Abstract
Objective
Care for fear of cancer recurrence (FCR) is considered the most common unmet need among cancer survivors. Yet the prevalence of FCR and predisposing factors remain inconclusive. To support targeted care, we provide a comprehensive overview of the prevalence and severity of FCR among cancer survivors and patients, as measured using the short form of the validated Fear of Cancer Recurrence Inventory (FCRI‐SF). We also report on associations between FCR and clinical and demographic characteristics.
Methods
This is a systematic review and individual participant data (IPD) meta‐analysis on the prevalence of FCR. In the review, we included all studies that used the FCRI‐SF with adult (≥18 years) cancer survivors and patients. Date of search: 7 February 2020. Risk of bias was assessed using the Joanna Briggs Institute critical appraisal tool.
Results
IPD were requested from 87 unique studies and provided for 46 studies comprising 11,226 participants from 13 countries. 9311 respondents were included for the main analyses. On the FCRI‐SF (range 0–36), 58.8% of respondents scored ≥13, 45.1% scored ≥16 and 19.2% scored ≥22. FCR decreased with age and women reported more FCR than men. FCR was found across cancer types and continents and for all time periods since cancer diagnosis.
Conclusions
FCR affects a considerable number of cancer survivors and patients. It is therefore important that healthcare providers discuss this issue with their patients and provide treatment when needed. Further research is needed to investigate how best to prevent and treat FCR and to identify other factors associated with FCR.
The protocol was prospectively registered (PROSPERO CRD42020142185).
Keywords: cancer, correlates, fear of recurrence, oncology, prevalence
1. BACKGROUND
Due to aging and improved diagnostic and treatment potential, the number of people living with and beyond cancer is rapidly increasing. 1 In 2018, the estimated number of cancer survivors diagnosed within the last five years was 43.8 million. 2 For this growing group, managing fear of cancer recurrence (FCR) has been reported as one of the most important unmet needs. 3 , 4 , 5 FCR is defined as “fear, worry, or concern relating to the possibility that cancer will come back or progress”. 6 Low levels of FCR can be helpful by promoting treatment compliance and healthy lifestyle adaptations. However, at clinical levels, FCR can limit quality of life and daily functioning and require professional help. 7 , 8 , 9 , 10 , 11 , 12 A 2019 Delphi study conceptualized four features as key characteristics of clinical FCR: "(a) high levels of preoccupation; (b) high levels of worry; (c) that are persistent; and (d) hypervigilance to bodily symptoms". 13 It is important to address FCR, because FCR may also lead to increased healthcare costs 14 and for most patients, it does not decrease over time without intervention. 3 , 7 , 11 , 15 , 16 Furthermore, several effective interventions to treat FCR have been developed. 17
In order to shape future healthcare provision, policy and research on FCR, it is crucial to know the prevalence and severity of FCR for the general cancer population and for different subgroups. This will help to estimate the burden of FCR and to target the type and intensity of interventions for those in need. Unfortunately, the precise prevalence of FCR remains unknown and estimates are wide ranging and inconclusive. For example, in a systematic review by Simard et al. (2013) studies found prevalences of 39%–97% for any level of FCR, 22%–87% for 'moderate to high' FCR and 0%–15% for ‘high' FCR. 3 Notably, part of this heterogeneity is caused by different studies using different scales. In the literature, the most commonly used measure of FCR is the Fear of Cancer Recurrence Inventory (FCRI). 18 Still, the comparability of studies is complicated by the use of different cut‐off scores across studies, namely 13, 16 and 22. 10 , 19 Scoring ≥13 indicates the possibility of clinical level FCR, scoring ≥16 indicates the likely presence of clinical level FCR and scoring ≥22 indicates a clinical severity of FCR that needs specialized intervention. 10 , 19
Several potential risk factors for FCR have been investigated. Predictive evidence is strongest for the presence of physical symptoms such as fatigue and pain, 3 sex, with women reporting higher levels of FCR than men, 20 and age, with younger patients more likely to report FCR than older patients. 3 , 9 , 21 However, the results of a recent review showed that the strength of the latter association decreased over the last decade. 22 Associations with other factors such as sleep quality, cancer type, and time since cancer diagnosis or treatment have also been investigated, but have yielded inconclusive results. 3 , 23
