Abstract
Background: Platinum-based chemotherapy, cisplatin (DDP) specifically, is the main strategy for treating lung cancer (LC). However, currently, there is a lack of predictive drug-resistance markers, and there is increased interest in the development of a reliable and sensitive panels of markers for DDP chemotherapy-effectiveness prediction. MicroRNAs represent a perspective pool of markers for chemotherapy effectiveness. Objectives: Data on miRNAs associated with LC DDP chemotherapy response are summarized and analyzed. Materials and methods: A comprehensive review of the data in the literature and an analysis of bioinformatics resources were performed. The gene targets of miRNAs, as well as their reciprocal relationships with miRNAs, were studied using several databases. Results and Discussion: The complex analysis of bioinformatics resources and the literature indicated that the expressions of 12 miRNAs have a high predictive potential for LC DDP chemotherapy responses. The obtained information was discussed from the point of view of the main mechanisms of LC chemoresistance. Conclusions: An overview of the published data and bioinformatics resources, with respect to the predictive microRNA markers of chemotherapy response, is presented in this review. The selected microRNAs and gene panel have a high potential for predicting LC DDP sensitiveness or DDP resistance as well as for the development of a DDP co-therapy.
Keywords: lung cancer, non-small cell lung cancer, cisplatin, DDP, chemotherapy, chemosensitivity, chemoresistance, therapeutic effectiveness markers, microRNA
1. Introduction
According to the GLOBOCAN data, lung cancer (LC) maintains its leading position and ranks first among men in both morbidity and mortality [1]. In 2020, 2.2 million new cases of lung cancer and 1.8 million deaths were registered. According to the modern classification, there are two main types of lung cancer: non-small cell lung cancer (NSCLC) which occurs in approximately 85% of patients and small cell lung cancer, which occurs in approximately 15% of patients [2]. There are three main subtypes of NSCLCs: squamous cell carcinoma (25% of lung cancers), adenocarcinoma (40% of lung cancers), and large cell carcinoma (10% of lung cancers); the other types of NSCLCs include neuroendocrine tumors and carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements [2]. Lung cancer is a severe disease that is difficult to treat. Currently, the most common treatment strategies for LC include surgery, chemotherapy, radiotherapy, and combinations of these treatments. The development of chemotherapy and radiotherapy resistance is a key issue in the progression of LC. As a result, in most countries, the five-year survival rate of patients with lung cancer is only about 10–20% [1].
2. Cisplatin and Lung Cancer Chemotherapy
The main method of LC treatment is chemotherapy. Platinum-based drugs are the gold-standard first-line treatments (ASCO and NCCN). The use of platinum-based drugs (cisplatin and carboplatin), including in combination with other chemotherapy agents (taxanes, pemetrexed, and antimetabolites), can achieve an overall survival for patients of 8 to 10 months on average [3]. A large and randomized comparison of four co-therapy regimens for stage IIIb and IV LC (cisplatin and paclitaxel, cisplatin and gemcitabine, cisplatin and docetaxel, and carboplatin and paclitaxel), which are the most commonly used regimens in clinical practice, showed that none of the used regimens had advantages over the others. The overall survival for all the studied regimens was about 10 months, with a one-year survival rate of 34% [4]. Patients with stage III LC undergo chemoradiotherapy (sequentially or simultaneously). This chemotherapy regimen is based on the basic drugs of the platinum group or their combination with other chemotherapy drugs, i.e., paclitaxel, etoposide, and vinblastine. The median progression-free survival of patients treated with chemoradiotherapy is poor (approximately eight months), with a five-year survival rate of only 15% [5]. An option to improve the situation is to switch to other treatment regimens, or example, treatment using the immunotherapy drug durvalumab, which is an inhibitor of the PDL 1 ligand that mobilizes the effector link of the antitumor immune system in a tumor microenvironment [6] or treatment of EGFR-positive LC patients using a combination of chemotherapy and tyrosine kinase inhibitors (platina-based chemotherapy and osemertinib) [7]. These chemotherapy schemes can achieve longer remission. However, these treatments have some limitations: immunotherapy is not available in all countries [1] and only about 15% of the general population of NSCLC patients have mutations in the EGFR gene [1,8].
Thus, platinum-based chemotherapy continues to be the main strategy for treating lung cancer [3,9]. Cisplatin (DDP) is the most widely used chemotherapy agent; it is an alkylating agent that effects inter- and intra-strand DNA cross-links, leading to cell-cycle arrest. However, drug resistance can develop, resulting in further development of a tumor and side effects such as myelosuppression, drug nephritis, nausea, vomiting, hearing loss, and polyneuropathy, which significantly reduce a patient’s quality of life [10]. Acquired chemoresistance during treatment is a major problem for clinicians and is a major cause of therapeutic failure [11]. Various mechanisms of tumor resistance to DDP have been described, and, most recently, these mechanisms have been classified as follows: (1) pre-target resistance (before cisplatin binds to DNA), (2) target resistance (directly associated with DNA-cisplatin adducts), (3) post-target resistance (associated with apoptosis caused by DDP-mediated DNA damage), and (4) off-target resistance (affecting molecular mechanisms that do not present obvious links to DDP-induced signals) [11]. Regardless of the resistance type, a tumor’s loss of sensitivity to DDP leaves a very short period of time for therapy correction aimed at increased patient survival. Clinical outcomes in the treatment of LC patients could be significantly improved through the introduction of non-invasive biomarker assays to predict and monitor the effectiveness of therapy [12]. However, there is a lack of reliable predictive drug-resistance markers and an urgent need to develop reproducible and highly sensitive panels of predictive markers for DDP-effectiveness assessment. Knowing a tumor’s response to cisplatin in advance would help clinicians, both before and during treatments, to select effective drugs and to adjust chemotherapy programs from one option to another in a timely manner. Efforts to identify such markers have primarily focused on the mechanisms underlying DDP resistance. DDP-resistance regulation represents a complicated network of many factors and signaling pathways. Obviously, a set of markers is needed to detect different types of tumors and, subsequently, to highlight the typical principal or driving aberrations specific to a particular tumor. MicroRNAs (miRNAs) could be promising candidate biomarkers for DDP resistance in LC, due to the multiple mechanisms by which they regulate the expression, and vice versa, for different target genes. Fortunately, there is considerable evidence on the association of aberrant miRNAs expression with DDP resistance in tumor cells (Tables S1 and S2).
3. MicroRNAs and Lung Cancer Chemotherapy
Since miRNAs regulate of a wide spectrum of physiological and pathological processes in cells, they are secreted from cells and enter the extracellular medium and biological fluids [13]. MicroRNAs have been shown to be rather stable in biological fluids, including blood or bronchial lavage, in which they circulate in tight complexes with biopolymers or are packed in membrane-coated vesicles [14,15] for review. Cell-free miRNAs (cfmiRNAs) can be released from different tumor areas or tumor nodes, and, therefore, a cfmiRNA profile reflects a patient’s generalized tumor phenotype. Considering the well-developed protocols for cfmiRNA isolation and evaluation of their sets and concentrations, cfmiRNAs could be promising diagnostic markers [16]. The availability of liquid media, such as blood, sputum, and saliva, and methods that do not require invasive procedures have provided an opportunity for using liquid biopsies in the diagnosis of cancers, including LC [17]. The correlation between changes in miRNA expression and tumor development during treatment (aggressiveness and chemoresistance) have prompted the development of miRNA diagnostic panels and the emergence of prognostic and predictive markers for monitoring cancer as well as the development of new strategic solutions for the treatment of platinum-resistant LC ([18,19,20] for review). In fact, miRNA dysregulation in LC and under LC chemotherapy is involved in the regulation of the genes crucial to chemoresistance development: DNA repair, apoptosis, cell-cycle regulation, epithelial–mesenchymal transition (EMT), hypoxia, autophagy, drug efflux, cancer stem-cells activation (CSCs), etc. [20,21,22].
Numerous studies have aimed at identifying the miRNAs that mediate DDP response by investigating miRNAs that induce resistance/sensitivity to DDP in tumor cells or through comparative analyses of miRNA expressions in chemo-resistant and chemo-sensitive samples (cell lines and the tissues or biofluids of DDP-resistant and -sensitive LC patients). However, there are fewer studies that have aimed at exploring miRNAs differentially expressed under DDP chemotherapy.
In the present study, we aim to propose a preliminary panel of miRNAs for predicting the effectiveness of DDP chemotherapy via analysis of experimental data on the miRNAs’ involvement in DDP response and their cross analysis with the data of the bioinformatic resources that describe miRNAs and genes mediating DDP-response interconnection. Studies for analysis were selected from the PubMed and Science Direct databases based on the selection criteria described below.
Inclusion Criteria
Studies that described an association between miRNA expression and responses to DDP chemotherapy in lung adenocarcinoma and non-small cell lung cancer patients, including liquid biopsy studies as well as in models of cultivated cells and xenografts, were included for consideration.
The inclusion criteria were:
Studies that reported a change in miRNA expression in response to DDP chemotherapy in NSCLC;
Studies that reported the influence of miRNA expression on resistance/sensitivity to DDP;
Studies that aimed to compare miRNA expression in DDP-resistant and DDP-sensitive samples (tissues, serum of LC patients, cell lines, and xenograft models).
The exclusion criteria were:
Studies published in languages other than English;
Letters to the editor, case studies, or review articles;
Bioinformatics data without experimental approval.
4. Comparison of miRNA Expression in DDP-Resistant and DDP-Sensitive Samples from LC Patients, Cell Lines, and Xenograft Models
Comparative studies of miRNA expression in therapy-resistant and -sensitive cancer cells of different origins represent a basic method of resistance markers’ identification. There are numerous such studies (Tables S1 and S2), however, only a few of these studies have used large-scale methods such as NGS. Most studies have analyzed the expression of a few miRNAs in LC DDP-sensitive and DDP-resistant cells and tumor-tissue samples using RT-PCR; the RT-PCR approach presents limited information on individual miRNA-expression differences (Tables S1 and S2), relationships with other miRNAs regulating the same gene targets or pathways, and interconnections of the pathways involved in response to DDP. In addition, there are only a few studies on cell-free miRNAs from blood plasma and miRNAs circulating in blood exosomes [23,24] or exosomes from a cell culture [25,26,27]. MicroRNAs packed in microvesicles, including exosomes, represent a convenient source of cell-free miRNAs for tumor diagnostics [28,29]. The miRNAs up- or downregulated in resistant samples represent perspective markers of resistance/sensitivity, correspondingly, however, they need to be additionally checked in this respect, for example, by directly studying their effect on DDP resistance by in vitro/ex vivo models.
5. Direct Effects of miRNA Overexpression/Depletion on DDP Sensitivity and Resistance
Changes in miRNA-expression levels in cells enable the direct evaluation of their effects on sensitivity/resistance to DPP chemotherapy. The results of studies of this type on sensitive and resistant cell lines and mice-xenograft models are presented in Tables S1 and S2. Parameters of chemosensitivity, such as the half-maximal inhibitory concentration (IC50), proliferation and apoptosis rates, cell-cycle arrest, cell viability, and migration and colony-formation ability, as well as tumor volume and weight, are usually assessed. Several miRNAs have been shown to be involved in DDP-resistance regulation using this approach, and many of these studies have included the study of the expression of the target genes that are crucial for a response to DDP (Tables S1 and S2) using luciferase reporter assay, qRT-PCR, and Western blot analysis. These investigations have helped to identify the network of miRNA–gene interactions involved in DDP-resistance development and to identify the principal miRNA players in the response to DPP. These miRNA and genes represent a valid set of preliminary prognostic markers.
6. Influence of DDP Chemotherapy on miRNA Expression
DDP chemotherapy, both as a powerful anticancer treatment and as a strong stressful intervention in organisms, causes significant changes in miRNA expression in tumor cells, as well as in many different normal cell types. However, until now, limited attention has been given to the analysis of the effect of DDP chemotherapy on the expression of miRNAs (Table 1).
Table 1.
The effect of DDP on miRNA level in LC samples.
| No. | DDP R/DDP S miRNA |
Downstream Regulated Target | In DDP R vs. DDP S Samples | Effect of DDP on miRNA Level | ↑ of miRNA Expression → Chemoresistance | ↓ of miRNA Expression → Chemosensitivity | Model: R/S Cells; Mice Xenografts | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Gene, Main Function/Pathway | Methods | ||||||||
| 1 | miR-33b-3p | P21 | luciferase assay, RT-PCR, Western blot | ↓ in cells | ↑ cell viability, proliferation, promoted G1/S transition, DNA-damage response | ↓ cell viability, G1 arrest, | S: A549 R: A549/DDP |
[30] | |
| 2 | miR-425-3p | AKT1, autophagy | luciferase assay, qRT-PCR, Western blot |
↑ in cells, cells exosomes | ↑ in serum exosomes, cell exosomes | ↑ cell viability, ↓ apoptosis in S cells | ↓ cell viability, ↑ apoptosis in R cells | S: A549 R: A549/DDP |
[26] |
| 3 | miR-3195 | ↑ in cells, cell exosomes | S: A549 | [25] | |||||
| 4 | miR-3676-5p | ↑ in cells, cell exosomes | S: A549 | ||||||
| 5 | miR-4443 | ↑ in cells, cell exosomes | S: A549 | ||||||
| 6 | Let7 (let-7a,-7b,-7c,-7d,-7e, -7f,-7g,-7i) | LIN28A,B | luciferase assay, IHC, RT-PCR, Western blot | ↓ in tissues, cells | ↓ in cells | ↓ cell viability in R cells | ↑ cell viability in S cells | S: A549 R: A549/DDP | [31] |
| 7 | miR-29c | AKT2 | RT-PCR | ↓ in tissues | ↑ in cells | ↓ cell viability in S ↓ tumor volume, proliferation (ki-67,AKT2) in X |
↑ cell viability | S: SPC-A-1, A549 X: A549 |
[32] |
| 8 | miR-32 | TRIM29 | ↓ in plasma | ↑ in plasma | [24] | ||||
| 9 | miR-181a |
↑ in cells | ↑ percentage of A549 cells with a G0-G1 DNA content ↑ proteolytic maturation of caspase-9 and caspase-3 triggered by CDDP ↑ proapoptotic member of the Bcl-2 family Bax |
No effect | S: A549 | [11] | |||
| 10 | miR-1244 | Bax, MEF2D, cyclin D1, p53 | qRT-PCR, Western blot | ↓ in cells | ↓ proliferation, ↑ apoptosis | S: A549, H522 | [33] | ||
DDP—cisplatin; DDP-S miRNA—miRNA associated with DDP sensitivity; DDP-R miRNA—miRNA associated with DDP resistance; R—chemotherapy-resistant cell line; S—chemotherapy-sensitive cell line; X—mice xenograft based on LC cell lines.
