Abstract
In the intestinal tracts of animals, methanogenesis from CO2 and other C1 compounds strictly depends on the supply of electron donors by fermenting bacteria, but sources and sinks of reducing equivalents may be spatially separated. Microsensor measurements in the intestinal tract of the omnivorous cockroach Blaberus sp. showed that molecular hydrogen strongly accumulated in the midgut (H2 partial pressures of 3 to 26 kPa), whereas it was not detectable (<0.1 kPa) in the posterior hindgut. Moreover, living cockroaches emitted large quantities of CH4 [105 ± 49 nmol (g of cockroach)−1 h−1] but only traces of H2. In vitro incubation of isolated gut compartments, however, revealed that the midguts produced considerable amounts of H2, whereas hindguts emitted only CH4 [106 ± 58 and 71 ± 50 nmol (g of cockroach)−1 h−1, respectively]. When ligated midgut and hindgut segments were incubated in the same vials, methane emission increased by 28% over that of isolated hindguts, whereas only traces of H2 accumulated in the headspace. Radial hydrogen profiles obtained under air enriched with H2 (20 kPa) identified the hindgut as an efficient sink for externally supplied H2. A cross-epithelial transfer of hydrogen from the midgut to the hindgut compartment was clearly evidenced by the steep H2 concentration gradients which developed when ligated fragments of midgut and hindgut were placed on top of each other—a configuration that simulates the situation in vivo. These findings emphasize that it is essential to analyze the compartmentalization of the gut and the spatial organization of its microbiota in order to understand the functional interactions among different microbial populations during digestion.
Methanogenesis is an important electron sink in the intestinal tracts of terrestrial arthropods such as Diplopoda (millipedes), Scarabaeidae (scarab beetles), Blattidae (cockroaches), and Isoptera (termites) (12). It has been postulated that termites contribute substantially to global methane fluxes (9, 17, 26). According to recent estimates, termites may account for about 4 to 10% of the global production of this greenhouse gas (1, 19). The contribution of all methane-producing arthropods together is likely to be much higher (12, 13).
Methanogenic archaea in arthropods are generally restricted to the hindgut, where they occur free-floating in the gut lumen, attached to chitinous structures of the gut wall, or as intracellular symbionts of gut-dwelling anaerobic protists (12). The most likely electron donors for intestinal methanogens are H2, formate, and methanol; there is no indication that aceticlastic methanogenesis plays a major role. Hydrogen is the by far most prominent among these potential substrates, particularly in termites. All termites investigated to date are characterized by gut segments with high H2 partial pressure (10, 20). In (phylogenetically) lower termites, which harbor large numbers of protozoa in their hindguts (3), the carbohydrate fermentation by anaerobic flagellates is likely to be the major source of H2 (14, 16). In (phylogenetically) higher termites, which possess a largely prokaryotic gut microbiota and typically do not host any flagellates in their hindgut, and also in other arthropods, the organisms and metabolic pathways that are responsible for the formation of H2 remain to be identified.
If methane-producing and hydrogen-consuming processes were homogeneously distributed in the gut segments that emit methane, low hydrogen partial pressures would be expected throughout the gut. Notably, microsensor measurements in the methane-emitting hindgut of the lower termite Reticulitermes flavipes have shown that hydrogen accumulates to high partial pressures at the gut center, whereas only small fluxes of hydrogen emanate from the hindgut (10). This is due to the presence of significant hydrogen-consuming activities at the periphery of the hindgut and hydrogen-producing activities apparently prevailing in the central region. In other words, the spatial organization of the hydrogen-producing and hydrogen-consuming microbiota controls hydrogen metabolism and methanogenesis in the hindgut of lower termites (5, 10, 24).
In the highly differentiated intestinal tract of higher termites (Cubitermes spp.), hydrogen-emitting and methane-producing gut compartments can be discriminated. The latter exhibit a significant hydrogen uptake activity when provided with external hydrogen (20). Based on the proximity of the hydrogen-producing and the hydrogen-consuming, methane-producing gut segments in vivo, a cross-epithelial hydrogen transfer has been postulated (6, 20). Since a striking compartmentalization of the intestinal tract is also present in other methane-producing arthropods (8), it can be assumed that this phenomenon is not restricted to higher termites. Here we describe the cross-epithelial transfer of hydrogen in Blaberus sp., a large, omnivorous cockroach with a highly differentiated intestinal tract.
MATERIALS AND METHODS
Organisms.
