Summary
Background
Hidradenitis suppurativa (HS) is a chronic autoinflammatory skin condition and is associated with several comorbidities. Previous studies report variable prevalence rates of HS, depending on the methodology. However, the exact prevalence remains unknown.
Objectives
To estimate the prevalence of HS in a large population‐based cohort in the Northern Netherlands, and to compare patients with HS to the general population, investigate characteristics and identify potential associated comorbidities.
Methods
Data were collected through a cross‐sectional survey‐based study within the Lifelines Cohort Study (n = 167 729), based on the general population located in the Northern Netherlands. A digital self‐reported questionnaire was developed consisting of validated questions for determining HS.
Results
Among 56 084 respondents, the overall prevalence of HS was 2.1% [95% confidence interval (CI) 2.0–2.2]. The respondents with HS had lower socioeconomic status than the controls (P < 0.001) and more frequently currently smoked (P < 0.001). Several new significant associations in patients with HS were revealed, such as fibromyalgia (OR 2.26, 95% CI 1.64–3.11), irritable bowel syndrome (OR 1.63, 95% CI 1.18–2.26), chronic fatigue syndrome (OR 1.72, 95% CI 1.06–2.78) and migraine (OR 1.48, 95% CI 1.11–1.96). Fibromyalgia and chronic fatigue syndrome remained significantly associated with HS in the multivariate analysis after adjusting for age, sex, body mass index, smoking status and socioeconomic status.
Conclusions
Our study showed a higher prevalence of HS in the Northern Netherlands compared with the overall estimated prevalence of 1% and identified several new associated comorbidities.
What is already known about this topic?
The overall prevalence of hidradenitis suppurativa (HS) is approximately 1%; however, the exact prevalence remains unclear.
HS is associated with several systemic comorbidities, including other immune‐mediated inflammatory diseases such as Crohn disease, diabetes mellitus and rheumatoid diseases.
What does this study add?
The overall questionnaire‐based prevalence of HS was 2.1% (95% confidence interval 2.0–2.2), which indicates underdiagnosis of this debilitating skin disease.
New associations between HS and fibromyalgia, irritable bowel syndrome, chronic fatigue syndrome and migraine were identified.
Overall prevalence of HS in exact numbers remains unclear, however this study found an overall questionnaire‐based prevalence of HS of 2.1% [95% CI 2.0 to 2.2], which indicates underdiagnosis of this debilitating skin disease. Moreover, HS is associated with several systemic comorbidities, and in our cohort study new associations between HS and fibromyalgia, irritable bowel syndrome, chronic fatigue syndrome and migraine were identified.#10;

Linked Comment: N. Kearney and B. Kirby. Br J Dermatol 2022; 186:767–768.
Plain language summary available online
Hidradenitis suppurativa (HS) is a chronic auto‐inflammatory skin disease, with debilitating effects on the quality of life of patients. 1 Patients experience stigmatization and feelings of shame. 2 The long diagnostic delay of up to 10 years, in which the disease can progress, contributes to the burden of HS. 3 , 4 Furthermore, HS is associated with smoking and low socioeconomic status (SES). 5 , 6 HS has also been associated with several inflammatory comorbidities such as Crohn disease and spondyloarthropathies, and metabolic comorbidities including metabolic syndrome and diabetes mellitus, with a predominantly chronic nature. 7 , 8 , 9 , 10 , 11 , 12 Earlier diagnosis and initiation of treatment, including lifestyle interventions, could mitigate the burden for patients with HS and may benefit the healthcare system as well.
The prevalence of HS is estimated to be approximately 1% in the general population. However, reported prevalences vary widely from 0.02% 1 to 4.10%, 2 due to underlying differences in the studied populations and in the applied research methodologies. 13 , 14 Three methodological approaches have been used to estimate the prevalence of HS, including (i) registry‐based studies, where information is collected from national registry or insurance databases; (ii) hospital‐based studies, in which HS diagnosis is based on physical examination; and (iii) population‐based self‐reported studies. In registry‐based studies, the prevalence estimates can be confounded due to the immortal time, selection bias, misdiagnosis, incorrect registry and data management miscoding, and patients who were not covered by insurance. Hospital‐based studies are often confounded by selection bias, as only patients reaching the doctors at a specific hospital are included. Hence, these approaches are perhaps not the most suited for assessing the prevalence of HS in the general population, although this is disputed.
Population‐based self‐reported studies also have the chance of misdiagnosis and the potential to over‐represent diagnosis, and subsequently prevalence estimates. Nonetheless, population‐based studies are likely to be the most suitable method to assess HS prevalence, and to trace undiagnosed cases. Therefore, we employed the unique large population‐based Lifelines cohort study to determine the prevalence of HS in the general adult population in the Northern Netherlands. Additionally, we assessed the potential factors and comorbidities associated with HS.
Patients and methods
A nested cross‐sectional study within the framework of the Lifelines Cohort Study was performed, employing an add‐on study for which a digital questionnaire was developed consisting of HS‐related questions. 15 Participants were ascertained as having HS in two ways (Appendix S1; see Supporting Information). Firstly, if ‘yes’ was answered to the question ‘Did you ever (during your life) get the diagnosis hidradenitis suppurativa (HS)?’. Diagnoses could be made by a general practitioner, medical specialist or other type of physician, or HS could be self‐diagnosed. Later on, these cases will be referred to as ‘reported HS diagnosis’. Secondly, if ‘no’ was answered, participants were asked two validated questions introduced by Esmann et al. (validated in a cohort of 74 cases) and Vinding et al. (validated in a cohort of 30 cases) for self‐diagnosing HS (Appendix S1). 16 , 17 If ‘yes’ was answered to both questions, the participant was identified as a patient having HS.
For self‐diagnosing HS, the first question (‘Do you have painful, recurring abscesses or boils in your armpits, groin, buttocks or on other locations, as seen in the images below?’) has a sensitivity of 97% and specificity of 82%, with a positive predictive value of 85%. The second question (‘Did you have at least two outbreaks of abscesses or boils within a period of 6 months?’) has a sensitivity of 90%, a specificity of 97%, a positive predictive value of 96% and a negative predictive value of 92%. Additionally, images showing HS lesions corresponding to the three Hurley stages were shown to the participants, enabling potential patients to perform a self‐assessment of the presence and stage of HS (Figure S1; see Supporting Information). Moreover, participants’ demographic characteristics, disease characteristics, smoking status, SES and comorbidities were collected. Appendix S2 in the Supporting Information details the design, analysis, multiple imputations and regression analyses.
