Abstract
Background
Paediatric peripheral intravenous catheter (PIVC) insertion using traditional landmark insertion technique can be difficult.
Aim
To systematically review the evidence comparing landmark to ultrasound guidance for PIVC insertion in general paediatric patients.
Study design
Cochrane methodology to systematically search for randomised controlled trials comparing landmark to ultrasound‐guided PIVC insertion.
Data sources
Cochrane Central Register of Controlled Trials, US National Library of Medicine, Cumulative Index to Nursing and Allied Health, Embase.
Data extraction
English‐language, paediatric trials published after 2000, reporting first‐attempt insertion success, overall PIVC insertion success, and/or time to insert were included. Central venous, non‐venous and trials including only difficult intravenous access were excluded. Data were independently extracted and critiqued for quality using GRADE by three authors, and analysed using random effects, with results expressed as risk ratios (RR), mean differences (MD) and 95% confidence intervals (CI). Registration (CRD42020175314).
Results
Of 70 titles identified, 5 studies (995 patients; 949 PIVCs) were included. There was no evidence of an effect of ultrasound guidance, compared to landmark, for first‐attempt insertion success (RR 1.27; 95% CI 0.90–1.78; I 2 = 88%; moderate quality evidence), overall insertion success (RR 1.14; 95% CI 0.90–1.44; I 2 = 82%; low quality evidence), or time to insertion (mean difference −3.03 min; 95% CI −12.73 to 6.67; I 2 = 92%; low quality evidence).
Limitations
Small sample sizes, inconsistent outcomes and definitions in primary studies precluded definitive conclusions.
Conclusions
Large clinical trials are needed to explore the effectiveness of ultrasound guidance for PIVC insertion in paediatrics. Specifically, children with difficult intravenous access might benefit most from this technology.
Keywords: catheterisation, peripheral, peripheral venous catheter, systematic review, ultrasonography, vascular access device
Key Points.
Insertion of peripheral intravenous catheters (PIVCs) in children can be difficult and procedural failure is high.
There was no clear evidence of improved first‐time PIVC insertion success when ultrasound guidance was used, in comparison to landmark in paediatric patients.
There is an urgent need for large randomised controlled trials with standardised outcome measures to determine the efficacy of ultrasound guidance to improve first‐time PIVC insertion success in paediatric patients.
Background
Peripheral intravenous catheters (PIVCs) are small, hollow tubes inserted into veins of the upper or lower limbs in children and neonates used to deliver short‐term intravenous therapy. 1 , 2 Most children admitted to hospital require a PIVC; 3 however, practitioners and health‐care consumers (patients and parents) often report this procedure to be one of the most challenging aspects of hospitalisation. 4 Clinically, this may be due to children's smaller, less visible veins, reduced procedural co‐operation, increased adiposity and anxiety compared to their adult counterparts. These challenges, in combination with limited practitioner training and technical skill, result in a first‐attempt insertion failure rate of approximately 50%. 4 Patients consistently describe PIVC insertion to be the most painful inpatient procedure 4 and failed insertion results in substantial negative sequelae including: trauma and harm to both the patient and their vasculature, increased risk of infiltration and extravasation, increased pain and anxiety, increased morbidity and mortality due to delayed treatment, and wastage of scarce health‐care resources. 5
Traditional methods for PIVC insertion involve palpation and visualisation of a suitable vein, followed by ‘blind’ insertion, however children's physiology, and/or the presence or history of chronic illness mean an appropriate vessel is not always easily identified. Clinicians are then forced to rely on their knowledge of advanced vascular anatomy to guide insertion choice and practices. 6 This landmark‐based insertion technique may contribute to the current high insertion failure rate. Patients at highest risk of PIVC insertion failure are those with difficult intravenous access (DIVA) and this risk might be reduced with the use of innovative technology and practices. 7 , 8 , 9 Technologies to assist PIVC insertion have evolved including transilluminators, near infra‐red light devices and ultrasound, all designed to improve vein identification and/or intra‐procedural guidance. 10 , 11
International organisations (e.g. Infusion Nurses Society, 12 Emergency Nurses' Association of the USA, 13 The Australian Commission on Quality and Safety in Healthcare, 14 Royal College of Nursing 15 and Association of Anaesthetists of Great Britain and Ireland Safe Vascular Access 2016 16 ) all recommend the use of technology to improve PIVC first‐attempt insertion success. Despite positive findings regarding ultrasound guidance to improve first‐attempt insertion success in adults, particularly those identified as DIVA, evidence in paediatric patients appears inconclusive. 10 Heinrichs et al. 10 attempted to answer this unresolved question 8 years ago, through a systematic review and meta‐analysis of PIVC insertion with technology (e.g. transilluminators and near‐infrared light devices). Despite the utility of near‐infrared light devices in children with DIVA, they concluded no overall clinical improvement (risk ratio (RR) 0.99; 95% confidence interval (CI) 0.74–1.33). At the time of that review, ultrasound‐guided PIVC insertion was in its infancy and there were no randomised controlled trials (RCTs) evaluating its clinical safety and efficiency. Since then, multiple small clinical trials have been undertaken comparing the safety and efficiency of ultrasound to insert PIVC compared to traditional techniques with inconsistent results. 17 , 18 , 19 , 20 , 21 To date, there has been no synthesis of these trials to explore the clinical benefit of ultrasound guidance to improve PIVC insertion in paediatrics. Therefore, the objective of this systematic review and meta‐analysis was to assess the effect of ultrasound guidance to improve first‐attempt insertion success, overall PIVC insertion success and time to PIVC insertion.
