Abstract
Aims
To evaluate the efficacy and safety of oral semaglutide versus comparators by patient characteristic subgroups in patients with type 2 diabetes.
Materials and Methods
Change from baseline in glycated haemoglobin (HbA1c) and body weight, and achievement of HbA1c <7.0% with oral semaglutide 7 mg, oral semaglutide 14 mg, flexibly dosed oral semaglutide (flex) and comparators were assessed across baseline subgroups (age, race, ethnicity, diabetes duration, body mass index and HbA1c) from the PIONEER programme. Treatment differences were analysed using a mixed model for repeated measurements for continuous variables and a logistic regression model for the binary endpoint. Pooled safety data were analysed descriptively.
Results
Changes from baseline in HbA1c and body weight, and the odds of achieving HbA1c <7.0%, were greater with oral semaglutide 14 mg/flex (n = 1934) and higher or similar with oral semaglutide 7 mg (n = 823) versus comparators (n = 2077) across most subgroups. Changes in HbA1c with oral semaglutide 14 mg/flex were greater for patients with higher baseline HbA1c (HbA1c >9.0%: –1.7% to –2.6%; HbA1c <8.0%: –0.7% to –1.2%). In some trials, Asian patients experienced greater HbA1c reductions with oral semaglutide 14 mg/flex (–1.5% to –1.8%) than other racial groups (–0.6% to –1.6%). The overall incidence of adverse events (AEs) with oral semaglutide was similar to that with comparators and was consistent across subgroups. More gastrointestinal AEs were observed with oral semaglutide, versus comparators, across subgroups.
Conclusions
Oral semaglutide demonstrated consistently greater HbA1c and body weight reductions across a range of patient characteristics, with greater HbA1c reductions seen at higher baseline HbA1c levels.
Keywords: antidiabetic drug, GLP‐1 analogue, glycaemic control, incretin therapy, type 2 diabetes, weight control
1. INTRODUCTION
Current guidelines emphasize the need for a patient‐centric approach in type 2 diabetes (T2D), with treatment individualized to a patient's clinical characteristics. 1 , 2 The efficacy and safety response to a diabetes therapy may vary according to patient characteristics, such as age, body mass index (BMI) and race, as well as indicators of disease status, such as glycated haemoglobin (HbA1c) and duration of disease. 3 , 4 , 5 , 6 , 7 Clinical trials often enrol patient populations with a broad range of characteristics and provide mean data for the whole population studied. However, each patient a physician sees is an individual and is rarely, if ever, representative of the mean. It is, therefore, important to understand how different patients may respond to new therapies so that physicians can better understand what response to expect and thereby have better informed conversations with individual patients. Analyses of trials according to patient characteristic subgroups can help to inform physicians on how the efficacy and safety of therapies might vary in patients with different clinical characteristics.
Oral semaglutide is the first glucagon‐like peptide‐1 analogue available in a tablet that has been approved in Europe, Japan and the United States for the treatment of T2D. 8 , 9 , 10 The efficacy and safety of oral semaglutide have been established in patients with T2D in the global phase 3a PIONEER programme. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 To provide physicians with data to support the decision‐making process and to better understand what responses they might expect with oral semaglutide in different patient groups, a comprehensive exploratory analysis of the PIONEER programme detailing the glycaemic and weight loss efficacy, and safety of oral semaglutide versus comparators by patient subgroups was conducted.
2. MATERIALS AND METHODS
2.1. Trial designs
The design of trials within the global PIONEER phase 3a programme have been reported previously. 11 , 12 , 13 , 14 , 15 , 16 , 17 The PIONEER efficacy trials (PIONEER 1–5, 7 and 8) were used for the present analyses; PIONEER 6 was a cardiovascular (CV) outcomes trial 18 and was, therefore, not included in the subgroup analyses.
PIONEER 1, 4, 5 and 8 were placebo‐controlled trials and PIONEER 2, 3, 4 and 7 were active‐controlled trials (empagliflozin, sitagliptin or liraglutide; Table S1). PIONEER 1, 3 and 8 investigated three once‐daily doses of oral semaglutide (3, 7 and 14 mg), PIONEER 2, 4 and 5 assessed oral semaglutide 14 mg, and PIONEER 7 employed a flexible dose adjustment approach (flex), where the dose could be increased or decreased according to predefined efficacy or tolerability criteria. Here, we report oral semaglutide 14 mg/flex data (with 7 mg data in Appendix S1). The 3 mg dose is for the purposes of dose escalation and is not a maintenance dose, 8 , 9 and was therefore not included in the analysis. Treatment durations were 26 weeks for PIONEER 1 and 5, 52 weeks for PIONEER 2, 4, 7 and 8 and 78 weeks for PIONEER 3.
The trials were performed according to relevant local regulatory guidance, and complied with the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice.
For further details on the primary endpoint and patient populations in the PIONEER trials, please refer to Table S1.
2.2. Subgroup analyses
All patients receiving oral semaglutide 7 or 14 mg (PIONEER 1–5 and 8), flex (PIONEER 7) or comparators were included in the subgroup analyses. Subgroup analyses were conducted for the following endpoints:
Change from baseline in HbA1c to end of treatment
Change from baseline in body weight to end of treatment
Achievement of HbA1c <7.0% at end of treatment.
The PIONEER programme employed two estimands, which have been described in detail elsewhere. 11 Efficacy data for the trial product estimand (on trial product without rescue medication dataset) from the PIONEER 1–5, 7 and 8 trials were analysed for the following subgroups:
Age: <45 years, ≥45 to <65 years, and ≥65 years
Race: White, Black/African American, and Asian
Ethnicity: Hispanic/Latino and non‐Hispanic/Latino
Diabetes duration: <5 years, ≥5 to <10 years, and ≥10 years
BMI: <25 kg/m2, ≥25 to <30 kg/m2, ≥30 to <35 kg/m2, and ≥35 kg/m2
Baseline HbA1c: ≤8%, >8 to ≤9%, and >9%.
Due to the low numbers of some racial and ethnic subgroups in PIONEER 5, this trial was excluded from the racial and ethnic efficacy subgroup analyses.
2.3. Statistical analyses
Data on the changes from baseline in HbA1c and body weight were analysed using a mixed model for repeated measurements, with treatment, region, strata (PIONEER 3–8), subgroup, interaction strata (PIONEER 5 and 8) and interaction between treatment and subgroup as categorical fixed effects and baseline value as covariate, all nested within visit and an unstructured residual covariance matrix.