Results
Analysis set
Our questionnaire was sent to 135 950 adult Lifelines participants, of whom 58 198 filled out the our questionnaire, resulting in a response rate of 42.8%. Of the adult participants, 1356 respondents did not answer the HS‐related questions. Five pairs of patients were familial related, and one of each of these pairs was excluded, as we wished to include only randomly selected cases. This left 56 084 respondents for analysis (Figure 1).
Figure 1.
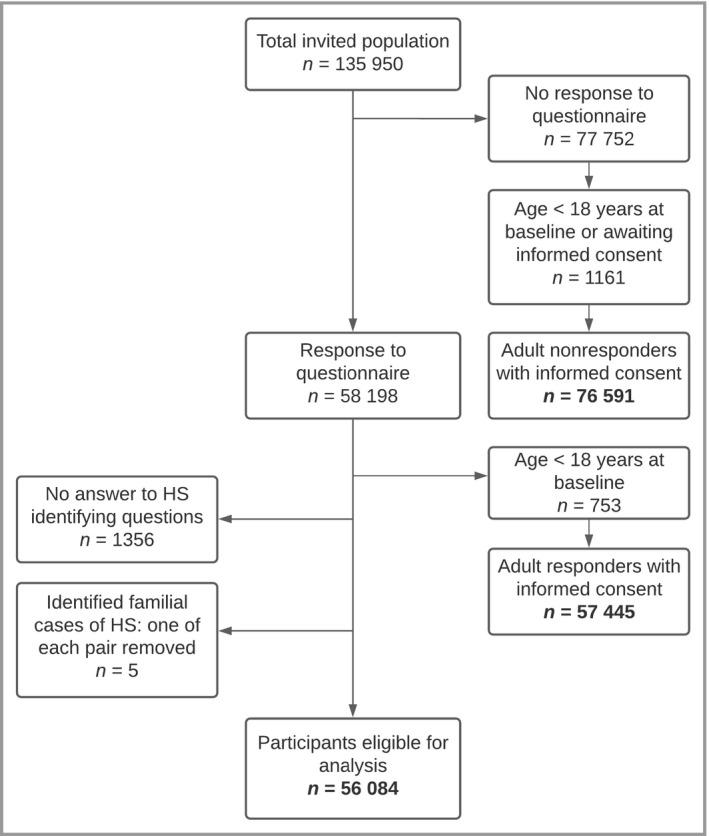
Flowchart demonstrating which participants with hidradenitis suppurativa (HS) were eligible for analysis.
Comparisons between baseline population characteristics in respondents and nonrespondents
For details on the comparisons of the total invited population to our questionnaire, see Table 1 and Appendix S3 (see Supporting Information).
Table 1.
Comparisons between respondents and nonrespondents in the total population invited to complete the self‐reported hidradenitis suppurativa questionnaire
| Total (n = 134 036) | Respondents (n = 57 445) | Nonrespondents (n = 76 591) | P‐valuec | |
|---|---|---|---|---|
| Sex, n (%)a | ||||
| Female | 78 451 (58.5) | 34 661 (60.3) | 43 790 (57.2) | < 0.001 |
| Male | 55 585 (41.5) | 22 784 (39.7) | 32 801 (42.8) | |
| Age (years), mean (SD) | 52.8 (12.5) | 55.8 (12.2) | 50.5 (12.3) | < 0.001 |
| Female | 52.3 (12.5) | 54.8 (12.1) | 50.3 (12.4) | < 0.001 |
| Male | 53.5 (12.6) | 57.3 (12.2) | 50.8 (12.2) | < 0.001 |
| Socioeconomic status,b mean (SD) | −0.62 (1.07) | −0.56 (1.06) | −0.66 (1.07) | < 0.001 |
| Smoking (last month), n (%)a, b | ||||
| No | 103 455 (78.9) | 47 164 (83.0) | 56 291 (75.7) | |
| Yes | 27 709 (21.1) | 9672 (17.0) | 18 037 (24.3) | < 0.001 |
| Missing | 2872 | 609 | 2263 | |
aThe first variable was used as the reference for analysis. bSelf‐reported. cAssociations with responder status.
Prevalence
In total, 448 respondents self‐reported having a diagnosis of HS. The combination of the two other diagnostic questions was positively answered by 708 respondents. This identified a total of 1156 prevalent cases of HS out of 56 084 respondents at the baseline, resulting in an overall prevalence of 2.1% [95% confidence interval (CI) 2.0–2.2] of HS. When we performed bootstrapping analysis, we observed an overall prevalence of HS of 2.1% (95% CI 1.8–2.4), which is similar to the prevalence obtained from the total cohort.
Of the respondents with HS, 73.5% (n = 850) were female, compared with 60.1% in the control group, leading to a prevalence of HS of 2.5% (95% CI 2.35–2.69) in women. In addition, 26.5% (306) of the cases of HS were male, leading to a prevalence of 1.3% (95% CI 1.17–1.47) in men, and a female‐to‐male ratio of 2.8.
When calculating with only the medically diagnosed cases of HS at the time of inclusion in the Lifelines cohort study, the prevalence of HS would be estimated as 0.80% (95% CI 0.73–0.88). Of those people with medically diagnosed cases of HS, 330 were female and 118 were male, with estimated prevalences of 1.0% (95% CI 0.88–1.09) and 0.5% (95% CI 0.42–0.61), respectively.
Subanalysis in participants with low socioeconomic status
The results of the subanalysis of participants with low SES and the estimated prevalence of HS are detailed in Appendix S3 (see Supporting Information).
Comparisons between participants with and without hidradenitis suppurativa
In total, 1156 nonfamilial, adult participants with HS at baseline were randomly matched by age group to 5000 population‐based controls. Univariate regression analysis showed that female sex was associated with increased risk of HS disease [odds ratio (OR) 1.84, 95% CI 1.60–2.13]. In the HS group, the mean (SD) age was 52.1 (11.8) years, compared with 56.0 (12.0) years for the control group. The HS respondents had a significantly lower SES than the control group (P < 0.001). Furthermore, almost one‐third of the HS group currently smoked (31.9%) or formerly smoked (31.9%), and both were significantly associated with HS (P ≤ 0.001). In the control group, 51.8% of respondents did not smoke (Table 2).
