Abstract
Herein, we disclose cyclic(alkyl)(amino)carbenes (CAACs) to be one‐electron reductants under the formation of a transient radical cation as indicated by EPR spectroscopy. The disclosed CAAC reducing reactivity was used to synthesize acyclic(amino)(aryl)carbene‐based Thiele and Chichibabin hydrocarbons, a new class of Kekulé diradicaloids. The results demonstrate CAACs to be potent organic reductants. Notably, the acyclic(amino)(aryl)carbene‐based Chichibabin's hydrocarbon shows an appreciable population of the triplet state at room temperature, as evidenced by both variable‐temperature NMR and EPR spectroscopy.
Keywords: carbene, electrochemistry, EPR spectroscopy, radical cation, reductant
CAAC donates electron and acts as reductant: Cyclic(alkyl)(amino)(carbene) CAAC donates one electron during the formation of transient radical‐cation evidenced by cyclic voltammetry and electron paramagnetic resonance (EPR) spectroscopy. Subsequently, the one electron‐reductant property of the disclosed CAAC was used to synthesize acyclic(amino)(aryl)carbene‐based Thiele and Chichibabin hydrocarbons. Notably, the acyclic(amino)(aryl)carbene‐based Chichibabin's hydrocarbon shows an appreciable population of the triplet state at room temperature, as evidenced by both variable‐temperature NMR and EPR spectroscopy.

Introduction
Since the report of cyclic(alkyl)(amino)carbenes (CAACs) in 2005 by Bertrand's group [1] such carbenes have been vastly used in different research areas ranging from advanced synthetic chemistry, [2] organometallic chemistry [3] to catalysis [4] and material chemistry. [5] In most cases it has been used as Lewis base, that is, a two‐electron σ‐donor accompanied by π‐acceptor properties. In comparison with N‐heterocyclic carbenes (NHCs), CAACs are considered as both more nucleophilic (stronger σ‐donors) as well as more electrophilic (stronger π‐acceptors) in nature due to the differences in substitution at the carbenic carbon center: two electronegative/π‐donating amino substituents versus one electronegative/π‐donating amino substituent and one σ‐donating alkyl group. [6] On the other hand, CAACs have a relatively smaller singlet‐triplet energy gap compared to NHCs. [6] As a result, CAACs are known to share one‐electron with a reaction partner to form a covalent bond under the formation of various compounds including C‐center based diradicals. [7] However, unlike for NHCs the formation of radical‐cations of CAACs have not been reported, not even in reactions with Ph3C+X− (X=PF6 and B(C6F5)4) [8] or B(C6F5)3. [9] In the case of NHCs I the formation of radical cations II (Scheme 1) was observed with various oxidizing reagents. [10] Recently, the formation of NHC‐derived radical cations II and respective single electron transfer events were studied comprehensively by UV/Vis spectroscopy. [11] NHC‐derived radical cations have limited stability; either they abstract available hydrogen atoms to yield III or they dimerize to the dication of NHC‐NHC dimer IV (Scheme 1), based on the N‐substituents. [10] The radical cations of parent carbenes (CH2) as well as diaryl carbenes have been of particular interest for a long time during which they have been studied as transient species. [12] Thus, we were interested in investigating the possibility of utilizing a CAAC V (Scheme 1) as one‐electron reductant.
Scheme 1.
One‐electron oxidation of N‐heterocyclic carbene I (top) and cyclic(alkyl)(amino)carbene V (bottom) (R=monoanionic organic substituent and the counter anions are omitted for clarity).
Herein, we report the one‐electron donor property of CAAC V under the formation of radical cation VI, which was indicated by EPR spectroscopy, despite its limited stability and swift hydrogen [13] abstraction to VII (Scheme 1). To address this unprecedented one‐electron donation of the CAAC V, it was utilized as reducing reagent for the synthesis of acyclic(amino)(aryl)carbene [14] ‐based Thiele and Chichibabin hydrocarbons, [15] a new class of Kekulé diradicaloids. [16] Notably, the latter shows an appreciable population of the triplet state at room temperature, as evidenced by both variable‐temperature NMR and EPR spectroscopy.
