Abstract
Objectives
Several recent clinical trials have shown that docosahexaenoic acid (DHA) supplements have a significant effect on cognition in cognitively impaired older adults. This randomised controlled trial aimed to investigate the cognitive effects of a DHA fish oil supplement in older adults with mild cognitive impairment, and to examine the moderating effect of the apolipoprotein E (APOE) ɛ4 allele on cognition and well‐being.
Methods/Design
Seventy‐two older adults between the ages of 60 and 90 from New Zealand were given a DHA supplement equivalent to 1491 mg DHA + 351 mg eicosapentaenoic acid per day or a placebo for a period of 12 months. Outcome measures included cognition, wellbeing and self‐rated quality of life as well as height, weight, blood pressure and APOE genotyping.
Results
The final analysis (n = 60) found no evidence of a treatment effect on cognitive measures, although did find a treatment effect on systolic blood pressure (p = 0.03, ƞ 2 = 0.08), and a treatment interaction for APOE ɛ4 carriers on depression (p = 0.04, ƞ 2 = 0.07) and anxiety (p = 0.02, ƞ 2 = 0.09) scores in favour of the DHA supplement.
Conclusions
Despite no effect on cognition, the positive result in APOE ɛ4 carriers on depression and anxiety scores and on systolic blood pressure justifies further DHA trials. It may be a prudent step going forward for more studies to replicate the design elements (dose, duration and cognitive measures) of previous DHA trials to help understand why not all older adults appear to benefit from taking a fish oil supplement.
Keywords: APOE ɛ4 carriers, DHA, EPA, mild cognitive impairment
Key points
Diet underlies the main risk factors for dementia including diabetes and hypertension.
Docosahexaenoic acid (DHA) has been shown to have a significant effect on cognition in impaired older adults.
Older adults who carry the apolipoprotein E (APOE) ɛ4 allele are at higher risk of cognitive decline.
DHA and eicosapentaenoic acid can decrease depression and anxiety scores in APOE ɛ4 carriers.
1. INTRODUCTION
Mild cognitive impairment (MCI) is a state or phase of cognitive decline between normal ageing and dementia. 1 This phase has been used to estimate conversion rates to dementia, to predict clinical outcomes, and to investigate modifiable risk factors and preventative treatments for dementia. 2 Investigating the effects of lifestyle factors such as diet and nutrition on cognition at this early phase is of interest, especially since diet underlies the main risk factors for dementia including diabetes, midlife obesity, hypertension and depression. 3
The two nutrients that have received the most attention in preventing cognitive decline are the omega‐3 polyunsaturated fatty acids (n‐3 PUFAs) docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). DHA and EPA are referred to as ‘conditionally essential fatty acids’ because humans cannot easily convert the essential fatty acid precursor alpha‐linolenic acid to DHA, and therefore, DHA and EPA usually need to be consumed in the diet or as a supplement. Nutritional research shows that older adults are not eating enough seafood to reach recommended daily amounts of DHA and EPA, 4 and epidemiological research shows positive associations between fish consumption and higher scores on cognitive tests 5 and a slower rate in cognitive decline. 6 DHA and EPA produce docosanoids and eicosanoids, which reduce inflammation and blood clotting, whereas DHA has been shown to be crucial for the structure and function of neuronal cell membranes and to have potent neuro‐protective and tissue regeneration effects. 7
The results from clinical trials investigating the cognitive effects of n‐3 PUFAs in older adults have been inconsistent and at times conflicting. Trials with cognitively healthy adults have found no treatment effects except on attention and reasoning tasks for apolipoprotein E (APOE) ɛ4 carriers, 8 , 9 , 10 , 11 and yet several smaller studies have found treatment effects on recall and working memory. 12 , 13 , 14 In older adults with Alzheimer's disease (AD) one trial found a lower decline in the fish oil group for APOE non‐ɛ4 carriers only, 15 and several trials have found positive results in milder forms of dementia 16 , 17 , 18 and in older adults with subjective memory complaints. 19 , 20 A meta‐analysis of n‐3 PUFAs trials in older adults concluded that n‐3 PUFAs provide a significant benefit on cognition but only in older adults with Cognitive Impairment no Dementia (CIND) in immediate recall and attention/processing speed, and in high‐functioning CIND and dementia participants (Mini‐Mental State Exam (MMSE) ≥26) in delayed verbal recall. 21
To date there have been several studies specifically in older adults with MCI. Sinn et al. 22 divided 50 participants with MCI into an EPA group (1670 mg of EPA + 160 mg of DHA), a DHA group (1550 mg of DHA + 400 mg of EPA) and a control group for 6 months. The trial found that DHA and EPA groups' scores improved significantly on the Geriatric Depression Scale, and the DHA group significantly improved in initial letter fluency compared to the control group. The following year a 12‐month trial was published by Lee et al. 23 where 36 MCI older adults were given a dose of 1290 mg of DHA + 450 mg of EPA. At the 12‐month mark, the DHA + EPA treatment group showed significant improvement in short‐term memory and working memory, immediate verbal memory, and delayed recall. Two further MCI trials found treatment effects on the MMSE and in perceptual speed and working memory. 24 , 25
The APOE ɛ4 allele has been shown to be the most inefficient of the three alleles at delivering PUFAs to neurons, where greater increases in cerebrospinal fluid levels of EPA and DHA have been found in non‐ɛ4 carriers than ɛ4 carriers after DHA supplementation. 26 The ɛ4 allele has been associated with faster myelin breakdown, greater hippocampal atrophy, reduced neuroplasticity capacity and increased inflammation. 27 , 28 Older adults who carry the ɛ4 allele are at higher risk of cognitive decline 29 whereas the ɛ2 allele has been shown to have a protective role on cognition. 30 The objective of this 12‐month randomised controlled trial was to replicate components (dose, duration, measures) of the two trials by Sinn et al. and Lee et al, and to extend with the addition of APOE genotyping. The primary aim was to investigate whether a high DHA fish oil supplement can improve cognitive performance and well‐being scores in older adults with MCI, and the secondary aim was to investigate whether the ɛ4 allele has a moderating effect on scores.