Nevertheless, miRNAs deregulated by DDP therapy represent a pool of prospective biomarkers for the development of a co-therapy, because they may reflect the development of a secondary resistance to DDP, which, in turn, may be influenced by changes in miRNA-expression levels. Liquid-biopsy studies [26] are of special interest, because they allow continuous observation of changes in miRNA-expression levels during LC therapy. However, such studies are only at their starting point and require normal-tissue DDP-response filtering.
There are also some studies that have aimed to associate miRNA expression with simultaneous resistance/sensitivity to different chemotherapies, including DDP (Table 2). They include investigations of DDP and other platinum-based drugs, taxanes, cytostatic vincristine, and cetuximab (IgG1 against epidermal growth factor, Table 2). The results of such studies have indicated that miRNAs are involved in the regulation of drug resistance via both common and different drug mechanisms, including multidrug resistance.
Table 2.
miRNA and resistance to DDP and other chemotherapies.
| No. | DDP R/DDP S miRNA | ↑ of miRNA Expression | ↓ of miRNA Expression | Model: (1) R/S Cells (2) Mice Xenografts | Drug | Reference |
|---|---|---|---|---|---|---|
| 1 | miR-181a | ↑ migration, invasion, EMT in S | ↓ migration, invasion, EMT in R cells | S: A549 R: A549/PTX, A549/DDP |
DDP, paclitaxel | [34] |
| 2 | Let7f miR-29a |
↓ cell viability in S, R | S: H2030 cells | DDP, carboplatin | [35] | |
| 3 | miR-34c-3p | ↓ cell viability, migration; ↑ apoptosis in cells ↓ tumor weight in X |
S: A549, H1299 X: A549 mice |
DDP, taxol | [36] | |
| 4 | miR-137 | ↓ cell proliferation, migration, induced cell apoptosis, arrested cell cycle in G1 phase and reversed drug resistance in R cells; ↓ tumor volume, weight, VEGF (angiogenesis) in X |
↑ cell growth, migration, cell survival, cell-cycle G1/S transition, resistance (CCK-8 assay) in S cells | S: A549 R: A549/CDDP X: A549/CDDP |
DDP, paclitaxel | [37] |
| 5 | miR-200c | ↓ cell viability, proliferation invasion, EMT; ↑ apoptosis | S: H1299, H596, and H522 | DDP, cetuximab | [38] | |
| 6 | miR-202 | ↓ cell viability, IC50; ↑ apoptosis in S; ↓ tumor volume in X |
S: NCI-H441, A549 X: A549 |
DDP | [32] | |
| ↓ IC50 in S | Oxaliplatin, carboplatin | |||||
| 7 | miR-216b | ↓ IC50; ↓ tumor weight in X | ↑ IC50 | S: A549, PC9 | DDP, carboplatin, oxaliplatin | [39] |
| 8 | miR-495 | ↓ cell viability, intracellular DDP accumulation in S, R | ↑ cell viability | S: A549 R: A549/DDP |
DDP, carboplatin, trans-/-diaminocyclohexaneoxalatoplatinum | [40] |
| 9 | miR-497 | ↓ cell viability, ↑ apoptosis in R | ↑ cell viability in S | S: A549 R: A549/DDP |
DDP, vincristine | [41] |
DDP—cisplatin; DDP-S miRNA—miRNA associated with DDP sensitivity; DDP-R miRNA—miRNA associated with DDP resistance; R—chemotherapy-resistant cell line; S—chemotherapy-sensitive cell line; X—mice xenograft based on LC cell lines.
7. Implication of miRNAs and Their Target Genes in Mechanisms of DDP LC Resistance
Well-known mechanisms of chemotherapy resistance (including lung-cancer DDP resistance) include apoptosis inhibition, cell-cycle progression, autophagy, drug transportation/detoxication (decreased drug uptake, activation of detoxification systems, and drug efflux), response to hypoxia, DNA repair, epithelial–mesenchymal transition (EMT), and cancer stem-cell (CSC) activation [42]. MicroRNAs have been shown to be involved in DDP-resistance development as well as to play an essential role in all the above-mentioned mechanisms.
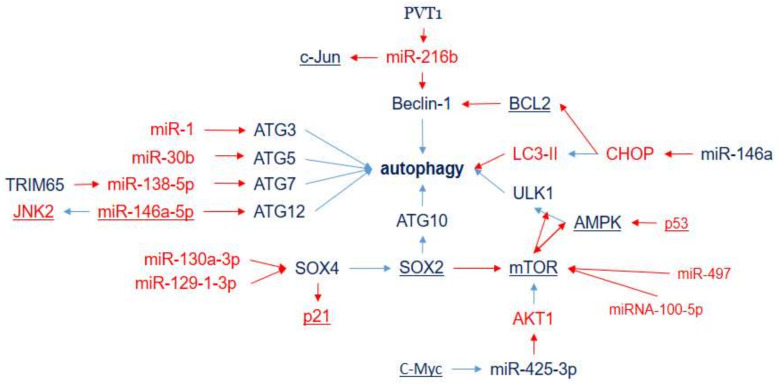
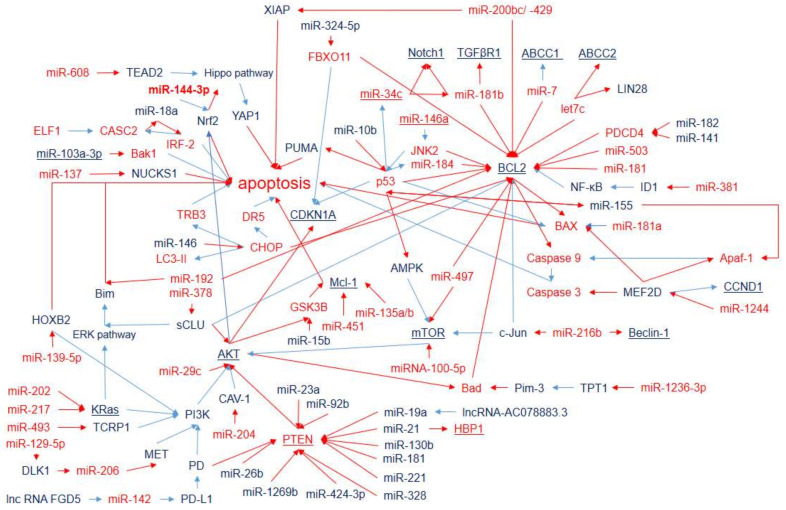
Autophagy implements the rearrangement of subcellular membranes for the subsequent autophagosome formation and lysosomal degradation of cytoplasmic contents and organelles [43]. Autophagy plays a dual suppressive or promoting role, which depends on the environmental context and the stage of tumorigenesis. During the early stages of cancer development, autophagy mainly acts as a survival pathway and a quality-control mechanism that may suppress cancer initiation and progression. At the late stage of cancer and under environmental stresses (such as hypoxia, starvation, hypoxia, heat stress, and accumulation of reactive oxygen species), autophagy promotes tumor survival, growth, and aggressiveness via metastasis [44]. Autophagy contributes to the response of cancer cells to chemotherapy agents: either as a protective mechanism for mediating resistance in response to chemotherapy or, in contrast, by inducing autophagic cell death, leading to sensitivity to chemotherapy [45]. However, when chemotherapy represents a strong stress, according to the published data (Tables S1 and S2), autophagy is mainly involved in DDP-resistance development. It has been shown that miRNAs, associated with the response to DDP therapy, are involved in the regulation of genes, which is crucial for all stages of autophagosome formation from initiation to maturation via autophagy-related genes (ATG), SOX4, SOX2, BCL2, Beclin-1, ULK, CHOP, etc. (Figure 1). Chemotherapy, both as a powerful anticancer treatment and as a strong stressful intervention in organisms, causes significant changes in miRNA expression in tumor cells as well as in many different normal cell types. However, until now, limited attention has been given to the analysis of the effect of DDP chemotherapy on the expression of miRNAs (Table 1).
Figure 1.
MicroRNAs and target genes implicated in LC DDP resistance via autophagy. Downregulation is shown by red arrows, and upregulation is shown by blue arrows. Overexpressed miRNAs and genes associated with DDP resistance (blue) and DDP sensitivity (red).
For example, autophagy activating kinase 1 (ULK1) takes part in the serine/threonine protein kinase ULK1 complex, which is in the upstream position during phagophore assembly [46]. Beclin-1 (regulated by miR-216b) participates in the regulation of the formation of the autophagosomal membrane. Double-membrane autophagosomes are assembled under the control of the Atg12 (regulated by miR-146a), Atg5 (regulated by miR-30b), and Atg16 conjugation system and catalyzed by Atg7 (regulated by miR-138) and Atg10 (regulated by SOX2). LC3-phosphatidylethanolamine conjugate (LC3-II) is recruited to autophagosomal membranes. SOX2, influenced by SOX4 (regulated by miR-130a and miR-129), targets Atg10 to induce autophagy.
Although most of the interconnections (Figure 1) are clear and represent the summation of miRNA parallel and sequential effects, there are some uncertainties. On the one hand, for example, SOX2 initiates autophagy by repressing mTOR. Then, the repressed mTOR results in activation of the AMPK-ULK pathway. On the other hand, low expressions of miR-100-5p and miR-497 result in enhancement of mTOR expression associated with DDP-resistance development. The mechanistic target of rapamycin (mTOR, regulated by miR-100-5p and miR-497) plays the role of the core inhibitor of autophagy and has been shown to be regulated by miRNAs associated with DDP resistance. However, it seems that mTOR has dual action with respect to DDP resistance, since it activates DDP-resistance development via activation of cell proliferation and survival. It may reflect the dual role of autophagy in DDP resistance, as mentioned earlier. This example indicates the necessity for a complex analysis of miRNA-integrative effects that excludes ambiguous potential markers.
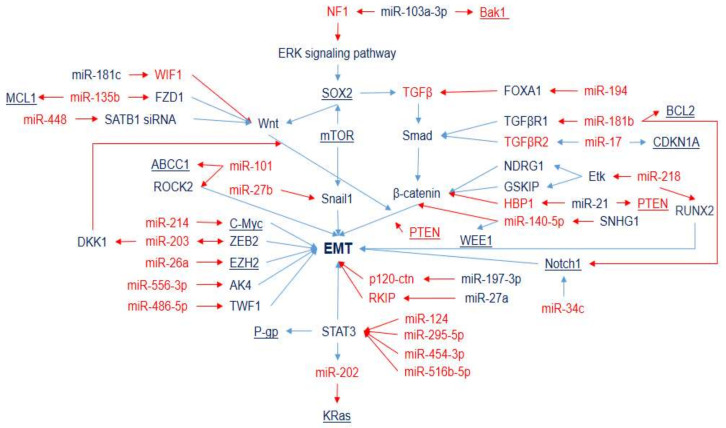
The epithelial–mesenchymal transition is an important step for cancer metastasis. It is a fundamental process in which epithelial cells gradually lose cell polarity and intercellular junctions, and acquire mesenchymal-cell phenotypic traits. Snail (regulated by miR-27b) is a significant EMT promoter in NSCLC [47]. The involvement of miRNAs in the DDP response via EMT regulation includes the ERK signaling pathway (miR-103a-3p), the PI3K/Akt signaling pathway, the TGF signaling pathway (miR-194, miR-181b, and miR-17), the Wnt/β-catenin signaling pathway (miR-181c, miR-135b, miR-448, miR-218, miR-21, and miR-140-5p), the Notch signaling pathway (miR-34c), the STAT signaling pathway (miR-125, miR-195-5p, miR-454-3p, and miR-516b-5p), and the enhancement of expression of EMT-related genes such as c-Myc (miR-214), ZEB2 (miR-203), EZH2 (miR-26a), AK4 (miR-556) TWF1 (miR-486-5p), and ROCK2 (miR-101) (Figure 2). EMT has also been associated with cancer stem cell (CSC) properties, for example, Myc (regulated by miR-214) mediates both cancer stem cells and EMT changes [48]. Moreover, there are genes (for example, BCL2 and c-Myc) that contribute to several key processes of DDP-resistance development simultaneously (they are presented in several figures and are highlighted by underlining). The miRNAs that affect such genes seem to have a synergetic effect and represent a very prospective pool of DDP-resistance markers.
Figure 2.
