Abstract
Two fundamental goals of endodontic treatment are to prevent or treat apical periodontitis. From a predictive perspective, several variables can affect the outcome of root canal treatment. Some of these variables depend on intraoperative factors, which include irrigation technique, size of the apical preparation, use of intracanal medicaments or the number of appointments necessary to complete the treatment. However, the outcome may also be affected by host and microbial factors. The intensity of periradicular bone loss or tissue damage, the presence of preoperative pain and associated conditions such as mechanical allodynia and central sensitization, the anatomical complexity of the apical portion of the canal, and the virulence and longevity of the bacterial infection can all have a profound influence on the outcome. Furthermore, numerous medical conditions have been reported to decrease the capability of the immune system to heal the periapical tissues. It is the clinician's responsibility to analyse these variables and incorporate them into the disinfection strategy to maximize the chances of healing. This narrative review will focus on the present status of intracanal medicaments, the clinical indications for their use and future directions for research.
Keywords: antimicrobial peptides, apical periodontitis, biofilms, calcium hydroxide
INTRODUCTION
Apical periodontitis (AP) is an inflammatory disease caused by a polymicrobial infection of the root canal (Ricucci et al., 2016, 2018; Ricucci & Siqueira, 2010). Early recognition of the infectious aetiology of AP led to the development of a range of antimicrobial strategies spanning the evolution of endodontics. At the beginning of the twentieth century, the recommended treatment was limited to the insertion of strong antiseptics in the pulp chamber of teeth affected by pulp necrosis. Due to the poor understanding of the role of mechanical and chemical debridement, it was desirable that the intracanal drug be both powerful and penetrating (Grossman, 1967; Schilder, 1974). Many toxic medicaments were used to accomplish the goal of disinfection (Grossman, 1967).
Unfortunately, overreliance on intracanal medicaments prolonged treatment in the form of numerous appointments without obtaining the desired outcome. Currently, it is known that the efficacy of intracanal medicaments is unpredictable in the presence of debris and therefore cannot substitute for the thorough debridement of the root canal space (Haapasalo et al., 2007; Portenier et al., 2002; Portenier Waltimo et al., 2006). In addition, the use of intracanal medicaments with a fixative action is no longer recommended as they are unlikely to be effective and have a questionable safety margin (Block et al., 1980).
During the last 30 years, the introduction of enhanced magnification, heat‐treated nickel–titanium instruments and new irrigation protocols have improved the ability of clinicians to manage complex cases and associated symptoms in a single visit (see Figure 1). However, not all the cases presented in dental practice will present a favourable prognosis. The prognosis may become questionable or unfavourable because of the presence of a long‐standing infection (see Figure 2), the inability to reach microorganisms in inaccessible areas (i.e. complex apical anatomy or the presence of extraradicular infection), the presence of large apical cysts or, in some cases, a patient's decreased immunocompetence. This last variable includes genetic or acquired predisposition to develop persistent apical periodontitis (Fouad & Burleson, 2003; Morsani et al., 2011). In other cases, the prognosis is affected by compounding factors (multifactorial). In these cases, the use of an intracanal medicament after proper debridement presents a clinical advantage, as improvement in symptoms can be confirmed before treatment completion (Chong & Pitt Ford, 1992).
FIGURE 1.
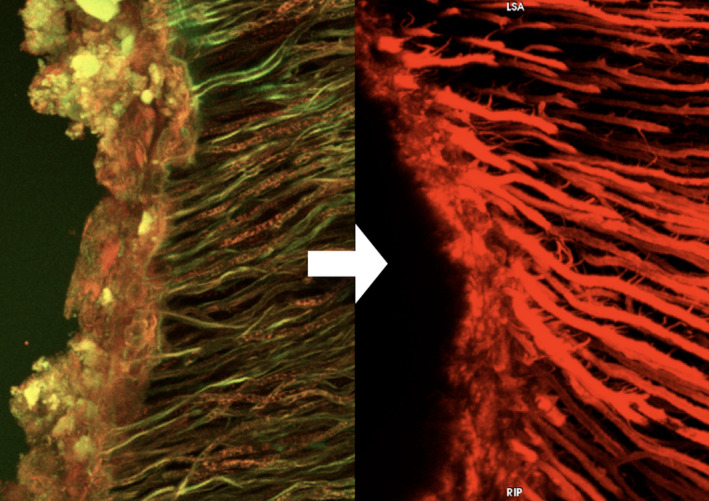
Endodontic triad model is based on the cleaning, shaping and filling of the root canal space. This model only includes the technical and microbiological aspects of the endodontic procedure. Clinical and patient's dependent information is not considered. Bacterial biofilms (left) must be removed by mechanical or chemical means to produce an environment that is suitable for the filling stage (right). Confocal laser scanning microscopy shows a dense biofilm layer infecting the dentine surface (left). A rhodamine B‐labelled sealer was used to show the adaptation of a resin‐based sealer into the dentinal tubules (right). Observe the irregular surface of dentine substrate
FIGURE 2.
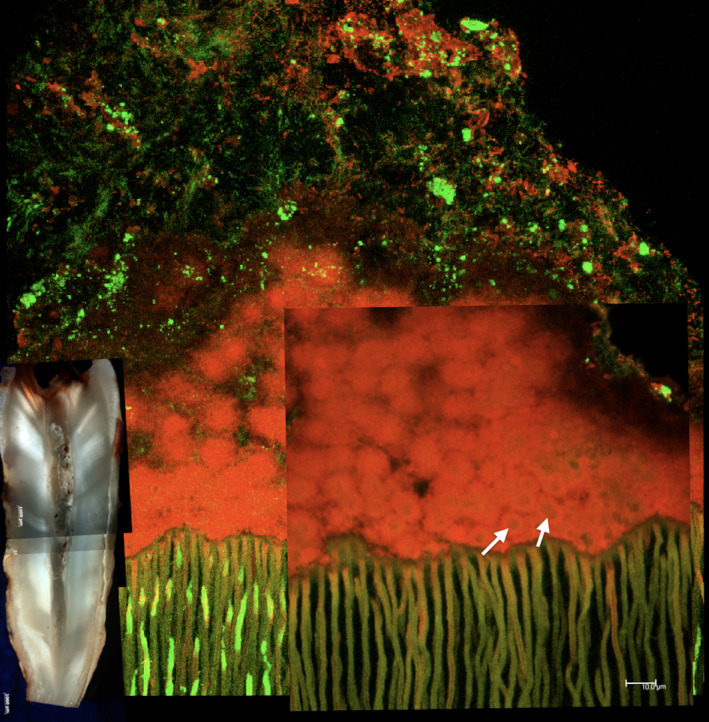
Confocal laser scanning microscopy image showing a typical biofilm architecture found in a single‐rooted tooth diagnosed with pulp necrosis. The biofilm is firmly attached to the dentine structure. Observe how the biofilm presents multiple layers. Close to the substrate, microbial cells showing viability are surrounded by dead cells labelled in red (arrows). Several bacterial morphotypes can be observed several micrometres away from the root canal wall. These cells are not permeable to the propidium dye and present intact membranes. Live/Dead staining technique (courtesy of Ronald Ordinola‐Zapata, Marco A.H Duarte and Marcia S. Graeff; bars represent 10 micrometres)
The use of an intracanal medicament gives the clinician the opportunity to test the effect of fundamental endodontic procedures; if the short‐term outcomes are satisfactory to the operator and the patient, the case can be completed and restored. Resolution of a sinus tract, pain relief, the absence of percussion and/or palpation sensitivity are favourable clinical signs of effective root canal disinfection. On the contrary, unsatisfactory results after the initial disinfection appointment give the patient the opportunity to decide whether accepting other treatment options such as endodontic surgery, intentional replantation, or opting for extraction and tooth replacement is the most advantageous treatment for the clinical scenario. Financial considerations also play an important role in a patient's acceptance and their expectations of treatment outcomes. Thus, contemporary intracanal medicaments such as calcium hydroxide are useful not only for the elimination of microorganisms and inactivation of their by‐products (Byström et al., 1985; Safavi & Nichols, 1993; Shuping et al., 2000) but also for the confirmation of initial signs of healing or symptom resolution before treatment completion (Chong & Pitt Ford, 1992).
Despite these positive attributes, the speciality is surrounded by some ambiguity about the use of intracanal medicaments (Kvist et al., 2004; Molander et al., 2007; Paredes‐Vieyra & Jimenez Enriquez, 2012; Penesis et al., 2008; Sathorn et al., 2005; Trope et al., 1999). Many clinical studies that have compared the use of multiple versus a single appointment protocol have used surrogate outcomes (i.e. short‐term postoperative pain assessment or bacterial reduction). In addition, randomized clinical trials in endodontics (Molander et al., 2007; Paredes‐Vieyra & Jimenez Enriquez, 2012; Penesis et al., 2008; Trope et al., 1999) have rarely measured the effect of demographic or prognostic factors in the study design, which increases the effect of confounding factors and bias. Multiple sources of bias can affect the result of clinical studies including attrition or loss to follow‐up (Penesis et al., 2008), selective bias and lack of power (Trope et al., 1999) amongst others. Thus, it is not surprising that comparing single and multiple visit appointment strategies involving cases with a favourable preoperative prognosis (i.e. asymptomatic anterior teeth) would not find any meaningful differences. The aim of this review is focused on the clinical and basic science aspects supporting the use of intracanal medicaments in endodontics.
IS APICAL PERIODONTITIS A DISEASE WITH MULTIPLE STAGES?
The current lack of perceived benefits associated with the use of intracanal medicaments noted in clinical studies (1 visit versus 2 visit references) could be attributed to how the binary AP disease model has been described and assessed in different studies (presence or absence). Efforts to classify severity of signs in AP cases can be found in the literature (Estrela et al., 2008; Ørstavik et al., 1986). These periapical indices were introduced to define the amount of bone loss by using two‐dimensional images and cone‐beam computed tomography. One limitation observed is that apical disease was described as a radiolucency surrounding the periapical tissues. A more thorough classification must include important bone loss characteristics that can describe the severity and ‘geography’ of the AP‐related bone loss such as the presence of lateral root lesions, the presence of furcation involvement of endodontic origin, erosion of cortical plates, the presence of through‐and‐through lesions, apico‐marginal defects or proximity of periapical disease to other anatomical structures. It is important to establish a treatment protocol based on the severity of the disease because an asymptomatic 2 × 2 mm lesion may require a different treatment strategy than a 10 × 10 mm periapical lesion that affects both cortical plates.
In order to maximize treatment efforts and prognosis, a method known as ‘staging’ was developed in the early 1960s by oncologists for measuring disease severity. This concept has been applied extensively for medical and surgical problems to classify patients. In staging, diseases are generically divided into categories of increasing levels of severity (Gonnella et al., 1976, 1984; Gonnella & Louis, 1987): for example, during neoplastic illnesses there are discrete ‘stages’ that can be defined and detected clinically. These stages reflect the severity of the disease and most importantly will impact the prognosis and choice of treatment modality (Gonnella & Louis, 1987; Markson et al., 1991).
To assess the advantages of intracanal medicaments in the future, new classifications of apical periodontitis should not only reflect the presence of pathosis and its extent but also reflect the impact on prognosis and choice of treatment modality by pre‐stratifying the severity of the disease (see Figure 3). According to Gonnella et al. (1984), a diagnosis should document the four elements required to define a disease: location of the problem, clinical manifestations, aetiology and severity. Symptomatic apical periodontitis may very well be an incomplete description. Symptomatic apical periodontitis with furcation involvement secondary to pulp necrosis in a cracked tooth provides much more specific information. These compounding factors allow the clinician and researchers to separate isolated apical disease and the presence of pathosis with multiple compounding factors. According to the staging criteria, apical periodontitis and associated signs (bone loss) and symptoms (mechanical allodynia, pain) might not be a single manifestation of pulp necrosis but a family of signs that may include different stages.
FIGURE 3.
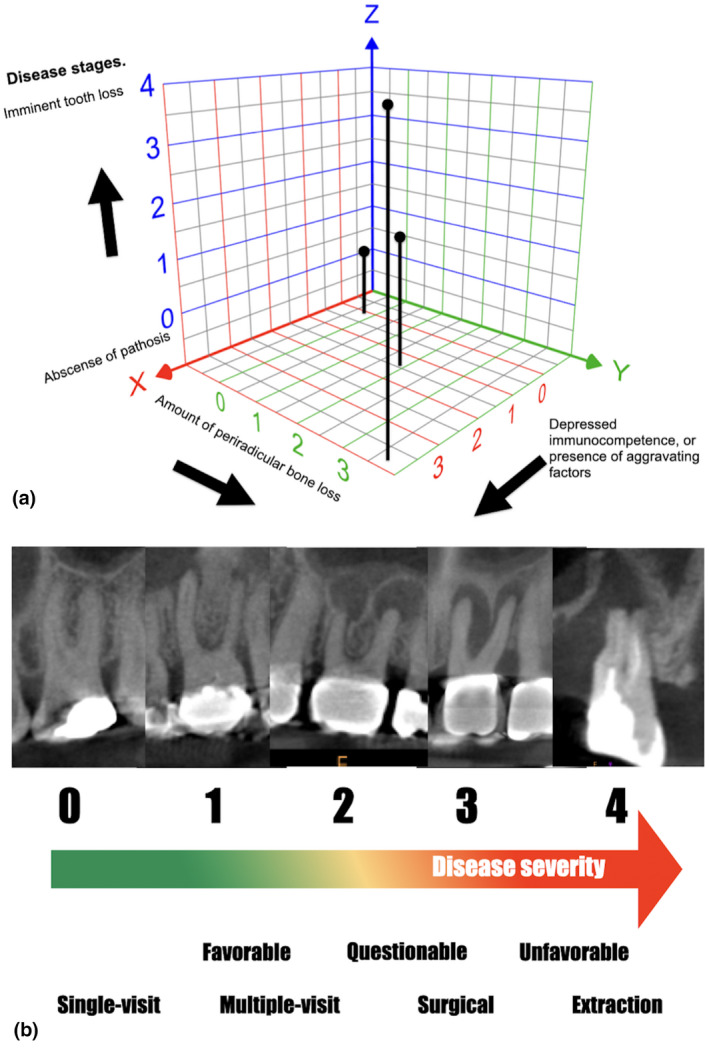
(a) Staging is a measurement of the severity of a patient's condition at any given point. The model is based on the extension of tissue/organ injury and host's factors. At the time of intervention, the severity of the apical periodontitis condition will affect not only the prognosis but also the use of treatment resources (i.e. number of visits, root‐end procedure and guided tissue regeneration). Observe how the interaction of periradicular bone loss and immune factors can increase the severity of the disease's stage. An extensive amount of periradicular bone loss in an immunocompromised patient reflects the presence of an advance stage in the grading system compared with an immunocompetent patient with minimal or no periradicular bone loss. (b) The severity of periradicular bone loss has been reported as a predictor factor for lack of complete healing. Note the different stages of bone loss; the severity of the disease not only affects the periapex but also the furcation and lateral root areas in advance stages (3–4). The extension of bone loss combined with the patient's medical information and other aggravating factors can affect the prognosis and treatment plan decisions. Graph modified from Gonella et al. (1984)
In order to establish an infection control strategy, the diagnosis must reflect severity in terms of the risk of tooth loss, disease progression or the development of acute systemic complications. Disease staging can also provide the framework to determine what resources and strategies need to be implemented, including single or multiple appointments, decompression, root‐end surgery with or without guided tissue regeneration, intentional replantation or extraction. A future diagnostic classification inclusive of staging is necessary to respond to differences in the nature of a patient's health requirements and provide clinical data relevant to analyse prognosis (see Figure 3). In staging, diseases are generically divided into categories of increasing levels of severity (Gonnella et al., 1984), and a modification for apical disease is suggested:
Stage 1, conditions with no complications or problems of minimal severity, including the absence of or minimal changes in the apical tissues.
