Abstract
Introduction
Women and girls affected by haemophilia, including haemophilia carriers (WGH) are at risk of bleeding symptoms that may go unrecognized, including heavy menstrual bleeding (HMB) and musculoskeletal bleeding. Terminology continues to evolve.
Aim
To describe the current recommendations for nomenclature surrounding WGH, and the current understanding of HMB, iron deficiency, and musculoskeletal complaints in these patients.
Methods
Literature was reviewed and summarized.
Results
With regards to nomenclature, women with factor levels less than 50% should be classified as having haemophilia, while carriers with normal levels should be characterized accordingly to symptomatology. HMB and resultant iron deficiency are common among WGH, have a multitude of downstream effects, and maybe overlooked due to stigma around menstruation. Musculoskeletal bleeding and resultant joint changes are increasingly recognized in this population but do not necessarily correlate with factor levels.
Conclusion
Although progress has been made in the care of WGH, much work remains to further improve their care.
Keywords: bleeding, hemarthrosis, hemophilia, menstruation, nomenclature, von willebrand disease, women's health

1. SUMMARY
Women and girls affected by haemophilia (WGH) are at an increased risk of bleeding. Furthermore, lack of recognition and misidentification is an ongoing issue. In 2021 we published our proposal stating that carriers should be initially stratified based on baseline factor levels. If in the normal range they would labelled as either asymptomatic or symptomatic carriers depending on their bleeding tendency (ISTH Bleeding Assessment Tool) acknowledging this could evolve in either direction over their lifetime. If their levels were in the haemophilia severity range, they should be labelled as so (mild, moderate, or severe deficiency) irrespective of their gender, although this does not fully address the haemophilia carrier diagnostic quandary (Figure 1).
FIGURE 1.
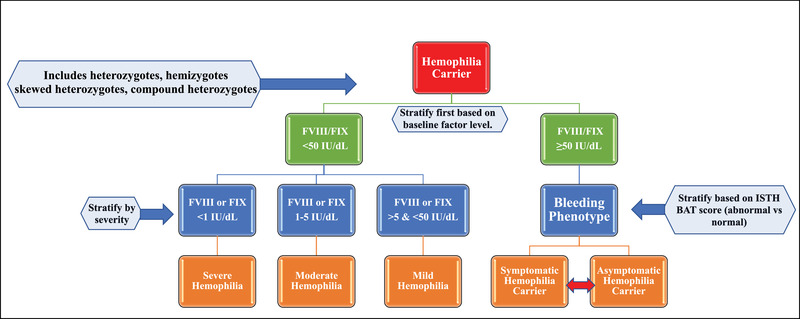
Revised nomenclature for haemophilia carriers. The original proposal did not allow for inclusion of transgender persons, used a factor threshold of 40% rather than 50% and did not provide context on bleeding tendency
WGH, as well as other bleeding disorders, are at risk for heavy menstrual bleeding (HMB) and iron deficiency anaemia (IDA). HMB is the most common symptom in women with inherited bleeding disorders (IBD). Menstruation is stigmatized within society and patients, as well as providers, may not know what a normal menstrual cycle is, or maybe hesitant to discuss this topic. 33 Appropriate identification and management are necessary as HMB and IDA are both associated with decreased quality of life.
In addition to menstrual bleeding, WGH are also at risk for musculoskeletal symptoms once thought to only affect their male counterparts. Although this type of bleeding is increasingly described, it is complicated by the lack of correlation between bleeding and factor levels, uncertainty of a mitigation plan and further study is warranted.
2. INTRODUCTION
2.1. Nomenclature for women with haemophilia and haemophilia carriers
The precept that haemophilia only affects males and leads to an unaffected pathological state in women and girls has led to decades of marginalization in haemophilia carriers. Some of the first descriptions of excessive bleeding in haemophilia carriers were reported in 1951 by Merskey and Macfarlane followed by mostly limited case series up until the 1980s. 1 When a haemophilia carrier does present with bleeding, typically alternative diagnoses are sought out‐delaying acknowledgement of the diagnosis. 2 , 3 The bleeding tendency of WGH has been well described over the last 20 years, yet the community still feels marginalized and not heard. 4 In response, programs and community efforts like Peer/Advocacy groups from HFA sisterhood, WFH publications “Symptomatic Carriers of Hemophilia” and “Carriers and Women with Hemophilia” and national meetings focused on providers and patients such as the World Federation of Hemophilia Global Summit on Women and Girls with Bleeding Disorders and patient‐centred meetings such as organized by Comprehensive Health Education Services, National Hemophilia Foundation and Hemophilia Foundation of Michigan to name a few.
The prevalence of haemophilia carriers is not fully known but it is estimated that for each male with haemophilia there are approximately three to five possible carriers and 1.5 definite carriers per affected male. 2 Nearly 1/3 of haemophilia carriers or heterozygotes will have a level in the haemophilia (FVIII and FIX <50%) range 5 while nearly one in five persons with mild haemophilia is a female. 6 Since the majority of haemophilia carriers with a factor deficiency have mild haemophilia, there should be a concerted effort to characterize this group with observational and interventional prospective studies. The majority of sponsored haemophilia trials conducted are in males with moderate to severe disease, mostly focused on demonstrating hemostatic efficacy to achieve regulatory drug approval. In these studies on males with moderate or severe disease, rarely are females allowed participation. Some of this exclusion is due to lack of drug toxicity data in neonates or in pregnant women (or of reproductive age) thus double barrier birth control protection is required or females are just excluded altogether. We certainly can do better and should advocate for more studies focused on the non‐severe deficiency state as many future hemostatic therapies may continue to be out of reach of many non‐severe patients.
