualization (supporting); formal analysis (supporting); funding acquisition (lead); supervision (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Peter Ferenci: Conceptualization (lead); data curation (equal); formal analysis (lead); methodology (lead); project administration (lead); writing – original draft (lead); writing – review and editing (lead). All authors approved the final version of the manuscript.
AUTHORSHIP
Guarantor of the article: Peter Ferenci.
Supporting information
Figure S1
ACKNOWLEDGEMENT
Declaration of personal interests: HZ received speaker honoria from the Abbvie, Bayer, BMS, Falk Foundation, Gilead, Intercept, Merck, MSD, Novartis, Pierre‐Fabre, Pharmacosmos, Vifor; he has advised for Abbvie, Bayer, Eisai, Gilead, Intercept, MSD, Novartis, Shire, Pierre‐Fabre, Pharmacosmos, Vifor. He further received travel grants from Abbvie, Bayer, Gilead and Intercept and research grants from Abbvie, Gilead, MSD, Novartis, Pharmacosmos and Vifor. EA served as a speaker and consultant for Gilead, and received travel support from Gilead. PM served as a speaker and/or consultant and/or advisory board member for MSD, AbbVie, Intercept and Gilead, and received travel support from Gilead and AbbVie. MT received speaker honoria from Falk Foundation, Gilead, Intercept and MSD; he has advised for Albireo, BiomX, Boehringer Ingelheim, Falk Pharma GmbH, Genfit, Gilead, Intercept, Jannsen, MSD, Novartis, Phenex, Regulus and Shire. He further received travel grants from Abbvie, Falk, Gilead and Intercept and research grants from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD Takeda and UltraGenyx. He is co‐inventor of patents on the medical use of NorUDCA filed by the Medical Universities of Graz and Vienna. IG received speaker honoria from AbbVie, Gilead, Intercept and consulting/advisory board fees from AbbVie, Gilead, Intercept and Eli Lilly. GM received grants from AbbVie, Gilead and MSD; speaking honoraria/advisory board fees from AbbVie, Gilead, MSD, Janssen, BMS, Roche, Intercept, Norgine, AstraZeneca, Falk and Shionogi. MM served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Gilead, and W. L. Gore & Associates and received travel support from AbbVie, Bristol‐Myers Squibb and Gilead. TR received grant support from Abbvie, Boehringer‐Ingelheim, Gilead, Gore, Intercept, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant and Siemens; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer‐Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Abbvie, Boehringer‐Ingelheim, Gilead and Roche. PF received an unrestricted research grant from Gilead, was member of the safety review Committee for MyrPharma, speaking honoraria from Gilead and Abbvie, consulting/advisory board fee from Vivaraxx. MJ, SC, TB, LH, KD, StA, AFS, KK, DB, HH: have nothing to disclose.
Declaration of funding interests: This study was supported by a restricted grant by MYR Pharmaceuticals (now Gilead Sciences) awarded to TR. The company had no influence on the study's design, antiviral treatment or the writing of this manuscript.
Jachs M, Schwarz C, Panzer M, Binter T, Aberle SW, Hartl L, et al. Response‐guided long‐term treatment of chronic hepatitis D patients with bulevirtide—results of a “real world” study. Aliment Pharmacol Ther. 2022;56:144–154. 10.1111/apt.16945
Thomas Reiberger and Peter Ferenci share the last authorship.
The Handling Editor for this article was Professor Grace Wong, and it was accepted for publication after full peer‐review.
Funding informationMJ and TR were supported by a restricted research grant from MYR Pharmaceuticals and Gilead Sciences. This study has been supported by a restricted grant (ULTRA) by MYR GmbH and Gilead Sciences.