The binary endpoint (achievement of HbA1c <7.0%) was analysed using a logistic regression model with treatment, region, strata (PIONEER 3–8), subgroup, interaction strata (PIONEER 5 and 8) and interaction between treatment and subgroup as categorical fixed effects and baseline value as covariate for each of the 1000 imputed complete datasets and pooled by Rubin's rule to draw inference. Missing values for continuous endpoints that enter the binary endpoint were imputed using an analysis of covariance‐based sequential multiple imputation model and the categorical endpoint derived from there. For the race (but not ethnicity) analyses, region was not included as a categorical fixed effect.
Subgroup interaction tests (5% significance level) were based on the estimated treatment differences for oral semaglutide versus comparator for the change from baseline endpoints and the estimated odds ratios (ORs) for the achievement of HbA1c <7.0%.
Because of the potential for type I errors due to multiple comparisons, findings should be interpreted as exploratory.
Safety data were pooled for all trials and analysed descriptively. For the age subgroup analyses, subgroups of <65 years and ≥65 years were used to assess for increased risk of adverse outcomes in patients aged ≥65 years with T2D.
3. RESULTS
In the PIONEER 1–5, 7 and 8 trials, the numbers of patients randomized were: oral semaglutide 14 mg/flex, N = 1934; oral semaglutide 7 mg, N = 823; and comparators, N = 2077 (placebo, n = 665 11 , 15 , 16 , 17 ; empagliflozin, n = 410 12 ; liraglutide, n = 284 15 ; sitagliptin, n = 718 13 , 14 ). Detailed baseline characteristics are described by study and patient subgroup in Table 1.
TABLE 1.
Baseline characteristics by study and subgroup
| Trial | Subgroup | Patients, N | Mean age, y | Mean duration of diabetes, y | Mean HbA1c, % | Mean body weight, kg |
|---|---|---|---|---|---|---|
| Age | ||||||
| PIONEER 1 | <45 years | 130 | 38 | 1.8 | 8.0 | 99.9 |
| ≥45–<65 years | 430 | 55 | 3.2 | 8.0 | 87.7 | |
| ≥65 years | 143 | 69 | 6.2 | 7.8 | 78.7 | |
| PIONEER 2 | <45 years | 94 | 40 | 4.1 | 8.4 | 101.1 |
| ≥45–<65 years | 512 | 56 | 6.6 | 8.2 | 92.3 | |
| ≥65 years | 215 | 69 | 10.8 | 7.9 | 85.9 | |
| PIONEER 3 | <45 years | 183 | 39 | 4.8 | 8.4 | 102.1 |
| ≥45–<65 years | 1179 | 56 | 8.2 | 8.4 | 91.7 | |
| ≥65 years | 501 | 69 | 11.0 | 8.1 | 86.2 | |
| PIONEER 4 | <45 years | 86 | 40 | 5.3 | 8.1 | 100.5 |
| ≥45–<65 years | 475 | 55 | 7.0 | 8.0 | 94.9 | |
| ≥65 years | 150 | 69 | 10.9 | 7.8 | 87.5 | |
| PIONEER 5 | <45 years | – | – | – | – | – |
| ≥45–<65 years | 65 | 59 | 11.1 | 8.0 | 102.7 | |
| ≥65 years | 259 | 73 | 14.7 | 8.0 | 87.8 | |
| PIONEER 7 | <45 years | 46 | 39 | 4.9 | 8.3 | 90.7 |
| ≥45–<65 years | 321 | 55 | 8.0 | 8.3 | 90.6 | |
| ≥65 years | 137 | 69 | 11.8 | 8.2 | 83.5 | |
| PIONEER 8 | <45 years | 43 | 40 | 9.5 | 8.3 | 88.5 |
| ≥45–<65 years | 410 | 57 | 13.2 | 8.2 | 88.9 | |
| ≥65 years | 278 | 70 | 18.6 | 8.1 | 81.1 | |
| Race | ||||||
| PIONEER 1 | White | 262 | 52 | 2.4 | 7.9 | 93.0 |
| Black/AA | 20 | 59 | 3.6 | 7.6 | 92.2 | |
| Asian | 60 | 59 | 7.6 | 8.0 | 67.2 | |
| PIONEER 2 | White | 708 | 58 | 7.5 | 8.1 | 92.1 |
| Black/AA | 59 | 55 | 6.9 | 8.4 | 97.0 | |
| Asian | 49 | 54 | 7.3 | 8.2 | 77.4 | |
| PIONEER 3 | White | 650 | 58 | 8.6 | 8.2 | 94.4 |
| Black/AA | 84 | 56 | 7.6 | 8.3 | 94.9 | |
| Asian | 120 | 57 | 11.0 | 8.2 | 73.4 | |
| PIONEER 4 | White | 519 | 57 | 7.5 | 8.0 | 98.0 |
| Black/AA | 29 | 54 | 8.7 | 8.1 | 96.8 | |
| Asian | 94 | 53 | 8.3 | 8.0 | 79.0 | |
| PIONEER 7 | White | 381 | 57 | 8.2 | 8.3 | 91.8 |
| Black/AA | 47 | 60 | 9.9 | 8.4 | 91.5 | |
| Asian | 72 | 57 | 11.8 | 8.2 | 71.2 | |
| PIONEER 8 | White | 192 | 62 | 13.5 | 8.2 | 92.7 |
| Black/AA | 24 | 59 | 14.1 | 8.3 | 98.6 | |
| Asian | 131 | 59 | 15.3 | 8.2 | 71.0 | |
| Ethnicity | ||||||
| PIONEER 1 | Hispanic/Latino | 97 | 51 | 2.5 | 8.0 | 85.2 |
| Non‐Hispanic/Latino | 243 | 55 | 3.8 | 7.9 | 89.9 | |
| PIONEER 2 | Hispanic/Latino | 199 | 56 | 7.9 | 8.3 | 85.9 |
| Non‐Hispanic/Latino | 622 | 58 | 7.3 | 8.1 | 93.4 | |
