Abstract
Objectives
To identify titanium particles (TPs) in biopsy specimens harvested from peri‐implantitis lesions and secondarily to study the histopathological characteristics in peri‐implantitis compared to periodontitis, in order to evaluate whether the presence of TPs could alter respective inflammatory patterns.
Material and methods
Biopsies containing granulation tissue were harvested during routine surgical treatment in 39 peri‐implantitis cases and 35 periodontitis controls. Serial sections were obtained using titanium‐free microtome blades. The first and last sections of the peri‐implantitis specimens were used for identification of TPs by scanning electron microscopy coupled with dispersive X‐ray spectrometry. Intermediate sections and periodontitis specimens were processed for descriptive histological study using haematoxylin–eosin staining and for immunohistochemical analysis using CD68, IL‐6, Nf‐kB and VEGF markers.
Results
TPs were identified in all peri‐implantitis specimens as free metal bodies interspersed within granulation tissue. However, presence of macrophages or multinucleated giant cells engulfing the TPs were not identified in any specimen. Peri‐implantitis granulations were characterized by a chronic inflammatory infiltrate rich in neutrophils. About half of peri‐implantitis patients exhibited a subacute infiltrate characterized with lymphocytes interweaved with neutrophils and eosinophils. When compared to periodontitis, peri‐implantitis tissues showed higher proportions of macrophages and a more intense neovascularization, based on significantly higher expression of CD68 and VEGF respectively.
Conclusion
TPs were identified in all peri‐implantitis specimens, but without evidencing any foreign body reaction suggestive for direct pathological effects of TPs. The peri‐implantitis granulation tissue was characterized by intense neovascularization and presence of a chronic inflammatory infiltrate dominated by plasma cells, neutrophils and macrophages.
Keywords: biopsy, cd68, dental implant, granulation tissue, immunohistochemistry, nf‐kb, peri‐implantitis, titanium, VEGF
1. INTRODUCTION
Dental implants represent the current gold standard treatment for the rehabilitating of tooth loss, providing the patients an improved oral health quality of life, as well as long‐term functional and aesthetic outcomes (Nickenig et al., 2008). However, in spite of the high long‐term survival rates (>90%) of implant‐supported restorations (Moraschini et al., 2015), the increasing incidence of implant complications and peri‐implant diseases has become a growing concern in modern implant dentistry (J. Derks et al., 2016; Figuero et al., 2014). Peri‐implantitis is defined as a plaque‐associated pathological condition occurring in tissues around dental implants, characterized with inflammation in the peri‐implant mucosa and subsequent progressive loss of supporting bone (Berglundh et al., 2018). Peri‐implantitis has an estimated patient‐based prevalence of around 20% (J. Derks et al., 2016), although there is a high heterogeneity in this prevalence data, between 4% and 45%, depending on the case definition used across studies (Jan Derks & Tomasi, 2015; Rakic et al., 2018). Without appropriate therapy, this condition frequently leads to the implant failure (J. Derks et al., 2016; Schwarz et al., 2018), while the current therapies, although able to arrest disease progression, have resulted in a low predictability of disease resolution (Figuero et al., 2014; Koldsland et al., 2018).
The aetiology and pathogenesis of peri‐implantitis bear clear similarities with that of periodontitis, although there are also important differences, not only related to the more aggressive pattern of progression in peri‐implantitis (Schwarz et al., 2018), but also to the clear differences in histopathological characteristics of the affected tissues (Berglundh Tord et al., Berglundh et al., 2018). These differential features have prompted some authors to question the role of bacteria as the primary aetiological agent and to attribute these peri‐implant lesions to implant‐related factors, such as release of titanium particles (TPs), considering these particles the primary source of peri‐implant pathology (Albrektsson et al., 2014). Although titanium, which is the main material in modern dental implants, is praised for good corrosive resistance, thanks to the titanium oxide layer present in sterile implants, this layer may dissolve in acid environment and allow the leakage of by‐products, which may elicit a chronic inflammatory reaction (Delgado‐Ruiz & Romanos, 2018). In vitro studies have shown accelerated Ti corrosion in presence of lipopolysaccharide (LPS) at low pH (Barão et al., 2011), what has suggested that the acidogenic environment resulting from chronic anaerobic peri‐implant infection may contribute to dissolution of the bioinert oxide layer followed by Ti leakage and enhanced peri‐implant inflammation (Wachi et al., 2015). The exact mechanism of interaction between TPs and the surrounding tissues remains, however, controversial. Some authors have reported that TPs will stimulate an inflammatory response (M. Pettersson et al., 2017; Swiatkowska et al., 2019), while others have suggested that the tissue response will occur as a foreign body reaction (FBR) with macrophages and multinucleated giant cells (MNGCs) engulfing the TPs (Albrektsson et al., 2014). Even though in experimental in vivo and in vitro studies, TPs have shown to dysregulate local immunity, mainly through impairing antigen‐presenting cells and secondarily promoting a pro‐inflammatory reaction (Heyman et al., 2018; M. Pettersson et al., 2017; Wachi et al., 2015), this effect has not been demonstrated in humans. In fact, there are few clinical studies evaluating the possible deleterious impact of TPs in the pathogenesis of peri‐implantitis (Tobias Fretwurst et al., 2016; Olmedo et al., 2013; Mattias Pettersson et al., 2019; Wilson et al., 2015). These studies have primarily aimed to identify TPs within the peri‐implant tissues and to study the histopathological characteristics of the affected tissues, but they have not been able to associate the presence of these TPs with the peri‐implant tissue pathology (T. Fretwurst et al., 2018; Tobias Fretwurst et al., 2016). So far, the exact role of TPs in peri‐implantitis has not been fully elucidated and remains unclear whether TPs may aggravate peri‐implant inflammation and lead to the end‐stage foreign body reaction (FBR).
The working hypothesis of the present study was that TPs could elicit a specific inflammatory response modifying the patterns of chronic inflammation and leading to an end‐stage FBR.
It was, therefore, the primary purpose of this observational study to identify TPs in peri‐implant granulation tissue harvested from peri‐implantitis lesions, and secondarily to investigate the histopathological patterns of peri‐implantitis compared to periodontitis specimens to evaluate whether presence of TPs could induce an effect on the inflammatory patterns.
2. MATERIAL AND METHODS
2.1. Study design and population
This study was designed as cross‐sectional case–control study evaluating the histopathological and immunohistochemical characteristic of tissue biopsies retrieved either from peri‐implantitis lesions (cases). As reference controls, tissue biopsies from periodontitis lesions were used (Figure 1). The population, source of the specimens was patients attending the Clinic for Maxillofacial, Oral Surgery and Implantology, Military Medical Academy, Belgrade, Serbia from September 2012 until November 2019. These patients were systemically healthy with a diagnosis of either peri‐implantitis or severe periodontitis according to the case definitions (Page & Eke, 2007; Sanz, and Chapple, 2012).