Results and Discussion
For this study, CAAC 1 (Figure 1) was chosen and the investigation of its one‐electron oxidation first addressed through electrochemical methods. The cyclic voltammogram (CV) of 1 exhibits an irreversible anodic peak potential which is similarly pronounced in the oxidative differential pulse voltammogram (DPV; Figure 1a, insert). [17] While in the reductive DPV a small back transition can be observed, this is absent on the CV time scale indicating that an electrochemical‐chemical (EC) mechanism accompanies the oxidation of 1. Due to the immediate reactivity of 1 with either ferrocene or decamethylferrocene we were not able to use an internal standard. Therefore, the given potentials are referenced only externally. [10d]
Figure 1.
a) Cyclic voltammogram of 1 in THF (0.1 M Bu4NPF6) measured at a scan rate of 20 mV s−1. Inset: Differential pulse voltammograms (DPV) of 1 in THF at a scan rate of 20 mV s−1 with 0.2 M Bu4NPF6). b) Experimental X‐band EPR spectrum of a solution of 2 in toluene generated in situ at 200 K. The isotropic signal has a g value of 2.0043 and a 14N hyperfine coupling of a(N)=16.7 MHz (5.9 G).
Subsequently, the chemical oxidation of 1 with NO[SbF6] was carried out in toluene by mixing pre‐cooled solutions of both at −78 °C (Scheme 2). Immediately, a pink coloration of the solution appeared which persists at −78 °C. Upon slowly increasing the temperature of the reaction mixture the color gradually transforms to a light‐brown tinge at room temperature. The 1H NMR spectrum of the crude reaction mixture shows the formation of 3[SbF6], a hydrogen abstracted product of the putative radical cation 2. Most likely it is formed through the one‐electron oxidation of 1 to radical cation 2 by NO[SbF6] followed by hydrogen abstraction. From this reaction 3[SbF6] was isolated as light‐brown crystalline solid with 68 % yield. The formation of 3[SbF6] was confirmed by solution state NMR spectroscopy and a solid‐state single crystal X‐ray diffraction study (Figure S50 in the Supporting Information). [18] A similar observation was also observed when we performed the oxidation using NO2[BF4]. During the course of the reaction, we did not observe any indication for the dimerization of 2 (dication of CAAC–CAAC dimer). [19] This is most likely due to the steric hindrance at the N‐center. Also, we did not observe any reaction between 1 and 3[SbF6] which is in contrast with that of the N‐iPr analogue. [20]
Scheme 2.
Oxidation of 1 (Dip=2,6‐iPr2C6H3).
Using in‐situ EPR spectroscopy, we found indication for the formation of a transient CAAC radical cation at low temperature. Monitoring the reaction at 200 K revealed the emergence of a triplet signal, [21] with a g value of 2.0043 and a nitrogen hyperfine coupling constant of a(N)=5.9 G (Figure 1b), that only persisted at this temperature and readily disappeared at higher temperature. [22] The spin density plot of the optimized theoretical structure of 2 locates most of the unpaired electron on the carbenic carbon (Figure S56). The hyperfine coupling constant of a(N) in 2 was calculated at various levels of theory. [17] However, in all these calculations, the hyperfine coupling constants for a(N) are determined to be smaller than the experimentally observed value (the closest comp. a(N)=3.7 G; exp. a(N)=5.9 G; Table S9). Therefore, the anomaly observed between experimental and calculated hyperfine coupling constant a(N) can be attributed to the accuracy of the computational methods. [23]
Consequently, to study its utility as a reducing reagent CAAC 1 was reacted with amino(aryl)‐based mono cation 4, and p‐phenylene and p,p’‐biphenylene bridged dications 5 and 6 (Scheme 3). [17] The 1 : 1 reaction of 1 and 4 at −78 °C leads to the immediate appearance of an intense red color which steadily changes to light brown at room temperature. From the reaction mixture we were able to isolate 3[OTf] in a 91 % yield, which implies that CAAC 1 is first oxidized to the CAAC radical cation 2 and then forms the hydrogen abstracted product. The final product composition for the reduction of 4 was not conclusive. The 1H NMR spectrum of the reaction mixture indicates the formation of an interactive mixture of products, most plausibly the initial formation of neutral radical species 7 with a limited stability (the reduction of 4 also has been confirmed by the cyclic voltammetry study, which shows a reversible reduction wave: Figure 4, below). The in situ recorded EPR spectrum at 290 K (sample was prepared at 243 K and EPR spectrometer was precooled at about 250 K) shows a broad and unresolved signal (Figure S47). We tentatively assign the observed signal to the neutral radical species 7 or follow up decomposed products as we know that the corresponding CAAC‐derived radical cation 2 is too reactive to be detected at more than 200 K and it shows a triplet signal.
