Abstract
Background
Insecticide resistance threatens the effectiveness of malaria vector control, calling for an urgent need to design suitable resistance management strategies. Here, we established the resistance profiling of an Ugandan Anopheles gambiae population to insecticides using WHO procedures and assessed the potential restoration of susceptibility in the hybrid line Mayuge/KISUMU in an insecticide-free environment for eighteen (18) generations.
Results
This An gambiae population exhibited a very high intensity of resistance to permethrin, deltamethrin, and alphacypermethrin with a consistent loss of efficacy of all long-lasting insecticidal nets (LLINs) tested including PBO-based and new generation nets Interceptor G2 (IG2) and Royal guard. Molecular analysis revealed a fixation of the L1014S-kdr mutation together with the overexpression of some P450 metabolic genes (CYP6Z1, CYP9K1, CYP6P1, 3 & 4) besides the cuticular resistance-related genes (CYP4G16) and sensorial appendage proteins (SAP1, SAP2, and SAP3) but no GSTe2 overexpression. In the absence of selection pressure, the mortality rate after exposure to insecticides increased significantly over generations, and restoration of susceptibility was observed for most of the insecticides in less than 10 generations. Accordingly, a significant reduction in the frequency of KdrE was observed after 13 generations coupled with reduced expression of most metabolic resistance genes.
Conclusions
The results of this study show that the high intensity of pyrethroid resistance observed in An gambiae from Uganda associated with the loss of efficacy of LLINs could compromise vector control efforts. The study also highlights that an early rotation of insecticides could help manage resistance to insecticides by restoring the susceptibility. However, the persistence of Kdr mutation together with overexpression of some metabolic genes after many generations in the absence of selection pressure indicates the potential implication of modifiers alleviating the cost of resistance which needs to be further investigated.
Introduction
Insecticide resistance is an increasing challenge for disease control [1]. Of particular concern is resistance to pyrethroid insecticides, given the high reliance on this class for malaria vector control. Populations of Anopheles gambiae, the main malaria vector, are exhibiting increasingly high levels of pyrethroid resistance, commonly measured by the knockdown resistance (kdr) mutations. This phenomenon leads to extensive loss of efficacy of LLINs including PBO-pyrethroid nets which is a growing problem in Uganda [2–5] and many other African countries [6–10].
Point mutations in the para-orthologous sodium channel gene disrupt insecticide binding to the voltage-gated sodium channels [11]. In this genomic region, two kdr mutations have been shown to be strongly associated with pyrethroid resistance in Anopheles gambiae including the Leucine to phenylalanine (L1014F-kdr west) and Leucine to Serine (L1014S-kdr east) mutations. Besides the Kdr, metabolic resistance is very common resistance mechanism in mosquitoes and considered to be more likely to cause control failure [12]. Compared to the well-characterised kdr, with available DNA-based diagnostic tools [13, 14], metabolic resistance still had only few molecular diagnostic tools preventing to assess the fitness cost associated with this resistance mechanism. This hampered the design of a suitable resistance management strategy to efficiently control the malaria vectors.
Polymorphism maintained at the kdr locus and metabolic genes in field populations of vectors at fine spatial scales despite strong insecticide selection pressure, is indicative of a fitness cost in the absence of insecticide [15, 16]. Although the availability of molecular marker for kdr helped to demonstrate evidence of such fitness costs in resistant mosquitoes [17–21], there is limited empirical evidence demonstrating restoration of pyrethroid susceptibility in insecticide-free environment in malaria vectors. Instead, studies tend to focus on selection towards resistance, describing increases in both kdr allele frequencies with pyrethroid exposure [15, 18, 22].
In this study, we extensively investigated the resistance profile of An. gambiae population from Mayuge (Eastern Uganda) and evaluated the fitness cost associated with the L1014S-kdr mutation in this population. Furthermore, we assessed potential restoration of susceptibility to the four recommended insecticide classes in the absence of selection pressure.
Materials and methods
Mosquito collection
Indoor resting and blood-fed female Anopheles mosquitoes were collected in Bubbalya (0°23′10.8′′N, 33°37′16.5′′E) in Mayuge (eastern Uganda) in February and October 2020. Mosquitoes were collected using electric aspirators morphologically identified as belonging to An. funestus group or An. gambiae s.l complex according to morphological keys [23]. These mosquitoes were kept in carton cups and fed with sugar until they became fully gravid prior to forced egg-laying in 1.5 ml microcentrifuge tubes and larvae reared to adults as previously described [24].
Molecular identification of field-collected females
Oviposited and non oviposited females An. gambiae s.l were dissected into head plus thorax and abdomen for Genomic DNA (gDNA) extraction using the Livak method [25]. The SINE PCR assay [26] was used for the identification of the An. gambiae species.