Peri‐implantitis defined by presence of probing depths (PD) ≥ 5mm, bleeding on probing (BOP)>1 and radiographic bone loss (RXBL) ≥2 mm measured from the implant shoulder to the first detectable bone to implant contact.
Severe periodontitis defined by presence of >2 interproximal sites with clinical attachment loss (CAL) >6 mm and >1 interproximal site with PD >5 mm not on the same teeth.
FIGURE 1.
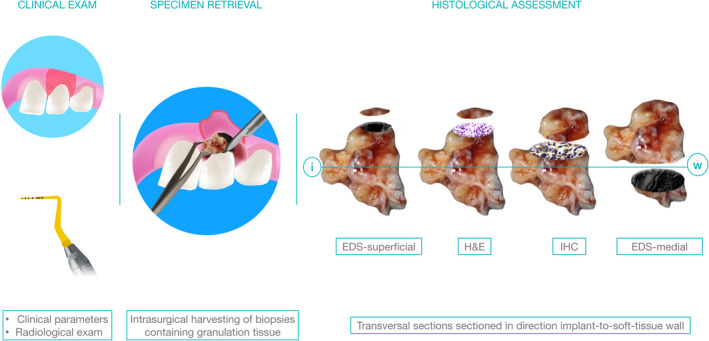
Study design. Diagnostic specimen represented granulation tissue harvested within routine surgical treatment of peri‐implantitis/periodontitis using Ti‐free instruments. In order to ensure true‐positive outcomes, the elemental analysis was performed using scanning electron microscopy (SEM) and dispersive X‐ray spectrometry (EDS) in the first and last (medial) sections following consecutive sectioning for haematoxylin and eosin staining (H&E), and immunostaining. Histopathological assessment was performed in H&E sections, while the nuclear factor kappa‐B (Nf‐kB), interleukin‐6 (IL‐6), Cluster of Differentiation 68 (CD68) and vascular endothelial growth factor (VEGF) were used to compare inflammatory patterns between the groups
In both groups of patients, surgical treatment was indicated, once the non‐surgical therapy did not meet the treatment objectives. Biopsies from patients were excluded if having systemic diseases that could alter the periodontal/peri‐implant tissue response.
The study was conducted according to the Helsinki declaration for human studies (World Medical Association, 2013) and the use of patient's tissue specimens was approved by the institutional ethics committee (Ethics Committee of the Medical Military Academy, Belgrade, Republic of Serbia, permission references: # VMA10‐12‐A.1, #2/2021). The patients were informed about study characteristics and accepted to donate their tissue specimens as part of the excised tissues during peri‐implant or periodontal surgery by signing an informed consent.
2.2. Tissue harvesting and histological processing
Diagnostic specimens represented peri‐implant and periodontal granulation tissues harvested during standard surgical treatment, following infiltrative anaesthesia (Ubistesin Forte, 3 M Espe, 4% articaine/ epinephrine 1/100,000, Deutschland GmbH,) and exposure of the soft tissue pocket wall by elevation of full‐thickness mucoperiosteal flap. An effort was made to minimize contact with the root/implant surface during the biopsy procedure, thus the specimens were excised from the pocket wall using a scalpel blade (15c, Aesculap AG,). Specimens were rinsed in sterile saline and further placed in cassettes (Tissue‐Tek Paraform Sectionable Cassette System by Sakura Finetek Europe, Netherlands) and containers containing 3.5% buffered formalin for immediate transportation to the laboratory. To orientate the specimen, the biopsies were placed with the dissection plane oriented towards the bottom of the cassette. Specimens were subsequently dehydrated and embedded in paraffin using standard protocols, and further sectioned using microtome (sections of 5 μm thickness) and mounted on glass slides (SuperFrost, Menzel Braunschweig,). In the peri‐implantitis specimens, Ti‐free instruments and blades were used for tissue harvesting and sectioning to prevent any false positivity due to possible contamination. The first and last (medial) peri‐implantitis sections were used for dispersive X‐ray spectrometry (EDS). The remaining sections and all periodontitis specimens were stained with haematoxylin–eosin (HE) or processed for immunohistochemistry (IHC).
Sections for IHC were immune‐stained with the following antibodies: CD68 (1:200, sc‐20060, Santa Cruz Biotechnology,), IL6 (1:100, sc‐130326, Santa Cruz Biotechnology,), NF‐κB (1:100, sc‐8008, Santa Cruz Biotechnology,) and VEGF (1:100, sc‐7269, Santa Cruz Biotechnology,) for identification of macrophages, markers of inflammation and neovascularization respectively. The standard settings protocol was performed for deparaffinization, rehydration and antigen retrieval, endogenous peroxidase activity was further blocked (Dako Dual Endogenous Enzyme Block,) while the IHC staining was performed using commercial kit according to the manufacturer's instructions (EnVision System‐ HRP; DAB, DakoCytomation,). The slides were counterstained with haematoxylin and later manually dehydrated, mounted and cover‐slipped.
2.3. Histological evaluation
HE sections were examined by an experienced pathologist (G.J.B.) blinded for specimen affiliation, using brightfield microscopy (BX50 Olympus, Inc,) equipped with imaging system (Q‐500 MC; Leica, Wetzlar, Germany) with the objective of evaluating the cell content of the inflammatory infiltrate, as well as possible signs of foreign body reaction (FBR).
IHC sections were counterstained with haematoxylin and subsequently dehydrated and mounted manually. Using the x40 objective, 4–5 representative fields of interest (300 × 200 μm) were selected depending on the dimension of the specimen and exported for identification and quantification of positive cell markers using the automated NIH ImageJ software (NIH, Bethesda, Maryland; https://imagej.nih.gov/ij/) equipped with the IHC profiler plugin (Varghese et al., 2014). The results were averaged per sample and expressed as proportion (%) of positive cells and relative ratio of positive and negative cells. The assessment was performed by two examiners blinded for the type of specimen, while their calibration was performed by double examination of 10 randomly selected immune‐stained sections. An inter‐examiner and intra‐examiner agreement were measured using linear weighted κ coefficient (inter‐examiner κ: 0.975; intra‐examiner κ G.B.J.: 0.968, M.R.:0.961).