MicroRNAs and target genes implicated in LC DDP resistance via EMT. Downregulation is shown by red arrows and upregulation is shown by blue arrows. Overexpressed miRNAs and genes associated with DDP resistance (blue) and DDP sensitivity (red).
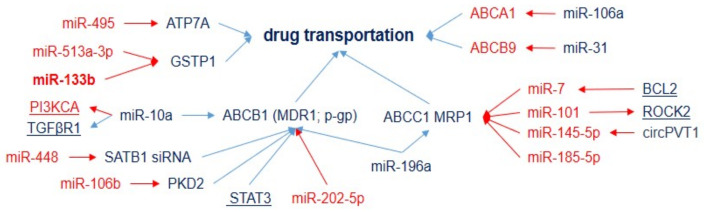
The inhibition of drug uptake, enhancement of drug efflux, and detoxification are other key mediators of drug resistance. Transporters such as ATP7A and ATP7B contribute to the sequestration and efflux of platinum compounds that mediate resistance to DDP. According to the current data, miRNAs associated with DDP response act via several efflux-pump classes: the ATP-binding cassette (ABC) superfamily, multidrug-resistance protein (MDR, P-glycoprotein), and multidrug-resistance-associated proteins (MRPs, Figure 3).
Figure 3.
MicroRNAs inhibiting drug uptake or enhancing drug efflux and drug detoxification in DDP-resistant tumors. Downregulation is shown by red arrows, and upregulation is shown by blue arrows. MicroRNAs and genes associated with DDP resistance (blue) and DDP sensitivity (red).
For example, MDR1 (regulated by miR-448, miR-106b, miR-202-5, miR-196a, and by several miRNAs (miR-124, 295-5p, miR-454-3p, and miR-516b-5p) via STAT3) encodes a multidrug efflux pump that plays a crucial role in the development of resistance to a vast number of drugs, including platinum-based drugs and taxanes, and, thus, is considered to be a key molecular target for effectively attenuating drug resistance [49]. The elevation of copper-transporting P-type adenosine triphosphatases ATP7A (regulated by miR-495) has been associated with resistance to platinum drugs [50,51]; moreover, it has been identified as a negative prognostic factor for patients with NSCLC with platinum-based chemotherapy [51]. The expression of the ABC superfamily of transport proteins, such as MRP1/ABCC1 (regulated by miR-7, miR-101, miR-145-5p, miR-185-5p, and miR-196a), ABCCA1 (downregulated by miR-106a), and ABCB9 (downregulated by miR-31), also correlates with DDP and multidrug resistance (Tables S1 and S2). Other miRNAs (miR-133b and miR-513a) influence DDP resistance via glutathione S-transferase gene 1, a member of the family of dimeric phase II metabolic enzymes, which acts as a catalyst for the binding of intermediary metabolites to cofactors, transforming them into more hydrophilic molecules and, thus, facilitating their detoxification. GSTP1 has been shown to be associated with lung-cancer expansion; it may cause resistance by binding/inactivating cisplatin (platinum-glutathione conjugates), enhancing DNA repair, or reducing cisplatin-induced oxidative stress [52].
Cisplatin causes cytotoxicity by DNA damage with formation of cisplatin–DNA adducts. As a consequence, DNA-repair mechanisms are enhanced in DDP-resistant cells and, conversely, suppressed in DDP-sensitive cells. For example, miR-488 contributes to DDP resistance via suppression of eIF3a expression and, consequently, activation of XPC and RPA14 of the NER, which are involved in DNA-repair pathways in organisms including prevention of gene mutation and repair-DNA distortion [53]. The miR-92 family associated with DDP sensitivity downregulates RAD21, which is crucial for the homologous recombination of DNA during double-strand-break repair. DNA damage activates the stimulation of checkpoint mechanisms and leads to DNA repair and further cell-cycle progression or to cell death by apoptosis. Thus, DNA-damage-inducible transcript 3, induced in response to certain stressors, contributes to endoplasmic reticulum stress-induced apoptosis. However, under DDP therapy, miR-146a expression may be enhanced and lead to the suppression of CHOP and CHOP-induced apoptosis [54].
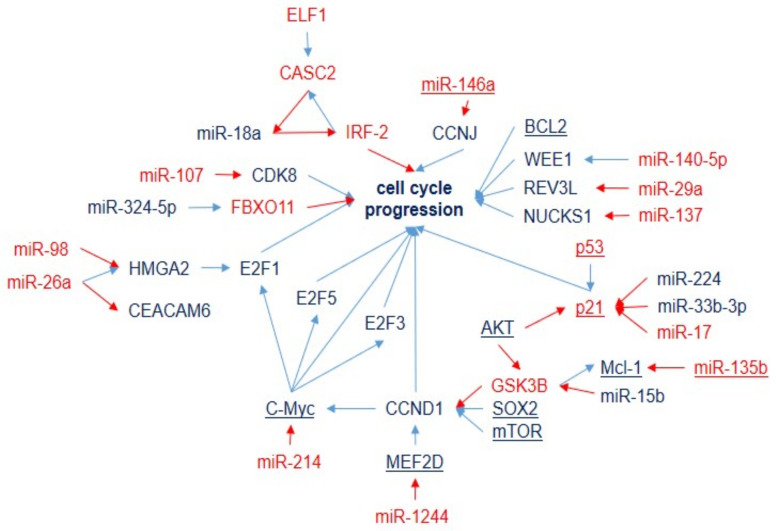
The miRNAs involved in DDP sensitivity/resistance via cell-cycle and apoptosis regulation are presented in Figure 4 and Figure 5. However, they are tightly related to each other and are separated artificially for easier identification.
Figure 4.
Cell-cycle regulation by miRNAs and target genes in LC-DDP-resistant tumors. Downregulation is shown by red arrows, and upregulation is shown by blue arrows. MicroRNAs and genes associated with DDP resistance (blue) and DDP sensitivity (red).
Figure 5.
MicroRNAs and target genes regulating apoptosis in LC-DDP-resistant cells. Downregulation is shown by red arrows, and upregulation is shown by blue arrows. MicroRNAs and genes associated with DDP resistance (blue) and DDP sensitivity (red) are shown.
Various studies have suggested that miRNAs are responsible for cell-cycle control by activating or inhibiting the expression of proteins involved in the response to DNA damage. Members of the BCL2 family have been the most frequently described target molecules among the different miRNAs associated with apoptosis related to DDP resistance in NSCLC cells (Table S2 and Figure 5). The complicated network of genes and miRNAs involved in the regulation of DDP resistance via BCL2 confirmed this. BCL2 is also considered to be an important anti-apoptotic protein that is involved in cisplatin-induced apoptosis (Figure 5). Other members of the BCL family, i.e., pro-apoptotic Bak1 and Bax, are regulated by miR-103a and miR-181a, correspondingly, and suppress their expression results in DDP resistance. Other widely represented mechanisms of miRNA influence on DDP resistance are the PTEN gene and the PI3K/Akt signaling pathway. This pathway is regulated by several miRNAs associated with DDP resistance (Figure 5) and influences apoptosis via NRF2 and BCL2 and the cell cycle via CDKN1.
The MiRNAs involved in CDDP-resistance of NSCLC cells, which are mainly assigned to apoptosis pathways, are summarized in Figure 5.
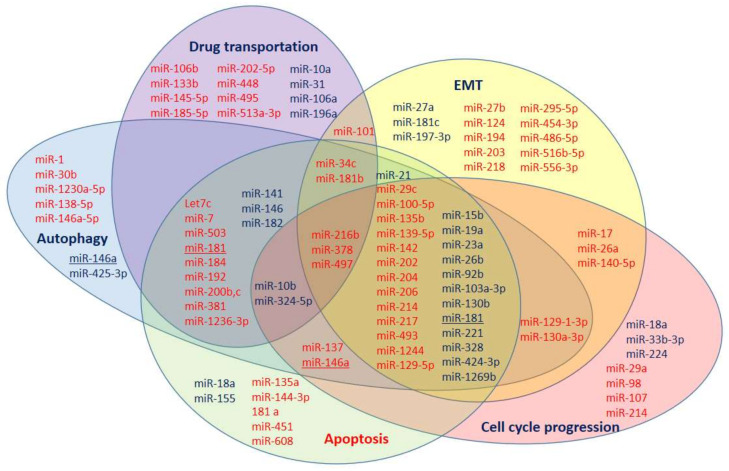
The data on the involvement of miRNAs in processes of DDP resistance from Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5 are summarized in Figure 6. Some miRNAs have multidirectional effects on DDP-resistance development (miR-146a and miR-181). Thus, they seem to be a doubtful choice as DDP-resistance markers. However, three of the analyzed miRNAs (miR-216b, miR-378, and miR-497) are involved in all the main processes (EMT, cell-cycle progression, drug transportation, apoptosis, and autophagy), and, therefore, have the highest potential as nonspecific DDP-resistance markers. Moreover, the distribution of miRNAs in Figure 6 evidence that all analyzed processes are crucial for DDP resistance and the development of a co-therapy for the DDP-resistance reversal should influence all of them.
Figure 6.
MicroRNA involvement in DDP-resistance development through EMT, drug transportation, apoptosis, cell cycle, and autophagy regulation. MicroRNAs, in which low expression is associated with DDP resistance development (red) and miRNAs, in which high expression is associated with DDP resistance development (blue).
8. Bioinformatics Analysis of miRNAs Involved in DDP-Response Regulation
MicroRNAs found in a few independent studies with different experimental designs are considered to be more valid as potential markers for the DDP response. To select such miRNAs, only miRNAs that were shown to be involved in the regulation of the ADT therapy response in three different types of experiments were considered for further analysis. The group of such miRNAs consisted of 9 miRNAs associated with DDP resistance and 23 miRNAs associated with DDP sensitivity (Table 3).
Table 3.
miRNAs regulating DDP-therapy response, confirmed in three different types of experiments (data based on DDP-resistance studies and extracted from Tables S1 and S2).
| DDP R/DDP S miRNA | Downstream Regulation | Reference |
|---|---|---|
| miR-21 | PTEN | [55,56,57,58] |
| miR-27a | RKIP | [59,60] |
| miR-92b-3p | PTEN | [23,61] |
| miR-130b | PTEN | [62] |
| miR-146a | CHOP | [54] |
| miR-224 | p-21 | [62] |
| miR-324-5p | FBXO11 | [63] |
| miR-425-3p | AKT1 | [26] |
| miR-1269b | PTEN | [64] |
| let7 a,b,c,d,e,f,g,i | LIN28 | [36,48] |
| miR-29c | AKT2 | [65] |
| miR-30b-5p | LRP8 | [66] |
| miR-34c-3p | Notch | [35] |
| miR-100-5p | mTOR | [25] |
| miR-101 | ABCC1, ROCK2 | [67,68] |
| miR-145-5p | ABCC1 | [69] |
| miR-146a | JNK2, CEACAM6, CCNJ | [70,71,72] |
| miR-181b | BCL2, TGFβR1, Notch2 | [73,74,75,76] |
| miR-193 | LRRC1 | [77] |
| miR-378 | sCLU | [78] |
| miR-379 | EIF4G2 | [79] |
| miR-381 | NFkB | [80] |
| miR-451a | MCL-1 | [81,82,83] |
| miR-486-5p | TWF1 | [84] |
| miR-613 | GJA1 | [85,86] |
DDP—cisplatin; DDP-S miRNA—miRNA associated with DDP sensitivity; DDP-R miRNA—miRNA associated with DDP resistance.
These miRNAs are involved in all key processes underlying PCA development: cell proliferation, epithelial–mesenchymal transition (EMT), apoptosis, cell-cycle progression, angiogenesis, metastasis, and invasion regulation (Table 4).
Table 4.
The involvement of selected miRNAs in crucial steps of tumorigenesis.
| DDPR/DDP S miRNA | Apop-tosis | EMT | Cell Cycle Progression | Auto-Phagy | Proliferation | Cell Growth | Angio-Genesis | Meta-Stasis | Invasion | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| miR-21 | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | [87,88] | |||
| miR-27a | ↑ | [60] | ||||||||
| miR-130b | ↑ | ↑ | ↑ | ↑ | ↑ | [88,89,90] | ||||
| Let7-a | ↓ | ↓ | ↓ | [91,92,93] | ||||||
| Let7-f | ||||||||||
| Let7-g | ↑ | ↓ | ↓ | [94,95] | ||||||
| Let7-i | ↓ | [96] | ||||||||
| miR-29c | ↓ | [65] | ||||||||
| miR-181b | ↑ | ↓ | ↓ | ↓ | [73,76,97,98,99] | |||||
| miR-193 | ↓ | ↓ | ↓ | [100] | ||||||
| miR-200b | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | [101,102,103,104] | |||
| miR-378 | ↓ | ↓ | ↓ | ↓ | ↓ | [105,106,107] |
DDP—cisplatin; DDP-S miRNA—miRNA associated with DDP sensitivity; DDP-R miRNA—miRNA associated with DDP resistance; ↑ the increased expression of selected miRNA is associated with upregulation of process; ↓ the increased expression of selected miRNA is associated with downregulation of process.
The Diana MirPath database presents processes regulated by all of these miRNAs. Twenty-four of the selected miRNAs, according to the Diana database, are involved in NSCL regulation (39 genes); there are eight miRNAs that are not involved: miR-146a, miR-200b, miR-203, miR-219a, miR-379, miR-381, miR-486, and miR-613. Moreover, all 32 selected miRNAs are involved in cell-cycle regulation (101 genes), the FoxO signaling pathway (95 genes), the Wnt signaling pathway (86 genes), the TNF signaling pathway (80 genes), the MAPK signaling pathway (156 genes), the TGF-beta signaling pathway (66 genes), the p53 signaling pathway (56 genes), the AMPK signaling pathway (89 genes), and the mTOR signaling pathway (46 genes).