2. MATERIALS AND METHODS
2.1. Participants and procedure
A total of 183 older people underwent telephone screening and 72 older adults were found to fit the eligibility criteria: between the age of 60 and 90, having a subjective memory complaint which was confirmed by a friend or family member, scoring 1–1.5 SD points below the test mean on at least one cognitive measure at baseline, preserved cognition/IQ and living independently. 31 Participants were excluded if they had taken a fish oil supplement within the last 6 months, ate more than two servings of fish/seafood per week, had problems swallowing soft‐gel capsules or had an allergy to fish or seafood. Participants were also excluded if they took blood thinning medications, had any major health conditions including head injury, had recent or upcoming surgery, as well as anything else that would interfere with the ability to participate in the trial, for example, travel plans during the testing sessions or heavy work commitments. A flow chart of the participants is shown in Figure 1.
FIGURE 1.
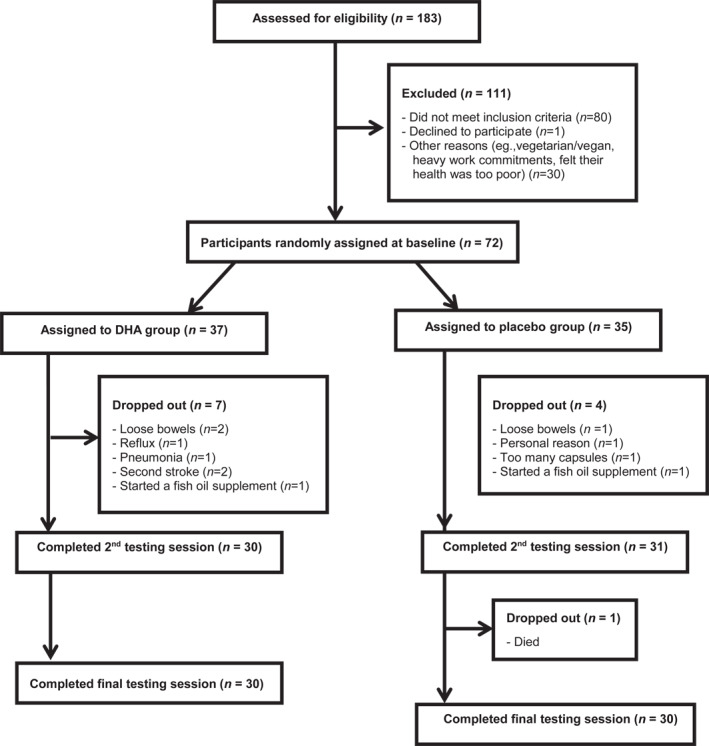
Participant recruitment, drop‐outs and completers
A paid research assistant generated a list of random participant numbers from Random.org, assigned participant numbers to capsule groups and labelled capsule bottles, and was the only person to know the grouping arrangements until the end of the testing. The cognitive, well‐being and physiological measures were administered at baseline, and after 6 and 12 months of taking the capsules. Participants were reimbursed for participating in each testing session. The trial was approved by the New Zealand Health and Disability Ethics Committee (14/CEN/148) and written informed consent was obtained from all participants. The trial was registered with the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au; request number: 366439).
3. INTERVENTION
The DHA group were given three capsules per day equating to a daily dosage of 1491 mg of triglyceride DHA and 351 mg of EPA. The placebo group were also given three capsules per day equating to a daily dose of 1857 mg of linoleic acid (LA) per day. The two types of capsules were identical in size, colour and shape, and both contained vitamin E (1.15 mg of vitamin E 1300 IU/g per capsule) as an anti‐oxidant and orange oil (10 mg per capsule) to help mask the taste of the oils. The high or pharmacologic dose of DHA and EPA was based on the two trials by Sinn et al. and Lee et al. and was equivalent to eating approximately 4–5 portions of oily fish per week.
3.1. Primary outcomes: cognitive, well‐being and physiological measures
The cognitive measures used were the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), 32 the National Adult Reading Test (NART), 33 the Coin Rotation Task (CRT), 34 the California Older Adult Stroop Test (COAST), 35 the Trail Making Test A and B (TMT) 36 and the Digit Span Backwards Test (DSBT) from the Weschler Adult Intelligence Scale 3 (WAIS‐III). 37 The well‐being measures included the Geriatric Depression Scale (GDS), 38 the Geriatric Anxiety Inventory (GAI) 39 and 36‐Item Short Form Health survey questionnaire (SF‐36). 40
3.2. Secondary outcomes: APOE status and fatty acid profiles
The physiological measures included weight which was measured using a dial scale, and height using a wall‐mounted stadiometer. Systolic and diastolic blood pressure were measured on the non‐dominant arm and after the participant has been seated for at least 15 min, using an automated sphygmomanometer (Sure Signs VM4; Philips Medical Systems).
Two fasting blood samples were taken throughout the course of the trial. The first sample was taken after the baseline testing session and was analysed for red blood cell (RBC) fatty acid profile status, vitamin B12 and ferritin levels. The second sample was taken after the 12‐month testing session and was analysed for RBC fatty acid profile status and APOE genotyping. APOE genotyping was performed using a polymerase chain reaction in a DNA Thermal Cycler (Perkin Elmer Cetus).
The RBC profile test measured concentrations of 38 fatty acids with a unit measure of mg/ml as well as total % fatty acid. The EPA and DHA values were used to calculate an omega‐3 index = EPA + DHA/total % fatty acids, and to calculate arachidonic acid (ARA) ratios including ARA:EPA and ARA:DHA. Fatty acid analysis was run using a gas chromatograph (Shimadzu G‐2010 plus).
3.3. Demographic measures
At the baseline testing session, a list of the participant's medications and supplements, existing health conditions and family history of dementia was compiled along with general demographic information. Participants completed a brief n‐3 PUFA FFQ during the baseline testing session to assess weekly fish (including fatty fish) and seafood consumption.
3.4. Statistical analysis
A power analysis was performed to calculate the required number of participants. Based on the calculation of 4 groups (Fish oil/ɛ4, Fish oil/non‐ɛ4, Placebo/ɛ4 and Placebo/non‐ɛ4) using an ANCOVA (with fixed effects, main effects and interactions) with an estimated effect size of f = 0.40, to provide a statistical power equal to 0.8 and an α level of 0.05 (two‐tailed), the minimum total sample size required was 64, 16 participants in each of the four groups. Differences between participant completers and drop‐outs, and between the two groups at baseline were analysed using two‐tailed independent samples t‐tests and chi‐square tests. ANCOVAs were used to test for treatment effects on the measures, with treatment group as the main factor and with age, gender and years of education as covariates. All assumptions were met for the linear model analyses including Levene's test of equality of error variances, and Bonferroni corrections were used for multiple analyses. Alpha was set at 0.05 for all analyses, all of which were performed using IBM SPSS statistics program version 25. Cohen's d values were calculated using the formula and ƞ 2 values were calculated with SPSS using the formula ƞ 2 = SS effect/SS total.