Scheme 3.
Reactions of 1 with 4, 5, and 6.
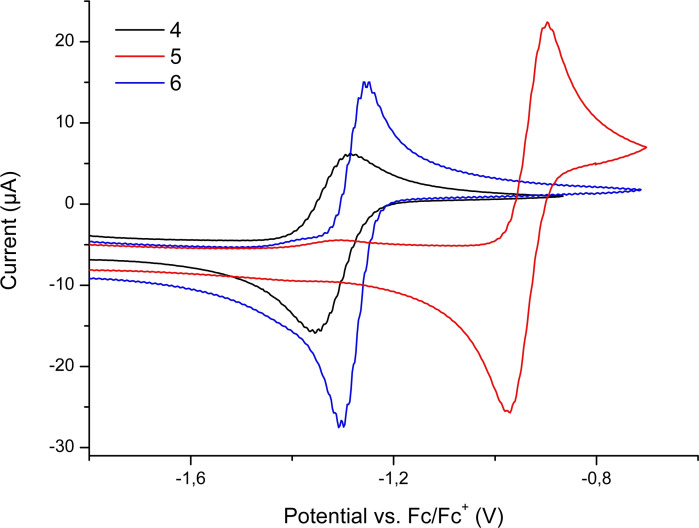
Figure 4.
Cyclic voltammograms of 4, 5, and 6 in CH3CN/0.1 M Bu4NPF6 measured at a GC working electrode.
The 1 : 2 reaction of 5 and 1 at −78 °C in THF immediately turns the solution red in color (Scheme 3). Crystallization in hexane after work up leads to pure product 8 (43 %) as red colored compound which is well soluble in hexane, toluene, and THF. Compound 8 in hexane solution exhibits a strong absorption at λ max (ϵ)=487 nm (33 900 L mol−1 cm−1) in the UV/Vis spectrum. In solution it is present as both syn and anti isomers in a 36 : 64 ratio. Quantum chemical calculations suggest that the syn isomer is more stable than the anti isomer by only 0.2 kcal mol−1. In the crystalline solid state, only the anti isomer was observed by X‐ray diffraction structural analysis (Figure S54). [18]
The 1 : 2 reaction of 6 and 1 at −78 °C in THF leads to the immediate appearance of a bluish green color (Scheme 3). Crystallization in hexane upon work up leads to pure product 9 (59 %) as green colored compound which is soluble in hexane, toluene, and THF. The reactions of 1 with 5 and 6 lead to the formation of 8 and 9, respectively, highlighting the CAAC as a potent organic reducing reagent. [24] Compound 9 in hexane exhibits a strong absorption in the UV/Vis spectrum at λ max (ϵ)=608 nm (75 000 L mol−1cm−1).
At room temperature the 1H NMR spectrum of 9 exhibits broad resonances for the aromatic protons in [D8]THF. This is an indication for a substantial population of the triplet state at room temperature. By lowering the temperature, the resonances for the aromatic protons gradually appear and at 203 K the resonances of the aromatic protons sharpen considerably (Figure 2a). In both solution and the solid state, 9 exhibits a broad unresolved EPR signal centered around g=2.0026 which increases in intensity with increasing temperature (Figure 2b). Fitting of the temperature‐dependent double‐integral intensity to the Bleaney–Bowers model gives a singlet‐triplet gap of 2 J=−257 cm−1 (ΔE ST=−0.74 kcal mol−1), thus indicating a thermally excited triplet state. This is close to that of anionic boron‐ and carbon‐based hetero‐diradicaloids spanned by a p‐phenylene bridge. [25]
Figure 2.
