Abstract
Objective
This study aimed to determine whether chronic metformin use interferes with the improvements in insulin resistance (IR) and cardiorespiratory fitness with aerobic training in people with hyperglycemia and metabolic syndrome (MetS).
Methods
A total of 63 middle‐aged (53 [7] years) individuals with MetS and obesity (BMI = 32.8 [4.5] kg/m2) completed 16 weeks of supervised high‐intensity interval training (3 d/wk, 43 min/session). Participants were either taking metformin (EXER+MET; n = 29) or were free of any pharmacological treatment for their MetS factors (EXER; n = 34). Groups were similar in their initial cardiorespiratory fitness (maximal oxygen uptake [VO2MAX]), age, percentage of women, BMI, and MetS factors (z score). The effects of exercise training on IR (homeostatic model assessment of insulin resistance [HOMA‐IR]), MetS z score, VO2MAX, maximal fat oxidation during exercise, and maximal aerobic power output were measured.
Results
Fasting insulin and HOMA‐IR decreased similarly in both groups with training (EXER+MET: −4.3% and −10.6%; EXER: −5.3% and −14.5%; p value for time = 0.005). However, metformin use reduced VO2MAX improvements by half (i.e., EXER+MET: 12.7%; EXER: 25.3%; p value for time × group = 0.012). Maximal fat oxidation during exercise increased similarly in both groups (EXER+MET: 20.7%; EXER: 25.3%; p value for time = 0.040). VO2MAX gains were not associated with HOMA‐IR reductions (EXER+MET: r = −0.098; p = 0.580; EXER: r = −0.255; p = 0.182).
Conclusions
Metformin use was associated with attenuated VO2MAX improvements but did not affect fasting IR reductions with aerobic training in individuals with hyperglycemia and high cardiovascular risk (i.e., MetS).
Study Importance.
What is already known?
-
►
Exercise and metformin are first‐line therapeutic options for the treatment of prediabetes and type 2 diabetes.
-
►
Nevertheless, there is evidence of interfering effects of metformin on the insulin‐sensitizing and cardiorespiratory fitness improvements induced by exercise training.
What does this study add?
-
►
Chronic metformin treatment was associated with blunted gains in maximal oxygen uptake (VO2MAX) with intense aerobic exercise training in people with metabolic syndrome and hyperglycemia.
-
►
However, the associated blunted VO2MAX development did not prevent exercise‐induced improvements in fasting insulin resistance.
How might these results change the focus of clinical practice?
-
►
Although fitness improvements may be blunted by the chronic use of metformin in people with metabolic syndrome, this does not hinder the clinical benefits of exercise training on reducing fasting insulin resistance; therefore, clinical practice should include exercise advice.
INTRODUCTION
Metabolic syndrome (MetS) is characterized by central obesity, dyslipidemia, hypertension, and hyperglycemia (1), which increase cardiovascular disease risk and all‐cause mortality (2). Some authors have supported insulin resistance (IR) as the triggering factor for MetS (3, 4). IR by itself is a predictor of disease, disability, and all‐cause mortality (5). On the other hand, longitudinal studies have shown that moderate and high levels of cardiorespiratory fitness ([CRF] assessed by maximal oxygen uptake [VO2MAX]) confer protection against developing type 2 diabetes mellitus and its related hyperglycemia (6, 7). It has been shown that aerobic exercise training that improves CRF also reduces IR (8). However, the concomitant loss of body weight with exercise training may be partially responsible for the IR improvements (6, 9). Body weight loss, and specifically visceral abdominal fat loss, reduces cardiovascular risk (10); therefore diet, in conjunction with aerobic exercise, is recommended to regain glycemic control in people with IR or hyperglycemia (11).
Metformin (i.e., 1, 1‐dimethyl‐biguanide) is the most‐prescribed oral medicine in the world to reduce hyperglycemia. The primary target tissue of metformin is the liver, but it also influences metabolic processes in skeletal muscle, adipose tissue, the intestines, the brain, and the cardiovascular system (12). Although the main metabolic effect of metformin may be a reduction in hepatic glucose production by inhibiting gluconeogenesis (13), other authors have suggested that metformin also increases skeletal muscle glucose uptake (14). Metformin has been found to reduce mitochondrial respiration by inhibiting complex I of the electron transport chain (ETC) (15), which could result in energy unbalance. Through this mechanism, but also independently of the ETC, metformin activates AMP‐activated protein kinase (AMPK) (16), which phosphorylates and inhibits the mechanistic target of rapamycin complex 1 (mTORC1). AMPK and mTORC1 are major cellular regulators of lipid and glucose metabolism (17); therefore, inhibition of mTORC1 could mediate the stimulation of carbohydrate metabolism with metformin.
Metformin inhibition of ETC complex I could limit the development of mitochondrial respiration with exercise training. It has been reported that metformin treatment blunts the exercise training improvements in VO2MAX in young adults (18), older adults (19), and adults with prediabetes (20). Furthermore, Konopka et al. (19) found that metformin completely abolishes the exercise‐induced improvements in mitochondrial respiration (i.e., complex I‐linked respiration). Given the strong association between low CRF and IR (6), it is possible that training that increases VO2MAX could alleviate IR. On the other hand, metformin treatment could limit training increases in VO2MAX and, subsequently, improvements in IR with training (18, 19, 20, 21). Conversely, if metformin actions on improving glucose metabolism are based on limiting mitochondrial function, the potentiation of mitochondrial development with exercise training may blunt metformin actions on glucose metabolism.
To our knowledge, there is only one study addressing the effects of metformin on training adaptations and MetS risk factors. In that study, 32 adults with glucose intolerance were separated into four groups (22), with two groups taking metformin and two groups completing exercise training for 12 weeks using continuous aerobic and resistance training. In this study, the groups taking metformin experienced initial body weight loss (4 kg), something that is typical when metformin prescription is first started. That body weight loss may have influenced the comparison between the groups that exercise trained with or without metformin. In this study, we propose to study the interactions between exercise training (specifically, high‐intensity interval training [HIIT]) and metformin in a sample of individuals chronically medicated with metformin. In this way, we will avoid the confusing effect of metformin initially lowering body weight. Our hypothesis is that chronic metformin treatment would restrain the VO2MAX increases induced by 16 weeks of HIIT and, in parallel, the expected reductions in IR in people with hyperglycemia and MetS.
METHODS
Participants
A total of 63 middle‐aged (53 [7] years) volunteers (31 women and 32 men with overweight and obesity [BMI = 32.8 (4.5) kg/m2] and MetS) completed the study. MetS was defined as the presence of three of the following five risk factors: elevated waist circumference (≥94 cm for men and ≥80 cm for women); elevated blood pressure (≥130 mm Hg for systolic and/or ≥85 mm Hg for diastolic); elevated fasting blood glucose (≥100 mg/dL); elevated triglycerides (TG; ≥150 mg/dL); and reduced high‐density lipoprotein cholesterol (HDL‐c; ≤40 mg/L for men and ≤50 mg/dL for women) (1). Participants were previously sedentary, as assessed by a 7‐day International Physical Activity Questionnaire (IPAQ) (23), with less than 120 min/wk of moderate‐intensity activity (24). Exclusion criteria included the following: untreated cardiovascular disease (including severe hypertension, defined as systolic blood pressure ≥ 180 mm Hg and/or diastolic blood pressure ≥ 120 mm Hg); renal disease; or any disease associated with exercise intolerance. All individuals provided written, witnessed, informed consent in accordance with a protocol approved by the local Virgen de la Salud Hospital’s Ethics Committee of Toledo (reference #170) and according to the Declaration of Helsinki.