Table 2.
Characteristics of participants with hidradenitis suppurativa (HS)
| Total (n = 6156) | Self‐reported HS (n = 1156) | No HS (n = 5000)c | P‐valued | |
|---|---|---|---|---|
| Sex, n (%)a | ||||
| Female | 3855 (62.6) | 850 (73.5) | 3005 (60.1) | < 0.001 |
| Male | 2301 (37.4) | 306 (26.5) | 1995 (39.9) | |
| Age (years), mean (SD) | 55.2 (12.1) | 52.1 (11.8) | 56.0 (12.0) | < 0.001 |
| Female | 54.1 (12.0) | 50.9 (11.4) | 55.0 (12.1) | < 0.001 |
| Male | 57.2 (11.9) | 55.5 (12.3) | 57.4 (11.9) | 0.011 |
| Socioeconomic status,b mean (SD) | −0.57 (1.08) | −0.65 (1.11) | −0.55 (1.07) | 0.013 |
| Missing | 783 | 139 | 644 | |
| Smoking status, n (%)a, b | ||||
| No | 2428 (49.0) | 319 (36.1) | 2109 (51.8) | |
| Yes | 853 (17.2) | 282 (31.9) | 571 (14.0) | < 0.001 |
| Former | 1671 (33.7) | 282 (31.9) | 1389 (34.1) | 0.001 |
| Missing | 1204 | 273 | 931 | |
aThe first variable was used as the reference for analysis. bSelf‐reported. cRandomly matched controls. dAssociations with HS status, analysed via univariable analysis.
Characteristics of participants with hidradenitis suppurativa
The overall median age at onset of HS symptoms was 25.0 years [interquartile range (IQR) 17.8–40.0] (Table 3). The median disease duration was 22.0 years (IQR 11.0–33.0) for women and 19.0 years (IQR 8.0–34.0) for men. In women, the genitals were more frequently affected than in men (36.2% vs. 10.8%). In contrast, in men the anal region was affected in 31.4%, while in women 22.7% reported involvement.
Table 3.
Self‐reported characteristics of patients with hidradenitis suppurativa (HS), overall and by sex
| Total (n = 1156) | Female (n = 850) | Male (n = 306) | |
|---|---|---|---|
| Age at beginning of HS (years), median (IQR) | 25.0 (17.8–40.0) | 24.0 (16.0–38.0) | 30.0 (19.0–45.0) |
| Missing | 41 | 21 | 20 |
| Disease duration (years), median (IQR) | 21.0 (10.8–33.0) | 22.0 (11.0–33.0) | 19.0 (8.0–34.0) |
| Missing | 41 | 21 | 20 |
| Affected areas | |||
| Armpit(s) | 348 (30.1) | 272 (32.0) | 76 (24.8) |
| Under the breasts | 71 (6.1) | 70 (8.2) | < 10 (< 3.3) |
| Groin | 599 (51.8) | 509 (59.9) | 90 (29.4) |
| Sexual organs | 341 (29.5) | 308 (36.2) | 33 (10.8) |
| Anal region | 289 (25.0) | 193 (22.7) | 96 (31.4) |
| Other | 266 (23.0) | 128 (15.1) | 138 (45.1) |
| Self‐reported Hurley stage | |||
| Hurley I | 817 (72.0) | 602 (71.7) | 215 (73.1) |
| Hurley II | 249 (22.0) | 191 (22.7) | 58 (19.7) |
| Hurley III | 68 (6.0) | 47 (5.6) | 21 (7.1) |
| Missing | 22 | 10 | 12 |
| Disease course | |||
| Improvement | 468 (40.9) | 357 (42.3) | 111 (37.0) |
| Deterioration | 145 (12.7) | 104 (12.3) | 41 (13.7) |
| Not better or worse | 452 (39.5) | 329 (39.0) | 123 (41.0) |
| Remission | 38 (3.3) | 26 (3.1) | 12 (4.0) |
| Other | 41 (3.6) | 28 (3.3) | 13 (4.3) |
| Missing | 12 | < 10 | < 10 |
| Family members with HS | |||
| Yes | 110 (25.2) | 87 (26.9) | 23 (20.5) |
| No | 154 (35.3) | 116 (35.8) | 38 (33.9) |
| Do not know | 172 (39.4) | 121 (37.3) | 51 (45.5) |
| Missing | 720 | 526 | 194 |
| Diagnosed by | |||
| General practitioner | 204 (46.6) | 151 (46.5) | 53 (46.9) |
| Dermatologist | 154 (35.2) | 110 (33.8) | 44 (38.9) |
| Surgeon | 27 (6.2) | 20 (6.2) | 7 (6.2) |
| Plastic surgeon | < 10 (< 0.9) | < 10 (< 1.2) | < 10 (< 3.3) |
| Gynaecologist | < 10 (< 0.9) | < 10 (< 1.2) | < 10 (< 3.3) |
| Emergency room doctor | < 10 (< 0.9) | < 10 (< 1.2) | < 10 (< 3.3) |
| Patient | 32 (7.3) | 25 (7.7) | <10 (< 3.3) |
| Other | < 10 (< 0.9) | < 10 (< 1.2) | < 10 (< 3.3) |
| Missing | 718 | 525 | 193 |
The data are reported as n or n (%) unless stated otherwise. IQR, interquartile range.
Guided by pictures, 72.0% staged themselves as having mild disease (Hurley I), 22.0% as having Hurley II and 6.0% as having Hurley III. Participants had previously been treated by a general practitioner in 70.8% of cases or a dermatologist in 34.3% (Table 4). When looking at the disease course over time, 40.9% reported a decrease in HS symptoms. In 25.2% of participants a positive family history was reported. Participants with a reported HS diagnosis (448 of 1156) were diagnosed by a general practitioner in 46.6% of cases and by a dermatologist in 35.2%. At the time of completing the questionnaire, 49 participants (4.2%; 11.3% of those with data) were receiving treatment for their HS, of whom 30 were being treated by a dermatologist.
Table 4.