Stage 2, problems limited to the periapical area, significantly increased risk of complications over stage 1. It is suggested that the increase in lesion size >5 mm and the presence of aggravating factors (tooth complexity, sinus tract) would fit this category.
Stage 3, extensive bone loss extending beyond the immediate apical region. ‘Through‐and‐through’ osseous defects, large radiolucencies, furcation involvement and J‐shaped lesions would be examples of this stage.
Stage 4, tooth loss or development of severe complications is imminent. Unfavourable prognosis.
A staging approach can also incorporate elements related to the systemic health of the patient since many diseases can affect bone healing. Understanding the multifaceted aspects of apical periodontitis is necessary to comprehend the advantages of a two‐step treatment model and the use of intracanal medicaments. The overall prognosis for healing of apical periodontitis can be affected by immune status (Marending et al., 2005; Morsani et al., 2011), size of the bone lesion (Ng et al., 2008; Sundqvist et al., 1998), diversity of the invading microbiome (see Figure 4), the presence of persistent infection (Sjögren et al., 1997), tooth type and the presence of cracks (Krell & Caplan, 2018), amongst other factors.
FIGURE 4.
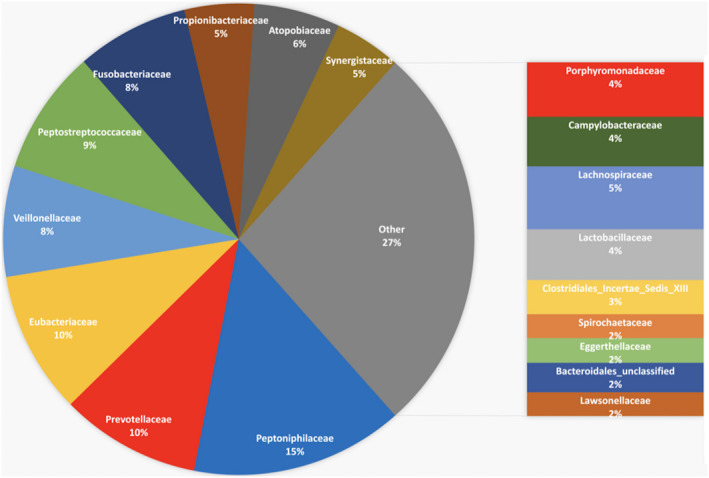
Microbial community found in primary endodontic infections (N = 31) after 16S rRNA next‐generation sequencing analysis. The microbiome is composed mainly of anaerobic bacteria. No Enterococcus spp were found in these samples. The data highlight the complexity of the endodontic microbiome and the necessity to challenge new antimicrobials with a mixed infection model (Unpublished data, Endodontic Division University of Minnesota)
WHEN TO USE AN INTRACANAL MEDICAMENT?
Understanding the clinical factors that contribute to treatment failure may influence the decision of the clinician to complete a case in a single session, favouring the use of an intracanal medicament, or to include a surgical approach in the treatment plan. Although most published data on the antimicrobial activity of different medicaments were obtained under laboratory conditions, it is important to note that the goal of laboratory studies is the discovery of the mechanisms involved in the development of pulpal and periapical disease. In many cases, these models are used to rank antimicrobials challenged under different conditions (see Figures 4 and 5). However, because the information derived from laboratory or animal studies on root canal disinfection cannot predict clinical outcomes (Oxford, Evidence Based Medicine, accessed on February 2022), the analysis of known preoperative prognostic factors linked to failure must be considered during the planning of the disinfection strategy. In this way, clinicians can use the prior information obtained from prospective studies to identify those factors that directly influence the prognosis of treatment.
FIGURE 5.
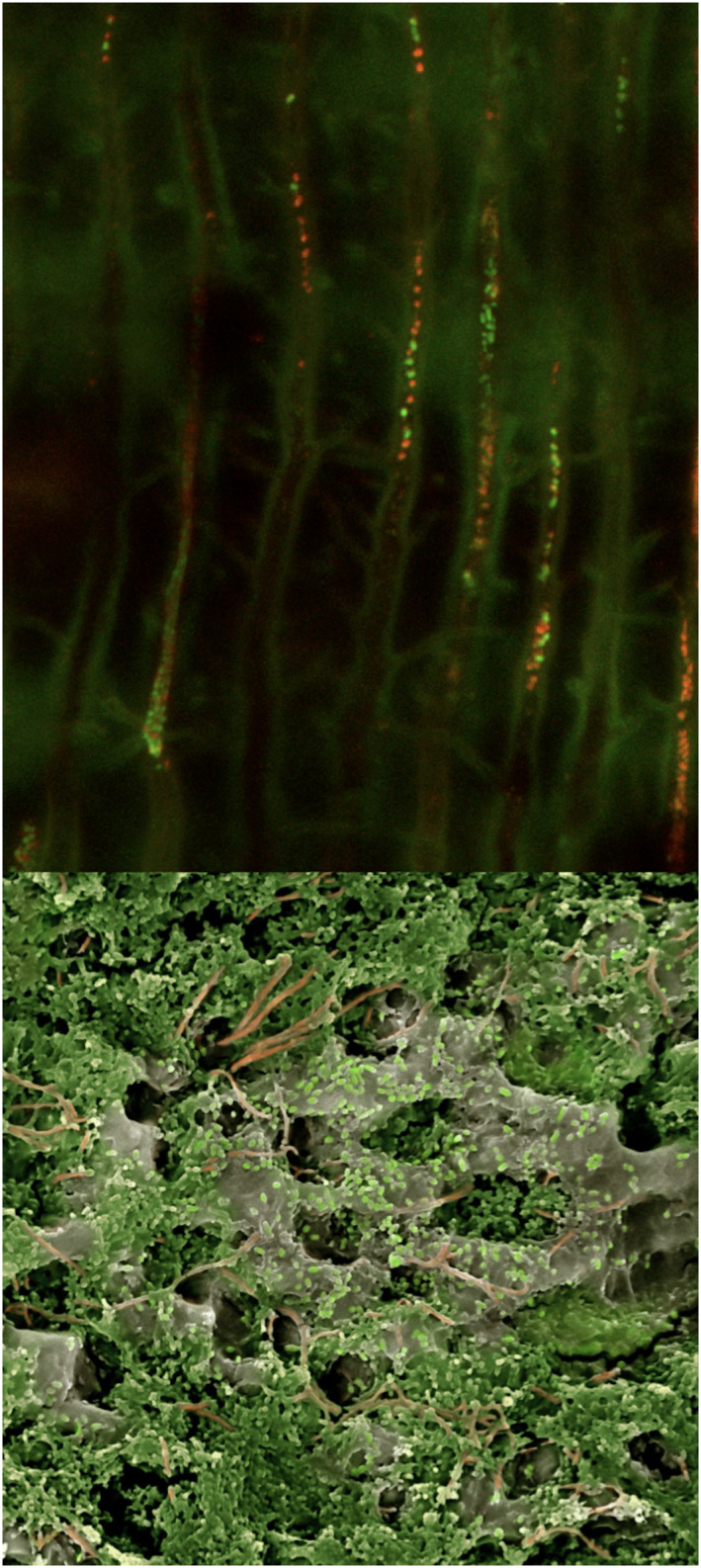
(top) Enterococcus faecalis colonizing dentinal tubules, a common model to assess the antimicrobial activity of endodontic medicaments. From Zapata et al. (2008). (Bottom) Bacteria from dental plaque colonizing the dentinal surface; observe the complexity of the later dentine infection including the presence of multiple bacterial morphotypes (SEM; courtesy of Ronald Ordinola‐Zapata, David Jaramillo, Diogo Guerreiro and Claudia Biguetti)
Adolfsson and Steineck (2000) defined a prognostic factor as a patient characteristic that identifies subgroups of untreated patients having different outcomes. For example, uncontrolled type II diabetes or the presence of a sinus tract in a tooth with a long‐standing infection can affect the resolution of apical periodontitis, thus increasing the chances of failure. In this scenario, it is the increased probability of endodontic failure, which drives the clinical strategy, use of intracanal medicament, use of adjunct surgical procedures or the number of appointments required to complete treatment. In a prospective study, Ng et al. (2011a, 2011b) monitored eight hundred patients receiving root canal treatment in single or multiple appointments with different intracanal medicaments for at least 2 years. The following prognostic factors were linked to lower healing rates or tooth loss:
The presence of apical periodontitis,
Size of the lesion,
Preoperative sinus tract,
The presence of interappointment flare‐up,
The presence of diabetes or systemic steroid therapy,
The presence of preoperative pain and
Narrow and deep periodontal probing depth.
Despite the fact that these studies provided information on relevant clinical factors, the cohort prospective design presents limitations. For example, cohort studies may suffer from selection bias because the treatment modality (use of medication or not) is not predetermined. This can increase the risk of selective bias because providers can select patients with favourable prognosis for the single appointment group, and more complex cases for the multiple appointment group for whom intracanal medicaments are used. If the effect of the intracanal medicaments is to be accurately determined, procedures to prevent the chance of imbalance between treatment groups with respect to important clinical prognostic factors or apical periodontitis stage categories should be considered in the design of future randomized clinical trials.
Preoperative pain and increased chance for flare‐up
A significant clinical variable to support the use of an intracanal medicament is the presence of moderate or severe preoperative pain. This is especially true for patients with symptomatic apical periodontitis or acute apical abscesses. Overall, the intensity of preoperative pain has been determined to be a prognostic factor of postoperative pain or flare‐up (Law et al., 2014, 2015; Nixdorf et al., 2012, 2016; Torabinejad et al., 1988). A prospective study revealed that approximately 19% of patients undergoing root canal treatment will present with severe pain 1 week after treatment (Law et al., 2015). Numerous predictive factors were associated with postoperative symptoms and must be considered before completion of treatment such as pain intensity at baseline, pain made worse by stress, pain interfering with daily activities and cases with symptomatic apical periodontitis. In a similar study, Nixdorf et al. (2016) reported the presence of chronic pain after root canal treatment. Chronic pain was defined as pain present 6 months after endodontic intervention. The authors reported that pain following root canal treatment is not uncommon (10%), and a greater number of days of pain in the week prior to treatment were predictive of persistent pain at 6 months.
Yoldas et al. (2004) evaluated the evidence and level of postoperative pain in retreatment cases completed in one versus two visits. Two hundred and eighteen cases that required retreatment were included in this study. The two‐visit retreatment with intracanal medication using calcium hydroxide was found to be effective in reducing postoperative pain of previously symptomatic teeth and decreased the number of flare‐ups in all retreatment cases. Torabinejad et al. (1988) determined the presence of interappointment emergencies in teeth with pulp necrosis. The authors found that patients in the age range of 40–59 years were more susceptible to developing interappointment emergencies. Patients with preoperative pain, retreatments, and women older than 40 years were also susceptible to developing interappointment emergencies. Trope (1991) compared the flare‐up rate for single‐visit treatment. The author found that the overall flare‐up rate was between 1.4% and 1.8%. However, patients with apical periodontitis requiring retreatment had a flare‐up occurrence of 13.6%. The cumulative evidence suggests that symptomatic cases and retreatments are prone to have a higher risk of postoperative pain. A treatment regimen including cleaning, shaping and medication with calcium hydroxide in these situations can assist the clinician in determining whether final resolution of the symptoms is possible by root canal treatment alone, or whether other treatment options need to be implemented to manage the patient's symptoms.
Conversely, previous data have also revealed that the use of intracanal medicaments does not influence the occurrence of postoperative pain (Sathorn et al., 2008). However, the outcome was measured according to binary definitions such as the presence or absence of symptoms. Patients presenting with acute or chronic dental pain can have a mixture of dental signs and symptoms. In some cases, referred pain, mechanical allodynia and central sensitization may be present due to pulpal and periapical inflammation (Owatz et al., 2007). Completing root canal treatment for symptomatic patients in one appointment prevents additional evaluation by the dentist and may result in failure to fulfil the patient's expectations (patient's centered outcome).
Lesion size and the presence of sinus tract
The number of residual walls in a bone defect of periodontal origin is recognized as a prognostic factor for the management of periodontal disease (Machtei et al., 1997). However, the complexity of the bone loss pattern caused by apical periodontitis, including the size and number of bone walls and treatment modality, has rarely been reported as a prognostic factor in root canal treatment outcome studies or randomized clinical trials (Molander et al., 2007; Paredes‐Vieyra & Jimenez Enriquez, 2012; Penesis et al., 2008; Sathorn et al., 2005).
In a study addressing treatment outcomes of non‐surgical retreatment cases, Sundqvist et al. (1998) found that the size of the periapical lesion can influence the treatment outcome. Lesions that healed were in the range of 2–6.5 mm compared with lesions that persisted that were in the range of 2.5–13 mm. This difference was statistically significant. It has also been found that teeth with periapical lesions 1–5 mm in diameter had a success rate of 86.6%, and in cases where the lesion was larger than 5 mm, the rate of success was 78.2% (Ricucci et al., 2011). In another study, teeth with small periradicular lesions (≤5 mm) had a resolution rate of 88.2%, whilst lesions that were large (5–10 mm) or very large (≥10 mm) completely healed in 72.7% and 54.5% of the cases, respectively (Artaza et al., 2021). Ng et al. (2011a) showed that the success rates for the treatment of teeth with and without a sinus tract, a condition associated with the pathologic perforation of the cortical plate, were 66.7% and 85%, respectively. Readers should also recognize that this information may apply to the patterns of bone loss characteristic of endo‐perio lesions. In addition, other demographic factors should be considered such as age and immunocompetence.