| PIONEER 3 | Hispanic/Latino | 168 | 55 | 9.0 | 8.4 | 84.4 |
| Non‐Hispanic/Latino | 743 | 58 | 8.8 | 8.2 | 92.4 | |
| PIONEER 4 | Hispanic/Latino | 40 | 56 | 9.6 | 8.0 | 86.2 |
| Non‐Hispanic/Latino | 671 | 56 | 7.5 | 8.0 | 94.5 | |
| PIONEER 7 | Hispanic/Latino | 105 | 56 | 8.7 | 8.4 | 86.4 |
| Non‐Hispanic/Latino | 399 | 58 | 8.8 | 8.3 | 89.2 | |
| PIONEER 8 | Hispanic/Latino | 55 | 61 | 13.0 | 8.3 | 82.4 |
| Non‐Hispanic/Latino | 293 | 61 | 14.4 | 8.2 | 85.5 | |
| Duration of diabetes | ||||||
| PIONEER 1 | <5 years | 529 | 53 | 1.3 | 7.9 | 90.9 |
| ≥5–<10 years | 108 | 58 | 7.1 | 7.9 | 82.2 | |
| ≥10 years | 66 | 62 | 15.7 | 8.0 | 75.8 | |
| PIONEER 2 | <5 years | 347 | 54 | 2.6 | 8.1 | 96.3 |
| ≥5–<10 years | 274 | 58 | 7.4 | 8.1 | 90.3 | |
| ≥10 years | 200 | 63 | 15.8 | 8.2 | 85.3 | |
| PIONEER 3 | <5 years | 577 | 54 | 2.8 | 8.2 | 96.5 |
| ≥5–<10 years | 687 | 58 | 7.5 | 8.3 | 91.1 | |
| ≥10 years | 599 | 62 | 15.5 | 8.3 | 86.3 | |
| PIONEER 4 | <5 years | 278 | 54 | 2.6 | 7.9 | 96.5 |
| ≥5–<10 years | 238 | 55 | 7.5 | 8.1 | 95.1 | |
| ≥10 years | 195 | 61 | 14.7 | 7.9 | 89.0 | |
| PIONEER 5 | <5 years | 30 | 69 | 3.6 | 7.9 | 95.4 |
| ≥5–<10 years | 82 | 69 | 7.5 | 7.9 | 92.9 | |
| ≥10 years | 212 | 71 | 18.0 | 8.0 | 89.4 | |
| PIONEER 7 | <5 years | 168 | 53 | 2.9 | 8.3 | 94.6 |
| ≥5–<10 years | 153 | 57 | 7.4 | 8.3 | 88.6 | |
| ≥10 years | 183 | 61 | 15.4 | 8.3 | 83.3 | |
| PIONEER 8 | <5 years | 69 | 55 | 3.3 | 8.2 | 92.1 |
| ≥5–<10 years | 145 | 57 | 7.8 | 8.2 | 87.1 | |
| ≥10 years | 517 | 62 | 18.6 | 8.2 | 84.7 | |
| Baseline BMI | ||||||
| PIONEER 1 | <25 kg/m2 | 95 | 61 | 7.5 | 8.0 | 60.6 |
| ≥25–<30 kg/m2 | 198 | 56 | 3.3 | 8.0 | 76.1 | |
| ≥30–<35 kg/m2 | 223 | 54 | 2.6 | 8.0 | 90.3 | |
| ≥35 kg/m2 | 187 | 50 | 2.9 | 7.9 | 112.3 | |
| PIONEER 2 | <25 kg/m2 | 52 | 58 | 9.6 | 8.2 | 68.0 |
| ≥25–<30 kg/m2 | 241 | 60 | 8.6 | 8.1 | 76.6 | |
| ≥30–<35 kg/m2 | 265 | 58 | 7.2 | 8.2 | 90.6 | |
| ≥35 kg/m2 | 262 | 56 | 6.2 | 8.2 | 111.0 | |
| PIONEER 3 | <25 kg/m2 | 185 | 61 | 11.2 | 8.2 | 62.8 |
| ≥25–<30 kg/m2 | 532 | 60 | 9.6 | 8.3 | 78.1 | |
| ≥30–<35 kg/m2 | 557 | 58 | 8.2 | 8.4 | 90.7 | |
| ≥35 kg/m2 | 589 | 55 | 7.3 | 8.3 | 112.6 | |
| PIONEER 4 | <25 kg/m2 | 50 | 58 | 8.8 | 7.9 | 66.0 |
| ≥25–<30 kg/m2 | 210 | 58 | 8.8 | 8.0 | 78.7 | |
| ≥30–<35 kg/m2 | 217 | 56 | 7.4 | 7.9 | 93.5 | |
| ≥35 kg/m2 | 234 | 55 | 6.5 | 8.0 | 114.2 | |
| PIONEER 5 | <25 kg/m2 | 20 | 74 | 17.4 | 7.8 | 67.6 |
| ≥25–<30 kg/m2 | 90 | 72 | 15.7 | 7.9 | 80.8 | |
| ≥30–<35 kg/m2 | 129 | 71 | 13.7 | 8.0 | 89.2 | |
| ≥35 kg/m2 | 84 | 67 | 12.0 | 8.0 | 109.7 | |
| PIONEER 7 | <25 kg/m2 | 61 | 62 | 11.9 | 8.3 | 66.3 |
| ≥25–<30 kg/m2 | 164 | 60 | 9.8 | 8.2 | 78.4 | |
| ≥30–<35 kg/m2 | 162 | 56 | 8.6 | 8.3 | 92.0 | |
| ≥35 kg/m2 | 117 | 54 | 6.1 | 8.4 | 110.0 | |
| PIONEER 8 | <25 kg/m2 | 139 | 62 | 17.1 | 8.0 | 60.8 |
| ≥25–<30 kg/m2 | 217 | 62 | 16.3 | 8.2 | 76.5 | |
| ≥30–<35 kg/m2 | 199 | 60 | 14.0 | 8.2 | 91.2 | |
| ≥35 kg/m2 | 176 | 59 | 13.1 | 8.3 | 111.3 | |
| Baseline HbA1c | ||||||
| PIONEER 1 | ≤8% | 409 | 55 | 3.5 | 7.5 | 89.0 |
| >8–≤9% | 244 | 53 | 3.5 | 8.5 | 87.0 | |
| >9% | 50 | 54 | 3.7 | 9.3 | 86.8 | |
| PIONEER 2 | ≤8% | 457 | 59 | 7.2 | 7.4 | 91.3 |
| >8–≤9% | 211 | 57 | 7.9 | 8.5 | 92.9 | |
| >9% | 153 | 55 | 7.4 | 9.7 | 90.9 | |
| PIONEER 3 | ≤8% | 850 | 59 | 8.4 | 7.5 | 90.6 |
| >8–≤9% | 593 | 57 | 8.7 | 8.5 | 91.4 | |
| >9% | 420 | 56 | 8.8 | 9.7 | 92.4 | |
| PIONEER 4 | ≤8% | 403 | 57 | 7.5 | 7.4 | 92.8 |
| >8–≤9% | 248 | 56 | 7.8 | 8.5 | 94.1 | |
| >9% | 60 | 53 | 7.2 | 9.3 | 101.2 | |
| PIONEER 5 | ≤8% | 188 | 71 | 13.1 | 7.5 | 91.5 |
| >8–≤9% | 108 | 70 | 15.7 | 8.5 | 89.2 | |
| >9% | 28 | 69 | 13.4 | 9.3 | 92.6 | |
| PIONEER 7 | ≤8% | 201 | 58 | 8.6 | 7.7 | 87.3 |
| >8–≤9% | 246 | 57 | 8.9 | 8.5 | 88.2 | |
| >9% | 57 | 56 | 8.7 | 9.4 | 95.2 | |
| PIONEER 8 | ≤8% | 329 | 62 | 15.6 | 7.5 | 83.6 |
| >8–≤9% | 296 | 60 | 14.9 | 8.5 | 87.6 | |
| >9% | 106 | 58 | 13.5 | 9.3 | 88.1 | |
Note: PIONEER 5 was not included in the race or ethnicity analyses due to small numbers of patients in some subgroups.