2.4. Scanning electron microscopy and X‐ray spectrometry (EDS)
The first and last medial section of test specimen was used for identification of metal particles (MP) using brightfield and polarized microscopy in HE‐stained sections (Tobias Fretwurst et al., 2016; Grosse et al., 2015) (Figure 2a,b) and scanning electron microscopy (SEM) (Leo 1450 VP, Zeiss, Oberkochen, Germany) (Figure 2c,d). Two‐section verification approach was applied to avoid false Ti positivity in superficial layers related to instrumental manipulation, thus only the samples presenting the TPs in the middle layer have been considered Ti positive. Characterization of the elemental composition of these MP was performed using dispersive X‐ray spectrometry (EDS) (Figure 2e,f). Samples were metalized for SEM analysis using carbon to avoid interference between palladium/gold and metals. The obtained specimens were examined using accelerating voltage (20kV) SEM and scanned with a 100 μm × 100 μm beam size at varying magnifications for the identification of particle‐like structures. Regions of interests where the particles were identified were magnified and tested using EDS to determine their elemental composition. Between 5 and 10 spectres per sample were analysed and the elemental composition was expressed as mean mass percentage of elements (mass %) by spectre.
FIGURE 2.
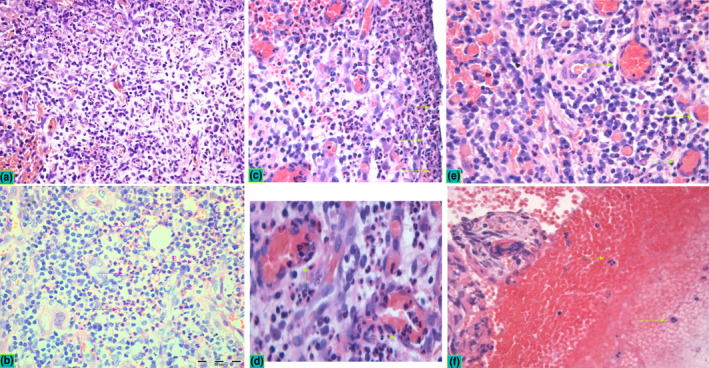
Histopathological profile in peri‐implantitis. Inflammatory infiltrate with focal presence of neutrophils was observed in all samples suggesting the infection‐induced chronic inflammation as a common pathological diagnosis of peri‐implantitis. Two patterns of inflammation were observed in PI, including chronic lymphocyte infiltrate with abundant plasma cells (a) and subacute infiltrate (b) characterized with chronic infiltrate interweaved with zones of neutrophil (N) granulocytes and sporadic eosinophils (E) (violet arrows indicate pink zones of granulocyte infiltrates within chronic infiltrate). Neutrophils were observed in all specimens in the lining contact zone towards dental implant (c, green arrows), the squeezed neutrophils were observed in the endothelium and vessel lumen (d) as well as in the extravasated blood content (e, f). Peri‐implantitis lesions exerted dense vascular networks with hyperaemic vessels that were frequently associated with micro‐bleeding characterized with focal erythrocyte infiltrates (f). All micrographs have been captured at x40 magnification, while the d and e represent zoomed crops of the same micrographs aiming at better visualization of specific histopathological details
2.5. Data analysis
The proportion of IHC‐positive cells and their relative ratio +/− per sample were expressed as median ± standard deviation and were further compared using Mann–Whitney U‐test between the groups. Statistical analysis was performed using commercial software (SPSS v.25.0; SPSS Inc.,). The current study was reported according to the STROBE guidelines.
3. RESULTS
Thirty‐nine peri‐implantitis and 35 periodontitis specimens were available for histological and IHC evaluation. Their characteristics regarding periodontal and peri‐implant parameters as well as age, gender and smoking habits are depicted in Table 1.
TABLE 1.
Demographic and periodontal characteristics of the groups
| Peri‐implantitis n = 39 | Periodontitis n = 35 | |
|---|---|---|
| Gender | ||
| Females (n) | 16 | 18 |
| Male (n) | 23 | 17 |
| Age (years; mean and range) | 52.5 (24–60) | 53.14 (32–62) |
| Smokers (n) | 18 | 21 |
| Number of teeth (n; mean and range) | 16.1 (0–25) | 18.2 (6–25) |
| Full‐mouth dental plaque index (% mean ± SD) | 20.4 ± 9.2 | 25.3 ± 3.9 |
| Full‐mouth dental bleeding on probing (% means ± SD) | 18.0 ± 5.3 | 20.5 ± 4.5 |
| Full‐mouth dental probing depth (mm; mean ± SD) | 3.9 ± 0.9 | 4.8 ± 3.2* |
| Full‐mouth CAL (mm; mean ± SD) | 2.9 ± 1.1 | 3.9 ± 2.1* |
| Implant‐site parameters | Periodontal‐site parameters | |
| Plaque index (% mean ± SD) | 81.88 ± 18.31 | 85.7 ± 15.4 |
| Bleeding on probing (% mean ± SD) | 97.1 ± 15.2 | 96.25 ± 12.45 |
| Probing depth (mm; mean ± SD) | 6.1 ± 1.9 | 7.8 ± 3.81 |
| Suppuration (n and %) | 12 (30.76) | 6 (17.14) |
*p < .05.
3.1. Descriptive histopathology
In both peri‐implantitis and periodontitis specimens, a chronic inflammatory infiltrate with focal presence of neutrophils comprised most of the tissue content. However, in peri‐implantitis specimens, the inflammatory infiltrate was denser and richer in vascular networks and hyperaemic vessels, usually associated with micro‐bleeding resulting in focal erythrocyte infiltrates (Figure 2). The peri‐implantitis specimens depicted two main patterns of inflammatory infiltrate: one with a predominant infiltrate of lymphocytes mainly plasma cells (Figure 2a) and the other with zones rich in neutrophil granulocytes and sporadic eosinophils interweaved with the chronic lymphocytic infiltrate (Figure 2b). Neutrophils were observed in all specimens in the area in close vicinity to the implant surface (Figure 2c), within the endothelium and vessel lumen (Figure 2d) and transmigrating through the endothelium (Figure 2e,f).
3.2. IHC patterns
Figure 3 depicts the differential expression of IHC markers between peri‐implantitis and periodontitis specimens, while the percentage of total positive cells and respective relative ratio +/− cells between the groups are provided in the Table 2. Both demonstrated intensive staining for inflammatory markers (CD68, Nf‐kB and IL‐6), although peri‐implantitis sections showed a significantly higher total positivity and relative ratio of CD68 compared to periodontitis sections (total positivity: p = .040; relative ratio: p < .021). VEGF also showed significantly higher expression in peri‐implantitis sections (total positivity: p = .036; relative ratio: p < .001). In fact, this marker was scarcely expressed in periodontitis sections.
FIGURE 3.
