Abstract
Sex allocation theory in simultaneous hermaphrodites predicts that optimal sex allocation is influenced by local sperm competition, which occurs when related sperm compete to fertilize a given set of eggs. Different factors, including the mating strategy and the ability to self‐fertilize, are predicted to affect local sperm competition and hence the optimal SA. Moreover, since the local sperm competition experienced by an individual can vary temporally and spatially, this can favour the evolution of sex allocation plasticity. Here, using seven species of the free‐living flatworm genus Macrostomum, we document interspecific variation in sex allocation, but neither their mating strategy nor their ability to self‐fertilize significantly predicted sex allocation among these species. Since we also found interspecific variation in sex allocation plasticity, we further estimated standardized effect sizes for plasticity in response to (i) the presence of mating partners (i.e. in isolation vs. with partners) and (ii) the strength of local sperm competition (i.e. in small vs. large groups). We found that self‐fertilization predicted sex allocation plasticity with respect to the presence of mating partners, with plasticity being lower for self‐fertilizing species. Finally, we showed that interspecific variation in sex allocation is higher than intraspecific variation due to sex allocation plasticity. Our study suggests that both sex allocation and sex allocation plasticity are evolutionarily labile, with self‐fertilization predicting the latter in Macrostomum.
Keywords: hypodermic insemination, local mate competition, phenotypic plasticity, reciprocal mating, self‐fertilization, sperm competition
Interspecific variation in both sex allocation and sex allocation plasticity in the hermaphroditic flatworm genus Macrostomum, including among closely related species, suggests that both sex allocation and sex allocation plasticity are evolutionarily labile. Moreover, self‐fertilization, but not mating strategy, predicts the evolution of sex allocation plasticity in Macrostomum.

1. INTRODUCTION
Sex allocation (SA) theory in simultaneous hermaphrodites predicts the optimal allocation of resources towards the male versus female function (Charnov, 1982). An important factor that can favour a female‐biased optimal SA in simultaneous hermaphrodites is local sperm competition (LSC) (Schärer, 2009; Schärer & Pen, 2013). LSC occurs when related sperm (usually from the same individual) compete for access to a given set of eggs, and it can be thought of as the inverse of sperm competition (Parker, 1970, 1998). LSC can be considered analogous to local mate competition (Hamilton, 1967), that is the competition between related males for access to mates, which can lead to a female‐biased sex ratio in species with separate sexes (and which Fischer, 1981, 1984, tried to extend to hermaphrodites). However, LSC does not require mates to be related or a spatial population structure to be present, since the local competition occurs among sperm, not males. An example of a factor that can affect LSC, and hence the optimal SA, is the mating group size (MGS) (Charnov, 1980, 1982), denoted as K+1, where K is the number of sperm donors from which a sperm recipient receives sperm at the time its eggs are fertilized. Specifically, Charnov's MGS model predicts that the optimal proportion of resources an individual allocates to the male function should increase with the number of sperm donors that are present in a local mating group.
The link between optimal SA and LSC can be visualized in terms of male fitness gain curves, which describe how much fitness is gained through the male function per unit resource investment (Charnov, 1982; Lloyd, 1984; Schärer, 2009). Under monogamy, LSC is necessarily very high, since the competing sperm are maximally related to each other, resulting in the male fitness gain curve saturating quickly. Thus, any investment into the production of more sperm than are required to fertilize the partner's eggs is a waste of resources, as the related sperm simply compete among themselves for fertilizations. Instead, these resources could be more profitably invested into the individual's own female function (Charnov, 1982), since the female fitness gain curve is often assumed to be linear (Campbell, 2000; Rademaker & de Jong, 1999; Schärer, 2009), and may sometimes even be accelerating (Rosas & Domínguez, 2009). Consequently, under monogamy, a simultaneous hermaphrodite is expected to have a highly female‐biased SA (Charnov, 1980, 1982). Yet, as the MGS increases—that is with more (unrelated) competitors contributing sperm to a given recipient—an individual's (related) sperm are more and more competing with these unrelated sperm. So, it now pays off to invest into the male function to gain a greater share of the fertilizations, and the male fitness gain curve is predicted to linearize, with a subsequent shift towards a more equal SA (Charnov, 1982; Schärer, 2009; Schärer & Pen, 2013).
An interesting question that arises then is whether and how interspecific variation in reproductive biology, including different mating strategies or the ability to self‐fertilize, could influence the strength of LSC and hence the optimal SA. For example, hermaphrodites often evolve different mating strategies in response to sexual conflict over mating roles, that is over whether to mate as a sperm donor and/or as a sperm recipient (Charnov, 1979; Michiels, 1998; Schärer et al., 2015). One such mating strategy is reciprocal mating (also called reciprocal copulation), in which both partners mate in both mating roles, and thus donate and receive sperm simultaneously. This strategy can lead to individuals being willing to engage in mating in order to obtain an opportunity to donate sperm, and this general willingness likely results in reduced precopulatory sexual selection. The ensuing higher mating rates could in turn result in increased sperm competition (and thus a decrease in LSC) and more intense postcopulatory sexual selection. Interestingly, however, the presence of certain postcopulatory processes could also increase LSC (Schärer, 2009). Indeed, theoretical studies have predicted that different postcopulatory sexual selection processes, such as sperm displacement, sperm digestion and cryptic female choice, can lead to the removal of sperm of one or multiple donors from competition, resulting in changes in LSC and hence the predicted optimal SA (Charnov, 1996; Greeff & Michiels, 1999; Pen & Weissing, 1999; Schärer & Pen, 2013; van Velzen et al., 2009).
In addition, it has been suggested that postcopulatory sexual selection and sexual conflict among simultaneous hermaphrodites can also favour the evolution of mating strategies that allow donors to bypass these postcopulatory processes and fertilize the eggs more directly (Charnov, 1979; Michiels, 1998). One such mating strategy is forced unilateral insemination, in which one partner mates only in the male role and donates sperm, for example via traumatic or hypodermic insemination (Charnov, 1979; Lange et al., 2013; Reinhardt et al., 2015), whereas the other partner only mates in the female role, likely often against its interests. It is currently difficult to predict the direction and magnitude of changes in LSC resulting from such a shift in mating strategy—and the effects that such shifts may thus have on optimal SA—since we still know relatively little about the particular postcopulatory processes involved in these mating strategies. In species with reciprocal mating, there may be some control over who or how many partners an individual receives and stores sperm from, and there could be strategies that lead to the (conceivably selective) removal of received sperm (van Velzen et al., 2009), possibly leading to increased LSC. In species with hypodermic insemination, however, such control might be more limited, since hypodermic sperm might function in a less easily controlled environment, with sperm from multiple donors thus mixing more randomly in a fair‐raffle‐type sperm competition. This would be expected to lower the strength of LSC (Schärer & Janicke, 2009).
In addition to the contrast between reciprocal copulation and hypodermic insemination, species that employ self‐fertilization might also differ in optimal SA from species that primarily outcross, because self‐fertilization greatly increases the strength of LSC. Thus, self‐fertilization leads to diminishing fitness returns from investing in the male function, favouring the evolution of a more female‐biased optimal SA compared with outcrossing species (Charlesworth & Charlesworth, 1981; Charnov, 1982, 1987), which is supported by empirical work in both plants and animals (Johnston et al., 1998; Lemen, 1980; Lemen, 1980; McKone, 1987; Schoen, 1982). In general, we would expect any process that has an effect on LSC to affect the shape of the male fitness gain curve. Also, this in turn leads to different predictions for the optimal SA (Schärer, 2009), as shown in Figure 1a.
FIGURE 1.
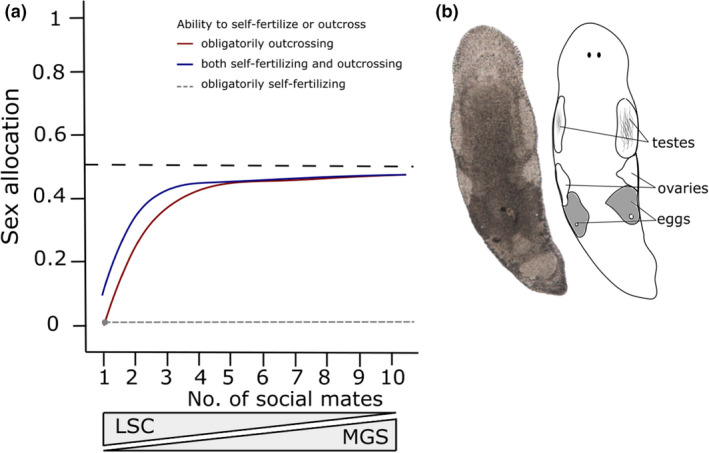
(a) A visualization of our hypotheses for the effect of the number of available social mates on the predicted sex allocation for species that either obligatorily outcross (red), species that both self‐fertilize and outcross simultaneously (blue), or species that obligatorily self‐fertilize (grey). The shown estimate of sex allocation is based on testis size / (testis size + ovary size) so that larger sex allocation values represent more strongly male‐biased allocation. As the number of social mates increases, the so‐called mating group size (MGS, i.e. the number of actual mates plus one) is expected to increase, whereas the level of local sperm competition (LSC) is expected to decrease. Note that for species that obligatorily self‐fertilize, MGS always remains one and LSC is always maximal, leading to a highly female‐biased sex allocation (i.e. the minimal male allocation to allow for full self‐fertility), independently of the number of social mates (indicated by grey dot and stippled line). For species that both self‐fertilize and outcross simultaneously, the MGS is already increased when the number of mates is one, since own sperm will compete with the partner's sperm. Also, in species that outcross only, the prediction of when the number of mates is one is only the minimal male allocation to allow for full outcross fertility. Note that these SA predictions are only approximate, since the degree to which MGS increases with the number of social mates will likely vary, and the extent of self‐fertilization and outcrossing is unclear in the species that show both. (b) Photograph and schematic drawing of an adult Macrostomum cliftonense (total length ~1.2 mm), showing the typical location of testes and ovaries (and the eggs formed from the ovaries)
Although most SA models investigate the effect of LSC on SA over evolutionary timescales, LSC can also vary temporally and spatially within an individual's lifetime. In hermaphrodites, altering the SA in response to the current conditions can influence the immediate reproductive success of an individual, favouring the evolution of SA plasticity. Indeed, plastic SA has been suggested to be one of the advantages of hermaphroditism over separate‐sex species (Charnov, 1982; Michiels, 1998, 1999). However, for species that do not often experience variation in LSC during their lifetime, we may not expect high levels of plasticity, particularly if there are costs to plasticity (Auld et al., 2010; DeWitt et al., 1998; Siljestam & Östman, 2017). Interestingly, although SA plasticity has now been documented in many hermaphroditic species (Hoch et al., 2016; Schärer, 2009; Yusa et al., 2013), its evolution is still comparatively poorly understood, with few studies having investigated variation in SA plasticity across animal species in a controlled experimental context (Schleicherová et al., 2014). Also finally, as SA can vary both between species (e.g. due to differences in mating strategy over evolutionary timescales) and within species (e.g. due to phenotypic plasticity), it is interesting to examine the relative magnitude of the interspecific versus intraspecific variation in SA.