Methods
Design
A systematic review and meta‐analysis were undertaken, based primarily on Cochrane Collaboration systematic review methods. 22 The review was prospectively registered with PROSPERO (CRD42020175314) and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 23
Inclusion and exclusion criteria
Studies were eligible for inclusion if they met pre‐defined criteria: (i) RCT design; (ii) participants were aged <18 years; (iii) ultrasound‐guided insertion was compared to landmark insertion techniques; (iv) reported PIVC insertion primary and secondary outcomes (described below). Studies were excluded if they reported only DIVA, central venous (e.g. peripherally inserted central catheters) or non‐venous (e.g. arterial) devices, were published before 2000 or were not written in English.
Primary and secondary outcomes
The primary outcome was first‐attempt PIVC insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).
Secondary outcomes were: total number of insertion attempts (i.e. number of skin punctures required to successfully insert PIVC), overall PIVC insertion success, time to insert the PIVC (i.e. procedural time; as defined by study author), PIVC dwell time (i.e. functional dwell time measured in hours), patient/parent satisfaction (as defined by study author e.g. Likert scale), health‐care worker satisfaction (as defined by study author e.g. Likert scale) and PIVC associated bloodstream infection (as defined by study authors).
Interventions
Ultrasound‐guided PIVC insertion was defined as the use of ultrasound to locate and select an appropriate vein, with the PIVC inserted under direct ultrasound visualisation by advancing the needle into the vein whilst moving the ultrasound probe in the direction of needle advancement. 24 Traditional landmark insertion was defined as insertion of PIVC by palpating and/or visualising an appropriate vein.
Systematic search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), US National Library of Medicine (MEDLINE), Cumulative Index to Nursing and Allied Health and Embase databases between 2000 and 2020 was undertaken on 23 March 2020 and updated on 25 October 2021. Medical Subject Heading terms (e.g. ‘Paediatrics’) and relevant keywords and their variants (e.g. ‘peripheral intravenous catheter’, ‘peripheral venous catheter’) were used. Search terms were developed in collaboration with a health‐care librarian. Additional studies were identified through hand searches of bibliographies. An outline of the systematic search strategy can be found in Table S1, Supporting Information.
Data extraction
Data regarding the study setting, population, number of participants, primary outcome and definition, and secondary outcomes and definitions were extracted independently by three review authors (TMK, JS, RP) using a standardised data extraction form, managed in Microsoft Excel.
Risk of bias
Studies fulfilling the inclusion criteria were assessed for their methodological quality by two review authors (TMK, JS) utilising the Cochrane Risk of Bias (RoB2). 25 , 26 Trials' risk of bias was assessed using the five following domains (random sequence generation, allocation concealment, blinding, attrition and reporting bias). 26 Grading of Recommendations Assessment, Development and Evaluation (GRADE) 27 approach was used for assessment of the overall quality of evidence for each outcome (Table 1). Individual RCTs began at high quality, we downgraded the level of evidence by one for ‘serious’ or two for ‘very serious’ study limitations (high risk of bias, serious inconsistency, publication bias or indirectness of evidence). Any disagreements between the review authors were resolved by discussion with a third reviewer (RP).
Table 1.