Experimental design
This was a prospective intervention (exercise training) parallel group study (treated or untreated with metformin). The exercise training intervention took place from early October 2019 to February 2020. Investigators were blinded to group allocation during data collection and analysis. Volunteers were recruited and screened, and they completed the treatment and testing in the order presented in Figure 1, in compliance with Consolidated Standards of Reporting Trials (CONSORT). A total of 34 individuals with MetS under chronic metformin treatment (>6 months of treatment, 1,279 [489] mg/d) composed the metformin group (EXER+MET). Another 29 individuals with MetS who were not taking metformin, or any other pharmacological treatment, composed the control group (EXER).
FIGURE 1.
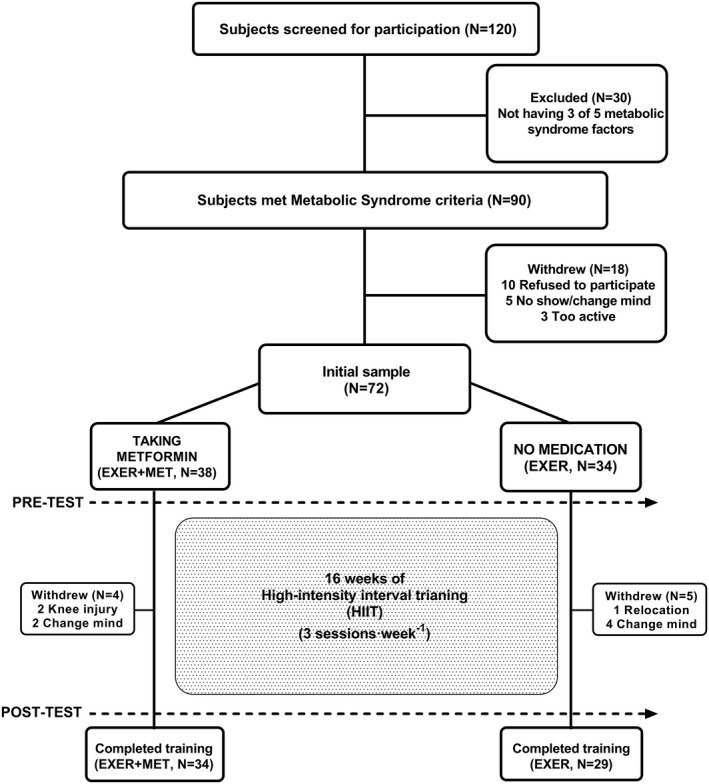
CONSORT (Consolidated Standards of Reporting Trials) schematic representation of the study procedures
At baseline and after 4 months of HIIT, participants attended the laboratory in the morning (7 am to 9 am) after 8 to 10 hours of overnight fasting and 48 hours after the last training bout. Participants were instructed to maintain their usual medication (i.e., EXER+MET group), physical activity, and dietary nutrition patterns during the duration of the study. Participants were not requested to record their diet because we were concerned that this might impact individuals’ normal feeding pattern through a Hawthorne effect (25), potentially confounding the effects of exercise training on body composition assessment.
Exercise intervention
Participants completed 16 weeks of a supervised HIIT cycling program. HIIT sessions were composed of four 4‐minute intervals at 90% of maximum heart rate (HRMAX) interspersed with 3‐minute active recovery at 70% HRMAX plus warm‐up and cooldown periods (26) for a total of 43 minutes. During all training sessions, HR was continuously displayed on a large screen (Seego Realtrack Systems, Almeria, Spain), and participants self‐adjusted the workload to reach their individually prescribed percentage of HRMAX. Monthly, during a regular training session, HRMAX was reevaluated, and training workloads were adjusted accordingly to maintain training stimulus (26). The training monitoring program (Seego Realtrack Systems) recorded the time that individuals were in the target HR zone (i.e., 70%‐90% HRMAX) in each exercise session.
Clinical investigation
Before and after 16 weeks of training, participants arrived at the laboratory in the morning, well hydrated and after an overnight fast. Nude body weight (Hawk; Mettler Toledo, Columbus, Ohio), height (stadiometer; Seca 217; Seca GmbH, Hamburg, Germany), and waist circumference were assessed by the same researcher. Body composition was analyzed by bioelectrical impedance analysis (Tanita BC‐418; Tanita Corp., Tokyo, Japan), and sectional analysis of right‐leg fat‐free mass (FFM) was used as an indirect measure of muscle gains with cycling HIIT. After 20 minutes of supine rest on a gurney, brachial resting blood pressure was measured after 15 minutes of supine rest using an electrocardiography (ECG) gated electro‐sphygmomanometer (Tango; SunTech Medical Inc., Morrisville, North Carolina) as the average of three measurements. Afterward, 5 mL of blood was drawn from an antecubital vein for the determination of glucose, insulin, and lipids (i.e., TG and HDL‐c). Last, metabolic and CRF assessments were carried out. After training, all testing was repeated 48 hours after the last training session to measure the chronic effects rather than the acute effects of the last bout of training.
Metabolic fitness and CRF
Maximal fat oxidation rate (FOMAX) was assessed using a submaximal graded exercise test (GXT) on a calibrated cycle ergometer with breath‐by‐breath indirect calorimetry (Quark b2; COSMED srl, Rome, Italy) monitoring. Initial power output was set at 10 W for women and 30 W for men and was increased 10 or 15 W every 4 minutes for women and men, respectively (27). The last minute of each stage was averaged to calculate nonprotein respiratory quotient and fat oxidation rate (28). The FOMAX test terminated when respiratory exchange ratio surpassed 1.0.
After resting and hydration, VO2MAX was assessed using GXT, starting at 30 W for women and 50 W for men, with increases of 15 or 20 W for women and men, respectively, until volitional exhaustion. After a cooldown and 15 minutes of passive rest, a short (1‐3 minutes) verification test was performed at 110% of the maximum workload (POMAX) reached during the GXT (29). During tests, a 12‐lead standard ECG was continuously monitored (Quark T12, COSMED). The highest values of VO2 and HR during GXT or verification test were determined as VO2MAX and HRMAX (29).
Blood analysis
Plasma glucose was analyzed using the glucose oxidase peroxidase method, with intra‐interassay coefficient of variation (iCV) of 0.9% to 1.2%. HDL‐c was analyzed using the accelerator selective detergent method (iCV = 1.7%‐2.9%). TG was analyzed with the glycerol‐3‐phosphate oxidize method (iCV = 0.8%‐1.7%). All the analyses were run in an automated Mindray BS 400 Chemistry Analyzer (Mindray Medical Instrumentation Ltd., Shenzhen, China). Insulin concentration was measured in duplicate using chemoluminescent microparticle immunoassay (iCV = 2.0%‐2.8%) in an automated analyzer (Architect ci4100; Abbott Laboratories, Abbott Park, Illinois). IR was calculated using the homeostatic model assessment of IR (HOMA‐IR) (30), following the proposed criteria of metabolic dysfunction‐associated fatty liver disease (HOMA‐IR ≥ 2.5) (31).