Recently we proposed a novel nomenclature for haemophilia carriers in an effort to facilitate communication amongst providers, patients and community members fostering respect and improving shared‐decision making. 7 We sought to acknowledge the patient journey of these women and girls as well as reported bleeding tendency. The collaborative project of the Scientific and Standardization Committee of ISTH proposal was initially constructed to start this conversation and we desired to create a living document 7 To that end, our research group felt the need to provide some clarification and modifications shortly following publication. Firstly, we wanted to be inclusive of the complexity of haemophilia carrier genetics and transgender persons. Secondly, stratification of the haemophilia carrier remained based on baseline factor level. This divides haemophilia carriers into those with levels in the haemophilia severity range and those with levels above that range (FVIII/FIX ≥ 50%). Bleeding phenotype does not factor into categorization if factor levels are reduced, mirroring the construct used in males with haemophilia. We did adjust the diagnostic threshold to be more in line with the CDC guidelines on haemophilia with the upper limit of mild deficiency diagnosis being a FVIII or FIX of 50% rather than 40%. This is based on the increased bleeding tendency of haemophilia carriers being demonstrated in those with levels above 40%. 8 This creates three new categories that are in line with males with hemophilia, severe haemophilia (FVIII/FIX <1%), moderate haemophilia (FVIII/FIX 1%–5%) and mild haemophilia (FVIII/FIX >5 and <50%). Thirdly, we adjusted the term from women and girls with mild, moderate or severe haemophilia replacing simply with haemophilia as aforementioned. We feel that using the same nomenclature regardless of gender will allow women equal access to resources. Fourthly, we sought to clarify the difference between asymptomatic and symptomatic haemophilia carrier when the baseline factor level is 50% or higher (the normal range). Although, multiple groups have demonstrated the increased bleeding tendency of haemophilia carriers, particularly those with normal levels, a significant portion have little to no significant bleeding. Therefore, we propose a symptomatic haemophilia carrier as having an abnormal bleeding score of ≥6 in teenage or adult females and ≥3 in young girls using either the ISTH BAT or ISTH SELF‐BAT. 9
2.1.1. Genetics of haemophilia carriers
Our improved understanding of the complex genetics of haemophilia should supplement our usage of the most recent proposed nomenclature (See Figure 1). 7 The F8 and F9 genes reside on the long arm of the X chromosome. Males who carry a deleterious variant on their X chromosomes (XY) will then develop haemophilia leading to a hemizygote or hemizygous state. The severity is linked to the corresponding variant present and will be classified as severe if FVIII or FIX is <1%, moderate if 1%–5% and mild if >5% and <50% with some international guidelines using <40% as the upper limit of normal. 10
Most genetic females have two X chromosomes, of which one could be affected by a deleterious allele leading to a heterozygous state. Heterozygotes make up the largest portion of the affected haemophilia carriers in the world and the population we generally are referring to when we use the term haemophilia carrier. 6 Of course, a genetic female could have both of her X chromosomes affected leading to a homozygous state. Thus, they would have the same factor expression as their male counterparts, depending on the variant, with some potential differences if they inherit two different alleles from each parent (compound heterozygote). Like males, females can develop the hemizygous state if they have Turner Syndrome (XO) or have a single X chromosome, expressing only the unaffected or variant allele. Importantly, heterozygotes may develop skewed inactivation. X‐linked chromosome inactivation (XCI) is a process in which inactivation occurs in one of the two X‐chromosomes in females, allowing a single functional X chromosome matching the state in males. 11 This process occurs early in life in the embryonic stage and is theorized to be unbiased and random. The “silenced chromosome” is packaged in an inactive structure called heterochromatin. This leads to an XCI ratio of 50:50 in normal females. 12 When it is skewed to the affected allele, particularly an 80:20 ratio or more, a significant reduction in FVIII or FIX can occur in haemophilia carriers. This concept of X linked inactivation is likely critical to our understanding of the discrepant bleeding in haemophilia carriers particularly in heterozygotes. Garagiola et al. recently demonstrated a strong correlation between very low levels of FVIII or FIX (≤20%) and skewed inactivation where 93% of carriers with levels ≤20% had an XCI pattern of ≥80:20. 13 Moreover, carriers with a very low factor level (≤20%) and high degree of XCI (≥80:20) had a higher ISTH‐BAT score than the carriers with the opposite state (>20% and <80:20). Of note this does not explain the excessive bleeding seen in carriers with higher factor levels. Cygan's research group demonstrated that skewed XCI explains low FVIII but not the bleeding tendency, however they are exploring these concepts with larger groups. 14
2.1.2. Evaluation of haemophilia carriers