Insecticide susceptibility assays
The insecticide resistance profile of An gambiae s.l was assessed using the WHO tube bioassays [27]. After molecular identification, mosquitoes were split in two groups for bioassay including An. gambiae s.s., and An. arabiensis. Bioassay tests were performed with the pyrethroids type I (permethrin (0.75%)) and type II (deltamethrin and alphacypermethrin (0.05%)), the organochlorine DDT (4%), the carbamate bendiocarb (0.1%), and the organophosphate pyrimiphos-methyl (0.25%). Assays were performed at 25 ± 1° C and 70–80% relative humidity. For each test, four replicates of 20–25 F1 female mosquitoes, 2–5 day-old were exposed to insecticide-impregnated papers for 1h and final mortality recorded after a holding period of 24h. When resistance was observed with 1x (discriminant concentration (DC)) of pyrethroid (permethrin and deltamethrin), intensity bioassays were carried out with 5x DC and 10x DC of these insecticides. The intensity bioassays with 5x and 10x DC were performed following the WHO 2016 test procedure (WHO, 2016). Synergist assays with piperonyl butoxide (PBO; an inhibitor of cytochrome P450s) were performed for the potential involvement of P450’s genes.
Insecticide-treated bed nets bioefficacy assays
Following the WHO guidelines for cone bioassays [28], the efficacy of the following LLINs including Olyset® Net (permethrin 2%) and Olyset® Plus net roof (permethrin 2% plus PBO 1% in the roof); PermaNet® 2.0 (deltamethrin 0.18%) and PermaNet® 3.0 side (deltamethrin 0.28%) was estimated using cone test approach. An untreated mosquito net was used as a control. Five replicates of ten F1 2–5 days old females were placed in plastic cones enclosed with the mosquito net during 3 min exposure. Mosquitoes were then placed in small holding paper cups with cotton soaked in a 10% sugar solution. Mortality was determined 24 h later.
Fitness cost study
Establishment of the mosquito strains
To facilitate the rearing of field mosquitoes, crossing was performed in February 2020 between An gambiae from Mayuge (MYG-R) and the susceptible laboratory KISUMU (KIS). The progeny (MYG/KIS) was intercrossed for several generations for resistance reversal study. To perform the crossing, pupae of each strain were collected and put individually in falcon tubes 15ml for individual emergence then the males of the resistant strain were mixed in the same cage with the females of the susceptible colony for random mating to generate the first generation as previously described [29]. At F12, the hybrid colony was backcrossed with the field strain to refresh the genetic background and the fitness cost associated with the L1014S-KdrE was evaluated using the F3 generation of the backcross.
Life trait experiments
All parameters were evaluated by simultaneously comparing fitness parameters (fecundity and fertility, larval mortality and adult longevity) between the mutant (1014S-RR), heterozygotes (L1014S-RS) and wild homozygote (L1014-SS), reared together in the same containers and under the same environmental conditions such as larval density and feeding, temperature and light exposition as done previously [29–31].
Population cage experiments to assess a potential restoration of susceptibility
Cage experiments were conducted to assess a potential reversal to susceptibility. After crosses between male MYG-R and female KISUMU, the progeny obtained (MYG/KIS) were let in cages for intercrosses for eighteen (18) generations. In each generation, all mosquitoes irrespective of their genotypes were mixed in cages for intercrossing to generate the next generation. Each generation consisted in about 3 cages of at least 200 mosquitoes/cage of all genotypes. In the first generation, the frequency of the KdrE_R resistant allele was assessed and then monitored in following generations by genotyping a set of about 35 females aged between 2-5days old as well as expression on some candidate genes. Besides, bioassay was performed against permethrin 1x, 5x, 10x and 1x +PBO over generations (MYG/KIS F2, F4, F7 and F13) to confirm the restoration of susceptibility. Additional bioassays were performed at F7 and F13 for the four classes of insecticide commonly used and the susceptibility was compared to the field F1.
Genotyping of resistance markers in An. gambiae
TaqMan assays with two labeled fluorochromes probes FAM and HEX were used to genotype the L1014F/L1014S-kdr (7), and the N1575Y mutation (35) associated with DDT and pyrethroid resistance in An. gambiae s.l. Also, the G119S-ace-1 responsible for organophosphate and carbamate resistance in An. gambiae s.l. was also genotyped using TaqMan assays [32].
Expression profile of resistance genes using real time quantitative PCR
To investigate the fitness cost associated with metabolic enzymes, the transcription profile of major insecticide resistance genes families including P450 metabolic genes (CYP6Z1, CYP9K1, CYP6P1, 3 & 4), GSTs (GSTe2), cuticular resistance-related genes (CYP4G16 and CYP4G17) and sensorial appendage proteins (SAP1, SAP2, and SAP3) overexpression were established in F3, F7, and F13 of the crossing compared to field F1 using KISUMU as susceptible reference strain. Total RNA from three biological replicates of 10 adults 2–5 days olds F1 for each group and similarly from KISUMU (susceptible lab strain) was extracted using Picopure RNA Isolation Kit (Arcturus). One microgram of RNA from each of the three biological replicates was used as a template for cDNA synthesis using the superscript III (Invitrogen) with oligo-dT20 and RNase H, following the manufacturer’s instructions.The relative expression level and fold-change (FC) was calculated individually according the 2-ΔΔCT method [33] after normalisation with Ribosomal protein S7 (RSP7) and Elongation factor (EF).
Ethics statement
No permits were required for this work as the study only focused on mosquitoes with no involvement of human participants.