Cockroaches (Blaberus sp.) were bred at the University of Nijmegen. They were fed a commercial pelleted rabbit diet, supplemented by apples, raw potatoes, and water ad libitum. Only adult cockroaches were used for the experiments.
For measurements with isolated guts or ligated gut segments, cockroaches were anesthetized with N2-CO2 (80:20, vol/vol), decapitated, and dissected in Ringer's solution (7) to prevent dehydration of the gut. When midgut and hindgut were incubated separately, each gut segment was ligated at both ends with thin cotton or nylon thread using a pair of watchmaker's forceps and a stereomicroscope.
CH4 and H2 production rates.
Living cockroaches were placed in 250-ml glass bottles, which were sealed with butyl-rubber stoppers, and were incubated for several hours under air at room temperature. Ethane (1 ml) was added as an internal standard (11). At regular intervals, gas samples (200 μl) were taken using hypodermic needles and 1-ml syringes and analyzed for methane and hydrogen by gas chromatography (21, 22). As a rule, gases were measured at 3 h and 17 to 22 h after the start of the incubation.
Gut segments were incubated in 2 ml of HEPES buffer (50 mM HEPES, 50 mM NaCl, 5 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.0]) in 10-ml vials (21). Since pilot experiments had shown that under these conditions hydrogen production was linear for up to 29 h and the production of methane from endogenous substrates was linear for up to 48 h, gas production rates were analyzed using the same protocol as for living cockroaches.
Completely separated midgut and hindgut segments incubated in the same vial did not differ significantly with respect to gas production from intact guts merely ligated between midgut and hindgut; therefore, the data for all coincubations were pooled. Since moderate shaking of the vials did not stimulate the production of methane and hydrogen by isolated guts or gut segments, we concluded that gas diffusion from the buffer into the headspace was not limiting under the experimental conditions.
Hydrogen microsensor measurements.
Hydrogen concentration profiles were measured with polarographic H2 microsensors, which had basically the same design as Clark-type O2 microsensors (18) and were modified according to Witty (25). The microsensors were constructed in our laboratory in Konstanz and were tested and calibrated as described earlier (10). The detection limit for H2 was about 100 Pa; the stirring sensitivity was always <1% of the signal obtained at a H2 partial pressure of 20 kPa (calibration gas, N2-H2, 80:20 [vol/vol]). For profile measurements, the microsensors were mounted on a manual micromanipulator (10). Each set of experiments was reproduced with at least four different animals; the profiles shown in the figures represent typical examples.
For axial hydrogen profiles, cockroaches were dissected and the intact, fully extended gut was placed in an incubation chamber (16 by 16 mm, 100 mm long) and fixed with minutia pins on a thin silicone layer at the bottom of the chamber. The chamber was filled with air-saturated Ringer's solution and irrigated at a continuous flow rate (5 ml min−1). Under these experimental conditions, the dissected guts exhibited a moderate peristalsis that persisted for several hours, indicating that they were still physiologically active.
For radial hydrogen profiles, shorter, ligated sections of midgut or hindgut were embedded in a smaller chamber in Ringer's solution solidified with 0.5% agarose and incubated under a controlled gas headspace. The agarose layer above the gut did not exceed 2 mm to avoid the formation of a diffusion barrier. The chamber was continuously flushed with the desired gas mixture. To demonstrate cross-epithelial transfer of hydrogen, ligated sections of midgut and hindgut that were lying in proximity in vivo (Fig. 1) were positioned in direct contact with each other and embedded in Ringer's solution solidified with 0.5% agarose. The experimental setup was the same as for the other radial profiles; the gut sections were incubated under air. The details of the experimental setup have been described earlier (10).
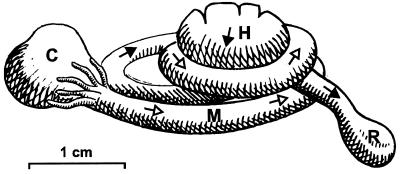
FIG. 1.
Semischematic presentation of the intestinal tract of an adult Blaberus sp. cockroach, illustrating the intimate intertwining of midgut (M) and hindgut (H) in the abdomen. Open (M) and closed (H) arrows indicate the direction of flow from crop (C) to rectum (R). The coiling of the individual loops in vivo is even tighter, especially in the larvae.
All hydrogen concentrations are reported as partial pressures. At atmospheric pressure, a partial pressure of 1 kPa equals a mixing ratio of 1% H2. At this partial pressure, pure water dissolves about 8.0 μmol of H2 per liter (20°C). However, we did not convert H2 partial pressures to molarities, since this would require knowledge of the exact solubility coefficient(s) for H2 in different gut contents.