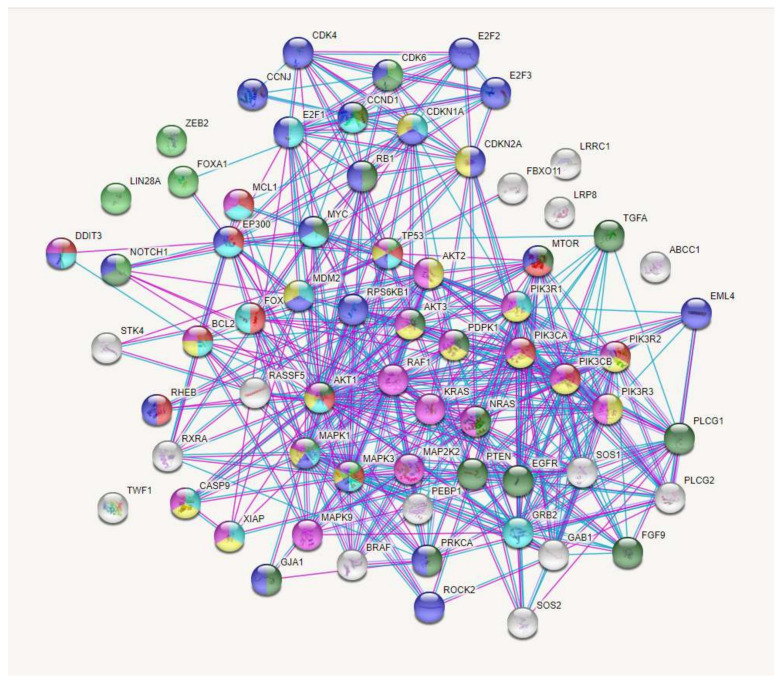
The interactions of proteins encoded by the genes regulated by selected miRNAs and involved in LC regulation (58 genes) were analyzed using the STRING database (string-db. org). The complicated and closed network organized by these genes and the interactions between them are presented in Figure 7.
Figure 7.
The interactions of proteins coded by genes that are regulated by miRNAs, which are the most valid as potential markers of DDP response and involved in lung-cancer regulation (STRING Database). Proteins involved in cellular response to DNA-damage stimulus (light blue); proteins involved in regulation of autophagy (red); proteins involved in stem-cell differentiation (green); proteins involved in regulation of epithelial-cell proliferation (dark green); proteins involved in cell-cycle regulation (blue); proteins involved in platinum drug resistance (yellow); proteins involved in apoptosis (pink).
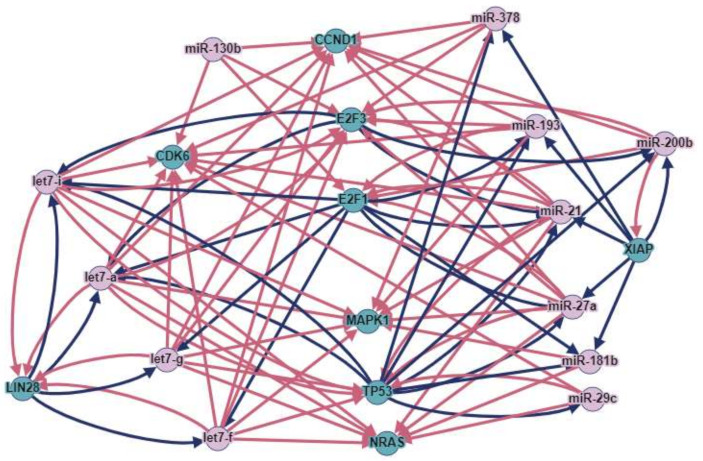
MicroRNAs and genes that are involved in a greater number of interactions represent the most robust potential markers of therapeutic effectiveness. To select such potential markers, we analyzed the regulation of genes by miRNAs and also whether transcription factors among selected genes could regulate miRNAs from the selected panel (using the TransmiR 2.0 database). The genes and miRNAs involved in the greatest number of interactions (more than 10) were then selected: let7a, i, g, f, miR-21, miR-27a, miR-29c, miR-130b, miR-181b, miR-193, miR-378, LIN28, CDK6, CCND1, E2F1, E2F3, MAPK1, TP53, NRAS, and XIAP (Figure 8).
Figure 8.
MicroRNAs and genes involved in DDP response, with the greatest number of interactions according to Diana and Targetscan databases. Red arrows represent downregulation, and blue arrows represent upregulation.
To understand whether the 39 dysregulated miRNAs were specific for LC DDP chemotherapy or not, a further analysis using both DIANA and miRPath was performed. It was revealed that the miRNAs played significant roles in the development of oncological diseases. For example, all of the miRNAs were involved in the development of small cell lung cancer (63 genes), prostate cancer (68 genes), colorectal cancer (48 genes), renal cell carcinoma (58 genes), bladder cancer (29 genes), thyroid cancer (21 genes), endometrial cancer (39 genes), pancreatic cancer (51 genes), and glioma (47 genes). Previously, we analyzed the involvement of miRNAs in the regulation of chemotherapy resistance of prostate cancer [108]. Most of the studies analyzed in this article had been based on investigations of miRNAs in the regulation of responses to taxanes (docetaxel and paclitaxel). The combined analysis of bioinformatics resources and the available literature indicated that the expressions of eight microRNAs and nine genes were associated with chemotherapy response and had a high potential for the prediction of the prostate cancer chemotherapy response [108]. Interestingly, three miRNAs (miR-21, miR-27a, and miR-181b) and three genes (CCND1, E2F3, and TP53) were part of both combined miRNA panels, i.e., for prostate-cancer chemotherapy resistance and for lung-cancer DDP resistance. This indicates that these miRNAs and genes may represent a panel for nonspecific-cancer chemotherapy-resistance assessment. The miRNAs from the selected panel are involved in all crucial mechanisms of chemotherapy resistance, and, therefore, they may be non-specific for the type of the cancer and also for the type of the chemotherapy. This assumption still needs to be investigated. However, if the change in miRNA-expression level from the selected panel is not a diagnostic criterion, then the fact that they occur in other types of cancer becomes insignificant.
9. Conclusions
The data presented indicate that the selected miRNA and gene panel are strongly involved in DDP response and represent a perspective tool for the assessment and prediction of DDP therapy effectiveness. Nevertheless, their prognostic efficacy remains to be further confirmed by studies of randomized cohorts of patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23147594/s1. References [25,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198] are cited in the Supplementary Materials.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
The research was carried out within the state assignment of Ministry of Health of Russian Federation (#121031300227-2 “Use of extracellular microRNA for non-invasive diagnostics of lung cancer”) and the Russian state budget project to the ICBFM SB RAS (#121030200173-6 “Diagnostics and therapy of oncological diseases”).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Travis W.D., Brambilla E., Nicholson A.G. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Fennell D.A., Summers Y., Cadranel J., Benepal T., Christoph D.C., Lal R., Das M., Maxwell F., Visseren-Grul C., Ferry D. Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat. Rev. 2016;44:42–50. doi: 10.1016/j.ctrv.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Schiller J.H., Harrington D., Belani C.P., Langer C., Sandler A., Krook J., Zhu J., Johnson D.H., for the Eastern Cooperative Oncology Group Comparison of Four Chemotherapy Regimens for Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 5.Butts C., Socinski M.A., Mitchell P.L., Thatcher N., Havel L., Krzakowski M. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 6.Spigel D.R., Faivre-Finn C., Gray J.E. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. J. Clin. Oncol. 2021;39:8511. doi: 10.1200/JCO.2021.39.15_suppl.8511. [DOI] [Google Scholar]
- 7.Wu Y.L., Tsuboi M., He J., John T., Grohe C., Majem M., Goldman J.W., Laktionov K., Kim S.W., Kato T., et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020;383:1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 8.Pennell N.A., Neal J.W., Chaft J.E., Azzoli C.G., Jänne P.A., Govindan R., Evans T.L., Costa D.B., Wakelee H.A., Heist R.S., et al. SELECT: A Phase II Trial of Adjuvant Erlotinib in Patients With Resected Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019;37:97–104. doi: 10.1200/JCO.18.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCCN Guidelines. 2020. [(accessed on 25 June 2020)]. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450.
- 10.Dasari S., Tchounwou B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L., Senovilla L., Vitale I., Michels L., Martins I., Kepp O., Castedo M., Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 12.Zandberga E., Kozirovskis V., Ābols A., Andrējeva D., Purkalne G., Linē A. Cell-free microRNAs as diagnostic, prognostic, and predictive biomarkers for lung cancer. Genes Chrom. Can. 2013;52:356–369. doi: 10.1002/gcc.22032. [DOI] [PubMed] [Google Scholar]
- 13.Liang H., Gong F., Zhang S., Zhang C.Y., Zen K., Chen X. The origin, function, and diagnostic potential of extracellular microRNAs in human body fluids. Wiley Interdiscip. Rev. RNA. 2014;5:285–300. doi: 10.1002/wrna.1208. [DOI] [PubMed] [Google Scholar]
- 14.Weber J.A., Baxter D.H., Zhang S., Huang D.Y., Huang K.H., Lee M.J., Galas D.J., Wang K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konoshenko M.Y., Lekchnov E.A., Vlassov A.V., Laktionov P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018;2018:8545347. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryzgunova O., Konoshenko M., Zaporozhchenko I., Yakovlev A., Laktionov P. Isolation of Cell-Free miRNA from Biological Fluids: Influencing Factors and Methods. Diagnostics. 2021;11:865. doi: 10.3390/diagnostics11050865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang W., Zhao Y., Huang W., Liang H., Zeng H., He J. Liquid biopsy for early stage lung cancer. J. Thorac. Dis. 2018;10:S876–S881. doi: 10.21037/jtd.2018.04.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadejeva I., Olschewski H., Hrzenjak A. MicroRNAs as regulators of cisplatin-resistance in non-small cell lung carcinomas. Oncotarget. 2017;8:115754–115773. doi: 10.18632/oncotarget.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J., Zhan Y., Feng J., Luo J., Fan S. MicroRNAs associated with therapy of non-small cell lung cancer. Int. J. Biol. Sci. 2018;14:390–397. doi: 10.7150/ijbs.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal M.A., Arora S., Prakasam G., Calin G.A., Syed M.A. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol. Aspects Med. 2019;70:3–20. doi: 10.1016/j.mam.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Lu L., Feng B., Chen Y., Lu L., Feng B., Han S., Cui S., Chu X., Chen L., et al. Non-coding RNAs as emerging regulators of epithelial to mesenchymal transition in non-small cell lung cancer. Oncotarget. 2017;8:36787–36799. doi: 10.18632/oncotarget.16375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan T., Wang W., Zhang B., Xu Y., Chen L., Pan S., Hu H., Geng Q. Regulatory mechanisms of microRNAs in lung cancer stem cells. SpringerPlus. 2016;15:1762. doi: 10.1186/s40064-016-3425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M., Shan W., Hua Y., Chao F., Cui Y., Lv L., Dou X., Bian X., Zou J., Li H., et al. Exosomal miR-92b-3p Promotes Chemoresistance of Small Cell Lung Cancer Through the PTEN/AKT Pathway. Front. Cell Dev. Biol. 2021;9:661602. doi: 10.3389/fcell.2021.661602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S., Li J., Chen L., Guo L., Ye M., Wu Y., Ji Q. Plasma miR-32 levels in non-small cell lung cancer patients receiving platinum-based chemotherapy can predict the effectiveness and prognosis of chemotherapy. Medicine. 2019;98:e17335. doi: 10.1097/MD.0000000000017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin X., Yu S., Zhou L., Shi M., Hu Y., Xu X., Shen B., Liu S., Yan D., Feng J. Cisplatin-resistant lung cancer cell-derived exosomes increase cisplatin resistance of recipient cells in exosomal miR-100-5p-dependent manner. Int. J. Nanomed. 2017;12:3721–3733. doi: 10.2147/IJN.S131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Y., Yuwen D., Chen J., Zheng B., Gao J., Fan M., Xue W., Wang Y., Li W., Shu Y., et al. Exosomal Transfer Of Cisplatin-Induced miR-425-3p Confers Cisplatin Resistance In NSCLC Through Activating Autophagy. Int. J. Nanomed. 2019;14:8121–8132. doi: 10.2147/IJN.S221383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X., Li M., Dai X., Yang Y., Peng Y., Xu C., Dai N., Wang D. Downregulation of exosomal miR-1273a increases cisplatin resistance of non-small cell lung cancer by upregulating the expression of syndecan binding protein. Oncol. Rep. 2020;44:2165–2173. doi: 10.3892/or.2020.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lekchnov E.A., Amelina E.V., Bryzgunova O.E., Zaporozhchenko I.A., Konoshenko M.Y., Yarmoschuk S.V., Murashov I.S., Pashkovskaya O.A., Gorizkii A.M., Zheravin A.A., et al. Searching for the Novel Specific Predictors of Prostate Cancer in Urine: The Analysis of 84 miRNA Expression. Int. J. Mol. Sci. 2018;19:4088. doi: 10.3390/ijms19124088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konoshenko M.Y., Lekchnov E.A., Bryzgunova O.E., Zaporozhchenko I.A., Yarmoschuk S.V., Pashkovskaya O.A., Pak S.V., Laktionov P.P. The Panel of 12 Cell-Free MicroRNAs as Potential Biomarkers in Prostate Neoplasms. Diagnostics. 2020;10:38. doi: 10.3390/diagnostics10010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S., Huang H., Chen Y.N., Deng Y.T., Zhang B., Xiong X.D., Yuan Y., Zhu Y., Huang H., Xie L., et al. DNA damage responsive miR-33b-3p promoted lung cancer cells survival and cisplatin resistance by targeting p21WAF1/CIP1. Cell Cycle. 2016;15:2920–2930. doi: 10.1080/15384101.2016.1224043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin J., Zhao J., Hu W., Yang G., Yu H., Wang R., Wang L., Zhang G., Fu W., Dai L., et al. Disturbance of the let-7/LIN28 double-negative feedback loop is associated with radio- and chemo-resistance in non-small cell lung cancer. PLoS ONE. 2017;12:e0172787. doi: 10.1371/journal.pone.0172787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun W., Ping W., Tian Y., Zou W., Liu J., Zu Y. miR-202 Enhances the Anti-Tumor Effect of Cisplatin on Non-Small Cell Lung Cancer by Targeting the Ras/MAPK Pathway. Cell. Physiol. Biochem. 2018;51:2160–2171. doi: 10.1159/000495835. [DOI] [PubMed] [Google Scholar]