4. RESULTS
4.1. Participant completers and drop‐outs
Seventy‐two participants were randomly assigned to receive the DHA supplement (n = 37) or a placebo (n = 35) for 12 months. Of the 72 participants, 11 participants withdrew (7 from the DHA group and 4 from the placebo group) and one participant died (placebo group) unrelated to the study, resulting in an attrition rate of 17%. There were no demographic, cognitive or well‐being differences between participants who dropped out and completed the trial, nor were there any differences between those who dropped out of the DHA group and the placebo group. The final analysis was performed on 60 participants—30 participants in each group.
4.2. Baseline characteristics of the participants
Baseline characteristics of the participants in the DHA and placebo groups are summarised in Table 1. The group mean on the NART was more than 1 SD above the age norm, indicating the participant group was above average in IQ. Twenty‐five participants scored 1–1.5 SD points below the test mean on a memory task, 25 participants on an attention or visuo‐spatial/constructional task and 10 on an executive function or language task. This equated to 25 amnestic MCI participants and 35 non‐amnestic MCI participants. The only significant difference between the two groups at baseline was in ARA concentrations, with significantly higher baseline ARA concentrations in the placebo group compared to the DHA group t(58) = 2.49, p = 0.02, d = 0.63.
TABLE 1.
Baseline characteristics of DHA and placebo groups
| Characteristic | DHA group (n = 30) | Placebo group (n = 30) |
|---|---|---|
| Gender‐Female (n/%) | 16/53 | 19/63 |
| NZ born ‐ European (n/%) | 22/73 | 25/83 |
| Right handed (n/%) | 27/90 | 23/77 |
| Married (n/%) | 14/47 | 11/37 |
| Hypertension meds (n/%) | 16/53 | 17/57 |
| APOE ɛ4 carriers (n/%) | 10/33 | 8/27 |
| APOE ɛ2 carriers (n/%) | 7/23 | 9/30 |
| Age (years) a | 72.33 (6.16) | 73.40 (6.96) |
| Education (years) | 15.56 (2.83) | 14.30 (3.19) |
| NART predicted IQ | 119.72 (4.76) | 119.02 (5.03) |
| BMI score (kg/m2) | 27.54 (4.16) | 26.01 (3.94) |
| Systolic BP (mmHg) | 145.90 (15.98) | 140.23 (21.91) |
| Diastolic BP (mmHg) | 83.67 (11.87) | 80.07 (13.77) |
| Alcohol (drinks/week) | 5.36 (5.87) | 4.88 (4.47) |
| Sleep (hours/night) | 7.11 (0.96) | 6.70 (1.16) |
| Exercise (days/week) | 4.17 (1.39) | 4.53 (1.17) |
| Number of meds (per day) | 2.26 (1.62) | 2.0 (1.52) |
| LA (%/TFA) b | 7.96 (1.03) | 7.95 (1.32) |
| ARA (%/TFA) | 18.87 (1.64) | 19.99 (1.83)* |
| EPA (%/TFA) | 1.00 (0.50) | 0.99 (0.44) |
| DPA (%/TFA) | 1.9 (0.35) | 1.85 (0.31) |
| DHA (%/TFA) | 4.1 (1.01) | 4.06 (0.96) |
| Omega‐3 index | 5.12 (1.29) | 5.06 (1.11) |
| Vitamin B12 (pmol/L) | 284.67 (112.08) | 325.86 (232.08) |
| Ferritin (μg/L) | 182.40 (152.36) | 150.30 (115.03) |
Note: Omega‐3 index = EPA + DHA / total % fatty acid.
Abbreviations: APOE, apolipoprotein E; ARA, arachidonic acid; BP, blood pressure; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; meds, medications; NART predicted IQ, predicted IQ scores from the National Adult Reading Test; NZ, New Zealand; TFA, total % fatty acid.
Values are means; SDs in parentheses.
Fatty acid profiles were measured from red blood cells.
*p < 0.05.
Of the 60 participants included in the final analysis, 30% were carriers of the ɛ4 allele and 27% were carriers of ɛ2. APOE ɛ4 carriers had significantly higher baseline GDS scores than non‐ɛ4 carriers t(58) = −2.26, p = 0.03, d = 0.62, and higher baseline GAI scores than non‐ɛ4 carriers t(58) = −1.86, p = 0.07, d = 0.53. APOE ɛ4 carriers had lower baseline SF‐36 Role physical scores than non‐ɛ4 carriers t(58) = 2.03, p = 0.05, d = 0.56, and lower baseline SF‐36 Physical functioning scores than non‐ɛ4 carriers t(58) = 2.44, p = 0.02, d = 0.68.
4.3. Primary outcome measures
The primary outcome was the change in cognitive and well‐being scores from baseline to 12 months. There were no significant differences between the DHA and placebo groups for any of the cognitive measures, see Table 2. The change in scores from baseline to 6 months were analysed but also showed no treatment effects.
TABLE 2.