a) Variable‐temperature 1H NMR spectra of 9 (selected region) in [D8]THF. b) Variable‐temperature X‐band EPR spectra of 9 in THF between 255 and 300 K. C) plots of double‐integral intensity (A) vs. T. The red line shows the fit to the Bleaney–Bowers equation (experimental parameters: microwave frequency=9.38 GHz, microwave power=0.2 mW, modulation amplitude=0.5 G, modulation frequency=100 kHz). d) Molecular structure of 9 with thermal ellipsoids at the 50 % probability level. All H atoms are omitted for clarity. Selected bond lengths [Å] and angles [°]: N1−C1 1.389(2), C1−C8 1.479(2), C1−C2 1.400(2), C2−C3 1.431(2), C3−C4 1.359(2), C4−C5 1.425(2), C5−C5’ 1.419(21), N1−C1−C8 114.44(14).
The solid‐state molecular structure of 9 (Figure 2d) [18] reveals that the C1–C2 bond length between the acyclic(amino)(aryl)carbene‐scaffolds and the p,p’‐biphenylene bridge as a π‐conjugated spacer is 1.400(2) Å, thereby shorter than those in Chichibabin's hydrocarbon (1.415 Å). [25] The ∠N1‐C1‐C8 is 114.44(14)° which is more acute than that of the first crystalline acyclic(amino)(aryl)carbene (2,6‐tBu2‐C6H3‐C(:)‐N(iPr)Me) (121°). [13a] The dihedral angle between the planes involving acyclic(amino)(aryl)carbene‐scaffolds (N1‐C1‐C8) and the adjacent phenyl spacer (C3‐C2‐C7) is 19.685(81)°. The arrangement of the two phenyl rings of the central p,p’‐biphenylene bridge is coplanar. The bond length alternation (BLA) of 9 is 0.07 Å, that is, larger than in Chichibabin's hydrocarbon (0.05 Å). [26]
In order to obtain deeper insights into the diradical's character and energies, DFT studies were performed of the singlet and triplet states of 9. [17] The adiabatic DFT calculations suggest that the singlet state has a lower energy than the triplet configuration by ΔE ST=−6.9 kcal mol−1 at the BP86‐D3/Def2‐SVP level of theory, whereas the experimentally derived EPR based ΔE ST was determined to be −0.74 kcal mol−1. Considering the effect of a torsional twist between two phenyl rings on the singlet‐triplet gap, the energy alteration of singlet and triplet states was investigated by scanning of the corresponding dihedral angle.
We have also employed modredundant calculations for broken‐symmetry singlet and triplet states (Figure 3). The calculations show that the torsional twist on the triplet state slightly affects the energy with up to ∼3.4 kcal mol−1. In the case of the singlet state, however, the influence is considerably high by at most ∼12.5 kcal mol−1. Therefore, the overestimation of the observed experimental and calculated ΔE ST gap may stem from the rotation of the C−C bond between two phenyl rings and/or the accuracy of the computational method.
Figure 3.

Energy scanning of the rotation of the C−C bond between the two phenyl rings of 9 for the broken‐symmetry singlet (orange) and the triplet (blue) states at the UBP86‐D3/Def2SVP level of theory.
Due to the reactivity of 1 with either ferrocene or decamethylferrocene we were not able to exactly reference its redox potential. To investigate the redox potential of 1 indirectly, cyclic voltammograms of 4, 5, and 6 were recorded. The CVs exhibit reversible redox waves at E 1/2=−1.32 (4), −0.93 (5), and −1.28 (6) V versus external Fc/Fc+, which we assigned to a one‐electron reduction for 4 and to two‐electron reductions for 5 and 6, respectively (Figures 4 and S40). As 4, 5, and 6 can be reduced using 1, it must have a redox potential E 1/2 of at least −1.3 V versus Fc/Fc+.