- 33.Li G.J., Zhao G.Q., Yang J.P., Zhou Y.C., Yang K.Y., Lei Y.J., Huang Y.C. Effect of miR-1244 on cisplatin-treated non-small cell lung cancer via MEF2D expression. Oncol. Rep. 2017;37:3475–3483. doi: 10.3892/or.2017.5624. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Zhang P., Sun X., Sun Y., Shi C., Liu H., Liu X. MicroRNA-181a regulates epithelial-mesenchymal transition by targeting PTEN in drug-resistant lung adenocarcinoma cells. Int. J. Oncol. 2015;47:1379–1392. doi: 10.3892/ijo.2015.3144. [DOI] [PubMed] [Google Scholar]
- 35.Yang L.Z., Lei C.C., Zhao Y.P., Sun H.W., Yu Q.H., Yang E.J., Zhan X. MicroRNA-34c-3p target inhibiting NOTCH1 suppresses chemosensitivity and metastasis of non-small cell lung cancer. J. Int. Med. Res. 2020;48:300060520904847. doi: 10.1177/0300060520904847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z., Liu C., Wu H., Xie Y., Gao H., Zhang X. CSB affected on the sensitivity of lung cancer cells to platinum-based drugs through the global decrease of let-7 and miR-29. BMC Cancer. 2019;19:948. doi: 10.1186/s12885-019-6194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen H., Wang L., Ge X., Jiang C.F., Shi Z.M., Li D.M., Liu W.T., Yu X., Shu Y.Q. MicroRNA-137 inhibits tumor growth and sensitizes chemosensitivity to paclitaxel and cisplatin in lung cancer. Oncotarget. 2016;7:20728–20742. doi: 10.18632/oncotarget.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceppi P., Mudduluru G., Kumarswamy R., Rapa I., Scagliotti G.V., Papotti M., Allgayer H. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol. Cancer Res. 2010;8:1207–1216. doi: 10.1158/1541-7786.MCR-10-0052. [DOI] [PubMed] [Google Scholar]
- 39.Huang G., Pan J., Ye Z., Fang B., Cheng W., Cao Z. Overexpression of miR-216b sensitizes NSCLC cells to cisplatin-induced apoptosis by targeting c-Jun. Oncotarget. 2017;8:104206–104215. doi: 10.18632/oncotarget.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song L., Li Y., Li W., Wu S., Li Z. miR-495 enhances the sensitivity of non-small cell lung cancer cells to platinum by modulation of copper-transporting P-type adenosine triphosphatase A (ATP7A) J. Cell. Biochem. 2014;115:1234–1242. doi: 10.1002/jcb.24665. [DOI] [PubMed] [Google Scholar]
- 41.Zhu W., Zhu D., Lu S., Wang T., Wang J., Jiang B., Shu Y., Liu P. miR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Med. Oncol. 2012;29:384–391. doi: 10.1007/s12032-010-9797-4. [DOI] [PubMed] [Google Scholar]
- 42.Min H.Y., Lee H.Y. Mechanisms of resistance to chemotherapy in non-small cell lung cancer. Arch. Pharm. Res. 2021;44:146–164. doi: 10.1007/s12272-021-01312-y. [DOI] [PubMed] [Google Scholar]
- 43.Levine B., Klionsky D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 44.Li X., He S., Ma B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer. 2020;19:12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jing Y., Liang W., Liu J., Zhang L., Wei J., Yang J., Zhang Y., Huang Z. Autophagy-mediating microRNAs in cancer chemoresistance. Cell Biol. Toxicol. 2020;36:517–536. doi: 10.1007/s10565-020-09553-1. [DOI] [PubMed] [Google Scholar]
- 46.Li Y.J., Lei Y.H., Yao N., Wang C.R., Hu N., Ye W.C., Zhang D.M., Chen Z.S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer. 2017;36:52. doi: 10.1186/s40880-017-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao W., Deng J.S., Li P.Y., Liang Y.C., Huang G.J. 3,4-Dihydroxybenzalactone Suppresses Human Non-Small Cell Lung Carcinoma Cells Metastasis via Suppression of Epithelial to Mesenchymal Transition, ROS-Mediated PI3K/AKT/MAPK/MMP and NFκB Signaling Pathways. Molecules. 2017;22:537. doi: 10.3390/molecules22040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin S., Cheryan V.T., Xu L., Rishi A.K., Reddy K.B. Myc mediates cancer stem-like cells and EMT changes in triple negative breast cancers cells. PLoS ONE. 2017;12:e0183578. doi: 10.1371/journal.pone.0183578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen D.W., Fojo A., Chin J.E., Roninson I.B., Richert N., Pastan I., Gottesman M.M. Human multidrug-resistant cell lines: Increased mdr1 expression can precede gene amplification. Science. 1986;232:643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- 50.Inoue Y., Matsumoto H., Yamada S., Kawai K., Suemizu H., Gika M., Takanami I., Iwazaki M., Nakamura M. Association of ATP7A expression and in vitro sensitivity to cisplatin in non-small cell lung cancer. Oncol. Lett. 2010;1:837–840. doi: 10.3892/ol_00000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z.H., Qiu M.Z., Zeng Z.L., Luo H.Y., Wu W.J., Wang F., Wang Z.Q., Zhang D.S., Li Y.H., Xu R.H. Copper-transporting P-type adenosine triphosphatase (ATP7A) is associated with platinum-resistance in non-small cell lung cancer (NSCLC) J. Transl. Med. 2012;10:21. doi: 10.1186/1479-5876-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goto S., Iida T., Cho S., Oka M., Kohno S., Kondo T. Overexpression of glutathione S-transferase pi enhances the adduct formation of cisplatin with glutathione in human cancer cells. Free Radic. Res. 1999;31:549–558. doi: 10.1080/10715769900301121. [DOI] [PubMed] [Google Scholar]
- 53.Fang C., Chen Y.X., Wu N.Y., Yin J.Y., Li X.P., Huang H.S., Zhang W., Zhou H.-H., Liu Z.Q. MiR-488 inhibits proliferation and cisplatin sensibility in non-small-cell lung cancer (NSCLC) cells by activating the eIF3a-mediated NER signaling pathway. Sci. Rep. 2017;7:40384. doi: 10.1038/srep40384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan W., Liao Y., Qiu Y., Liu H., Tan D., Wu T., Tang M., Zhang S., Wang H. miRNA 146a promotes chemotherapy resistance in lung cancer cells by targeting DNA damage inducible transcript 3 (CHOP) Cancer Lett. 2018;428:55–68. doi: 10.1016/j.canlet.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z.L., Wang H., Liu J., Wang Z.X. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol. Cell. Biochem. 2013;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]
- 56.Cao L., Chen J., Ou B., Liu C., Zou Y., Chen Q. GAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axis. Biomed. Pharmacother. 2017;93:570–579. doi: 10.1016/j.biopha.2017.06.089. [DOI] [PubMed] [Google Scholar]
- 57.Xu L., Huang Y., Chen D., He J., Zhu W., Zhang Y., Liu X. Downregulation of miR-21 increases cisplatin sensitivity of non-small-cell lung cancer. Cancer Genet. 2014;207:214–220. doi: 10.1016/j.cancergen.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Su C., Cheng X., Li Y., Han Y., Song X., Yu D., Cao X., Liu Z. MiR-21 improves invasion and migration of drug-resistant lung adenocarcinoma cancer cell and transformation of EMT through targeting HBP1. Cancer Med. 2018;7:2485–2503. doi: 10.1002/cam4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang Z., Yin J., Fu W., Mo Y., Pan Y., Dai L., Huang H., Li S., Zhao J. MiRNA 17 family regulates cisplatin-resistant and metastasis by targeting TGFbetaR2 in NSCLC. PLoS ONE. 2014;9:e94639. doi: 10.1371/journal.pone.0094639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J., Wang Y., Song Y., Fu Z., Yu W. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol. Cancer. 2014;13:193. doi: 10.1186/1476-4598-13-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y., Li L., Guan Y., Liu X., Meng Q., Guo Q. MiR-92b regulates the cell growth, cisplatin chemosensitivity of A549 non small cell lung cancer cell line and target PTEN. Biochem. Biophys. Res. Commun. 2013;440:604–610. doi: 10.1016/j.bbrc.2013.09.111. [DOI] [PubMed] [Google Scholar]
- 62.Wang H., Zhu L.J., Yang Y.C., Wang Z.X., Wang R. MiR-224 promotes the chemoresistance of human lung adenocarcinoma cells to cisplatin via regulating G₁/S transition and apoptosis by targeting p21(WAF1/CIP1) Br. J. Cancer. 2014;111:339–354. doi: 10.1038/bjc.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ba Z., Zhou Y., Yang Z., Xu J., Zhang X. miR-324-5p upregulation potentiates resistance to cisplatin by targeting FBXO11 signalling in non-small cell lung cancer cells. J. Biochem. 2019;166:517–527. doi: 10.1093/jb/mvz066. [DOI] [PubMed] [Google Scholar]
- 64.Yang W., Xiao W., Cai Z., Jin S., Li T. miR-1269b Drives Cisplatin Resistance of Human Non-Small Cell Lung Cancer via Modulating the PTEN/PI3K/AKT Signaling Pathway. OncoTargets Ther. 2020;13:109–118. doi: 10.2147/OTT.S225010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun D.M., Tang B.F., Li Z.X., Guo H.B., Cheng J.L., Song P.P., Zhao X. MiR-29c reduces the cisplatin resistance of non-small cell lung cancer cells by negatively regulating the PI3K/Akt pathway. Sci. Rep. 2018;8:8007. doi: 10.1038/s41598-018-26381-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu H., Shen X., Chen B., Chen T., Feng G., Chen S., Feng D., Xu Q. miR-30b-5p inhibits cancer progression and enhances cisplatin sensitivity in lung cancer through targeting LRP8. Apoptosis. 2021;26:261–276. doi: 10.1007/s10495-021-01665-1. [DOI] [PubMed] [Google Scholar]
- 67.Shao N., Song L., Sun X. Exosomal circ_PIP5K1A regulates the progression of non-small cell lung cancer and cisplatin sensitivity by miR-101/ABCC1 axis. Mol. Cell. Biochem. 2021;476:2253–2267. doi: 10.1007/s11010-021-04083-8. [DOI] [PubMed] [Google Scholar]
- 68.Ye Z., Yin S., Su Z., Bai M., Zhang H., Hei Z., Cai S. Downregulation of miR-101 contributes to epithelial-mesenchymal transition in cisplatin resistance of NSCLC cells by targeting ROCK2. Oncotarget. 2016;7:37524–37535. doi: 10.18632/oncotarget.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng F., Xu R. CircPVT1 contributes to chemotherapy resistance of lung adenocarcinoma through miR-145-5p/ABCC1 axis. Biomed. Pharmacother. 2020;124:109828. doi: 10.1016/j.biopha.2020.109828. [DOI] [PubMed] [Google Scholar]
- 70.Pang L., Lu J., Huang J., Xu C., Li H., Yuan G., Cheng X., Chen J. Upregulation of miR-146a increases cisplatin sensitivity of the non-small cell lung cancer A549 cell line by targeting JNK-2. Oncol. Lett. 2017;14:7745–7752. doi: 10.3892/ol.2017.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du H., Li Y., Sun R., Yuan Y., Sun S., Zhang Y. CEACAM6 promotes cisplatin resistance in lung adenocarcinoma and is regulated by microRNA-146a and microRNA-26a. Thorac. Cancer. 2020;11:2473–2482. doi: 10.1111/1759-7714.13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi L., Xu Z., Wu G., Chen X., Huang Y., Wang Y., Jiang W., Ke B. Up-regulation of miR-146a increases the sensitivity of non-small cell lung cancer to DDP by downregulating cyclin J. BMC Cancer. 2017;17:138. doi: 10.1186/s12885-017-3132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu W., Shan X., Wang T., Shu Y., Liu P. miR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int. J. Cancer. 2010;127:2520–2529. doi: 10.1002/ijc.25260. [DOI] [PubMed] [Google Scholar]