Change in cognitive, well‐being and physiological measures by group from baseline to 12 months
| DHA group (n = 30) | Placebo group (n = 30) | DHA compared to placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Change | Baseline | 12 months | Change | Difference | Treatment effect | ApoE ɛ4 x treatment | |||
| p | ƞ b | p | ƞ b | ||||||||
| RTot | 101.9 (11.9) a | 108.4 (12.8) | 6.5 (5.8) | 100.3 (11.9) | 105.5 (11.2) | 5.2 (7.4) | 1.3 (−4.7, 2.1) b | 0.37 c | 0.02 | 0.64 | 0.00 |
| RIM | 104.8 (16.2) | 110 (13.6) | 5.2 (8.7) | 105.3 (13.9) | 109.9(13.8) | 4.5 (12.3) | 0.6 (−6.1, 4.8) | 0.68 | 0.01 | 0.66 | 0.00 |
| RVC | 96.6 (9.6) | 98.4 (8.8) | 1.7 (9.1) | 92.9 (12.6) | 91.4 (11.8) | −1.4 (13.1) | 3.1 (−9.0, 2.7) | 0.28 | 0.02 | 0.99 | 0.00 |
| RL | 103.9 (13.8) | 107.0 (101.1) | 3.1 (13.1) | 103.2 (12.8) | 106.7 (7.8) | 3.5 (11.7) | −0.4 (−5.9, 6.8) | 0.86 | 0.00 | 0.83 | 0.00 |
| RA | 103.7 (13.8) | 110.4 (15.3) | 6.7 (8.1) | 102.9 (13.3) | 109.8 (13.9) | 6.9 (10.5) | −0.2 (−4.6, 5.0) | 0.93 | 0.00 | 0.73 | 0.00 |
| RDM | 98.2 (11.3) | 104.6 (11.9) | 6.3 (7.9) | 98.3 (12.7) | 102.8 (12.9) | 4.5 (10.3) | 1.8 (−6.5, 2.9) | 0.45 | 0.01 | 0.38 | 0.01 |
| CR | −0.33 (1.2) | −0.003 (1.4) | 0.33 (0.7) | −0.25 (1.2) | −0.12 (1.5) | 0.13 (0.9) | 0.1 (−0.6, 0.24) | 0.38 | 0.01 | 0.50 | 0.01 |
| COA | 57.8 (22.9) | 65.2 (21.5) | 7.4 (16.5) | 53.7 (22.8) | 64.8 (22.4) | 11.1 (13.3) | −3.6 (−4.1, 11.4) | 0.35 | 0.02 | 0.23 | 0.03 |
| DSF | 75.4 (24.3) | 84.5 (19.1) | 9.1 (20.9) | 63.2 (26) | 77.1 (22.1) | 13.9 (24) | −4.7 (−6.8, 16.4) | 0.42 | 0.01 | 0.58 | 0.01 |
| DSBT | 56.1 (32.5) | 63.6 (31.1) | 7.6 (17.3) | 49.5 (30.5) | 51.4 (30.1) | 1.86 (24.5) | 5.7 (−16.6, 5.3) | 0.30 | 0.02 | 0.56 | 0.01 |
| DSTot | 12.4 (3.4) | 13.6 (3.4) | 1.2 (2.3) | 11.2 (3.2) | 12.1 (3) | 0.9 (2.2) | 0.3 (−1.4, 0.8) | 0.60 | 0.01 | 0.40 | 0.01 |
| TMTB | 41.9 (30.4) | 48.0 (31.1) | 6.1 (26.2) | 49.8 (31.6) | 56.8 (32.3) | 6.9 (37) | −0.8 (−15.7, 17.4) | 0.91 | 0.00 | 0.81 | 0.00 |
| GDS | 1.97 (2) | 1.97 (2.7) | 0.0 (1.4) | 2.2 (2.4) | 2.1 (2.2) | 0.03 (2.3) | −0.03 (−0.9, 1.0) | 0.95 | 0.00 | 0.04* | 0.07 |
| GAI | 2.2 (3.0) | 1.83 (3.1) | 0.4 (2.4) | 2.6 (3.6) | 1.9 (2.4) | 0.7 (2.6) | −0.3 (−0.9, 1.6) | 0.64 | 0.00 | 0.02* | 0.09 |
| SFPF | 50.0 (6.9) | 50.0 (6.6) | 0.03 (2.9) | 51.3 (5.9) | 49.8 (6.4) | −1.5 (4) | 1.5 (−0.2,3.3) | 0.09 | 0.05 | 0.83 | 0.00 |
| SFBP | 50.5 (7.7) | 52.1 (7.4) | 1.6 (8.9) | 52.6 (8.8) | 49.9 (8.1) | −2.6 (11.1) | 4.2 (−0.9, 9.5) | 0.09 | 0.05 | 0.58 | 0.01 |
| SFSF | 52.3 (6.7) | 53.5 (5.7) | 1.17 (6.3) | 51.6 (6) | 49.9 (9.3) | −1.5 (9.6) | 2.6 (−1.5, 6.8) | 0.21 | 0.03 | 0.71 | 0.00 |
| SBP | 145.9 (15.9) | 147.7 (16.7) | 1.8 (16.2) | 140.2 (21.9) | 150.9 (25.1) | 10.7 (14.5) | 8.8 (−0.9, 16.8) | 0.03* | 0.08 | 0.47 | 0.01 |
| DBP | 83.6 (11.9) | 84 (12.9) | 0.3 (10.9) | 80.1 (13.7) | 81.9 (12.1) | 1.83 (10) | 1.5 (−3.9, 6.9) | 0.57 | 0.01 | 0.35 | 0.02 |
Abbreviations: COA, COAST incongruent score; CR, coin rotation; DBP, diastolic blood pressure; DSBT, Digit Span Backward Test; DSF, digit span forward; DSTot, digit span total; GAI, Geriatric Anxiety Inventory; GDS, Geriatric Depression Scale; RA, RBANS Attention; RDM, RBANS Delayed memory; RIM, RBANS Immediate memory; RL, RBANS Language; RTot, RBANS Total score; RVC, RBANS Visuospatial/Constructional; SBP, systolic blood pressure; SFBP, SF‐36 bodily pain; SFPF, SF‐36 physical functioning; SFSF, SF‐36 social functioning; TMTB, Trial Making Test B.
Values are means; SDs in parentheses.
Values are means; 95% CIs in parentheses.
p values were derived by using ANCOVA (adjusted for age, gender and years of education) with APOE as an independent variable to test APOE ɛ4 x treatment interaction. None of the analyses violated Levene's test of equality of error variances.
*p < 0.05.
4.4. Secondary outcome measures
The secondary outcomes were the change in physiological measurements and fatty acid profiles from baseline to 12 months, and the moderating effect of the ɛ4 allele on cognitive and wellbeing scores. Table 2 shows that there was a significant treatment effect on systolic blood pressure, where systolic blood pressure increased more in the placebo group over the 12‐month period than in the DHA group F(1, 55) = 4.97, p = 0.03, ƞ 2 = 0.08.
There was a significant effect for APOE ɛ4 x treatment on GDS and GAI scores, see Figures 2 and 3. GDS scores significantly improved in APOE ɛ4 carriers in the DHA group compared to ɛ4 carriers in the placebo group F(1, 53) = 4.16, p = 0.04, ƞ 2 = 0.07, and GAI scores significantly improved in ɛ4 carriers in the DHA group compared to ɛ4 carriers in the placebo group F(1, 53) = 5.41, p = 0.02, ƞ 2 = 0.09. GDS and GAI scores were correlated (r = 0.56, p < 0.01) across all participants. There were no other ɛ4 x treatment effects on the cognitive and well‐being measures. Overall, RBANS immediate memory scores improved significantly more in non‐ɛ4 carriers than in ɛ4 carriers F(1, 54) = 4.25, p = 0.04, ƞ 2 = 0.07, and RBANS delayed memory score improved significantly more in non‐ɛ4 carriers than in ɛ4 carriers F(1, 54) = 6.03, p = 0.02, ƞ 2 = 0.10.