RESULTS
Axial hydrogen profiles and emission rates of H2 and CH4.
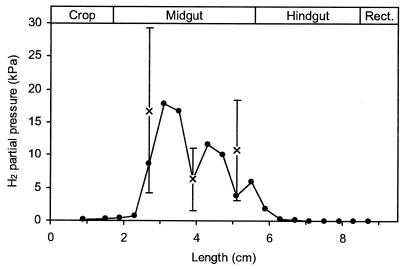
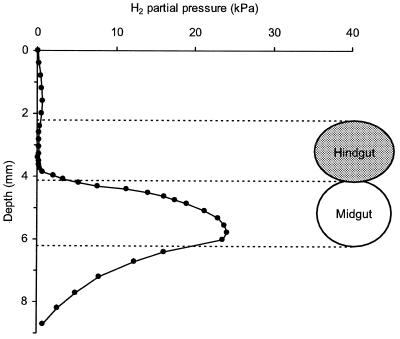
Axial hydrogen profiles of the intact guts of Blaberus sp. revealed large differences among the various gut segments (Fig. 2). While hydrogen partial pressures were always below the detection limit of the microsensor (0.1 kPa) in the posterior hindgut of all animals investigated, the midgut segments accumulated substantial amounts of H2. Hydrogen profiles of the midgut varied considerably among individual animals (data not shown), ranging from 3 to 26 kPa H2. The average partial pressures of hydrogen in the anterior, median, and posterior regions of the midgut were 17, 6, and 11 kPa (n = 7), respectively. In most cases, the accumulation of hydrogen extended into the anterior hindgut. However, hydrogen accumulation was never observed in the posterior part of the hindgut or the crop.
FIG. 2.
Typical axial hydrogen profile (●) of an intact, fully extended gut of Blaberus sp. and average H2 partial pressures (×) in the anterior, median, and posterior midguts of different animals (means ± standard deviations; n = 7). Hydrogen was measured with a microsensor; all readings were taken at the gut center.
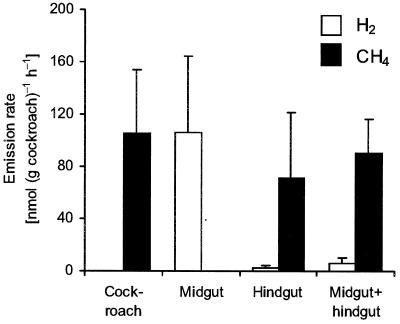
Measurement of hydrogen and methane emissions by intact, living cockroaches (Fig. 3) showed that the animals emitted only traces of H2 but large quantities of CH4 [105 ± 49 nmol (g of cockroach)−1 h−1 (n = 12)]. Notably, isolated midguts incubated in vitro emitted significant amounts of H2 at constant rates [106 ± 58 nmol (g of cockroach)−1 h−1], whereas isolated hindguts emitted only CH4 [71 ± 50 nmol (g of cockroach)−1 h−1]. When intact intestinal tracts with ligated midgut and hindgut segments were incubated in the same vial, methane emission increased over that of isolated hindguts [91 ± 26 nmol (g of cockroach)−1 h−1], whereas the hydrogen concentrations in the headspace remained low (Fig. 3).
FIG. 3.
Hydrogen and methane emission rates of whole insects, intact guts, and ligated midgut and hindgut segments of Blaberus sp. Values are means (plus standard deviations) of 12 different assays each. The large variance is due to the individual differences among the animals.
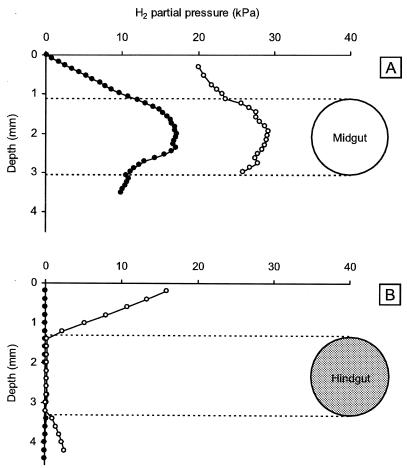
Radial hydrogen profiles.