Treatment of hidradenitis suppurativa (HS), overall and by sex
| Total (n = 1156) | Female (n = 850) | Male (n = 306) | |
|---|---|---|---|
| Current treatment | |||
| Yes, by | 49 (11.3) | 36 (11.2) | 13 (11.6) |
| General practitioner | 18 (37) | 14 (39) | 4 (31) |
| Dermatologist | 30 (61) | 21 (58) | 9 (69) |
| Other specialist | < 10 (< 20) | < 10 (< 28) | < 10 (< 77) |
| No, reason | 384 (88.7) | 285 (88.8) | 99 (88.4) |
| HS in remission | 131 (34.1) | 90 (31.6) | 41 (41) |
| Currently no boils | 193 (50.3) | 153 (53.7) | 40 (40) |
| Medication has no effect | 32 (8.3) | 23 (8.1) | < 10 (< 10) |
| Other | 28 (7.3) | 20 (7.0) | 8 (8) |
| Missing | 723 | 529 | 194 |
| Treated in the past by | |||
| General practitioner | 819 (70.8) | 598 (70.4) | 221 (72.2) |
| Dermatologist | 397 (34.3) | 291 (34.2) | 106 (34.6) |
| Other specialists | 408 (35.3) | 328 (38.6) | 80 (26.1) |
| None | 206 (17.8) | 157 (18.5) | 49 (16.0) |
| Other | 15 (1.3) | 10 (1.2) | < 10 (< 3.3) |
All data are reported as n (%).
Comparisons between reported hidradenitis suppurativa (HS) diagnosis and self‐diagnosed HS
For details on the comparisons between participants with HS with a reported HS diagnosis vs. self‐diagnosed HS, see Table 5 and Appendix S3 (see Supporting Information).
Table 5.
Self‐reported characteristics of patients with hidradenitis suppurativa (HS), reported diagnosis vs. self‐diagnosis
| Total HS (n = 1156) | Reported HS diagnosis (n = 448) | Self‐diagnosed HS (n = 708) | P‐valuec | |
|---|---|---|---|---|
| Age at beginning of HS (years), median (IQR) | 25.0 (17.8–40.0) | 25.0 (16.0–40.0) | 25.0 (18.0–40.0) | 0.51 |
| Missing | 41 | 28 | 13 | |
| Disease duration (years), median (IQR) | 21.0 (10.8–33.0) | 24.0 (13.0–35.0) | 20.0 (9.0–32.0) | < 0.001 |
| Missing | 41 | 28 | 13 | |
| Affected areasa | ||||
| Armpit(s) | 348 (30.1) | 153 (34.1) | 195 (27.5) | |
| Under the breasts | 71 (6.1) | 39 (8.7) | 32 (4.5) | |
| Groin | 599 (51.8) | 233 (52.0) | 366 (51.7) | |
| Sexual organs | 341 (29.5) | 140 (31.3) | 201 (28.4) | |
| Anal region | 289 (25.0) | 102 (22.8) | 187 (26.4) | |
| Other | 266 (23.0) | 120 (26.8) | 146 (20.4) | |
| Self‐reported Hurley stageb | ||||
| Hurley I | 817 (72.0) | 313 (73.0) | 504 (71.5) | |
| Hurley II | 249 (22.0) | 86 (20.0) | 163 (23.1) | 0.28 |
| Hurley III | 68 (6.0) | 30 (7.0) | 38 (5.4) | 0.35 |
| Missing | 22 | 19 | < 10 | |
| Disease courseb | ||||
| Improvement | 468 (40.9) | 224 (51.3) | 244 (34.5) | |
| Deterioration | 145 (12.7) | 61 (14.0) | 84 (11.9) | 0.22 |
| Not better or worse | 452 (39.5) | 109 (24.9) | 343 (48.4) | < 0.001 |
| Remission | 38 (3.3) | 28 (6.4) | 10 (1.4) | 0.53 |
| Other | 41 (3.6) | 15 (3.4) | 26 (3.7) | 0.001 |
| Missing | 12 | 11 | < 10 | |
The data are reported as n or n (%) unless stated otherwise. aNo univariable analysis could be performed due to overlap of the answers. bThe first variable was used as the reference for univariable analysis. cAssociations with HS diagnosis, analysed via univariable analysis. IQR, interquartile range.
Comorbidities
Of the respondents, participants with HS were more likely to be obese (body mass index ≥ 30 kg m−2) than the control group (OR 2.02, 95% CI 1.70–2.40) (Table 6). In the HS group significantly more participants had skin diseases, including acne (OR 3.07, 95% CI 2.53–3.73), psoriasis (OR 2.34, 95% CI 1.93–2.84) and alopecia areata (OR 2.63, 95% CI 1.15–6.03), than in the control group. Furthermore, univariate regression analysis revealed significant associations between HS and diabetes mellitus type 2 (OR 1.87, 95% CI 1.18–3.00), rheumatoid arthritis (OR 1.56, 95% CI 1.06–2.29), fibromyalgia (OR 2.26, 95% CI 1.64–3.11), bladder dysfunction (for example cystitis) (OR 1.87, 95% CI 1.42–2.45), kidney disease (OR 1.70, 95% CI 1.08–2.69) and polycystic ovary syndrome (PCOS) (OR 2.29, 95% CI 1.48–3.55). As for lung diseases, both chronic obstructive pulmonary disease (COPD) and asthma were also significantly associated with HS status, with ORs of 1.74 (95% CI 1.35–2.23) and 1.44 (95% CI 1.17–1.77), respectively.
Table 6.