A detailed genetic pedigree should be created to identify potential and obligate haemophilia carriers in an affected family. Although there are not definitive guidelines regarding the ideal diagnostic timing for a potential or obligate haemophilia carrier, it is reasonable to consider hemostatic testing of heterozygotes prior to consideration of a procedure, following development of bleeding and prior to menstruation. 10 Typical hemostatic testing should include a one‐stage FVIII and chromogenic FVIII assay for haemophilia A carriers or a one‐stage FIX assay for haemophilia B carriers. In homozygotes or hemizygotes, hemostatic testing should be performed at birth, if possible via an umbilical cord blood sample. Timely assessment of the baseline factor level apart from genetic evaluation is critical to diagnose and prevent unnecessary bleeding. 15 Since FVIII is an acute phase reactant, repeat testing at a steady health state should be encouraged. Possible haemophilia carriers should be offered genetic testing prior to their first pregnancy. Knowledge of this carrier status allows appropriate genetic counselling, awareness of reproductive options and time to cope with the diagnosis and potential risk of haemophilia in offspring. 15
2.1.3. Bleeding tendency and haemophilia carriers
As aforementioned, there is a general lack of correlation of bleeding to the female with factor deficiency. WGH have an increased bleeding phenotype as characterized by a growing number of studies. 16 , 17 , 18 , 19 Bleeding tendency as measured by validated instruments (ISTH Bleeding Assessment Tool) is increased in WGH compared to unaffected females, notable even in the 40%–60% baseline level range. Typically, carriers will have bleeding with minor injuries, bleeding following medical interventions, HMB, muscle hematomas and joint bleeding, ranging from 8% to 16%. 8 , 17 , 18 , 20 , 21 Of note the bleeding tendency is not mostly mucosal in location compared to females with moderate to severe Von Willebrand disease (VWD) with bleeding most notable in muscles and in joints (see below section on musculoskeletal bleeding for more details). We should acknowledge that there are a significant number of haemophilia carriers with normal levels associated with a bleeding tendency.
2.2. Heavy menstrual bleeding and iron deficiency
HMB in WGH can lead to iron deficiency with or without anaemia. Little research has been done on HMB in WGH specifically, with much more data collected on other IBD, especially VWD. HMB is a common complaint in the general population, affecting approximately ∼40% of reproductive age women. 22 Objectively, HMB is defined as total blood loss per menstrual cycle exceeding 80 ml although accurate quantification is difficult and not practical in clinical practice. More recently, a more practical definition has been suggested as regularly excessive menstrual blood loss (MBL) that affects a patient's quality of life. In addition to decreased quality of life, HMB is associated with missed work and school, financial cost, poor sleep, fatigue, and depression. 23 , 24 , 25 , 26 , 27
HMB can be the earliest sign of a bleeding disorder. One in five adults and one in three adolescents with HMB are found to have an underlying bleeding disorder. 28 , 29 Conversely, HMB is the most common symptom in women with IBD, and occurs in 64% of haemophilia carriers. 18 When looking at all IBDs, about 50% of menstruating individuals present with HMB at menarche. 30 Others may present with HMB once their cycles become ovulatory. Overall, up to 90% of women with IBDs will experience HMB. 31 Menstrual bleeding patterns in women and girls with IBDs may also vary compared to the general population with longer menstrual duration, increased frequency of flooding, and heavy bleeding throughout menstruation. 32 Women and girls with IBD may also experience bleeding with ovulation which can lead to hemorrhagic ovarian cysts as well as hemoperitoneum.
It is important to obtain a detailed menstrual history in all menstruating patients. Given the stigma around menstruation, patients may not volunteer their symptoms or recognize them as abnormal. 33 Specific details that signal the need for further evaluation include: bleeding longer than seven days, menstrual flow that requires double protection, soaking more than five sanitary product per day, flow that requires sanitary product change overnight, passing clots greater than an inch in diameter, the sensation of gushing, personal history of anaemia, personal history of excessing bleeding or bruising, and family history of a bleeding disorder. 34 , 35 The pictorial bleeding assessment chart, which was recently validated using modern sanitary products, can help quantify bleeding with a cutoff of 100 signalling HMB 36 The workup of HMB depends on the age of the patient. In adolescents, the most common causes of HMB are nonstructural and include anovulatory cycles, coagulopathies (both acquired and IBD), endometrial disorders and iatrogenic causes. In adult women, structural causes are more common including polyps, adenomyosis, leiomyosis, and malignancy/hyperplasia. As such, imaging is more commonly performed in their evaluation. Although IBDs can be the underlying aetiology of HMB, the presence of a known IBD should not preclude additional workup for other etiologies as indicated by the patient's clinical presentation and risk factors. Laboratory workup for all patients should include a pregnancy test, complete blood count (CBC) and ferritin level to assess for iron deficiency which may be present without anaemia or microcytosis. In patients suspected to have an underlying bleeding disorder, an international working group suggests tier 1 testing include activated partial thromboplastin time, prothrombin time, Von Willebrand factor (VWF) panel, fibrinogen level or thrombin time, and platelet aggregation, with factor levels falling into tier 2, only to be tested in select patients. 37 As mentioned above, both one‐stage and chromogenic FVIII levels should be measured when Haemophilia A is suspected. Testing for a coagulopathy in the setting of HMB can be complicated by elevations of VWF in the setting of HMB, as well as delayed platelet aggregation in the setting of anaemia (haemoglobin <10 g/dl). 38 , 39 As such, repeat testing may be needed outside of the acute setting.