Conclusion
In conclusion, a cyclic(alkyl)(amino)carbene (CAAC) acts as a potent one‐electron reductant transforming to a radical cation in the process. The formation of the transient radical cation was indicated by EPR spectroscopy. The CAAC was then used as a reducing reagent for the synthesis of acyclic(amino)(aryl)carbene‐based Thiele and Chichibabin hydrocarbons, a new class of Kekulé diradicaloids. Variable‐temperature NMR and EPR spectroscopy studies show that the acyclic(amino)(aryl)carbene‐based Chichibabin's hydrocarbon has an appreciable population of the triplet state at room temperature. The newly disclosed properties of CAACs might stimulate respective further exploration in new directions, such as single electron transfer processes [27] and use as n‐type dopant/organic reductant. [28]
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
We acknowledge generous support of the Department of Atomic Energy, Government of India, under Project Identification No. RTI 4007 and SERB (CRG/2019/003415), India. The numerical calculations reported in this paper were partially performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources). We are grateful to the reviewers for their critical insights to improve the manuscript. Open Access funding enabled and organized by Projekt DEAL.
Dedicated to Professor A. Ajayaghosh on the occasion of his 60th birthday
A. Maiti, B. J. Elvers, S. Bera, F. Lindl, I. Krummenacher, P. Ghosh, H. Braunschweig, C. B. Yildiz, C. Schulzke, A. Jana, Chem. Eur. J. 2022, 28, e202104567.
Contributor Information
Prof. Prasanta Ghosh, Email: ghosh@pghosh.in.
Prof. Dr. Holger Braunschweig, Email: h.braunschweig@uni-wuerzburg.de.
Prof. Cem B. Yildiz, Email: cemburakyildiz@aksaray.edu.tr.
Prof. Dr. Carola Schulzke, Email: carola.schulzke@uni-greifswald.de.
Dr. Anukul Jana, Email: ajana@tifrh.res.in.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1. Lavallo V., Canac Y., Präsang C., Donnadieu B., Bertrand G., Angew. Chem. Int. Ed. 2005, 44, 5705–5709; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 5851–5855. [Google Scholar]
- 2.Selected reviews:
- 2a. Melaimi M., Jazzar R., Soleilhavoup M., Bertrand G., Angew. Chem. Int. Ed. 2017, 56, 10046–10068; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 10180–10203; [Google Scholar]
- 2b. Soleilhavoup M., Bertrand G., Acc. Chem. Res. 2015, 48, 256–266; [DOI] [PubMed] [Google Scholar]
- 2c. Kundu S., Sinhababu S., Chandrasekhar V., Roesky H. W., Chem. Sci. 2019, 10, 4727–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a. Lavallo V., Canac Y., DeHope A., Donnadieu B., Bertrand G., Angew. Chem. Int. Ed. 2005, 44, 7236–7239; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 7402–7405; [Google Scholar]
- 3b. Ung G., Rittle J., Soleilhavoup M., Bertrand G., Peters J. C., Angew. Chem. Int. Ed. 2014, 53, 8427–8431; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 8567–8571; [Google Scholar]
- 3c. Ung G., Peters J. C., Angew. Chem. Int. Ed. 2015, 54, 532–535; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 52–545. [Google Scholar]
- 4.
- 4a. Morvan J., Mauduit M., Bertrand G., Jazzar R., ACS Catal. 2021, 11, 1714–1748; [Google Scholar]
- 4b. Yazdani S., Junor G. P., Peltier J. L., Gembicky M., Jazzar R., Grotjahn D. B., Bertrand G., ACS Catal. 2020, 10, 5190–5201; [Google Scholar]
- 4c. Marx V. M., Sullivan A. H., Melaimi M., Virgil S. C., Keitz B. K., Weinberger D. S., Bertrand G., Grubbs R. H., Angew. Chem. Int. Ed. 2015, 54, 1919–1923; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 1939–1943; [Google Scholar]
- 4d. Anderson D. R., Lavallo V., O'Leary D. J., Bertrand G., Grubbs R. H., Angew. Chem. Int. Ed. 2007, 46, 7262–7265; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 7400–7403. [Google Scholar]
- 5.