Grading of recommendations assessment, development and evaluation
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Ultrasound | Landmark technique | Relative (95% CI) | Absolute (95% CI) | ||
| First‐attempt insertion success | ||||||||||||
| 4 | Randomised trials | Not serious | Very serious† | Not serious | Serious‡ | None | 263/396 (66.4%) | 269/411 (65.5%) | RR 1.10 (0.86–1.40) | 65 more per 1000 (from 92 fewer to 262 more) |
⨁◯◯◯ very low |
Critical |
| Insertion success | ||||||||||||
| 4 | Randomised trials | Not serious | Serious§ | Not serious | Serious‡ | None | 38/284 (13.4%) | 47/290 (16.2%) | RR 0.63 (0.22–1.80) | 60 fewer per 1000 (from 126 fewer to 130 more) |
⨁⨁◯◯ low |
Critical |
| Time to cannulation (min) | ||||||||||||
| 2 | Randomised trials | Not serious | Very serious† | Not serious | Serious‡ | None | 162 | 171 | — | MD 3.03 lower (12.73 lower to 6.67 higher) |
⨁◯◯◯ very low |
Important |
Heterogeneity I 2 > 80%.
Wide confidence interval.
Heterogeneity I 2 70–80%.
CI, confidence interval; MD, mean difference; RR, risk ratio.
Data analysis and synthesis
Where two or more trials with sufficient evidence of study homogeneity with respect to trial interventions and population were identified, meta‐analysis using RevMan 5 (version 5.4.1) 28 with random effects was conducted. Where there was evidence of significant heterogeneity among eligible trials or their samples, a narrative analysis of the findings was provided. The primary analysis involved comparison of treatment effect using the primary outcome measure, and RR with 95% CI were used to measure intervention effect for PIVC insertion success rate. Mean difference (MD) and 95% CI for continuous outcomes (e.g. time for insertion) were calculated and the standardised MD (difference between experimental and control groups across trials) reported as the summary statistic. Descriptive statistics were used to summarise information regarding study population, interventions and results. Given the heterogeneity of study populations, subgroup analyses were planned for emergent versus non‐emergent PIVC insertion, and PIVC inserted by health‐care practitioners specialising in vascular access versus other health‐care professionals. However, there were insufficient discrete data to undertake these subgroup analyses.
Results
Search strategy
Figure 1 describes the flow of inclusion and exclusion for study selection, in accordance with the PRISMA guidelines. 29 Following removal of duplicates, 70 records were identified, with 32 justifying full‐text review. Finally, five studies were included in the review. 17 , 18 , 19 , 20 , 21
Fig. 1.
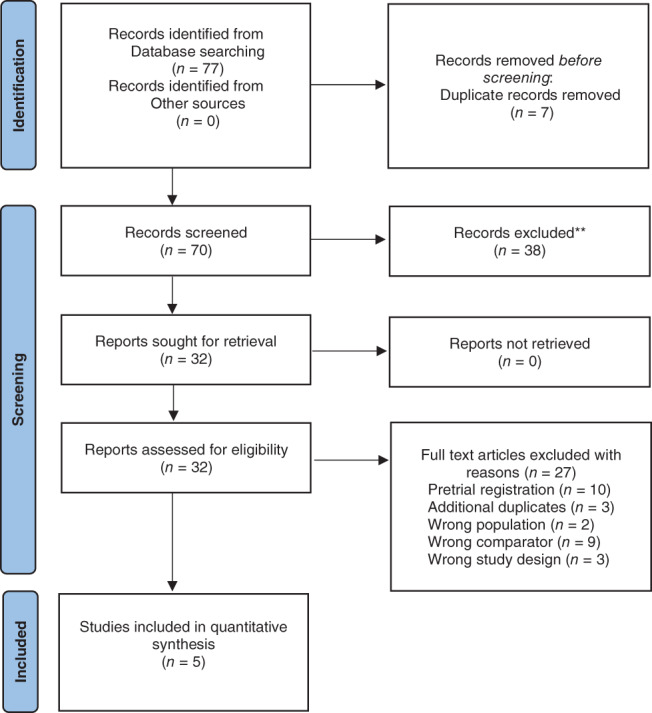
PRISMA flow chart.