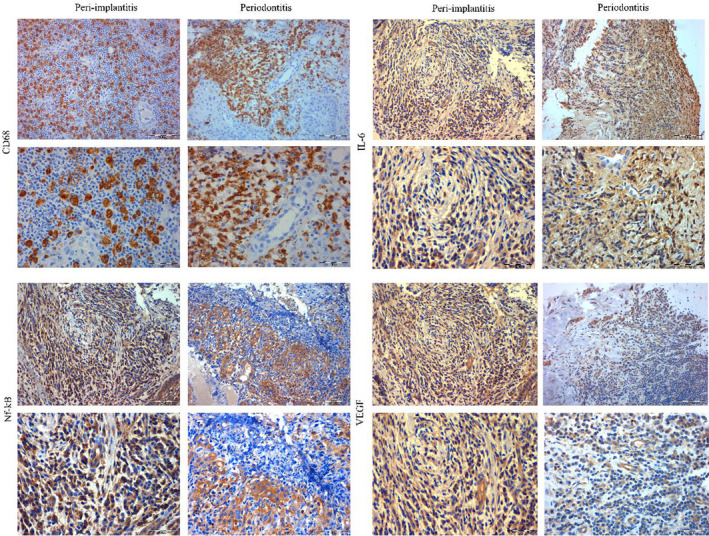
Expression of immunohistochemical markers between peri‐implantitis and periodontitis. Peri‐implantitis and periodontitis granulation tissue exhibited intensive staining for CD68, Nf‐kB and IL‐6, while the CD68 was the single marker significantly more expressed in peri‐implantitis. VEGF was significantly more expressed in peri‐implantitis when compared to periodontitis where it was lightly expressed. Micrographs were captured at two magnifications x200 and x400 per each experimental and control sample
TABLE 2.
Quantitative characteristics of immunohistochemical markers between the groups
| Periodontitis | Peri‐implantitis | |||
|---|---|---|---|---|
| Total + cells | +/− ratio | Total + cells | +/− ratio | |
| CD68 | 27.11 ± 38.17 | 1.81 ± 0.32 | 73.85 ± 35.28* | 4.32 ± 1.01* |
| Nf‐kB | 66.23 ± 10.16 | 2.62 ± 0.15 | 68.77 ± 11.57 | 1.54 ± 0.41 |
| IL−6 | 43.32 ± 7.63 | 0.84 ± 0.15 | 38.37 ± 15.12 | 0.71 ± 0.3.21 |
| VEGF | 34,24 ± 8.24 | 0,19 ± 0.15 | 43,095 ± 21.03* | 1,67 ± 5.45* |
CD68 ‐ cluster of differentiation 68; Nf‐kB ‐ nuclear factor kappa‐B; IL‐6 ‐ interleukin 6; VEGF ‐ vascular endothelial growth factor; * p < .05.
3.3. Presence of metal particles within the granulation tissue in test specimens
TPs were identified in all PI biopsies (100%) with variable size and shape, and all specimens presented TPs in the central zones (inner medial sections) confirming the lack of false positivity due to instrumental manipulation within tissue harvesting. Two trends of distribution were observed: in the vicinity of the implant surface as small spot‐like particles diffusely dispersed (Figure 4a,b), and as single particles with higher diameter present in the central zones (Figure 4c) with average particle size of 8.9 ± 24.8 μm2. TPs were observed as a free inter‐cellular content without depicting any sign of specific inflammatory reaction (FBR). Phagocytes with engulfed particles or MNGCs were not observed in any specimen. The elemental characterization confirmed the presence of titanium in all peri‐implantitis specimens, together with identification of Si, Fe, Rb and Yr in some specimens (Figure 4d,e). Other organic and non‐organic elements probably originating from biological fluids and carbon metallization such as C, O, K, Mg, Si, S, P were also identified, as well as Ca suggesting the presence of resorbing bone fragments within the inflammatory tissue (Figure 4f).
FIGURE 4.
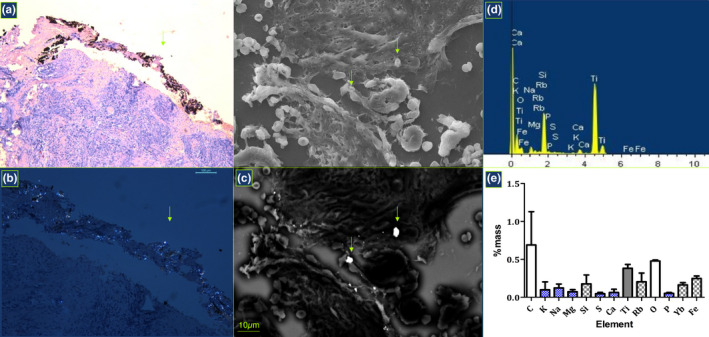
Identification of Ti particles. Metal particles were identified in H&E sections using light microscopy (a) and polarized light (b), and further using scanning electron microscopy using different conditions (c) while they were confirmed using dispersive X‐ray spectrometry (d) and elemental analysis was performed to estimate a mean mass percentage of identified elements with respective SD (e). Smaller dispersed titanium particles were observed in the boundary zone (a) while the solitary particles with higher diameter were rather observed in central zones (d). In addition to Ti, elemental analysis confirmed the presence of other constitutive elements of dental materials and instruments in some specimens, including Si, Fe, Rb and Yr (d, e), as well as other organic and non‐organic elements probably originating from biological fluids and carbon metallization such as C, O, K, Mg, Si, S and P
4. DISCUSSION
The results from this investigation have evidenced the presence of TPs in all peri‐implantitis specimens studied. However, these particles did not show any sign of biological activity around them nor were present within scavenger cells. Furthermore, there were no signs of frustrated phagocytosis and presence of MNGCs, so the typical histological signs of a FBR were not identified in any specimen. In comparison with the periodontitis tissues, peri‐implantitis tissues demonstrated a more severe inflammatory pattern with a higher degree of vascularization. Furthermore, a clear neutrophil infiltration within the granulation tissue was clearly identified in most of the peri‐implantitis specimens. These findings were also apparent when the tissues were studied with immunohistochemistry, with peri‐implantitis tissues demonstrating a significantly higher macrophage afflux and more expressed neovascularization.
The present study has confirmed the previously established histopathological features of peri‐implantitis lesions characterized by aggregation of neutrophils, particularly expressed in the lining zone facing the implant within a mixed inflammatory infiltrate composed of lymphocytes and macrophages, with predominance of plasma cells (O. Carcuac & Berglundh, 2014; Gualini & Berglundh, 2003; Wilson et al., 2015). Moreover, the varying grade of inflammation from chronic to subacute (O. Carcuac & Berglundh, 2014; Gualini & Berglundh, 2003) was confirmed in the present study since 50% patients presented mixed subacute infiltrate consisted of granulocytes, thus future studies should investigate whether these subacute histopathological patterns are associated with the most severe form of peri‐implantitis. The comparative evaluation of the histopathological findings between peri‐implantitis and periodontitis confirmed previously established observations regarding the bacterially induced chronic inflammatory pattern in both diseases, being the most prominent macroscopic and microscopic differences the larger lesion size and more expressed neovascularization in peri‐implantitis, what confirms previous descriptions comparing both lesions (O. Carcuac & Berglundh, 2014).