Results
Vector composition
In February, An funestus s.l was the predominant malaria vector (87.2%: 1636/1877) followed by An gambiae s.l. (12.8%: 241/1877) but in October, An. gambiae s.l was the main vector collected (80.1%: 1700/2121). Molecular identification of 100 An. gambiae s.l from Mayugue revealed that 78 (81.2%) were An. gambiae whereas the remaining (18.8%) were An. arabiensis.
Insecticide susceptibility assays
F1 progeny from field-collected females An gambiae in October showed extremely high resistance to permethrin, deltamethrin and alphacypermethrin. F1 females from Mayuge showed 1.52 ± 1.52%, 3.19 ± 1.96% and 0% mortality 24h after exposure to permethrin 1x, deltamethrin 1x and alphacypermethrin 1x respectively (Fig 1A). This An. gambiae population was also resistant to carbamate bendiocarb 1x, with mortality rate of 70.21 ± 1.79% (Fig 1A). High resistance was noted against the organochlorine, DDT (mortality = 15.51 ± 1.88% (Fig 1A). A full susceptibility was observed with the organophosphate, pyrimiphos-methyl 1x with a 100% mortality rate. Due to low sample size, An arabiensis was tested only to permethrin exhibiting a mortality of 18.33 ± 1.67%.
Fig 1. Susceptibility profile of An. gambiae s.l population from Mayuge.
A) susceptibility profile of females An. gambiae; B) resistance intensity with 5× and 10× the diagnostic concentrations of permethrin and deltamethrin and Alphacypermethrin C) effect of pre-exposure to synergist PBO against pyrethroids. D) Susceptibility profile and intensity of females An. arabiensis and E) bio-efficacy of different commercial LLINs against An. gambiae. Results are average of percentage mortalities ± SEM; Results are average of percentage mortalities from four replicates each ± SEM.
This population exhibited a mortality rate of 26.3 ± 3.4% and 91.12 ± 2.2% to permethrin 5x and 10x respectively (Fig 1B) showing a high intensity of resistance to permethrin. The mortality of 45.8 ± 4.2% and 70.5 ± 5.8% was observed after exposure to deltamethrin 5x and 10x respectively indicating a high intensity of resistance to deltamethrin in Mayuge (Fig 1B). Similar observations were made for alphacypermethrin with mortality rates of 13.6 ± 4.1% and 59.6 ± 1.3% for 5x and 10x (Fig 1B). An. arabiensis also displayed a high intensity of resistance to permethrin 5x with mortality of 51.3 ± 3.3% (Fig 1D). Synergist assays performed with PBO revealed partial recovery of susceptibility after exposure to permethrin (from 1.52 ± 1.52% to 37.4 ± 15.9% mortality) and greater recovery with deltamethrin and alphacypermethrin with mortality rate of 78.33 ± 11.7% and 85.0 ± 5.0% respectively (Fig 1C).
Bioefficacy of insecticide-treated bed nets
Standard nets (Olyset and PermaNet 2.0, DuraNet and Interceptor) showed very low efficacy against this population of malaria vector with mortality rate of less than 15% for all these nets (Fig 1E). However, the PBO-based net PermaNet 3.0 showed optimal efficacy with 100% mortality observed (Fig 1E). In contrast, the Olyset plus (PBO-based net) showed very low efficacy (mortality rate = 35.12 ± 12.1%) confirming the very low mortality recorded with Permethrin + PBO in this site. New generation nets (NGN) (Interceptor G2 and Royal Guard) which combine a pyrethroid and non-pyrethroid insecticides induced very low mortality against this population (Fig 1E). However, beside the direct killing effect, NGN such as Royal acts by sterilising the female which could help reduce the vector density.
Distribution of insecticide resistance markers in field collected An. gambiae
The L1014F-KdrW mutation was completely absent in both An. gambiae and An. arabiensis from Mayuge. However, the L1014S-KdrE mutation was fixed in An gambiae (100% RR) and completely absent in An. arabiensis. The N1575Y-kdr mutation conferring pyrethroid resistance and the G119S-Ace1 mutation conferring carbamate resistance were completely absent in both species.
Fitness cost associated with the L1014S mutation
In the hybrid strain MYG/KIS, mosquitoes with wild allele (L1014) displayed significant greater ability to lay eggs in the second gonotrophic cycle compared to those with the 1014S mutant allele (χ2 = 54.8; P<0.0001). However, no difference was found in the number of eggs laid by mosquitoes with mutant genotype (RR) (Mean = 77) compared to heterozygote RS (Mean = 64±6.3) and those with wild genotype (SS) (Mean = 55±6.4) as well as the hatch rate probably due to very low samples size of mosquitoes with RR genotype (Fig 2A). Assessment of the odds ratio (OR) showed that the ability of SS mosquitoes to lay eggs was higher compared to RR (OR = 25.7; confidence interval (CI) 95%: 9.1–72.6; P < 0.0001) but suggesting an association between the L1014S mutation and reduced fecundity although no significant difference was observed when compared to RS (OR = 1.7; CI 95%: 0.8–3.4; p = 0.09). Heterozygote also showed a higher ability to lay eggs compared to RR (OR = 15.09; CI 95%: 5.3–43.1; P < 0.0001) (Fig 2B) confirming the burden of the 1014S allele on the female fecundity.
Fig 2. Influence of of the L1014S-KdrE on key life traits of An. gambiae.