2.2.1. Iron deficiency and iron deficiency anaemia
Iron deficiency is the most common cause of anaemia, accounting for more than 60% of cases and is of particular concern in girls and women of reproductive age, 40 especially those with HMB. Iron deficiency is associated with a plethora of symptoms including fatigue, dizziness, dyspnea, loss of concentration, headaches, restless legs syndrome, hair loss and pica. 41 Serum ferritin is the most specific and sensitive test to identify iron deficiency. A ferritin level of <30 μg/L is consistent with iron deficiency although the optimal cutoff is debated with some recent studies advocating for a cutoff of 25 μg/L in non‐pregnant women. 42 , 43 Ferritin is an acute phase reactant so care should be taken in the evaluation of patients with underlying inflammation. Treatment of iron deficiency with or without anaemia in patients with HMB is two‐pronged. Iron must be replaced, and HMB must be managed. Without suppression of HMB, it is incredibly difficult to fully replenish iron stores. It is important to treat iron deficiency regardless of the presence of anaemia, as studies have shown improvements in fatigue and sleep with iron repletion. 44 , 45 Many patients are treated with oral iron as it is safe, effective, easily accessible, and relatively inexpensive. Numerous oral iron formulations are available. Daily or every other day dosing results in maximal absorption given the regulatory effects of hepcidin. 46 , 47 However, up to 70% of patients experience gastrointestinal side effects. 48 Current intravenous iron formulations have improved safety profiles and are appropriate for patients who do not tolerate oral supplementation, those with ongoing blood loss and those with malabsorption. Ferric carboxymaltose, ferumoxytol, low molecular weight iron dextran, and ferric derisomaltose all allow for full repletion in one to two doses.
2.2.2. Management of HMB
Medical treatment of HMB in patients with IBD includes hormonal and non‐hormonal therapies. Regarding hormonal therapies, there are combined estrogen‐progesterone formulations (including combined oral contraceptives [COCs], transdermal patch, transvaginal ring) and progesterone only formulations (progesterone only pills [POPs], intrauterine devices [IUDs], and injectables depot medroxyprogesterone acetate [DPMA]). COCs typically make bleeding more regular, lighter, reduce dysmenorrhea, and provide contraception. Randomized trials in the general population have found reductions in MBL ranging from 35% to 69%. 49 COCs can be prescribed cyclically or continuously and those with shorter hormone free intervals are associated with less withdrawal bleeding. 50 The levonorgestrel intrauterine system has been found to be safe and efficacious in patients with IBD although there are concerns about higher rates of expulsion. 51 , 52 Antifibrinolytic therapy following placement may help avoid this problem. 53 Non‐hormonal treatments include antifibrinolytics, desmopressin, and factor replacement. Tranexamic acid (TXA) is the most studied antifibrinolytic for HMB and has been shown to decrease MBL by 26%–54%. 49 Tranexamic acid is particularly helpful in those wishing to conceive and those with heavy bleeding with normal duration. A retrospective study by Chaudhury et al found that antifibrinolytics were the most utilized treatment (used in 50%) for HMB in WGH, followed by desmopressin and factor concentrates (used in 29% and 14%, respectively). 54 Non‐steroidal anti‐inflammatory drugs (NSAIDs) are used for HMB in the general population but are not recommended in patients with IBD given their antiplatelet effects. Desmopressin can be used in patients with VWD or Haemophilia A who have been shown to be responsive. The most recent international guidelines on management of VWD suggest the use of hormonal therapies or TXA over the use of desmopressin. 53 This recommendation was based on expert opinion, a randomized clinical trial that found a greater decrease in MBL with the use of TXA over desmopressin, and an observational study comparing desmopressin and hormonal therapy. 55 , 56 Similar guidelines for management of HMB in WGH do not currently exist. Factor replacement can also be used for the management of HMB and has been shown to be effective in patients with a range of factor deficiencies. 54 , 57 , 58 , 59 , 60 , 61
2.3. Bleeding and musculoskeletal issues among WGH
Contrary to historical belief, non‐vaginal bleeding symptoms in WGH appear to be similar to those experienced by males with haemophilia. 62 In fact, musculoskeletal bleeding is increasingly described amongst WGH. 21 , 63 , 64 Our understanding of type, severity, impact and mechanism (in those with normal factor levels) of bleeding experienced by these women is in evolution, and we will likely play knowledge acquisition catch‐up for years to come as many women remain undiagnosed in the absence of a family history in males. 65 , 66 , 67 , 68
It is becoming increasingly clear that severity of haemophilia carrier bleeding poorly correlates with factor activity levels. 16 , 69 , 70 , 71 A study conducted by Plug et al., reported that carriers with clotting levels between .05 and .60 IU had prolonged bleeding from minor wounds, and after tooth extraction, tonsillectomy, and surgery. 5 Another study conducted by Miesbach et al., showed that haemophilia A carriers with higher factor VIII levels, up to 60%, are also at increased risk of bleeding. 72 A survey conducted by Paroskie et al. found that haemophilia A carriers self‐reported a higher frequency of bleeding compared to existing literature. 73 More recently, a study by James et al. reported that haemophilia carriers had a higher mean bleeding score using the International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH‐BAT) compared to age‐matched controls (5.7 vs. 1.43, p < .0001). 18 Moreover, others have published that bleeding appears to impact carrier health‐related quality of life (HR‐QoL).63,74 The impact of bleeding in carriers and lack of correlation with plasma factor activity levels was further highlighted by haemophilia A carriers with normal levels of factor VIII:C (median: .79 IU/ml) having significantly poorer scores in “Pain” and “General Health” categories in the Rand 26‐Health Survey 1.0, 63 and significantly lower scores in the mental health, general health and social functioning domains compared to normal controls. 74 Disparity between factor levels and impact on health among carriers, while confusing for health care providers, must be acknowledged—as independent investigators have come to the same conclusions time and time again. Thus, comprehensive and meaningful care for WGH requires a leap of faith away from dogma rooted in the coagulation lab test results.