The immunohistochemical study specifically focused on presence of macrophages, inflammatory markers and markers of neovascularization. Macrophages were selected since these cells are primary drivers of biological response on titanium implants (Zhang et al., 2018), both responsible for osteointegration in presence of biocompatible surface as well as for detection of harmful TPs, which is followed by stimulation of T‐helper type‐1 cells (TH1), promoting a M1‐macrophage response directed to eliminate them (Klopfleisch & Jung, 2017). We selected the marker CD68 as an universal macrophage marker, commonly used to estimate the intensity of inflammatory reaction against biomaterials (Miron et al., 2016). The IHC results from the present study demonstrated significantly increased expression of CD68 in peri‐implantitis, compared to periodontitis, what clearly suggests an increased macrophage activity in peri‐implantitis. This finding has been reported in previous histopathological studies (O. Carcuac & Berglundh, 2014; Tobias Fretwurst et al., 2020; Galarraga‐Vinueza et al., 2020). In fact, recent studies have confirmed the predominance of pro‐inflammatory M1‐macrophages over M2‐macrophages in peri‐implantitis (Tobias Fretwurst et al., 2020; Galarraga‐Vinueza et al., 2020; Garaicoa‐Pazmino et al., 2019).
Markers of inflammation used in this investigation (Nf‐κB and IL‐6) did not demonstrate significant differences between peri‐implantitis and periodontitis lesions. Moreover, the Nf‐κB was selected as representing an intrinsic transcription factor in the biosynthesis of pro‐inflammatory mediators, routinely used for estimation of the inflammatory response intensity (Zhang et al., 2018). The lack of differences in the intensity of this marker between both lesions may indicate the lack of specific inflammatory enhancers in peri‐implantitis, collectively supporting the similar etiopathogenesis in periodontitis and peri‐implantitis, characterized with non‐resolving chronic inflammation in response to a dysbiotic biofilm. IL‐6 was selected as a key pro‐inflammatory cytokine associated with bone resorption and with frustrated phagocytosis (Anderson et al., 2008) (Klopfleisch & Jung, 2017). In that context, the similar pattern of IL‐6 expression between peri‐implantitis and periodontitis is in concordance with the lack of clear macrophage overstimulation in response to the TPs identified in the peri‐implantitis specimens. VEGF was estimated as representing a major macrophage chemoattractant and mediator of neovascularization (Harding et al., 2019). This factor was significantly more expressed in peri‐implantitis tissues, what also corroborates previous investigations reporting an enhanced neovascularization in peri‐implantitis lesions (O. Carcuac & Berglundh, 2014; Gualini & Berglundh, 2003). Since neovascularization relates to the metabolic activity within pathological process and related severity (Kumar et al., 2016), this is suggestive for more severe inflammatory process in peri‐implantitis. Overexpression of VEGF has been directly correlated to TPs exposure in a dose‐ and time‐depending manner (Miyanishi et al., 2003; Spanogle et al., 2006); however, in this investigation, the increased VEGF could not be assigned to the presence of TPs since there was a lack of histopathological signs associated with the TPs in the assessed specimens. However, other investigations have reported possible interference of TPs with local immunological networks leading to pro‐inflammatory upregulation (M. Pettersson et al., 2017). There is, therefore, a need for future research on the possible association between TPs and inflammatory upregulation.
The post‐market material‐vigilance represents the backbone for safe and effective use of medical devices (Kramer et al., 2013; Schmalz & Galler, 2017), and therefore, it is sound to study the potential leakage of TPs and their potential harmful effects when studying the biological complications of dental implants. In the present study, we have evaluated not only the presence of the typical pathological signs of FBR associated with the TPs (presence of multinucleated cells around the TPs), but also the inflammatory patterns and its intensity in aid of IHC with carefully selected biomarkers reflecting the intensity of the inflammatory response (Nf‐kB) and neovascularization (VEGF). Furthermore, we have used as controls, granulation tissue from periodontitis specimens with the assumption that these tissues were completely TPs free. Regarding the first objective, we could not identify the presence of multinucleated cells and other characteristic pathological signs of a FBR associated with the presence of the identified TPs. The intensity of the inflammation measured by the Nf‐kB expression did not demonstrate any significant difference when comparing peri‐implantitis versus periodontitis granulation tissues, although the increased number of macrophages and higher neovascularization patterns in the peri‐implantitis granulation tissue indicate a more severe pattern of destruction in this disease.
The screening of TPs in both superficial and medial granulation layers excluded the possible false positivity due to instrumentation. Other studies have shown that during submarginal debridement detached particles from scaling instruments may remain within peri‐implant tissues (Eger et al., 2017) what may have interfered with an accurate interpretation of the study outcomes.
TPs seemingly represent a common finding in peri‐implant tissues that may originate from drilling, friction and instrumental manipulation during routine dental procedures (Kister et al., 2017; Kotsakis et al., 2020; Suárez‐López Del Amo et al., 2018). Moreover, a bacterially contaminated microenvironment and the use of corrosive antiseptics may facilitate the process of TPs detachment (Kotsakis et al., 2016; Mombelli et al., 2018). However, there are only few published studies that have identified TPs in soft tissue biopsies and granulation tissue from peri‐implantitis patients (Tobias Fretwurst et al., 2016; Mattias Pettersson et al., 2019; Wilson et al., 2015). These studies have also reported a high prevalence of TPs associated with similar histopathological characteristics, as those reported in the present investigation. The main discrepancy, however, lays in the finding of MNGCs in more than 10% of samples, as reported by Wilson et al. (Wilson et al., 2015). These cells, however, were not identified in the present investigation in the vicinity of the TPs. The role of MNGCs in peri‐implant tissues is controversial since these cells can be found in tissues from both healthy osseointegrated implants as well as from peri‐implantitis specimens (Bosshardt, Chappuis, & Buser, 2017; Salata et al., 2007). Furthermore, these cells cannot be accurately distinguished from activated osteoclasts, unless using specific markers since they originate from the same precursor monocyte/macrophage lineage (Miron et al., 2016). In fact, activated osteoclasts represent a common finding in peri‐implantitis tissues, being their number a differential factor when compared with periodontitis lesions (Olivier Carcuac et al., 2013). Albrektsson et al. have proposed a theory to explain the pathogenesis of peri‐implantitis based on an unfavourable FBR towards dental implants and released TPs, while suggesting the secondary role of bacterial infection in peri‐implantitis (Albrektsson et al., 2014,2019). This theory cannot be corroborated with the results from this investigation since the signs of FBR such as presence of MNGCs or frustrated phagocytosis in the vicinity of the TPs were not identified in any of the specimens.