Although the presence or absence of apical periodontitis is associated with the presence of microorganisms in the root canal space, the number of microbial taxa per canal has been found to be in direct proportion to the lesion size. Small lesions (<5 mm) harboured 11.7 taxa, lesions from 5 to 10 mm harboured 16 taxa, and lesions larger than 10 mm harboured about 20 species (Rôças & Siqueira, 2008). The differences in species diversity between different lesion sizes help to explain the long‐held concept that root canal treatment of teeth with large lesions has a lower success rate than treatment of teeth with small or no lesions (Ricucci et al., 2011; Rôças & Siqueira, 2008). More severe disease conditions can negatively impact the outcome and should present symptom's resolution after initial debridement of the root canal space and prior to treatment completion.
The medical condition of the host and presence of comorbidities
Occasionally, studies have shown that teeth with an unfavourable prognosis can be treated successfully using non‐surgical treatment approaches (Calişkan, 2004; Southard & Rooney, 1984). Unfortunately, many of these studies did not control for an important factor, the patient's demographics. The ability of connective tissues to fully regenerate in young patients compared with older adults is well known. Bone turnover and healing is rapid in growing children and slows in adulthood (Lindaman, 2001). Although medical conditions can occur at any age, they are more common in the older adult population. For example, 80% of older adults in the United States have at least one chronic disease and 60% have at least two comorbidities (Center for Disease Control & Prevention, 2017; Divo et al., 2014). Because the number of older adults (>65 years) is estimated to grow in industrialized countries (Divo et al., 2014), the number of these patients seeking endodontic services will undoubtedly increase. Ageing effects including comorbidities have been rarely considered a factor that affects the outcome of root canal treatment. It is known that ageing decreases health and survival of an individual. Ageing can be associated with an elevated concentration of several cytokines such as interleukins and tumour necrosis factor‐alpha (Brüünsgaard & Pedersen, 2003; Bruunsgaard et al., 2000). These pro‐inflammatory mediators are also influenced by inflammation‐related diseases such as type II diabetes, cardiovascular disease and autoimmune disorders, amongst others. Elevated levels of inflammation‐related biomarkers in the blood of older individuals are risk factors for age‐related conditions and are often described by the term ‘inflammageing’ (Ferrucci & Fabbri, 2018). At the local level, these patients can also be affected by caries and periodontal disease. In general, these older adult populations with complex medical histories probably will have quite different apical periodontitis progression patterns compared with healthier older adults, young adults and children.
Although the effect of systemic diseases on the outcome of root canal treatment has not been extensively researched, it is known that diabetes increases the chances of root canal treatment failure by three times (Fouad & Burleson, 2003). One retrospective study (Marending et al., 2005) found that an impaired non‐specific immune system, such as in cases of patients with insulin‐dependent diabetes, renal insufficiency, rheumatoid arthritis and ulcerative colitis, amongst others, influenced the persistence of apical periodontitis after treatment. Presently, it is accepted that immunologically impaired patients and the medications they take to treat their diseases may play an important role in the resolution of apical periodontitis. More importantly, many factors can be present in the same patient, which increase uncertainty in the prognosis. For example, glucocorticoids used to treat autoimmune diseases and bisphosphonates disrupt osteoclast function (Novack & Teitelbaum, 2008). In arthritic joints, inflammatory cytokines, largely via the NFκB pathway, enhance osteoclastogenesis and cause local osteolysis (Novack & Teitelbaum, 2008). In addition, estrogen deficiency stimulates osteoclast differentiation and survival, both directly and indirectly, leading to postmenopausal osteoporosis (Novack & Teitelbaum, 2008).
A previous case–control study assessing the healing pattern of 19 patients taking TNF‐α inhibitors with a baseline periapical index of 2.8 showed satisfactory healing 2 years after root canal treatment (Cotti et al., 2018). These data show that the systemic condition might not be relevant if the severity of apical periodontitis is low, because small lesions are generally associated with less complex microbiota. It is also important to note that the reduction in the microbial level necessary to obtain healing in an immunocompetent patient may be completely different compared with an immunocompromised patient. Overall, patients with diseases impeding immune function or those taking immunosuppressive medications may experience greater risk for developing apical periodontitis or exhibit persistent apical periodontitis resistant to traditional endodontic treatment (Marending et al., 2005). Genetic factors can also protect individuals from or predispose them to the untoward effects of a microbial challenge. An association between allele distribution and the polymorphism in RANK has been reported (Petean et al., 2019). Subjects who carry the T allele had a lower risk of having persistent apical periodontitis. These findings suggest that polymorphisms in RANK and RANKL genes are associated with PAP and that root canal treatment is intimately related to the host response (Petean et al., 2019). In general, teeth with a questionable prognosis are candidates for additional visits to enhance the antimicrobial control and to assess its effect on the patient's symptoms. A series of cases are presented in Figures 6, 7, 8, 9, 10, 11, 12, 13, 14.
FIGURE 6.
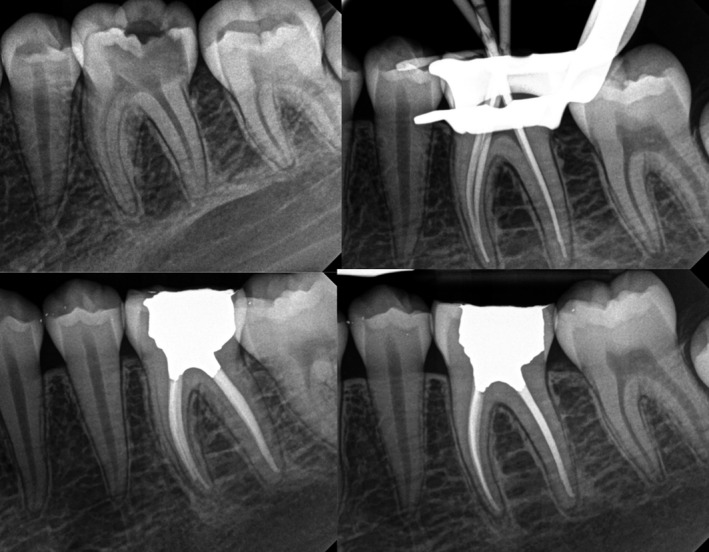
Mandibular first molar with asymptomatic irreversible pulpitis and minimal apical changes in a teenager patient. Case was completed in a single appointment since no aggravating causes were recorded other than restorative factors. It is accepted that cases with normal apical tissues present better prognosis than cases affected by apical periodontitis. A full‐coverage restoration was recommended
FIGURE 7.
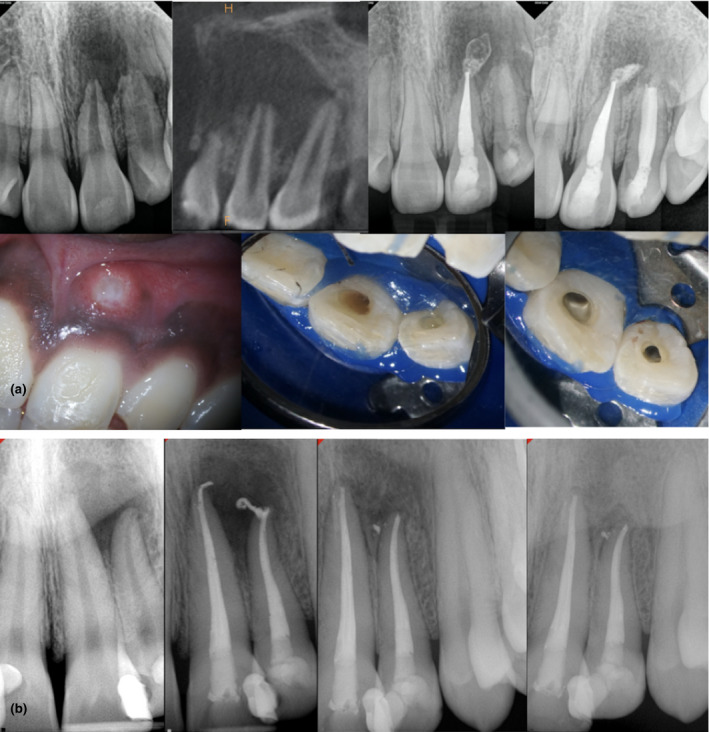
(a) Acute apical abscess in a pre‐teenager female. Pulp necrosis developed after trauma of teeth #9 and 10 (maxillary and lateral central incisors). Ill‐defined radiolucency surrounding the apices of affected teeth. The limited field‐of‐view cone‐beam computed tomography shows the extension of the periapical lesion. Observe the purulent exudate after the pulp chamber access. The case was treated in two visits using a calcium hydroxide medication. Observe the significant healing only 6 months after the initial debridement visit. The prognosis is favourable. (b) Asymptomatic apical periodontitis in teeth #9 and 10 in a young immunocompetent adult; both teeth were completed in a single appointment; observe how the sealer extrusion did not impair the healing. Follow‐up images were taken at 6 and 13 months. Clinical factors in favour of this case were lack of symptomatology, low anatomical challenge, lack of systemic condition and favourable age. The case can be considered as healing
FIGURE 8.
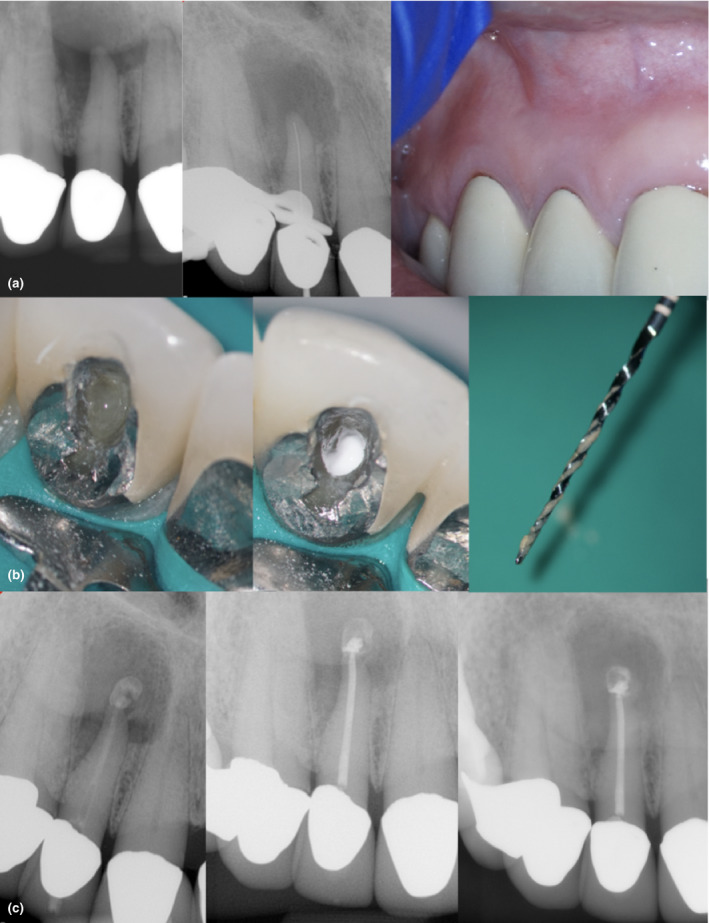
Acute apical abscess associated with a maxillary lateral incisor in an older adult patient. The patient was on a monoclonal antibody therapy to manage an autoimmune disorder. Note the extension of the radiolucency (>10 mm) and the intraoral swelling (a). The prognosis is unfavourable because of the presence of an impaired immune system and the existence of severe apical bone loss. The goals of treatment were to manage the infection, to reduce the lesion size and to create the conditions necessary for a root‐end resection (if necessary). Suppuration noted after the pulp chamber access (a), calcium hydroxide (b) placed after the initial debridement and irrigation with 6% sodium hypochlorite (35.04). The symptoms did not resolve at the 1‐week follow‐up visit, and a decompression procedure was performed following Hoen, LaBounty and Strittmatter technique (Hoen et al., 1990). Systemic antibiotics were indicated. The case was filled with gutta–percha and sealer (c) once the symptoms resolved (pain and swelling). In total, three visits of cleaning, irrigation and medication (c) were used (bottom left). A 4‐month follow‐up, periapical image shows decrease in the size of the periapical lesion (bottom right). A future surgical intervention was not discarded. The case shows the compounding effect of an extensive periradicular lesion and an acute infection in a patient taking modulators of the immune system
FIGURE 9.
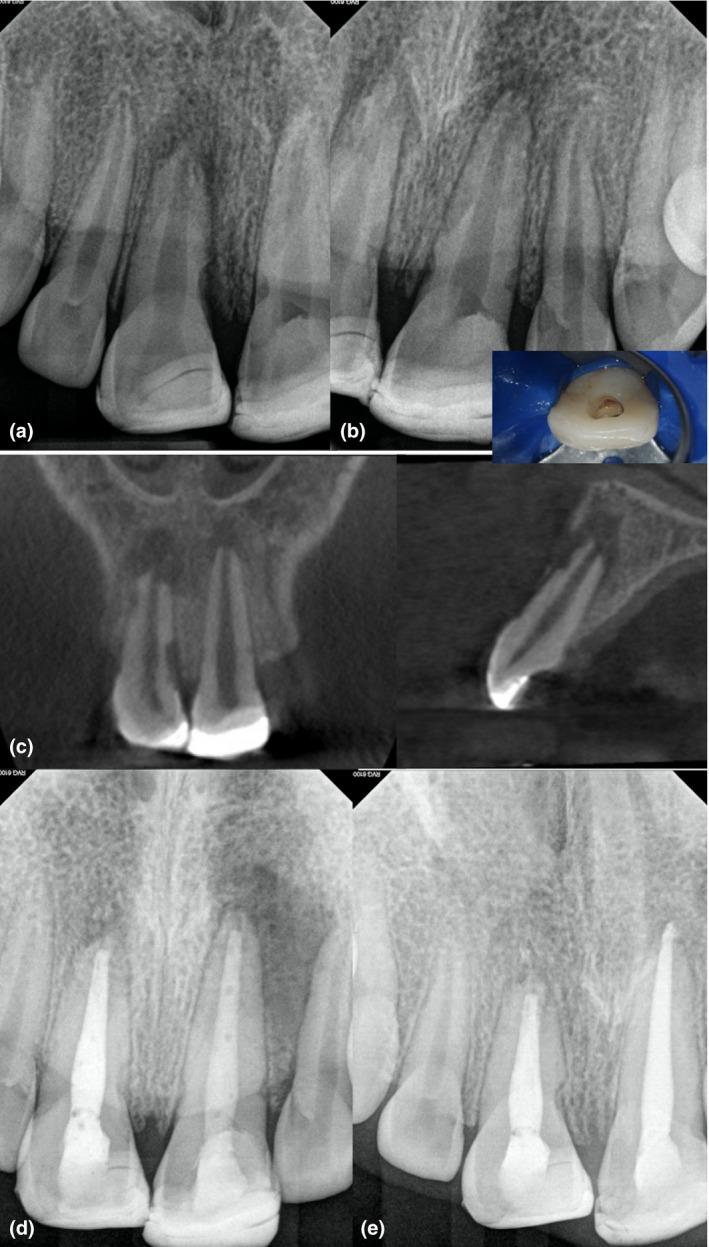
Eleven‐year‐old patient with pulp necrosis, symptomatic apical periodontitis and external inflammatory root resorption. These conditions are consequence of a previous dental trauma in both maxillary central incisors. The pulp chamber access confirmed the presence of a necrotic pulp. Observe the presence of radiopaque restorations located at the coronal level and ill‐defined radiolucencies (a–b). Multiple resorptive defects are observed in the coronal and sagittal cone‐beam computed tomography sections (c). Case treated in multiple visits using calcium hydroxide (d). The 12‐month follow‐up shows healing of the resorptive defects. The periapical tissues can be considered as healing (e)
FIGURE 10.