Abbreviations: AA, African American, BMI, body mass index; HbA1c, glycated haemoglobin; y, years.
3.1. Age subgroups
Baseline body weight tended to be higher in younger patients, while baseline HbA1c was similar across age groups (Table 1). HbA1c reductions with oral semaglutide 14 mg/flex were similar across age subgroups, and were larger with oral semaglutide 14 mg/flex than comparators, irrespective of age (Figure 1A). There were no significant subgroup interactions for treatment differences in HbA1c by age. Across age groups, the glycaemic efficacy of oral semaglutide 7 mg was greater than that of placebo (PIONEER 1 and 8), and similar to, or greater than, that of sitagliptin (PIONEER 3; Figure S1A). Across trials and age subgroups, estimated ORs consistently favoured oral semaglutide 7 and 14 mg, and flex, over comparators for the achievement of the HbA1c <7.0% target at the end of treatment (Figure S2), and there was no interaction of subgroups on the estimated ORs.
FIGURE 1.
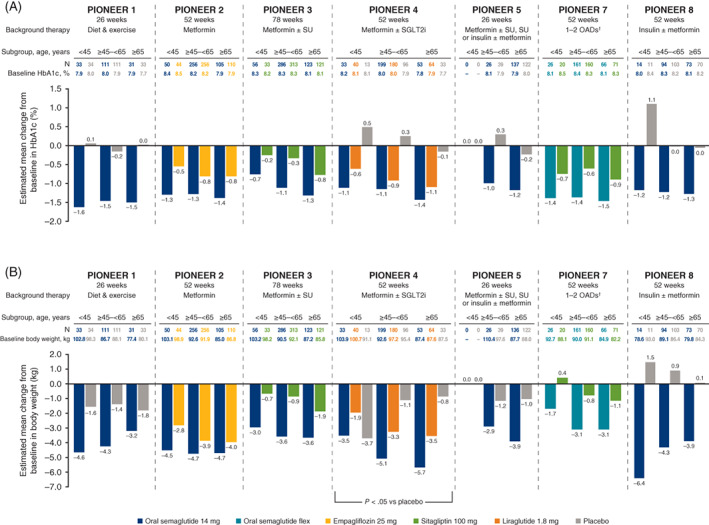
Change from baseline in A, glycated haemoglobin (HbA1c), and B, body weight, by baseline age. Abbreviations: flex, flexibly dosed; OAD, oral antidiabetes drug; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea. †Including metformin, an SU, an SGLT2i or a thiazolidinedione. Analyses were conducted at the end of treatment on data from the full analysis set using the trial product estimand. The P value indicates a significant treatment‐by‐subgroup interaction with regard to the estimated treatment differences for that trial (two‐sided significance test)
Body weight reductions were generally greater with oral semaglutide 14 mg/flex than with comparators, largely without association between age subgroup and reduction in body weight, except for oral semaglutide 14 mg versus placebo in PIONEER 4 (Figure 1B). Oral semaglutide 7 mg was also associated with larger decreases in body weight than placebo and sitagliptin across age subgroups (Figure S1B).
3.2. Racial and ethnic subgroups
Baseline characteristics were similar across race and ethnicity subgroups in each study except for body weight, which was generally lower in the Asian versus other racial subgroups (Table 1). HbA1c reductions were generally greater with oral semaglutide 14 mg/flex versus comparators across racial and ethnic subgroups (Figures S3A and S4A). In the placebo‐controlled PIONEER 1, 4 and 8 trials, a significant interaction was seen between treatment and race, with the greatest HbA1c estimated treatment difference with oral semaglutide 14 mg in the Asian subgroup (Figure S3A). There were no significant subgroup interactions in the ethnicity analyses (Figure S4A). HbA1c reductions with oral semaglutide 7 mg were greater than with placebo and were similar to, or greater than, those observed with sitagliptin (Figures S5A and S6A). The odds of achieving HbA1c <7.0% by baseline race or ethnicity subgroup across the PIONEER trials generally favoured oral semaglutide over comparators, and there were no significant subgroup‐by‐treatment interactions (Figures S7 and S8).
Oral semaglutide 14 mg/flex generally reduced body weight to a greater extent than comparators, regardless of race or ethnicity, with no clear pattern in change across trials and racial subgroups (Figures S3B and S4B); only one significant interaction was seen for ethnicity in PIONEER 7. Body weight reductions with oral semaglutide 7 mg were generally greater than with placebo and were similar to, or greater than, those observed with sitagliptin (Figures S5B and S6B).
3.3. Diabetes duration subgroups
Mean age was lower and mean body weight was higher in the subgroup with the shortest diabetes duration (<5 years) at baseline. Mean HbA1c was similar at baseline across the diabetes duration subgroups within each trial (Table 1).
Across the PIONEER trials, the efficacy of oral semaglutide 14 mg/flex in reducing HbA1c was consistently greater than for comparators, irrespective of diabetes duration, with no treatment interactions across subgroups (Figure 2A). Oral semaglutide 7 mg was also associated with larger decreases in HbA1c than placebo, and larger or similar decreases compared with sitagliptin, across diabetes duration subgroups (Figure S9A). Across trials and diabetes duration subgroups, estimated ORs consistently favoured oral semaglutide 7 and 14 mg, and flex, over comparators for the achievement of HbA1c <7.0% (Figure S10), with no significant subgroup interaction observed.
FIGURE 2.