- 5a. Hamze R., Peltier J. L., Sylvinson D., Jung M., Cardenas J., Haiges R., Soleilhavoup M., Jazzar R., Djurovich P. I., Bertrand G., Thompson M. E., Science 2019, 363, 601–606; [DOI] [PubMed] [Google Scholar]
- 5b. Ullrich T., Pinter P., Messelberger J., Haines P., Kaur R., Hansmann M. M., Munz D., Guldi D. M., Angew. Chem. Int. Ed. 2020, 59, 7906–7914; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 7980–7988; [Google Scholar]
- 5c. Bakker A., Freitag M., Kolodzeiski E., Bellotti P., Timmer A., Ren J., Lammers B. S., Moock D., Roesky H. W., Mönig H., Amirjalayer S., Fuchs H., Glorius F., Angew. Chem. Int. Ed. 2020, 59, 13643–13646; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 13745–13749. [Google Scholar]
- 6.
- 6a. Lavallo V., Canac Y., Donnadieu B., Schoeller W. W., Bertrand G., Angew. Chem. Int. Ed. 2006, 45, 3488–3491; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 3568–3571; [Google Scholar]
- 6b. Frey G. D., Lavallo V., Donnadieu B., Schoeller W. W., Bertrand G., Science 2007, 316, 439–441; [DOI] [PubMed] [Google Scholar]
- 6c. Denk K., Sirsch P., Herrmann W. A., J. Organomet. Chem. 2002, 649, 219–224. [Google Scholar]
- 7.
- 7a. Mondal K. C., Roesky H. W., Schwarzer M. C., Frenking G., Tkach I., Wolf H., Kratzert D., Herbst-Irmer R., Niepötter B., Stalke D., Angew. Chem. Int. Ed. 2013, 52, 1801–1805; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 1845–1850; [Google Scholar]
- 7b. Zhu K., Dutta S., Han W., Wang C., Lee J., Tan G., Koley D., So C.-W., Li Y., Inorg. Chem. 2021, 60, 7143–7149; [DOI] [PubMed] [Google Scholar]
- 7c. Roy S., Mondal K. C., Roesky H. W., Acc. Chem. Res. 2016, 49, 357–369; [DOI] [PubMed] [Google Scholar]
- 7d. Mondal K. C., Roy K. C. S., Roesky H. W., Chem. Soc. Rev. 2016, 45, 1080–1111. [DOI] [PubMed] [Google Scholar]
- 8. Mondal K. C., Samuel P. P., Roesky H. W., Niepoetter B., Herbst-Irmer R., Stalke D., Ehret F., Kaim W., Maity B., Koley D., Chem. Eur. J. 2014, 20, 9240–9245. [DOI] [PubMed] [Google Scholar]
- 9. Thakur A., Vardhanapu P. K., Vijaykumar G., Bhatta S. R., J. Chem. Sci. 2016, 128, 613–620. [Google Scholar]
- 10.
- 10a. Shaikh A. C., Veleta J. M., Moutet J., Gianetti T. L., Chem. Sci. 2021,12, 4841–4849; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Pell T. P., Couchman S. A., Ibrahim S., Wilson D. J. D., Smith B. J., Barnard P. J., Dutton J. L., Inorg. Chem. 2012, 51, 13034–13040; [DOI] [PubMed] [Google Scholar]
- 10c. Dutton J. L., Tabeshi R., Jennings M. C., Lough A. J., Ragogna P. J., Inorg. Chem. 2007, 46, 8594–8602; [DOI] [PubMed] [Google Scholar]
- 10d. Ramnial T., McKenzie I., Gorodetsky B. E., Tsang M. W., Clyburne J. A. C., Chem. Commun. 2004, 1054–1055. [DOI] [PubMed] [Google Scholar]
- 11. Dong Z., Pezzato C., Sienkiewicz A., Scopelliti R., Fadaei-Tirani F., Severin K., Chem. Sci. 2020, 11, 7615–7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.
- 12a. Sharma R. B., Semo N. M., Koski W. S., J. Phys. Chem. 1987, 91, 4127–4131; [Google Scholar]
- 12b. Parker V. D., Bethell D., J. Am. Chem. Soc. 1987, 109, 5066–5072; [Google Scholar]
- 12c. Stoub D., Goodman G. V., J. Am. Chem. Soc. 1997, 119, 11110–11111; [Google Scholar]
- 12d. Bethell D., Parker V. D., Acc. Chem. Res. 1988, 21, 400–407. [Google Scholar]
- 13.