REFERENCES
- 1. Miao Z, Zhang S, Ou X, Li S, Ma Z, Wang W, et al. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection. J Infect Dis. 2020;221:1677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jachs M, Binter T, Schmidbauer C, Hartl L, Strasser M, Laferl H, et al. Hepatitis D virus (HDV) prevalence in Austria is low but causes considerable morbidity due to fast progression to cirrhosis. United European Gastroenterol J. 2021;9:1119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stockdale AJ, Kreuels B, Henrion MYR, Giorgi E, Kyomuhangi I, de Martel C, et al. The global prevalence of hepatitis D virus infection: systematic review and meta‐analysis. J Hepatol. 2020;73:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Urban S, Neumann‐Haefelin C, Lampertico P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult‐to‐treat disease. Gut Published Online First: 08 June 2021. doi: 10.1136/gutjnl-2020-323888 [DOI] [PMC free article] [PubMed]
- 5. Wedemeyer H, Yurdaydin C, Hardtke S, Caruntu FA, Curescu MG, Yalcin K, et al. Peginterferon alfa‐2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT‐II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 2019;19:275–86. [DOI] [PubMed] [Google Scholar]
- 6. Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J Hepatol. 2016;65:490–8. [DOI] [PubMed] [Google Scholar]
- 7. Li W, Urban S. Entry of hepatitis B and hepatitis D virus into hepatocytes: basic insights and clinical implications. J Hepatol. 2016;64(Suppl 1):S32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Fälth M, et al. Hepatitis B and D viruses exploit sodium taurocholate co‐transporting polypeptide for species‐specific entry into hepatocytes. Gastroenterology. 2014;146:1070–83. [DOI] [PubMed] [Google Scholar]
- 9. Wedemeyer H, Schöneweis K, Bogomolov PO, Voronkova N, Chulanov V, Stepanova T, et al. Final results of a multicenter, open‐label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in combination with PEG‐interferon alpha 2a in patients with chronic HBV/HDV co‐infection. J Hepatol. 2019;70(Suppl 1):e81. [Google Scholar]
- 10. Wedemeyer H, Blank A, Allweiss L, Dandri‐Petersen M, Bremer B, Voronkova N, et al. Final results of a multicenter, open‐label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with tenofovir in patients with chronic HBV/HDV co‐infection. J Hepatol. 2018;68(Suppl 1):S3. [Google Scholar]
- 11. Asselah T, Loureiro D, Le Gal F, Narguet S, Brichler S, Bouton V, et al. Early virological response in six patients with hepatitis D virus infection and compensated cirrhosis treated with Bulevirtide in real‐life. Liver Int. 2021;41:1509–17. [DOI] [PubMed] [Google Scholar]
- 12. Kang C. Syed YY. Bulevirtide: first approval. Drugs. 2020;80:1601–5. [DOI] [PubMed] [Google Scholar]
- 13. Wedemeyer H, Aleman S, Andreone P, Blank A, Brunetto M, Pavel Bogomolov P, et al. Bulevirtide Monotherapy at low and high doses in patients with chronic Hepatitis Delta: 24‐week interim data of the phase 3 MYR301 study. J Hepatol. 2021;75(Suppl 2):S294. [Google Scholar]
- 14. Asselah T, Arama SS, Bogomolov P, Bourliere M, Fontaine H, Gherlan GS, et al. Safety and efficacy of bulevirtide monotherapy and in combination with peginterferon Alfa‐2a in patients with chronic hepatitis delta: 24 weeks interim data of MYR204 phase 2b study. J Hepatol. 2021;75(Suppl 2):S291. [Google Scholar]
- 15. Le Gal F, Gordien E, Affolabi D, Hanslik T, Alloui C, Deny P, et al. Quantification of Hepatitis Delta virus RNA in serum by consensus real‐time PCR indicates different patterns of Virological response to interferon therapy in chronically infected patients. J Clin Microbiol. 2005;43:2363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loglio A, Ferenci P, Uceda Renteria SC, Tham CYL, van Bommel F, Borghi M, et al. Excellent safety and effectiveness of high‐dose Myrcludex‐B monotherapy administered for 48weeks in HDV‐related compensated cirrhosis: a case report of 3 patients. J Hepatol. 2019;71:834–9. [DOI] [PubMed] [Google Scholar]
- 17. Wedemeyer H, Tüzün A, Zachou K, Dalekos N, Pehlivan S, Zeuzem S, et al. Association between level of hepatitis D virus RNA at week 24 of pegylated interferon therapy and outcome. Clin Gastroenterol Hepatol. 2015;13:2342–2349.e2. [DOI] [PubMed] [Google Scholar]
- 18. Lok AS, Francesco Negro F, Asselah T, Farci P, Rizzetto M. Concise review: endpoints and new options for treatment of chronic hepatitis D. Hepatology. 2021;74:3479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwabl P, Bota S, Salzl P, Mandorfer M, Payer BA, Ferlitsch A, et al. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int. 2015;35:381–90. [DOI] [PubMed] [Google Scholar]
- 20. Reiberger T, Schwabl P, Trauner M, Peck‐Radosavljevic M, Mandorfer M. Measurement of the hepatic venous pressure gradient and Transjugular liver biopsy. J Vis Exp. 2020. (160):e58819. doi: 10.3791/58819. [DOI] [PubMed] [Google Scholar]