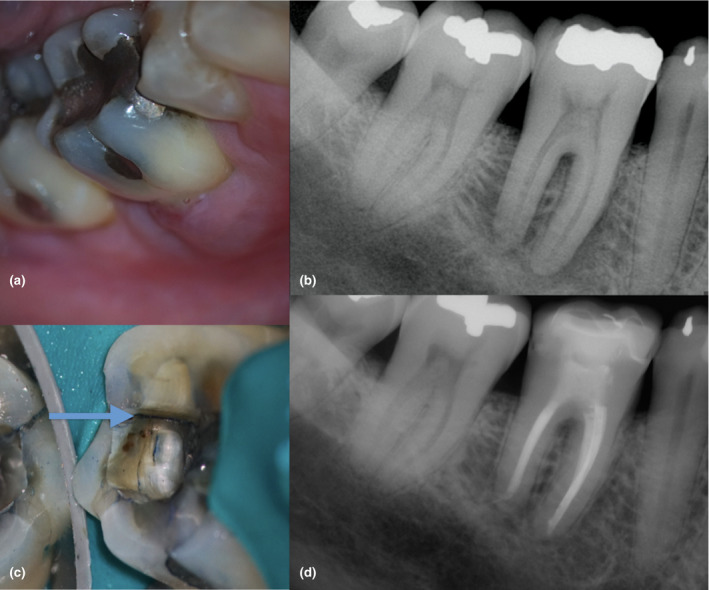
Mandibular first molar with pulp necrosis, chronic apical abscess, and furcation involvement in an immunocompetent older adult (a). The periapical digital image shows a radiopaque restoration consistent with an amalgam occlusal filling in the mandibular first, second and third molar, an ill‐defined radiolucency with a lateral root lesion, and furcation involvement associated with #30 (b). Observe the presence of a crack in the distal wall (c). Three prognostic factors were identified in this case: presence of a distal crack, periapical pathosis and probing depth higher than 4 mm (Krell & Caplan, 2018). Prognosis was unfavourable. The treatment plan option accepted by the patient was debridement, placement of calcium hydroxide medication and a temporary crown in the affected molar. The case was completed 3 months later after initial signs of healing were confirmed. Despite the unfavourable prognosis, the 18‐month follow‐up radiograph shows significant healing (d). The case was classified as healing
FIGURE 11.
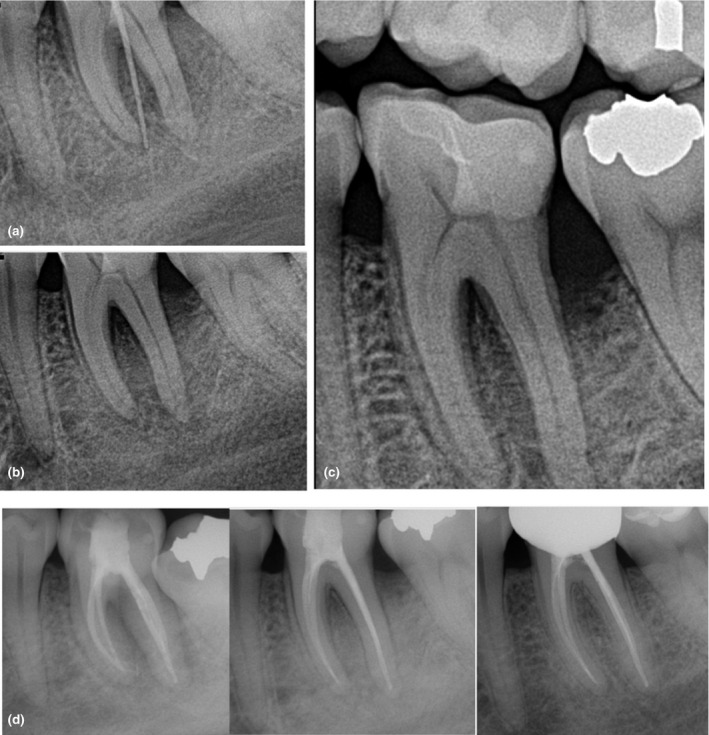
Forty‐year‐old patient with pulp necrosis and acute apical abscess in a mandibular first molar. Extraoral swelling was present at the initial consultation. The dental history included a cuspal fracture, a crown lengthening procedure and delivery of an indirect restoration. The abscess developed a few weeks after restorative treatment. (a–c) Observe the ill‐defined radiolucency associated with the first molar, furcation involvement, lateral root lesions and moderate vertical bone loss associated with the distal root. The prognosis was unfavourable due to the extensive bone loss. The treatment included 2 visits of debridement and intracanal medication until signs of healing were observed. Antibiotics were used at the initial visit to manage the extraoral swelling. Calcium hydroxide was used as intracanal medicament. (d) Postoperative image, 3‐month follow‐up and 18‐month recall showing recovery of the bone architecture and healing of apical tissues. A new full‐coverage restoration was placed to avoid a biomechanical failure
FIGURE 12.
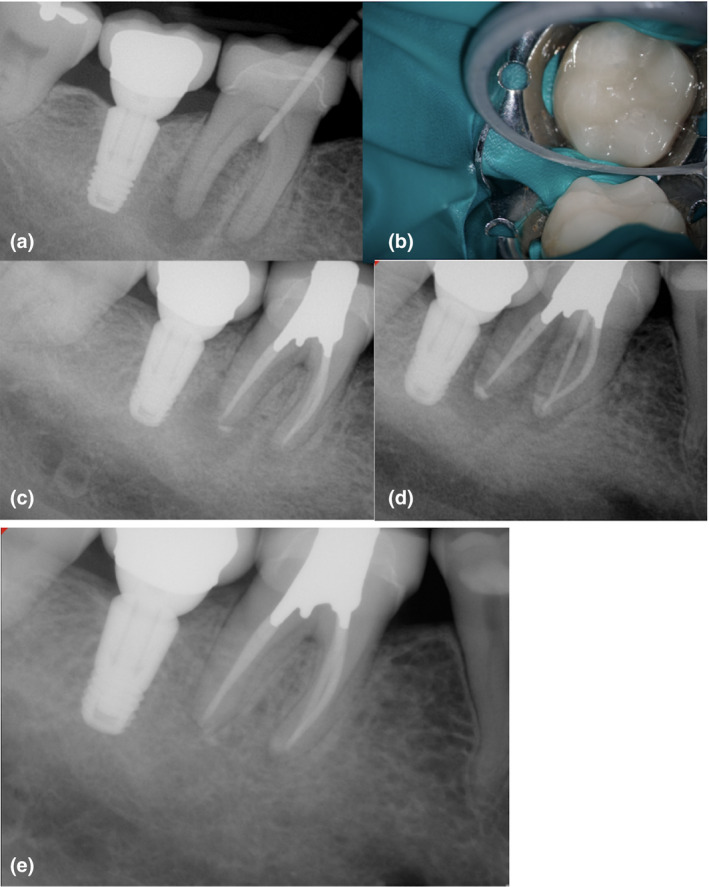
Mandibular first molar with pulp necrosis and chronic apical abscess in an immunocompetent older adult (a). Observe the J‐shaped lesion surrounding the distal root, furcation involvement and a calcified pulp chamber. The prognosis was questionable. The presence of an indirect restoration did not avoid a conservative treatment (b). The case was completed after the sinus tract healed and probing depth was <4 mm. A two‐visit model was used to manage this chronic infection (c‐d). The 12‐month recall shows satisfactory healing of the furcation area and periapical tissues. Signs of healing avoided a future surgical intervention in this case with complex bone loss pattern and a possible apico‐marginal defect (e)
FIGURE 13.
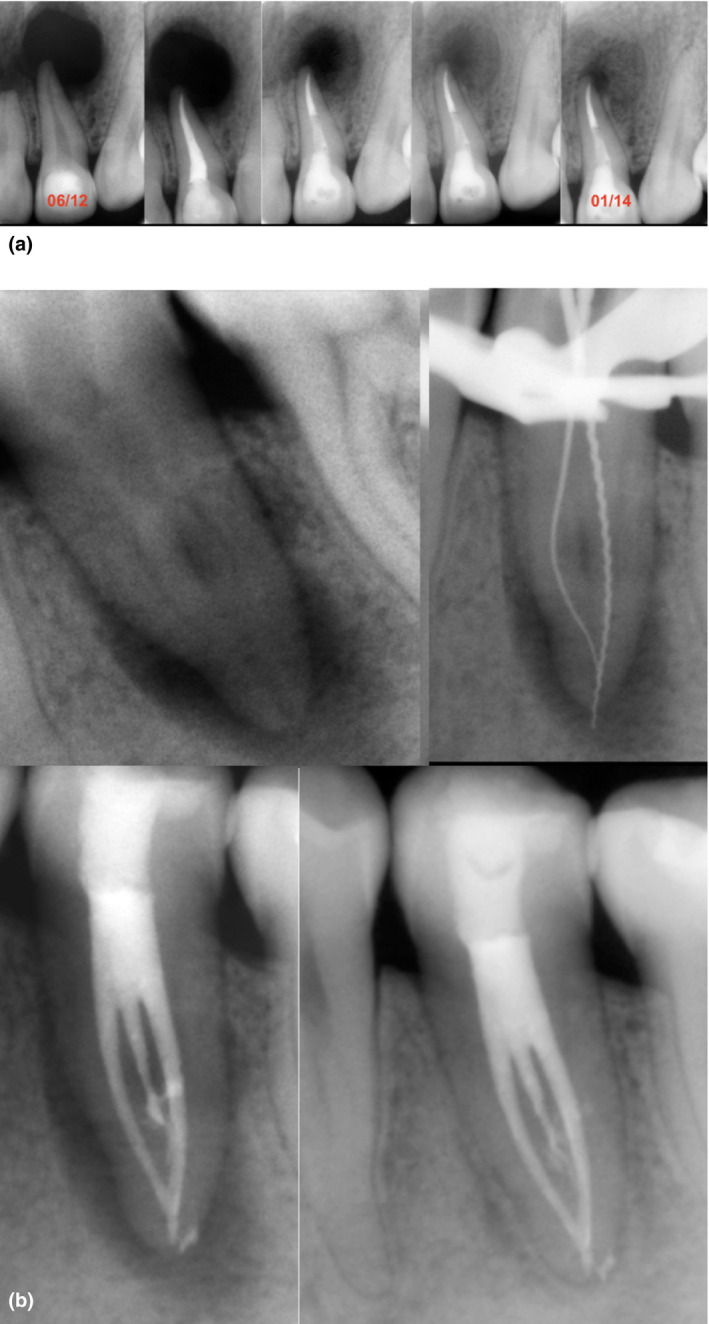
(a) Symptomatic apical periodontitis associated with a maxillary central incisor with pulp necrosis in a 28‐year‐old patient. Observe the well‐defined large radiolucency and the radiographic characteristic of a ‘through‐and‐through bone defect’. The case was treated in multiple visits using a calcium hydroxide medication. This protocol made an invasive surgical procedure unnecessary. The follow‐up periapical digital image shows significant bone recovery. The case can be classified as healing. (b) A mandibular second premolar with complex anatomy presenting an ill‐defined periapical radiolucency with furcation involvement and a J‐shaped lesion. The case was completed using an intracanal medication. The 36‐month follow‐up periapical image shows healing of the periapical tissues
FIGURE 14.