Comorbidities in patients with hidradenitis suppurativa (HS) vs. controls
| Demographic data, n (%) | Univariable analysis | Multivariable analysisc | |||||
|---|---|---|---|---|---|---|---|
| Total (n = 6156) | HS (n = 1156) | Not HS (n = 5000) | P‐valueb | OR (95% CI) | P‐valueb | OR (95% CI) | |
| BMI (kg m−2)a | |||||||
| ≤ 25 | 2632 (42.8) | 422 (36.5) | 2210 (44.2) | ||||
| 25–30 | 2476 (40.2) | 443 (38.3) | 2033 (40.7) | 0.079 | 1.14 (0.99–1.32) | ||
| ≥ 30 | 1048 (17.0) | 291 (25.2) | 757 (15.1) | < 0.001 | 2.02 (1.70–2.40) | ||
| Skin disorders | |||||||
| Acne | 491 (8.0) | 189 (16.3) | 302 (6.0) | < 0.001 | 3.07 (2.53–3.73) | < 0.001 | 3.13 (2.71–3.62) |
| Psoriasis | 532 (8.6) | 175 (15.1) | 357 (7.1) | < 0.001 | 2.34 (1.93–2.84) | < 0.001 | 2.37 (2.10–2.69) |
| Eczema | 655 (10.6) | 207 (17.9) | 448 (9.0) | 0.023 | 0.79 (0.65–0.97) | ||
| Alopecia areata | 24 (0.4) | < 10 (< 0.9) | 15 (0.3) | 0.022 | 2.63 (1.15–6.03) | ||
| Metabolic diseases | |||||||
| Diabetes type 2 | 161 (2.6) | 44 (3.8) | 117 (2.3) | 0.007 | 1.87 (1.18–3.00) | 0.005 | 2.66 (1.88–3.75) |
| Hypertension | 1359 (22.1) | 280 (24.2) | 1079 (21.6) | 0.045 | 1.17 (1.00–1.36) | ||
| Hypercholesterolaemia | 830 (13.5) | 139 (12.0) | 691 (13.8) | 0.17 | 1.15 (0.94–1.40) | ||
| Heart diseases | |||||||
| Heart failure | 61 (1.0) | 13 (1.1) | 48 (1.0) | 0.24 | 1.42 (0.79–2.55) | ||
| Heart attack | 56 (0.9) | < 10 (< 0.9) | 48 (1.0) | 0.39 | 0.72 (0.34–1.53) | ||
| Lung diseases | |||||||
| COPD | 331 (5.4) | 92 (8.0) | 239 (4.8) | < 0.001 | 1.74 (1.35–2.23) | 0.003 | 1.63 (1.38–1.92) |
| Asthma | 561 (9.1) | 136 (11.6) | 425 (8.5) | < 0.001 | 1.44 (1.17–1.77) | ||
| Gastrointestinal disorders | |||||||
| Crohn disease | 30 (0.5) | 12 (1.0) | 18 (0.4) | 0.011 | 2.69 (1.25–5.79) | ||
| Ulcerative colitis | 47 (0.8) | 8 (0.7) | 39 (0.8) | 0.59 | 0.80 (0.36–1.78) | ||
| Irritable bowel syndrome | 647 (10.5) | 176 (15.2) | 471 (9.4) | 0.003 | 1.63 (1.18–2.26) | ||
| Musculoskeletal disorders | |||||||
| Rheumatoid arthritis | 168 (2.7) | 44 (3.8) | 124 (2.5) | 0.026 | 1.56 (1.06–2.29) | ||
| Fibromyalgia | 261 (4.2) | 86 (7.4) | 175 (3.5) | < 0.001 | 2.26 (1.64–3.11) | 0.044 | 1.51 (1.23–1.86) |
| Neurological disorders | |||||||
| Migraine | 1286 (20.9) | 296 (25.6) | 990 (19.8) | 0.007 | 1.48 (1.11–1.96) | ||
| Chronic fatigue syndrome | 99 (1.6) | 30 (2.6) | 69 (1.4) | 0.028 | 1.72 (1.06–2.78) | 0.010 | 2.16 (1.19–3.90) |
| Urological disorders | |||||||
| Kidney disease | 93 (1.5) | 26 (2.2) | 67 (1.3) | 0.023 | 1.70 (1.08–2.69) | < 0.001 | 2.36 (1.69–3.30) |
| Bladder dysfunction | 268 (4.4) | 79 (6.8) | 189 (3.8) | < 0.001 | 1.87 (1.42–2.45) | 0.001 | 1.83 (1.54–2.17) |
| Gynaecological disorders | |||||||
| Polycystic ovary syndrome | 91 (1.5) | 31 (2.7) | 60 (1.2) | < 0.001 | 2.29 (1.48–3.55) | ||
| Mental disorders | |||||||
| Depression | 754 (12.2) | 228 (19.7) | 526 (10.5) | < 0.001 | 2.03 (1.43–2.79) | ||
| Anxiety | 386 (6.3) | 106 (9.2) | 280 (5.6) | 0.005 | 1.60 (1.15–2.22) | ||
| Malignancies of any kind | 390 (6.3) | 76 (6.6) | 314 (6.3) | 0.71 | 1.05 (0.81–1.36) | ||
Comorbidities were self‐reported except for body mass index (BMI). CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio. aBMI ≤ 25 kg m−2 was used as the reference for univariable and multivariable analysis. bAssociations with HS status. cCorrected for sex, age, BMI, smoking status and socioeconomic status. Multivariable data are shown only for significant results.
Crohn disease (OR 2.69, 95% CI 1.25–5.79) and irritable bowel syndrome (OR 1.63, 95% CI 1.18–2.26) were significantly more common in the participants with HS, while ulcerative colitis was negatively associated with HS disease (OR 0.80, 95% CI 0.36–1.78); however, the latter result was not significant. For neurological disorders, like migraine (OR 1.48, 95% CI 1.11–1.96) and chronic fatigue syndrome (OR 1.72, 95% CI 1.06–2.78), as well as mental disorders, like depression (OR 2.03, 95% CI 1.43–2.79) and anxiety (OR 1.60, 95% CI 1.15–2.22), significantly more patients in the HS group were affected than controls. Malignancies and the different subtypes of cancer were not significantly associated with HS (P = 0.71).
Several comorbidities remained significantly associated with HS in the multivariate model, such as acne (OR 3.13, 95% CI 2.71–3.62), chronic fatigue syndrome (OR 2.16, 95% CI 1.19–3.90) and fibromyalgia (OR 1.51, 95% CI 1.23–1.86) (Table 6).
Comorbidities compared between male and female patients with hidradenitis suppurativa
For details on the comparisons of comorbidities in male and female participants with HS, see Table S1 (see Supporting Information) and Appendix S3.
Discussion
In our study, we aimed to determine the prevalence of HS in the general adult population in the Northern Netherlands and to assess the potential factors and comorbidities associated with HS. We found an overall prevalence of 2.1% of HS in the general population of the Northern Netherlands. Moreover, we newly identified fibromyalgia, irritable bowel syndrome, chronic fatigue syndrome and migraine to be associated with HS.