Isolated, agarose-embedded midguts showed the highest hydrogen accumulation at the center of the gut (Fig. 4A). In the gut periphery, H2 diffused across the gut epithelium into the surrounding agarose. When midgut sections were incubated under a headspace of air containing 20 kPa of H2, the hydrogen concentration in the midgut lumen increased, and the shape of hydrogen profiles did not show any evidence of hydrogen uptake activities in the midgut periphery. Isolated hindgut sections, on the other hand, showed no detectable hydrogen accumulation, confirming the results of the axial profiles (Fig. 4B). Notably, all hindgut sections tested (n = 12) were efficient hydrogen sinks when incubated under air containing 20 kPa of H2.
FIG. 4.
Typical radial hydrogen profiles of agarose-embedded midgut (A) and hindgut (B) sections of Blaberus sp. incubated under a gas headspace of air (●) or air enriched with 20 kPa of H2 (○). Hydrogen was measured with a microsensor.
To test whether hydrogen is transferred across the gut epithelium from midgut to hindgut, short, ligated sections of midgut and hindgut that were also in direct contact in vivo (Fig. 1) were placed on top of each other and embedded in agarose. Radial hydrogen profiles revealed an accumulation of hydrogen at the center of the midgut sections and a diffusion of hydrogen into the surrounding agarose. Towards the hindgut sections, however, steep diffusive gradients of hydrogen developed (Fig. 5). Despite its high accumulation in the midgut, hydrogen was completely consumed within the first 100 μm beyond the hindgut epithelium. In the central portion of the hindgut lumen, there was no evidence of any accumulation of hydrogen. Comparable profiles were obtained irrespective of the positioning of the gut sections, i.e., whether the midgut was placed on top of hindgut or vice versa (data not shown). Steep hydrogen gradients developed across the midgut-hindgut interface of all gut sections which were tested in this experimental setup (n = 6).
FIG. 5.
Typical radial hydrogen profile through ligated midgut and hindgut sections placed in direct contact with each other and embedded in agarose, mimicking the in vivo arrangement of the sections (Fig. 1). Guts were incubated under air, and hydrogen was measured with a microsensor.
DISCUSSION
The strong accumulation of hydrogen in the midgut of Blaberus sp. and its emission from isolated midgut segments contrasts strongly with the nearly complete absence of H2 in the breath of cockroaches. This implies that almost all H2 produced in the midgut is consumed inside the animal. Considering the anatomy of the intestinal tract and the juxtaposition of hydrogen-producing and hydrogen-consuming gut segments in vivo (Fig. 1), it is tempting to assume that the hydrogen formed in the midgut diffuses across the gut epithelia into the hindgut, where it is removed by a hydrogenotrophic microbiota. Such a model has already been proposed to explain a similar phenomenon in soil-feeding termites (20) and is supported by the stimulation of methane emission by externally applied hydrogen observed in living termites (10, 20) and cockroaches (J. H. P. Hackstein and T. van Alen, unpublished results).
This is the first time, however, that the cross-epithelial transfer of endogenously produced hydrogen was measured directly. Coincubation experiments and radial hydrogen concentration profiles of isolated midgut and hindgut segments of Blaberus sp. clearly identify the presence of a strong hydrogen source and the absence or insignificance of hydrogen-consuming processes in the midgut and the presence of a highly efficient hydrogen sink in the hindgut. The cross-epithelial transfer of hydrogen is clearly evidenced by the radial hydrogen profiles obtained when ligated fragments of midgut and hindgut were placed on top of each other, a configuration that simulates the in vivo situation (Fig. 1).
Notably, H2 did not accumulate in the headspace when midgut and hindgut were coincubated in vitro, whereas the methane production increased considerably (Fig. 3). This observation strongly argues for a preferential stimulation of methanogenesis by cross-epithelial hydrogen transfer. The hydrogen emission rates of isolated midguts are about 5.5-fold higher than the corresponding stimulation of methanogenesis during coincubation. Assuming that H2 consumed by the hindgut is used exclusively for methanogenesis from CO2, a 4:1 stoichiometry would be expected. Therefore, it is possible that other hydrogen-consuming processes, e.g., reductive acetogenesis, are stimulated as well. The large variation in the emission rates between individual cockroaches, which also depends on developmental stage, sex, and diet (15) (unpublished observations), does not allow a definite conclusion based on this data set. Nevertheless, the stimulation of reductive acetogenesis by exogenous H2 has been clearly demonstrated in the hindgut of soil-feeding termites with radiotracer techniques (23).