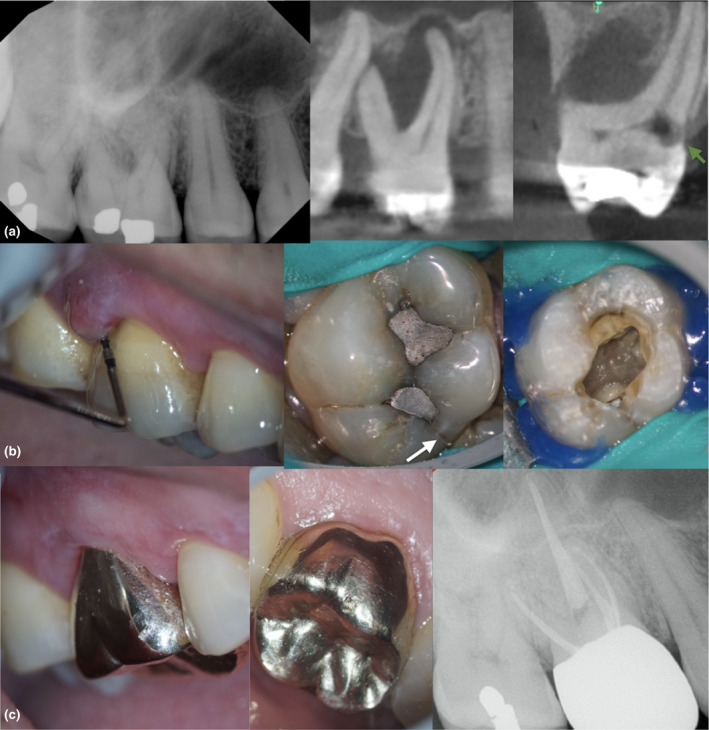
Fifty‐six‐year‐old patient with a chronic apical abscess and pulp necrosis in a maxillary first molar. No systemic conditions were recorded. The periapical image shows the presence of periapical radiolucencies and furcation involvement. Limited field‐of‐view CBCT sagittal and coronal sections revealed the presence of an extensive furcation defect, a lateral root lesion and external invasive cervical resorption (EICR) in the palatal side (a–b). The clinical inspection revealed the presence of a crack in the distal marginal ridge associated with occlusal amalgam restorations. The inspection of the pulp floor revealed that the crack did not extend below the CEJ level. The prognosis was unfavourable because of the presence of several factors including extensive bone loss, EICR and a crack in the distal marginal ridge. The treatment plan options included extraction, debridement and intracanal medication or no treatment. Patient elected the non‐surgical treatment option, including the use of intracanal dressing. The common understanding between the provider and the patient was that the treatment will be completed after showing initial signs of healing (decrease in probing depth) and closure of the sinus tract. Occlusion was reduced after treatment, and a full‐coverage restoration was recommended after 3 months of follow‐ups. (c) The 18‐month recall shows that the apical tissues are healing
ANTIMICROBIAL ACTIVITY OF CALCIUM HYDROXIDE
It is widely accepted that there is a strong association between infection of the root canal space and the development of apical periodontitis. Oral biofilms can colonize the main root canal and lateral anatomy including apical ramifications (Ricucci et al., 2018; Ricucci & Siqueira, 2010, 2010a). Initially, intracanal medicaments were indicated for almost all cases of pulp necrosis. The old concept of what is removed from the root canal is of greater significance with regard to success than what is placed to fill the root canal system is still valid presently. According to Chong and Pitt Ford (1992), intracanal medicaments are recommended when the root canal is extensively infected and when interappointment intervals are long. The authors stressed that medicaments should not be used as an alternative to thorough cleaning and shaping.
Many studies have found numerous advantages of calcium hydroxide as the medicament of choice, primarily its high alkalinity, tissue dissolution capability, ability to neutralize endotoxins and antibacterial properties. Calcium hydroxide (Ca(OH)2) is a widely used intracanal medicament. According to Siqueira and Lopes (1999), the pH of Ca(OH)2 is ~12.5. Hydroxyl ions create free radicals that destroy components of bacterial cell membranes. These free radicals react with bacterial DNA inhibiting DNA replication and cell activity, and cause mutations. The alkaline pH of calcium hydroxide also alters enzyme activity, disrupting cellular metabolism and structural proteins. Hydroxyl ions can diffuse across dentine increasing the pH to 9.0, also known as trans‐dentinal medication (Tronstad et al., 1981). This effect may be important in controlling bacterial reservoirs in dentinal tubules. Calcium hydroxide can also act as a physical barrier, limiting the proliferation of residual microorganisms and preventing reinfection due to coronal leakage (Siqueira & Lopes, 1999). Calcium hydroxide also has the ability to dissolve residual tissue. This medicament can dissolve necrotic tissue alone, or can be used to pretreat tissues to increase their dissolution rate when sodium hypochlorite is used as irrigant (Hasselgren et al., 1988). An updated version of this study revealed that pretreatment with calcium hydroxide increased the tissue dissolving efficacy of 0.5% sodium hypochlorite plus ultrasonic irrigation to the level achieved with full‐strength sodium hypochlorite (Türkün & Cengiz, 1997).
Safavi and Nichols (1994) concluded that calcium hydroxide hydrolysed the lipid A moiety of bacterial LPS, resulting in the release of free hydroxyl fatty acids. This result suggests that calcium hydroxide‐mediated degradation of LPS may be an important reason for the beneficial effects observed with calcium hydroxide use in clinical endodontics. Several laboratory studies have demonstrated the potential of calcium hydroxide medication to inactivate the LPS molecule (Safavi & Nichols, 1993, 1994). Using an animal model, Nelson‐Filho et al. (2002) reported that LPS did not induce periapical inflammatory reactions in dog's teeth when the LPS was mixed with calcium hydroxide and then placed inside the root canals. On the contrary, when the canals were filled with the endotoxin alone it induced a severe inflammatory process. Following the same methodology, Tanomaru et al. (2003) and Silva et al. (2004) evaluated the ability of three concentrations of sodium hypochlorite (1%, 2.5% and 5%) and 2% chlorhexidine to detoxify the root canal. The results revealed that only canals medicated with calcium hydroxide were associated with absent or mild inflammatory infiltrate at the apical third, normal periodontal ligament thickness and low degree of cementum resorption.
The vehicles to deliver the medication have been classified as aqueous, viscous and oily according to their consistency and ability to allow for calcium hydroxide dissociation (Fava & Saunders 1999). Since the action of calcium hydroxide is pH‐dependent, the ideal vehicle should allow ionic dissociation of this medicament. Ca(OH)2 is not equally effective against all bacteria and cannot substitute for proper debridement (Fava & Saunders, 1999). For example, bacteria such as Enterococcus or Streptococcus can tolerate high pH levels in the range of 9–11 (Chávez de Paz et al., 2007; Weckwerth et al., 2013). In addition, bacteria may survive after intracanal medication for many reasons. They may be intrinsically resistant to the medicament or may be enclosed within anatomical variations of the canal space that are inaccessible to debridement efforts. Furthermore, the effects of dentine, organic debris and tissue fluids can buffer the pH of Ca(OH)2, affecting its properties (Haapasalo et al., 2007; Portenier et al., 2001). It is also questionable whether viscous or oily vehicles are clinically beneficial, as they do not allow high dissociation and consequent release of hydroxyl ions, which are responsible for the main biologic effects of calcium hydroxide (Siqueira & Lopes, 1999). To compensate for some of these deficiencies, the association of calcium hydroxide dressing with other antimicrobials has been advocated. These include the use of paramonochlorophenol camphorate (Silveira et al., 2011), chlorhexidine (Gomes et al., 2006) or iodine potassium iodide (Tello‐Barbaran et al., 2010).
The removal of intra‐radicular biofilm has been recognized as the main focus of treatment of teeth with pulp necrosis. In the clinical scenario, the primary end‐point in prospective studies is the clinical and radiographic evidence of apical periodontitis resolution (Ørstavik et al., 1986). In clinical studies, the assessment of the effectiveness of treatment is limited by the lengthy response time before an end‐point occurs such as periradicular bone regeneration. This is one of the motivating factors for the use of surrogate outcomes. Researchers have sought numerous outcomes that are sensitive to differences between root canal treatment regimens (use of intracanal medications or not). In the interest of practicality, these changes in measures are often recorded within a short period after root canal treatment is initiated, for example at the end of the disinfection procedure (culture samples) or 1 week after the completion of root canal treatment (postoperative pain). Briefly, a surrogate is an outcome that substitutes for a definitive clinical end‐point, such as bone regeneration. In the case of microbiological samples, two conditions are necessary to validate this biomarker as a surrogate: (1) the biologic marker must be correlated with the clinical end‐point; and (2) the marker must fully capture the net effect of the intervention on the clinical efficacy end‐point (Hunter et al., 2010). For example, in microbiological studies, positive bacterial cultures before the canal fill have been used as a surrogate for persistence of apical periodontitis.
Examples of a specific study design that has served as the basis to support the link between bacterial negative culture and radiographic healing are the studies of Sjögren et al. (1997) and Kvist et al. (2004). These authors found that cleaning and shaping in the absence of an intracanal medication is linked to only 68% success compared with cases in which bacteria were completely removed (Sjögren et al 1997). In the two‐visit model, healing of apical periodontitis was considered successful in 95% of cases. It is important to highlight that the Sjögren et al. (1997) study was completed in single‐rooted teeth and cases were irrigated with 0.5% NaOCl.
Another series of clinical studies addressing the antimicrobial efficacy of different instrumentation and medication protocols were published between 1998 and 2005 (Card et al., 2002; Dalton et al., 1998; McGurkin‐Smith et al., 2005; Shuping et al., 2000). In the first study (Dalton et al., 1998), the effect of rotary instrumentation on microbial reduction in teeth with necrotic pulps was compared with the conventional step‐back technique. The results revealed that both instrumentation techniques were equivalent regarding microbial reduction. Of 48 individuals who were included in this study, 13 were associated with no quantifiable organisms after the instrumentation procedure. The study stressed the necessity of using antimicrobial adjuncts to increase microbial reduction. In a subsequent study (Shuping et al., 2000), the authors included the use of an antimicrobial irrigant (1% NaOCl) and an intracanal dressing (calcium hydroxide). The microbiologic samples were obtained from forty‐two individuals after the root canals were enlarged to different apical sizes and irrigated with 1% NaOCl. In addition, the canals were medicated with calcium hydroxide for at least 7 days. Using this protocol, 61% of the cases were bacteria‐free at the end of instrumentation; when calcium hydroxide medication was used, 92% of the cases did not have any positive culture. The results revealed that the apical size of the preparation and the use of the intracanal medication contributed to the reduction in the microbial load. Other clinical studies show that on average, between 20 and 30% of the canals still have viable microorganisms after medication with Ca (OH)2 (Ørstavik et al., 1991).
Clinical studies have also shown that the efficacy of Ca (OH)2 in combination with other antimicrobials is not entirely conclusive. Some studies revealed that the antimicrobial effect of Ca (OH)2 improved when chlorhexidine was incorporated in the paste (Paiva et al., 2013). In contrast, others have shown no significant increase in antimicrobial activity (Manzur et al., 2007; Zerella et al., 2005). Zerella et al. (2005) reported that intracanal dressing with a mixture of 2% chlorhexidine and Ca (OH)2 was at least as effective as Ca (OH)2 in an inert vehicle in disinfecting root canal‐treated teeth with apical periodontitis. Paiva et al. (2013) used molecular methods to evaluate the clinical antimicrobial effects of one‐week intracanal medication with Ca (OH)2 mixed with 2% chlorhexidine. The authors revealed that intracanal medication promoted a significant decrease in bacterial load to levels significantly below those achieved by the instrumentation and irrigation alone.
In a randomized clinical trial, Manzur et al. (2007) assessed the antimicrobial efficacy of intracanal medication with Ca (OH)2/saline, 2% chlorhexidine gel (CHX) and a combination of both Ca (OH)2 and CHX and concluded that the antibacterial efficacy of three medications was comparable. In a further study, Menakaya et al. (2015) compared the efficacy of Ca (OH)2 powder mixed with 0.2% CHX or mixed with normal saline as an intracanal medicament in the treatment of apical periodontitis and reported no significant difference in the outcome between groups. It is important to note that despite their practicality, surrogate outcomes have some limitations. Specifically, the results based on surrogate end‐points are less certain than results based on long‐term follow‐up because postoperative factors are not taken into account. The effectiveness of calcium hydroxide in decreasing the number of positive cultures after treatment has been shown to be inconsistent. To achieve the desired effect, the intracanal medication should also be maintained for at least 7 days. Sjögren et al. (1991) evaluated the antimicrobial effectiveness of calcium hydroxide when used as a short‐term intracanal dressing in vivo. The authors revealed that a calcium hydroxide dressing efficiently eliminated microorganisms, which may survive biomechanical instrumentation, and that predictable results can be achieved by dressing the canal for 7 days.
INTRACANAL MEDICAMENTS IN REGENERATIVE ENDODONTICS
Cleaning and removal of intracanal infection in teeth with incomplete root formation can be considered a challenge that requires individual disinfection strategies when compared to treatment of teeth with complete root formation. A variety of medicaments were recommended for the management of teeth with an ‘open apex’ including Frank's paste (apexification procedure) (Frank, 1966), and the use of antibiotics for guided endodontic repair. Antibiotics have been used as intracanal medication in root canal treatment at least since the 1951s (Grossman polyantibiotic paste) (Parhizkar et al., 2018). However, local application of antibiotics in the root canal space has been restricted because of the high microbial diversity of the intracanal bacterial population and the risks of adverse effects. The triple antibiotic paste has been recommended for the management of teeth with incomplete root formation. The paste is prepared by mixing three antibiotics: ciprofloxacin, minocycline and metronidazole, with sterile distilled water (Hoshino et al., 1996). The antimicrobial efficacy was evaluated by Hoshino et al. (1996) and Ordinola‐Zapata et al. (2013). The results revealed the ability of the paste to eliminate microorganisms infecting dentine compared with calcium hydroxide and 2% chlorhexidine gel. In addition, the experiment explored whether the residual microorganisms could recolonize the chemically treated biofilms. The results revealed that the triple antibiotic paste does not significantly increase the number of live organisms in comparison with calcium hydroxide (Ordinola‐Zapata et al., 2013)
Despite the recognized antimicrobial activity of this antibiotic dressing, it should be emphasized that the use of antibiotic‐based intracanal medications can result in clinical and biologic side effects, including tooth structure staining (Ordinola‐Zapata et al., 2013). Cohenca et al. (2010) and da Silva et al. (2010) evaluated in vivo the apical repair in immature dog's teeth with experimentally induced apical periodontitis after root canal instrumentation and intracanal medication with the triple antibiotic paste compared with the use of negative apical pressure irrigation. The authors found a significantly more intense inflammatory cell infiltrate and a less advanced repair process when the paste was used.
It is important to highlight that there is a global concern regarding antibiotic resistance. The emergence of drug resistance in bacterial populations is undermining the effectiveness of antibiotics and the ability to treat infectious diseases, particularly in immunocompromised patients, including older adults, patients with cancer and patients receiving organ transplants (Boucher et al., 2009). The Center for Disease Control and Prevention (CDC) estimates that drug‐resistant bacteria cause 23 000 deaths each year in the United States and are a leading cause of death worldwide with 1.2 million deaths in 2019 (Antimicrobial Resistance Collaborators, 2022; Sweileh, 2021). It has been projected that there will be more deaths due to antimicrobial resistance than cancer by 2050. Antibiotic resistance is a growing concern because of the potential limitations placed on the medical community to safely perform other medical procedures including chemotherapy, surgery and organ transplants (Boucher et al., 2009; Sweileh, 2021; Antimicrobial Resistance Collaborators, 2022). Thus, the use of antibiotics as a routine intracanal medicament is discouraged.
FUTURE DIRECTIONS: NANOPARTICLES AND ANTIMICROBIAL PEPTIDES
The control of multi‐drug‐resistant pathogenic bacteria has become a priority for the World Health Organization (WHO). Therefore, there has been an increasing demand for emerging non‐conventional new antimicrobial therapies (Prestinaci et al., 2015). Antimicrobial peptides (AMPs) and nanoparticle‐based medicaments have been explored for use in dentistry.