(A) and (B) Schematic representation of the impact of L1014S genotypes on egg-laying success with odd ratio (OR); (C) Distribution of the L1014S genotypes at different developmental stages of the hybrid MYG/KIS; D) the proportion of pupae obtained in D7, D9 and D11 of development; E) influence of L1014S on the adult longevity of An. gambiae.
No difference was observed concerning the mortality rate from the larvae to the adult for the three genotypes (Fig 2C). Assessment of the rate of pupae formation by comparing the frequency of the genotypes of the pupae obtained in D7, D9 and D11 showed that mosquitoes with the wild genotype developed significantly faster than homozygote mutants and heterozygote mosquitoes as their frequency decreased significantly from D7 (38%) and D9 (51%) to D11 (26%) (χ2 = 4.4; p = 0.03) when that of RS and RR were increasing indicating a fitness cost of the resistant allele (Fig 2B). Genotyping of thirty (30) live mosquitoes at D1, D10, and D20 post emergence to assess the association between the L1014S mutation and adult longevity revealed an heterozygote advantage. Comparison of genotypes frequency showed a decrease proportion of mosquitoes with both SS and RR genotypes from D1 to D20 (χ2 = 21.2; p = 0.0017) (Fig 2B and 2D) compared to heterozygote indicating that heterozygote mosquitoes live longer than those with homozygote genotypes.
Restoration of susceptibility in the hybrid strain MYG/KIS
At F2 generation, the hybrid strain MYG/KIS exhibited a mortality rate of 26.82 ± 13.18%, 75.50 ± 0.5% and 89.0 ± 1.0% to permethrin 1x, 5x and 10x respectively (Fig 3). Synergist test performed at this generation revealed that P450 monoxygenases are playing a major role in the resistance with a significant recovery of susceptibility (mortality rate = 92 ± 4%) after PBO exposure.
Fig 3. Susceptibility profile of the hybrid MYG/KIS to permethrin over 13 generation in the absence of selection.
Results of WHO tube bioassays with permethrin 1x, 5x and 10x and PBO+ permethrin 1x. Results are average of percentage mortalities ± SEM of four replicates.
Genotyping of L1014F mutation across generations revealed that the frequency of 50% of the 1014S mutant allele obtained in F1 generation of the crossing decreased significantly in the next generations (Fig 4A & 4B). A significant and consistent increase in the proportion of wild and heterozygote mosquitoes was observed from F2 to F13 moving from 50% to 15% (χ2 = 9.6; p = 0.002) with a predominance of heterozygotes indicating a fitness cost associated with the 1014S allele which lead to reversal to susceptibility (Fig 4A & 4B). Accordingly the deviation to Hardy-Weinberg equilibrium was noticed in F4 (χ2 = 8; P < 0.001), F9 (χ2 = 3.8; P < 0.05), and F10 (χ2 = 17.5; P < 0.0001) confirming the fitness cost associated with the kdr mutation. However, the equilibrium was restored in some generation including F5-F9 and F12 to above (P>0.05) highlighting the implication of genetic modifiers alleviating the cost associated with the 1014S-kdr resistant allele. Nevertheless, restoration of susceptibility was observed after bioassay performed with permethrin over generations (Fig 3).
Fig 4. Evaluation of the reversal to susceptibility in the hybrid colony MYG/KIS.
Changes in the L1014S genotypes (A) and allele (B) over13 generations in the insecticides free-environment; F represents each generation.
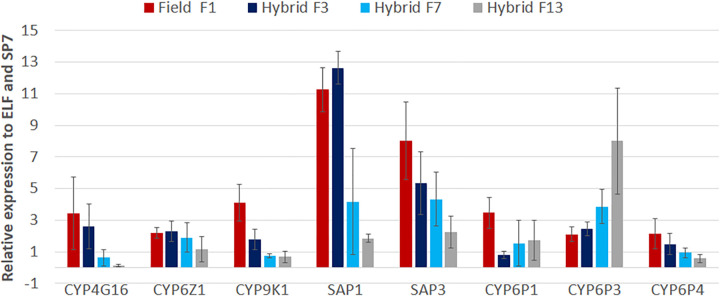
For metabolic genes, qPCR results revealed a significant overexpression of several P450 resistant genes (CYP6Z1 (2.2± 0.3), CYP9K1 (4.1± 1.1), CYP6P1 (3.5 ± 1.0), CYP6P3(2.1± 0.5) & CYP6P4(2.1± 0.9)) besides the cuticular genes (CYP4G16 (3.4± 2.3)) and sensorial appendage proteins (SAP1(11.2± 1.4), SAP2(2.9 ± 1.2), and SAP3(8.01± 2.5)) with no overexpression of GSTe2 (0.5± 0.1) (Table 1). The level of expression of these genes decreased significantly over generations (from F3-F13) (except for CYP6P3) suggesting a recovery of susceptibility as observed with the kdr-E marker (Fig 5). The expression of decreased by 3.3, 1.1, 3.1, 9.4, 6.9, 1.7 and 1.52 folds for CYP4G16, CYP6Z1, CYP9K1, SAP1, SAP3, CYP6P1 and CYP6P3 respectively.