FIGURE 2.
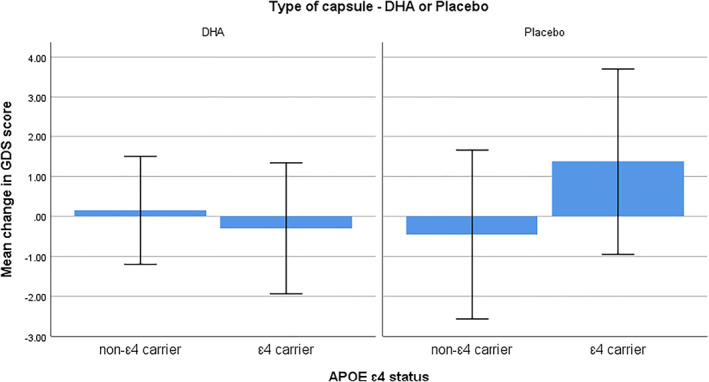
Mean change in Geriatric Depression Scale scores from baseline to 12 months in apolipoprotein E ɛ4 carriers and non‐ɛ4 carriers. Values are means with 1 standard deviation represented by vertical bars. A decrease in score indicates an improvement
FIGURE 3.
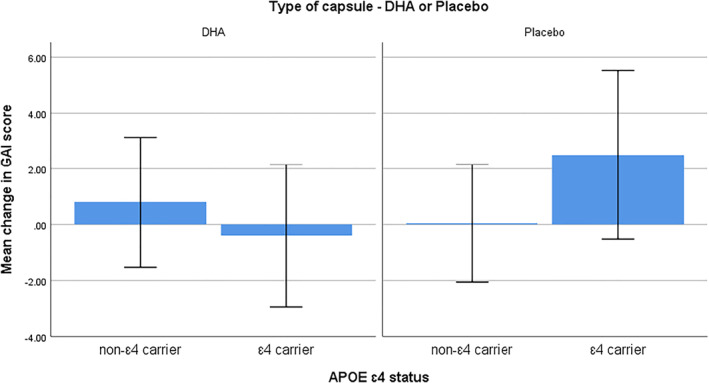
Mean change in Geriatric Anxiety Inventory scores from baseline to 12 months in apolipoprotein E ɛ4 carriers and non‐ɛ4 carriers. Values are means with 1 standard deviation represented by vertical bars. A decrease in score indicates an improvement
RBC fatty acid concentrations of EPA (d = 1.00), DHA (d = 1.76) and omega‐3 index levels (d = 1.72) increased in the DHA group compared to the placebo group (all p < 0.01), and both the ARA:EPA (d = 1.07) and ARA:DHA (d = 1.68) ratios decreased in the DHA group compared to the placebo group (both p < 0.01), see Table 3.
TABLE 3.
Change in PUFA concentrations of DHA and placebo groups from baseline to 12 months
| PUFA | DHA group (n = 30) | Placebo group (n = 30) | Difference | p | d | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Change | Baseline | 12 months | Change | DHA to placebo | |||
| LA a | 7.97 (1.06) b | 7.41 (1.04) | −0.55 (0.87) | 7.95 (1.32) | 7.78 (1.53) | −0.16 (0.99) | −0.39 (−0.87, −0.09) c | 0.12 | 0.42 |
| ALA | 0.00 | 0.00 | 0.00 | 0.08 (0.21) | 0.00 | −0.08 (0.21) | 0.08 (0.004, 0.15) d | ||
| ARA | 18.87 (1.64) | 13.32 (2.88) | −5.55 (2.91) | 19.99 (1.83) | 15.21 (3.78) | −4.78 (3.81) | −0.77 (−0.98, 2.52) | 0.38 | 0.20 |
| NA | 4.44 (0.84) | 5.54 (0.77) | 1.09 (1.02) | 4.40 (0.96) | 5.51 (0.70) | 1.11 (0.92) | 0.02 (−0.48, 0.52) | 0.94 | 0.19 |
| EPA | 1.00 (0.50) | 1.10 (0.49) | 0.09 (0.61) | 0.99 (0.44) | 0.56 (0.32) | −0.44 (0.47) | 0.54 (0.26, 0.82) | <0.01** | 1.00 |
| DPA | 1.92 (0.35) | 1.44 (0.43) | −0.48 (0.50) | 1.85 (0.31) | 1.54 (0.53) | −0.32 (0.65) | −0.16 (−1.14, 0.46) | 0.29 | 0.28 |
| DHA | 4.11 (1.01) | 6.24 (1.78) | 2.13 (1.87) | 4.06 (0.96) | 3.4 (1.36) | −0.66 (1.19) | 2.79 (1.97, 3.59) | <0.01** | 1.76 |
| ARA:EPA | 22.87 (9.47) | 12.59 (5.37) | −8.95 (10.30) | 24.07 (10.18) | 26.41 (7.95) | 2.32 (10.57) | −11.25 (−16.8, −5.73) | <0.01** | 1.07 |
| ARA:DHA | 4.81 (1.04) | 2.27 (0.70) | −2.54 (1.15) | 5.21 (1.40) | 5.03 (1.92) | −0.17 (1.63) | −2.33 (−3.09, −1.63) | <0.01** | 1.68 |
| Omega‐3 | 5.12 (1.29) | 7.34 (2.20) | 2.22 (2.29) | 5.06 (1.11) | 3.96 (1.56) | −1.10 (1.47) | 3.38 (2.39, 4.37) | <0.01** | 1.72 |
Note: ARA:EPA, ratio of arachidonic acid to eicosapentaenoic acid; ARA:DHA, ratio of arachidonic acid to docosahexaenoic acid; Omega‐3 = EPA + DHA/total % fatty acid.
Abbreviations: ALA, alpha‐linolenic acid; ARA, arachidonic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; LA, linoleic acid; EPA, eicosapentaenoic acid; NA, nervonic acid.
Fatty acid profiles were measured from red blood cells.
Values are means (as percentage of fatty acids); SDs in parentheses.
Values are means; 95% CIs in parentheses.
Unable to calculate due to zero values.
**p < 0.01.
4.5. Compliance, seafood consumption and fatty acid concentrations
Compliance rates at the end of the trial were on average 93% across the groups, with no significant difference between the DHA group and the placebo group t(58) = 1.27, p = 0.21, d = 0.32. This compliance was confirmed by changes in RBC fatty acids concentrations.