Compared with traditional intracanal medicaments, antimicrobial peptides (AMPs) are promising alternatives with high antimicrobial potency, good biocompatibility and low bacterial resistance. AMPs are mostly cationic oligopeptides either derived from natural sources (e.g. bacteria, fungi, plants, and animals) or designed by computational methods. Several online databases summarized the reported AMPs; for example, the Antimicrobial Peptide Database 3 (APD3) contain 3324 AMPs (last accessed on 5 February 2022, https://aps.unmc.edu/) (Wang et al., 2016) and the Database of Antimicrobial Activity and Structure of Peptides, version 3.0 (DBAASP v3), contain 18 433 peptides with 14 085 AMPs targeting Gram‐positive microorganisms and 14 927 AMPs targeting Gram‐negative microorganisms (last accessed on 5 February 2022; https://dbaasp.org/) (Pirtskhalava et al., 2021). As E. faecalis is, until now, the most reported organism found in secondary endodontic infections, the antimicrobial activity of AMPs as intracanal medications has been primarily tested against E. faecalis. Compared with the conventional calcium hydroxide treatment, AMPs showed significantly increased activity in eliminating E. faecalis and eradicating biofilms (Lee & Baek, 2012; Lee et al., 2013; Winfred et al., 2014). By substituting all amino acids of AMPs to D‐enantiomers, the antimicrobial activity was further enhanced against E. faecalis compared with L‐enantiomers (Hirt et al., 2018).
The mechanism of AMPs eliminating bacteria is still a matter of debate (Bechinger & Gorr 2017). Due to the amphipathic and cationic properties of AMPs, the antimicrobial mechanism was determined to be closely related to the interactions between AMPs and negatively charged bacterial membrane bilayers. Several models have been proposed to introduce the concept of transmembrane pore formation leading to bacterial lysis, including ‘barrel‐stave’, ‘carpet’ and ‘toroidal‐pore’ models (Brogden, 2005). Ye and co‐workers studied the relationships between the antimicrobial activity and the self‐assembly of AMPs using a model AMP, GL13K, derived from a human parotid secretory protein (Ye & Aparicio, 2019, 2022; Ye et al., 2021). In their studies, the peptides with higher potency in forming self‐assembled nanofibers presented stronger antimicrobial activity, which might be caused by the strong interactions between the self‐assembled AMPs and the bacterial lipid bilayer and other cell envelope components. There has been increasing speculation that AMPs might also target other intracellular components, such as protein, DNA and RNA (Brogden, 2005). Moreover, the immunomodulatory properties of AMPs have also been recently studied to simulate the immune system (Haney & Hancock, 2013). Given that AMPs do not have specific protein targets in bacteria, it is more difficult to develop widespread bacterial resistance against AMPs compared with conventional antibiotics. Further understanding of the antimicrobial mechanisms of AMPs would help design more effective and specific AMPs as intracanal medications.
Another effective antimicrobial agent as an intracanal medication is the use of nanoparticles, including metal, polymeric and ceramic nanoparticles. These nanoparticles are commonly mixed in the calcium hydroxide paste as additives to enhance the antimicrobial potency of calcium hydroxide (Sy Agossa et al., 2021). One of the most studied metal nanoparticles against E. faecalis is silver nanoparticle (AgNP) due to its broad‐spectrum antimicrobial activity and simple fabrication procedures (Afkhami et al., 2015; Halkai et al., 2018; Noronha et al., 2017; Wu et al., 2014; Zheng et al., 2018). Other antimicrobial metal nanoparticles used in endodontics included copper nanoparticle (CuNP) (Rojas et al., 2021), selenium nanoparticle (SeNP) (Miglani & Tani‐Ishii, 2021; Shahmoradi et al., 2021) and zinc oxide nanoparticle (ZnONP) (Aguiar et al., 2015; Guerreiro‐Tanomaru et al., 2013; Samiei et al., 2018). Polymeric nanoparticles can be applied either as a direct antimicrobial agent, such as chitosan nanoparticles (Del Carpio‐Perochena et al., 2017; Suresh et al., 2021), or as a biodegradable nanocarrier for antimicrobial drugs, such as poly(D,L‐lactide‐co‐glycolide) (PLGA) nanoparticles (Arafa et al., 2020; Elmsmari et al., 2021; Makkar & Patri, 2017). Ceramic nanoparticles, such as mesoporous calcium silicate nanoparticles, could be loaded with antimicrobial agents (e.g. Ag, Zn and chlorhexidine) (Fan et al., 2014, 2016; Leng et al., 2020). Compared with some traditional intracanal medications, the treatment of nanoparticles had no significant influence on the mechanical properties of dentine (Suzuki et al., 2019; Zhu et al., 2017).
The combination of antimicrobial agents with different mechanisms of delivery may represent the future direction of intracanal medicaments. The very simple example was the combination of two common intracanal medicaments; examples included the combinations of AMPs with intracanal irrigants (Tong et al., 2014), chitosan with chlorhexidine gluconate (Savitha et al., 2019), and AgNPs with chlorhexidine gluconate (Charannya et al., 2018). The nanocomposites of AMP‐AgNP had a significant increase in antimicrobial activity compared with either single AMP or single AgNP (Pal et al., 2016, 2019; Ruden et al., 2009; Ye et al., 2022). This might be attributed to the synergistic effect that AMPs create by inducing bacterial transmembrane pore formation for the access of AgNPs to the internal targets. With multi‐antimicrobial agents, the incidence of bacterial resistance would also be significantly lower than that using single antimicrobial agents.
CONCLUSIONS
Intracanal medicament is essential part of the endodontic armamentarium. Although their use appears to be diminishing, they are useful in cases with questionable or unfavourable prognosis. There is the necessity of well‐designed prospective studies to assess the long‐term outcomes of root canal treatment modalities. Clinical studies lack proper stratification of relevant patient's factors including age, the presence of comorbidities that may impair healing, size of the lesion and the presence of endo‐perio defects. Ideally, studies need to embrace the advantages of using a stage disease model. The use of nanoparticles and antimicrobial peptides as intracanal medicaments is promissory, and more research in this area is encouraged.
CONFLICT OF INTEREST
The authors deny any conflicts of interest related to this study.
ETHICS STATEMENT
The article is a narrative review and did not involve human participants.
AUTHOR CONTRIBUTIONS
Ronald Ordinola‐Zapata: Conceptualization, writing, editing, funding acquisition. Jorge Vera: Writing, editing. W. Craig Noblett: Writing, editing. Alejandro Perez Ron: Writing, editing. Zhou Ye: Writing, editing.
ACKNOWLEDGEMENTS
The research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1‐TR002494.
Ordinola‐Zapata, R. , Noblett, W.C. , Perez‐Ron, A. , Ye, Z. & Vera, J. (2022) Present status and future directions of intracanal medicaments. International Endodontic Journal, 55(Suppl. 3), 613–636. Available from: 10.1111/iej.13731
REFERENCES
- Adolfsson, J. & Steineck, G. (2000) Prognostic and treatment‐predictive factors‐is there a difference? Prostate Cancer and Prostatic Diseases, 3, 265–268. [DOI] [PubMed] [Google Scholar]
- Afkhami, F. , Pourhashemi, S.J. , Sadegh, M. , Salehi, Y. & Fard, M.J. (2015) Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. Journal of Dentistry, 43, 1573–1579. [DOI] [PubMed] [Google Scholar]
- Aguiar, A.S. , Guerreiro‐Tanomaru, J.M. , Faria, G. , Leonardo, R.T. & Tanomaru‐Filho, M. (2015) Antimicrobial activity and pH of calcium hydroxide and zinc oxide nanoparticles intracanal medication and association with chlorhexidine. Journal of Contemporary Dental Practice, 16, 624–629. [DOI] [PubMed] [Google Scholar]
- Antimicrobial Resistance Collaborators . (2022) Global burden of antimicrobial resistance in 2019: a systematic analysis. Lancet, 399, 629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafa, M.G. , Mousa, H.A. & Afifi, N.N. (2020) Preparation of PLGA‐chitosan based nanocarriers for enhancing antibacterial effect of ciprofloxacin in root canal infection. Drug Delivery, 27, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artaza, L. , Campello, A. , Soimu, G. , Alves, F.R.F. , Rôças, I.N. & Siqueira, J.F. (2021) Clinical and radiographic outcome of the root canal treatment of infected teeth with associated sinus tract: a retrospective study. Australian Endodontic Journal, 47, 599–607. [DOI] [PubMed] [Google Scholar]
- Bechinger, B. & Gorr, S.U. (2017) Antimicrobial Peptides: Mechanisms of Action and Resistance. Journal of Dental Research, 96, 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block, R.M. , Lewis, R.D. , Hirsch, J. , Coffey, J. & Langeland, K. (1980) Systemic distribution of N2 paste containing 14C paraformaldehyde following root canal therapy in dogs. Oral Surgery, Oral Medicine, Oral Pathology, 50, 350–360. [DOI] [PubMed] [Google Scholar]
- Boucher, H.W. , Talbot, G.H. , Bradley, J.S. , Edwards, J.E. , Gilbert, D. , Rice, L.B. et al. (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clinical Infectious Diseases, 48(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Brogden, K.A. (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature Reviews Microbiology, 3, 238–250. [DOI] [PubMed] [Google Scholar]
- Brüünsgaard, H. & Pedersen, B.K. (2003) Age‐related inflammatory cytokines and disease. Immunology and Allergy Clinics of North America, 23, 15–39. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard, H. , Skinhøj, P. , Pedersen, A.N. , Schroll, M. & Pedersen, B.K. (2000) Ageing, tumour necrosis factor‐alpha (TNF‐alpha) and atherosclerosis. Clinical & Experimental Immunology, 121, 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byström, A. , Claesson, R. & Sundqvist, G. (1985) The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endodontics Dental Traumatology, 1, 170–175. [DOI] [PubMed] [Google Scholar]
- Calişkan, M.K. (2004) Prognosis of large cyst‐like periapical lesions following nonsurgical root canal treatment: a clinical review. International Endodontic Journal, 37, 408–416. [DOI] [PubMed] [Google Scholar]
- Card, S.J. , Sigurdsson, A. , Ørstavik, D. & Trope, M. (2002) The effectiveness of increased apical enlargement in reducing intracanal bacteria. Journal of Endodontics, 28, 779–783. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention . (2017) Healthy aging: promoting well‐being in older adults. Available from: https://www.cdc.gov/grand‐rounds/pp/2017/20170919‐senior‐aging.html [Accessed February 1st 2022]. [Google Scholar]
- Charannya, S. , Duraivel, D. , Padminee, K. , Poorni, S. , Nishanthine, C. & Srinivasan, M.R. (2018) Comparative evaluation of antimicrobial efficacy of silver nanoparticles and 2% chlorhexidine gluconate when used alone and in combination assessed using agar diffusion method: an in vitro study. Contemporary Clinical Dentistry, 9, S204–S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez de Paz, L.E. , Bergenholtz, G. , Dahlén, G. & Svensäter, G. (2007) Response to alkaline stress by root canal bacteria in biofilms. International Endodontic Journal, 40, 344–355. [DOI] [PubMed] [Google Scholar]
- Chong, B.S. & Pitt Ford, T.R. (1992) The role of intracanal medication in root canal treatment. International Endodontic Journal, 25, 97–106. [DOI] [PubMed] [Google Scholar]
- Cohenca, N. , Heilborn, C. , Johnson, J.D. , Flores, D.S. , Ito, I.Y. & da Silva, L.A. (2010) Apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing on root canal disinfection in dog teeth. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 109, e42–e46. [DOI] [PubMed] [Google Scholar]
- Cotti, E. , Mezzena, S. , Schirru, E. , Ottonello, O. , Mura, M. , Ideo, F. et al. (2018) Healing of apical periodontitis in patients with inflammatory bowel disease and under anti‐tumor necrosis factor alpha therapy. Journal of Endodontics, 44, 1777–1782. [DOI] [PubMed] [Google Scholar]
- Dalton, B.C. , Ørstavik, D. , Phillips, C. , Pettiette, M. & Trope, M. (1998) Bacterial reduction with nickel‐titanium rotary instrumentation. Journal of Endodontics, 24, 763–767. [DOI] [PubMed] [Google Scholar]
- Del Carpio‐Perochena, A. , Kishen, A. , Felitti, R. , Bhagirath, A.Y. , Medapati, M.R. , Lai, C. et al. (2017) Antibacterial properties of chitosan nanoparticles and propolis associated with calcium hydroxide against single‐ and multispecies biofilms: an in vitro and in situ study. Journal of Endodontics, 43, 1332–1336. [DOI] [PubMed] [Google Scholar]
- Divo, M.J. , Martinez, C.H. & Mannino, D.M. (2014) Ageing and the epidemiology of multimorbidity. European Respiratory Journal, 44, 1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmsmari, F. , González Sánchez, J.A. , Duran‐Sindreu, F. , Belkadi, R. , Espina, M. , García, M.L. et al. (2021) Calcium hydroxide‐loaded PLGA biodegradable nanoparticles as an intracanal medicament. International Endodontic Journal, 54, 2086–2098. [DOI] [PubMed] [Google Scholar]
- Estrela, C. , Bueno, M.R. , Azevedo, B.C. , Azevedo, J.R. & Pécora, J.D. (2008) A new periapical index based on cone beam computed tomography. Journal of Endodontics, 34, 1325–1331. [DOI] [PubMed] [Google Scholar]
- Fan, W. , Li, Y. , Sun, Q. , Ma, T. & Fan, B. (2016) Calcium‐silicate mesoporous nanoparticles loaded with chlorhexidine for both anti‐ Enterococcus faecalis and mineralization properties. Journal of Nanobiotechnology, 14, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, W. , Wu, D. , Tay, F.R. , Ma, T. , Wu, Y. & Fan, B. (2014) Effects of adsorbed and templated nanosilver in mesoporous calcium‐silicate nanoparticles on inhibition of bacteria colonization of dentin. International Journal of Nanomedicine, 9, 5217–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava, L.R. & Saunders, W.P. (1999) Calcium hydroxide pastes: classification and clinical indications. International Endodontic Journal, 32, 257–282. [DOI] [PubMed] [Google Scholar]