A recent meta‐analysis of FCRI results found that 53.9% of cancer survivors and patients scored above the ≥13 cut‐off, 43.3% above the ≥16 cut‐off, and 30% above the ≥22 cut‐off on the FCRI severity subscale. 23 In this meta‐analysis, only the cut‐offs reported in the individual articles could be considered and studies reporting different cut‐offs could not be analyzed together. For example, studies reporting only the ≥13 cut‐off could not be analyzed together with studies reporting only the ≥22 cut‐off. Also, the meta‐analysis included studies that selected patients based on their level of FCR, and thus does not reflect the general cancer population. To obtain more precise estimates of the prevalence of FCR, we have conducted a systematic review and individual participant data (IPD) meta‐analysis. In IPD analyses, researchers from each study are asked to share the original research data, so that these data can be combined and re‐analyzed. Using IPD analyses, we could look at all cut‐offs for all provided study data, unrestricted by the cut‐offs reported by the authors of the individual studies. Also, we were able to conduct subgroup analyses that would not be possible with smaller sample sizes. Our main aim was to provide a comprehensive overview of the prevalence and severity of FCR among cancer survivors [no active cancer present] and patients [active cancer present] and to identify associations with clinical and demographic characteristics. In addition, we report the clinical and demographic characteristics of groups with different levels of FCR severity.
2. METHODS
A systematic review and IPD meta‐analysis on the prevalence of FCR was conducted. The research plan was developed in collaboration with an international board of experts (the ‘advisory board’) who have specialized in psycho‐oncology (AS, GH, NK, RZ, SL, SS, WL) and published in advance on the Open Science Framework * (OSF) and Prospero (CRD42020142185).
2.1. Selection of variables
Several tools to measure FCR 3 , 24 have been developed. The FCRI was selected to assess the main outcome because it has good psychometric properties, is widely used, and is available in 10 different languages, 18 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 increasing sample diversity. The FCRI includes seven subscales: FCR severity, coping, functioning impairments, triggers, psychological distress, insight, and reassurance. The severity subscale (range 0–36) is widely used as a short form of the FCRI (FCRI‐SF) and was also used as the primary outcome in this study, because the total score includes several aspects other than severity. 23 It contains nine items (range 0–4), for example, “I am afraid of cancer recurrence”, “I believe it is normal to be worried or anxious about the possibility of cancer recurrence” and “How much time per day do you spend thinking about the possibility of cancer recurrence?”. Using the FCRI‐SF allowed for the inclusion of studies that collected data using only this subscale and not the total scale. If repeated measures were available, only baseline data were included. Since the different cut‐offs represent different levels of FCR severity (see introduction), we examined all three cut‐offs in this study.
In this study we distinguish between people who have active disease and those who no longer have active disease, by stratifying the results by these groups and calling them patients and survivors, respectively.
In collaboration with the advisory board and based on clinical experience and literature, we identified variables that we expected could correlate with FCR, would be clinically relevant, and for which we expected many studies to have collected data. The following variables were selected for inclusion in the study: age, sex, time since cancer diagnosis, cancer type, and continent where the study was conducted.
2.2. Eligibility criteria
Data from all participants from all studies that used the FCRI‐SF from adult (≥18 years) cancer survivors and patients were eligible. Data from studies that selected patients based on the severity of their FCR were not included in the main outcome analyses, but were included for the analyses of the characteristics of groups with different levels of FCR.
2.3. Search and selection strategy
PubMed, MEDLINE, PsycINFO, Embase, EMcare, CINAHL and Scopus were searched on 7 Feb 2020 using the following terms:
“Fear of cancer recurrence inventory”
“FCRI" AND (fear OR worry OR concern OR anxiety)
Since the FCRI has only existed since 2009, 18 there was no time restriction. A forward search was done using all articles describing the development of a new translation of the FCRI. We expected that studies that use a questionnaire would always reference the article describing its development. Therefore, we expected this forward search would allow us to find all articles that used the FCRI.