4.6. Blinding and adverse effects
Over 75% of participants stated they had ‘no idea’ which type of capsule they had been given through‐out the course of the 12‐month period, 66.6% (n = 20) from the DHA group and 86.6% (n = 26) from the placebo group. Two participants reported tasting an orange flavour (one DHA, one placebo). The capsules were well tolerated except for a one gastrointestinal adverse effect in the DHA group.
5. DISCUSSION
The trial did not find a significant effect for DHA supplementation on cognition in older adults with MCI despite a significant increase in DHA and EPA concentrations. The trial was designed to replicate the two trials by Sinn et al. and Lee et al., and to examine the moderating effect of the APOE ɛ4 allele. Sinn et al. found an improvement in GDS scores after 6 months, which the current trial failed to replicate, although the trial did find a moderating treatment effect from the ɛ4 allele. Lee et al. noted that participants from their trial were from a low socio‐economic background where consumption of foods high in n‐3 PUFAs were low due to financial constraints. Participants in Lee et al.‘s trial began with approximately half the DHA and EPA plasma levels of the baseline DHA and EPA RBC concentrations in the current trial. This supports the idea that participants with lower levels at baseline may benefit more from taking supplementation.
The current trial used similar cognitive measures to Sinn et al. and Lee et al., except Sinn et al. used an initial letter fluency task whereas in this trial semantic fluency was combined with picture naming into a RBANS language quotient score. Unfortunately, because reliability coefficients are higher for indexes than for subtests, the RBANS subtest scores could not be reported. Lee et al. found a large effect size using digit span total (ƞ p 2 = 0.25), which was not replicated in this trial. Lee et al. also found medium effect sizes for immediate verbal memory (ƞ p 2 = 0.11) and delayed recall (ƞ p 2 = 0.12) which were again not replicated in this trial. The MMSE was used in two of the MCI trials including the trial by Lee et al., and both found treatment effects. The issue with the MMSE as a cognitive measure is that it often results in a ceiling effect, especially in high functioning or highly educated older adults, whereas measures involving word lists and digit spans show no ceiling effects. 41 It may be more useful to include a pre‐morbid functioning task rather than a global scale such as the MMSE to gauge the baseline level functioning of participants.
Research indicates that measuring pre‐morbid functioning is more reliable than using years of education, because the quantity of education is not always an indication of quality as years of education can be influenced by many ‘non‐cognitive factors’ such as gender, income and ethnicity. 42 Lee et al. observed that participants were ‘mildly impaired’ implying the positive results were due to the level of cognitive impairment. The null result of the current trial is consistent with trials in cognitively healthy older adults. 9 , 10 , 11 , 12 This raises the question about whether the participants were sufficiently impaired and therefore less likely to benefit from the intervention. The mean IQ score of participants was high at 119, yet baseline scores for RBANS indexes averaged a quotient of 100 (considering the participants were a mix of amnestic and non‐amnestic MCI) indicating that overall the group were cognitively impaired. Research shows that people with high IQ scores tend to have a greater cognitive reserve which is the ability to compensate for brain damage and more efficiently make use of available brain networks. 43 It is unclear whether a greater cognitive reserve prevented a treatment effect. It would be helpful for further DHA trials to involve MRI scanning to account for this possibility.
The trial found that GDS and GAI scores improved in ɛ4 carriers in the DHA group, whereas GDS and GAI scores worsened in ɛ4 carriers in the placebo group. This result indicates that ɛ4 carriers may benefit from a DHA supplement with alleviating depression and anxiety symptoms. The link between genetics, and both depression and anxiety is well established. 44 Depression and carrying the ɛ4 allele are both associated with higher levels of inflammation, and therefore it is possible that the fish oil improved depression scores due to the interaction of these two factors. The DHA group showed a smaller increase (a stabilising effect) in systolic blood pressure than the placebo group. The mechanisms by which n‐3 PUFAs reduce blood pressure are via prostaglandins which cause vasodilation, anti‐platelet aggregation, and control sodium and water retention. 45 A meta‐analysis of 31 fish oil RCTs found that reduction in blood pressure was dose‐responsive and was strongest in those with hypertension. 46 In the current trial the mean baseline systolic blood pressure was 143 and the mean diastolic blood pressure was 81, which supports these findings. Over half of participants were taking hypertension medication, and yet those who took the DHA supplement had a smaller increase in systolic blood pressure over the 12‐month period, which suggests that fish oil may provide additional benefit alongside other treatments.
One possible explanation for the difference in results could be that the DHA group only showed a 51.8% increase in DHA RBC concentrations, whereas Sinn et al. found a 90.1% increase in DHA RBC levels in the DHA group and Lee et al. a 83.7% increase in plasma DHA levels. It is unclear why the increase was so small in comparison, considering the high dose of DHA and similar compliance percentages. The difference may be due to impaired digestion or some other factor such as inadequate levels of other nutrients such as vitamin B which is required for n‐3 PUFAs to be incorporated into cells. 30 It is also unclear why ARA levels decreased in the placebo group since LA is a precursor to ARA, although LA conversion to ARA has been shown to minimal in human adults. 47 , 48 Despite a high dose of LA, the placebo group showed a decrease in LA concentrations, which suggests that LA may have been converted to fatty acids other than ARA or it could reflect deposition in tissues undetected by RBC analysis. Baseline DHA concentrations in both the current trial and in Sinn et al. were higher than most Western countries indicating the participants had a high dietary intake. Whether this can explain why neither trial found an effect on memory is unclear.
Why the results from clinical trials have been inconsistent is of interest, especially when epidemiological evidence is strong. The only treatment effect detected in the current trial was in systolic blood pressure, although the results showed a trend towards an improvement in SF‐36 bodily pain scores (p = 0.09, ƞ 2 = 0.05) in the DHA group. DHA may work via homeostatic mechanisms, and in this case, the n‐3 PUFAs were used to repair damage within the cardiovascular and musculoskeletal systems rather than within the neuronal system. A previous trial found that in healthy older adults, fish oil supplementation resulted in significant increases in fractional anisotropy, mean diffusivity and radial diffusivity, an indication of superior white matter structural integrity. 14 The trial also found that the supplement increased grey matter regional volume whereas a trial in AD found no changes in total brain volume using MRI. 16 This suggests that supplementation may result in structural and functional changes within the neuronal system, but only at the pre‐pathological stage. It is possible that a regular intake of EPA and DHA could help to strengthen neuronal pathways as a result of normal wear and tear, but not more severely damaged neuronal pathways. It is clear that genetic factors play a part in the physical and cognitive ageing process, although it is unclear whether EPA and DHA improve cognitive outcomes via genes or by targeting specific areas of the brain (and therefore specific cognitive domains), or via broad‐spectrum mechanisms such as reducing inflammation, and by improving synaptic transmission and cerebral flow. Understanding how EPA and DHA work to improve cognition needs to be explored further to understand who may benefit the most from taking a fish oil supplement.