Study characteristics
Included studies reported outcomes in a total of 995 patients and 949 successful PIVC insertions. All included studies were RCTs, undertaken in Brazil, 17 France, 18 Canada 19 or the USA. 20 , 21 The age of trial participants ranged from birth to 18 years of age. All children included in the reviewed studies required hospital admission either via the emergency department 19 , 20 or for a surgical procedure. 17 , 18 , 21 Of the studies undertaken in children requiring a surgical procedure (n = 3), two were undertaken in the operating theatre post inhalational gas, 18 , 21 the third study included fully conscious surgical inpatients. The majority of studies were undertaken within the last 10 years 18 , 21 , 30 with the exception of Doniger et al. 20 (undertaken between 2006 and 2007) and Avelar et al. 17 (dates of data collection were not clear); however, the results were published in 2013. Ultrasound‐guided technology was the intervention described in all studies with one study 23 also including use of the vein viewer as a concomitant intervention. Table 2 describes the populations and PIVC insertion characteristics of the included studies. All trials described evidence of ethical review board approval and participant consent for trial participation. No trial acknowledged industry support, either in part or in full to undertake the trial. 19
Table 2.
Characteristics of included studies
| Author; country | Method | Participants | Indication for PIVC | Intervention | Comparator | PIVC inserter/operator USG and landmark PIVC | Outcomes |
|---|---|---|---|---|---|---|---|
| Avelar et al. 17 ; Brazil | Single centre RCT | N = 355; 1 day to 18 years; hospitalised | Admitted to a paediatric surgical unit | Ultrasound | Traditional landmark | Trained nurse | Successful insertion of PIVC on first puncture; catheter dwell time; absence of identification of signs of local intravenous therapy complications |
| Benkhadra et al. 18 ; France | Single centre RCT | N = 40; less than 3 years; operating room suite | Induction of anaesthesia | Ultrasound | Traditional landmark | Anaesthetist | First‐time insertion success; overall PIVC insertion success; time to cannulation, total number of punctures and type of catheter used |
| Curtis et al. 19 ; Canada | Single centre RCT | N = 418; less than 16 years; emergency department | Required PIVC as part of their treatment | Ultrasound and near infrared light | Traditional landmark | Not stated | First‐attempt PIVC insertion success; number of attempts to successful PIVC placement; time to successful PIVC insertion |
| Doniger et al. 20 ; USA | Single centre RCT | N = 50; less than 10 years; emergency department | Required PIVC as part of their treatment | Ultrasound | Traditional landmark | Emergency department nurse or physician | Number of attempts; overall PIVC insertion success; overall procedure time; number of needle redirections and the necessity for alternative methods of vascular access |
| Hanada et al. 21 ; USA | Single centre RCT | N = 102; weighing greater than or equal to 3 kg and aged less than 4 years; operating room suite | Maintenance of anaesthesia | Ultrasound | Traditional landmark | Anaesthetist | First‐attempt insertion success; success rate of PIVC insertion within 10 min |
PIVC, paediatric peripheral intravenous catheter; RCT, randomised controlled trial.
Study quality
The quality of the studies was mixed, with incomplete reporting of denominators of outcomes and poor outcome definition consistency. Most domains were assessed as low risk of bias. There was some risk of bias concerns regarding blinding of participants and personnel to the intervention, which was assessed as high risk in all studies due to the nature of the intervention. Three trial investigators reported the use of computer‐generated randomisation 17 , 19 , 21 and two studies stated that sealed, opaque envelopes were used however they did not report use of a tamper seal 18 , 20 and only one study reported that the envelopes were numbered. 18 Figure 2 illustrates the risk of bias for each domain across all trials.
Fig. 2.
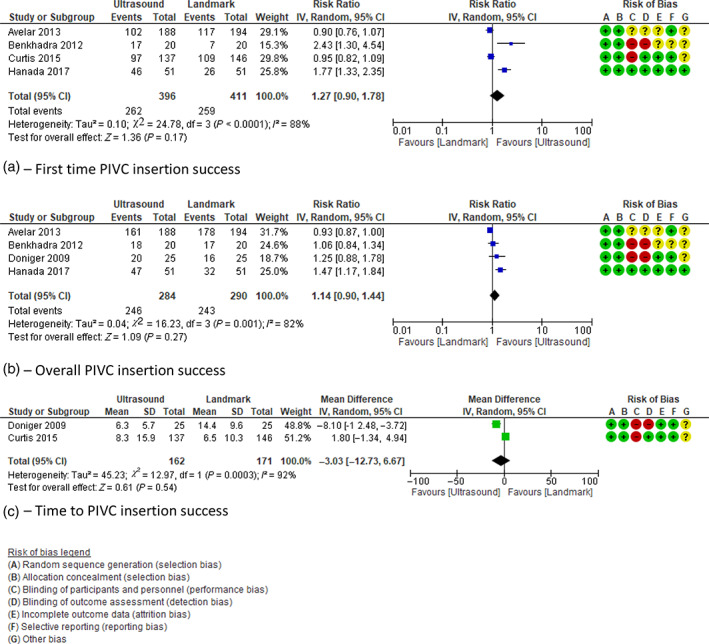
Meta‐analysis of studies reporting (a) first‐time PIVC insertion success, (b) overall PIVC insertion success, (c) time to PIVC insertion success. CI, confidence interval; PIVC, paediatric peripheral intravenous catheter.