An excellent model system for testing the effect of the mating strategy and the ability to self‐fertilize on SA and SA plasticity is the free‐living flatworm genus Macrostomum (Macrostomorpha, Platyhelminthes) (Figure 1b). This genus contains many species exhibiting one of at least two different mating strategies: one involving reciprocal mating and the other hypodermic insemination (Brand et al., 2022a; Schärer et al., 2011; Singh et al., 2022). In reciprocally mating species, a facultative postcopulatory suck behaviour has been observed, in which an individual places its pharynx on top of its female antrum (the sperm‐receiving and egg‐laying organ) and appears to suck, and this has been hypothesized to remove received ejaculate components (Schärer, Joss, et al., 2004; Singh et al., 2022; Vizoso et al., 2010). No such postcopulatory suck behaviour has been documented in hypodermically inseminating species (Singh et al., 2022), which presumably exhibit forced unilateral mating, with sperm being hypodermically injected into the partner via a needle‐like male copulatory organ (Brand et al., 2022a; Schärer et al., 2011). Moreover, both reciprocally mating and hypodermically inseminating species possess a female antrum, but in the latter, this organ has a simple morphology and presumably serves only for egg laying, whereas in the former, it is usually more complex and used both for egg‐laying and for receiving sperm from the partner (Brand et al., 2022a; Schärer et al., 2011). Interestingly, hypodermic insemination is also associated with a suite of morphological traits that potentially facilitate self‐fertilization (Giannakara & Ramm, 2017; Ramm et al., 2012, 2015), although self‐fertilization has recently also been documented in at least one reciprocally mating species, M. mirumnovem (Singh, Vellnow, et al., 2020).
Here, we collected literature data on SA estimates from a range of experiments performed in six Macrostomum species, and we present additional data from a new and currently still undescribed species, Macrostomum sp. 22 (see Supplementary Information S1). In all studies, the experimental design generally consisted in raising worms from juveniles in three different group sizes (isolated, pairs or octets) and then obtaining estimates of their SA once the worms had reached maturity. In M. lignano, the group size (i.e. the number of available social mates) has been shown to be a good proxy for the MGS (Janicke et al., 2013). To facilitate SA plasticity comparisons between species, we calculated standardized effect sizes for SA plasticity in response to (i) the presence of mating partners (i.e. comparing isolated worms vs. worms with partners) and (ii) the strength of LSC (i.e. comparing paired worms vs. octet worms). Using SA estimates and these SA plasticity effect sizes, we then examined whether the mating strategy and ability to self‐fertilize predicted SA or SA plasticity, while accounting for the phylogenetic interrelationships. Lastly, considering the interspecific SA differences and the extensive SA plasticity shown by some of the species, we examined how much of the variation in SA occurs between species versus within species, by partitioning the SA variance into its interspecific and intraspecific components.
2. MATERIALS AND METHODS
2.1. Study species
To examine how SA and SA plasticity evolve across the Macrostomum genus, we gathered data on SA and SA plasticity in seven Macrostomum species (we use the ‘Genus species Author, Year’ format to refer to binomials and include citations to the relevant taxonomic works). Three species are hypodermically inseminating, namely M. pusillum Ax, 1951 (Ax, 1951), M. hystrix Örsted, 1843 sensu Luther, 1905 (Luther, 1905; Örsted, 1843; Schärer et al., 2011), and the currently undescribed species Macrostomum sp. 22, with the SA data reported in, respectively, Giannakara and Ramm (2017), Winkler and Ramm (2018), and Supplementary Information S1. An additional four species are reciprocally mating, namely M. janickei Schärer et al., 2020, M. cliftonense Schärer et al., 2020, and M. mirumnovem Schärer et al., 2020 (Schärer et al., 2020; Zhang et al., 2021), with the SA data reported in Singh, Vellnow, et al. (2020), plus studies in M. lignano Ladurner et al., 2005 (Ladurner et al., 2005), with the SA data reported in several studies (see below). Details of all the SA studies are given in Table 1.
TABLE 1.
Details of sex allocation experiments, including species, mating strategy, ability to self‐fertilize, sample sizes and SA estimates for each group size, weighted SA (across group sizes), resulting SA plasticity effect sizes (for the presence of mating partners and the strength of LSC), inclusion of enclosure size in the experimental design, and reference
| Species | Mating strategy | Ability to self‐fertilize | Sample sizes for each group size | SA for each group size (95% CI) | Weighted SA (mean ± SD) | SA plasticity effect size (mean ± SD) | Enclosure size included | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated | Pair | Octet | Isolated | Pair | Octet | Presence of mating partner | Strength of LSC | ||||||
| M. janickei | Reciprocal | No | 57 | 63 | 57 | 0.50 (0.48–0.52) | 0.65 (0.63–0.67) | 0.68 (0.66–0.70) | 0.61 ± 0.07 | 1.87 ± 0.18 | 0.36 ± 0.18 | Yes | Singh et al. (2020) |
| M. cliftonense | Reciprocal | No | 56 | 57 | 60 | 0.55 (0.54–0.56) | 0.73 (0.71–0.74) | 0.77 (0.76–0.78) | 0.68 ± 0.09 | 3.62 ± 0.25 | 0.82 ± 0.19 | Yes | Singh et al. (2020) |
| M. mirumnovem | Reciprocal | Yes | 57 | 51 | 59 | 0.45 (0.43–0.47) | 0.51 (0.48–0.54) | 0.52 (0.50–0.55) | 0.49 ± 0.03 | 0.69 ± 0.16 | 0.11 ± 0.19 | Yes | Singh et al. (2020) |
| Macrostomum sp. 22 | Hypodermic | Yes | 58 | 59 | 57 | 0.36 (0.33–0.40) | 0.38 (0.35–0.41) | 0.45 (0.41–0.48) | 0.39 ± 0.03 | 0.41 ± 0.16 | 0.51 ± 0.18 | Yes | This study |
| M. pusillum | Hypodermic | Yes | 31 | 32 | 29 | 0.37 (0.34–0.40) | 0.37 (0.34–0.40) | 0.36 (0.32–0.40) | 0.36 ± 0.01 | −0.05 ± 0.21 | −0.14 ± 0.25 | No | Giannakara and Ramm (2017) |
| M. hystrix | Hypodermic | Yes | 30 | 48 | 27 | 0.67 (0.63–0.71) | 0.77 (0.73–0.81) | 0.79 (0.73–0.85) | 0.74 ± 0.04 | 0.83 ± 0.22 | 0.20 ± 0.23 | No | Winkler and Ramm (2018) |
| M. lignano | – | – | 11 | 12 | 0.57 (0.54–0.60) | 0.63 (0.62–0.64) | 0.60 ± 0.03 | – | 1.77 ± 0.47 | Yes | Schärer and Ladurner, (2003) | ||
| – | – | 9 | 12 | 0.55 (0.52–0.58) | 0.6 (0.58–0.62) | 0.57 ± 0.02 | – | 1.46 ± 0.47 | Yes | Schärer et al., (2004) | |||
| – | – | 46 | 46 | 0.63 (0.60–0.66) | 0.73 (0.71–0.75) | 0.68 ± 0.05 | – | 1.18 ± 0.22 | No | Janicke and Schärer, (2009) | |||
| 56 | 62 | – | 0.48 (0.46–0.50) | 0.62 (0.60–0.65) | 0.55 ± 0.07 | 1.69 ± 0.21 | – | No | Schärer and Janicke, (2009) | ||||
| – | – | 16 | 10 | 0.58 (0.55–0.61) | 0.75 (0.67–0.82) | 0.64 ± 0.08 | – | 2.09 ± 0.48 | No | Janicke et al. (2013) | |||
| – | – | 19 | 25 | 0.60 (0.58–0.62) | 0.67 (0.65–0.69) | 0.64 ± 0.03 | – | 1.43 ± 0.33 | No | Marie‐Orleach et al. (2014) | |||
| 37 | 38 | 37 | 0.41 (0.38–0.43) | 0.57 (0.54–0.60) | 0.65 (0.62–0.67) | 0.54 ± 0.09 | 2.37 ± 0.25 | 0.86 ± 0.23 | No | Ramm et al. (2019) | |||
| M. lignano (weighted mean) | Reciprocal | No | – | – | – | 0.45 | 0.59 | 0.68 | 0.59 ± 0.08 | 1.97 ± 0.17 | 1.25 ± 0.13 | ||
For Macrostomum lignano, data from seven independent experiments and the weighted mean SA estimates and SA plasticity effect sizes across all experiments are given. Note that the largest estimates are printed in bold type.