MetS z score
Sex‐specific MetS z score provided information on the continuous evolution of MetS risk factors with the treatments. The sum of the z score for each MetS component was divided by five to compile the MetS risk score with units of standard deviation (SD) (32). The equations used to calculate MetS z score were the following:
| (1) |
| (2) |
Statistical analysis
Per‐protocol analysis was used, and only individuals who completed the protocol were included in the statistical analysis. A Kolmogorov–Smirnov test revealed that all variables showed a normal distribution except for TG, HDL‐c, HOMA‐IR, glucose, and insulin. Those variables were log‐transformed for statistical analysis. Data are reported as mean (SD). Data in figures are mean (SEM) for easier display. Additionally, 95% CI was calculated. At baseline, variables were compared between groups with unpaired Student t test. Mixed‐design ANOVA with baseline HOMA‐IR as a covariate was used to detect differences within and between groups before and after training. Mixed‐design ANOVA was used to detect differences on IR variables within and between groups before and after training. When a time by group interaction existed, a Bonferroni post hoc test was performed to identify the time point at which groups significantly differed. A McNemar test was used to evaluate the prevalence of MetS factors. Pearson coefficients of correlation (r) were conducted to test the association among measured variables. All statistical procedures were run on SPSS version 24 (IBM Corp., Armonk, New York) with statistical significance set at p ≤ 0.05.
RESULTS
Baseline participant characteristics
Participants were White individuals living in Southern Europe, with similar age between groups (EXER+MET: 54 [6] years and EXER: 51 [8] years; p = 0.189). At baseline, EXER+MET and EXER groups were similar in body weight (86.8 [13.6] kg and 90.1 [15.4] kg, respectively; p = 0.392), number of MetS factors (3.9 [0.9] and 3.6 [0.6], respectively; p = 0.106), percentage of women in each group (50% and 48%, respectively; p = 0.891), and CRF (VO2MAX: 22.2 [5.3] mL/min/kg and 21.1 [4.3] mL/min/kg, respectively; p = 0.351). Data were analyzed without sex differentiation because all women were postmenopausal and were not taking hormonal replacement, and their responses did not differ from men’s responses. All participants in the EXER+MET group maintained their dose of metformin treatment during the 4‐month intervention (i.e., average of 1,279 [489] mg/d). A similar number of individuals, with characteristics similar to their respective groups (number and MetS factors z score), withdrew from the experiment in each group (Figure 1). Therefore, the statistical analysis was performed per protocol. The training monitoring program (Seego Realtrack Systems) revealed similar fulfillment of the training target HR and similar numbers of minutes trained between groups (p = 0.731 and p = 0.752, respectively). As expected, groups differed in their basal glucose concentration and HOMA‐IR, which were higher in the EXER+MET group. In contrast, mean arterial pressure (MAP) and systolic and diastolic blood pressure were initially higher in the EXER group than in the EXER+MET group (102.4 [10.0] mm Hg and 95.3 [11.0] mm Hg, 126 [16] mm HG and 135 [12] mm Hg, and 80 [11] mm Hg and 86 [10] mm Hg, respectively; p = 0.009, p = 0.016, and p = 0.018; Table 1).
TABLE 1.
Changes in body composition and MetS factors with a 16‐week HIIT program
| EXER+MET | EXER | Baseline | Time | Time × group | |||
|---|---|---|---|---|---|---|---|
| Baseline | 16 weeks | Baseline | 16 weeks | p value | p value | p value | |
| Age (y) | 54 ± 6 | 51 ± 8 | 0.189 | ||||
| Women (%) | 50 | 48 | 0.891 | ||||
| Body weight (kg) | 86.8 ± 13.6 | 85.5 ± 12.6 | 90.1 ± 15.4 | 88.3 ± 14.6 | 0.364 | 0.907 | 0.191 |
| BMI (kg/m2) | 32.5 ± 5.2 | 32.0 ± 5.2 | 33.1 ± 3.5 | 32.5 ± 3.4 | 0.585 | 0.833 | 0.163 |
| Fat mass (kg) | 31.9 ± 7.9 | 31.0 ± 7.8 | 33.9 ± 7.0 | 33.3 ± 7.0 | 0.286 | 0.203 | 0.606 |
| FFM (kg) | 54.9 ± 9.9 | 54.6 ± 9.7 | 56.2 ± 11.3 | 55.1 ± 11.3 | 0.635 | 0.368 | 0.771 |
| Right‐leg FFM (kg) | 10.2 ± 3.5 | 10.1 ± 3.3 | 9.8 ± 3.0 | 9.6 ± 2.8 | 0.655 | 0.204 | 0.626 |
| Waist circumference (cm) | 106.5 ± 9.8 | 103.9 ± 9.8 | 106.3 ± 10.7 | 103.9 ± 10.0 | 0.926 | 0.372 | 0.717 |
| (MetS prevalence) | (100%) | (100%) | (100%) | (97%) | |||
| Glucose (mg/dL) | 152.3 ± 47.3 | 145.3 ± 43.2 | 104.4 ± 10.1 | 103.2 ± 17.3 | <0.001 | 0.344 | 0.452 |
| (MetS prevalence) | (100%) | (100%) | (72%) | (59%) | |||
| Triglycerides (mg/dL) | 142.2 ± 85.5 | 138.3 ± 75.5 | 148.8 ± 83.6 | 138.7 ± 74.7 | 0.758 | 0.356 | 0.685 |
| (MetS prevalence) | (30%) | (27%) | (41%) | (34%) | |||
| HDL‐c (mg/dL) | 43.7 ± 11.1 | 44.2 ± 10.4 | 44.0 ± 12.8 | 43.9 ± 10.5 | 0.925 | 0.988 | 0.992 |
| (MetS prevalence) | (64%) | (55%) | (69%) | (62%) | |||
| Mean arterial pressure (mm Hg) | 95.3 ± 11.0 a | 91.3 ± 10.7 b | 102.4 ± 10.0 | 93.8 ± 8.9 b | 0.009 | 0.005 | 0.021 |
| (MetS prevalence) | (45%) | (30%) | (76%) | (41%) b | |||
| Systolic blood pressure (mm Hg) | 126 ± 16 a | 121 ± 14 b | 135 ± 12 | 124 ± 13 b | 0.016 | 0.019 | 0.027 |
| Diastolic blood pressure (mm Hg) | 80 ± 11 a | 76 ± 10 | 86 ± 10 | 78 ± 8 | 0.018 | 0.013 | 0.092 |
| MetS z score | 0.62 ± 0.65 | 0.43 ± 0.74 | 0.44 ± 0.43 | 0.19 ± 0.47 | 0.218 | 0.673 | 0.273 |
| MetS factors | 3.9 ± 0.9 | 3.7 ± 0.9 | 3.6 ± 0.6 | 3.1 ± 1.1 | 0.106 | 0.156 | 0.264 |
Data are presented as mean ± SD for 63 patients with MetS divided into the EXER+MET and EXER groups. Baseline HOMA‐IR was used as a covariate in the statistical analysis.