Table 1. Relative expression of metabolic resistance gene in F1 An gambiae from Mayuge.
| Genes | Fold-change | SEM |
|---|---|---|
| CYP4G16 | 3.42 | 2,30 |
| CYP4G17 | 1.62 | 1,32 |
| CYP6M2 | 0,78 | 0,36 |
| CYP6Z1 | 2,21 | 0,33 |
| CYP6Z2 | 0,42 | 0,08 |
| CYP9K1 | 4,11 | 1,15 |
| GSTe2 | 0,45 | 0,16 |
| SAP1 | 11,25 | 1,40 |
| SAP2 | 2,91 | 1,18 |
| SAP3 | 8,01 | 2,46 |
| CYP6P1 | 3,46 | 0,99 |
| CYP6P3 | 2,10 | 0,48 |
| CYP6P4 | 2,14 | 0,94 |
Fig 5. Differential expression by quantitative reverse-transcription polymerase chain reaction of key metabolic genes in the hybrid MYG/KIS over generation compared to field F1 unexposed.
Histograms represent the fold-change of the genes in the hybrid MYG/KIS mosquitoes across generations relative to the pyrethroid-susceptible KISUMU laboratory strain.
After seven generations in insecticide-free environment (F7), the hybrid strain became susceptible to most of the insecticides tested including pyrethroids, carbamates and pyrimiphos methyl (Fig 6). A full susceptibility to all the insecticides tested was noticed at the 13th generation except for DDT and alphacypermethrin where a mortality rate of 77.60% and 80.40% was observed (Fig 6) indicating potential implication of genetic modifiers alleviating the cost of resistance to these insecticides and showing that reversal for these insecticides needs more time. For this reason, the strain was maintained in the insectary until F18 where a mortality rate of 93.1% was noted for alphacypermethrin and 90.2% for DDT.
Fig 6. Evaluation of the restoration of susceptibility in the hybrid colony MYG/KIS.
Determination of susceptibility to all the four classes of insecticides, resistance intensity with 5× and 10× the diagnostic concentrations of pyrethroids and effect of pre-exposure to synergist PBO against pyrethroids type I and type II in comparison with field F0. Results are average of percentage mortalities from four replicates each ± SEM.
Discussion
In this study, we extensively investigated the resistance profile of An. gambiae population from Mayuge (Eastern Uganda) and evaluated the fitness cost associated with the kdr mutation and metabolic genes in this population. Furthermore, we assessed potential restoration of susceptibility to the four recommended insecticide classes in the absence of selection pressure.
Elevated resistance intensity in the An gambiae population from Uganda
The Ugandan An. gambiae in this study exhibited extremely high level of resistance to pyrethroid with drastical loss of efficacy of insecticidal treated LLINs including new generation nets. A similar high level of resistance was reported recently in An. funestus s.s. populations from for the same location [34]. Such high intensity with loss in the efficacy of standard and PBO-based nets observed in An gambiae from Mayuge is similar to the observations of Okia et al. (2018) in Tororo a location in Eastern Uganda. The escalation of pyrethroid and DDT resistance observed in the Mayuge An. gambiae population is higher than resistance reported in other locations such as Kome, southern Chad (permethrin, 26.7% mortality, deltamethrin, 25.4% and DDT, 41.7%) [10]; and in Auyo, northern Nigeria (deltamethrin, 78.4% mortality and DDT, 44%); and in Djenne [35]. This increase may be associated with insecticide selective pressure imposed by the massive use of LLINs in Uganda since 2006 combined with agriculture. The same level of resistance was reported in An. coluzzii Cameroon and Chad [10], An. funestus in Cameroon [7] for which no mortality was noticed after exposure to the synergist net Olyset Plus. This was reported also a few years ago in Southern Mozambique where a complete loss of the efficacy of Olyset® Net, and PermaNet® 2.0, the two most distributed LLINs across Africa [8, 36], was noticed. Resistance was noted also for the carbamate bendiocarb althought the Ace1 mutation was absent in this population showing that such insecticide could not be an alternative to pyrethroid for IRS. This justifies the deployment of the new generation net Royal guard in the location which acts by sterilizing the female mosquitoes. The full susceptibility to the organophosphate pyrimiphos-methyl, seeing in this location suggests that this insecticide class is the most suitable for IRS against An gambiae and supports the use of Actellic for IRS in many districts of the country.
Genotyping of resistant markers revealed a fixation of the L1014S-kdr mutation showing that this mechanism is responsible in majority for pyrethroids resistance in these An gambiae mosquitoes particularly to permethrin for which a lower recovery of susceptibility was obtained after PBO pre-exposure. The same synergist assay with PBO helped to significantly recover the susceptibility to deltamethrin and alphacypermethrin showing that many P450s are involved in the extremely high intensity of resistance observed to type II pyrethroid in this location. qPCR analysis on the F1 progeny from field collected females revealed the overexpression of some P450 metabolic genes (CYP6Z1, CYP9K1, CYP6P1,3&4) besides the cuticular genes (CYP4G16) and sensorial appendage proteins (SAP1, SAP2, and SAP3) with almost no overexpression of GSTe2. This indicates that the 1014S resistant allele combines with P450, cuticular and sensorial genes to accelerate the intensity of resistance in this location.