The species M. mirumnovem (Singh, Vellnow, et al., 2020) and all three hypodermically inseminating species (Giannakara & Ramm, 2017; Ramm et al., 2012, 2015, this study) can self‐fertilize and were classified accordingly. Moreover, we classified species as either reciprocally mating or hypodermically inseminating using both behavioural and morphological data. Behavioural data showed the presence of both reciprocal mating and the postcopulatory suck behaviour in M. lignano, M. janickei, M. cliftonense and M. mirumnovem, whereas neither of these behaviours were observed in the hypodermically inseminating M. pusillum, M. hystrix and Macrostomum sp. 22 (Schärer, Joss, et al., 2004a; Schärer et al., 2020; Singh et al., 2022). In addition, a classification of species using only morphological traits, which are known to be correlated with the mating strategy in Macrostomum (Schärer et al., 2011; Singh et al., 2022), corroborated our above classification based on behaviour. Thus, species could also be classified morphologically as reciprocally mating or hypodermically inseminating depending on their male and female genital morphology, the sperm morphology and the location of (received) allosperm (for details, see Brand et al., 2022a).
2.2. SA estimates and SA plasticity effect sizes across species
For most species, the experimental procedure was similar to that described for Macrostomum sp. 22 (Supplementary Information S1); that is, worms were raised from juveniles in three different group sizes (isolated, pairs or octets) until adulthood, when testis and ovary size was measured in one randomly chosen worm per replicate as a proxy for an individual's male and female allocation. The SA was then estimated as testis size/(testis size + ovary size). In some experiments, the density of worms was varied independently of group size using two enclosure sizes (small and large) (for details and sample sizes, see Table 1). We expect that the experiments are nevertheless comparable, since all experiments where density was included as a factor revealed that it did not have a significant effect on the estimate of SA.
For each experiment, we calculated the mean SA and respective standard deviation across the group sizes, weighted by the sample size for each group size, using the R package ‘Hmisc’ (Harrell, 2020). To facilitate interspecies comparisons of the SA plasticity estimates, we calculated standardized effect sizes and their standard deviation, using Cohen's d (Cohen, 1988) with Hedges’ correction for small sample size (Hedges, 1981), including confidence intervals (Howell, 2011) of the effect sizes using the R package ‘effsize’ (Torchiano, 2017). Specifically, Cohen's d with Hedges’ correction was calculated as the difference between the means of the two different contrasts of interest divided by the pooled standard deviation for the data. For each species, we calculated two (orthogonal) effect sizes for SA plasticity to compare the effect of the (usually) three treatment groups, isolated worms (I), worms in pairs (P) and worms in octets (O). Specifically, we calculated an effect size with respect to the presence of mating partners, by comparing isolated worms (I) vs. worms with partners (both P and O). Also, we calculated an effect size with respect to the strength of LSC, by comparing worms in pairs (P) vs. worms in octets (O). Pairs represent a condition with high LSC, whereas octets represent a condition with low LSC. Studies in M. lignano have shown that, in larger groups, individuals mate with many of the available partners (Janicke et al., 2013; Janicke & Schärer, 2009a), that there is sperm displacement (Marie‐Orleach et al., 2014) and that paternity is usually shared (Marie‐Orleach et al., 2016; Vellnow et al., 2018).
For M. lignano, we collected data sets from multiple published experiments (up to 25 November 2019), and calculated SA and SA plasticity effect sizes for each of them separately. In total, we found two and six studies, respectively, where we could extract the effects of the presence of mating partners (Ramm et al., 2019; Schärer & Janicke, 2009) and the strength of LSC (Janicke et al., 2013; Janicke & Schärer, 2009b; Marie‐Orleach et al., 2014; Ramm et al., 2019; Schärer & Ladurner, 2003; Schärer, Ladurner, et al., 2004). For Schärer and Ladurner (2003), we calculated SA from the available data, since that study only reported values for testis and ovary size. We excluded one previous study (Janicke & Schärer, 2010) from our analysis, since the worms used in that study came from the same experiment as Janicke and Schärer (2009), and so these did not represent fully independent data sets (we used the latter study since it included more replicates). In addition, in Marie‐Orleach et al. (2014) we combined the data from both inbred lines (HUB1 and DV1), since they did not differ in any of the traits measured. Also, it is noted that in Schärer and Janicke (2009), there are only isolated and paired worms, and hence, the estimated effect size for the presence of mating partners does not include the effect of octets. This should not have a large effect on the calculated effect size, since in most cases the difference between the isolated and paired treatments is much larger than the difference between paired and octet treatments (Figure 2).
FIGURE 2.
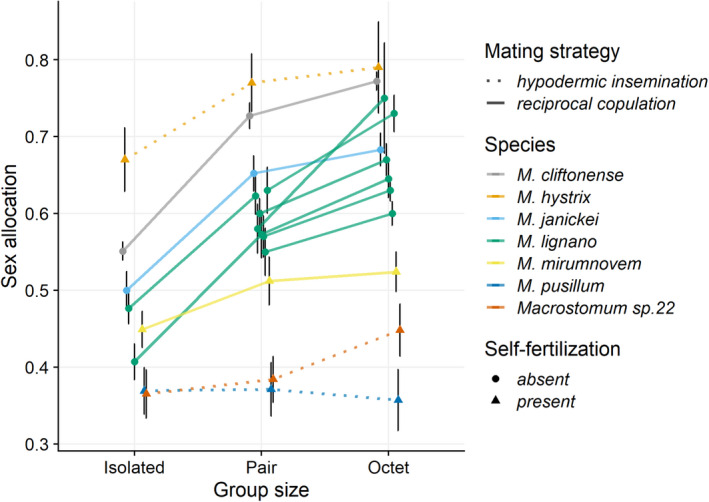
Effect of group size on estimates of sex allocation in seven different Macrostomum species (given by different colours). The line types represent the two mating strategies, and the symbols represent the ability to self‐fertilize. The plots show means and 95% confidence intervals of the raw (untransformed) data. The data have been jittered along the x‐axis to decrease overlap. Note that for M. lignano, data from seven independent experiments are shown
For M. lignano, a weighted mean across experiments was used to estimate the SA, and SA plasticity effect sizes (Turner & Bernard, 2006), for both the presence of mating partners and the strength of LSC. For SA, we took the mean of the SA across experiments, weighted by the sample size of each experiment. To weight each effect size, it was multiplied by the inverse of its variance (inverse variance weight, IVW), which allowed us to take into consideration the differences in sample size across experiments, namely:
Also, its standard error was calculated as:
2.3. Evolution of SA and SA plasticity
For the phylogenetically corrected analyses of the SA and SA plasticity effect sizes, we trimmed (ape package; Paradis et al., 2004) a recently generated ultrametric phylogenomic tree obtained from Brand, Viktorin, et al. (2022). This tree had been constructed using an alignment containing 385 protein‐coding genes (and 74 175 variable sites) from 98 transcriptome‐sequenced species, and calculated with a maximum‐likelihood approach (called H‐IQ‐TREE in Brand, Viktorin, et al., 2022). All bipartitions in this trimmed tree had maximal support (Figure 3), and trees constructed using a range of phylogenetic approaches had identical topologies with respect to the seven species studied here (Brand, Viktorin, et al., 2022), thus obviating the need to account for phylogenetic uncertainty in the following analyses.
FIGURE 3.
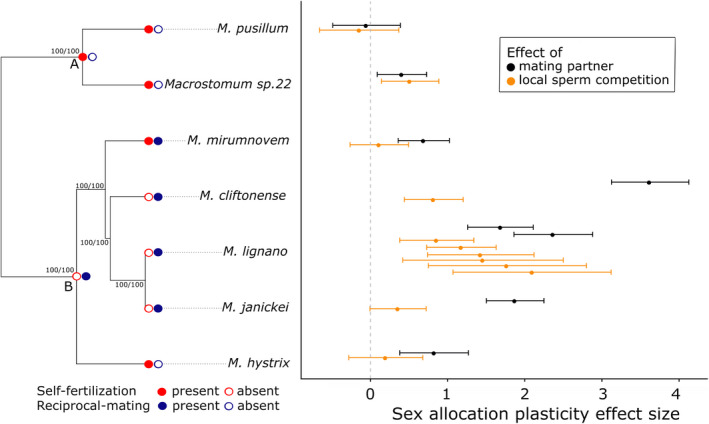
Standardized SA plasticity effect sizes for the effect of the presence of mating partners (i.e. comparing isolated worms vs. worms with partners) and the strength of local sperm competition (i.e. comparing worms in pairs vs. octets) among seven Macrostomum species (right side). The error bars represent 95% confidence intervals. For M. lignano, data from multiple experiments are shown. Also indicated is whether self‐fertilization (P. Singh, personal observations) or/and reciprocal mating behaviour (Singh et al., 2022) is present or absent in a species (left side), and these traits are mapped onto a trimmed maximum‐likelihood phylogeny of the genus (i.e. the H‐IQ‐TREE phylogeny from Brand et al., 2022b, which is based on 385 genes in 98 Macrostomum species), and all the shown bipartitions in this tree had maximal support, as indicated by ultrafast bootstrap support (first number) and approximate likelihood‐ratio tests (second number), respectively. A and B represent the inferred ancestral states at important internal nodes, suggesting that there are independent origins of hypodermic insemination and self‐fertilization in the genus (see also Methods and Figure S1 for details)
A recent study, using morphological traits, has shown that there are at least nine independent origins of hypodermic insemination in Macrostomum (Brand et al., 2022a). Although a similarly unequivocal analysis for multiple independent origins of self‐fertilization is currently not yet available, self‐fertilization was not readily observed in multiple reciprocally mating species that we also cultured in the laboratory, including M. spirale Ax, 1956 (Ax, 1956; Schärer et al., 2011), M. axi Papi, 1959 (Papi, 1959), M. clavituba Ax, 2008 (Ax, 2008) and M. poznaniense Kolasa, 1973 (Kolasa, 1973), which were held in isolation for extended periods of time for behavioural experiments (P. Singh, personal observations). All of these species show reciprocal mating behaviour (Singh et al., 2022), and they fall outside of the subclade containing both M. mirumnovem and M. hystrix (Figure S1). Using data for our study and these additional species, we reconstructed the ancestral state at the internal nodes of the phylogenetic tree (H‐IQ‐TREE) using a continuous‐time Markov model with MBASR (MrBayes Ancestral States with R, Heritage, 2021), with a sampling setting of 500 for both the presence of reciprocal mating behaviour and the presence of self‐fertilization (Figure S1a,b). Our analysis showed that M. hystrix clearly represents an independent origin of hypodermic insemination in an otherwise largely reciprocally mating clade, when compared to the other two hypodermically inseminating species, M. pusillum and Macrostomum sp. 22, which belong to a uniformly hypodermically inseminating clade (Figure S1a and Figure 3). Additionally, M. hystrix and M. mirumnovem represent two independent origins of self‐fertilization relative to those observed in M. pusillum and Macrostomum sp. 22 (Figure S1b).