- 74.Wang X., Chen X., Meng Q., Jing H., Lu H., Yang Y., Cai L., Zhao Y. MiR-181b regulates cisplatin chemosensitivity and metastasis by targeting TGFβR1/Smad signaling pathway in NSCLC. Sci. Rep. 2015;5:17618. doi: 10.1038/srep17618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu H.N., Qie P., Yang G., Song Y.B. miR-181b inhibits chemoresistance in cisplatin-resistant H446 small cell lung cancer cells by targeting Bcl-2. Arch. Med. Sci. 2018;14:745–751. doi: 10.5114/aoms.2018.73131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X., Meng Q., Qiao W., Ma R., Ju W., Hu J., Lu H., Cui J., Jin Z., Zhao Y., et al. miR-181b/Notch2 overcome chemoresistance by regulating cancer stem cell-like properties in NSCLC. Stem Cell Res. Ther. 2018;9:327. doi: 10.1186/s13287-018-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu H., Mu X., Liu L., Wu H., Hu X., Chen L., Liu J., Mu Y., Yuan F., Liu W., et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020;11:801. doi: 10.1038/s41419-020-02962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X., Jiang Y., Huang Z., Li D., Chen X., Cao M., Meng Q., Pang H., Sun L., Zhao Y., et al. miRNA-378 reverses chemoresistance to cisplatin in lung adenocarcinoma cells by targeting secreted clusterin. Sci. Rep. 2016;6:19455. doi: 10.1038/srep19455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hao G.J., Hao H.J., Ding Y.H., Wen H., Li X.F., Wang Q.R., Zhang B.B. Suppression of EIF4G2 by miR-379 potentiates the cisplatin chemosensitivity in nonsmall cell lung cancer cells. FEBS Lett. 2017;591:636–645. doi: 10.1002/1873-3468.12566. [DOI] [PubMed] [Google Scholar]
- 80.Huang R.S., Zheng Y.L., Zhao J., Chun X. microRNA-381 suppresses the growth and increases cisplatin sensitivity in non-small cell lung cancer cells through inhibition of nuclear factor-κB signaling. Biomed. Pharmacother. 2018;98:538–544. doi: 10.1016/j.biopha.2017.12.092. [DOI] [PubMed] [Google Scholar]
- 81.Cheng D., Xu Y., Sun C., He Z. MicroRNA-451 sensitizes lung cancer cells to cisplatin through regulation of Mcl-1. Mol. Cell. Biochem. 2016;423:85–91. doi: 10.1007/s11010-016-2827-6. [DOI] [PubMed] [Google Scholar]
- 82.Wang L., Shang X., Feng Q. LncRNA TATDN1 contributes to the cisplatin resistance of non-small cell lung cancer through TATDN1/miR-451/TRIM66 axis. Cancer Biol. Ther. 2019;20:261–271. doi: 10.1080/15384047.2018.1529091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bian H.B., Pan X., Yang J.S., Wang Z.X., De W. Upregulation of microRNA-451 increases cisplatin sensitivity of non-small cell lung cancer cell line (A549) J. Exp. Clin. Cancer Res. 2011;30:20. doi: 10.1186/1756-9966-30-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin X., Pang W., Zhang Q., Huang H. MicroRNA-486-5p improves nonsmall-cell lung cancer chemotherapy sensitivity and inhibits epithelial-mesenchymal transition by targeting twinfilin actin binding protein 1. J. Int. Med. Res. 2019;47:3745–3756. doi: 10.1177/0300060519850739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li D., Meng D., Niu R. Exosome-Reversed Chemoresistance to Cisplatin in Non-Small Lung Cancer through Transferring miR-613. Cancer Manag. Res. 2020;12:7961–7972. doi: 10.2147/CMAR.S254310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo J., Jin Y., Li M., Dong L. Tumor suppressor miR-613 induces cisplatin sensitivity in non-small cell lung cancer cells by targeting GJA1. Mol. Med. Rep. 2021;23:385. doi: 10.3892/mmr.2021.12024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Bica-Pop C., Cojocneanu-Petric R., Magdo L., Raduly L., Gulei D., Berindan-Neagoe I. Overview upon miR-21 in lung cancer: Focus on NSCLC. Cell. Mol. Life Sci. 2018;75:3539–3551. doi: 10.1007/s00018-018-2877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu D.J., Zhong M., Wang W.L. Long noncoding RNA CASC15 is upregulated in non-small cell lung cancer and facilitates cell proliferation and metastasis via targeting miR-130b-3p. Eur. Rev. Med. Pharmacol. Sci. 2019;23:7943–7949. doi: 10.26355/eurrev_201909_19010. [DOI] [PubMed] [Google Scholar]
- 89.Tian J., Hu L., Li X., Geng J., Dai M., Bai X. MicroRNA-130b promotes lung cancer progression via PPARγ/VEGF-A/BCL-2-mediated suppression of apoptosis. J. Exp. Clin. Cancer Res. 2016;35:105. doi: 10.1186/s13046-016-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Hirono T., Jingushi K., Nagata T., Sato M., Minami K., Aoki M., Takeda A.H., Umehara T., Egawa H., Nakatsuji Y., et al. MicroRNA-130b functions as an oncomiRNA in non-small cell lung cancer by targeting tissue inhibitor of metalloproteinase-2. Sci. Rep. 2019;9:6956. doi: 10.1038/s41598-019-43355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qi L., Liu F., Zhang F., Zhang S., Lv L., Bi Y., Yu Y. lncRNA NEAT1 competes against let-7a to contribute to non-small cell lung cancer proliferation and metastasis. Biomed. Pharmacother. 2018;103:1507–1515. doi: 10.1016/j.biopha.2018.04.053. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y.Y., Ren T., Cai Y.Y., He X.Y. MicroRNA let-7a inhibits the proliferation and invasion of nonsmall cell lung cancer cell line 95D by regulating K-Ras and HMGA2 gene expression. Cancer Biother. Radiopharm. 2013;28:131–137. doi: 10.1089/cbr.2012.1307. [DOI] [PubMed] [Google Scholar]
- 93.Shi Z., Liu J., Sun D. Let-7a targets Rsf-1 to modulate radiotherapy response of non-small cell lung cancer cells through Ras-MAPK pathway. J. Buon. 2021;26:1422–1431. [PubMed] [Google Scholar]
- 94.Park S., Minai-Tehrani A., Xu C.X., Chang S.H., Woo M.A., Noh M.S., Lee E.S., Lim H.T., An G.H., Lee K.H., et al. Suppression of A549 lung cancer cell migration by precursor let-7g microRNA. Mol. Med. Rep. 2010;3:1007–1013. doi: 10.3892/mmr.2010.373. [DOI] [PubMed] [Google Scholar]
- 95.Kumar M.S., Erkeland S.J., Pester R.E., Chen C.Y., Ebert M.S., Sharp P.A., Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc. Natl. Acad. Sci. USA. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li C., Chen L., Song W., Peng B., Zhu J., Fang L. DICER activates autophagy and promotes cisplatin resistance in non-small cell lung cancer by binding with let-7i-5p. Acta Histochem. 2021;123:151788. doi: 10.1016/j.acthis.2021.151788. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Y., Zheng X., Chen L.J., Xu B., Jiang J.T. microRNA-181b suppresses the metastasis of lung cancer cells by targeting sex determining region Y-related high mobility group-box 6 (Sox6) Pathol. Res. Pract. 2019;215:335–342. doi: 10.1016/j.prp.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 98.Li X., Han J., Zhu H., Peng L., Chen Z. miR-181b-5p mediates TGF-β1-induced epithelial-to-mesenchymal transition in non-small cell lung cancer stem-like cells derived from lung adenocarcinoma A549 cells. Int. J. Oncol. 2017;51:158–168. doi: 10.3892/ijo.2017.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J., Xing Y., Rong L. miR-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the PTEN/PI3K/AKT pathway. Oncol. Rep. 2018;39:1631–1639. doi: 10.3892/or.2018.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xun G., Ma M., Li B., Zhao S. miR-138 and miR-193 target long non-coding RNA UCA1 to inhibit cell proliferation, migration, and invasion of lung cancer. Thorac. Cancer. 2020;11:2681–2689. doi: 10.1111/1759-7714.13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feng B., Wang R., Song H., Chen L.-B. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer. 2012;118:3365–3376. doi: 10.1002/cncr.26560. [DOI] [PubMed] [Google Scholar]
- 102.Tang Q., Li M., Chen L., Bi F., Xia H. miR-200b/c targets the expression of RhoE and inhibits the proliferation and invasion of non-small cell lung cancer cells. Int. J. Oncol. 2018;53:1732–1742. doi: 10.3892/ijo.2018.4493. [DOI] [PubMed] [Google Scholar]
- 103.Chen J.H., Zhou L.Y., Xu S., Zheng Y.L., Wan Y.F., Hu C.P. Overexpression of lncRNA HOXA11-AS promotes cell epithelial-mesenchymal transition by repressing miR-200b in non-small cell lung cancer. Cancer Cell Int. 2017;17:64. doi: 10.1186/s12935-017-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu C., Hu W., Li L.-L., Wang Y.-X., Zhou Q., Zhang F., Li D.-J. Roles of miR-200 family members in lung cancer: More than tumor suppressors. Future Oncol. 2018;14:2875–2886. doi: 10.2217/fon-2018-0155. [DOI] [PubMed] [Google Scholar]
- 105.Ji K.X., Cui F., Qu D., Sun R.Y., Sun P., Chen F.Y., Wang S.L., Sun H.S. MiR-378 promotes the cell proliferation of non-small cell lung cancer by inhibiting FOXG1. Eur. Rev. Med. Pharmacol. Sci. 2018;22:1011–1019. doi: 10.26355/eurrev_201802_14383. [DOI] [PubMed] [Google Scholar]
- 106.Chen L.T., Xu S.D., Xu H., Zhang J.F., Ning J.F., Wang S.F. MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med. Oncol. 2012;29:1673–1680. doi: 10.1007/s12032-011-0083-x. [DOI] [PubMed] [Google Scholar]
- 107.Skrzypek K., Tertil M., Golda S., Ciesla M., Weglarczyk K., Collet G., Guichard A., Kozakowska M., Boczkowski J., Was H., et al. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid. Redox Signal. 2013;19:644–660. doi: 10.1089/ars.2013.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Konoshenko M., Laktionov P. The miRNAs involved in prostate cancer chemotherapy response as chemoresistance and chemosensitivity predictors. Andrology. 2022;10:51–71. doi: 10.1111/andr.13086. [DOI] [PubMed] [Google Scholar]
- 109.Huang T., Ren K., Ding G., Yang L., Wen Y., Peng B., Wang G., Wang Z. miR-10a increases the cisplatin resistance of lung adenocarcinoma circulating tumor cells via targeting PIK3CA in the PI3K/Akt pathway. Oncol. Rep. 2020;43:1906–1914. doi: 10.3892/or.2020.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun W., Ma Y., Chen P., Wang D. MicroRNA-10a silencing reverses cisplatin resistance in the A549/cisplatin human lung cancer cell line via the transforming growth factor-β/Smad2/STAT3/STAT5 pathway. Mol. Med. Rep. 2015;11:3854–3859. doi: 10.3892/mmr.2015.3181. [DOI] [PubMed] [Google Scholar]
- 111.Lin C.-C., Liao W.-T., Yang T.-Y., Lu H.-J., Hsu S.-L., Wu C.-C. MicroRNA-10b modulates cisplatin tolerance by targeting p53 directly in lung cancer cells. Oncol. Rep. 2021;46:1–13. doi: 10.3892/or.2021.8118. [DOI] [PubMed] [Google Scholar]
- 112.Lu T., Lu W., Jia C., Lou S., Zhang Y. Knockdown of miR-15b partially reverses the cisplatin resistance of NSCLC through the GSK-3β/MCL-1 pathway. Adv. Clin. Exp. Med. 2021;30:849–857. doi: 10.17219/acem/135701. [DOI] [PubMed] [Google Scholar]
- 113.Xiao X.-H., He S.-Y. ELF1 activated long non-coding RNA CASC2 inhibits cisplatin resistance of non-small cell lung cancer via the miR-18a/IRF-2 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3130–3142. doi: 10.26355/eurrev_202003_20680. [DOI] [PubMed] [Google Scholar]
- 114.Xing S., Qu Y., Li C., Huang A., Tong S., Wu C., Fan K. Deregulation of lncRNA-AC078883.3 and microRNA-19a is involved in the development of chemoresistance to cisplatin via modulating signaling pathway of PTEN/AKT. J. Cell. Physiol. 2019;234:22657–22665. doi: 10.1002/jcp.28832. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y., Wang J., Hui B., Sun W., Li B., Shi F., Che S., Chai L., Song L. Pristimerin enhances the effect of cisplatin by inhibiting the miR-23a/Akt/GSK3β signaling pathway and suppressing autophagy in lung cancer cells. Int. J. Mol. Med. 2019;43:1382–1394. doi: 10.3892/ijmm.2019.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liang N., Zhou X., Zhao M., Zhao D., Zhu Z., Li S., Yang H. Retracted: Down-regulation of microRNA-26b modulates non-small cell lung cancer cells chemoresistance and migration through the association of PTEN. Acta Biochim. Biophys. Sin. 2015;47:530–538. doi: 10.1093/abbs/gmv046. [DOI] [PubMed] [Google Scholar]