The biological response on TPs has been attributed to several factors, such as particle size, surface topography and chemistry as well as the presence of contaminating biomolecules (Pajarinen et al., 2013; Zhang et al., 2018). The TPs identified in this investigation demonstrated a higher degree of biocompatibility since no biological reaction could be identified around them (Sakamoto et al., 2016). Furthermore, the similar pattern of expression of IL6 and Nf‐kB between peri‐implantitis and periodontitis tissues indicates that TPs may not be responsible for this hyperinflammatory tissue response.
The results of this study, however, must be interpreted with caution since this cross‐sectional observational study can only identify associations and never lead to casual interpretations. Another limitation of this study is the lack of SEM and EDS analysis of periodontitis specimens, since we assumed that they were TPs free. Furthermore, descriptive histology always bears a certain degree of subjective bias and IHC is always subject to a high variability due to methodological difficulties in tissular antigen–antibody expression. Therefore, the reported findings on lack of biological activity around TPs, the higher macrophage afflux and more expressed neovascularization in peri‐implantitis tissues require further research in well‐designed studies.
5. CONCLUSION
Within limitations of this study, TPs are common finding in peri‐implantitis, but do not evoke a marked biological response. Peri‐implantitis granulations demonstrated clear differences when compared to periodontitis, mainly a different pattern of the inflammatory infiltrate with presence of subacute infiltration in about half of the samples and a higher macrophage afflux and neovascularization.
AUTHOR CONTRIBUTION
Mia M. Rakic: Conceptualization (lead); Funding acquisition (lead); Investigation (lead); Methodology (lead); Project administration (lead); Resources (equal); Writing – original draft (lead); Writing – review & editing (equal). Milena Radunovic: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Writing – original draft (equal). Zoran Tatic: Data curation (equal); Investigation (equal); Validation (equal); Writing – review & editing (equal). Aleksandra Petkovic‐Curcin: Data curation (equal); Investigation (equal); Writing – review & editing (equal). Gordana Basta‐Jovanovic: Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – original draft (equal); Writing – review & editing (equal). Mariano Sanz‐Alonso: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – original draft (equal); Writing – review & editing (equal).
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the Ministry of Education and Science, Republic of Serbia, Belgrade, Serbia (project references #41008), Medical Military Academy, Republic of Serbia (MFVMA 07/22‐24) and Serbian Academy of Sciences and Arts.
Rakic, M. , Radunovic, M. , Petkovic‐Curcin, A. , Tatic, Z. , Basta‐Jovanovic, G. , & Sanz, M. (2022). Study on the immunopathological effect of titanium particles in peri‐implantitis granulation tissue: A case–control study. Clinical Oral Implants Research, 33, 656–666. 10.1111/clr.13928
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Albrektsson, T. , Dahlin, C. , Jemt, T. , Sennerby, L. , Turri, A. , & Wennerberg, A. (2014). Is Marginal bone loss around oral implants the result of a provoked foreign body reaction? Clinical Implant Dentistry and Related Research, 16(2), 155–165. 10.1111/cid.12142 [DOI] [PubMed] [Google Scholar]
- Albrektsson, T. , Jemt, T. , Mölne, J. , Tengvall, P. , & Wennerberg, A. (2019). On inflammation‐immunological balance theory—A critical apprehension of disease concepts around implants: Mucositis and marginal bone loss may represent normal conditions and not necessarily a state of disease. Clinical Implant Dentistry and Related Research, 21(1), 183–189. 10.1111/cid.12711 [DOI] [PubMed] [Google Scholar]
- Anderson, J. M. , Rodriguez, A. , & Chang, D. T. (2008). Foreign body reaction to biomaterials. Seminars in Immunology, 20(2), 86–100. 10.1016/j.smim.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barão, V. A. , Mathew, M. T. , Assunção, W. G. , Yuan, J. C. , Wimmer, M. A. , & Sukotjo, C. (2011). The role of lipopolysaccharide on the electrochemical behavior of titanium. Journal of Dental Research, 90(5), 613–618. 10.1177/0022034510396880 [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Armitage, G. , Araujo, M. G. , Avila‐Ortiz, G. , Blanco, J. , Camargo, P. M. , Chen, S. , Cochran, D. , Derks, J. , Figuero, E. , Hämmerle, C. H. F. , Heitz‐Mayfield, L. J. A. , Huynh‐Ba, G. , Iacono, V. , Koo, K. T. , Lambert, F. , McCauley, L. , Quirynen, M. , Renvert, S. , … Zitzmann, N. (2018). Peri‐implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl. 20), S286–S291. 10.1111/jcpe.12957 [DOI] [PubMed] [Google Scholar]
- Bosshardt, D. D. , Chappuis, V. , & Buser, D. (2017). Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000, 73(1), 22–40. 10.1111/prd.12179 [DOI] [PubMed] [Google Scholar]
- Carcuac, O. , Abrahamsson, I. , Albouy, J. P. , Linder, E. , Larsson, L. , & Berglundh, T. (2013). Experimental periodontitis and peri‐implantitis in dogs. Clinical Oral Implants Research, 24(4), 363–371. 10.1111/clr.12067 [DOI] [PubMed] [Google Scholar]
- Carcuac, O. , & Berglundh, T. (2014). Composition of human peri‐implantitis and periodontitis lesions. Journal of Dental Research, 93(11), 1083–1088. 10.1177/0022034514551754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐Ruiz, R. , & Romanos, G. (2018). Potential causes of titanium particle and ion release in implant dentistry: A systematic review. International Journal of Molecular Sciences, 19(11), 3585. 10.3390/ijms19113585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks, J. , Schaller, D. , Hakansson, J. , Wennstrom, J. L. , Tomasi, C. , & Berglundh, T. (2016). Effectiveness of implant therapy analyzed in a swedish population: Prevalence of peri‐implantitis. Journal of Dental Research, 95(1), 43–49. 10.1177/0022034515608832 [DOI] [PubMed] [Google Scholar]