- Ferrucci, L. & Fabbri, E. (2018) Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology, 15, 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad, A.F. & Burleson, J. (2003) The effect of diabetes mellitus on endodontic treatment outcome: data from an electronic patient record. Journal American Dental Association, 134, 43–51; quiz 117–118. [DOI] [PubMed] [Google Scholar]
- Frank, A.L. (1966) Therapy for the divergent pulpless tooth by continued apical formation. Journal American Dental Association, 72, 87–93. [DOI] [PubMed] [Google Scholar]
- Gomes, B.P. , Vianna, M.E. , Sena, N.T. , Zaia, A.A. , Ferraz, C.C. & de Souza Filho, F.J. (2006) In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 102, 544–550. [DOI] [PubMed] [Google Scholar]
- Gonnella, J.S. , Hornbrook, M.C. & Louis, D.Z. (1984) Staging of disease. A case‐mix measurement. JAMA, 251, 637–644. [PubMed] [Google Scholar]
- Gonnella, J.S. , Louis, D.Z. & McCord, J.J. (1976) The staging concept–an approach to the assessment of outcome of ambulatory care. Medical Care, 14, 13–21. [DOI] [PubMed] [Google Scholar]
- Gonnella, J.S. & Louis, D.Z. (1987) Disease staging classification system. Medical Care, 25(4), 360. [DOI] [PubMed] [Google Scholar]
- Grossman, L.I. (1967) Rationale of endodontic treatment. Dental Clinics of North America, 483–490. [PubMed] [Google Scholar]
- Guerreiro‐Tanomaru, J.M. , Pereira, K.F. , Nascimento, C.A. , Bernardi, M.I. & Tanomaru‐Filho, M. (2013) Use of nanoparticulate zinc oxide as intracanal medication in endodontics: pH and antimicrobial activity. Acta Odontologica Latinoamericana, 26, 144–148. [PubMed] [Google Scholar]
- Haapasalo, M. , Qian, W. , Portenier, I. & Waltimo, T. (2007) Effects of dentin on the antimicrobial properties of endodontic medicaments. Journal of Endodontics, 33, 917–925. [DOI] [PubMed] [Google Scholar]
- Halkai, K.R. , Mudda, J.A. , Shivanna, V. , Rathod, V. & Halkai, R. (2018) Evaluation of antibacterial efficacy of fungal‐derived silver nanoparticles against Enterococcus faecalis . Contemporary Clinical Dentistry, 9, 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney, E.F. & Hancock, R.E. (2013) Peptide design for antimicrobial and immunomodulatory applications. Biopolymers, 100, 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselgren, G. , Olsson, B. & Cvek, M. (1988) Effects of calcium hydroxide and sodium hypochlorite on the dissolution of necrotic porcine muscle tissue. Journal of Endodontics, 14, 125–127. [DOI] [PubMed] [Google Scholar]
- Hirt, H. , Hall, J.W. , Larson, E. & Gorr, S.U. (2018) A D‐enantiomer of the antimicrobial peptide GL13K evades antimicrobial resistance in the Gram positive bacteria Enterococcus faecalis and Streptococcus gordonii. PLoS One, 13, e0194900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen, M.M. , LaBounty, G.L. & Strittmatter, E.J. (1990) Conservative treatment of persistent periradicular lesions using aspiration and irrigation. Journal of Endodontics, 16, 182–186. [DOI] [PubMed] [Google Scholar]
- Hoshino, E. , KuriharaAndo, N. , Sato, I. , Uematsu, H. , Sato, M. , Kota, K. et al. (1996) In‐vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. International Endodontic Journal, 29, 125–130. [DOI] [PubMed] [Google Scholar]
- Hunter, D. , Losina, E. , Guermazi, A. , Burstein, D. , Lassere, M. & Kraus, V. (2010) A pathway and approach to biomarker validation and qualification for osteoarthritis clinical trials. Current Drug Targets, 11, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell, K.V. & Caplan, D.J. (2018) 12‐month success of cracked teeth treated with orthograde root canal treatment. Journal of Endodontics, 44, 543–548. [DOI] [PubMed] [Google Scholar]
- Kvist, T. , Molander, A. , Dahlén, G. & Reit, C. (2004) Microbiological evaluation of one‐ and two‐visit endodontic treatment of teeth with apical periodontitis: a randomized, clinical trial. Journal of Endodontics, 30, 572–576. [DOI] [PubMed] [Google Scholar]
- Law, A.S. , Nixdorf, D.R. , Rabinowitz, I. , Reams, G.J. , Smith, J.A. Jr , Torres, A.V. et al. (2014) Root canal therapy reduces multiple dimensions of pain: a national dental practice‐based research network study. Journal of Endodontics, 40, 1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law, A.S. , Nixdorf, D.R. , Aguirre, A.M. , Reams, G.J. , Tortomasi, A.J. , Manne, B.D. et al. (2015) Predicting severe pain after root canal therapy in the National Dental PBRN. Journal of Dental Research, 94, 37S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.K. , Park, Y.J. , Kum, K.Y. , Han, S.H. , Chang, S.W. , Kaufman, B. et al. (2013) Antimicrobial efficacy of a human β‐defensin‐3 peptide using an Enterococcus faecalis dentine infection model. International Endodontic Journal, 46, 406–412. [DOI] [PubMed] [Google Scholar]
- Lee, S.H. & Baek, D.H. (2012) Antibacterial and neutralizing effect of human β‐defensins on Enterococcus faecalis and Enterococcus faecalis lipoteichoic acid. Journal of Endodontics, 38, 351–356. [DOI] [PubMed] [Google Scholar]
- Leng, D. , Li, Y. , Zhu, J. , Liang, R. , Zhang, C. , Zhou, Y. et al. (2020) The antibiofilm activity and mechanism of nanosilver‐ and nanozinc‐incorporated mesoporous calcium‐silicate nanoparticles. International Journal of Nanomedicine, 15, 3921–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindaman, L.M. (2001) Bone healing in children. Clinics in Podiatric Medicine and Surgery, 18, 97–108. [PubMed] [Google Scholar]
- Machtei, E.E. , Dunford, R. , Hausmann, E. , Grossi, S.G. , Powell, J. , Cummins, D. et al. (1997) Longitudinal study of prognostic factors in established periodontitis patients. Journal of Clinical Periodontology, 24, 102–109. [DOI] [PubMed] [Google Scholar]
- Makkar, H. & Patri, G. (2017) Fabrication and appraisal of poly (Lactic‐Co‐Glycolic Acid) ‐ Moxifloxacin nanoparticles using vitamin E‐TPGS: a potential intracanal drug delivery agent. Journal of Clinical and Diagnostic Research, 11, ZC05–ZC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzur, A. , González, A.M. , Pozos, A. , Silva‐Herzog, D. & Friedman, S. (2007) Bacterial quantification in teeth with apical periodontitis related to instrumentation and different intracanal medications: a randomized clinical trial. Journal of Endodontics, 33, 114–118. [DOI] [PubMed] [Google Scholar]
- Marending, M. , Peters, O.A. & Zehnder, M. (2005) Factors affecting the outcome of orthograde root canal therapy in a general dentistry hospital practice. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 99, 119–124. [DOI] [PubMed] [Google Scholar]
- Markson, L.E. , Nash, D.B. , Louis, D.Z. & Gonnella, J.S. (1991) Clinical outcomes management and disease staging. Evaluation & the Health Professions, 14, 201–227. [DOI] [PubMed] [Google Scholar]
- McGurkin‐Smith, R. , Trope, M. , Caplan, D. & Sigurdsson, A. (2005) Reduction of intracanal bacteria using GT rotary instrumentation, 5.25% NaOCl, EDTA, and Ca(OH)2. Journal of Endodontics, 31, 359–363. [DOI] [PubMed] [Google Scholar]
- Menakaya, I.N. , Oderinu, O.H. , Adegbulugbe, I.C. & Shaba, O.P. (2015) Incidence of postoperative pain after use of calcium hydroxide mixed with normal saline or 0.2% chlorhexidine digluconate as intracanal medicament in the treatment of apical periodontitis. Saudi Dent Journal, 27, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglani, S. & Tani‐Ishii, N. (2021) Biosynthesized selenium nanoparticles: characterization, antimicrobial, and antibiofilm activity against. PeerJ, 9, e11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander, A. , Warfvinge, J. , Reit, C. & Kvist, T. (2007) Clinical and radiographic evaluation of one‐ and two‐visit endodontic treatment of asymptomatic necrotic teeth with apical periodontitis: a randomized clinical trial. Journal of Endodontics, 33, 1145–1148. [DOI] [PubMed] [Google Scholar]
- Morsani, J.M. , Aminoshariae, A. , Han, Y.W. , Montagnese, T.A. & Mickel, A. (2011) Genetic predisposition to persistent apical periodontitis. Journal of Endodontics, 37, 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson‐Filho, P. , Leonardo, M.R. , Silva, L.A. & Assed, S. (2002) Radiographic evaluation of the effect of endotoxin (LPS) plus calcium hydroxide on apical and periapical tissues of dogs. Journal of Endodontics, 28, 694–696. [DOI] [PubMed] [Google Scholar]
- Ng, Y.L. , Mann, V. , Rahbaran, S. , Lewsey, J. & Gulabivala, K. (2008) Outcome of primary root canal treatment: systematic review of the literature – Part 2. Influence of clinical factors. International Endodontic Journal, 41, 6–31. [DOI] [PubMed] [Google Scholar]
- Ng, Y.L. , Mann, V. & Gulabivala, K. (2011a) A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: part 1: periapical health. International Endodontic Journal, 44, 583–609. [DOI] [PubMed] [Google Scholar]
- Ng, Y.L. , Mann, V. & Gulabivala, K. (2011b) A prospective study of the factors affecting outcomes of non‐surgical root canal treatment: part 2: tooth survival. International Endodontic Journal, 44, 610–625. [DOI] [PubMed] [Google Scholar]
- Nixdorf, D.R. , Law, A.S. , Look, J.O. , Rindal, D.B. , Durand, E.U. , Kang, W. et al. (2012) Large‐scale clinical endodontic research in the National Dental Practice‐Based Research Network: study overview and methods. Journal of Endodontics, 38, 1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf, D.R. , Law, A.S. , Lindquist, K. , Reams, G.J. , Cole, E. , Kanter, K. et al. (2016) Frequency, impact, and predictors of persistent pain after root canal treatment: a national dental PBRN study. Pain, 157, 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha, V.T. , Paula, A.J. , Durán, G. , Galembeck, A. , Cogo‐Müller, K. , Franz‐Montan, M. et al. (2017) Silver nanoparticles in dentistry. Dental Materials, 33, 1110–1126. [DOI] [PubMed] [Google Scholar]
- Novack, D.V. & Teitelbaum, S.L. (2008) The osteoclast: friend or foe? Annual Review Pathology: Mechanisms of Disease, 3, 457–484. [DOI] [PubMed] [Google Scholar]
- Ordinola‐Zapata, R. , Bramante, C.M. , Minotti, P.G. , Cavenago, B.C. , Garcia, R.B. , Bernardineli, N. et al. (2013) Antimicrobial activity of triantibiotic paste, 2% chlorhexidine gel, and calcium hydroxide on an intraoral‐infected dentin biofilm model. Journal of Endodontics, 39, 115–118. [DOI] [PubMed] [Google Scholar]
- Ørstavik, D. , Kerekes, K. & Eriksen, H.M. (1986) The periapical index: a scoring system for radiographic assessment of apical periodontitis. Endodontics Dental Traumatology, 2, 20–34. [DOI] [PubMed] [Google Scholar]
- Ørstavik, D. , Kerekes, K. & Molven, O. (1991) Effects of extensive apical reaming and calcium hydroxide dressing on bacterial infection during treatment of apical periodontitis: a pilot study. International Endodontic Journal, 24, 1–7. [DOI] [PubMed] [Google Scholar]
- Owatz, C.B. , Khan, A.A. , Schindler, W.G. , Schwartz, S.A. , Keiser, K. & Hargreaves, K.M. (2007) The incidence of mechanical allodynia in patients with irreversible pulpitis. Journal of Endodontics, 33, 552–556. [DOI] [PubMed] [Google Scholar]
- Oxford Centre for Evidence‐Based Medicine . Oxford Centre for Evidence‐Based Medicine: levels of evidence (March 2009). Available from: https://www.cebm.ox.ac.uk/resources/levels‐of‐evidence/oxford‐centre‐for‐evidence‐based‐medicine‐levels‐of‐evidence‐march‐2009 [Accessed February 1st 2022]. [Google Scholar]
- Paiva, S.S. , Siqueira, J.F. , Rôças, I.N. , Carmo, F.L , Leite, D.C.A. , Ferreira, D.C. et al. (2013) Clinical antimicrobial efficacy of NiTi rotary instrumentation with NaOCl irrigation, final rinse with chlorhexidine and interappointment medication: a molecular study. International Endodontic Journal, 46, 225–233. [DOI] [PubMed] [Google Scholar]
- Pal, I. , Bhattacharyya, D. , Kar, R.K. , Zarena, D. , Bhunia, A. & Atreya, H.S. (2019) A peptide‐nanoparticle system with improved efficacy against multidrug resistant bacteria. Scientific Reports, 9, 4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, I. , Brahmkhatri, V.P. , Bera, S. , Bhattacharyya, D. , Quirishi, Y. , Bhunia, A. et al. (2016) Enhanced stability and activity of an antimicrobial peptide in conjugation with silver nanoparticle. Journal of Colloid and Interface Science, 483, 385–393. [DOI] [PubMed] [Google Scholar]
- Paredes‐Vieyra, J. & Enriquez, F.J. (2012) Success rate of single‐versus two‐visit root canal treatment of teeth with apical periodontitis: a randomized controlled trial. Journal of Endodontics, 38, 1164–1169. [DOI] [PubMed] [Google Scholar]
- Parhizkar, A. , Nojehdehian, H. & Asgary, S. (2018) Triple antibiotic paste: momentous roles and applications in endodontics: a review. Restorative Dentistry and Endodontics, 43, e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penesis, V.A. , Fitzgeralg, P.I. , Fayad, M. , Wenckus, C.S. , BeGole, E.A. & Johnson, B.R. (2008) Outcome of one‐visit and two‐visit endodontic treatment of necrotic teeth with apical periodontitis: a randomized controlled trial with one‐year evaluation. Journal of Endodontics, 34, 251–257. [DOI] [PubMed] [Google Scholar]
- Petean, I.B.F. , Küchler, E.C. , Soares, I.M.V. , Segato, R.A.B. , Silva, L.A.B.D. , Antunes, L.A.A. et al. (2019) Genetic polymorphisms in RANK and RANKL are associated with persistent apical periodontitis. Journal of Endodontics, 45, 526–531. [DOI] [PubMed] [Google Scholar]