The current trial had several limitations. The lack of educational norms for the RBANS was a major limitation, especially given that the participant group was highly educated and showed high pre‐morbid functioning. As a result, it was difficult to gauge the exact level of cognitive impairment of the participant group, which is important as it has been suggested that the negative results from trials could be due to participants not being impaired enough or having a high cognitive reserve. Participants in the trial were a combination of amnestic and non‐amnestic MCI due to low recruitment numbers, so including only one MCI subgroup and therefore using a memory test as a cut‐off for eligibility, should be considered for future trials. This variability of memory deficits at baseline may have contributed to the lack of treatment effect considering previous DHA trials in older adults have mostly found positive results in the areas of memory and attention.
6. CONCLUSION
The trial found no evidence of a treatment effect on cognition, although did find a treatment interaction for APOE ɛ4 carriers on GDS and GAI scores in favour of DHA. This result indicates that ɛ4 carriers may benefit from taking an EPA and DHA supplement to alleviate depression and anxiety symptoms. Failure to replicate the results from Sinn et al. and Lee et al. may be due to differences in baseline concentrations of EPA and DHA, or due to the severity or type of cognitive impairment or a greater cognitive reserve. Future studies should consider utilising brain imaging techniques, pre‐morbid testing and isolating by MCI subtypes to account for these discrepancies.
CONFLICT OF INTEREST
The authors have no actual or potential conflicts of interest. Dr. Mengelberg reports grants from the Neurological Foundation, Oakley Mental Health Foundation, HOPE Selwyn Foundation scholarships for Research on Ageing, and Massey University Research Fund during the conduct of the study. Dr. Leathem has nothing to disclose. Dr. Podd has nothing to disclose. Dr. Hill has nothing to disclose. Dr. Conlon has nothing to disclose.
ETHICS STATEMENT
The trial was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving participants were approved by the New Zealand Central Health and Disability Ethics Committee (14/CEN/148). Written informed consent was obtained from all participants. The trial was registered with the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au; request number: 366439).
AUTHOR CONTRIBUTIONS
Alexia Mengelberg was in charge of designing and conducting the trial, performing the statistical analysis, and had primary responsibility for the content of the manuscript. Janet Leathem was involved in designing the trial, analysing data and revising the manuscript. John Podd was involved in designing the trial, statistical analysis and revising the manuscript. Stephen Hill was involved in the statistical analysis and revising the manuscript. Cathryn Conlon was involved in designing the trial and revising the manuscript.
ACKNOWLEDGEMENTS
This work was supported by funding from the Oakley Mental Health Research Foundation, the Neurological Foundation, HOPE Selwyn Foundation scholarships for Research on Ageing, and a Massey University Research Fund. These funders had no role in the design, analysis or writing of the manuscript. No grant numbers were assigned to the funding.
Open access publishing facilitated by Massey University, as part of the Wiley ‐ Massey University agreement via the Council of Australian University Librarians.
Mengelberg A, Leathem J, Podd J, Hill S, Conlon C. The effects of docosahexaenoic acid supplementation on cognition and well‐being in mild cognitive impairment: a 12‐month randomised controlled trial. Int J Geriatr Psychiatry. 2022;1‐12. 10.1002/gps.5707
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303‐308. [DOI] [PubMed] [Google Scholar]
- 2. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240‐246. [DOI] [PubMed] [Google Scholar]
- 3. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population‐based perspective. Alzheimers Dement. 2015;11:718‐726. [DOI] [PubMed] [Google Scholar]
- 4. Bourre J, Paquotte P. Seafood (wild and farmed) for the elderly: contribution to the dietary intakes of iodine, selenium, DHA and vitamins B12 and D. J Nutr Health Aging. 2008;12:186‐192. [DOI] [PubMed] [Google Scholar]
- 5. Dangour A, Allen E, Elbourne D, Fletcher A, Richards M, Uauy R. Fish consumption and cognitive function among older people in the UK: baseline data from the OPAL study. J Nutr Health Aging. 2009;13:198‐202. [DOI] [PubMed] [Google Scholar]
- 6. Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849‐1853. [DOI] [PubMed] [Google Scholar]
- 7. Valenzuela A. Docosahexaenoic acid (DHA), an essential fatty acid for the proper functioning of neuronal cells: their role in mood disorders. Int J Fats Oils. 2009;60:203‐212. [Google Scholar]
- 8.van de; Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71:430‐438. [DOI] [PubMed] [Google Scholar]
- 9. Dangour AD, Allen E, Elbourne D, et al. Effect of 2‐y n−3 long‐chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double‐blind, controlled trial. Am J Clin Nutr. 2010;91:1725‐1732. [DOI] [PubMed] [Google Scholar]
- 10. Danthiir V, Hosking DE, Nettelbeck T, et al. An 18‐mo randomized, double‐blind, placebo‐controlled trial of DHA‐rich fish oil to prevent age‐related cognitive decline in cognitively normal older adults. Am J Clin Nutr. 2018;107:754‐762. [DOI] [PubMed] [Google Scholar]
- 11. Moran C, di Palumbo AS, Bramham J, et al. Effects of a six‐month multi‐ingredient nutrition supplement intervention of omega‐3 polyunsaturated fatty acids, vitamin D, resveratrol, and whey protein on cognitive function in older adults: a randomised, double‐blind, controlled trial. J Prev Alzheimers Dis. 2018;5:175‐183. [DOI] [PubMed] [Google Scholar]
- 12. Nilsson A, Radeborg K, Salo I, Björck I. Effects of supplementation with n‐3 polyunsaturated fatty acids on cognitive performance and cardiometabolic risk markers in healthy 51 to 72 years old subjects: a randomized controlled cross‐over study. Nutr J. 2012;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Witte AV, Kerti L, Hermannstädter HM, et al. Long‐chain omega‐3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2013;24:3059‐3068. [DOI] [PubMed] [Google Scholar]