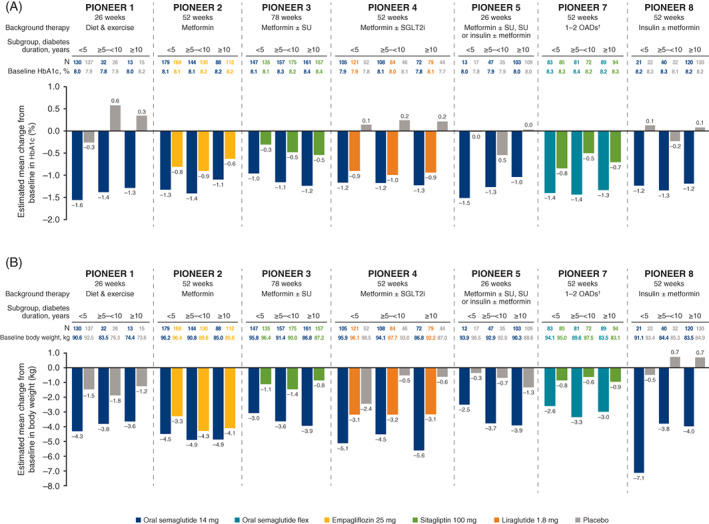
Change from baseline in A, glycated haemoglobin (HbA1c), and B, body weight, by duration of diabetes. Abbreviations: flex, flexibly dosed; OAD, oral antidiabetes drug; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea. †Including metformin, an SU, an SGLT2i or a thiazolidinedione. Analyses were conducted at the end of treatment on data from the full analysis set using the trial product estimand. No significant treatment‐by‐subgroup interactions were observed with regard to the estimated treatment differences for each trial (two‐sided significance test)
Body weight reductions were generally greater with oral semaglutide 14 mg/flex than with comparators, without any significant association between treatment and diabetes duration subgroups (Figure 2B). Oral semaglutide 7 mg was also associated with larger decreases in body weight than placebo and sitagliptin across diabetes duration subgroups (Figure S9B).
3.4. Baseline BMI subgroups
Across BMI subgroups, HbA1c levels were similar at baseline. In line with the observations from the age and diabetes duration subgroups characteristics, patients with higher BMI tended to be younger and have a shorter diabetes duration (Table 1). Reductions in HbA1c with oral semaglutide 14 mg/flex were greater than with comparators across BMI subgroups and across trials (Figure S11A), and there was no significant interaction between subgroups and treatment differences. HbA1c reductions with oral semaglutide 7 mg were greater than placebo and similar to, or greater than, sitagliptin (Figure S12A). There were no significant subgroup interactions for the odds of achieving HbA1c <7.0% by baseline BMI subgroup across the PIONEER trials, and ORs generally favoured oral semaglutide over comparators (Figure S13).
Oral semaglutide 14 mg/flex also reduced body weight more than comparators across the PIONEER trials and across BMI subgroups. One significant subgroup‐by‐treatment interaction was observed for oral semaglutide 14 mg compared with liraglutide in PIONEER 4, but there was no clear pattern across the BMI subgroups: treatment differences in the <25 and ≥30 to <35 kg/m2 subgroups were larger than in the ≥25 to <30 and ≥35 kg/m2 subgroups (Figure S11B). Body weight reductions with oral semaglutide 7 mg were generally greater than with placebo and sitagliptin (Figure S12B).
3.5. Baseline HbA1c subgroups
At baseline, mean age, diabetes duration and body weight were generally similar across HbA1c subgroups (Table 1). Changes in HbA1c with oral semaglutide 14 mg/flex were greater for patients with higher baseline HbA1c (HbA1c >9%: –1.7% to –2.6%; HbA1c <8%: –0.7% to –1.2%; Figure 3A), and the HbA1c reductions were generally greater than with comparators across trials and subgroups of baseline HbA1c. When comparing subgroups, treatment differences with oral semaglutide 14 mg/flex were numerically greater in patients with higher baseline HbA1c, although only one significant treatment‐by‐subgroup interaction was observed for oral semaglutide 14 mg versus placebo in PIONEER 4 (Figure 3A). Because a consistent pattern of HbA1c responses with oral semaglutide 14 mg across subgroups was observed in all PIONEER trials, a pooled analysis of the placebo‐controlled trials (only performed at Week 26 as this was the latest common timepoint) was performed to investigate further. This additional analysis confirmed the observed pattern with a significant treatment‐by‐subgroup interaction across the placebo‐controlled trials (P < .0001), with the greatest HbA1c treatment differences in those with highest baseline HbA1c levels (Figure S14). Oral semaglutide 7 mg was also associated with larger decreases in HbA1c than placebo and larger, or similar, decreases compared with sitagliptin across HbA1c subgroups (Figure S15A).
FIGURE 3.
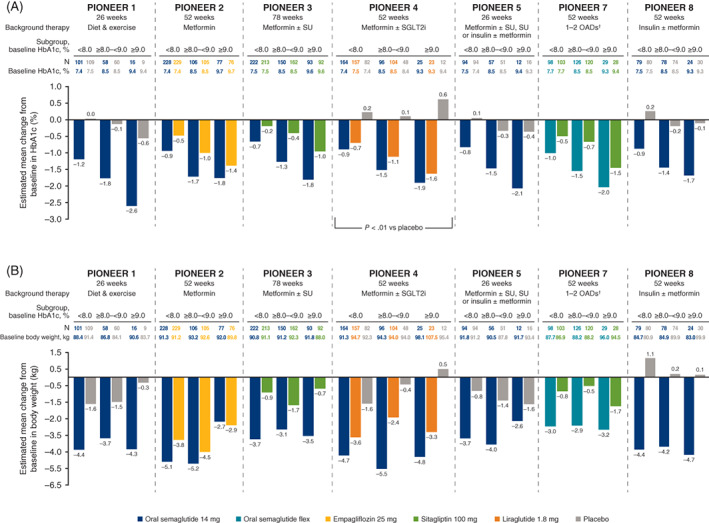
Change from baseline in A, glycated haemoglobin (HbA1c), and B, body weight, by baseline HbA1c. Abbreviations: flex, flexibly dosed; OAD, oral antidiabetes drug; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea. †Including metformin, an SU, an SGLT2i or a thiazolidinedione. Analyses were conducted at the end of treatment on data from the full analysis set using the trial product estimand. The P value indicates a significant treatment‐by‐subgroup interaction with regard to the estimated treatment differences for that trial (two‐sided significance test)
Across trials and baseline HbA1c subgroups, estimated ORs consistently favoured oral semaglutide 7 and 14 mg, and flex, over comparators for the achievement of HbA1c <7.0% (Figure S16), with no significant interaction across HbA1c subgroups.
Reductions in body weight were generally greater with oral semaglutide 14 mg/flex versus comparators. However, there was no consistent relationship between change in body weight and baseline HbA1c (Figure 3B). Oral semaglutide 7 mg was also associated with larger decreases in body weight than placebo and sitagliptin across HbA1c subgroups (Figure S15B).
3.6. Safety
The proportions of patients with adverse events (AEs) were generally similar across subgroups and between oral semaglutide and comparators (Table 2), and specific active comparators (Table S2).
TABLE 2.