Abbreviations: EXER, exercise; EXER+MET, exercise + metformin; FFM, fat‐free mass; HDL‐c, high‐density lipoprotein cholesterol; HIIT, high‐intensity interval training; HOMA‐IR, homeostatic model assessment of insulin resistance; MetS, metabolic syndrome.
Significant difference from EXER group at that time point (p < 0.05).
Significant change from baseline within each group.
Body weight and composition
Evolution of body weight and composition following training is depicted in Table 1. After 16 weeks of HIIT, no time or time per group effect, using baseline HOMA‐IR as covariate, was found in body weight (p value for time = 0.907, p value for time × group = 0.191), BMI (p value for time = 0.833, p value for time × group = 0.163), fat mass (p value for time = 0.203, p value for time × group = 0.606), FFM (p value for time = 0.093, p value for time × group = 0.129), and right‐leg FFM (p value for time = 0.368, p value for time × group = 0.771).
MetS components
Evolution of MetS components after 16 weeks of training is depicted in Table 1. After 16 weeks of training, MAP was reduced in both groups (EXER+MET: −3.9, 95% CI: −6.5 to −1.3 mm Hg, p = 0.003; EXER: −8.5, 95% CI: −11.3 to −5.7 mm Hg, p < 0.001). In addition, systolic blood pressure was reduced in both groups (EXER+MET: −4.8, 95% CI: −8.3 to −1.4 mm Hg, p = 0.007; EXER: −10.8, 95% CI: −14.6 to −7.1 mm Hg, p < 0.001). However, the MAP‐ and systolic blood pressure‐lowering effects of training were larger in the EXER group (p = 0.021 and p = 0.027, respectively). No time or time per group effect was found in waist circumference, TG, HDL‐c, MetS z score, or MetS factors. A McNemar test revealed that MAP was reduced after training only in the EXER group (p = 0.002).
IR
Evolution of fasting glucose, insulin, and IR (i.e., HOMA‐IR) after 16 weeks of training is depicted in Figure 2. Plasma glucose concentration and HOMA‐IR were higher in the EXER+MET group before training (p < 0.001 and p = 0.003, respectively), whereas plasma insulin was similar among groups (p = 0.534). After 16 weeks of HIIT, fasting insulin and HOMA‐IR decreased similarly (p value for time = 0.005) in the EXER+MET (‐1.01, 95% CI: ‐2.45 to 0.43 µIU/mL and ‐0.61, 95% CI: ‐1.23 to 0.02, respectively) and EXER groups (‐1.82, 95% CI: ‐3.38 to ‐0.26 µIU/mL and ‐0.47, 95% CI: ‐1.14 to 0.21, respectively). However, plasma glucose concentration was not affected by training; therefore, no time or time per group effect was found.
FIGURE 2.
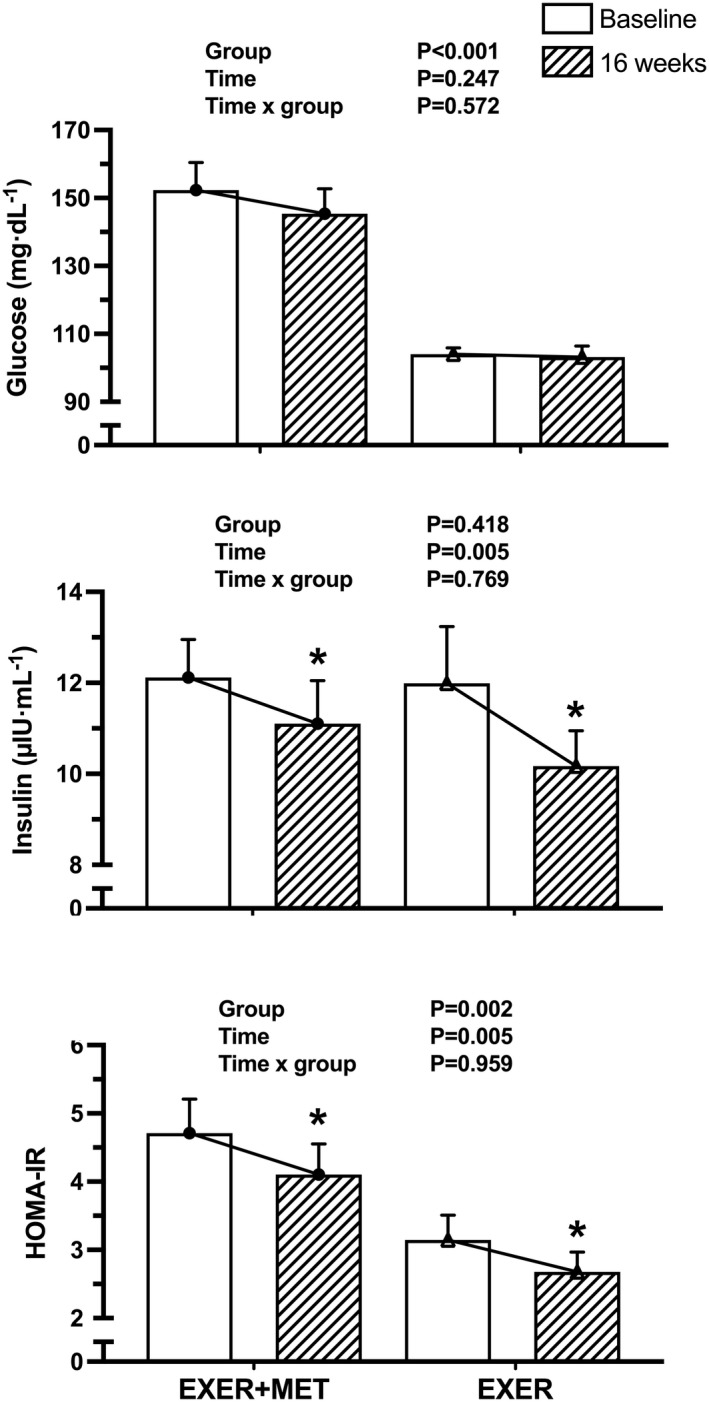
Effect of 16 weeks of high‐intensity interval training on fasting hyperglycemia, insulin concentrations in plasma, and fasting insulin resistance, calculated with HOMA‐IR, in the EXER+MET (n = 34) and EXER (n = 29) groups. Data are presented as mean ± SEM. *Significant change from baseline within each group. EXER, exercise group; EXER+MET, exercise + metformin group; HOMA‐IR, homeostatic model assessment of insulin resistance
Metabolic fitness and CRF
CRF (i.e., VO2MAX) after 16 weeks of training is shown in Figure 3. Before training, VO2MAX (EXER+MET: 1.94 [0.59] L/min, 22.2 [5.3] mL/kg/min, 35.0 [6.9] mL/kg FFM/min; EXER: 1.91 [0.55] L/min, 21.1 [4.3] mL/kg/min, 34.0 [5.8] mL/kg FFM/min), FOMAX (EXER+MET: 0.22 [0.07] g/min; EXER: 0.23 [0.07] g/min), POMAX (EXER+MET: 153 [57] W; EXER: 155 [47] W), and HRMAX (EXER+MET : 153 [17] beats/min; EXER: 156 [15] beats/min) were similar between groups (p > 0.05). After 16 weeks of training, absolute VO2MAX improved in both groups (EXER+MET: 0.23, 95% CI: 0.13‐ 0.33 L/min, p < 0.001; EXER: 0.43, 95% CI: 0.32‐0.54 L/min, p < 0.001). Moreover, relative VO2MAX improved in both groups (EXER+MET: 2.97, 95% CI: 1.76‐4.18 mL/kg/min, p < 0.001 and 4.44, 95% CI: 2.73‐6.15 mL/kg FFM/min, p < 0.001; EXER: 5.17, 95% CI: 3.85‐6.48 mL/kg/min, p < 0.001 and 8.19, 95% CI: 6.32‐10.05 mL/kg FFM/min, p < 0.001). There was a time per group interaction, with higher VO2MAX improvements in the EXER group when VO2MAX was expressed in absolute terms, relative to body weight or FFM terms (p = 0.012, p = 0.021, and p = 0.006, respectively). Likewise, POMAX improved further in the EXER group (interaction p = 0.05; EXER+MET: 29, 95% CI: 20‐38 W, p < 0.001; EXER: 42, 95% CI: 32‐52 W, p < 0.001). In contrast, FOMAX increased similarly (p value for time = 0.040, p value for time × group = 0.698) in both groups (EXER+MET: 0.05, 95% CI: 0.01‐0.08 g/min; EXER: 0.06, 95% CI: 0.02‐0.09 g/min). Last, HRMAX had a similar response in both group after training (p value for time = 0.006, p value for time × group = 0.335).