While it is clear that bleeding is driving the impact on HR‐QoL, until recently this connection was thought to be mainly due to secondary IDA. However, Gilbert et al. noted that women with haemophilia A/carriers also experience subclinical joint bleeding leading to structural joint damage akin to affected men. 64 This was demonstrated by soft tissue and osteochondral changes on joint magnetic resonance imaging (MRI). 64 Also, women with factor VIII deficiency also seem to have reduced joint range of motion even in those with factor activity levels above 40%. 64 All of this data suggests that women with haemophilia experience musculoskeletal bleeding and sequelae related to this, including diminished bone density and structural bone health. In male haemophilia patients, many studies have identified a high prevalence of low bone mineral density (BMD) and increased risk for osteoporosis. 75 , 76 , 77 , 78 The pathophysiology of low BMD in haemophilia has yet to be elucidated. It may be possible that factor VIII itself is responsible for the maintenance of bone integrity. 79 However, the underlying mechanism is likely multifactorial as recurrent musculoskeletal bleeding leads to chronic inflammation, structural changes and secondary functional impairments. 75 , 78
3. CONCLUSION
It is clear that implementation of the recently proposed nomenclature for haemophilia carriers will be useful in facilitating communication amongst providers, patients and community members fostering respect and improving shared‐decision making. Acknowledging the patient journey of these patients is crucial as well as accepting the reported bleeding tendency. The proposal is simply a start of the conversation and we hope it is a living document.
A nomenclature system can help assist the provider and patients in diagnosis but certainly it will take a change of provider attitudes to improve the relationship between the same providers and carriers. Moving forward we should demand carrier inclusion in more industry sponsored drug studies and developing studies focused on the non‐severe state.
Appropriate identification and management of HMB, as well as the iron deficiency associated with it, is crucial to the care of WGH. Education around normal menses and normalization of discussion of this topic may help identify issues earlier and improve quality of life.
The health‐related consequences of haemophilia have been extensively studied in men and we are beginning to evaluate the multifaceted impact of haemophilia‐related bleeding among women as well. Additional observational data is needed to evaluate for objective evidence of musculoskeletal bleeding as it seems that the culture shift towards improving the care of carriers is slow to turn. An example of an important knowledge gap is whether carriers are at increased risk of diminished bone density. This is important because osteoporosis is associated with future risk of fracture and premature mortality. We are currently conducting a prospective Canadian cohort study to compare bone mineral density in adult perimenopausal symptomatic haemophilia A carriers with age and body mass index‐matched controls via dual‐energy X‐ray absorptiometry. Our hypothesis is that carriers will have lower bone mass relative to control women of similar age and if we are right, the connection between carriership and impaired musculoskeletal health will be indisputably confirmed which should help change the paradigm of clinical care offered to these women.
Although considerable advances have been made in the care of women and girls with bleeding disorders, much work is left to be done.
Weyand AC, Sidonio RF, Sholzberg M. Health Issues in Women and Girls Affected by Hemophilia with a Focus on Nomenclature, Heavy Menstrual Bleeding, and Musculoskeletal Issues. Haemophilia. 2022;28(Suppl. 4):18–25. 10.1111/hae.14535
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Merskey, C , The occurrence of haemophilia in the human female. Q J Med. 1951;20(79):299‐312. [PubMed] [Google Scholar]
- 2. MacLean, PE , Fijnvandraat K, Beijlevelt M, Peters M, The impact of unaware carriership on the clinical presentation of haemophilia. Haemophilia, 2004;10(5):560‐564. [DOI] [PubMed] [Google Scholar]
- 3. Arya, S , Wilton P, Page D, et al., “Everything was blood when it comes to me”: understanding the lived experiences of women with inherited bleeding disorders. J Thromb Haemost, 2020. 18(12):3211‐3221. [DOI] [PubMed] [Google Scholar]
- 4. Arya, S , Wilton P, Page D, et al., “They don't really take my bleeds seriously”: barriers to care for women with inherited bleeding disorders. J Thromb Haemost, 2021;19(6):1506‐1514. [DOI] [PubMed] [Google Scholar]
- 5. Plug, I , Mauser‐Bunschoten EP, Bröcker‐Vriends AHJT, et al., Bleeding in carriers of hemophilia. Blood, 2006. 108(1):525‐526. [DOI] [PubMed] [Google Scholar]
- 6. Miller, CH , Bean CJ, Genetic causes of haemophilia in women and girls. Haemophilia, 2021. 27(2):e164‐e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Galen, KPM , d'Oiron R, James P, et al., A new hemophilia carrier nomenclature to define hemophilia in women and girls: communication from the SSC of the ISTH. J Thromb Haemost, 2021;19(8):1883‐1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mauser Bunschoten, EP , van Houwelingen JC, Visser EJS, van Dijken PJ, Kok AJ, Sixma JJ. Bleeding symptoms in carriers of hemophilia A and B. Thromb Haemost, 1988;59(3):349‐352. [PubMed] [Google Scholar]