Adverse events across subgroups in the PIONEER trials
| Patients, N | Patients with ≥1 AE, % | Patients with an AE leading to trial product discontinuation, % | Patients with ≥1 GI AE, % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subgroup factor | Subgroup | Oral semaglutide a | Comparator b | Oral semaglutide a | Comparator b | Oral semaglutide a | Comparator b | Oral semaglutide a | Comparator b |
| Age, y | <65 | 2519 | 1451 | 72.8 | 71.8 | 7.5 | 3.9 | 36.9 | 24.5 |
| ≥65 | 1058 | 623 | 79.1 | 74.5 | 13.0 | 6.0 | 44.6 | 22.5 | |
| Race c | White | 2579 | 1562 | 74.1 | 72.1 | 9.4 | 4.6 | 37.9 | 21.9 |
| Black/AA | 248 | 146 | 70.3 | 72.1 | 7.3 | 7.2 | 37.9 | 24.9 | |
| Asian | 576 | 269 | 76.4 | 72.6 | 7.5 | 2.5 | 42.8 | 29.6 | |
| Ethnicity d | H/L | 592 | 370 | 74.3 | 71.5 | 8.7 | 2.2 | 38.7 | 20.2 |
| Non‐H/L | 2903 | 1681 | 74.7 | 72.8 | 9.2 | 5.1 | 39.1 | 24.5 | |
| Duration of diabetes, y | <5 | 1259 | 736 | 70.9 | 70.3 | 7.0 | 4.5 | 34.5 | 23.3 |
| ≥5–<10 | 1087 | 600 | 75.5 | 71.6 | 9.1 | 4.0 | 39.4 | 23.2 | |
| ≥10 | 1231 | 738 | 78.1 | 75.6 | 11.2 | 4.9 | 44.0 | 25.1 | |
| Baseline BMI, kg/m2 | <25 | 413 | 187 | 75.2 | 73.1 | 13.5 | 5.6 | 44.6 | 28.2 |
| ≥25–<30 | 1046 | 603 | 76.9 | 73.9 | 9.5 | 5.5 | 41.5 | 26.2 | |
| ≥30–<35 | 1086 | 665 | 73.6 | 69.0 | 8.6 | 2.8 | 37.0 | 20.1 | |
| ≥35 | 1030 | 619 | 73.2 | 75.3 | 7.6 | 5.1 | 36.9 | 24.5 | |
| Baseline HbA1c, % | ≤8 | 1769 | 1066 | 75.1 | 71.6 | 10.5 | 4.4 | 39.8 | 23.8 |
| >8–≤9 | 1221 | 723 | 73.1 | 72.8 | 8.3 | 4.4 | 39.7 | 23.2 | |
| >9 | 587 | 285 | 77.1 | 75.3 | 6.6 | 5.4 | 36.2 | 25.7 | |
Note: Data are the on‐treatment safety analysis set. Percentages are the Cochran–Mantel–Haenszel adjusted proportion of patients.
Abbreviations: AA, African American, AE, adverse event; BMI, body mass index; GI, gastrointestinal, H, Hispanic; HbA1c, glycated haemoglobin; L, Latino; y, years.
Oral semaglutide included data from all three oral semaglutide doses (3, 7 and 14 mg).
Comparators included placebo, empagliflozin 25 mg, sitagliptin 100 mg or liraglutide 1.8 mg, depending on the trial.
Due to local regulations, collection of data on race was not permitted at French and Brazilian trial sites.
Due to local regulations, collection of data on ethnicity was not permitted at French sites.
Within individual subgroups, the proportion of patients with an AE leading to treatment discontinuation and the proportion of patients with ≥1 gastrointestinal (GI) AE were greater in the oral semaglutide treatment arm versus comparators, regardless of subgroup (Table 2). It should be noted that, in many of the PIONEER trials, at least one of the comparators was placebo, and the proportion of patients discontinuing trial product due to an AE or with ≥1 GI AE tended to be lowest in patients receiving placebo (Table S2).
The incidence of AEs leading to treatment discontinuation and the incidence of GI AEs was generally greater with oral semaglutide than with comparators. In patients taking oral semaglutide, there was a slight tendency for AEs leading to treatment discontinuation to occur more often in subgroups of patients who were older versus younger, in subgroups with a longer versus a shorter diabetes duration, in subgroups with a lower versus a higher baseline HbA1c and in subgroups with a lower versus a higher BMI (Table 2). This pattern of more discontinuations due to AEs in older versus younger patients was also seen for the active comparators (Table S2). In the oral semaglutide treatment group, the incidence of GI AEs was higher in the subgroups with older versus younger patients, and in subgroups with a longer versus a shorter diabetes duration, and was somewhat greater in subgroups of patients with a lower versus a higher BMI (Table 2).
4. DISCUSSION
The PIONEER trials assessed the efficacy and safety of oral semaglutide across a wide range of patients reflective of those seen in clinical practice, 11 , 12 , 13 , 14 , 15 , 16 , 17 representing a large database for physicians to understand how responses might vary by patient characteristics (Tables 1 and 2, Figures 1, 2, 3). The efficacy of oral semaglutide was generally consistent across the age and diabetes duration subgroups studied. Notably, patients with higher baseline HbA1c experienced greater estimated treatment differences in HbA1c with oral semaglutide compared to placebo. A greater glycaemic response at higher baseline HbA1c levels is a known phenomenon as baseline HbA1c is often a predictor of glycaemic response for many glucose‐lowering treatments, 19 including other glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) 20 , 21 , 22 , 23 and once‐weekly subcutaneous semaglutide. 24 Since this phenomenon also applies to other glucose‐lowering treatments, it is not surprising that HbA1c level had less impact on the magnitude of estimated treatment differences in trials with an active comparator.
No significant subgroup interactions were seen for change in HbA1c by baseline BMI with oral semaglutide. This may be explained by the long half‐life and adequate systemic exposure achieved with the 7 and 14 mg doses over a wide range of body weights (40–188 kg). 9 The lack of interaction for BMI subgroups on HbA1c treatment differences is consistent with a real‐world study with liraglutide. 25 A meta‐analysis of data with liraglutide suggested that a modest interaction between baseline BMI and change in HbA1c may be present for the 1.8 mg dose, but the authors suggested that reductions in HbA1c with liraglutide were largely independent of baseline BMI. 26
Analyses of trials with once‐weekly subcutaneous semaglutide have suggested a trend to greater absolute body weight loss in patients with a higher BMI. In addition, the observation of similar proportions of patients achieving body weight loss of ≥5% or ≥10% across BMI subgroups 27 would suggest that body weight loss may be relative to baseline. A real‐world study has also reported a significant trend for greater weight reduction with liraglutide in patients with higher baseline BMI. 25 In the present analyses with oral semaglutide, body weight reductions were seen across BMI subgroups, with a general pattern towards greater reductions in body weight in patients with higher baseline BMI within several of the PIONEER trials. However, when looking at treatment differences between oral semaglutide and comparators, a significant interaction between BMI subgroup and treatment differences in body weight was not shown in most PIONEER trials, indicating that BMI subgroup did not impact differences in body weight reductions versus comparators.