Restoration of susceptibility in the resistant An. gambiae hybrid line
For successful insecticide resistance management, regular monitoring of resistance development and underlying mechanisms are key. Among the insecticide-resistant management strategies, rotational and mixture of insecticides to retard or reverse the spread of resistance are the most efficient and they are mainly based on the assumption of resistance having a fitness cost in the absence of selection. Knowledge of the reversal rate for insecticides such as pyrethroids is therefore crucial before implementing any resistance management strategies in the field based on rotation/mixture of insecticides.
In this study, the hybrid strain MYG/KIS raised in the absence of insecticide selection pressure became progressively more susceptible compared to the parental population. This suggests that resistance under field conditions can diminish after few generations without insecticide pressure. This implies a higher fitness of the susceptible phenotypes relative to resistant phenotypes in the untreated environment as observed for the kdr on some life traits such as fecundity, larval development and longevity. Some cases of reversal of insecticide resistance of culicines (Aedes aegypti and Culex pipiens) and Anopheles gambiae raised in the absence of insecticide in semi-field and laboratory conditions have been reported [15, 16, 37, 38]. The same reversal has been observed in pests such as cotton bollworm (Helicoverpa armigera) in Benin West Africa [39]. In this species, when the use the level of resistance increased quickly during the application of insecticides and decreased when treatment was suspended in the field. On the other hand, it was reported an increasing phenotypic resistance coupled to increase frequency of kdr resistant alleles and over-expression of monoxygenases/esterase in An. gambiae subjected to deltamethrin selection pressure in the lab [15, 16] indicating the rapid development of resistance in the environment undergoing continues insecticide use.
The frequency and the degree of dominance of resistant genes in a population is one of the factors able to influence the intensity and development of resistance in a population [40]. In this study, the frequency of 1014S mutant allele decreased consistently over generations from F2 to F13 indicating the importance of kdr marker in monitoring insecticide resistance development in an environment experiencing intensive use of insecticides. These results agree with the assumption that observed reversal of insecticide resistance in the absence of insecticide pressure is associated to fitness costs as shown previously [41]. Accordingly, a high fitness cost was observed in mosquitoes harbouring the resistant allele for the kdr marker although a heterozygote advantage was observed for some traits. Such heterozygote advantage was previously reported for the mating competitiveness in An gambiae for the kdr and Rdl target site resistance [42]. This could help maintaining the resistant allele in the population which could be rapidly re-selected if the selection pressure increases. Because of the lack of molecular markers for metabolic resistance in An gambiae, it was not possible to study the fitness cost associated with this mechanism as done previously for GSTs and P450s in An. funestus [29–31]. Nevertheless, we observed that the expression level of monooxygenases, cuticular and sensorial appendage proteins decreased significantly over generations in this hybrid strain maintained without insecticide pressure, indicating that metabolic resistance decreased in the absence of insecticide selection pressure likely as a consequence of related fitness cost.
Furthermore, we observed that when the hybrid resistant strain was pre-exposed to PBO before exposure to permethrin, reversal of susceptibility to this insecticide was significantly faster, confirming the key role of metabolic resistance in the observed resistance. The restoration of susceptibility in a population showing high resistance levels with the use of PBO shows that the incorporation of synergists with the future control strategies may be useful in restoring the effectiveness of the existing tools. All these show that using insecticides of differing modes of action on a selective basis with the addition of synergists for public health/agriculture use, could help to manage resistance in the target populations.
Conclusions
High intensity of resistance associated with the loss of efficacy of impregnated bed nets was observed in An. gambiae from Mayuge. This represents a serious threat for vector control. Interestingly, we noticed in the absence of selection pressure a significant reduction in the frequency of Kdr-E together with the expression of key metabolic resistance genes leading to restoration of susceptibility in less than 10 generations supporting that rotation of insecticide can improve vector control against current and importantly for novel insecticides.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Renewal Wellcome Trust Senior Research Fellowship in Biomedical Sciences to Charles S Wondji (217188/Z/19/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? The Lancet. 2016;387(10029):1785–8. doi: 10.1016/S0140-6736(15)00417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramphul U, Boase T, Bass C, Okedi LM, Donnelly MJ, Müller P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103(11):1121–6. doi: 10.1016/j.trstmh.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 3.Verhaeghen K, Van Bortel W, Roelants P, Okello PE, Talisuna A, Coosemans M. Spatio-temporal patterns in kdr frequency in permethrin and DDT resistant Anopheles gambiae ss from Uganda. The American journal of tropical medicine and hygiene. 2010;82(4):566–73. doi: 10.4269/ajtmh.2010.08-0668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulamba C, Irving H, Riveron JM, Mukwaya LG, Birungi J, Wondji CS. Contrasting Plasmodium infection rates and insecticide susceptibility profiles between the sympatric sibling species Anopheles parensis and Anopheles funestus s.s: a potential challenge for malaria vector control in Uganda. Parasit Vectors. 