It should be pointed out that there is so far no satisfactory explanation for the fate of the hydrogen which apparently emanates from the side of the midgut which is not in contact with the hindgut in situ (Fig. 5). Most likely, oxygen supply to the gut, which is effected by the tracheal system in the living cockroach (a combination of advective and diffusive transport in the gas phase and only a short-range diffusive transport in the aqueous phase), is restricted by embedding the gut in agarose. The resulting diffusion limitation would create a significant oxygen deficit, which would stimulate the formation of H2 in the midgut (10). Therefore, it is possible that in vivo fluxes of H2 from the midgut are lower than those determined under experimental conditions—or that intercompartmental transfer of H2 is not the only reason for the absence of hydrogen emission in living cockroaches.
The presence of highly differentiated gut segments in a variety of methane-producing arthropods (12) suggests that a cross-epithelial transfer of reducing equivalents between different gut compartments is likely to occur in other animals as well. The diffusion of hydrogen is facilitated by the inherent permeability of the intestinal epithelia to gases; transepithelial gas exchange between gut and bloodstream (or between gut and tracheal system) is well established in humans (4) and in a number of arthropods (2). Nevertheless, it should be considered that formate and methanol are also potential substrates for methanogenesis in the hindgut. The hemolymph of soil-feeding termites, for example, contains appreciable concentrations of formate (23). Formate seems to be produced by microbial fermentations in the midgut and stimulates methanogenesis in the hindgut (20). A similar situation is present in the larvae of scarab beetles (Pachnoda sp.) (T. Lemke and A. Brune, unpublished results). Moreover, methanol is formed as the demethylation product of pectins in the midgut of several species of cockroaches and the larvae of Pachnoda (21; J. H. P. Hackstein, J. A. de Gouw, and C. Warneke, unpublished results). It remains to be shown that methanol is transported between the gut compartments, but there is evidence that Methanomicrococcus blatticola, an obligately methanol-reducing, methanogenic bacterium, is a major methanogenic organism in the hindgut of the cockroach Periplaneta americana (21). While methanol should be able to pass through the epithelial barrier easily, formate and other charged metabolites would require specific transport systems.
The results of the present study emphasize that we are just beginning to understand the interdependence of microbial processes in arthropod guts. Any such gut, no matter how small it is or how simple it seems to be at first glance, is a complex and highly structured environment (6). In order to understand the physiology of the digestive tract and to resolve the functional interactions among the different microbial populations, it is essential to analyze the compartmentalization of the gut and the spatial organization of its microbiota in more detail.
REFERENCES
- 1.Bignell D E, Eggleton P, Nunes L, Thomas K L. Termites as mediators of carbon fluxes in tropical forests: budgets for carbon dioxide and methane emissions. In: Watt A B, Stork N E, Hunter M D, editors. Forests and insects. London, United Kingdom: Chapman and Hall; 1997. pp. 109–134. [Google Scholar]
- 2.Bijnen F G C, Harren F J M, Hackstein J H P, Reuss J. Intracavity CO laser photoacoustic trace gas detection: cyclic CH4, H2O, and CO2 emission by cockroaches and scarab beetles. Appl Optics. 1996;35:5357–5368. doi: 10.1364/AO.35.005357. [DOI] [PubMed] [Google Scholar]
- 3.Breznak J A, Brune A. Role of microorganisms in the digestion of lignocellulose by termites. Annu Rev Entomol. 1994;39:453–487. [Google Scholar]
- 4.Brighenti F, Casiraghi M C, Pellegrini N, Riso P, Simonetti P, Testolin G. Comparison of lactulose and inulin as reference standard for the study of resistant starch fermentation using hydrogen breath test. Ital J Gastroenterol. 1995;27:122–128. [PubMed] [Google Scholar]
- 5.Brune A. Termite guts: the world's smallest bioreactors. Trends Biotechnol. 1998;16:16–21. [Google Scholar]