FIGURE 3.
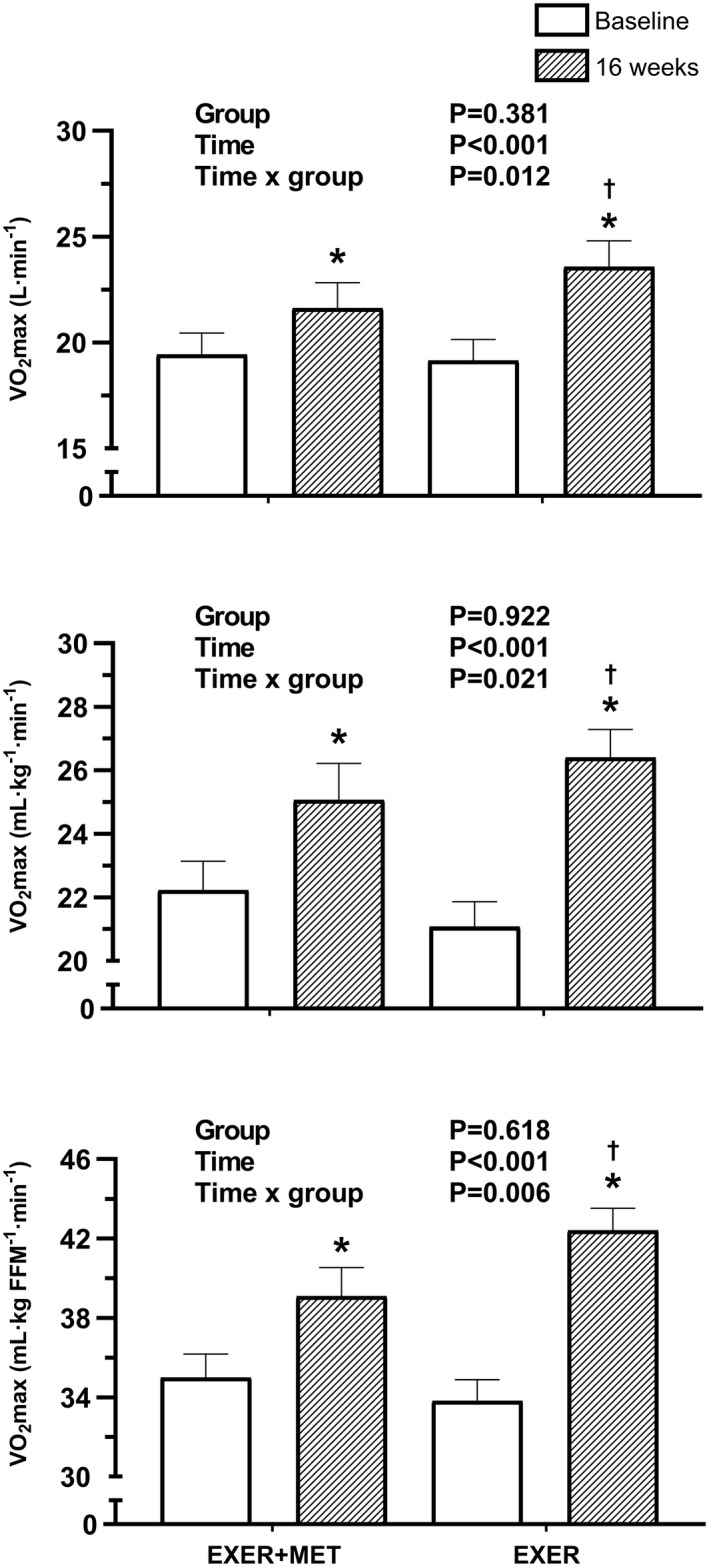
Absolute and relative (i.e., divided by body weight and FFM) VO2MAX after 16 weeks ofhigh‐intensity interval training in the EXER+MET (n = 34) and EXER (n = 29) groups. Data are presented using baseline homeostatic model assessment of insulin resistance as a covariate in the statistical analysis. *Significant change from baseline within each group. †Significant difference in the VO2MAX improvement between groups (p < 0.05). EXER, exercise group; EXER+MET, exercise + metformin group; FFM, fat‐free mass; VO2MAX, maximal oxygen uptake
Predictors of improved IR
Reductions in body weight were significantly correlated with reductions in fasting insulin and HOMA‐IR in the EXER+MET (r = 0.618 and 0.740; p < 0.001) and EXER (r = 0.508 and 0.551; p < 0.05) groups. Reductions in body weight were also significantly correlated with reductions in glucose in the EXER+MET group (r = 0.580; p < 0.001), but not in the EXER group (r = 0.245; p = 0.201). Moreover, metformin dose, as an indicator of diabetes severity, was not correlated with improvements (i.e., post minus pre) in glucose (r = 0.195; p = 0.373), insulin (r = 0.190; p = 0.384), and HOMA‐IR (r = 0.239; p = 0.272) following training. Metformin dose was not associated with improvements in VO2MAX (r = −0.360; p = 0.092), FOMAX (r = −0.299; p = 0.165), and POMAX (r = −0.409; p = 0.053) in the EXER+MET group. Metformin dose was not associated with FOMAX at baseline (r = −0.137; p = 0.533). VO2MAX improvements with training, in the EXER+MET or EXER groups, were not correlated with reductions in fasting glucose (r = −0.032; p = 0.858 and r = −0.358, p = 0.056), insulin levels (r = −0.134; p = 0.449 and r = −0.162; p = 0.402), or HOMA‐IR (r = −0.098; p = 0.580 and r = −0.255; p = 0.182). Finally, we did not find an association between FOMAX and HOMA‐IR at baseline (EXER+MET: r = −0.073, p = 0.683; EXER: r = 0.230, p = 0.230) or between FOMAX and HOMA‐IR reductions after 16 weeks of training (EXER+MET: r = −0.001, p = 0.995; EXER: r = −0.153, p = 0.429) in any of the groups.