2014;7:71. doi: 10.1186/1756-3305-7-71 ; PubMed Central PMCID: PMC3937429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okia M, Hoel DF, Kirunda J, Rwakimari JB, Mpeka B, Ambayo D, et al. Insecticide resistance status of the malaria mosquitoes: Anopheles gambiae and Anopheles funestus in eastern and northern Uganda. Malaria journal. 2018;17(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riveron JM, Watsenga F, Irving H, Irish SR, Wondji CS. High Plasmodium Infection Rate and Reduced Bed Net Efficacy in Multiple Insecticide-Resistant Malaria Vectors in Kinshasa, Democratic Republic of Congo. J Infect Dis. 2018;217(2):320–8. doi: 10.1093/infdis/jix570 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menze BD, Wondji MJ, Tchapga W, Tchoupo M, Riveron JM, Wondji CS. Bionomics and insecticides resistance profiling of malaria vectors at a selected site for experimental hut trials in central Cameroon. Malaria journal. 2018;17(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riveron JM, Huijben S, Tchapga W, Tchouakui M, Wondji MJ, Tchoupo M, et al. Escalation of pyrethroid resistance in the malaria vector Anopheles funestus induces a loss of efficacy of piperonyl butoxide–based insecticide-treated nets in Mozambique. The Journal of infectious diseases. 2019;220(3):467–75. doi: 10.1093/infdis/jiz139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchouakui M, Fossog BT, Ngannang BV, Djonabaye D, Tchapga W, Njiokou F, et al. Investigation of the influence of a glutathione S-transferase metabolic resistance to pyrethroids/DDT on mating competitiveness in males of the African malaria vector, Anopheles funestus. Wellcome open research. 2019;4. doi: 10.12688/wellcomeopenres.15013.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim SS, Fadel AN, Tchouakui M, Terence E, Wondji MJ, Tchoupo M, et al. High insecticide resistance in the major malaria vector Anopheles coluzzii in Chad Republic. Infectious Diseases of Poverty. 2019;8(1):100. doi: 10.1186/s40249-019-0605-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soderlund D, Knipple D. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect biochemistry and molecular biology. 2003;33(6):563–77. doi: 10.1016/s0965-1748(03)00023-7 [DOI] [PubMed] [Google Scholar]
- 12.Hemingway J. The role of vector control in stopping the transmission of malaria: threats and opportunities. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130431. doi: 10.1098/rstb.2013.0431 ; PubMed Central PMCID: PMC4024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect molecular biology. 1998;7(2):179–84. Epub 1998/04/16. doi: 10.1046/j.1365-2583.1998.72062.x . [DOI] [PubMed] [Google Scholar]
- 14.Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9(5):491–7. doi: 10.1046/j.1365-2583.2000.00209.x . [DOI] [PubMed] [Google Scholar]
- 15.Machani MG, Ochomo E, Zhong D, Zhou G, Wang X, Githeko AK, et al. Phenotypic, genotypic and biochemical changes during pyrethroid resistance selection in Anopheles gambiae mosquitoes. Scientific reports. 2020;10(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman MK, Uc-Puc V, Rodriguez J, Cutler DJ, Morran LT, Manrique-Saide P, et al. Restoration of pyrethroid susceptibility in a highly resistant Aedes aegypti population. Biology letters. 2018;14(6):20180022. doi: 10.1098/rsbl.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins AJ, Ribeiro CDeM, Bellinato DF, Peixoto AA, Valle D, Lima JBP. Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PloS one. 2012;7(3):e31889. doi: 10.1371/journal.pone.0031889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brito LP, Linss JG, Lima-Camara TN, Belinato TA, Peixoto AA, Lima JB, et al. Assessing the effects of Aedes aegypti kdr mutations on pyrethroid resistance and its fitness cost. PLoS One. 2013;8(4):e60878. doi: 10.1371/journal.pone.0060878 ; PubMed Central PMCID: PMC3620451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diniz DF, de Melo-Santos MA, Santos EM, Beserra EB, Helvecio E, de Carvalho-Leandro D, et al. Fitness cost in field and laboratory Aedes aegypti populations associated with resistance to the insecticide temephos. Parasit Vectors. 2015;8:662. doi: 10.1186/s13071-015-1276-5 ; PubMed Central PMCID: PMC4696322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanai D, Hardstone Yoshimizu M, Scott JG. The Insecticide Resistance Allele kdr-his has a Fitness Cost in the Absence of Insecticide Exposure. J Econ Entomol. 2018;111(6):2992–5. doi: 10.1093/jee/toy300 . [DOI] [PubMed] [Google Scholar]
- 21.Nkahe DL, Kopya E, Djiappi-Tchamen B, Toussile W, Sonhafouo-Chiana N, Kekeunou S, et al. Fitness cost of insecticide resistance on the life-traits of a Anopheles coluzzii population from the city of Yaounde, Cameroon. Wellcome Open Res. 2020;5:171. doi: 10.12688/wellcomeopenres.16039.2 ; PubMed Central PMCID: PMC7525343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez-Gonzalez LC, Briceno A, Ponce-Garcia G, Villanueva-Segura OK, Davila-Barboza JA, Lopez-Monroy B, et al. Assessing the effect of selection with deltamethrin on biological parameters and detoxifying enzymes in Aedes aegypti (L.). Pest Manag Sci. 2017;73(11):2287–93. doi: 10.1002/ps.4609 . [DOI] [PubMed] [Google Scholar]
- 23.Gillies M, Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;55:1–143. [Google Scholar]
- 24.Morgan JC, Irving H, Okedi LM, Steven A, Wondji CS. Pyrethroid resistance in an Anopheles funestus population from Uganda. PLoS One. 2010;5(7):e11872. Epub 2010/08/06. doi: 10.1371/journal.pone.0011872 ; PubMed Central PMCID: PMC2912372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107(4):611–34. Epub 1984/08/01. PubMed Central PMCID: PMC1202380. doi: 10.1093/genetics/107.4.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malaria Journal. 2008;7(1):163. doi: 10.1186/1475-2875-7-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2016. [Google Scholar]
- 28.World Health Organization. Guidelines for laboratory and field-testing of long-lasting insecticidal nets. Geneva. Switzerland. 2013.