Primary outcome: First‐time PIVC insertion success
Of the four trials that assessed the review's primary outcome of first‐attempt insertion success 17 , 18 , 19 , 21 (Fig. 2a), we found no evidence of an effect of ultrasound guidance, in comparison to landmark (RR 1.27; 95% CI 0.90–1.78). There was moderate quality evidence for this outcome, with high statistical heterogeneity (I 2 = 88%).
Secondary outcomes
Overall PIVC insertion success
The incidence of overall PIVC insertion success was reported in four studies. 17 , 18 , 20 , 21 Compared to landmark technique, Figure 2b demonstrates no evidence of an overall effect when ultrasound guidance was used to insert PIVCs (RR 1.14; 95% CI 0.90–1.44). Evidence was of low quality and statistical heterogeneity high (I 2 = 82%).
Time to successful insertion
Similarly no overall effect (MD 3.03 min; 95% CI −12.73 to 6.67) was demonstrated for time to PIVC insertion (Fig. 2c) when ultrasound guidance was used. 20 , 31 Statistical heterogeneity was high (I 2 92%) and the quality of evidence was low.
Other outcomes
We found insufficient trials reporting dwell time, patient/parent satisfaction, health‐care worker satisfaction and infection.
Discussion
In this systematic review and meta‐analysis of ultrasound‐guided PIVC insertion for general paediatric patients compared to landmark technique, there was no clear improvement in first‐attempt insertion success, overall insertion success or time to PIVC insertion. However, lack of standard outcome definitions, inclusion criteria and study populations limit interpretation. Given the heterogeneity of the studies from which the data were drawn, pooled results should be interpreted with caution. The data characterise the best available evidence for clinical care, help identify sources of variability in results across studies, and inform future sample size calculations. Due to the heterogenous trial population, we estimate a sample size of 7000 patients (3500 per group) is required to demonstrate superiority with 80% power assuming a two‐sided χ 2 test and significance level (α) of 0.05. 32 Acknowledging the limited feasibility of achieving this sample size a more realistic and clinically relevant focus might be on patients most likely to benefit from ultrasound‐guided PIVC insertion such as children with DIVA.
Clinically, ultrasound might provide a valuable adjunct to PIVC insertion in the paediatric population where first‐attempt insertion failure using landmark technique is high. 4 This is likely due to the child's presenting pathology, smaller vessel size, increased adiposity and reduced procedural compliance. 33 However, as with all new medical procedures there is a learning curve that leads practitioners along the novice to expert continuum. In an observational study (n = 1077), 33 newly trained inserters reached 80% first‐attempt insertion success after four ultrasound PIVC insertions in adult patients. 34 The learning curve to achieve greater proficiency then steepens markedly, requiring 60 insertions to achieve 94% first‐attempt insertion success. Presumably, the numbers required to achieve this might be greater in children. Practically, ultrasound should provide thorough real‐time assessment of the vein depth, diameter and quality, including valves, venous bifurcation, blood flow and the presence of other structures such as arteries and nerves. The extent to which ultrasonography is used for venous assessment and PIVC insertion is operator dependent and can lead to varying outcomes based on skill level. Only a few studies, including those included in this review have systematically explored the association between different methods of PIVC insertion and the incidence of adverse events such as pain, anxiety, haematoma or nerve injury. In contrast, some observational studies have reported increased complications related to ultrasound‐guided PIVC due to infiltration, dislodgement and thrombosis. 35 , 36 , 37 Therefore, to improve insertion success and reduce complications during PIVC dwell, further large, paediatric cohort studies are needed to investigate the relationship between these two important outcomes.