- 14. Külzow N, Witte AV, Kerti L, et al. Impact of omega‐3 fatty acid supplementation on memory functions in healthy older adults. J Alzheimers Dis. 2016;51:713‐725. [DOI] [PubMed] [Google Scholar]
- 15. Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freund‐Levi Y, Eriksdotter‐Jönhagen M, Cederholm T, et al. ω‐3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double‐blind trial. Arch Neurol. 2006;63:1402‐1408. [DOI] [PubMed] [Google Scholar]
- 17. Kotani S, Sakaguchi E, Warashina S, et al. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res. 2006;56:159‐164. [DOI] [PubMed] [Google Scholar]
- 18. Chiu C‐C, Su K‐P, Cheng T‐C, et al. The effects of omega‐3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double‐blind placebo‐controlled study. Progr Neuropsychopharmacol Biol Psychiatry. 2008;32:1538‐1544. [DOI] [PubMed] [Google Scholar]
- 19. Yurko‐Mauro K, McCarthy D, Rom D, et al. Beneficial effects of docosahexaenoic acid on cognition in age‐related cognitive decline. Alzheimers Dementia. 2010;6:456‐464. [DOI] [PubMed] [Google Scholar]
- 20. Vakhapova V, Cohen T, Richter Y, Herzog Y, Korczyn AD. Phosphatidylserine containing ω–3 fatty acids may improve memory abilities in non‐demented elderly with memory complaints: a double‐blind placebo‐controlled trial. Dement Geriatr Cogn Disord. 2010;29:467‐474. [DOI] [PubMed] [Google Scholar]
- 21. Mazereeuw G, Lanctot K, Chau S, et al. Effects of omega‐3 fatty acids on cognitive performance: a meta‐analysis. Neurobiol Aging. 2012;33:1482.e17‐1482.e29. [DOI] [PubMed] [Google Scholar]
- 22. Sinn N, Milte CM, Street SJ, et al. Effects of n‐3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6‐month randomised controlled trial. Br J Nutr. 2012;107:1682‐1693. [DOI] [PubMed] [Google Scholar]
- 23. Lee L, Shahar S, Chin AV, Yusoff NAM. Docosahexaenoic acid‐concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): a 12‐month randomised, double‐blind, placebo‐controlled trial. Psychopharmacology. 2013;225:605‐612. [DOI] [PubMed] [Google Scholar]
- 24. Rondanelli M, Opizzi A, Faliva M, et al. Effects of a diet integration with an oily emulsion of DHA‐phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr Neurosci. 2012;15:46‐54. [DOI] [PubMed] [Google Scholar]
- 25. Yacong B, Xueyuan Z, Youli W, et al. The n‐3 polyunsaturated fatty acids supplementation improved the cognitive function in the Chinese elderly with mild cognitive impairment: a double‐blind randomized controlled trial. Nutrients. 2017;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arellanes IC, Choe N Solomon V. Brain delivery of supplemental docosahexaenoic acid (DHA): a randomized placebo‐controlled clinical trial. EBioMedicine. 2020;59:102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barberger‐Gateau P, Samieri C, Feart C, et al. Dietary omega 3 polyunsaturated fatty acids and Alzheimer’s disease: interaction with apolipoprotein E genotype. Curr Alzheimer Res. 2011;8:479‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187‐222. [DOI] [PubMed] [Google Scholar]
- 29. Chhetri JK, de Souto Barreto P, Cantet C, et al. Trajectory of the MAPT‐PACC‐preclinical Alzheimer cognitive composite in the placebo group of a randomized control trial: results from the MAPT study: lessons for further trials. J Prev Alzheimers Dis. 2018;5:31‐35. [DOI] [PubMed] [Google Scholar]
- 30. Berlau DJ, Corrada MM, Head E, et al. APOE ε2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72:829‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183‐194. [DOI] [PubMed] [Google Scholar]
- 32. Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Psychological Corporation; 1998. [DOI] [PubMed] [Google Scholar]
- 33. Nelson H. The Nelson Adult Reading Test (NART) manual. Windsor, England. NFER‐Nelson. 1982;530:531. [Google Scholar]
- 34. Mendoza JE, Apostolos GT, Humphreys JD, et al. Coin Rotation Task (CRT): a new test of motor dexterity. Arch Clin Neuropsychol. 2009;24:287‐292. [DOI] [PubMed] [Google Scholar]
- 35. Pachana NA, Thompson LW, Marcopulos BA, et al. California Older Adult Stroop Test (COAST) development of a Stroop test adapted for geriatric populations. Clin Gerontol. 2004;27:3‐22. [Google Scholar]
- 36. US Army . Army Individual Test Battery. Manual of Directions and Scoring. War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 37. Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. Psychological Corporation; 1997. [Google Scholar]
- 38. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol J Aging Ment Health. 1986;5:165‐173. [Google Scholar]
- 39. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19:103‐114. [DOI] [PubMed] [Google Scholar]
- 40. Ware JE, Jr , Gandek B. The SF‐36 Health Survey: development and use in mental health research and the IQOLA Project. Int J Ment Health. 1994;23:49‐73. [Google Scholar]
- 41. Bartels C, Wegrzyn M, Wiedl A, et al. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahl RE, Beiser A, Seshadri S, et al. Defining MCI in the Framingham Heart Study Offspring: education vs. WRAT‐based norms. Alzheimer Dis Assoc Disord. 2013;27:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tucker MA, Stern Y. Cognitive reserve in aging. Curr Alzheimer Res. 2011;8:354‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta‐analysis. Am J Psychiatry. 2000;157:1552‐1562. [DOI] [PubMed] [Google Scholar]
- 45. Das UN. Nutritional factors in the pathobiology of human essential hypertension. Nutrition. 2001;17:337‐346. [DOI] [PubMed] [Google Scholar]
- 46. Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta‐analysis of controlled trials. Circulation. 1993;88:523‐533. [DOI] [PubMed] [Google Scholar]
- 47. Nichaman MZ, Olson RE, Sweeley CC. Metabolism of linoleic acid‐1‐14C in normolipemic and hyperlipemic humans fed linoleate diets. Am J Clin Nutr. 1967;20:1070‐1083. [DOI] [PubMed] [Google Scholar]
- 48. Liou YA, Innis SM. Dietary linoleic acid has no effect on arachidonic acid, but increases n‐6 eicosadienoic acid, and lowers dihomo‐γ‐linolenic and eicosapentaenoic acid in plasma of adult men. Prostagl Leukot Essent Fat Acids. 2009;80:201‐206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