As our results demonstrated an overall prevalence of 2.1% of HS in our Dutch cohort, we argue that the previously estimated prevalence of 1% is an underestimation of the actual prevalence, especially in Northern European countries. 14 , 18 , 19 Nonetheless, it should be taken into consideration that we determined the prevalence of self‐reported HS, which raised the risk of misdiagnoses and might result in an overestimated prevalence. However, taking into account that the Lifelines cohort consists largely of older participants, the actual prevalence might be higher, considering that HS generally appears around late puberty or early adulthood. 20 It should also be noted that the mean age of the HS cohort was quite high compared with the usual age of people with HS reported in large populations, 21 , 22 possibly due to the high age of the Lifelines cohort in general, which might have resulted in an underestimation of the HS prevalence as well.
When comparing the respondents and the nonrespondents to our questionnaire, the respondent group of participants was more likely to have higher SES and not to smoke. Again, this indicates that the overall found prevalence could still be an underestimation of the real prevalence of HS, as HS is associated with low SES and smoking in the past, and therefore our respondent group had a lower risk of developing HS. 23 , 24 Therefore, we reanalysed our data with participants with only low SES and indeed found a higher estimated prevalence of 2.4% of HS. Similarly, we found in our cohort associations between HS and low SES, and HS and smoking. However, the causality of both low SES and smoking and the association with HS could not be determined in this study because of the cross‐sectional design. For example, a low SES could be a consequence of the disease, but also a risk factor for the development of HS. It has been reported that HS is a female‐dominant disease as women outnumbered men with HS by nearly 3 to 1, 25 which is identical to our findings with a female‐to‐male ratio of 2.8.
Prior studies exploring the prevalence of HS based their study populations on selected groups. 23 , 24 , 26 Others used insurance or healthcare data, in which cases can be missed due to miscoding, uninsured patients and underdiagnosis, while self‐reported studies, like our study, have a higher chance of misdiagnosis. 13 , 19 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 In the current study, the prevalence of HS would be 0.80% using only previously diagnosed cases of HS. This indicates that HS is subject to underdiagnosis in the Netherlands, which could be due to feelings of shame consequently preventing patients from seeing a doctor or due to lack of recognition of HS by the treating physician. Nevertheless, in 2015 Blok et al. showed that only 19% of patients with HS were diagnosed by a general practitioner, while our current study demonstrates that 46.6% of the participants with HS were diagnosed by a general practitioner. 37 This suggests an increased awareness of HS among general practitioners, possibly due to improved knowledge of HS as a result of education and a prominent patient association.
Despite the above, as more than half of the identified cases of HS were self‐reported, we still have not reached the majority of patients with HS in the Northern Netherlands. Our results showed that participants who self‐diagnosed their HS had comparable ages at onset of disease and similar self‐reported severity of HS to the HS‐diagnosed participants. As the main characteristics of participants with self‐reported HS vs. diagnosed HS were similar, this indirectly validated once more the diagnostic questions for HS by Esmann et al. and Vinding et al. that we used in our study. 16 , 17 However, there were still some slight differences between the two groups that should be mentioned. The respondents with self‐diagnosed HS more frequently reported a stable course of HS and had a significantly shorter disease duration than the group with reported HS diagnosis: 20.0 years vs. 24.0 years, respectively (P < 0.001). Both stable HS disease and shorter disease duration could explain why no physician was consulted yet or no diagnosis was made. However, a median disease duration of 20.0 years is still a long time for not seeking medical care. As an earlier diagnosis could prevent progression of disease and could contribute to a lower burden of disease, increased awareness is needed for identifying the undiagnosed patients with HS.
We confirmed previously reported associated comorbidities, such as rheumatoid arthritis, depression and anxiety. 38 , 39 Our findings are also consistent with those of previous studies on the association between HS and Crohn disease, diabetes mellitus type 2 and PCOS. 40 , 41 , 42 In addition, COPD, asthma, kidney disease and bladder dysfunction (of any kind) were also associated with HS. 43 While occurrences of fistulas in the urinary tracts and bladder are mentioned in literature, this is unlikely the cause of any bladder dysfunction in our cohort. Hence, the majority classified themselves as having stage I disease, where sinus tracts have not yet developed. Interestingly, we identified new associations between HS and other diseases, such as fibromyalgia, chronic fatigue syndrome and migraine. Irritable bowel syndrome was also significantly more common in the HS group, which is a compelling finding as both irritable bowel syndrome and HS are associated with metabolic disease. 44 In addition, subanalysis stratifying the HS cohort based on sex showed significant associations, as more women with HS had irritable bowel syndrome and fibromyalgia than men.
In contrast to previous findings, our results from the total cohort showed that heart diseases and malignancies were not significantly associated with HS. 45 , 46 A possible explanation for this discrepancy could be that Egeberg et al. studied patients and controls in a hospital setting. 45 Moreover, Tannenbaum et al. did not assess the severity of HS in their study population. 46 Considering that HS was predominantly reported as mild in our population, this could be the reason for not finding associations with heart disease and malignancies among our cohort with HS.
Our study was limited by the self‐reported HS diagnosis. However, validated questions and images of HS were used to minimize the chance of false positive cases. Nevertheless, the validations of both questions by Esmann et al. and Vinding et al. took place in small cohorts of 74 cases and 30 cases, respectively, with similar number of noncases. 16 , 17 This could result in an overestimated positive predictive value, and in a higher chance of false‐positive identified cases of HS. In contrast, as the mean age of our cohort was relatively high, we could have missed cases of younger people with HS. However, as no significant differences between the groups with HS diagnosed by a medical practitioner and self‐diagnosed HS were found for most disease characteristics, it is convincing that the self‐diagnosed HS group could be considered representative for our investigated HS cohort.
To investigate the association of HS with comorbid conditions, we adjusted for a set of commonly known covariates. It was not our intention to study comorbidities specifically, as HS was used as the outcome, and not the comorbidities themselves. To explain in more detail: we studied the existence of associations between certain comorbidities and HS, adjusting for the most commonly known covariates. Nevertheless, we think it is very interesting to study additional covariates that may influence each of the comorbid conditions. However, to answer that specific research question, a different study design with comorbidity‐specific analyses must be used, which was not the focus of this study. For this study, data from the Lifelines baseline assessment were partially used, which were collected up to 10 years before our questionnaire was filled out. The respondent characteristics might have changed over time, which could have influenced our results. However, when possible, we used the most recent data available. Also, nonresponse was high for several different questions, which could interfere with the outcomes, as well as the lack of possibility to answer ‘no’ if a certain comorbidity was not present. The main strengths of our study are the very large sample size, the population‐based setting and results that are generalizable to Northern European countries. 47
In conclusion, our study demonstrates an overall prevalence of 2.1% of HS in the general population of the Northern Netherlands, indicating that underdiagnosis is still an issue in the Netherlands. As the majority of cases of HS were not diagnosed, this emphasizes the need for more awareness among physicians. Furthermore, we identified fibromyalgia, irritable bowel syndrome, chronic fatigue syndrome and migraine as new associated conditions with HS. HS appears to be associated with even more comorbidities, stressing the need for early diagnosis and initiation of treatment.