- 6.Brune A, Friedrich M. Microecology of the termite gut: structure and function on a microscale. Curr Opin Microbiol. 2000;3:263–269. doi: 10.1016/s1369-5274(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 7.Brune A, Emerson D, Breznak J A. The termite gut microflora as an oxygen sink: microelectrode determination of oxygen and pH gradients in guts of lower and higher termites. Appl Environ Microbiol. 1995;61:2681–2687. doi: 10.1128/aem.61.7.2681-2687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazemier A E, Op den Camp H J M, Hackstein J H P, Vogels G D. Fibre digestion in arthropods. Comp Biochem Physiol. 1997;118A:101–109. [Google Scholar]
- 9.Collins N M, Wood T G. Termites and atmospheric gas production. Science. 1984;224:84–86. doi: 10.1126/science.224.4644.84. [DOI] [PubMed] [Google Scholar]
- 10.Ebert A, Brune A. Hydrogen concentration profiles at the oxic-anoxic interface: a microsensor study of the hindgut of the wood-feeding lower termite Reticulitermes flavipes (Kollar) Appl Environ Microbiol. 1997;63:4039–4046. doi: 10.1128/aem.63.10.4039-4046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gijzen H J, Broers C A M, Barugahare M, Stumm C K. Methanogenic bacteria as endosymbionts of the ciliate Nyctotherus ovalis in the cockroach hindgut. Appl Environ Microbiol. 1991;57:1630–1634. doi: 10.1128/aem.57.6.1630-1634.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackstein J H P, Stumm C K. Methane production in terrestrial arthropods. Proc Natl Acad Sci USA. 1994;91:5441–5445. doi: 10.1073/pnas.91.12.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hackstein J H P, Langer P, Rosenberg J. Genetic and evolutionary constraints for the symbiosis between animals and methanogenic bacteria. Environ Monit Assess. 1996;42:59–76. doi: 10.1007/BF00394041. [DOI] [PubMed] [Google Scholar]
- 14.Hungate R E. Experiments on the nutrition of Zootermopsis. III. The anaerobic carbohydrate dissimilation by the intestinal protozoa. Ecology. 1939;20:230–245. [Google Scholar]
- 15.Kane M D, Breznak J A. Effect of host diet on production of organic acids and methane by cockroach gut bacteria. Appl Environ Microbiol. 1991;57:2628–2634. doi: 10.1128/aem.57.9.2628-2634.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odelson D A, Breznak J A. Nutrition and growth characteristics of Trichomitopsis termopsidis, a cellulolytic protozoan from termites. Appl Environ Microbiol. 1985;49:614–621. doi: 10.1128/aem.49.3.614-621.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen R A, Khalil M A K. Global production of methane by termites. Nature. 1983;301:704–705. [Google Scholar]
- 18.Revsbech N P. An oxygen microelectrode with a guard cathode. Limnol Oceanogr. 1989;34:472–476. [Google Scholar]
- 19.Sanderson M G. Biomass of termites and their emissions of methane and carbon dioxide: a global database. Global Biogeochem Cycles. 1996;10:543–557. [Google Scholar]
- 20.Schmitt-Wagner D, Brune A. Hydrogen profiles and localization of methanogenic activities in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.) Appl Environ Microbiol. 1999;65:4490–4496. doi: 10.1128/aem.65.10.4490-4496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprenger W W, van Belzen M C, Rosenberg J, Hackstein J H P, Keltjens J T. Methanomicrococcus blatticola gen. nov., sp. nov., a methanol- and methylamine-reducing methanogen from the hindgut of the cockroach Periplaneta americana. Int J Syst Evol Microbiol. 2000;50:1989–1999. doi: 10.1099/00207713-50-6-1989. [DOI] [PubMed] [Google Scholar]
- 22.Teunissen M J, Op den Camp H J M, Orpin C G, Huis in't Veld J H J, Vogels G D. Comparison of growth characteristics of anaerobic fungi from ruminant and non-ruminant herbivores during cultivation in a defined medium. J Gen Microbiol. 1991;137:1401–1408. doi: 10.1099/00221287-137-6-1401. [DOI] [PubMed] [Google Scholar]
- 23.Tholen A, Brune A. Localization and in situ activities of homoacetogenic bacteria in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.) Appl Environ Microbiol. 1999;65:4497–4505. doi: 10.1128/aem.65.10.4497-4505.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tholen A, Brune A. Impact of oxygen on metabolic fluxes and in situ rates of reductive acetogenesis in the hindgut of the wood-feeding termite Reticulitermes flavipes. Environ Microbiol. 2000;2:436–449. doi: 10.1046/j.1462-2920.2000.00127.x. [DOI] [PubMed] [Google Scholar]
- 25.Witty J F. Microelectrode measurements of hydrogen concentrations and gradients in legume nodules. J Exp Bot. 1991;42:765–771. [Google Scholar]
- 26.Zimmerman P R, Greenberg J P, Wandiga S O, Crutzen P J. Termites: a potentially large source of atmospheric methane, carbon dioxide, and molecular hydrogen. Science. 1982;218:563–565. doi: 10.1126/science.218.4572.563. [DOI] [PubMed] [Google Scholar]