- 117.Dong Z., Zhong Z., Yang L., Wang S., Gong Z. MicroRNA-31 inhibits cisplatin-induced apoptosis in non-small cell lung cancer cells by regulating the drug transporter ABCB9. Cancer Lett. 2014;343:249–257. doi: 10.1016/j.canlet.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 118.Wang Q., Zhong M., Liu W., Li J., Huang J., Zheng L. Alterations of microRNAs in Cisplatin-resistant Human Non-small Cell Lung Cancer Cells (A549/DDP) Exp. Lung Res. 2011;37:427–434. doi: 10.3109/01902148.2011.584263. [DOI] [PubMed] [Google Scholar]
- 119.Wu H., Zhou J., Mei S., Wu D., Mu Z., Chen B., Xie Y., Ye Y., Liu J. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. J. Cell. Mol. Med. 2016;21:1228–1236. doi: 10.1111/jcmm.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou D.H., Wang X., Feng Q. EGCG enhances the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC A549 cells. Nutr. Cancer. 2014;66:636–644. doi: 10.1080/01635581.2014.894101. [DOI] [PubMed] [Google Scholar]
- 121.Zhu H., Yang J., Yang S. MicroRNA-103a-3p potentiates chemoresistance to cisplatin in non-small cell lung carcinoma by targeting neurofibromatosis 1. Exp. Ther. Med. 2020;19:1797–1805. doi: 10.3892/etm.2020.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang H., Huang H., Wang L., Liu Y., Wang M., Zhao S., Lu G., Kang X. Cancer-associated fibroblasts secreted miR-103a-3p suppresses apoptosis and promotes cisplatin resistance in non-small cell lung cancer. Aging. 2021;13:14456–14468. doi: 10.18632/aging.103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma Y., Li X., Cheng S., Wei W., Li Y. MicroRNA 106a confers cisplatin resistance in non small cell lung cancer A549 cells by targeting adenosine triphosphatase binding cassette A1. Mol. Med. Rep. 2015;11:625–632. doi: 10.3892/mmr.2014.2688. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Q., Zhang B., Sun L., Yan Q., Zhang Y., Zhang Z., Su Y., Wang C. MicroRNA-130b targets PTEN to induce resistance to cisplatin in lung cancer cells by activating Wnt/β-catenin pathway. Cell Biochem. Funct. 2018;36:194–202. doi: 10.1002/cbf.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fu W.-F., Chen W.-B., Dai L., Yang G.-P., Jiang Z.-Y., Pan L., Zhao J., Chen G. Inhibition of miR-141 reverses cisplatin resistance in non-small cell lung cancer cells via upregulation of programmed cell death protein 4. Eur. Rev. Med. Pharmacol. Sci. 2016;20:2565–2572. [PubMed] [Google Scholar]
- 126.Bar J., Gorn-Hondermann I., Moretto P., Perkins T.J., Niknejad N., Stewart D.J., Goss G.D., Dimitroulakos J. miR Profiling Identifies Cyclin-Dependent Kinase 6 Downregulation as a Potential Mechanism of Acquired Cisplatin Resistance in Non–Small-Cell Lung Carcinoma. Clin. Lung Cancer. 2015;16:e121–e129. doi: 10.1016/j.cllc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 127.Van Roosbroeck K., Fanini F., Setoyama T., Ivan C., Rodriguez-Aguayo C., Fuentes-Mattei E., Xiao L., Vannini I., Redis R.S., D’Abundo L., et al. Combining Anti-Mir-155 with Chemotherapy for the Treatment of Lung Cancers. Clin. Cancer Res. 2017;23:2891–2904. doi: 10.1158/1078-0432.CCR-16-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zang Y.-S., Zhong Y.-F., Fang Z., Li B., An J. MiR-155 inhibits the sensitivity of lung cancer cells to cisplatin via negative regulation of Apaf-1 expression. Cancer Gene Ther. 2012;19:773–778. doi: 10.1038/cgt.2012.60. [DOI] [PubMed] [Google Scholar]
- 129.Zhang H., Hu B., Wang Z., Zhang F., Wei H., Li L. miR-181c contributes to cisplatin resistance in non-small cell lung cancer cells by targeting Wnt inhibition factor 1. Cancer Chemother. Pharmacol. 2017;80:973–984. doi: 10.1007/s00280-017-3435-1. [DOI] [PubMed] [Google Scholar]
- 130.Ning F.L., Wang F., Li M.L., Yu Z.S., Hao Y.Z., Chen S.S. MicroRNA-182 modulates chemosensitivity of human non-small cell lung cancer to cisplatin by targeting PDCD4. Diagn. Pathol. 2014;9:143. doi: 10.1186/1746-1596-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang F., Li Y., Wu H., Qi K., You J., Li X., Zu L., Pan Z., Wang Y., Li Y., et al. MiR-192 confers cisplatin resistance by targeting Bim in lung cancer. Zhongguo Fei Ai Za Zhi. 2014;17:384–390. doi: 10.3779/j.issn.1009-3419.2014.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li J.-H., Luo N., Zhong M.-Z., Xiao Z.-Q., Wang J.-X., Yao X.-Y., Peng Y., Cao J. Inhibition of microRNA-196a might reverse cisplatin resistance of A549/DDP non-small-cell lung cancer cell line. Tumor Biol. 2015;37:2387–2394. doi: 10.1007/s13277-015-4017-7. [DOI] [PubMed] [Google Scholar]
- 133.Yang T., Li H., Chen T., Ren H., Shi P., Chen M. LncRNA MALAT1 Depressed Chemo-Sensitivity of NSCLC Cells through Directly Functioning on miR-197-3p/p120 Catenin Axis. Mol. Cells. 2019;42:270–283. doi: 10.14348/molcells.2019.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang N., Zhu C., Xu Y., Qian W., Zheng M. Negative Regulation of PTEN by MicroRNA-221 and Its Association with Drug Resistance and Cellular Senescence in Lung Cancer Cells. BioMed Res. Int. 2018;2018:7908950. doi: 10.1155/2018/7908950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo S., Zhang L., Zhang Y., Wu Z., He D., Li X., Wang Z. Long non-coding RNA TUG1 enhances chemosensitivity in non-small cell lung cancer by impairing microRNA-221-dependent PTEN inhibition. Aging. 2019;11:7553–7569. doi: 10.18632/aging.102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang C., Wang S., Ma F., Zhang W. miRNA-328 overexpression confers cisplatin resistance in non-small cell lung cancer via targeting of PTEN. Mol. Med. Rep. 2018;18:4563–4570. doi: 10.3892/mmr.2018.9478. [DOI] [PubMed] [Google Scholar]
- 137.Hao G.-J., Ding Y.-H., Wen H., Li X.-F., Zhang W., Su H.-Y., Liu D.-M., Xie N.-L. Attenuation of deregulated miR-369-3p expression sensitizes non-small cell lung cancer cells to cisplatin via modulation of the nucleotide sugar transporter SLC35F5. Biochem. Biophys. Res. Commun. 2017;488:501–508. doi: 10.1016/j.bbrc.2017.05.075. [DOI] [PubMed] [Google Scholar]
- 138.Lu C., Wang H., Chen S., Yang R., Li H., Zhang G. Baicalein inhibits cell growth and increases cisplatin sensitivity of A549 and H460 cells via miR-424-3p and targeting PTEN/PI3K/Akt pathway. J. Cell. Mol. Med. 2018;22:2478–2487. doi: 10.1111/jcmm.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Galluzzi L., Morselli E., Vitale I., Kepp O., Senovilla L., Criollo A., Servant N., Paccard C., Hupé P., Robert T., et al. miR-181a and miR-630 Regulate Cisplatin-Induced Cancer Cell Death. Cancer Res. 2010;70:1793–1803. doi: 10.1158/0008-5472.CAN-09-3112. [DOI] [PubMed] [Google Scholar]
- 140.Yang L., Lin Z., Wang Y., Gao S., Li Q., Li C., Xu W., Chen J., Liu T., Song Z., et al. MiR-5100 increases the cisplatin resistance of the lung cancer stem cells by inhibiting the Rab6. Mol. Carcinog. 2017;57:419–428. doi: 10.1002/mc.22765. [DOI] [PubMed] [Google Scholar]
- 141.Hua L., Zhu G., Wei J. MicroRNA-1 overexpression increases chemosensitivity of non-small cell lung cancer cells by inhibiting autophagy related 3-mediated autophagy. Cell Biol. Int. 2018;42:1240–1249. doi: 10.1002/cbin.10995. [DOI] [PubMed] [Google Scholar]
- 142.Liu H., Wu X., Huang J., Peng J., Guo L. miR-7 modulates chemoresistance of small cell lung cancer by repressing MRP1/ABCC1. Int. J. Exp. Pathol. 2015;96:240–247. doi: 10.1111/iep.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Çetintaş V.B., Vardarlı A.T., Düzgün Z., Kaymaz B.T., Açıkgöz E., Aktuğ H., Can B.K., Gündüz C., Eroğlu Z. miR-15a enhances the anticancer effects of cisplatin in the resistant non-small cell lung cancer cells. Tumor Biol. 2015;37:1739–1751. doi: 10.1007/s13277-015-3950-9. [DOI] [PubMed] [Google Scholar]
- 144.Zhao J., Fu W., Liao H., Dai L., Jiang Z., Pan Y., Huang H., Mo Y., Li S., Yang G., et al. The regulatory and predictive functions of miR-17 and miR-92 families on cisplatin resistance of non-small cell lung cancer. BMC Cancer. 2015;15:1–14. doi: 10.1186/s12885-015-1713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang J., Hua X., Qi N., Han G., Yu J., Yu Y., Wei X., Li H., Chen X., Leng C., et al. MiR-27b suppresses epithelial–mesenchymal transition and chemoresistance in lung cancer by targeting Snail1. Life Sci. 2019;254:117238. doi: 10.1016/j.lfs.2019.117238. [DOI] [PubMed] [Google Scholar]
- 146.Chen X., Zhu H., Ye W., Cui Y., Chen M. MicroRNA-29a enhances cisplatin sensitivity in non-small cell lung cancer through the regulation of REV3L. Mol. Med. Rep. 2018;19:831–840. doi: 10.3892/mmr.2018.9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luo S., Shen M., Chen H., Li W., Chen C. Long non-coding RNA TP73-AS1 accelerates the progression and cisplatin resistance of non-small cell lung cancer by upregulating the expression of TRIM29 via competitively targeting microRNA-34a-5p. Mol. Med. Rep. 2020;22:3822–3832. doi: 10.3892/mmr.2020.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao Z., Liu J., Wang C., Wang Y., Jiang Y., Guo M. MicroRNA-25 regulates small cell lung cancer cell development and cell cycle through cyclin E2. Int. J. Clin. Exp. Pathol. 2014;7:7726–7734. [PMC free article] [PubMed] [Google Scholar]
- 149.Seidl C., Panzitt K., Bertsch A., Brcic L., Schein S., Mack M., Leithner K., Prinz F., Olschewski H., Kornmueller K., et al. MicroRNA-182-5p regulates hedgehog signaling pathway and chemosensitivity of cisplatin-resistant lung adenocarcinoma cells via targeting GLI2. Cancer Lett. 2019;469:266–276. doi: 10.1016/j.canlet.2019.10.044. [DOI] [PubMed] [Google Scholar]
- 150.Xiang Q., Tang H., Yu J., Yin J., Yang X., Lei X. MicroRNA-98 sensitizes cisplatin-resistant human lung adenocarcinoma cells by up-regulation of HMGA2. Die Pharm. 2013;68:274–281. [PubMed] [Google Scholar]
- 151.Hu B., Zhang H., Wang Z., Zhang F., Wei H., Li L. LncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance in non-small-cell lung cancer cell line by targeting SOX4. Cancer Biol. Ther. 2017;18:974–983. doi: 10.1080/15384047.2017.1385679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu S., Qin X., Chen T., Zhou L., Xu X., Feng J. MicroRNA-106b-5p regulates cisplatin chemosensitivity by targeting polycystic kidney disease-2 in non-small-cell lung cancer. Anti-Cancer Drugs. 2017;28:852–860. doi: 10.1097/CAD.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Z., Zhang L., Yin Z.-Y., Fan X.-L., Hu B., Wang L.-Q., Zhang D. miR-107 regulates cisplatin chemosensitivity of A549 non small cell lung cancer cell line by targeting cyclin dependent kinase 8. Int. J. Clin. Exp. Pathol. 2014;7:7236–7241. [PMC free article] [PubMed] [Google Scholar]
- 154.Qi M.-M., Ge F., Chen X.-J., Tang C., Ma J. MiR-124 changes the sensitivity of lung cancer cells to cisplatin through targeting STAT3. Eur. Rev. Med. Pharmacol. Sci. 2019;23:5242–5250. doi: 10.26355/eurrev_201906_18190. [DOI] [PubMed] [Google Scholar]