DISCUSSION
We found that metformin treatment does not blunt the beneficial effects of 16 weeks of HIIT on reducing fasting IR (HOMA‐IR) and fasting hyperinsulinemia (Figure 2). This finding contrasts with our hypothesis and the current literature, which has suggested that metformin prevents the improvements in glucose uptake after 8 to 12 weeks of aerobic training in people with prediabetes (19, 20, 21, 33). We recruited people with MetS already treated with metformin and compared them with another group of people with MetS whose fasting hyperglycemia (104.4 [10.1] mg/dL; Table 1) had not yet progressed to require metformin prescription. Both groups were similar as far as cardiovascular disease risk (i.e., MetS z score; Table 1), but their type 2 diabetes stage was different. The prevailing view of metformin interfering with training adaptations could discourage metformin users from joining an exercise program. Our data importantly balance that view, suggesting that metformin does not interfere with training reductions in fasting IR (i.e., HOMA‐IR) when prescription has been ongoing for more than 6 months.
Malin et al. (21) found that insulin sensitivity (tested 28 hours after training using euglycemic‐hyperinsulinemic clamp) increased after 12 weeks of aerobic‐resistance training (EXER group), and that adding metformin to training (EXER+MET group) tended to blunt this training effect. This happened despite a larger body weight loss in the EXER+MET group than in the EXER group (i.e., 4 kg vs. 0.5 kg). Although the information that prevailed from that paper was that metformin blunts the full effect of exercise training in improving insulin’s glucose disposal, other relevant measurements of glucose metabolism did not differ between groups. For instance, fasting insulin decreased similarly in both groups (−1.7 [0.7] µIU/mL and −2.9 [1.5] µIU/mL for the EXER+MET and EXER groups, respectively) (22), similar to the present study (−1.0 [3.1] µIU/mL and −1.8 [5.2] µIU/mL; Table 1). Furthermore, HOMA‐IR showed similar reductions in both groups (−24% and −12% for the EXER+MET and EXER groups, respectively (21), similar to the present study (−1.0 [3.1] µIU/mL and −1.8 [5.2] µIU/mL; Figure 2). Therefore, the blunting effect of metformin on reducing IR was evident only when insulin was elevated at 187 μIU/mL during the hyperinsulinemic clamp technique (21). Those insulin concentrations were observed only after ingestion of a high glucose load (75‐g oral glucose tolerance test) in people with prediabetes in the phase of insulin overproduction (34). Therefore, metformin intake may not negatively affect exercise improvements in glucose transport at lower, more typical insulin concentrations and definitely not at fasting insulin concentrations (Figure 2).
We observed that chronic metformin treatment interfered with the VO2MAX and POMAX gains of exercise training, as previously described (Figure 3) (19, 20). We hypothesized that the lower improvement in VO2MAX would also restrict exercise reductions in fasting IR in the EXER+MET group. However, this was not the case, and the gains in VO2MAX and the reductions in IR with training were not associated (Figure 4). Longitudinal studies have shown that aerobic exercise training that improves VO2MAX also reduces IR (8, 35, 36). Nevertheless, the underlying mechanism supporting this positive association is not well defined. Our data suggest that CRF and glucose metabolism improve simultaneously with exercise training, without a clear relationship between them (Figure 4).
FIGURE 4.
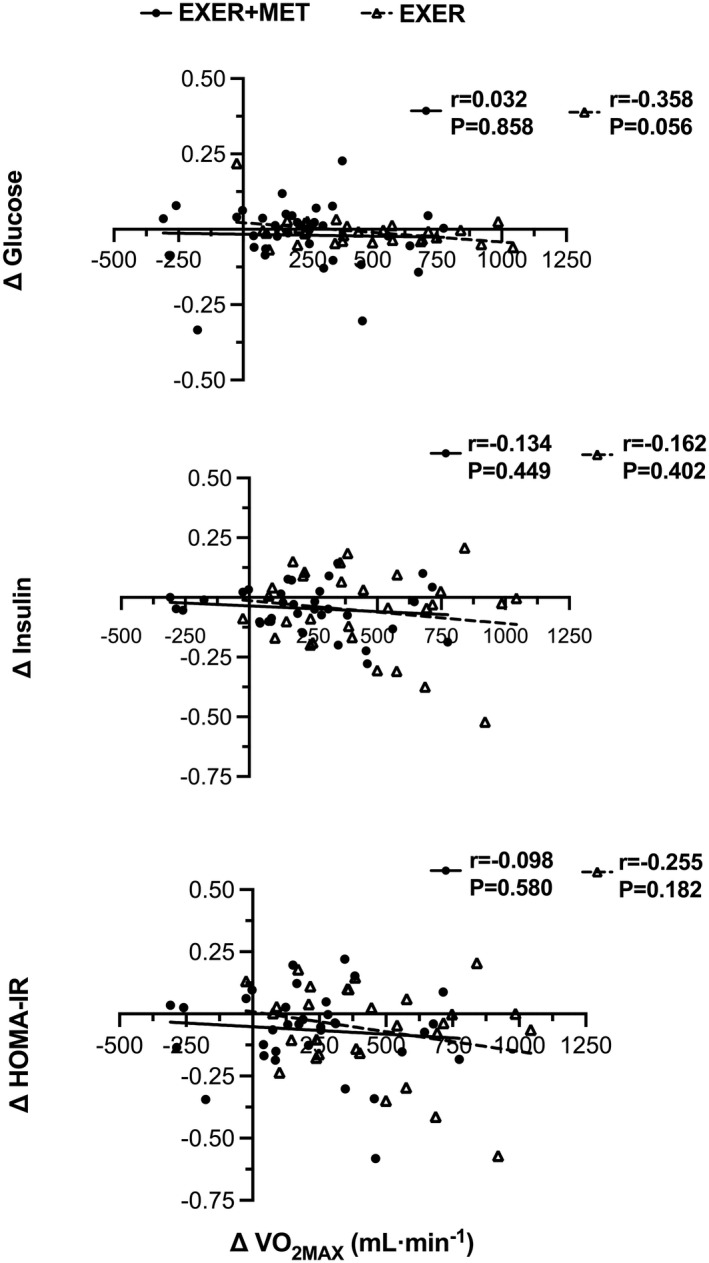
Pearson correlation between gains in cardiorespiratory fitness after 16 weeks of high‐intensity interval training (∆VO2MAX) and changes in fasting glucose, insulin, and HOMA‐IR in EXER+MET (n = 34) and EXER (n = 29) groups. EXER, exercise group; EXER+MET, exercise + metformin group; HOMA‐IR, homeostatic model assessment of insulin resistance; VO2MAX, maximal oxygen uptake
In our training intervention study, only body weight loss correlated with the reductions in HOMA‐IR in both groups of individuals. It was shown that, when the energy expended during training was reimbursed by increasing food intake to prevent body weight loss, glucose uptake during a clamp was not improved with training (37). Other investigators showed that exercise training reduced IR independently of body weight losses by improving cellular signaling mechanisms (i.e., insulin receptor and glucose transporter type 4 [GLUT4]) (35). We have recently found using an intravenous glucose load test that a minimal weight loss (i.e., >2%) is required for exercise training to reduce IR in people with MetS (9). In this study, we confirm a link between body weight losses and the reductions in IR.