- Derks, J. , & Tomasi, C. (2015). Peri‐implant health and disease. A systematic review of current epidemiology. Journal of Clinical Periodontology, 42(Suppl. 16), S158–S171. 10.1111/jcpe.12334 [DOI] [PubMed] [Google Scholar]
- Eger, M. , Sterer, N. , Liron, T. , Kohavi, D. , & Gabet, Y. (2017). Scaling of titanium implants entrains inflammation‐induced osteolysis. Scientific Reports, 7, 39612. 10.1038/srep39612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figuero, E. , Graziani, F. , Sanz, I. , Herrera, D. , & Sanz, M. (2014). Management of peri‐implant mucositis and peri‐implantitis. Periodontology 2000, 66(1), 255–273. [DOI] [PubMed] [Google Scholar]
- Fretwurst, T. , Buzanich, G. , Nahles, S. , Woelber, J. P. , Riesemeier, H. , & Nelson, K. (2016). Metal elements in tissue with dental peri‐implantitis: A pilot study. Clinical Oral Implants Research, 27(9), 1178–1186. 10.1111/clr.12718 [DOI] [PubMed] [Google Scholar]
- Fretwurst, T. , Garaicoa‐Pazmino, C. , Nelson, K. , Giannobile, W. V. , Squarize, C. H. , Larsson, L. , & Castilho, R. M. (2020). Characterization of macrophages infiltrating peri‐implantitis lesions. Clinical Oral Implants Research, 31(3), 274–281. 10.1111/clr.13568 [DOI] [PubMed] [Google Scholar]
- Fretwurst, T. , Nelson, K. , Tarnow, D. P. , Wang, H. L. , & Giannobile, W. V. (2018). Is metal particle release associated with peri‐implant bone destruction? an emerging concept. Journal of Dental Research, 97(3), 259–265. 10.1177/0022034517740560 [DOI] [PubMed] [Google Scholar]
- Galarraga‐Vinueza, M. E. , Obreja, K. , Ramanauskaite, A. , Magini, R. , Begic, A. , Sader, R. , & Schwarz, F. (2020). Macrophage polarization in peri‐implantitis lesions. Clinical Oral Investigations, 25(4), 2335–2344. 10.1007/s00784-020-03556-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaicoa‐Pazmino, C. , Fretwurst, T. , Squarize, C. H. , Berglundh, T. , Giannobile, W. V. , Larsson, L. , & Castilho, R. M. (2019). Characterization of macrophage polarization in periodontal disease. Journal of Clinical Periodontology, 46(8), 830–839. 10.1111/jcpe.13156 [DOI] [PubMed] [Google Scholar]
- Grosse, S. , Haugland, H. K. , Lilleng, P. , Ellison, P. , Hallan, G. , & Høl, P. J. (2015). Wear particles and ions from cemented and uncemented titanium‐based hip prostheses‐a histological and chemical analysis of retrieval material. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 103(3), 709–717. 10.1002/jbm.b.33243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualini, F. , & Berglundh, T. (2003). Immunohistochemical characteristics of inflammatory lesions at implants. Journal of Clinical Periodontology, 30(1), 14–18. 10.1034/j.1600-051X.2003.300103.x [DOI] [PubMed] [Google Scholar]
- Harding, J. S. , Herbath, M. , Chen, Y. , Rayasam, A. , Ritter, A. , Csoka, B. , Hasko, G. , Michael, I. P. , Fabry, Z. , Nagy, A. , & Sandor, M. (2019). VEGF‐a from granuloma macrophages regulates granulomatous inflammation by a non‐angiogenic pathway during mycobacterial infection. Cell Reports, 27(7), 2119–2131. 10.1016/j.celrep.2019.04.072 [DOI] [PubMed] [Google Scholar]
- Heyman, O. , Koren, N. , Mizraji, G. , Capucha, T. , Wald, S. , Nassar, M. , Tabib, Y. , Shapira, L. , Hovav, A. H. , & Wilensky, A. (2018). Impaired differentiation of langerhans cells in the murine oral epithelium adjacent to titanium dental implants. Frontiers in Immunology, 9, 1712. 10.3389/fimmu.2018.01712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kister, F. , Specht, O. , Warkentin, M. , Geis‐Gerstorfer, J. , & Rupp, F. (2017). Peri‐implantitis cleaning instrumentation influences the integrity of photoactive nanocoatings. Dental Materials, 33(2), e69–e78. 10.1016/j.dental.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Klopfleisch, R. , & Jung, F. (2017). The pathology of the foreign body reaction against biomaterials. Journal of Biomedical Materials Research. Part A, 105(3), 927–940. 10.1002/jbm.a.35958 [DOI] [PubMed] [Google Scholar]
- Koldsland, O. C. , Wohlfahrt, J. C. , & Aass, A. M. (2018). Surgical treatment of peri‐implantitis: Prognostic indicators of short‐term results. Journal of Clinical Periodontology, 45(1), 100–113. 10.1111/jcpe.12816 [DOI] [PubMed] [Google Scholar]
- Kotsakis, G. A. , Black, R. , Kum, J. , Berbel, L. , Sadr, A. , Karoussis, I. , Simopoulou, M. , & Daubert, D. (2020). Effect of implant cleaning on titanium particle dissolution and cytocompatibility. Journal of Periodontology, 92(4), 580–591. 10.1002/JPER.20-0186 [DOI] [PubMed] [Google Scholar]
- Kotsakis, G. A. , Lan, C. , Barbosa, J. , Lill, K. , Chen, R. , Rudney, J. , & Aparicio, C. (2016). Antimicrobial agents used in the treatment of peri‐implantitis alter the physicochemistry and cytocompatibility of titanium surfaces. Journal of Periodontology, 87(7), 809–819. 10.1902/jop.2016.150684 [DOI] [PubMed] [Google Scholar]
- Kramer, D. B. , Tan, Y. T. , Sato, C. , & Kesselheim, A. S. (2013). Postmarket surveillance of medical devices: A comparison of strategies in the US, EU, Japan, and China. PLoS Med, 10(9), e1001519. 10.1371/journal.pmed.1001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, N. P. , Banurekha, V. V. , Nair, D. , & Babu, S. (2016). Circulating angiogenic factors as biomarkers of disease severity and bacterial burden in pulmonary tuberculosis. PLoS One, 11(1), e0146318. 10.1371/journal.pone.0146318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron, R. J. , Zohdi, H. , Fujioka‐Kobayashi, M. , & Bosshardt, D. D. (2016). Giant cells around bone biomaterials: Osteoclasts or multi‐nucleated giant cells? Acta Biomaterialia, 46, 15–28. 10.1016/j.actbio.2016.09.029 [DOI] [PubMed] [Google Scholar]
- Miyanishi, K. , Trindade, M. C. D. , Ma, T. , Goodman, S. B. , Schurman, D. J. , & Smith, R. L. (2003). Periprosthetic osteolysis: Induction of vascular endothelial growth factor from human monocyte/macrophages by orthopaedic biomaterial particles. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research, 18(9), 1573–1583. 10.1359/jbmr.2003.18.9.1573 [DOI] [PubMed] [Google Scholar]