Evaluation by racial subgroups suggests potentially greater HbA1c reductions and greater treatment differences for semaglutide versus placebo and liraglutide in Asian subgroups compared to that seen in other populations. Asian patients with T2D are known to have greater β‐cell dysfunction and to respond differently to treatment compared with European and US populations. 28 Indeed, the PIONEER 9 and 10 trials, conducted in Japanese patients, demonstrated reductions in HbA1c with oral semaglutide that were greater than those typically observed in the global PIONEER trials. 29 , 30 This could be attributable to the lower mean baseline body weight of patients in Japanese compared with global PIONEER trials, 29 , 30 which has been associated with higher exposure with semaglutide, 31 , 32 although other mechanisms cannot be excluded.
The safety and tolerability profile of oral semaglutide was generally consistent across the subgroups studied, and had a similar overall incidence of AEs versus comparators. Elderly patients may be more likely to have comorbidities and to be receiving multiple medications, 33 so the safety of diabetes treatment in these patients is of particular interest. Consistent with published subgroup analyses by age with once‐weekly subcutaneous semaglutide 34 and dulaglutide, 35 the proportion of patients reporting AEs with oral semaglutide was generally similar across age subgroups. Treatment discontinuations due to an AE tended to be more common in older patients for both oral semaglutide and comparators, with the proportion discontinuing being greater for oral semaglutide than comparators. This is consistent with the observations from the subgroup analysis by age for once‐weekly subcutaneous semaglutide. 34
As expected for a GLP‐1RA, GI AEs occurred more frequently with oral semaglutide than with comparators. This was consistent across the subgroups analysed and similar to subgroup analyses with once‐weekly subcutaneous semaglutide. 24 , 34 Younger patients and those with a shorter duration of diabetes tended to experience fewer GI AEs than older patients and those with longer‐standing disease. Clinical guidelines increasingly advocate the earlier consideration of GLP‐1RAs for reasons of efficacy and CV protection in those at high CV risk, 1 , 36 and it could be argued that this approach is also sensible from a tolerability perspective, given that patients who are younger and whose T2D is relatively newly diagnosed may be expected to be more robust, and thus perhaps better equipped to deal with any side effects of medication. Nevertheless, in the PIONEER trials, GI AEs were usually mild or moderate in severity and subsided over time without the need to discontinue therapy. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
The main strength of the subgroup analyses in this paper is that the large and heterogeneous population included reflects a wide range of patients treated in everyday clinical practice. Treatment‐by‐subgroup interaction tests were based on the estimated treatment differences for oral semaglutide versus comparator, rather than the change from baseline, which is an additional strength of these analyses. Certain limitations should be taken into account when assessing our observations. Although subgroup analyses can provide useful information, they should be interpreted with caution because of the multiple comparisons being made, which can result in false‐positive findings. 37 The subgroup analyses were exploratory and therefore may not have sufficient power to detect differences between individual subgroups. Patient numbers were low in some subgroups, which may have affected the variability and robustness of those observations, and prevented investigation of certain other subgroups of interest, such as in patients aged >75 years. Finally, although the PIONEER programme included a broad range of patients, some patient groups might be underrepresented.
Although Week 26 was the primary endpoint for most PIONEER trials, the present subgroup analyses focused on the end of treatment to capture the full effect on HbA1c and body weight during these trials. Importantly, the reductions in HbA1c observed at Week 26 following treatment with oral semaglutide in the PIONEER trials were generally sustained and similar to those at the end of treatment, with peak effect on body weight occurring after Week 26. Therefore, conclusions regarding the effects of patient subgroups on change in HbA1c in this analysis are generally consistent with the data previously reported for the PIONEER trials.
In conclusion, the PIONEER programme illustrates the heterogeneity of T2D, with differences in clinical characteristics most noticeable by age and by duration of diabetes: younger patients and those with shorter duration of diabetes were more obese, indicating distinct patient care goals and therapeutic needs for this population. The quantitative assessments of efficacy and tolerability by patient subgroups in the PIONEER programme suggest that oral semaglutide is efficacious at reducing HbA1c and body weight consistently more than most comparators, and is well tolerated across a diverse set of adults of different ages, who have had T2D for different lengths of time, with a range of baseline HbA1c levels, BMI values, races and ethnicities. Physicians may expect particularly greater glycaemic efficacy in patients with high HbA1c and in patients of Asian descent when receiving oral semaglutide. Moreover, better tolerability may be observed in patients with shorter diabetes duration, and in younger patients. Given the broad heterogeneity of T2D, understanding expected responses based on individual patient characteristics, as conducted in this study, may further support patient‐centred dialogue and care.
AUTHOR CONTRIBUTIONS
Erik Christiansen and Robert Bauer were involved in the conduct of trials and data collection, with Robert Bauer analysing the data for the post hoc analyses. All authors were involved in interpreting the data and writing the manuscript.
CONFLICTS OF INTEREST
V.R.A. reports receiving consultancy fees from Applied Therapeutics, Fractyl, Novo Nordisk A/S, Pfizer and Sanofi, and research grant support (to institution) from Applied Therapeutics/Medpace; Eli Lilly; Premier/Fractyl, Novo Nordisk and Sanofi/Medpace. V.R.A.'s spouse is employed by Janssen. E.M. reports scientific advisory board, consulting, lecturing and/or research grants from AstraZeneca, Menarini, Merck Sharp & Dohme, Novo Nordisk and Sanofi. M.H. has served as an advisory board member for AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Novo Nordisk and Sanofi; a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Mundipharma, Novartis, Novo Nordisk and Sanofi; and received research support from Eli Lilly and Sanofi. J.R. has served on advisory panels for Applied Therapeutics, Boehringer Ingelheim, Eli Lilly, Intarcia, Novo Nordisk, Oramed, Sanofi and Zealand, and has received research support from Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Genentech, GlaxoSmithKline, Intarcia, Janssen, Lexicon, Merck and company, Novo Nordisk, Oramed, Pfizer and Sanofi. J.J.M. has received grants from Merck and company, Novo Nordisk and Sanofi, and lecture/other fees from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Merck and company, Novo Nordisk, Sanofi and Servier. R.B., E.C. and K.K. are employees of Novo Nordisk. R.B. and E.C are shareholders in Novo Nordisk.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14710.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGEMENTS
The authors would like to thank the patients who participated, the investigators, all trial site staff and all Novo Nordisk employees involved in these trials. The authors would also like to thank Graham Allcock, PhD, of Axis, a division of Spirit Medical Communications Group Limited, for medical writing support (funded by Novo Nordisk A/S), in accordance with Good Publication Practice 3 (GPP3) guidelines (www.ismpp.org/gpp3).