Acknowledging the significant heterogeneity between the studies included in this review, the resultant lack of effect is unsurprising. Similarly, conflicting results were observed in early adult reviews that initially reported contradictory or inconclusive results when ultrasound guidance was compared to landmark PIVC insertion. 20 Most recently, however, 19 a systematic review and meta‐analysis (5 RCTs; 3 cohort studies; 1660 patients) reported definitive results demonstrating an overall insertion success of 81% when ultrasound was used, compared to 70% with landmark technique (odds ratio (OR) 2.49, 95% CI 1.37–4.52, P = 0.003). They also reported significantly fewer attempts, reduced time to insertion and increased patient satisfaction. For patients with DIVA, evidence for ultrasound guidance for PIVC insertion was particularly compelling with 75% versus 49% first‐attempt insertion success (OR 3.23, 95% CI 1.35–7.72, P = 0.008). The promising results demonstrated in large adult RCTs confirm the need for similarly large, paediatric clinical trials which might demonstrate consistent results.
Although an overall effect was not demonstrated, this review has substantial clinical and research implications. Some paediatric studies 20 , 21 support ultrasound guidance for DIVA which is consistent with the adult literature. 38 Incorporating validated tools to identify patients where difficulty is predicted prior to a failed PIVC insertion attempt would enable earlier escalation to expert practitioner and/or technology; however, a validated escalation pathway to ensure the right skilled clinician makes the first insertion attempt is lacking. 39 , 40 Rippey et al. 41 demonstrated statistically that clinician gestalt (gut instinct) is a predictor of PIVC insertion success. This has important implications for not only patient assessment (risk of DIVA) but self‐assessment prior to PIVC insertion. Further research is urgently needed combining assessment and identification of potential DIVA patients coupled with an escalation pathway with recommendation for skilled clinician (novice, intermediate or expert) and requirement for technology‐assisted insertion.
This review has several limitations. Firstly, the shortcomings in the number, sample size, design quality and heterogeneity of the included studies. The setting and total number of included patients varied widely. For example, the extremes in patient groups included patients presenting to the emergency department unwell, unco‐operative and anxious, 19 , 20 compared to other studies that reported PIVC insertion whilst the child was anaesthetised. 21 Future studies should stratify for this difference to ensure equal allocation. In addition, some studies reported DIVA patients but lacked a definition of how DIVA was ascribed limiting our ability to undertake subgroup analysis. Second, we limited our inclusion criteria to English language which might have excluded some trials with important outcomes.
This study also has important strengths. To our knowledge, this is the first systematic review and meta‐analysis in paediatrics comparing ultrasound‐guided PIVC insertion to traditional landmark technique. This is important because of the unique challenges associated with the use of this new technology in this vulnerable patient cohort. Although based on a small number of studies, our review only included RCTs which provide the highest quality evidence, and we used best practice methods for systematic review methodology.
Conclusion
This review has demonstrated large, sufficiently powered RCTs are needed to explore the effectiveness of ultrasound guidance compared to landmark to insert PIVC in children. Future studies should consider patient‐centred outcome measures such as pain, anxiety, patient and clinician satisfaction as well as focussing the evaluation of this intervention towards the population that may most benefit, for example, children with DIVA.
Supporting information
Table S1 Summary of search strategy.
Acknowledgement
Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Conflict of interest: TMK's employer, Griffith University has received on her behalf unrestricted investigator‐initiated research or educational grants from product manufacturers (BD‐Bard). Griffith University has received consultancy payments on her behalf from product manufacturers (3M, Medical Specialties Australia, Smiths Medical and Vygon). JS's employer, Griffith University has received unrestricted investigator‐initiated research or educational grants on her behalf from product manufacturers (BD‐Bard). CMR's employer, Griffith University has received unrestricted investigator‐initiated research or educational grants on her behalf from product manufacturers (BD‐Bard; Cardinal Health). In addition, Griffith University has received consultancy payments from manufacturers (3M, BD‐Bard). Griffith University received a donation of products from ICU Medical. AJU's employer, Griffith University has received unrestricted investigator‐initiated research or educational grants on her behalf from product manufacturers (3M; BD‐Bard). Griffith University has indicated she does not have any financial relationships relevant to this article to disclose.