Author contributions
Lisette Maria Prens: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Klasiena Bouwman: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Lisa Troelstra: Data curation (equal); formal analysis (equal); investigation (equal). Errol P Prens: Conceptualization (equal); supervision (equal); writing – review and editing (equal). Behrooz Z Alizadeh: Supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Barbara Horvath: Conceptualization (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
Supporting information
Figure S1 Examples of Hurley stages I–III.
Appendix S1 Overview of questions used for identifying hidradenitis suppurativa status.
Appendix S2 Supplementary methods.
Appendix S3 Supplementary results.
Table S1 Comorbidities in female and male patients with hidradenitis suppurativa.
Video S1 Author video.
Funding sources Novartis Pharma B.V., project code CAIN457ANL01T. The Lifelines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport; the Dutch Ministry of Economic Affairs; the University Medical Center Groningen, University of Groningen; and the Northern Provinces of the Netherlands.
Conflicts of interest B.H. reports fees from Janssen‐Cilag (advisory boards, educational grants, consultations, investigator initiative studies), AbbVie (advisory boards, educational grants, consultations, investigator initiative studies), Novartis Pharma (advisory boards, consultations, investigator initiative studies), UCB Pharma (advisory boards, consultations), LEO Pharma (consultations), Solenne B.V. (investigator initiative studies), Celgene (consultations, investigator initiative studies), Akari Therapeutics (consultations, investigator initiative studies), Philips (consultation), Roche (consultation), Regeneron (consultation) and Sanofi (consultation), fees for which were paid to the institution. L.M.P., K.B., L.D.T., E.P.P. and B.Z.A. declare they have no conflicts of interest regarding this study.
Data availability statement The data that support the findings of this study are available from Lifelines. Restrictions apply to the availability of these data, which were used under licence for this study. Data are available from the authors with the permission of Lifelines.
L.M.P. and K.B. are joint first authors.
Plain language summary available online
References
- 1. Sabat R, Jemec GBE, Matusiak Ł et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020; 6:18. [DOI] [PubMed] [Google Scholar]
- 2. Matusiak Ł. Profound consequences of hidradenitis suppurativa: a review. Br J Dermatol 2020; 183:171–7. [DOI] [PubMed] [Google Scholar]
- 3. Saunte DM, Boer J, Stratigos A et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015; 173:1546–9. [DOI] [PubMed] [Google Scholar]
- 4. Garg A, Neuren E, Cha D et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol 2020; 82:366–76. [DOI] [PubMed] [Google Scholar]
- 5. Deckers IE, Janse IC, van der Zee HH et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross‐sectional reference study. J Am Acad Dermatol 2016; 75:755–9. [DOI] [PubMed] [Google Scholar]
- 6. Garg A, Papagermanos V, Midura M, Strunk A. Incidence of hidradenitis suppurativa among tobacco smokers: a population‐based retrospective analysis in the U.S.A. Br J Dermatol 2018; 178:709–14. [DOI] [PubMed] [Google Scholar]
- 7. Janse IC, Koldijk MJ, Spekhorst LM et al. Identification of clinical and genetic parameters associated with hidradenitis suppurativa in inflammatory bowel disease. Inflamm Bowel Dis 2016; 22:106–13. [DOI] [PubMed] [Google Scholar]
- 8. Deckers IE, Benhadou F, Koldijk MJ et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross‐sectional study. J Am Acad Dermatol 2017; 76:49–53. [DOI] [PubMed] [Google Scholar]
- 9. Rondags A, van Straalen KR, Arends S et al. High prevalence of clinical spondyloarthritis features in patients with hidradenitis suppurativa. J Am Acad Dermatol 2019; 80:551–4. [DOI] [PubMed] [Google Scholar]
- 10. Rondags A, Arends S, Wink FR et al. High prevalence of hidradenitis suppurativa symptoms in axial spondyloarthritis patients: a possible new extra‐articular manifestation. Semin Arthritis Rheum 2019; 48:611–17. [DOI] [PubMed] [Google Scholar]
- 11. Garg A, Birabaharan M, Strunk A. Prevalence of type 2 diabetes mellitus among patients with hidradenitis suppurativa in the United States. J Am Acad Dermatol 2018; 79:71–6. [DOI] [PubMed] [Google Scholar]
- 12. Garg A, Hundal J, Strunk A. Overall and subgroup prevalence of Crohn disease among patients with hidradenitis suppurativa: a population‐based analysis in the United States. JAMA Dermatol 2018; 154:814–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zimman S, Comparatore MV, Vulcano AFF et al. Hidradenitis suppurativa: estimated prevalence, clinical features, concomitant conditions, and diagnostic delay in a university teaching hospital in Buenos Aires, Argentina. Actas Dermosifiliogr 2019; 110:297–302. [DOI] [PubMed] [Google Scholar]
- 14. Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996; 35:191–4. [DOI] [PubMed] [Google Scholar]
- 15. Scholtens S, Smidt N, Swertz MA et al. Cohort profile: LifeLines, a three‐generation cohort study and biobank. Int J Epidemiol 2015; 44:1172–80. [DOI] [PubMed] [Google Scholar]
- 16. Esmann S, Dufour DN, Jemec GBE. Questionnaire‐based diagnosis of hidradenitis suppurativa: specificity, sensitivity and positive predictive value of specific diagnostic questions. Br J Dermatol 2010; 163:102–6. [DOI] [PubMed] [Google Scholar]
- 17. Vinding GR, Miller IM, Zarchi K et al. The prevalence of inverse recurrent suppuration: a population‐based study of possible hidradenitis suppurativa. Br J Dermatol 2014; 170:884–9. [DOI] [PubMed] [Google Scholar]
- 18. Revuz JE, Canoui‐Poitrine F, Wolkenstein P et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case–control studies. J Am Acad Dermatol 2008; 59:596–601. [DOI] [PubMed] [Google Scholar]