As a preliminary analysis, we next examined whether there was an association between self‐fertilization and the mating strategy, since such an association would render these non‐independent predictor variables. For this, we used the DISCRETE model in BayesTraits V.3.0.1 to test for correlated evolution between the presence of self‐fertilization and the presence of hypodermic insemination using the reversible jump Markov chain Monte Carlo (RJ MCMC) approach (Meade & Pagel, 2016; Pagel, 1994; Pagel & Meade, 2006). To test support for correlated evolution, we compared the marginal likelihood of a dependent model, in which the presence of self‐fertilization depends on the presence of hypodermic insemination, with an independent model, in which hypodermic insemination and self‐fertilization evolve independently. We ran each RJ MCMC for ten million iterations, while discarding the first one million iterations as burn‐in, after which the chain was sampled every 1000th iteration. We placed 1000 stepping stones (iterating each for 10 000 times) and used a gamma hyperprior (gamma 0 1 0 1) to obtain the marginal likelihood values for the models. We performed three separate runs for both the independent and dependent models to check for the stability of the likelihood values and convergence. We established that the chains had converged using Gelman and Rubin's convergence diagnostic (Gelman & Rubin, 1992) and that the effective sample size was >200 for all parameters, using the R package coda (Plummer et al., 2006). In addition, we also confirmed that the acceptance rate was usually between 20% and 40% (ideal when the chain is at convergence and indicating good mixing; Pagel & Meade, 2006). We evaluated the alternative models using the log Bayes factor (BF) and used the convention that a BF value greater than 2 is considered as positive support for the best‐fit model (Pagel & Meade, 2006). We found only very weak support for the dependent model of evolution over the independent model for the association between self‐fertilization and mating strategy, with all three runs for each model providing similar values (mean marginal likelihood, independent = −9.59, dependent = −9.57, BF: 0.05; see also Table S2). This showed that the presence of self‐fertilization appears uncorrelated to the mating strategy in our data set, and we could therefore use the mating strategy and self‐fertilization as independent predictors for our analysis of SA and SA plasticity.
We estimated the phylogenetic signal using Blomberg's K (picante package, version 1.8.2; Blomberg et al., 2003; Kembel et al., 2010) and Pagel's λ (phytools package, version 0.7–70; Pagel, 1999; Revell, 2012), for both SA and the two SA plasticity effect sizes. A phylogenetic signal value close to zero is suggestive of a trait evolving independently of the phylogeny, whereas a value close to 1 suggests that the traits evolve under Brownian motion. In our case, the estimates for SA showed a phylogenetic signal, whereas the two SA plasticity effect sizes did not differ significantly from 0 (Table S3). Since our sample sizes are small, the likelihood‐ratio tests used to assess Pagel's λ can be unreliable (Boettiger et al., 2012), as the asymptotic properties of maximum‐likelihood estimation may not hold for small sample sizes. Thus, we present both phylogenetically corrected analyses and analyses without correcting for phylogeny.
For the phylogenetically corrected analyses, we compared the fit of different character evolution models (i.e. Brownian motion, Ornstein–Uhlenbeck and early‐burst) (geiger package, Harmon et al., 2008) for SA and the two SA plasticity effect sizes. Although it has been suggested that data sets with small sample sizes can erroneously favour Ornstein–Uhlenbeck models over simpler models such as Brownian motion (Cooper, Thomas, & FitzJohn, 2016; Cooper, Thomas, Venditti, et al., 2016), our small data set was found to be more consistent with a Brownian motion model (see Table S4). Thus, we used Brownian motion as the preferred model for the subsequent analysis. This does not necessarily imply, however, that the traits here actually evolve at random, but solely that a Brownian motion model fits our data better than the alternative Ornstein–Uhlenbeck or early‐burst models.
Using phylogenetic generalized least squares (PGLS) regressions and a Brownian motion model of character evolution (nlme package, version 3.1–152; Pinheiro et al., 2014), we tested whether the mating strategy (reciprocal mating vs. hypodermic insemination) or self‐fertilization (presence or absence) predicted SA and the two SA plasticity effect sizes (presence of mating partners and strength of LSC). PGLS allows us to account for the phylogenetic non‐independence of observations resulting from common evolutionary history of species. Furthermore, we also conducted an analysis without correcting for phylogeny, where we calculated Wilcoxon's rank‐sum tests to assess whether the mating strategy or self‐fertilization predicted the differences in SA or SA plasticity effect sizes. Although comparative studies usually consider only the mean value of a trait, this does not allow us to incorporate intraspecific variability, which can be a possible source of error (Boucher et al., 2012; Tonnabel et al., 2018). Here, we therefore incorporated intraspecific variation for both the PGLS and Wilcoxon's rank‐sum tests using a resampling approach (with 10 000 iterations). Each time, we generated a random value from a normal distribution with the observed means and standard deviations for each species (Table 1) and performed the analysis on this value. We report the mean values of PGLS in the main text and the distributions in Figure S2.
Finally, to partition the observed variance in SA into its between‐species and within‐species components, we used the mean SA (or weighted mean SA across all experiments for M. lignano) for each group size and species (i.e. 3 observations per species), and fit a linear mixed model (lme4 package; Bates et al., 2015) using species as a random effect and calculated the percentage of variance explained by species. Additionally, to explore the impact that the variable outcomes of the different experiments in M. lignano might have on these estimates, we picked one mean SA value per group size and experiment at random, and then redid the above analysis (resampling 10 000 times).
All statistical analyses were carried out using R version 4.0.5 (R Core Team), unless stated otherwise.
3. RESULTS
3.1. SA and effect sizes of SA plasticity for all species
We found variation in overall SA across the seven Macrostomum species (Figure 2, Table 1), with respect to both the mating strategy (solid vs. dotted lines) and self‐fertilization (circles vs. triangles), as is evident from the non‐overlapping confidence intervals (Knezevic, 2008). Two of the three hypodermically inseminating species (Macrostomum sp. 22 and M. pusillum) exhibited a low SA. Similarly, and in line with our predictions, three of the four self‐fertilizing species (Macrostomum sp. 22, M. pusillum and M. mirumnovem), exhibited a relatively low SA, likely indicating that SA in these species was female‐biased. Interestingly, the exception to these patterns was M. hystrix, which had the highest SA of all the studied species. There was also interspecific variation in the effect size estimates of SA plasticity, even among relatively closely related species (Figure 3, Table 1). For example, M. cliftonense and M. lignano exhibited the highest SA plasticity in response to the presence of mating partners and the strength of LSC, respectively. In addition, M. lignano exhibited SA plasticity across all experiments, and although the magnitude varied somewhat, the confidence intervals overlapped, indicating that the estimates were fairly consistent across experiments (Figure 3).
3.2. Evolution of SA and SA plasticity
The phylogenetic generalized least squares (PGLS) models showed that neither the mating strategy (mean: t5 = −0.56, p = 0.62) nor the ability to self‐fertilize (mean: t5 = −0.54, p = 0.61) significantly predicted SA (Figure S2a–d). Similar to SA, the mating strategy also did not significantly predict the SA plasticity effect size, neither due to the presence of mating partners (mean: t5 = 0.97, p = 0.38) nor due to the strength of LSC (mean: t5 = 0.29, p = 0.78) (Figure S2e,f,i,j). In contrast, self‐fertilization significantly predicted plasticity of SA in response to the presence of mating partners (mean: t5 = −2.98, p = 0.03, Figure S2g‐h), with this kind of plasticity being lower for selfing species (compare the black effect size estimates between selfing vs. non‐selfing species in Figure 3). No significant association between self‐fertilization and plasticity of SA was observed in response to the strength of LSC (mean: t5 = −0.72, p = 0.52, Figure S2k–l). Qualitatively similar results were obtained from the analyses that did not correct for phylogeny using Wilcoxon's rank‐sum tests (Table S5).
Lastly, the linear mixed model, using the weighted mean SA for M. lignano, showed that between‐species variance explained 73.6% of the total SA variance (interspecific variance = 0.0177, and residual variance = 0.0063), suggesting that the observed SA variance between species is nearly three times larger than the observed variance within species (and the resampling approach showed very similar results; Table S6).
4. DISCUSSION
Our study showed that there was interspecific variation in both SA and SA plasticity in Macrostomum, also among closely related species, suggesting that both SA and SA plasticity are evolutionarily labile. Furthermore, although the mating strategy predicted neither SA nor the SA plasticity effect sizes, self‐fertilizing species had a lower SA plasticity in response to the presence of mating partners. In the following, we discuss these findings in some detail.