- 13a. Solé S., Gornitzka H., Schoeller W. W., Bourissou D., Bertrand G., Science 2001, 292, 1901–1903; [DOI] [PubMed] [Google Scholar]
- 13b. Cattoen X., Gornitzka H., Bourissou D., Bertrand G., J. Am. Chem. Soc. 2004, 126, 1342–1343; [DOI] [PubMed] [Google Scholar]
- 13c. Vignolle J., Asay M., Miqueu K., Bourissou D., Bertrand G., Org. Lett. 2008, 10, 4299–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saito M., Kawamata Y., Meanwell M., Navratil R., Chiodi D., Carlson E., Hu P., Chen L., Udyavara S., Kingston C., Tanwar M., Tyagi S., McKillican B. P., Gichinga M. G., Schmidt M. A., Eastgate M. D., Lamberto M., He C., Tang T., Malapit C. A., Sigman M. S., Minteer S. D., Neurock M., Baran P. S., J. Am. Chem. Soc. 2021, 143, 7859–7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.
- 15a. Thiele J., Balhorn H., Ber. Dtsch. Chem. Ges. 1904, 37, 1463–1470; [Google Scholar]
- 15b. Tschitschibabin A. E., Ber. Dtsch. Chem. Ges. 1907, 40, 1810–1819. [Google Scholar]
- 16.
- 16a.M. Abe, Chem. Rev. 2013, 113, 7011–7088; [DOI] [PubMed]
- 16b.T. Stuyver, B. Chen, T. Zeng, P. Geerlings, F. D. Proft, R. Hoffmann, Chem. Rev. 2019, 119, 11291–11351; [DOI] [PubMed]
- 16c.B. M. Barry, R. G. Soper, J. Hurmalainen, A. Mansikkamäki, K. N. Robertson, W. L. McClennan, A. J. Veinot, T. L. Roemmele, U. Werner-Zwanziger, R. T. Boeré, H. M. Tuononen, J. A. C. Clyburne, J. D. Masuda, Angew. Chem. Int. Ed. 2018, 57, 749–754; Angew. Chem. 2018, 130, 757–762; [DOI] [PubMed]
- 16d.M. M. Hansmann, M. Melaimi, D. Munz, G. Bertrand, J. Am. Chem. Soc. 2018, 140, 2546–2554; [DOI] [PubMed]
- 16e.D. Rottschäfer, N. K. T. Ho, B. Neumann, H. -G. Stammler, M. von. Gastel, D. M. Andrada, R. S. Ghadwal, Angew. Chem. Int. Ed. 2018, 57, 5838–5842; Angew. Chem. 2018, 130, 5940–5944; [DOI] [PubMed]
- 16f.D. Rottschafer, B. Neumann, H.-G. Stammler, D. M. Andrada, R. S. Ghadwal, Chem. Sci. 2018, 9, 4970–4976; [DOI] [PMC free article] [PubMed]
- 16g.A. Maiti, J. Stubbe, N. I. Neuman, P. Kalita, P. Duari, C. Schulzke, V. Chandrasekhar, B. Sarkar, A. Jana, Angew. Chem. Int. Ed. 2020, 59, 6729–6734; Angew. Chem. 2020, 132, 6795–6800; [DOI] [PMC free article] [PubMed]
- 16h.A. Maiti, S. Chandra, B. Sarkar, A. Jana, Chem. Sci. 2020, 11, 11827–11833; [DOI] [PMC free article] [PubMed]
- 16i.A. Maiti, S. Sobottka, S. Chandra, D. Jana, P. Ravat, B. Sarkar, A. Jana, J. Org. Chem. 2021, 86, 16464–16472; [DOI] [PubMed]
- 16j.A. Mahata, S. Chandra, A. Maiti, D. K. Rao, C. B. Yildiz, B. Sarkar, A. Jana, Org. Lett. 2020, 22, 8332−8336; [DOI] [PubMed]
- 16k.A. Mahata, N. Chrysochos, I. Krummenacher, S. Chandra, H. Braunschweig, C. Schulzke, B. Sarkar, C. B. Yildiz, A. Jana, J. Org. Chem. 2021, 86, 10467–10473. [DOI] [PubMed]
- 17.See the Supporting Information for the experimental details, analytical data, NMR spectra, UV/Vis spectra, X-ray crystallographic details, and details of quantum chemical calculation.