Low CRF is strongly associated with elevated cardiovascular disease risk and mortality (38). Aerobic training is the cornerstone of lifestyle changes to promote CRF improvements in people with MetS (39). However, studies have suggested that metformin blunts training improvements in middle‐aged individuals with prediabetes (21, 40), older individuals (19), and even healthy, active individuals (18). However, no information is available for individuals with very low CRF (i.e., MetS; ~22 mL O2/kg/min) under chronic metformin treatment (>6 months) like those studied here. Our findings also corroborate that, in people with MetS, chronic metformin treatment blunts the improvement in CRF by 50% after 16 weeks of HIIT, a type of training shown to promote gains in CRF (41).
Metformin use during training (EXER+MET) did not affect the gains in FOMAX after 16 weeks of exercise training (i.e., 20%: EXER+MET group; 25%: EXER). FOMAX is used as a surrogate of mitochondrial function improvement with exercise training (42). Therefore, our results suggest that the reduced increase in VO2MAX in EXER+MET might not be related to impaired mitochondrial development. Konopka et al. (19) showed that metformin intake during training altered intrinsic mitochondrial function independently of influencing mitochondrial biogenesis or abundance. Thus, metformin, although influencing mitochondria function, may not alter training‐induced mitochondria biogenesis. Moreover, Malin and Braun showed that metformin did not inhibit training increases in fat oxidation and the concomitant reductions in carbohydrate use (20). Our data also suggest that metformin does not inhibit the metabolic training adaptations to increase exercise fat oxidation (i.e., FOMAX), which suggests normal mitochondria β oxidation development.
After 16 weeks of HIIT, the EXER+MET group lowered MAP by 4 mm Hg whereas the EXER group lowered it by 8 mm Hg (p = 0.02 for the interaction; Table 1). The higher initial blood pressure in the EXER group could have contributed to the larger blood pressure‐lowering effect of the same training program in this group (43). However, this comparative resistance to lower blood pressure with training in the EXER+MET group coincides with a previous report from Malin and Braun’s group (22). They suggest that metformin alters the effects of training on reducing vascular inflammation and improving endothelial function. Exercise combined with metformin was shown to lower AMPK activation in skeletal muscle (33), which is important for nitric oxide production and, therefore, for vasodilation. Ultimately, the limitation in vasodilation could affect blood flow to the contracting muscle and oxygen delivery, explaining the lower VO2MAX development with EXER+MET.
Walton et al. (44) reported that metformin may inhibit muscle hypertrophy via inhibition of mTORC1, leading to decreased muscle protein synthesis or increased autophagy. In their study, metformin attenuated the gains in one‐repetition‐maximum knee extension (placebo group: 23.1% [18.9%]; metformin group: 15.3% [18.5%]; p = 0.055). In contrast, Boule et al. (40) observed that training while medicated with metformin did not affect strength gains following aerobic training in middle‐aged patients with type 2 diabetes. Here we showed that metformin blunted the gains in cycling peak aerobic power (POMAX, watts) following 16 weeks of a HIIT program. The reduced development in cycling leg power output with training in the EXER+MET group may be the main reason for the lower increase in VO2MAX. It is unclear whether that effect was mediated by metformin restraining leg power because our indirect measure of leg FFM did not reveal changes after 16 weeks of HIIT (Table 1).
Study limitations include our indirect method to assess body composition and our lack of direct measure of mitochondrial respiration and function. Another potential limitation is that only per‐protocol statistical analysis was used. On the other hand, we used an available index of IR (HOMA‐IR) in a moderately large sample of individuals with hyperglycemia, among other cardiovascular risks (i.e., MetS). Of note, HOMA‐IR reflects liver glucose regulation, assuming a feedback loop between the liver and pancrea’ β‐cells, and it is less informative of IR in peripheral tissues. The strengths of the study are that we carefully measured whole‐body maximal fat oxidation during exercise (27) and used a verification test to confirm the attainment of VO2MAX (29). Moreover, to our knowledge, our study is unique in that the EXER+MET pharmacological dose (i.e., 1,279 [489] mg/d) was much less than the dose that other investigators have used (i.e., 2,000 mg/d), which may lead to hormesis effects (45, 46). Furthermore, by recruiting individuals who were chronically medicated, we avoided the confounding effects of metformin on body weight loss at the onset of prescription.
As shown by other authors, we found that chronic metformin treatment (1,279 [489] mg/d for at least 6 months) is associated with blunted gains in VO2MAX with intense aerobic exercise training in people with MetS and hyperglycemia. The blunted VO2MAX development did not prevent other health benefits of training. Specifically, metformin did not interfere with the exercise‐induced reductions in IR, at least at fasting insulin concentrations. Our study disputes that metformin interferes with training improvements in glucose metabolism (at least in resting IR), which could encourage metformin users to engage in exercise training.O
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Alfonso Moreno‐Cabañas and Ricardo Mora‐Rodriguez contributed to the conceptualization of the study, designed and refined the methods, collected the data, performed the statistical analyses pertaining, and developed and revised the manuscript; Felix Morales‐Palomo, Laura Alvarez‐Jimenez, and Juan Fernando Ortega contributed to the conceptualization of the study and collection of data, performed statistical analyses, and revised the manuscript. The authors have read and approved the final version of the manuscript and agreed with authorship order.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov identifier: NCT03019796.
ACKNOWLEDGMENTS
The authors recognize the invaluable contribution of the participants of the study. This work was partially funded by a grant from the Spanish Ministry of Science and Innovation (PID2020‐116159RB‐I00; MCIN/AEI/10.13039/501100011033).