- 155.Huang Q., Xing S., Peng A., Yu Z. NORAD accelerates chemo-resistance of non-small-cell lung cancer via targeting at miR-129-1-3p/SOX4 axis. Biosci. Rep. 2020;40:BSR20193489. doi: 10.1042/BSR20193489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ma Z., Cai H., Zhang Y., Chang L., Cui Y. MiR-129-5p inhibits non-small cell lung cancer cell stemness and chemoresistance through targeting DLK1. Biochem. Biophys. Res. Commun. 2017;490:309–316. doi: 10.1016/j.bbrc.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 157.Lin C., Xie L., Lu Y., Hu Z., Chang J. miR-133b reverses cisplatin resistance by targeting GSTP1 in cisplatin-resistant lung cancer cells. Int. J. Mol. Med. 2018;41:2050–2058. doi: 10.3892/ijmm.2018.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou L., Qiu T., Xu J., Wang T., Wang J., Zhou X., Huang Z., Zhu W., Shu Y., Liu P. miR-135a/b modulate cisplatin resistance of human lung cancer cell line by targeting MCL1. Pathol. Oncol. Res. 2013;19:677–683. doi: 10.1007/s12253-013-9630-4. [DOI] [PubMed] [Google Scholar]
- 159.Su W., Mo Y., Wu F., Guo K., Li J., Luo Y., Ye H., Guo H., Li D., Yang Z. miR-135b reverses chemoresistance of non-small cell lung cancer cells by downregulation of FZD1. Biomed. Pharmacother. 2016;84:123–129. doi: 10.1016/j.biopha.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 160.Pan X., Chen Y., Shen Y., Tantai J. Knockdown of TRIM65 inhibits autophagy and cisplatin resistance in A549/DDP cells by regulating miR-138-5p/ATG7. Cell Death Dis. 2019;10:429. doi: 10.1038/s41419-019-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Du H., Bao Y., Liu C., Zhong A., Niu Y., Tang X. miR-139-5p enhances cisplatin sensitivity in non-small cell lung cancer cells by inhibiting cell proliferation and promoting apoptosis via the targeting of Homeobox protein Hox-B2. Mol. Med. Rep. 2020;23:104. doi: 10.3892/mmr.2020.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shi S.L., Zhang Z.H. Long non-coding RNA SNHG1 contributes to cisplatin resistance in non-small cell lung cancer by regulating miR-140-5p/Wnt/β-catenin pathway. Neoplasma. 2019;66:756–765. doi: 10.4149/neo_2018_181218N980. [DOI] [PubMed] [Google Scholar]
- 163.Fu J., Cai H., Wu Y., Fang S., Wang D. Elevation of FGD5-AS1 contributes to cell progression by improving cisplatin resistance against non-small cell lung cancer cells through regulating miR-140-5p/WEE1 axis. Gene. 2020;755:144886. doi: 10.1016/j.gene.2020.144886. [DOI] [PubMed] [Google Scholar]
- 164.Zhu F., Niu R., Shao X., Shao X. FGD5-AS1 promotes cisplatin resistance of human lung adenocarcinoma cell via the miR-142-5p/PD-L1 axis. Int. J. Mol. Med. 2020;47:523–532. doi: 10.3892/ijmm.2020.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yin Y., Liu H., Xu J., Shi D., Zhai L., Liu B., Wang L., Liu G., Qin J. miR-144-3p regulates the resistance of lung cancer to cisplatin by targeting Nrf2. Oncol. Rep. 2018;40:3479–3488. doi: 10.3892/or.2018.6772. [DOI] [PubMed] [Google Scholar]
- 166.Yuwen D.-L., Sheng B.-B., Liu J., Wenyu W., Shu Y.-Q. MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2017;21:2650–2658. [PubMed] [Google Scholar]
- 167.Sui C., Meng F., Li Y., Jiang Y. miR-148b reverses cisplatin-resistance in non-small cell cancer cells via negatively regulating DNA (cytosine-5)-methyltransferase 1(DNMT1) expression. J. Transl. Med. 2015;13:1–9. doi: 10.1186/s12967-015-0488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tung M.-C., Lin P.-L., Cheng Y.-W., Wu D.-W., Yeh S.-D., Chen C.-Y., Lee H. Reduction of microRNA-184 by E6 oncoprotein confers cisplatin resistance in lung cancer via increasing Bcl-2. Oncotarget. 2016;7:32362–32374. doi: 10.18632/oncotarget.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pei K., Zhu J.-J., Wang C.-E., Xie Q.-L., Guo J.-Y. MicroRNA-185-5p modulates chemosensitivity of human non-small cell lung cancer to cisplatin via targeting ABCC1. Eur. Rev. Med. Pharmacol. Sci. 2016;20:4697–4704. [PubMed] [Google Scholar]
- 170.Wu Z., Gong Q., Yu Y., Zhu J., Li W. Knockdown of circ-ABCB10 promotes sensitivity of lung cancer cells to cisplatin via miR-556-3p/AK4 axis. BMC Pulm. Med. 2020;20:10. doi: 10.1186/s12890-019-1035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhu X., Li D., Yu F., Jia C., Xie J., Ma Y., Fan S., Cai H., Luo Q., Lv Z., et al. miR-194 inhibits the proliferation, invasion, migration, and enhances the chemosensitivity of non-small cell lung cancer cells by targeting forkhead box A1 protein. Oncotarget. 2016;7:13139–13152. doi: 10.18632/oncotarget.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhu W., Xu H., Zhu D., Zhi H., Wang T., Wang J., Jiang B.-H., Shu Y., Liu P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother. Pharmacol. 2011;69:723–731. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- 173.Shen J.-G., Xu S.-N., Yin L.-G. LncRNA NORAD/miR-202-5p regulates the drug resistance of A549/DDP to cisplatin by targeting P-gp. Gen. Physiol. Biophys. 2020;39:481–489. doi: 10.4149/gpb_2020027. [DOI] [PubMed] [Google Scholar]
- 174.Cheng R., Lu C., Zhang G., Zhang G., Zhao G. Overexpression of miR-203 increases the sensitivity of NSCLC A549/H460 cell lines to cisplatin by targeting Dickkopf-1. Oncol. Rep. 2017;37:2129–2136. doi: 10.3892/or.2017.5505. [DOI] [PubMed] [Google Scholar]
- 175.Duan X., Fu Z., Gao L., Zhou J., Deng X., Luo X., Fang W., Luo R. Direct interaction between miR-203 and ZEB2 suppresses epithelial–mesenchymal transition signaling and reduces lung adenocarcinoma chemoresistance. Acta Biochim. Biophys. Sin. 2016;48:1042–1049. doi: 10.1093/abbs/gmw099. [DOI] [PubMed] [Google Scholar]
- 176.Huang G., Lou T., Pan J., Ye Z., Yin Z., Li L., Cheng W., Cao Z. MiR-204 reduces cisplatin resistance in non-small cell lung cancer through suppression of the caveolin-1/AKT/Bad pathway. Aging. 2019;11:2138–2150. doi: 10.18632/aging.101907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen Q.-Y., Jiao D.-M., Wang J., Hu H., Tang X., Chen J., Mou H., Lu W. miR-206 regulates cisplatin resistance and EMT in human lung adenocarcinoma cells partly by targeting MET. Oncotarget. 2016;7:24510–24526. doi: 10.18632/oncotarget.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li Q.-Q., Xie Y.-K., Wu Y., Li L.-L., Liu Y., Miao X.-B., Liu Q.-Z., Yao K.-T., Xiao G.-H. Sulforaphane inhibits cancer stem-like cell properties and cisplatin resistance through miR-214-mediated downregulation of c-MYC in non-small cell lung cancer. Oncotarget. 2017;8:12067–12080. doi: 10.18632/oncotarget.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen L., Han X., Hu Z., Chen L. The PVT1/miR-216b/Beclin-1 regulates cisplatin sensitivity of NSCLC cells via modulating autophagy and apoptosis. Cancer Chemother. Pharmacol. 2019;83:921–931. doi: 10.1007/s00280-019-03808-3. [DOI] [PubMed] [Google Scholar]
- 180.Guo J., Feng Z., Huang Z., Wang H., Lu W. MicroRNA-217 functions as a tumour suppressor gene and correlates with cell resistance to cisplatin in lung cancer. Mol. Cells. 2014;37:664–671. doi: 10.14348/molcells.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xie J., Yu F., Li D., Zhu X., Zhang X., Lv Z. MicroRNA-218 regulates cisplatin (DPP) chemosensitivity in non-small cell lung cancer by targeting RUNX2. Tumor Biol. 2015;37:1197–1204. doi: 10.1007/s13277-015-3831-2. [DOI] [PubMed] [Google Scholar]
- 182.Rao C., Miao X., Zhao G., Zhang C., Shen H., Dong C., Yang M. MiR-219a-5p enhances cisplatin sensitivity of human non-small cell lung cancer by targeting FGF9. Biomed. Pharmacother. 2019;114:108662. doi: 10.1016/j.biopha.2019.108662. [DOI] [PubMed] [Google Scholar]
- 183.Dong Y., Xu T., Zhong S., Wang B., Zhang H., Wang X., Wang P., Li G., Yang S. Circ_0076305 regulates cisplatin resistance of non-small cell lung cancer via positively modulating STAT3 by sponging miR-296–5p. Life Sci. 2019;239:116984. doi: 10.1016/j.lfs.2019.116984. [DOI] [PubMed] [Google Scholar]
- 184.Deng S.-H., Wu D.-M., Li L., Liu T., Zhang T., Li J., Yu Y., He M., Zhao Y.-Y., Han R., et al. miR-324-3p reverses cisplatin resistance by inducing GPX4-mediated ferroptosis in lung adenocarcinoma cell line A549. Biochem. Biophys. Res. Commun. 2021;549:54–60. doi: 10.1016/j.bbrc.2021.02.077. [DOI] [PubMed] [Google Scholar]
- 185.Zhu X., Han J., Lan H., Lin Q., Wang Y., Sun X. A novel circular RNA hsa_circRNA_103809/miR-377-3p/GOT1 pathway regulates cisplatin-resistance in non-small cell lung cancer (NSCLC) BMC Cancer. 2020;20:1190. doi: 10.1186/s12885-020-07680-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ning M.-Y., Cheng Z.-L., Zhao J. MicroRNA-448 targets SATB1 to reverse the cisplatin resistance in lung cancer via mediating Wnt/β-catenin signalling pathway. J. Biochem. 2020;168:41–51. doi: 10.1093/jb/mvaa024. [DOI] [PubMed] [Google Scholar]
- 187.Zhao X., Li X., Zhou L., Ni J., Yan W., Ma R., Wu J., Feng J., Chen P. LncRNA HOXA11-AS drives cisplatin resistance of human LUAD cells via modulating miR-454-3p/Stat3. Cancer Sci. 2018;109:3068–3079. doi: 10.1111/cas.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jiang P., Xu C., Chen L., Chen A., Wu X., Zhou M., Haq I.U., Mariyam Z., Feng Q. EGCG inhibits CSC-like properties through targeting miR-485/CD44 axis in A549-cisplatin resistant cells. Mol. Carcinog. 2018;57:1835–1844. doi: 10.1002/mc.22901. [DOI] [PubMed] [Google Scholar]
- 189.Gu Y., Zhang Z., Yin J., Ye J., Song Y., Liu H., Xiong Y., Lu M., Zheng G., He Z. Epigenetic silencing of miR-493 increases the resistance to cisplatin in lung cancer by targeting tongue cancer resistance-related protein 1(TCRP1) J. Exp. Clin. Cancer Res. 2017;36:114. doi: 10.1186/s13046-017-0582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hou Z., Wang Y., Xia N., Lv T., Yuan X., Song Y. Pseudogene KRT17P3 drives cisplatin resistance of human NSCLC cells by modulating miR-497-5p/mTOR. Cancer Sci. 2020;112:275–286. doi: 10.1111/cas.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Qiu T., Zhou L., Wang T., Xu J., Wang J., Chen W., Zhou X., Huang Z., Zhu W., Shu Y., et al. miR-503 regulates the resistance of non-small cell lung cancer cells to cisplatin by targeting Bcl-2. Int. J. Mol. Med. 2013;32:593–598. doi: 10.3892/ijmm.2013.1439. [DOI] [PubMed] [Google Scholar]
- 192.Wu Y., Guo L., Liu J., Liu R., Liu M., Chen J. The reversing and molecular mechanisms of miR-503 on the drug-resistance to cisplatin in A549/DDP cells. Zhongguo Fei Ai Za Zhi. 2014;17:1–7. doi: 10.3779/j.issn.1009-3419.2014.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang X., Zhu J., Xing R., Tie Y., Fu H., Zheng X., Yu B. miR-513a-3p sensitizes human lung adenocarcinoma cells to chemotherapy by targeting GSTP1. Lung cancer. 2012;77:488–494. doi: 10.1016/j.lungcan.2012.05.107. [DOI] [PubMed] [Google Scholar]
- 194.Xu Y., Jiang T., Wu C., Zhang Y. CircAKT3 inhibits glycolysis balance in lung cancer cells by regulating miR-516b-5p/STAT3 to inhibit cisplatin sensitivity. Biotechnol. Lett. 2020;42:1123–1135. doi: 10.1007/s10529-020-02846-9. [DOI] [PubMed] [Google Scholar]
- 195.Deng H., Qianqian G., Ting J., Aimin Y. miR-539 enhances chemosensitivity to cisplatin in non-small cell lung cancer by targeting DCLK1. Biomed. Pharmacother. 2018;106:1072–1081. doi: 10.1016/j.biopha.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 196.Wang Y., Li F., Ma D., Gao Y., Li R., Gao Y. MicroRNA-608 sensitizes non-small cell lung cancer cells to cisplatin by targeting TEAD2. Mol. Med. Rep. 2019;20:3519–3526. doi: 10.3892/mmr.2019.10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jiang W., Zhao X., Yang W. MiR-647 promotes cisplatin-induced cell apoptosis via downregulating IGF2 in non-small cell lung cancer. Minerva Med. 2021;112:312–313. doi: 10.23736/S0026-4806.19.06240-2. [DOI] [PubMed] [Google Scholar]
- 198.Wang Z., Liu L., Guo X., Guo C., Wang W. microRNA-1236-3p Regulates DDP Resistance in Lung Cancer Cells. Open Med. 2019;14:41–51. doi: 10.1515/med-2019-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.