- 18.Deposition Numbers 2095380 (3 (SbF6)), 2095381 (4), 2032057 (D), 2032059 (5), 2095382 (6), 2032063 (8), and 2095383 (9) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 19. Nayak M. K., Suhr S., Chrysochos N., Rawat H., Schulzke C., Chandrasekhar V., Sarkar B., Jana A., Chem. Commun. 2021, 57,1210–1213. [DOI] [PubMed] [Google Scholar]
- 20. Mandal D., Dolai R., Kalita P., Narayanan R. S., Kumar R., Sobottka S., Sarkar B., Rajaraman G., Chandrasekhar V., Jana A., Chem. Eur. J. 2018, 24, 12722–12727. [DOI] [PubMed] [Google Scholar]
- 21.The byproduct NO also has a triplet signature but with different nitrogen hyperfine coupling constants.
- 22.This observation rules out the possible formation of CAAC-NO adduct. Please see the Supporting Information for the calculated EPR hyperfine coupling constant of CAAC-NO adduct (Table S8). For related NHC-NO adduct, please see: Park J., Song H., Kim Y., Eun B., Kim Y., Bae D. Y., Park S., Rhee Y. M., Kim W. J., Kim K., Lee E., J. Am. Chem. Soc. 2015, 137, 4642–4645.25844581 [Google Scholar]
- 23.
- 23a. Kirste B., Magn. Reson. Chem. 2016, 54, 835–841; [DOI] [PubMed] [Google Scholar]
- 23b. Pushkarevsky N. A., Chulanova E. A., Shundrin L. A., Smolentsev A. I., Salnikov G. E., Pritchina E. A., Genaev A. M., Irtegova I. G., Yu I., Bagryanskaya, Konchenko S. N., Gritsan N. P., Beckmann J., Zibarev A. V., Chem. Eur. J. 2019, 25, 806–816; [DOI] [PubMed] [Google Scholar]
- 23c. Rajca A., Shiraishi K., Boratyński P. J., Pink M., Miyasaka M., Rajca S., J. Org. Chem. 2011, 76, 8447–8457. [DOI] [PubMed] [Google Scholar]
- 24.
- 24a. Broggi J., Terme T., Vanelle P., Angew. Chem. Int. Ed. 2014, 53, 384–413; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 392–423; [Google Scholar]
- 24b. Liu W., Vianna A., Zhang Z., Huang S., Huang L., Melaimi M., Bertrand G., Yan X., Chem Catal. 2021, 1, 196–206; [Google Scholar]
- 24c. Wang C., Liu L., Org. Chem. Front. 2021, 8, 1454–1460; [Google Scholar]
- 24d. Ding Y. F., Yang C. Y., Huang C. X., Lu Y. Z., Yao F., Pan C. K., Wang J. Y., Pei J., Angew. Chem. Int. Ed. 2021, 60, 5816–5820; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 5880–5884. [Google Scholar]
- 25. Maiti A., Zhang F., Krummenacher I., Bhattacharyya M., Mehta S., Moos M., Lambert C., Engels B., Mondal A., Braunschweig H., Ravat P., Jana A., J. Am. Chem. Soc. 2021, 143, 3687–3692. [DOI] [PubMed] [Google Scholar]
- 26. Montgomery L. K., Huffman J. C., Jurczak E. A., Grendze M. P., J. Am. Chem. Soc. 1986, 108, 6004–6011. [DOI] [PubMed] [Google Scholar]
- 27.
- 27a. Merk A., Großekappenberg H., Schmidtmann M., Luecke M.-P., Lorent C., Driess M., Oestreich M., Klare H. F. T., Müller T., Angew. Chem. Int. Ed. 2018, 57, 15267–15271; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 15487–15492; [Google Scholar]
- 27b. Dong Z., Cramer H. H., Schmidtmann M., Paul L. A., Siewert I., Müller T., J. Am. Chem. Soc. 2018, 140, 15419–15424. [DOI] [PubMed] [Google Scholar]
- 28. Lu Y., Wang J.-Y., Pei J., Acc. Chem. Res. 2021, 54, 2871–2883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.