- 21. de Franchis R, Bosch J, Garcia‐Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty Baveno VII – renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–74. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loglio A, Ferenci P, Uceda Renteria SC, CYL Tham, Scholtes C, et al. Safety and effectiveness of up to 3 years' bulevirtide monotherapy in patients with HDV‐related cirrhosis. J Hepatol. 2022. Feb;76(2):464–9. doi: 10.1016/j.jhep.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 23. Mandorfer M, Kozbial K, Schwabl P, Chromy D, Semmler G, Stättermayer AF, et al. Changes in hepatic venous pressure gradient predict hepatic decompensation in patients who achieved sustained Virologic response to interferon‐free therapy. Hepatology. 2020;71:1023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwarz C, Chromy D, Bangert C, Schwarz M, Jachs M, Reiberger T, et al. Immediate‐type hypersensitivity reaction to bulevirtide and successful desensitization in a patient with HBV/HDV‐associated compensated cirrhosis. J Hepatol. 2022; (in press):S0168‐8278(22)00172‐6. doi: 10.1016/j.jhep.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 25. https://clinicaltrials.gov/ct2/show/NCT03852719
- 26. de Ledinghen V, Guyader D, Metivier S, Hilleret ML, Fontaine H, Roche B, et al. Safety and efficacy of 2 mg Bulevirtide in patients with chronic HBV/HDV co‐infection: first real‐world results. Hepatology 2021;74(Suppl. 1):16A [Google Scholar]
- 27. Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC, Public Policy Committee of the American Association for the Study of Liver Disease . Serum activity of alanine amino‐transferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–70. [DOI] [PubMed] [Google Scholar]
- 28. Senior JR. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury–past, present, and future. Clin Pharmacol Therap. 2012;92:332–9. [DOI] [PubMed] [Google Scholar]
- 29. Mandorfer M, Hernández‐Gea V, Reiberger TG, Pagán JC. Hepatic venous pressure gradient response in non‐selective Beta‐blocker treatment – is it worth measuring? Curr Hepatol Rep. 2019;18:174–86. [Google Scholar]
- 30. Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, et al. Sustained virologic response to interferon‐free therapies ameliorates HCV‐induced portal hypertension. J Hepatol. 2016;65:692–9. [DOI] [PubMed] [Google Scholar]
- 31. Broquetas T, Herruzo‐Pino P, Mariño Z, Naranjo D, Vergara M, Morillas RM, et al. Elastography is unable to exclude cirrhosis after sustained virological response in HCV‐infected patients with advanced chronic liver disease. Liver Int. 2021;41:2733–46. [DOI] [PubMed] [Google Scholar]
- 32. D'Ambrosio R, Aghemo A, Fraquelli M, Rumi MG, Donato MF, Paradis V, et al. The diagnostic accuracy of Fibroscan® for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol. 2013;59:251–6. 10.1016/j.jhep.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 33. Paternostro R, Pfeiffenberger J, Ferenci P, Stättermayer AF, Stauber RE, Wrba F, et al. Non‐invasive diagnosis of cirrhosis and long‐term disease monitoring by transient elastography in patients with Wilson disease. Liver Int. 2020;40:894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farci P, Roskams T, Chessa L, Peddis G, Mazzoleni AP, Scioscia R, et al. Long‐term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology. 2004;126:1740–9. [DOI] [PubMed] [Google Scholar]
- 35. Yurdaydin C, Keskin O, Kalkan Ç, Karakaya F, Çaliskan A, Kabaçam G, et al. Interferon treatment duration in patients with chronic delta hepatitis and its effect on the natural course of the disease. J Infect Dis. 2018;217:1184–92. [DOI] [PubMed] [Google Scholar]
- 36. Abdrakhman A, Ashimkhanova A, Almawi WY. Effectiveness of pegylated interferon monotherapy in the treatment of chronic hepatitis D virus infection: a meta‐analysis. Antiviral Res. 2021;185:104995. [DOI] [PubMed] [Google Scholar]
- 37. Zhenfeng Z, Urban S. New insights into HDV persistence: the role of interferon response and implications for upcoming novel therapies. J Hepatol. 2021;74:686–99. [DOI] [PubMed] [Google Scholar]
- 38. Wedemeyer H, Schöneweis K, Bogomolov PO, Chulanov V, Stepanova T, Viacheslav M, et al. 48 weeks of high dose (10 mg) Bulevirtide as monotherapy or with peginterferon alfa‐2a in patients with chronic HBV/HDV co‐infection. J Hepatol. 2020;73(Suppl1):S52–3. [Google Scholar]
- 39. Yurdaydin C, Keskin O, Yurdcu E, Çalişkan A, Önem S, Karakaya F, et al. A phase 2 dose‐finding study of lonafarnib and ritonavir with or without interferon alpha for chronic delta hepatitis. Hepatology. 2022, in press;12:695–700. [DOI] [PubMed] [Google Scholar]
- 40. Salhab A, Amer J, Lu Y, Safadi R. Sodium+/taurocholate cotransporting polypeptide as target therapy for liver fibrosis. Gut. 2021. Jul 15:gutjnl‐2020‐323345. doi: 10.1136/gutjnl-2020-323345. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Slijepcevic D, Roscam Abbing RLP, Fuchs CD, Haazen LCM, Beuers U, et al. Na+‐Taurocholate Cotransporting polypeptide inhibition has HepatoprotectiveEffects in cholestasis in mice. Hepatology. 2018;68:2057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cai SY, Ouyang X, Chen Y, Soroka CJ, Wang J, Mennone A, et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte‐specific inflammatory response. JCI Insight. 2017;2:e90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferenci P. Response guided therapy in patients with chronic hepatitis C – yesterday, today and tomorrow. Best Pract Res Clin Gastroenterol. 2012;26:463–9. [DOI] [PubMed] [Google Scholar]
- 44. Palom A, Sopena S, Riveiro‐Barciela M, Carvalho‐Gomes A, Madejón A, Rodriguez‐Tajes S, et al. One‐quarter of chronic hepatitis D patients reach HDV‐RNA decline or undetectability during the natural course of the disease. Aliment Pharmacol Ther. 2021;54:462–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