Aroda VR, Bauer R, Christiansen E, et al. Efficacy and safety of oral semaglutide by subgroups of patient characteristics in the PIONEER phase 3 programme. Diabetes Obes Metab. 2022;24(7):1338‐1350. doi: 10.1111/dom.14710
Funding information These trials were funded by Novo Nordisk A/S, Søborg, Denmark.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request. Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in datasets in a de‐identified/anonymized format.
REFERENCES
- 1. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Introduction: standards of medical care in diabetes‐2021. Diabetes Care. 2021;44(Suppl 1):S1‐S2. [DOI] [PubMed] [Google Scholar]
- 3. Hayashino Y, Izumi K, Okamura S, et al. Duration of diabetes and types of diabetes therapy in Japanese patients with type 2 diabetes: the Japan Diabetes Complication and its Prevention prospective study 3 (JDCP study 3). J Diabetes Investig. 2017;8:243‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Longo M, Bellastella G, Maiorino MI, Meier JJ, Esposito K, Giugliano D. Diabetes and aging: from treatment goals to pharmacologic therapy. Front Endocrinol (Lausanne). 2019;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weng W, Tian Y, Kimball ES, et al. Treatment patterns and clinical characteristics of patients with type 2 diabetes mellitus according to body mass index: findings from an electronic medical records database. BMJ Open Diabetes Res Care. 2017;5:e000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong L, Adler S. Glycemic control of type 2 diabetes mellitus across stages of renal impairment: information for primary care providers. Postgrad Med. 2018;130:381‐393. [DOI] [PubMed] [Google Scholar]
- 8. European Medicines Agency . Rybelsus® Summary of product characteristics 2020. https://www.ema.europa.eu/en/documents/product‐information/rybelsus‐epar‐product‐information_en.pdf. Accessed December 2, 2020.
- 9. Food and Drug Administration . Rybelsus® Prescribing information 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf. Accessed December 2, 2020.
- 10. Pharmaceuticals and Medical Devices Agency . New drugs approved in FY 2020. https://www.pmda.go.jp/files/000242574.pdf. Accessed April 20, 2022.
- 11. Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21:2203‐2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272‐2281. [DOI] [PubMed] [Google Scholar]
- 13. Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open‐label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:528‐539. [DOI] [PubMed] [Google Scholar]
- 15. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double‐blind, phase 3a trial. Lancet. 2019;394:39‐50. [DOI] [PubMed] [Google Scholar]
- 16. Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo‐controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515‐527. [DOI] [PubMed] [Google Scholar]
- 17. Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42:2262‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841‐851. [DOI] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose‐lowering therapies: a meta‐analysis of randomized clinical trials. Diabet Med. 2010;27:309‐317. [DOI] [PubMed] [Google Scholar]
- 20. Gallwitz B, Dagogo‐Jack S, Thieu V, et al. Effect of once‐weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20:409‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henry RR, Buse JB, Sesti G, et al. Efficacy of antihyperglycemic therapies and the influence of baseline hemoglobin A(1C): a meta‐analysis of the liraglutide development program. Endocr Pract. 2011;17:906‐913. [DOI] [PubMed] [Google Scholar]
- 22. Blonde L, Chava P, Dex T, Lin J, Nikonova EV, Goldenberg RM. Predictors of outcomes in patients with type 2 diabetes in the lixisenatide GetGoal clinical trials. Diabetes Obes Metab. 2017;19:275‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frías JP, Hardy E, Ahmed A, et al. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly alone, or dapagliflozin alone added to metformin monotherapy in subgroups of patients with type 2 diabetes in the DURATION‐8 randomized controlled trial. Diabetes Obes Metab. 2018;20:1520‐1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aroda VR, Capehorn MS, Chaykin L, et al. Impact of baseline characteristics and beta‐cell function on the efficacy and safety of subcutaneous once‐weekly semaglutide: a patient‐level, pooled analysis of the SUSTAIN 1‐5 trials. Diabetes Obes Metab. 2020;22:303‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chitnis AS, Ganz ML, Benjamin N, Langer J, Hammer M. Clinical effectiveness of liraglutide across body mass index in patients with type 2 diabetes in the United States: a retrospective cohort study. Adv Ther. 2014;31:986‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montanya E, Fonseca V, Colagiuri S, Blonde L, Donsmark M, Nauck MA. Improvement in glycated haemoglobin evaluated by baseline body mass index: a meta‐analysis of the liraglutide phase III clinical trial programme. Diabetes Obes Metab. 2016;18:707‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahrén B, Atkin SL, Charpentier G, et al. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes Metab. 2018;20:2210‐2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma RCW, Chan JCN. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yabe D, Nakamura J, Kaneto H, et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open‐label, randomised, active‐controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020;8:392‐406. [DOI] [PubMed] [Google Scholar]
- 30. Yamada Y, Katagiri H, Hamamoto Y, et al. Dose‐response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52‐week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020;8:377‐391. [DOI] [PubMed] [Google Scholar]
- 31. Carlsson Petri KC, Ingwersen SH, Flint A, Zacho J, Overgaard RV. Semaglutide s.c. once‐weekly in type 2 diabetes: a population pharmacokinetic analysis. Diabetes Ther. 2018;9:1533‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Overgaard RV, Navarria A, Inwersen SH, Bækdal TA, Kildemoes RJ. Clinical pharmacokinetics of oral semaglutide: analyses of data from clinical pharmacology trials. Clin Pharmacokinet. 2021;60:1335‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noale M, Veronese N, Cavallo Perin P, et al. Polypharmacy in elderly patients with type 2 diabetes receiving oral antidiabetic treatment. Acta Diabetol. 2016;53:323‐330. [DOI] [PubMed] [Google Scholar]
- 34. Warren M, Chaykin L, Trachtenbarg D, Nayak G, Wijayasinghe N, Cariou B. Semaglutide as a therapeutic option for elderly patients with type 2 diabetes: pooled analysis of the SUSTAIN 1‐5 trials. Diabetes Obes Metab. 2018;20:2291‐2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boustani MA, Pittman I 4th, Yu M, Thieu VT, Varnado OJ, Juneja R. Similar efficacy and safety of once‐weekly dulaglutide in patients with type 2 diabetes aged ≥65 and <65 years. Diabetes Obes Metab. 2016;18:820‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2021. Diabetes Care. 2021;44(Suppl 1):S111‐S124. [DOI] [PubMed] [Google Scholar]
- 37. European Medicines Agency . Guideline on the investigation of subgroups in confirmatory clinical trials 2019. https://www.ema.europa.eu/en/investigation-subgroups-confirmatory-clinical-trials. Accessed July 29, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
Data are available upon reasonable request. Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in datasets in a de‐identified/anonymized format.