- 9. Elbatarny, M , Mollah S, Grabell J, et al., Normal range of bleeding scores for the ISTH‐BAT: adult and pediatric data from the merging project. Haemophilia, 2014;20(6):831‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Srivastava, A , Santagostino E, Dougall A, et al., WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia, 2020;26 Suppl 6:1‐158. [DOI] [PubMed] [Google Scholar]
- 11. Amos‐Landgraf, JM , Cottle A, Plenge RM, et al., X chromosome‐inactivation patterns of 1,005 phenotypically unaffected females. Am J Hum Genet, 2006. 79(3):493‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carrel, L , Willard HF, X‐inactivation profile reveals extensive variability in X‐linked gene expression in females. Nature, 2005. 434(7031): 400‐404. [DOI] [PubMed] [Google Scholar]
- 13. Garagiola, I , Mortarino M, Siboni SM, et al., X Chromosome inactivation: a modifier of factor VIII and IX plasma levels and bleeding phenotype in Haemophilia carriers. Eur J Hum Genet, 2021;29(2):241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cygan, PH , Kouides PA, Regulation and importance of factor VIII levels in hemophilia A carriers. Curr Opin Hematol, 2021;28(5):315‐322. [DOI] [PubMed] [Google Scholar]
- 15. van Galen, K , Lavin M, Skouw‐Rasmussen N, et al., European principles of care for women and girls with inherited bleeding disorders. Haemophilia, 2021;27(5):837‐847. [DOI] [PubMed] [Google Scholar]
- 16. Olsson, A , Hellgren, M ., Berntorp, E , Ljung, R , Baghaei, F . Clotting factor level is not a good predictor of bleeding in carriers of haemophilia A and B. Blood Coagul Fibrinolysis, 2014;25(5):471‐475. [DOI] [PubMed] [Google Scholar]
- 17. Paroskie, A , Gailani D, DeBaun MR, Sidonio RF Jr, A cross‐sectional study of bleeding phenotype in haemophilia A carriers. Br J Haematol, 2015;170(2):223‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. James, PD , Mahlangu J, Bidlingmaier C, et al., Evaluation of the utility of the ISTH‐BAT in haemophilia carriers: a multinational study. Haemophilia, 2016;22(6):912‐918. [DOI] [PubMed] [Google Scholar]
- 19. Puetz, J , Cheng D, Descriptive analysis of bleeding symptoms in haemophilia carriers enrolled in the ATHNdataset. Haemophilia, 2021;27(6):1045‐1050. [DOI] [PubMed] [Google Scholar]
- 20. Sidonio, RF , D Mili F, Li T, et al., Females with FVIII and FIX deficiency have reduced joint range of motion. Am J Hematol, 2014;89(8):831‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilbert, L , Rollins L, Hilmes M, et al., Haemophilia A carriers demonstrate pathological and radiological evidence of structural joint changes . Haemophilia, 2014;20(6):e426‐e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haamid F, Sass AE, Dietrich JE. NASPAG clinical recommendation. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335‐340. [DOI] [PubMed] [Google Scholar]
- 23. Côté I, Jacobs P, Cumming D. Work loss associated with increased menstrual loss in the United States. Obstet Gynecol. 2002;100(4):683‐637. [DOI] [PubMed] [Google Scholar]
- 24. Wang W, Bourgeois T, Kilma J, Berlan ED, Fischer AN, O'Brien SH. Iron deficiency and fatigue in adolescent females with heavy menstrual bleeding. Haemophilia. 2013;19(2):225‐230. [DOI] [PubMed] [Google Scholar]
- 25. Kennedy KER, Onyeonwu C, Nowakowski S, et al. Menstrual regularity and bleeding is associated with sleep duration, sleep quality and fatigue in a community sample. J Sleep Res. 2021:e13434. [DOI] [PubMed] [Google Scholar]
- 26. McGrath M, Quint EH, Weyand AC. Depression in adolescents and young adults with heavy menstrual bleeding in a referral clinic setting. Am J Hematol. 2021;96(4):E105‐E108 [DOI] [PubMed] [Google Scholar]
- 27. Weyand AC, Fitzgerald KD, McGrath M, et al. Depression in female adolescents with heavy menstrual bleeding. J Pediatr. 2021:S0022‐3476(21)00880‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zia A, Jain S, Kouides P, et al. Bleeding disorder in adolescents with heavy menstrual bleeding in a multicenter prospective US cohort. Haematologica. 2020;105:1969‐1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA. Frequency of inherited bleeding disoders in women with menorrhagia. Lancet. 1998;351:485‐489. [DOI] [PubMed] [Google Scholar]
- 30. Dowlut‐McElroy T, Williams KB, Carpenter SL, Strickland JL. Menstrual patterns and treatment of heavy menstrual bleeding in adolescents with bleeding disorders. J Pediatr Adolesc Gynecol. 2015;28:499‐501. [DOI] [PubMed] [Google Scholar]
- 31. McLintock C. Women with bleeding disorders: clinical and psychological issues. Haemophilia. 2018;24(S6):20‐24. [DOI] [PubMed] [Google Scholar]
- 32. Kadir RA, Economides DL, Sabin CA, Pollard D, Lee CA. Assessment of menstrual blood loss and gynaecological problems in patients with inherited bleeding disorders. Haemophilia. 1999;5(1):40‐48. [DOI] [PubMed] [Google Scholar]
- 33. Weyand AC, James PD. Sexism in the management of bleeding disorders. Res Pract Thromb Haemost. 2020;5(1):51‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Hemophilia Foundation . Project Red Flag: Real Talk About Women's Bleeding Disorders. http://www.projectredflag.org/.