In the context of phenotypic plasticity, very few studies have explored predictions of SA theory across multiple species in hermaphroditic animals (Hoch & Levinton, 2012; Schleicherová et al., 2014), with most studies focussing solely on intraspecific comparisons (Schärer, 2009). Hoch and Levinton (2012) tested Charnov’s (1980, 1982) MGS model in two species of acorn barnacles, Semibalanus balanoides and Balanus glandula, by manipulating both the number and the density of individuals in a natural setting. They showed that although both species exhibit an increased male allocation (estimated using the sum of the mass of testes, sperm, seminal vesicle and penis) at higher densities, there was no clear effect on SA from either treatment. In part, this was because the species also responded in terms of their female allocation (estimated using egg mass), with both the number and density manipulation having a positive effect in S. balanoides—that is an effect in the opposite direction than predicted by the model—although there was no clear effect in B. glandula. The authors argued that this might result from interspecific differences in life‐history traits. In contrast to the acorn barnacles, a study on resource allocation in response to different numbers of mating partners in three related polychaete worms, Ophryotrocha diadema, O. adherens and O. gracilis, showed that there was no significant plasticity in male allocation (estimated as the number of sperm produced by an individual) across the species (Schleicherová et al., 2014), whereas the species differed in the plasticity in female allocation (using multiple estimates, such as resource investment in eggs, total number of egg cocoons and time interval between egg laying). The authors proposed that the magnitude of plasticity depended on the species‐specific costs of the sex function, and also on the mating system of the species, and that there might be considerable resource allocation to behaviours linked to the male role in at least one of these species (Lorenzi et al., 2006; Santi et al., 2018). So, although both of these studies document interspecific variation in SA plasticity, they only included two or three species, respectively, meaning that they lacked sufficient power to explore statistically whether interspecific differences in reproductive traits may affect the level of SA plasticity.
Our results showed that both SA and SA plasticity varied across the studied Macrostomum species. Both hypodermically inseminating and self‐fertilizing species tended to show a low (i.e. more female‐biased) SA, with the notable exception of M. hystrix. This is interesting, since M. hystrix has been shown to represent an independent origin of hypodermic insemination in the reciprocally mating clade (Schärer et al., 2011). It has previously been suggested that SA could become more male‐biased when a species shifts to hypodermic insemination, since hypodermic sperm might compete more in a fair‐raffle‐type sperm competition (Schärer & Janicke, 2009), unless selfing were to become the dominant mating mode. Although capable of self‐fertilization, M. hystrix is known to reach very high densities in the field (L. Schärer, pers. obs.), which could potentially favour the evolution of a high SA. In this context, it is also important to note that our estimates of male and female allocation are not absolute, such that when we obtain SA estimates >0.5, that probably does not suggest a male‐biased SA. This is because our SA estimate implicitly assumes that both testis and ovary area are similarly suitable and complete proxies for male and female reproductive allocation, respectively, across species. However, it is clear that these proxies do not provide absolute estimates of SA, for multiple reasons (see also Singh, Vellnow, et al., 2020). For example, the energetic expenditure per unit tissue could differ between the testes and ovaries (Schärer, 2009), or there could be other components of male and female allocation that are not quantified by assessing gonad size, such as substantial provisioning of developing oocytes with yolk, sex‐specific behaviours (Picchi & Lorenzi, 2019), or the production of seminal fluid (Patlar et al., 2019), components that could themselves also be plastic.
In M. lignano, the 95% confidence intervals of four of the six effect sizes in response to the strength of LSC do not overlap with those of M. janickei (Figure 3). This could indicate that M. lignano and M. janickei, which are sibling species capable of hybridization (Singh, Ballmer, et al., 2020), have evolved differences in SA plasticity. Variation in SA plasticity in species with similar reproductive biology could stem from different environmental conditions experienced by the species. For example, evolution of SA plasticity might not be favoured in species that inhabit relatively stable environments, especially if the maintenance of plasticity is costly (Auld et al., 2010; DeWitt et al., 1998; Siljestam & Östman, 2017). Even in the absence of maintenance costs, there could still be production costs of plasticity, although if the benefits of adjusting SA outweigh these costs, then phenotypic plasticity could still be maintained. This cost–benefit ratio of SA plasticity can vary across species (Steiner, 2007; Van Buskirk, 2002) and environments (Ratikainen & Kokko, 2019), leading to the retention or loss of plasticity in a given species. Moreover, such costs of SA plasticity might play an important role in both the evolution and the maintenance of simultaneous hermaphroditism (St. Mary, 1997), and could also constrain plastic changes in SA, potentially leading to a suboptimal SA. A study in O. diadema showed that there were no large short‐term fitness costs of sex adjustment for the species (Lorenzi et al., 2008), whereas in M. lignano, production costs of SA plasticity have previously been documented (Sandner, 2013), with an alternating group size environment leading to a lower hatchling production compared to a stable group size environment. We currently do not have data on production costs of plasticity in M. janickei when exposed to a stable vs. fluctuating environment.
Across all species, the estimates of plasticity effect sizes are larger for the presence of mating partners than for the strength of LSC (number of mating partners), and for M. cliftonense and M. janickei, the confidence intervals of the two effect size estimates do not overlap. This suggests that the increase in SA going from paired to octet groups is not as drastic as going from isolated worms to worms in larger groups. A similar phenomenon has been observed in the freshwater snail, Lymnaea stagnalis, where paired snails showed higher expression of six seminal fluid protein (SFP) genes than isolated snails, whereas the SFP expression of snails in larger (6 individuals) groups was similar to that of paired snails (Nakadera et al., 2019). In our study, this situation could potentially arise if in the presence of multiple partners in octets, worms do increase not only their testis size but also their rate of sperm production per unit testis size (Schärer & Vizoso, 2007), which in M. lignano has been shown to occur due to an increase in the speed of spermatogenesis (Giannakara et al., 2016). If such effects occur generally (but see Giannakara & Ramm, 2017 for M. pusillum, a species that lacks SA plasticity, see also below), we might underestimate the extent of plasticity in male allocation when solely measuring testis size. Alternatively, there could also be increased investment in other components of the male function (see above). A study in O. diadema showed that increased mating opportunities are accompanied by higher investment into behavioural components of male function, such as aggressive behaviour (Lorenzi et al., 2006; Santi et al., 2018).
The SA plasticity effect sizes did not differ significantly between species exhibiting the reciprocal and hypodermic mating strategies, whereas self‐fertilizing species had a lower SA plasticity (in response to the presence of mating partner, but not the strength of LSC). Self‐fertilizing species have an opportunity to donate sperm both in isolation and in the presence of mating partners, and so may be less sensitive to changes in the social environment, since they already allocate substantially to the male function when in isolation. Moreover, the response may also depend on the rate and pattern of self‐fertilization (see below). Interestingly, to date M. mirumnovem is the only reciprocally mating Macrostomum species in which self‐fertilization has been recorded (Singh, Vellnow, et al., 2020), whereas studies have usually found self‐fertilization in hypodermic species. Including M. mirumnovem in our study allowed us to disentangle the association between mating strategy and self‐fertilization with respect to SA plasticity effect sizes, clearly demonstrating how including additional species in a phylogenetically informed context leads to more informed interpretations.
Interesting questions that arise then concern the causes of the variation in SA and SA plasticity between the self‐fertilizing species. An effect of self‐fertilization on SA has been theoretically predicted, and although there has been both theoretical and empirical work exploring the effect of self‐fertilization on SA in plants (Brunet, 1992; Charlesworth & Charlesworth, 1981; Charnov, 1987; Lemen, 1980; McKone, 1987; Schoen, 1982), there have been few such studies in animals (Johnston et al., 1998). A comparative study on SA in plants showed that resource allocation to flower parts can differ depending on the mating systems, such as the pollen–ovule ratio increasing with the likelihood of cross‐pollination (Cruden, 1977, but see Cruden & Lyon, 1985).
In our study, one possible explanation for variation between self‐fertilizing species could be whether the species differ in their rate and pattern of self‐fertilization, which could have an effect on both optimal SA and optimal SA plasticity. For example, in obligatorily self‐fertilizing species, we would expect that they do not increase their SA with an increase in the number of social mates, since LSC would be expected to remain high irrespective of the number of individuals in the local group (Figure 1a). On the contrary, for species that exhibit facultative self‐fertilization, we might expect considerable SA plasticity despite a low overall SA. Our results do conform somewhat to this pattern, with M. pusillum—hypothesized to be obligately self‐fertilizing (Giannakara & Ramm, 2017)—showing both the lowest overall SA and essentially no SA plasticity. Its closest congener, Macrostomum sp. 22, showed a similarly female‐biased SA in isolated and paired worms, but significant SA plasticity between worms in pairs and octets. If worms shifted from self‐fertilization when isolated, to exclusively outcrossing when in the presence of a mating partner, then the strength of LSC would not be expected to change from when they are alone to being in a pair. We would, however, expect the strength of LSC to drop from worms in pairs to worms in octets, favouring an increase in male allocation and SA, a scenario that would match the observed SA plasticity patterns in this species. This could suggest that Macrostomum sp. 22 may only self in isolation. In contrast, M. hystrix—thought to be a preferentially outcrossing species, with studies showing costs of self‐fertilization (Giannakara & Ramm, 2020; Ramm et al., 2012) and delayed selfing in isolation, at least in some populations (Ramm et al., 2012)—was found to be more plastic than M. pusillum.