Corresponding authors of eligible articles who were approached to share their data were also asked if they had additional published or unpublished datasets using the FCRI (e.g., from screening patients prior to including only those with a certain level of FCR in a study). These datasets were included if the data were of high quality (e.g., systematically obtained and recorded) and sufficient information was available about recruitment, sampling, and data collection method.
The records identified in the searches were screened based on their titles and abstracts. Potentially eligible records were full text screened. If upon reading the full article, there was any doubt about whether the authors had collected data using the FCRI, authors were contacted. This includes protocol papers that stated they were intending to use the FCRI. Studies that included only part of the FCRI‐SF were not included.
The screening was done by two independent reviewers (YL and NT), using Covidence, a software system for managing systematic reviews (www.covidence.org).
2.4. Quality assessment
To evaluate risk of bias, two researchers (YL and NT) independently assessed each study using the Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data. Four out of nine domains were omitted due to lack of relevance for the present study. The domains that were used addressed the sample frame, the sampling method, the sample size, the description of subjects and setting and the response rate. For each domain, the researchers judged whether there was a risk of bias in answering the research question of the current study. Based on the available information in the published articles, they chose between "Yes", "No" and "Unclear". The risk of bias assessment is presented in Appendix A.
Domain 1 assessed the sample frames of the studies. Studies that excluded participants who score below one of the cut‐offs on the FCRI‐SF (e.g. RCTs on FCR interventions, requiring participants to have a certain level of FCR) do not reflect the general cancer population and were excluded for the analyses for the main outcome, due to a high risk of bias. Similarly, a study that excluded patients with sleeping disorders, which could correlate with FCR, was excluded for the main analyses. These studies, with a risk of bias on domain 1, were only used to describe the characteristics of groups with different levels of FCR (see appendix D). In these analyses, comparisons are made within rather than across FCR severity groups, eliminating this risk of bias. For domain 3, sample sizes below 30 were considered a risk of bias. For domain 5, a response rate of less than 50% was considered a risk of bias. These cut‐offs were selected in collaboration with the advisory board.
2.5. Collection of individual participant data
Corresponding authors of all eligible studies were contacted via e‐mail and asked whether they would be willing to share their data. Every author was reminded at least twice, when there was no response after 2 weeks. If there was still no response another author was approached to request the data.
Authors were asked to provide the following information: participants' eligibility criteria, recruitment methods, and definitions of survivors and patients used in the study. Authors were also asked to report any changes made to the original FCRI and whether times since diagnosis and end of curative treatment were obtained from medical record or from patient reporting. If available, authors were asked to share their study protocol. Finally, authors were asked to check their ethical protocols to ensure sharing individual data was permitted.
2.6. Statistical methods
All outcomes were predetermined in the protocol and published on PROSPERO and OSF. A one‐stage approach was used for all analyses. All outcomes were reported separately for cancer survivors and patients. All analyses were performed in R. 35
The primary study outcome was the prevalence of FCR. Prevalence of FCR per sex, age group (18–29, 30–44, 45–59, 60–74, ≥75), cancer type, time since cancer diagnosis (0–1 year, 2–5 years, 6–10 years, >10 years) and continent where the study was conducted were also reported. Prevalence estimates were reported as percentages of people scoring below, between, and above the various cut‐offs on the FCRI‐SF. Additionally, mean scores and confidence intervals were reported. When calculating mean scores, clustering effects per dataset were accounted for by adding a random intercept per study. 36
Second, associations between FCR severity and sex, age, cancer type, time since cancer diagnosis and continent where the study took place were assessed using multilevel regression analysis with fixed effects for all variables and a random intercept per study.
Finally, the characteristics of respondents with different levels of FCR were described. The number and percentage of people within each FCR severity category (<13, 13–15, 16–21, ≥22) who have the characteristics measured in this study (e.g., age, sex) were reported. Studies that screened on level of FCR prior to inclusion were included only for these analyses.
In order to compare the results of our IPD analysis to the results of the studies that did not provide IPD, we performed an aggregate data analysis. Two independent reviewers (YL and a research assistant) extracted the mean FCRI‐SF score and/or the percentage scoring ≥13, depending on what information was reported in the articles.