- Mombelli, A. , Hashim, D. , & Cionca, N. (2018). What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clinical Oral Implants Research, 29(Suppl. 18), 37–53. 10.1111/clr.13305 [DOI] [PubMed] [Google Scholar]
- Moraschini, V. , Poubel, L. A. D. C. , Ferreira, V. F. , & Barboza, E. D. S. P. (2015). Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow‐up period of at least 10 years: A systematic review. International Journal of Oral and Maxillofacial Surgery, 44(3), 377–388. 10.1016/j.ijom.2014.10.023 [DOI] [PubMed] [Google Scholar]
- Nickenig, H. J. , Wichmann, M. , Andreas, S. K. , & Eitner, S. (2008). Oral health‐related quality of life in partially edentulous patients: Assessments before and after implant therapy. Journal of Cranio‐Maxillo‐Facial Surgery: Official Publication of the European Association for Cranio‐Maxillo‐Facial Surgery, 36(8), 477–480. 10.1016/j.jcms.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Olmedo, D. G. , Nalli, G. , Verdú, S. , Paparella, M. L. , & Cabrini, R. L. (2013). Exfoliative cytology and titanium dental implants: A pilot study. Journal of Periodontology, 84(1), 78–83. 10.1902/jop.2012.110757 [DOI] [PubMed] [Google Scholar]
- Page, R. C. , & Eke, P. I. (2007). Case definitions for use in population‐based surveillance of periodontitis. Journal of Periodontology, 78(7 Suppl), 1387–1399. 10.1902/jop.2007.060264 [DOI] [PubMed] [Google Scholar]
- Pajarinen, J. , Kouri, V. P. , Jämsen, E. , Li, T. F. , Mandelin, J. , & Konttinen, Y. T. (2013). The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomaterialia, 9(11), 9229–9240. 10.1016/j.actbio.2013.06.027 [DOI] [PubMed] [Google Scholar]
- Pettersson, M. , Kelk, P. , Belibasakis, G. N. , Bylund, D. , Molin Thorén, M. , & Johansson, A. (2017). Titanium ions form particles that activate and execute interleukin‐1β release from lipopolysaccharide‐primed macrophages. Journal of Periodontal Research, 52(1), 21–32. 10.1111/jre.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson, M. , Pettersson, J. , Johansson, A. , & Molin Thorén, M. (2019). Titanium release in peri‐implantitis. Journal of Oral Rehabilitation, 46(2), 179–188. 10.1111/joor.12735 [DOI] [PubMed] [Google Scholar]
- Rakic, M. , Galindo‐Moreno, P. , Monje, A. , Radovanovic, S. , Wang, H. L. , Cochran, D. , Sculean, A. , & Canullo, L. (2018). How frequent does peri‐implantitis occur? A systematic review and meta‐analysis. Clinical Oral Investigations, 22(4), 1805–1816. 10.1007/s00784-017-2276-y [DOI] [PubMed] [Google Scholar]
- Sakamoto, M. , Watanabe, H. , Higashi, H. , & Kubosawa, H. (2016). Pseudotumor caused by titanium particles from a total hip prosthesis. Orthopedics, 39(1), e162–e165. 10.3928/01477447-20151218-12 [DOI] [PubMed] [Google Scholar]
- Salata, L. A. , Burgos, P. M. , Rasmusson, L. , Novaes, A. B. , Papalexiou, V. , Dahlin, C. , & Sennerby, L. (2007). Osseointegration of oxidized and turned implants in circumferential bone defects with and without adjunctive therapies: An experimental study on BMP‐2 and autogenous bone graft in the dog mandible. International Journal of Oral and Maxillofacial Surgery, 36(1), 62–71. 10.1016/j.ijom.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Sanz, M. , & Chapple, I. L. On behalf of Working Group 4 of the VIII European Workshop on Periodontology* (2012). Clinical research on peri‐implant diseases: Consensus report of working group 4. Journal of Clinical Periodontology, 39, 202–206. 10.1111/j.1600-051X.2011.01837.x [DOI] [PubMed] [Google Scholar]
- Schmalz, G. , & Galler, K. M. (2017). Biocompatibility of biomaterials—Lessons learned and considerations for the design of novel materials. Dental Materials: Official Publication of the Academy of Dental Materials, 33(4), 382–393. 10.1016/j.dental.2017.01.011 [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Derks, J. , Monje, A. , & Wang, H. L. (2018). Peri‐implantitis. Journal of Clinical Periodontology, 45(Suppl. 20), S246–S266. 10.1111/jcpe.12954 [DOI] [PubMed] [Google Scholar]
- Spanogle, J. P. , Miyanishi, K. , Ma, T. , Epstein, N. J. , Smith, R. L. , & Goodman, S. B. (2006). Comparison of VEGF‐producing cells in periprosthetic osteolysis. Biomaterials, 27(21), 3882–3887. 10.1016/j.biomaterials.2006.02.035 [DOI] [PubMed] [Google Scholar]
- Suárez‐López Del Amo, F. , Garaicoa‐Pazmiño, C. , Fretwurst, T. , Castilho, R. M. , & Squarize, C. H. (2018). Dental implants‐associated release of titanium particles: A systematic review. Clinical Oral Implants Research, 10.1111/clr.13372 [DOI] [PubMed] [Google Scholar]
- Swiatkowska, I. , Martin, N. , & Hart, A. J. (2019). Blood titanium level as a biomarker of orthopaedic implant wear. Journal of Trace Elements in Medicine and Biology, 53, 120–128. 10.1016/j.jtemb.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Varghese, F. , Bukhari, A. B. , Malhotra, R. , & De, A. (2014). IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One, 9(5), e96801. 10.1371/journal.pone.0096801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi, T. , Shuto, T. , Shinohara, Y. , Matono, Y. , & Makihira, S. (2015). Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology, 327, 1–9. 10.1016/j.tox.2014.10.016 [DOI] [PubMed] [Google Scholar]
- Wilson, T. G. , Valderrama, P. , Burbano, M. , Blansett, J. , Levine, R. , Kessler, H. , & Rodrigues, D. C. (2015). Foreign bodies associated with peri‐implantitis human biopsies. Journal of Periodontology, 86(1), 9–15. 10.1902/jop.2014.140363 [DOI] [PubMed] [Google Scholar]
- World Medical Association (2013). World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Wu, X. , Wang, G. , Liu, P. , Qin, S. , Xu, K. , Tong, D. , Ding, H. , Tang, H. , & Ji, F. (2018). Macrophage polarization, inflammatory signaling, and NF‐κB activation in response to chemically modified titanium surfaces. Colloids and Surfaces. B, Biointerfaces, 166, 269–276. 10.1016/j.colsurfb.2018.03.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