References
- 1. Kleidon T, Ullman A. Right device assessment and selection in pediatrics. In: Moureau NL, ed. Vessel Health and Preservation: The Right Approach for Vascular Access. Cham: Springer International Publishing; 2019; 181–95. [Google Scholar]
- 2. Ullman AJ, Bernstein SJ, Brown E, Aiyagari R, Doellman D, Faustino EVS, Gore B, Jacobs JP, Jaffray J, Kleidon T, Mahajan PV, McBride CA, Morton K, Pitts S, Prentice E, Rivard DC, Shaughnessy E, Stranz M, Wolf J, Cooper DS, Cooke M, Rickard CM, Chopra V The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics 2020;145(Suppl. 3):S269, S284. [DOI] [PubMed] [Google Scholar]
- 3. Ullman AJ, Takashima M, Kleidon T, Ray‐Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: A secondary analysis of 4206 catheters. J. Pediatr. Nurs. 2019; 50: e18–25. [DOI] [PubMed] [Google Scholar]
- 4. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: A quality improvement initiative. J. Paediatr. Child Health 2019; 55: 1214–23. [DOI] [PubMed] [Google Scholar]
- 5. Goff DA, Larsen P, Brinkley J et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp. Pediatr. 2013; 3: 185–91. [DOI] [PubMed] [Google Scholar]
- 6. Larsen P, Eldridge D, Brinkley J et al. Pediatric peripheral intravenous access: Does nursing experience and competence really make a difference? J. Infus. Nurs. 2010; 33: 226–35. [DOI] [PubMed] [Google Scholar]
- 7. Schults J, Rickard C, Kleidon T, Paterson R, Macfarlane F, Ullman A. Difficult peripheral venous access in children: An international survey and critical appraisal of assessment tools and escalation pathways. J. Nurs. Scholarsh. 2019; 51: 537–46. [DOI] [PubMed] [Google Scholar]
- 8. Vinograd AM, Chen AE, Woodford AL et al. Ultrasonographic guidance to improve first‐attempt success in children with predicted difficult intravenous access in the emergency department: A randomized controlled trial. Ann. Emerg. Med. 2019; 74: 19–27. [DOI] [PubMed] [Google Scholar]
- 9. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound: Peripheral intravenous catheter insertion. Chest 2020; 157: 369–75. [DOI] [PubMed] [Google Scholar]
- 10. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta‐analysis of new interventions for peripheral intravenous cannulation of children. Pediatr. Emerg. Care 2013; 29: 858–66. [DOI] [PubMed] [Google Scholar]
- 11. Parker SIA, Benzies KM, Hayden KA. A systematic review: Effectiveness of pediatric peripheral intravenous catheterization strategies. J. Adv. Nurs. 2017; 73: 1570–82. [DOI] [PubMed] [Google Scholar]
- 12. Infusion Nurses Society . Infusion therapy standards of practice. J. Infus. Nurs. 2016; 39: S63–S65. [Google Scholar]
- 13. Crowley M, Brim C, Proehl J et al. Emergency nursing resource: Difficult intravenous access. J. Emerg. Nurs. 2012; 38: 335–43. [DOI] [PubMed] [Google Scholar]
- 14. Keogh S, Mathew S, Alexandrou E et al. Peripheral Intravenous Catheters: A Review of Guidelines and Research. Canberrra: Australian Commission on Safety and Quality in Health Care; 2019. [Google Scholar]
- 15. Royal College of Nursing . Standards for Infusion Therapy. London, UK: Royal College of Nursing; 2020. [DOI] [PubMed] [Google Scholar]
- 16. Bodenham Chair A, Babu S, Bennett J et al. Association of Anaesthetists of Great Britain and Ireland: Safe vascular access 2016. Anaesthesia 2016; 71: 573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avelar AF, Peterlini MA, da Pedreira ML. Assertiveness and peripheral intravenous catheters dwell time with ultrasonography‐guided insertion in children and adolescents. Rev. Esc. Enferm. U.S.P. 2013; 47: 539–46. [DOI] [PubMed] [Google Scholar]
- 18. Benkhadra M, Collignon M, Fournel I et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: A prospective randomized study. Pediatr. Anesth. 2012; 22: 449–54. [DOI] [PubMed] [Google Scholar]
- 19. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near‐infrared vascular imaging to guide peripheral intravenous catheterization in children: A pragmatic randomized controlled trial. CMAJ 2015; 187: 563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doniger SJ, Ishimine P, Fox JC, Kanegaye JT. Randomized controlled trial of ultrasound‐guided peripheral intravenous catheter placement versus traditional techniques in difficult‐access pediatric patients. Pediatr. Emerg. Care 2009; 25: 154–9. [DOI] [PubMed] [Google Scholar]