Finally, our results indicate that there appears to be more variation in SA between species than within species in the sample of species we studied here, which is remarkable, since we saw extensive plasticity in SA in certain species in response to changes in group size, including across the multiple experiments in M. lignano. This result suggests that we can probably interpret variation in SA among field‐collected worms of different Macrostomum species as being at least partially due to interspecific variation, even if they happen to have been sampled from different group sizes, and may therefore also vary in part due to SA plasticity. Thus, although we cannot currently explain the observed interspecific variation in SA among the species studied here, our study will facilitate research in understanding the evolution of SA patterns across Macrostomum species, by allowing future studies to include SA estimates from field‐collected worms (such as in Brand et al., 2022b).
Collectively, our results suggest that there is interspecific variation in SA and SA plasticity in Macrostomum, with self‐fertilization being a significant predictor for the latter (although the relatively small number of species we could include in this comparative study of course needs to be taken into consideration). Future studies should explore how the extent of self‐fertilization, be it obligatorily or facultative, affects SA and SA plasticity using data from a greater number of species.
AUTHOR CONTRIBUTIONS
PS and LS designed the study, analysed the data and wrote the manuscript. PS performed the experiment. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors hereby declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jeb.14020.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Christian Felber for help with the morphological measurements of Macrostomum sp. 22, Lucas Marie‐Orleach, Steven Ramm and Tim Janicke for providing access to their data sets; Dita Vizoso and Kaja Wasik for help with collecting specimens of Macrostomum sp. 22, Jeremias Brand, Axel Wiberg and Nikolas Vellnow for helpful discussions; and Gudrun Viktorin, Daniel Lüscher, Jürgen Hottinger, Lukas Zimmerman and Urs Stiefel for technical support. This research was supported by grants 31003A_162543 and 310030_184916 of the Swiss National Science Foundation (SNSF) to LS. Open access funding provided by Universitat Basel.[[Correction added on 09 July 2022, after first online publication: CSAL funding statement has been added.]
Singh, P. , & Schärer, L. (2022). Evolution of sex allocation plasticity in a hermaphroditic flatworm genus. Journal of Evolutionary Biology, 35, 817–830. 10.1111/jeb.14020
DATA AVAILABILITY STATEMENT
All data in this manuscript will be available at https://doi.org/10.5061/dryad.ksn02v76b.
REFERENCES
- Auld, J. R. , Agrawal, A. A. , & Relyea, R. A. (2010). Re‐evaluating the costs and limits of adaptive phenotypic plasticity. Proceedings of the Royal Society B: Biological Sciences, 277, 503–511. 10.1098/rspb.2009.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ax, P. (1951). Die Turbellarien des Eulitorals der Kieler Bucht. Zoologische Jahrbuecher Abteilung Fuer Systematik Oekologie Und Geographie Der Tiere, 80, 277–378. [Google Scholar]
- Ax, P. (1956). Les Turbellariés des étangs côtiers du littoral méditerranéen de la France méridionale… Hermann et Cie. [Google Scholar]
- Ax, P. (2008). Plathelminthes aus Brackgewässern der Nordhalbkugel. Abhandlungen der Mathematisch‐naturwissenschaftligen Klasse (pp. 696). Verlag der Akademie der Wissenschaften und der Literatur. [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. M. , & Walker, S. C. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Blomberg, S. P. , Garland, T. , & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution, 57, 717–745. 10.1111/j.0014-3820.2003.tb00285.x [DOI] [PubMed] [Google Scholar]
- Boettiger, C. , Coop, G. , & Ralph, P. (2012). Is your phylogeny informative? Measuring the power of comparative methods. Evolution, 66, 2240–2251. 10.1111/j.1558-5646.2011.01574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher, F. C. , Thuiller, W. , Roquet, C. , Douzet, R. , Aubert, S. , Alvarez, N. , & Lavergne, S. (2012). Reconstructing the origins of high‐alpine niches and cushion life form in the genus Androsace S.L. (Primulaceae). Evolution, 66, 1255–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, J. N. , Harmon, L. J. , & Schärer, L. (2022a). Frequent origins of traumatic insemination involve convergent shifts in sperm and genital morphology. Evolution Letters, 6(1), 63–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, J. N. , Harmon, L. J. , & Schärer, L. (2022b). Mating behavior and reproductive morphology predict macroevolution of sex allocation in hermaphroditic flatworms. BMC Biology, 20, 35. 10.1186/s12915-022-01234-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, J. N. , Viktorin, G. , Wiberg, R. A. W. , Beisel, C. , & Schärer, L. (2022). Large‐scale phylogenomics of the genus Macrostomum (Platyhelminthes) reveals cryptic diversity and novel sexual traits. Molecular Phylogenetics and Evolution, 166, 107296. 10.1016/j.ympev.2021.107296 [DOI] [PubMed] [Google Scholar]
- Brunet, J. (1992). Sex allocation in hermaphroditic plants. Trends in Ecology & Evolution, 7, 79–84. 10.1016/0169-5347(92)90245-7 [DOI] [PubMed] [Google Scholar]
- Campbell, D. R. (2000). Experimental tests of sex‐allocation theory in plants. Trends in Ecology & Evolution, 15, 227–232. 10.1016/S0169-5347(00)01872-3 [DOI] [PubMed] [Google Scholar]
- Charlesworth, D. , & Charlesworth, B. (1981). Allocation of resources to male and female functions in hermaphrodites. Biological Journal of the Linnean Society, 15, 57–74. 10.1111/j.1095-8312.1981.tb00748.x [DOI] [Google Scholar]
- Charnov, E. L. (1979). Simultaneous hermaphroditism and sexual selection. Proceedings of the National Academy of Sciences of the United States of America, 76, 2480–2484. 10.1073/pnas.76.5.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov, E. L. (1980). Sex allocation and local mate competition in barnacles. Marine Biology Letters, 1, 269–272. [Google Scholar]
- Charnov, E. L. (1982). The theory of sex allocation. Monographs in population biology 18:1–355. Princeton University Press. [PubMed] [Google Scholar]
- Charnov, E. L. (1987). On sex allocation and selfing in higher plants. Evolutionary Ecology, 1, 30–36. 10.1007/BF02067266 [DOI] [Google Scholar]
- Charnov, E. L. (1996). Sperm competition and sex allocation in simultaneous hermaphrodites. Evolutionary Ecology, 10, 457–462. 10.1007/BF01237878 [DOI] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. L. Erlbaum Associates; [Google Scholar]
- Cooper, N. , Thomas, G. H. , & FitzJohn, R. G. (2016). Shedding light on the ‘dark side’ of phylogenetic comparative methods. Methods in Ecology and Evolution, 7, 693–699. 10.1111/2041-210X.12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, N. , Thomas, G. H. , Venditti, C. , Meade, A. , & Freckleton, R. P. (2016). A cautionary note on the use of Ornstein Uhlenbeck models in macroevolutionary studies. Biological Journal of the Linnean Society, 118, 64–77. 10.1111/bij.12701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden, R. W. (1977). Pollen‐ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution, 31. 10.2307/2407542 [DOI] [PubMed] [Google Scholar]
- Cruden, R. W. , & Lyon, D. L. (1985). Patterns of biomass allocation to male and female functions in plants with different mating systems. Oecologia, 66. 10.1007/BF00379868 [DOI] [PubMed] [Google Scholar]
- DeWitt, T. J. , Sih, A. , & Wilson, D. S. (1998). Costs and limits of phenotypic plasticity. Trends in Ecology & Evolution, 13, 77–81. 10.1016/S0169-5347(97)01274-3 [DOI] [PubMed] [Google Scholar]
- Fischer, E. A. (1981). Sexual allocation in a simultaneously hermaphroditic coral reef fish. The American Naturalist, 117, 64–82. 10.1086/283686 [DOI] [Google Scholar]
- Fischer, E. A. (1984). Local mate competition and sex allocation in simultaneous hermaphrodites. The American Naturalist, 124, 590–596. 10.1086/284298 [DOI] [Google Scholar]
- Gelman, A. , & Rubin, D. B. (1992). Inference from iterative simulation using multiple sequences. Statistical Science, 7, 457–472. 10.1214/ss/1177011136 [DOI] [Google Scholar]
- Giannakara, A. , & Ramm, S. A. (2017). Self‐fertilization, sex allocation and spermatogenesis kinetics in the hypodermically inseminating flatworm Macrostomum pusillum . The Journal of Experimental Biology, 220, 1568–1577. [DOI] [PubMed] [Google Scholar]
- Giannakara, A. , & Ramm, S. A. (2020). Evidence for inter‐population variation in waiting times in a self‐fertilizing flatworm. Invertebrate Reproduction & Development, 64, 158–168. 10.1080/07924259.2020.1732485 [DOI] [Google Scholar]
- Giannakara, A. , Schärer, L. , & Ramm, S. A. (2016). Sperm competition‐induced plasticity in the speed of spermatogenesis. BMC Evolutionary Biology, 16, 60. 10.1186/s12862-016-0629-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeff, J. M. , & Michiels, N. K. (1999). Sperm digestion and reciprocal sperm transfer can drive hermaphrodite sex allocation to equality. The American Naturalist, 153, 421–430. 10.1086/303184 [DOI] [PubMed] [Google Scholar]