- 35. Oleka C, Dietrich JE. HMB in the adolescent: a review of the modern approach to diagnosis and management. Clin Obstet Gynecol. 2020;63(3):553‐560. [DOI] [PubMed] [Google Scholar]
- 36. Spence M, de Repentigny K, Bowman M, Hopman W, Thibeault L, James P. Validation of the pictorial blood loss assessment chart using modern sanitary products. Haemophilia. 2021;27(5):e632‐e635. [DOI] [PubMed] [Google Scholar]
- 37. Zia A, Kouides P, Khodyakov D, et al, Standardizing care to manage bleeding disorders in adolescents with heavy menses—a joint project from the ISTH pediatric/neonatal and women's health SSCs. J Thromb Haemost. 2020;18(10):2759‐2774 [DOI] [PubMed] [Google Scholar]
- 38. Mokhtar GM, Ibrahim WE, Kassim NA, Ragab IA, Saad AA, Raheem HGA Alterations of platelet functions in children and adolescents with iron‐deficiency anemia and response to therapy, Platelets. 2015;26:5, 448‐452 [DOI] [PubMed] [Google Scholar]
- 39. Brown MC, White MH, Friedberg R, et al. Elevated von Willebrand factor levels during heavy menstrual bleeding episodes limit the diagnostic utility for von Willebrand disease. Res Pract Thromb Haemost. 2021;5(4):e12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 disease and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dugan C, MacLean B, Cabolis K, Abeysiri S, Khong A, Sajic M, Richards T. Women's Health research Collaborative. The misogyny of iron deficiency. Anaesthesia. 2021;76 Suppl 4:56‐62. [DOI] [PubMed] [Google Scholar]
- 42. Camaschella C. Iron‐deficiency anemia. N Engl J Med. 2015;372(19):1832‐43. [DOI] [PubMed] [Google Scholar]
- 43. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non‐pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross‐sectional study. Lancet Haematol. 2021;8(8):e572‐e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222‐7. [DOI] [PubMed] [Google Scholar]
- 45. Pittori C, Buser A, Gasser UE, et al. A pilot iron substitution programme in female blood donors with iron deficiency without anaemia. Vox Sang. 2011;100(3):303‐11. [DOI] [PubMed] [Google Scholar]
- 46. Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice‐daily split dosing in iron‐depleted women: two open‐label, randomised controlled trials. Lancet Haematol. 2017;4(11):e524‐e533. 10.1016/S2352-3026(17)30182-5. Epub 2017 Oct 9. PMID: 29032957 [DOI] [PubMed] [Google Scholar]
- 47. Stoffel NU, Zeder C, Brittenham GM, Moretti D, Zimmermann MB. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron‐deficient anemic women. Haematologica. 2020;105(5):1232‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side‐effects in adults: a systematic review and meta‐analysis. PLoS One. 2015;10(2):e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matteson KA, Rahn DD, Wheeler TL 2nd, et al. Nonsurgical management of heavy menstrual bleeding: a systematic review. Obstet Gynecol. 2013;121(3):632‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakajima ST, Archer DF, Ellman H. Efficacy and safety of a new 24‐day oral contraceptive regimen of norethindrone acetate 1 mg/ethinyl estradiol 20 micro g (Loestrin 24 Fe). Contraception. 2007;75(1):16‐22 [DOI] [PubMed] [Google Scholar]
- 51. Huguelet PS, Laurin JL, Thornhill D, Moyer G. Use of the levonorgestrel intrauterine system to treat heavy menstrual bleeding in adolescents and young adults with inherited bleeding disorders and Ehlers‐Danlos syndrome. J Pediatr Adolesc Gynecol. 2021:S1083‐3188(21)00341‐7. [DOI] [PubMed] [Google Scholar]
- 52. Campos RR, Baêta T, Silva‐Filho A, Rezende SM, Rocha ALL. Use of a levonorgestrel 52‐mg intrauterine system in the control of abnormal uterine bleeding in women with inherited bleeding disorders. Contraception. 2020;102(4):254‐258. [DOI] [PubMed] [Google Scholar]
- 53. Connell NT, Flood VH, Brignardello‐Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chaudhury A, Sidonio R Jr, Jain N, et al. Women and girls with haemophilia and bleeding tendencies: outcomes related to menstruation, pregnancy, surgery and other bleeding episodes from a retrospective chart review. Haemophilia. 2021;27(2):293‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Amesse LS, Pfaff‐Amesse T, Leonardi R, Uddin D, French JA 2nd. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD‐associated menorrhagia: a single‐institution study. J Pediatr Hematol Oncol. 2005;27(7):357‐363. [DOI] [PubMed] [Google Scholar]
- 56. Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145(2):212‐220. [DOI] [PubMed] [Google Scholar]