- 21. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound‐guided short‐axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: A randomised study. Anaesthesia 2017; 72: 1508–15. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JPT, Thomas J, Chandler J et al. Cochrane Handbook for Systematic Reviews of Interventions. London, UK: Cochrane Collaboration; 2020. [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Int. J. Surg. 2010; 8: 336–41. [DOI] [PubMed] [Google Scholar]
- 24. Chapman GA, Johnson D, Bodenham AR. Visualisation of needle position using ultrasonography. Anaesthesia 2006; 61: 148–58. [DOI] [PubMed] [Google Scholar]
- 25. JPT H, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, UK: The Cochrane Collaboration; 2011. [updated March 2011]. Available from: www.cochranehandbook.org. [Google Scholar]
- 26. Sterne JAC, Savović J, Page MJ et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 27. Atkins D, Best D, Briss PA. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diaz‐Hennessey S, O'Shea ER. Virtual reality: Augmenting the acute pain experience in children. Pediatr. Nurs. 2019; 45: 122–7. [Google Scholar]
- 29. Moher D, Shamseer L, Clarke M et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst. Rev. 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Curtis SJ, Craig W, Logue E, Vandermeer B, Hanson A, Klassen T. CAEP/ACMU 2014 scientific abstracts, May 31‐June 4, 2014, Ottawa, Ontario. CJEM 2014;16 Suppl 1:S19‐114. [DOI] [PubMed] [Google Scholar]
- 31. Curtis SJ, Craig W, Erin L, Vandermeer B, Hanson A, Klassen T. A randomized controlled trial comparing ultrasound, veinviewer and standard approach to peripheral intravenous catheter placement in the pediatric emergency department. Peadiatr. Child Health 2014; 19: e46–7. [Google Scholar]
- 32. Sealed Envelop Ltd . Power calculator for binary outcome superiority trial 2012. 2 December 2020. Available from: https://www.sealedenvelop.com/power/binary-superiority/
- 33. Moore C, Kollpainter R, Andrews L et al. AIUM practice parameter for the use of ultrasound to guide vascular access procedures. J. Ultrasound Med. 2019; 38: E4–E18. [DOI] [PubMed] [Google Scholar]
- 34. Stolz LA, Cappa AR, Minckler MR et al. Prospective evaluation of the learning curve for ultrasound‐guided peripheral intravenous catheter placement. J. Vasc. Access 2016; 17: 366–70. [DOI] [PubMed] [Google Scholar]
- 35. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First‐attempt success, longevity, and complication rates of ultrasound‐guided peripheral intravenous catheters in children. Pediatr. Emerg. Care 2018; 34: 376–80. [DOI] [PubMed] [Google Scholar]
- 36. Fields JM, Dean AJ, Todman RW et al. The effect of vessel depth, diameter, and location on ultrasound‐guided peripheral intravenous catheter longevity. Am. J. Emerg. Med. 2012; 30: 1134–40. [DOI] [PubMed] [Google Scholar]
- 37. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J. Infus. Nurs. 2017; 40: 176–82. [DOI] [PubMed] [Google Scholar]
- 38. van Loon FHJ, Buise MP, Claassen JJF, Dierick‐van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: A systematic review and meta‐analysis. Br. J. Anaesth. 2018; 121: 358–66. [DOI] [PubMed] [Google Scholar]
- 39. Riker MW, Kennedy C, Winfrey BS, Yen K, Dowd MD. Validation and refinement of the difficult intravenous access score: A clinical prediction rule for identifying children with difficult intravenous access. Acad. Emerg. Med. 2011; 18: 1129–34. [DOI] [PubMed] [Google Scholar]
- 40. Yen K, Riegert A, Gorelick MH. Derivation of the DIVA score: A clinical prediction rule for the identification of children with difficult intravenous access. Pediatr. Emerg. Care 2008; 24: 143–7. [DOI] [PubMed] [Google Scholar]
- 41. Rippey JCR, Carr PJ, Cooke M, Higgins N, Rickard CM. Predicting and preventing peripheral intravenous cannula insertion failure in the emergency department: Clinician ‘gestalt’ wins again. Emerg. Med. Australas. 2016; 28: 658–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary of search strategy.