- 29.Tchouakui M, Riveron Miranda J, Mugenzi LMJ, Djonabaye D, Wondji MJ, Tchoupo M, et al. Cytochrome P450 metabolic resistance (CYP6P9a) to pyrethroids imposes a fitness cost in the major African malaria vector Anopheles funestus. Heredity (Edinb). 2020;124(5):621–32. doi: 10.1038/s41437-020-0304-1 ; PubMed Central PMCID: PMC7171194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchouakui M, Mugenzi LMJ, Wondji MJ, Tchoupo M, Njiokou F, Wondji CS. Combined over-expression of two cytochrome P450 genes exacerbates the fitness cost of pyrethroid resistance in the major African malaria vector Anopheles funestus. Pestic Biochem Physiol. 2021;173:104772. doi: 10.1016/j.pestbp.2021.104772 ; PubMed Central PMCID: PMC8024743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchouakui M, Riveron JM, Djonabaye D, Tchapga W, Irving H, Soh Takam P, et al. Fitness Costs of the Glutathione S-Transferase Epsilon 2 (L119F-GSTe2) Mediated Metabolic Resistance to Insecticides in the Major African Malaria Vector Anopheles Funestus. Genes. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bass C, Nikou D, Blagborough AM, Vontas J, Sinden RE, Williamson MS, et al. PCR-based detection of Plasmodium in Anopheles mosquitoes: a comparison of a new high-throughput assay with existing methods. Malaria journal. 2008;7(1):1–9. doi: 10.1186/1475-2875-7-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature protocols. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 34.Tchouakui M, Mugenzi LMJ, D. Menze B, Khaukha JNT, Tchapga W, Tchoupo M, et al. Pyrethroid Resistance Aggravation in Ugandan Malaria Vectors Is Reducing Bednet Efficacy. Pathogens. 2021;10(4):415. doi: 10.3390/pathogens10040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim SS, Mukhtar MM, Irving H, Riveron JM, Fadel AN, Tchapga W, et al. Exploring the Mechanisms of Multiple Insecticide Resistance in a Highly Plasmodium-Infected Malaria Vector Anopheles funestus Sensu Stricto from Sahel of Northern Nigeria. Genes (Basel). 2020;11(4). doi: 10.3390/genes11040454 ; PubMed Central PMCID: PMC7230678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glunt KD, Abilio AP, Bassat Q, Bulo H, Gilbert AE, Huijben S, et al. Long-lasting insecticidal nets no longer effectively kill the highly resistant Anopheles funestus of southern Mozambique. Malar J. 2015;14:298. doi: 10.1186/s12936-015-0807-z ; PubMed Central PMCID: PMC4524426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi L, Hu H, Ma K, Zhou D, Yu J, Zhong D, et al. Development of resistance to pyrethroid in Culex pipiens pallens population under different insecticide selection pressures. PLoS neglected tropical diseases. 2015;9(8):e0003928. doi: 10.1371/journal.pntd.0003928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams J, Flood L, Praulins G, Ingham VA, Morgan J, Lees RS, et al. Characterisation of Anopheles strains used for laboratory screening of new vector control products. Parasites & vectors. 2019;12(1):1–14. doi: 10.1186/s13071-019-3774-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djihinto AC, Katary A, Prudent P, Vassal J-M, Vaissayre M. Variation in resistance to pyrethroids in Helicoverpa armigera from Benin Republic, West Africa. Journal of economic entomology. 2009;102(5):1928–34. doi: 10.1603/029.102.0525 [DOI] [PubMed] [Google Scholar]
- 40.Chareonviriyaphap T, Rongnoparut P, Juntarumporn P. Selection for pyrethroid resistance in a colony of Anopheles minimus species A, a malaria vector in Thailand. Journal of vector ecology: journal of the Society for Vector Ecology. 2002;27(2):222–9. [PubMed] [Google Scholar]
- 41.Berticat C, Bonnet J, Duchon S, Agnew P, Weill M, Corbel V. Costs and benefits of multiple resistance to insecticides for Culex quinquefasciatus mosquitoes. BMC evolutionary biology. 2008;8(1):1–9. doi: 10.1186/1471-2148-8-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt N, Kwiatkowska RM, Irving H, Diabaté A, Dabire R, Wondji CS. Target-site resistance mutations (kdr and RDL), but not metabolic resistance, negatively impact male mating competiveness in the malaria vector Anopheles gambiae. Heredity. 2015;115(3):243–52. Epub 04/22. doi: 10.1038/hdy.2015.33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.