- Hamilton, W. D. (1967). Extraordinary sex ratios. Science, 156, 477–488. 10.1126/science.156.3774.477 [DOI] [PubMed] [Google Scholar]
- Harmon, L. J. , Weir, J. T. , Brock, C. D. , Glor, R. E. , & Challenger, W. (2008). GEIGER: investigating evolutionary radiations. Bioinformatics, 24, 129–131. 10.1093/bioinformatics/btm538 [DOI] [PubMed] [Google Scholar]
- Harrell, F. E. (2020). Hmisc: Harrell miscellaneous. R package version 4.4‐0. [Google Scholar]
- Hedges, L. V. (1981). Distribution theory for glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 6, 107–128. 10.3102/10769986006002107 [DOI] [Google Scholar]
- Heritage, S. (2021). MBASR: Workflow‐simplified ancestral state reconstruction of discrete traits with MrBayes in the R environment (preprint). bioRxiv. 10.1101/2021.01.10.426107 [DOI] [Google Scholar]
- Hoch, J. M. , & Levinton, J. S. (2012). Experimental tests of sex allocation theory with two species of simultaneously hermaphroditic acorn barnacles. Evolution, 66, 1332–1343. 10.1111/j.1558-5646.2011.01548.x [DOI] [PubMed] [Google Scholar]
- Hoch, J. M. , Schneck, D. T. , & Neufeld, C. J. (2016). Ecology and evolution of phenotypic plasticity in the penis and cirri of barnacles. Integrative and Comparative Biology, 56, 728–740. 10.1093/icb/icw006 [DOI] [PubMed] [Google Scholar]
- Howell, D. C. (2011). Confidence intervals on effect size. University of Vermont. 10.1134/S0362119713030110 [DOI] [Google Scholar]
- Janicke, T. , Marie‐Orleach, L. , De Mulder, K. , Berezikov, E. , Ladurner, P. , Vizoso, D. B. , & Schärer, L. (2013). Sex allocation adjustment to mating group size in a simultaneous hermaphrodite. Evolution, 67, 3233–3242. 10.1111/evo.12189 [DOI] [PubMed] [Google Scholar]
- Janicke, T. , & Schärer, L. (2009a). Determinants of mating and sperm‐transfer success in a simultaneous hermaphrodite. Journal of Evolutionary Biology, 22, 405–415. 10.1111/j.1420-9101.2008.01660.x [DOI] [PubMed] [Google Scholar]
- Janicke, T. , & Schärer, L. (2009b). Sex allocation predicts mating rate in a simultaneous hermaphrodite. Proceedings of the Royal Society B: Biological Sciences, 276, 4247–4253. 10.1098/rspb.2009.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke, T. , & Schärer, L. (2010). Sperm competition affects sex allocation but not sperm morphology in a flatworm. Behavioral Ecology and Sociobiology, 64, 1367–1375. 10.1007/s00265-010-0951-y [DOI] [Google Scholar]
- Johnston, M. O. , Das, B. , & Hoeh, W. R. (1998). Negative correlation between male allocation and rate of self‐fertilization in a hermaphroditic animal. Proceedings of the National Academy of Sciences of the United States of America, 95, 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel, S. W. , Cowan, P. D. , Helmus, M. R. , Cornwell, W. K. , Morlon, H. , Ackerly, D. D. , Blomberg, S. P. , & Webb, C. O. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics, 26, 1463–1464. 10.1093/bioinformatics/btq166 [DOI] [PubMed] [Google Scholar]
- Knezevic, A. (2008). Overlapping confidence intervals and statistical significance (p. 73). StatNews: Cornell University Statistical Consulting Unit. [Google Scholar]
- Kolasa, J. (1973). Two new species of Macrostomum (Turbellaria), A redescription of an established species and new records from Poland. Bolletino Di Zoologia, 40, 181–200. [Google Scholar]
- Ladurner, P. , Schärer, L. , Salvenmoser, W. , & Rieger, R. M. (2005). A new model organism among the lower Bilateria and the use of digital microscopy in taxonomy of meiobenthic Platyhelminthes: Macrostomum lignano, n. sp. (Rhabditophora, Macrostomorpha). Journal of Zoological Systematics and Evolutionary Research, 43, 114–126. 10.1111/j.1439-0469.2005.00299.x [DOI] [Google Scholar]
- Lange, R. , Reinhardt, K. , Michiels, N. K. , & Anthes, N. (2013). Functions, diversity, and evolution of traumatic mating. Biological Reviews, 88, 585–601. 10.1111/brv.12018 [DOI] [PubMed] [Google Scholar]
- Lemen, C. (1980). Allocation of reproductive effort to the male and female strategies in wind‐pollinated plants. Oecologia, 45, 156–159. 10.1007/BF00346454 [DOI] [PubMed] [Google Scholar]
- Lloyd, D. G. (1984). Gender allocations in outcrossing cosexual plants. In Dirzo R., & Sarukhan J. (Eds.), Perspectives in plant population ecology (pp. 277–300). Sinauer. [Google Scholar]
- Lorenzi, M. C. , Schleicherová, D. , & Sella, G. (2006). Life history and sex allocation in the simultaneously hermaphroditic polychaete worm Ophryotrocha diadema: The role of sperm competition. Integrative and Comparative Biology, 46, 381–389. 10.1093/icb/icj042 [DOI] [PubMed] [Google Scholar]
- Lorenzi, M. C. , Schleicherova, D. , & Sella, G. (2008). Sex adjustments are not functionally costly in simultaneous hermaphrodites. Marine Biology, 153, 599–604. 10.1007/s00227-007-0833-7 [DOI] [Google Scholar]
- Luther, A. (1905). Zur Kenntnis der Gattung Macrostoma (vol. 5, 1–61, 64 plates). Festschrift für Palmén, Helsingfors. [Google Scholar]
- Marie‐Orleach, L. , Janicke, T. , Vizoso, D. B. , David, P. , & Schärer, L. (2016). Quantifying episodes of sexual selection: Insights from a transparent worm with fluorescent sperm. Evolution, 70, 314–328. 10.1111/evo.12861 [DOI] [PubMed] [Google Scholar]
- Marie‐Orleach, L. , Janicke, T. , Vizoso, D. B. , Eichmann, M. , & Schärer, L. (2014). Fluorescent sperm in a transparent worm: validation of a GFP marker to study sexual selection. BMC Evolutionary Biology, 14, 148. 10.1186/1471-2148-14-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKone, M. J. (1987). Sex allocation and outcrossing rate: A test of theoretical predictions using Bromegrasses (Bromus). Evolution, 41, 591. 10.2307/2409260 [DOI] [PubMed] [Google Scholar]
- Meade, A. , & Pagel, M. (2016). BayesTraits manual (version 3). http://www.evolution.rdg.ac.uk/BayesTraitsV3 [Google Scholar]
- Michiels, N. K. (1998). Mating conflicts and sperm competition in simultaneous hermaphrodites. In Birkhead T. R., & Møller A. P. (Eds.), Sperm competition and sexual selection (pp. 219–254). Elsevier. [Google Scholar]
- Michiels, N. K. (1999). Sexual adaptations to high density in hermaphrodites. Invertebrate Reproduction & Development, 36, 35–40. 10.1080/07924259.1999.9652675 [DOI] [Google Scholar]
- Nakadera, Y. , Giannakara, A. , & Ramm, S. A. (2019). Plastic expression of seminal fluid protein genes in a simultaneously hermaphroditic snail. Behavioral Ecology, 30, 904–913. 10.1093/beheco/arz027 [DOI] [Google Scholar]
- Örsted, A. S. (1843). Forsog til en ny classification af Planarierne (Planariea Duges) grundet paa mikroskopisk‐anatomiske Undersogelser. Naturhistorisk Tidsskrift, 4, 519–581. [Google Scholar]
- Pagel, M. (1994). Detecting correlated evolution on phylogenies: A general method for the comparative analysis of discrete characters. Proceedings of the Royal Society of London. Series B: Biological Sciences, 255, 37–45. [Google Scholar]
- Pagel, M. (1999). Inferring the historical patterns of biological evolution. Nature, 401, 877–884. 10.1038/44766 [DOI] [PubMed] [Google Scholar]
- Pagel, M. , & Meade, A. (2006). Bayesian analysis of correlated evolution of discrete characters by reversible‐jump Markov Chain Monte Carlo. The American Naturalist, 167, 808–825. 10.1086/503444 [DOI] [PubMed] [Google Scholar]
- Papi, F. (1959). Specie nuove o poco note del gen. Macrostomum (Turbellaria: Macrostomida) rinvenute in Italia. Monitore Zoologico Italiano, 66, 84–102. [Google Scholar]
- Paradis, E. , Claude, J. , & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290. 10.1093/bioinformatics/btg412 [DOI] [PubMed] [Google Scholar]
- Parker, G. A. (1970). Sperm competition and its evolutionary consequences in the insects. Biological Reviews, 45, 525–567. 10.1111/j.1469-185X.1970.tb01176.x [DOI] [Google Scholar]
- Parker, G. A. (1998). Sperm competition and the evolution of ejaculates: towards a theory base. In Blum M. S., & Blum N. A. (Eds.), Sperm competition and sexual selection (pp. 3–54). Academic Press. [Google Scholar]
- Patlar, B. , Weber, M. , & Ramm, S. A. (2019). Genetic and environmental variation in transcriptional expression of seminal fluid proteins. Heredity, 122, 595–611. 10.1038/s41437-018-0160-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pen, I. , & Weissing, F. J. (1999). Sperm competition and sex allocation in simultaneous hermaphrodites: A new look at Charnov’s invariance principle. Evolutionary Ecology Research, 517–525. [Google Scholar]
- Picchi, L. , & Lorenzi, M. C. (2019). Gender‐related behaviors: Evidence for a trade‐off between sexual functions in a hermaphrodite. Behavioral Ecology, 30, 770–784. 10.1093/beheco/arz014 [DOI] [Google Scholar]
- Pinheiro, J. , Bates, D. , DebRoy, S. , Sarkar, D. , & R Core Team . (2014). nlme: Linear and nonlinear mixed effects models. R package version 3.1–117. http://cranr‐projectorg/web/packages/nlme/index.html [Google Scholar]
- Plummer, M. , Best, N. , Cowles, K. , & Vines, K. (2006). CODA: Convergence diagnosis and output analysis for MCMC. R News, 6(1), 7–11. [Google Scholar]
- Rademaker, M. C. J. , & Jong, T. J. (1999). The shape of the female fitness curve for Cynoglossum officinale: Quantifying seed dispersal and seedling survival in the field. Plant Biology, 1, 351–356. 10.1111/j.1438-8677.1999.tb00263.x [DOI] [Google Scholar]