- Pirtskhalava, M. , Amstrong, A.A. , Grigolava, M. , Chubinidze, M. , Alimbarashvili, E. , Vishnepolsky, B. et al. (2021) DBAASP v3: database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Research, 49, D288–D297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenier, I. , Haapasalo, H. , Rye, A. , Waltimo, T. , Ørstavik, D. & Haapasalo, M. (2001) Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. International Endodontic Journal, 34, 184–188. [DOI] [PubMed] [Google Scholar]
- Portenier, I. , Haapasalo, H. , Ørstavik, D. , Yamauchi, M. & Haapasalo, M. (2002) Inactivation of the antibacterial activity of iodine potassium iodide and chlorhexidine digluconate against Enterococcus faecalis by dentin, dentin matrix, type‐I collagen, and heat‐killed microbial whole cells. Journal of Endodontics, 28, 634–637. [DOI] [PubMed] [Google Scholar]
- Portenier, I. , Waltimo, T. , Ørstavik, D. & Haapasalo, M. (2006) Killing of Enterococcus faecalis by MTAD and chlorhexidine digluconate with or without cetrimide in the presence or absence of dentine powder or BSA. Journal of Endodontics, 32, 138–141. [DOI] [PubMed] [Google Scholar]
- Prestinaci, F. , Pezzotti, P. & Pantosti, A. (2015) Antimicrobial resistance: a global multifaceted phenomenon. Pathogens and Global Health, 109, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricucci, D. , Candeiro, G.T. , Bugea, C. & Siqueira, J.F. (2016) Complex apical intraradicular infection and extraradicular mineralized biofilms as the cause of wet canals and treatment failure: report of 2 cases. Journal of Endodontics, 42, 509–515. [DOI] [PubMed] [Google Scholar]
- Ricucci, D. , Loghin, S. , Gonçalves, L.S. , Rôças, I.N. & Siqueira, J.F. (2018) Histobacteriologic conditions of the apical root canal system and periapical tissues in teeth associated with sinus tracts. Journal of Endodontics, 44, 405–413. [DOI] [PubMed] [Google Scholar]
- Ricucci, D. , Russo, J. , Rutberg, M. , Burleson, J.A. & Spångberg, L.S. (2011) A prospective cohort study of endodontic treatments of 1,369 root canals: results after 5 years. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 112, 825–842. [DOI] [PubMed] [Google Scholar]
- Ricucci, D. & Siqueira, J.F. (2010) Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. Journal of Endodontics, 36, 1277–1288. [DOI] [PubMed] [Google Scholar]
- Ricucci, D. & Siqueira, J.F. (2010a) Fate of the tissue in lateral canals and apical ramifications in response to pathologic conditions and treatment procedures. Journal of Endodontics, 36, 1–15. [DOI] [PubMed] [Google Scholar]
- Rôças, I.N. & Siqueira, J.F. (2008) Root canal microbiota of teeth with chronic apical periodontitis. Journal of Clinical Microbiology, 46, 3599–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, B. , Soto, N. , Villalba, M. , Bello‐Toledo, H. , Meléndrez‐Castro, M. & Sánchez‐Sanhueza, G. (2021) Antibacterial activity of copper nanoparticles (CuNPs) against a resistant calcium hydroxide multispecies endodontic biofilm. Nanomaterials, 11(9), 2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruden, S. , Hilpert, K. , Berditsch, M. , Wadhwani, P. & Ulrich, A.S. (2009) Synergistic interaction between silver nanoparticles and membrane‐permeabilizing antimicrobial peptides. Antimicrobial Agents and Chemotherapy, 53, 3538–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi, K.E. & Nichols, F.C. (1993) Effect of calcium hydroxide on bacterial lipopolysaccharide. Journal of Endodontics, 19, 76–78. [DOI] [PubMed] [Google Scholar]
- Safavi, K.E. & Nichols, F.C. (1994) Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. Journal of Endodontics, 20, 127–129. [DOI] [PubMed] [Google Scholar]
- Samiei, M. , Torab, A. , Hosseini, O. , Abbasi, T. , Abdollahi, A.A. & Divband, B. (2018) Antibacterial effect of two nano zinc oxide gel preparations compared to calcium hydroxide and chlorhexidine mixture. Iranian Endodontic Journal, 13, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathorn, C. , Parashos, P. & Messer, H.H. (2005) Effectiveness of single‐ versus multiple‐visit endodontic treatment of teeth with apical periodontitis: a systematic review and meta‐analysis. International Endodontic Journal, 38, 347–355. [DOI] [PubMed] [Google Scholar]
- Sathorn, C. , Parashos, P. & Messer, H. (2008) The prevalence of postoperative pain and flare‐up in single‐ and multiple‐visit endodontic treatment: a systematic review. International Endodontic Journal, 41, 91–99. [DOI] [PubMed] [Google Scholar]
- Savitha, A. , SriRekha, A. , Vijay, R. , Ashwija, C.C. & Jaykumar, T. (2019) An in vivo comparative evaluation of antimicrobial efficacy of chitosan, chlorhexidine gluconate gel and their combination as an intracanal medicament against Enterococcus faecalis in failed endodontic cases using real time polymerase chain reaction (qPCR). Saudi Dental Journal, 31, 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder, H. (1974) Cleaning and shaping the root canal. Dental Clinics North America, 18, 269–296. [PubMed] [Google Scholar]
- Shahmoradi, S. , Shariati, A. , Zargar, N. , Yadegari, Z. , Asnaashari, M. , Amini, S.M. et al. (2021) Antimicrobial effects of selenium nanoparticles in combination with photodynamic therapy against Enterococcus faecalis biofilm. Photodiagnosis and Photodynamic Therapy, 35, 102398. [DOI] [PubMed] [Google Scholar]
- Shuping, G.B. , Ørstavik, D. , Sigurdsson, A. & Trope, M. (2000) Reduction of intracanal bacteria using nickel‐titanium rotary instrumentation and various medications. Journal of Endodontics, 26, 751–755. [DOI] [PubMed] [Google Scholar]
- Silva, L.A. , Leonardo, M.R. , Assed, S. & Tanomaru Filho, M. (2004) Histological study of the effect of some irrigating solutions on bacterial endotoxin in dogs. Brazilian Dental Journal, 15, 109–114. [DOI] [PubMed] [Google Scholar]
- da Silva, L.A. , Nelson‐Filho, P. , da Silva, R.A. , Flores, D.S.H. , Heilborn, C. , Johnson, J.D. et al. (2010) Revascularization and periapical repair after endodontic treatment using apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing in dogs' teeth with apical periodontitis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 109, 779–787. [DOI] [PubMed] [Google Scholar]
- Silveira, C.F.D.S.M.P.D. , Malagutte, K.N.D.S. , Nogueira, B.F. , Reis, F.M. , Rodrigues, C.D.S.A. , Rossi, D.A.A. et al. (2011) Assessment of the antibacterial activity of calcium hydroxide combined with chlorhexidine paste and other intracanal medications against bacterial pathogens. European Journal of Dentistry, 5, 1–7. [PMC free article] [PubMed] [Google Scholar]
- Siqueira, J.F. & Lopes, H.P. (1999) Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. International Endodontic Journal, 32, 361–369. [DOI] [PubMed] [Google Scholar]
- Sjögren, U. , Figdor, D. , Spangberg, L. & Sundqvist, G. (1991) The antimicrobial effect of calcium hydroxide as a short‐term intracanal dressing. International Endodontic Journal, 24, 119–125. [DOI] [PubMed] [Google Scholar]
- Sjögren, U. , Figdor, D. , Persson, S. & Sundqvist, G. (1997) Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. International Endodontic Journal, 30, 297–306. [DOI] [PubMed] [Google Scholar]
- Southard, D.W. & Rooney, T.P. (1984) Effective one‐visit therapy for the acute periapical abscess. Journal of Endodontics, 10, 580–583. [DOI] [PubMed] [Google Scholar]
- Sundqvist, G. , Figdor, D. , Persson, S. & Sjögren, U. (1998) Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re‐treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 85, 86–93. [DOI] [PubMed] [Google Scholar]
- Suresh, N. , Subbarao, H.J. , Natanasabapathy, V. & Kishen, A. (2021) Maxillary anterior teeth with extensive root resorption treated with low‐level light‐activated engineered chitosan nanoparticles. Journal of Endodontics, 47, 1182–1190. [DOI] [PubMed] [Google Scholar]
- Suzuki, T.Y.U. , Gallego, J. , Assunção, W.G. , Briso, A.L.F. & Dos Santos, P.H. (2019) Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLoS One, 14, e0217750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweileh, W.M. (2021) Global research publications on irrational use of antimicrobials: call for more research to contain antimicrobial resistance. Globalization and Health, 17, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy, K. , Agossa, K. , Maton, M. , Chijcheapaza‐Flores, H. , Martel, B. , Siepmann, F. et al. (2021) How adding chlorhexidine or metallic nanoparticles affects the antimicrobial performance of calcium hydroxide paste as an intracanal medication: an in vitro study. Antibiotics, 10(11), 1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanomaru, J.M. , Leonardo, M.R. , Tanomaru Filho, M. , Bonetti Filho, I. & Silva, L.A. (2003) Effect of different irrigation solutions and calcium hydroxide on bacterial LPS. International Endodontic Journal, 36, 733–739. [DOI] [PubMed] [Google Scholar]
- Tello‐Barbaran, J. , Nakata, H.M. , Salcedo‐Moncada, D. , Bramante, C.M. & Ordinola‐Zapata, R. (2010) The antimicrobial effect of iodine‐potassium iodide after cleaning and shaping procedures in mesial root canals of mandibular molars. Acta Odontologica Latinoamericana, 23, 244–247. [PubMed] [Google Scholar]
- Tong, Z. , Huang, L. , Ling, J. , Mao, X. , Ning, Y. & Deng, D. (2014) Effects of intracanal irrigant MTAD Combined with nisin at sub‐minimum inhibitory concentration levels on Enterococcus faecalis growth and the expression of pathogenic genes. PLoS One, 9, e90235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabinejad, M. , Kettering, J.D. , McGraw, J.C. , Cummings, R.R. , Dwyer, T.G. & Tobias, T.S. (1988) Factors associated with endodontic interappointment emergencies of teeth with necrotic pulps. Journal of Endodontics, 14, 261–266. [DOI] [PubMed] [Google Scholar]
- Tronstad, L. , Andreasen, J.O. , Hasselgren, G. , Kristerson, L. & Riis, I. (1981) pH changes in dental tissues after root canal filling with calcium hydroxide. Journal of Endodontics, 7, 17–21. [DOI] [PubMed] [Google Scholar]
- Trope, M. (1991) Flare‐up rate of single‐visit endodontics. International Endodontic Journal, 24, 24–26. [DOI] [PubMed] [Google Scholar]
- Trope, M. , Delano, O. & Orstavik, D. (1999) Endodontic treatment of teeth with apical periodontitis: single vs. Multivisit treatment. Journal of Endodontics, 25, 345–350. [DOI] [PubMed] [Google Scholar]
- Türkün, M. & Cengiz, T. (1997) The effects of sodium hypochlorite and calcium hydroxide on tissue dissolution and root canal cleanliness. International Endodontic Journal, 30, 335–342. [DOI] [PubMed] [Google Scholar]
- Wang, G. , Li, X. & Wang, Z. (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Research, 44, D1087–D1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckwerth, P.H. , Zapata, R.O. , Vivan, R.R. , Tanomaru Filho, M. , Maliza, A.G. & Duarte, M.A. (2013) In vitro alkaline pH resistance of Enterococcus faecalis. Brazilian Dental Journal, 24, 474–476. [DOI] [PubMed] [Google Scholar]
- Winfred, S.B. , Meiyazagan, G. , Panda, J.J. , Nagendrababu, V. , Deivanayagam, K. , Chauhan, V.S. et al. (2014) Antimicrobial activity of cationic peptides in endodontic procedures. European Journal of Dentistry, 8, 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D. , Fan, W. , Kishen, A. , Gutmann, J.L. & Fan, B. (2014) Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. Journal of Endodontics, 40, 285–290. [DOI] [PubMed] [Google Scholar]
- Ye, Z. & Aparicio, C. (2019) Modulation of supramolecular self‐assembly of an antimicrobial designer peptide by single amino acid substitution: implications on peptide activity. Nanoscale Advances, 1, 4679–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Z. & Aparicio, C. (2022) Interactions of two enantiomers of a designer antimicrobial peptide with structural components of the bacterial cell envelope. Journal of Peptide Science, 28, e3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Z. , Ting, S. , Kun, L. , Nicholas, G.F. , Isha, M. & Constanza, E. (2022) Hybrid nanocoatings of self‐assembled organic‐inorganic amphiphiles for prevention of implant infections. Acta biomaterialia, 140, 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Z. , & Zhu X. Mutreja I. Boda S.K. Fischer N.G., Zhang A. et al. (2021) Biomimetic mineralized hybrid scaffolds with antimicrobial peptides. Bioactive Materials, 6, 2250–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoldas, O. , Topuz, A. , Isçi, A.S. & Oztunc, H. (2004) Postoperative pain after endodontic retreatment: single‐ versus two‐visit treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 98, 483–487. [DOI] [PubMed] [Google Scholar]
- Zapata, R.O. , Bramante, C.M. , Gomes de Moraes, I. , Bernardineli, N. , Gasparoto, T.H. , Graeff, M.S. et al. (2008) Confocal laser scanning microscopy is appropriate to detect Enterococcus faecalis in infected dentin. Journal of Endodontics, 34, 1198–1201. [DOI] [PubMed] [Google Scholar]
- Zerella, J.A. , Fouad, A.F. & Spångberg, L.S. (2005) Effectiveness of a calcium hydroxide and chlorhexidine digluconate mixture as disinfectant during retreatment of failed endodontic cases. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 100, 756–761. [DOI] [PubMed] [Google Scholar]
- Zheng, T. , Huang, X. , Chen, J. , Feng, D. , Mei, L. , Huang, Y. et al. (2018) A liquid crystalline precursor incorporating chlorhexidine acetate and silver nanoparticles for root canal disinfection. Biomaterials Science, 6, 596–603. [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Liang, R. , Sun, C. , Xie, L. , Wang, J. , Leng, D. et al. (2017) Effects of nanosilver and nanozinc incorporated mesoporous calcium‐silicate nanoparticles on the mechanical properties of dentin. PLoS One, 12, e0182583. [DOI] [PMC free article] [PubMed] [Google Scholar]