Moreno‐Cabañas A, Morales‐Palomo F, Alvarez‐Jimenez L, Ortega JF, Mora‐Rodriguez R. Effects of chronic metformin treatment on training adaptations in men and women with hyperglycemia: A prospective study. Obesity (Silver Spring). 2022;30:1219–1230. doi: 10.1002/oby.23410
Funding information
Spanish Ministry of Science and Innovation (PID2020‐116159RB‐I00; MCIN/AEI/10.13039/501100011033)
See Commentary, pg. 1141.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Alberti K, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 2. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113‐1132. [DOI] [PubMed] [Google Scholar]
- 3. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3:1‐58. doi: 10.1002/cphy.c110062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595‐1607. [DOI] [PubMed] [Google Scholar]
- 5. Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age‐related diseases. J Clin Endocrinol Metab. 2001;86:3574‐3578. [DOI] [PubMed] [Google Scholar]
- 6. Solomon TPJ, Malin SK, Karstoft K, et al. Association between cardiorespiratory fitness and the determinants of glycemic control across the entire glucose tolerance continuum. Diabetes Care. 2015;38:921‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130:89‐96. [DOI] [PubMed] [Google Scholar]
- 8. Winnick JJ, Sherman WM, Habash DL, et al. Short‐term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole‐body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab. 2008;93:771‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mora‐Rodriguez R, Ortega JF, Ramirez‐Jimenez M, Moreno‐Cabañas A, Morales‐Palomo F. Insulin sensitivity improvement with exercise training is mediated by body weight loss in subjects with metabolic syndrome. Diabetes Metab. 2020;46:210‐218. [DOI] [PubMed] [Google Scholar]
- 10. Sabag A, Way KL, Keating SE, et al. Exercise and ectopic fat in type 2 diabetes: a systematic review and meta‐analysis. Diabetes Metab. 2017;43:195‐210. [DOI] [PubMed] [Google Scholar]
- 11. American Diabetes Association . Summary of Revisions: Standards of Medical Care in Diabetes‐2021 . Diabetes Care. 2021;44(suppl 1):S4‐S6. [DOI] [PubMed] [Google Scholar]
- 12. Miller BF, Thyfault JP. Exercise‐pharmacology interactions: metformin, statins, and healthspan. Physiology (Bethesda). 2020;35:338‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madiraju AK, Qiu Y, Perry RJ, et al. Metformin inhibits gluconeogenesis via a redox‐dependent mechanism in vivo. Nat Med. 2018;24:1384‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867‐872. [DOI] [PubMed] [Google Scholar]
- 15. Wessels B, Ciapaite J, van den Broek NM, Nicolay K, Prompers JJ. Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a dose‐dependent manner. PLoS One. 2014;9:e100525. doi: 10.1371/journal.pone.0100525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP‐activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074‐2081. [DOI] [PubMed] [Google Scholar]
- 17. Zhou G, Myers R, Li Y, et al. Role of AMP‐activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braun B, Eze P, Stephens BR, et al. Impact of metformin on peak aerobic capacity. Appl Physiol Nutr Metab. 2008;33:61‐67. [DOI] [PubMed] [Google Scholar]
- 19. Konopka AR, Laurin JL, Schoenberg HM, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18:e12880. doi: 10.1111/acel.12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malin SK, Braun B. Effect of metformin on substrate utilization after exercise training in adults with impaired glucose tolerance. Appl Physiol Nutr Metab. 2013;38:427‐430. [DOI] [PubMed] [Google Scholar]
- 21. Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35:131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malin SK, Nightingale J, Choi SE, Chipkin SR, Braun B. Metformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose tolerant adults. Obesity (Silver Spring). 2013;21:93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381‐1395. [DOI] [PubMed] [Google Scholar]
- 24. Bennett JA, Winters‐Stone K, Nail LM, Scherer J. Definitions of sedentary in physical‐activity‐intervention trials: a summary of the literature. J Aging Phys Act. 2006;14:456‐477. [DOI] [PubMed] [Google Scholar]
- 25. Cook RF, Billings DW, Hersch RK, Back AS, Hendrickson A. A field test of a web‐based workplace health promotion program to improve dietary practices, reduce stress, and increase physical activity: randomized controlled trial. J Med Internet Res 2007;9:e17. doi: 10.2196/jmir.9.2.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morales‐Palomo F, Ramirez‐Jimenez M, Ortega JF, Lopez‐Galindo PL, Fernandez‐Martin J, Mora‐Rodriguez R. Effects of repeated yearly exposure to exercise‐training on blood pressure and metabolic syndrome evolution. J Hypertens. 2017;35:1992‐1999. [DOI] [PubMed] [Google Scholar]
- 27. Morales‐Palomo F, Ramirez‐Jimenez M, Ortega JF, Moreno‐Cabañas A, Mora‐Rodriguez R. Exercise training adaptations in metabolic syndrome individuals on chronic statin treatment. J Clin Endocrinol Metab. 2020;105:dgz304. doi: 10.1210/clinem/dgz304 [DOI] [PubMed] [Google Scholar]
- 28. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:628‐634. [DOI] [PubMed] [Google Scholar]
- 29. Moreno‐Cabañas A, Ortega JF, Morales‐Palomo F, et al. The use of a graded exercise test may be insufficient to quantify true changes in V̇o(2max) following exercise training in unfit individuals with metabolic syndrome. J Appl Physiol. 1985;2020:760‐767. [DOI] [PubMed] [Google Scholar]
- 30. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412‐419. [DOI] [PubMed] [Google Scholar]
- 31. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction‐associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202‐209. [DOI] [PubMed] [Google Scholar]
- 32. Mora‐Rodriguez R, Ortega JF, Guio de Prada V, et al. Effects of simultaneous or sequential weight loss diet and aerobic interval training on metabolic syndrome. Int J Sports Med. 2016;37:274‐281. [DOI] [PubMed] [Google Scholar]
- 33. Sharoff CG, Hagobian TA, Malin SK, et al. Combining short‐term metformin treatment and one bout of exercise does not increase insulin action in insulin‐resistant individuals. Am J Physiol Endocrinol Metab. 2010;298:E815‐E823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Savage PJ, Gorden P, Bennett PH, Miller M. Insulin responses to oral carbohydrate in true prediabetics and matched controls. Lancet 1975;1:300‐302. [DOI] [PubMed] [Google Scholar]
- 35. Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151‐E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Gorman DJ, Karlsson HKR, McQuaid S, et al. Exercise training increases insulin‐stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia. 2006;49:2983‐2992. [DOI] [PubMed] [Google Scholar]
- 37. Newsom SA, Schenk S, Thomas KM, et al. Energy deficit after exercise augments lipid mobilization but does not contribute to the exercise‐induced increase in insulin sensitivity. J Appl Physiol. 1985;2010:554‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all‐cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395‐2401. [DOI] [PubMed] [Google Scholar]
- 39. Wewege MA, Thom JM, Rye KA, Parmenter BJ. Aerobic, resistance or combined training: a systematic review and meta‐analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. 2018;274:162‐171. [DOI] [PubMed] [Google Scholar]
- 40. Boulé NG, Kenny GP, Larose J, Khandwala F, Kuzik N, Sigal RJ. Does metformin modify the effect on glycaemic control of aerobic exercise, resistance exercise or both? Diabetologia. 2013;56:2378‐2382. [DOI] [PubMed] [Google Scholar]
- 41. Tjønna AE, Lee SJ, Rognmo Ø, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Achten J, Jeukendrup AE. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20:716‐727. [DOI] [PubMed] [Google Scholar]
- 43. Mora‐Rodriguez R, Ortega JF, Morales‐Palomo F, Ramirez‐Jimenez M, Moreno‐Cabañas A, Alvarez‐Jimenez L. Endurance exercise training reduces blood pressure according to the Wilder's principle. Int J Sports Med. 2022;43:336‐343. doi: 10.1055/a-1548-6985. [DOI] [PubMed] [Google Scholar]
- 44. Walton RG, Dungan CM, Long DE, et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: a randomized, double‐blind, placebo‐controlled, multicenter trial: the MASTERS trial. Aging Cell 2019;18:e13039. doi: 10.1111/acel.13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang YU, An H, Liu T, et al. Metformin improves mitochondrial respiratory activity through activation of AMPK. Cell Rep. 2019;29:1511‐1523.e1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ortega JF, Hamouti N, Fernández‐Elías VE, de Prada MV, Martínez‐Vizcaíno V, Mora‐Rodríguez R. Metformin does not attenuate the acute insulin‐sensitizing effect of a single bout of exercise in individuals with insulin resistance. Acta Diabetol. 2014;51:749‐755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