- Ramm, S. A. , Lengerer, B. , Arbore, R. , Pjeta, R. , Wunderer, J. , Giannakara, A. , Berezikov, E. , Ladurner, P. , & Schärer, L. (2019). Sex allocation plasticity on a transcriptome scale: Socially sensitive gene expression in a simultaneous hermaphrodite. Molecular Ecology, 28, 2321–2341. 10.1111/mec.15077 [DOI] [PubMed] [Google Scholar]
- Ramm, S. A. , Schlatter, A. , Poirier, M. , & Schärer, L. (2015). Hypodermic self‐insemination as a reproductive assurance strategy. Proceedings of the Royal Society B: Biological Sciences, 282(1811), 20150660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm, S. A. , Vizoso, D. B. , & Schärer, L. (2012). Occurrence, costs and heritability of delayed selfing in a free‐living flatworm. Journal of Evolutionary Biology, 25, 2559–2568. 10.1111/jeb.12012 [DOI] [PubMed] [Google Scholar]
- Ratikainen, I. I. , & Kokko, H. (2019). The coevolution of lifespan and reversible plasticity. Nature Communications, 10, 538. 10.1038/s41467-019-08502-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, K. , Anthes, N. , & Lange, R. (2015). Copulatory wounding and traumatic insemination. Cold Spring Harbor Perspectives in Biology, 7, a017582. 10.1101/cshperspect.a017582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell, L. J. (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. 10.1111/j.2041-210X.2011.00169.x [DOI] [Google Scholar]
- Rosas, F. , & Domínguez, C. A. (2009). Male sterility, fitness gain curves and the evolution of gender specialization from distyly in Erythroxylum havanense . Journal of Evolutionary Biology, 22, 50–59. 10.1111/j.1420-9101.2008.01618.x [DOI] [PubMed] [Google Scholar]
- Sandner, P. (2013). Impacts of sperm competition on mating behaviour and life history traits in a simultaneous hermaphrodite. University of Basel. [Google Scholar]
- Santi, M. , Picchi, L. , & Lorenzi, M. C. (2018). Dynamic modulation of reproductive strategies in a simultaneous hermaphrodite and preference for the male role. Animal Behaviour, 146, 87–96. 10.1016/j.anbehav.2018.10.004 [DOI] [Google Scholar]
- Schärer, L. (2009). Tests of sex allocation theory in simultaneously hermaphroditic animals. Evolution, 63, 1377–1405. 10.1111/j.1558-5646.2009.00669.x [DOI] [PubMed] [Google Scholar]
- Schärer, L. , Brand, J. N. , Singh, P. , Zadesenets, K. S. , Stelzer, C.‐P. , & Viktorin, G. (2020). A phylogenetically informed search for an alternative Macrostomum model species, with notes on taxonomy, mating behavior, karyology, and genome size. Journal of Zoological Systematics and Evolutionary Research, 58, 41–65. [Google Scholar]
- Schärer, L. , & Janicke, T. (2009). Sex allocation and sexual conflict in simultaneously hermaphroditic animals. Biology Letters, 5, 705–708. 10.1098/rsbl.2009.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer, L. , Janicke, T. , & Ramm, S. A. (2015). Sexual conflict in hermaphrodites. Cold Spring Harbor Perspectives in Biology, 7, a017673. 10.1101/cshperspect.a017673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer, L. , Joss, G. , & Sandner, P. (2004). Mating behaviour of the marine turbellarian Macrostomum sp.: These worms suck. Marine Biology, 145, 373–380. 10.1007/s00227-004-1314-x [DOI] [Google Scholar]
- Schärer, L. , & Ladurner, P. (2003). Phenotypically plastic adjustment of sex allocation in a simultaneous hermaphrodite. Proceedings of the Royal Society B: Biological Sciences, 270, 935–941. 10.1098/rspb.2002.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer, L. , Ladurner, P. , & Rieger, R. M. (2004). Bigger testes do work more: experimental evidence that testis size reflects testicular cell proliferation activity in the marine invertebrate, the free‐living flatworm Macrostomum sp. Behavioral Ecology and Sociobiology, 56, 420–425. [Google Scholar]
- Schärer, L. , Littlewood, D. T. J. , Waeschenbach, A. , Yoshida, W. , & Vizoso, D. B. (2011). Mating behavior and the evolution of sperm design. Proceedings of the National Academy of Sciences of the United States of America, 108, 1490–1495. 10.1073/pnas.1013892108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer, L. , & Pen, I. (2013). Sex allocation and investment into pre‐ and post‐copulatory traits in simultaneous hermaphrodites: The role of polyandry and local sperm competition. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 368, 20120052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer, L. , & Vizoso, D. B. (2007). Phenotypic plasticity in sperm production rate: There’s more to it than testis size. Evolutionary Ecology, 21, 295–306. 10.1007/s10682-006-9101-4 [DOI] [Google Scholar]
- Schleicherová, D. , Sella, G. , Meconcelli, S. , Simonini, R. , Martino, M. P. , Cervella, P. , & Lorenzi, M. C. (2014). Does the cost of a function affect its degree of plasticity? A test on plastic sex allocation in three closely related species of hermaphrodites. Journal of Experimental Marine Biology and Ecology, 453, 148–153. 10.1016/j.jembe.2014.01.010 [DOI] [Google Scholar]
- Schoen, D. J. (1982). Male reproductive effort and breeding system in an hermaphroditic plant. Oecologia, 53, 255–257. 10.1007/BF00545673 [DOI] [PubMed] [Google Scholar]
- Siljestam, M. , & Östman, Ö. (2017). The combined effects of temporal autocorrelation and the costs of plasticity on the evolution of plasticity. Journal of Evolutionary Biology, 30, 1361–1371. 10.1111/jeb.13114 [DOI] [PubMed] [Google Scholar]
- Singh, P. , Ballmer, D. N. , Laubscher, M. , & Schärer, L. (2020). Successful mating and hybridisation in two closely related flatworm species despite significant differences in reproductive morphology and behaviour. Scientific Reports, 10, 12830. 10.1038/s41598-020-69767-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, P. , Brand, J. N. , & Schärer, L. (2022). Evolution and co‐evolution of the suck behaviour, a postcopulatory female resistance trait that manipulates received ejaculate. bioRxiv. 10.1101/2022.04.13.485945 [DOI] [Google Scholar]
- Singh, P. , Vellnow, N. , & Schärer, L. (2020). Variation in sex allocation plasticity in three closely related flatworm species. Ecology and Evolution, 10, 26–37. 10.1002/ece3.5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Mary, C. M. (1997). Sequential patterns of sex allocation in simultaneous hermaphrodites: Do we need models that specifically incorporate this complexity? The American Naturalist, 150, 73–97. 10.1086/286057 [DOI] [PubMed] [Google Scholar]
- Steiner, U. K. (2007). Investment in defense and cost of predator‐induced defense along a resource gradient. Oecologia, 152, 201–210. 10.1007/s00442-006-0645-3 [DOI] [PubMed] [Google Scholar]
- Tonnabel, J. , Schurr, F. M. , Boucher, F. , Thuiller, W. , Renaud, J. , Douzery, E. J. P. , & Ronce, O. (2018). Life‐history traits evolved jointly with climatic niche and disturbance regime in the genus Leucadendron (Proteaceae). The American Naturalist, 191, 220–234. [DOI] [PubMed] [Google Scholar]
- Torchiano, M. (2017). effsize: Efficient effect size computation. R package version 0.7, 1. [Google Scholar]
- Turner, H. M. , & Bernard, R. M. (2006). Calculating and synthesizing effect sizes. Contemporary Issues in Communication Science and Disorders, 33, 42–55. [Google Scholar]
- Van Buskirk, J. (2002). A comparative test of the adaptive plasticity hypothesis: Relationships between habitat and phenotype in Anuran larvae. The American Naturalist, 160, 87–102. 10.1086/340599 [DOI] [PubMed] [Google Scholar]
- van Velzen, E. , Schärer, L. , & Pen, I. (2009). The effect of cryptic female choice on sex allocation in simultaneous hermaphrodites. Proceedings of the Royal Society B: Biological Sciences, 276, 3123–3131. 10.1098/rspb.2009.0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellnow, N. , Marie‐Orleach, L. , Zadesenets, K. S. , & Schärer, L. (2018). Bigger testes increase paternity in a simultaneous hermaphrodite, independently of the sperm competition level. Journal of Evolutionary Biology, 31, 180–196. 10.1111/jeb.13212 [DOI] [PubMed] [Google Scholar]
- Vizoso, D. B. , Rieger, G. , & Schärer, L. (2010). Goings‐on inside a worm: Functional hypotheses derived from sexual conflict thinking. Biological Journal of the Linnean Society, 99, 370–383. 10.1111/j.1095-8312.2009.01363.x [DOI] [Google Scholar]
- Winkler, L. , & Ramm, S. A. (2018). Experimental evidence for reduced male allocation under selfing in a simultaneously hermaphroditic animal. Biology Letters, 14, 20180570. 10.1098/rsbl.2018.0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa, Y. , Takemura, M. , Sawada, K. , & Yamaguchi, S. (2013). Diverse, continuous, and plastic sexual systems in barnacles. Integrative and Comparative Biology, 53, 701–712. 10.1093/icb/ict016 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Shi, Y. , Zeng, Z. , Xin, F. , Deng, L. , & Wang, A. (2021). Two new brackish‐water species of Macrostomum (Platyhelminthes: Macrostomorpha) from China and their phylogenetic positions. Zoological Science, 38(3), 273–286. 10.2108/zs200121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All data in this manuscript will be available at https://doi.org/10.5061/dryad.ksn02v76b.