- 19. Ingram JR, Collins H, Atkinson MD, Brooks CJ. The prevalence of hidradenitis suppurativa is shown by the Secure Anonymised Information Linkage (SAIL) Databank to be one per cent of the population of Wales. Br J Dermatol 2020; 183:950–2. [DOI] [PubMed] [Google Scholar]
- 20. Albares MP, Belinchón I, Ramos JM et al. Epidemiologic study of skin diseases among immigrants in Alicante, Spain. Actas Dermosifiliogr 2012; 103:214–22. [DOI] [PubMed] [Google Scholar]
- 21. Calao M, Wilson JL, Spelman L et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population‐based cross‐sectional study. PLOS ONE 2018; 13:e0200683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shalom G, Freud T, Harman‐Boehm I et al. Hidradenitis suppurativa and metabolic syndrome: a comparative cross‐sectional study of 3207 patients. Br J Dermatol 2015; 173:464–70. [DOI] [PubMed] [Google Scholar]
- 23. Delany E, Gormley G, Hughes R et al. A cross‐sectional epidemiological study of hidradenitis suppurativa in an Irish population (SHIP). J Eur Acad Dermatol Venereol 2018; 32:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mintoff D, Camilleri L, Aquilina S et al. Prevalence of hidradenitis suppurativa in Malta: comparison with established epidemiological data. Clin Exp Dermatol 2020; 45:758–9. [DOI] [PubMed] [Google Scholar]
- 25. Garg A, Kirby JS, Lavian J et al. Sex‐ and age‐adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol 2017; 153:760–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fania L, Ricci F, Sampogna F et al. Prevalence and incidence of hidradenitis suppurativa: an exercise on indirect estimation from psoriasis data. J Eur Acad Dermatol Venereol 2017; 31:e410–11. [DOI] [PubMed] [Google Scholar]
- 27. Ingram JR, Jenkins‐Jones S, Knipe DW et al. Population‐based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018; 178:917–24. [DOI] [PubMed] [Google Scholar]
- 28. Shlyankevich J, Chen AJ, Kim GE, Kimball AB. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart‐verified case–control analysis. J Am Acad Dermatol 2014; 71:1144–50. [DOI] [PubMed] [Google Scholar]
- 29. Killasli H, Sartorius K, Emtestam L, Svensson Å. Hidradenitis suppurativa in Sweden: a registry‐based cross‐sectional study of 13,538 patients. Dermatology 2020; 236:281–8. [DOI] [PubMed] [Google Scholar]
- 30. Lee JH, Kwon HS, Jung HM et al. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: a nationwide population‐based study. J Eur Acad Dermatol Venereol 2018; 32:1784–90. [DOI] [PubMed] [Google Scholar]
- 31. Cosmatos I, Matcho A, Weinstein R et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol 2013; 68:412–19. [DOI] [PubMed] [Google Scholar]
- 32. Shahi V, Alikhan A, Vazquez BG et al. Prevalence of hidradenitis suppurativa: a population‐based study in Olmsted County, Minnesota. Dermatology 2014; 229:154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marvel J, Vlahiotis A, Sainski‐Nguyen A et al. Disease burden and cost of hidradenitis suppurativa: a retrospective examination of US administrative claims data. BMJ Open 2019; 9:e030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirsten N, Petersen J, Hagenström K, Augustin M. Epidemiology of hidradenitis suppurativa in Germany – an observational cohort study based on a multisource approach. J Eur Acad Dermatology Venereol 2020; 34:174–9. [DOI] [PubMed] [Google Scholar]
- 35. Shalom G, Babaev M, Freud T et al. Demographic and health care service utilization by 4417 patients with hidradenitis suppurativa. J Am Acad Dermatol 2017; 77:1047–52. [DOI] [PubMed] [Google Scholar]
- 36. Sung S, Kimball AB. Counterpoint: analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol 2013; 69:818–19. [DOI] [PubMed] [Google Scholar]
- 37. Blok JL, Boersma M, Terra JB et al. Surgery under general anaesthesia in severe hidradenitis suppurativa: a study of 363 primary operations in 113 patients. J Eur Acad Dermatol Venereol 2015; 29:1590–7. [DOI] [PubMed] [Google Scholar]
- 38. Cugno M, Gualtierotti R, Meroni PL, Marzano AV. Inflammatory joint disorders and neutrophilic dermatoses: a comprehensive review. Clin Rev Allergy Immunol 2018; 54:269–81. [DOI] [PubMed] [Google Scholar]
- 39. Machado MO, Stergiopoulos V, Maes M et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta‐analysis. JAMA Dermatol 2019; 155:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen WT, Chi CC. Association of hidradenitis suppurativa with inflammatory bowel disease: a systematic review and meta‐analysis. JAMA Dermatol 2019; 155:1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phan K, Charlton O, Smith SD. Hidradenitis suppurativa and metabolic syndrome – systematic review and adjusted meta‐analysis. Int J Dermatol 2019; 58:1112–17. [DOI] [PubMed] [Google Scholar]
- 42. Phan K, Charlton O, Smith SD. Hidradenitis suppurativa and polycystic ovarian syndrome: systematic review and meta‐analysis. Australas J Dermatol 2020; 61:e28–33. [DOI] [PubMed] [Google Scholar]
- 43. Magun R, Shalom G, Cohen AD. Hidradenitis suppurativa and lung diseases: a study of 3207 patients. J Am Acad Dermatol 2016; 74 (Suppl. 1):AB55. [Google Scholar]
- 44. Boda D. Tackling key immunological and immuno‐dermatological pathways and their link to treatment options. Exp Ther Med 2020; 20:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all‐cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol 2016; 152:429–34. [DOI] [PubMed] [Google Scholar]
- 46. Tannenbaum R, Strunk A, Garg A. Association between hidradenitis suppurativa and lymphoma. JAMA Dermatol 2019; 155:624–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klijs B, Scholtens S, Mandemakers JJ et al. Representativeness of the LifeLines cohort study. PLOS ONE 2015; 10:e0137203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Examples of Hurley stages I–III.
Appendix S1 Overview of questions used for identifying hidradenitis suppurativa status.
Appendix S2 Supplementary methods.
Appendix S3 Supplementary results.
Table S1 Comorbidities in female and male patients with hidradenitis suppurativa.
Video S1 Author video.