- 57. Gill JC, Castaman G, Windyga J, et al. Hemostatic efficacy, safety, and pharmacokinetics of a recombinant von Willebrand factor in severe von Willebrand disease. Blood. 2015 Oct 22;126(17):2038‐2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Katayama H, Nagao A, Hosokai R, Suzuki T. [Successful long‐term management of ovarian bleeding and menorrhagia with prothrombin complex concentrate in a patient with congenital factor X deficiency]. Rinsho Ketsueki. 2018;59(11):2428‐2431. Japanese. [DOI] [PubMed] [Google Scholar]
- 59. Napolitano M, Di Minno MN, Batorova A, et al. Women with congenital factor VII deficiency: clinical phenotype and treatment options from two international studies. Haemophilia. 2016;22(5):752‐759. [DOI] [PubMed] [Google Scholar]
- 60. Park YH, Lim JH, Yi HG, Lee MH, Kim CS. Factor V Deficiency in Korean Patients: clinical and laboratory features, treatment, and outcome. J Korean Med Sci. 2016;31(2):208‐213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rugeri L, Martinaud C, Beurrier P, et al. Gynecological and obstetric outcome in the French cohort of women with factor XIII deficiency. Thromb Res. 2020;191:22‐25 [DOI] [PubMed] [Google Scholar]
- 62. Raso S, Lambert C, Boban A, Napolitano M, Siragusa S, Hermans C. Can we compare haemophilia carriers with clotting factor deficiency to male patients with mild haemophilia? Haemophilia. 2020;26(1):117‐121. [DOI] [PubMed] [Google Scholar]
- 63. Canadian Hemophilia Society . Symptomatic Carriers of Hemophilia. All About Hemophilia. 2010; [Google Scholar]
- 64. Gilbert L, Paroskie A, Gailani D, Debaun MR, Sidonio RF. Haemophilia A carriers experience reduced health‐related quality of life. Haemophilia. 2015;21(6):761‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bryant P, Boukouvala A, McDaniel J, Nance D. Hemophilia A in females: considerations for clinical management. AHA. 2020;143(3):289–94. [DOI] [PubMed] [Google Scholar]
- 66. Khair K, Holland M, Pollard D. The experience of girls and young women with inherited bleeding disorders. Haemophilia. 2013;19(5):e276‐281. [DOI] [PubMed] [Google Scholar]
- 67. Mauser‐Bunschoten EP. Symptomatic carriers of hemophilia. Treatment of Hemophilia [Internet]. 2008. Available from: https://www1.wfh.org/publication/files/pdf‐1202.pdf
- 68. Di Michele DM, Gibb C, Lefkowitz JM, Ni Q, Gerber LM, Ganguly A. Severe and moderate haemophilia A and B in US females. Haemophilia. 2014;20(2):e136‐143. [DOI] [PubMed] [Google Scholar]
- 69. World Federation of Hemophilia . Carriers and women with hemophilia [Internet]. 2012. Available from: https://www.wfh.org/en/abd/carriers/carriers‐and‐females‐with‐hemophilia‐en.
- 70. Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1‐47. [DOI] [PubMed] [Google Scholar]
- 71. Carcao MD, van den Berg HM, Ljung R, et al. Correlation between phenotype and genotype in a large unselected cohort of children with severe hemophilia A. Blood. 2013;121(19):3946‐3952. [DOI] [PubMed] [Google Scholar]
- 72. Miesbach W, Alesci S, Geisen C, Oldenburg J. Association between phenotype and genotype in carriers of haemophilia A. Haemophilia. 2011;17(2):246‐251. [DOI] [PubMed] [Google Scholar]
- 73. Paroskie A, Oso O, Almassi B, DeBaun MR, Sidonio RF. Both hemophilia health care providers and hemophilia a carriers report that carriers have excessive bleeding. J Pediatr Hematol Oncol. 2014;36(4):e224‐e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Olsson A, Hellgren M, Berntorp E, Baghaei F. Association between bleeding tendency and health‐related quality of life in carriers of moderate and severe haemophilia. Haemophilia. 2015;21(6):742–746. [DOI] [PubMed] [Google Scholar]
- 75. Wells AJ, Mclaughlin P, Simmonds JV, et al. A case‐control study assessing bone mineral density in severe haemophilia A in the UK. Haemophilia. 2015;21(1):109‐115. [DOI] [PubMed] [Google Scholar]
- 76. Iorio A, Fabbriciani G, Marcucci M, Brozzetti M, Filipponi P. Bone mineral density in haemophilia patients: a meta‐analysis. Thromb Haemost. 2010;103(3):596‐603. [DOI] [PubMed] [Google Scholar]
- 77. Paschou SA, Anagnostis P, Karras S, et al. Bone mineral density in men and children with haemophilia A and B: a systematic review and meta‐analysis. Osteoporos Int. 2014;25(10):2399‐2407. [DOI] [PubMed] [Google Scholar]
- 78. Anagnostis P, Vakalopoulou S, Slavakis A, et al. Reduced bone mineral density in patients with haemophilia A and B in Northern Greece. Thromb Haemost. 2012;107(3):545‐551. [DOI] [PubMed] [Google Scholar]
- 79. Recht M, Liel MS, Turner RT, Klein RF, Taylor JA. The bone disease associated with factor VIII deficiency in mice is secondary to increased bone resorption. Haemophilia. 2013;19(6):908‐912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