2.7. Missing data
If researchers had applied imputation, they were asked to provide the imputed datasets. Still, almost all received datasets had missing data. In the combined dataset used for the main analyses, there was a total of 2.8% missing data. We therefore applied multilevel imputation using jomoImpute to impute both sporadic and systematic missing data. Multilevel imputation has been shown to lead to better outcomes than complete case analysis and traditional multiple imputation. 37 It can also be applied to both linear and non‐linear variables and even when some variables are entirely missing from some datasets. 37
It was not possible to impute all variables for all participants at the same time. Therefore, for the prevalence and severity calculations, variables were imputed separately, to include as many participants as possible. Still, for some variables, the imputations did not converge and the unimputed data was used. For the multilevel regression analysis, data of survivors and patients were imputed separately, in order to impute as many variables as possible. As a result, participants without a known patient or survivor status, including two entire datasets, were excluded from these analyses. For patients, we imputed the categorical "time since cancer diagnosis" variable, since the imputation with the continuous variable did not converge. For survivors, neither the categorical nor the continuous time since cancer diagnosis variable converged. Therefore, participants without this variable could not be included in the analyses.
3. RESULTS
The database searches revealed 746 studies. After duplicates were removed, 280 abstracts were screened, and 203 papers were screened in full‐text, resulting in final inclusion of 154 papers (87 unique studies; see Figure 1). There were 24 differences (0.92 agreement) between reviewers during the abstract screening and 9 (0.95 agreement) during the full text screening. All were easily resolved through discussion.
FIGURE 1.
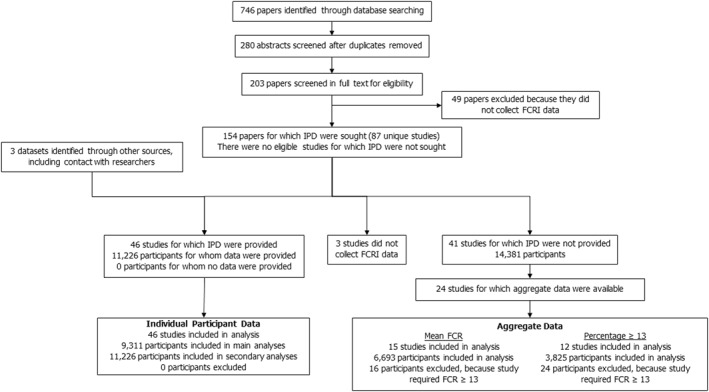
Flowchart of studies identified, screened, and included with individual participant data (IPD) or aggregate data
Authors of the 87 included studies were contacted to request participation in the IPD study and to provide data. Authors of 43 studies accepted and shared their datasets. In addition, 3 other unpublished datasets were provided by these authors. In total, data from 46 independent studies (11,226 participants) 15 , 16 , 18 , 25 , 26 , 27 , 28 , 29 , 32 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 were included in the IPD meta‐analysis. No important issues were identified in checking IPD.
For the remaining 44 studies, no data could be included. Three studies did not collect data using the FCRI. Reasons for not including the other 41 were: the author did not respond (n = 12), the author did not follow‐up after initial contact (n = 8), the university did not give permission (n = 7), the ethics committee did not give permission (n = 5), the data were not yet published (n = 5), the authors did not have time to participate (n = 3), and there were no contact details on the article (n = 1). Notably, the data were requested during the COVID‐19 pandemic, which may have impacted authors' opportunities to share data.
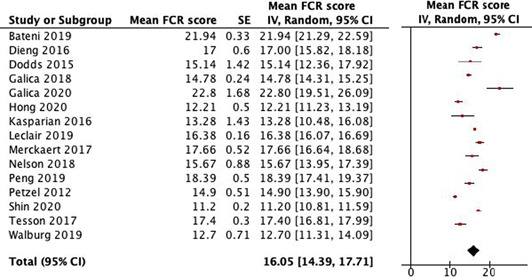
For 24 studies for which IPD was not available, aggregate data could be obtained from the articles. Fifteen studies reported data on the mean FCR score 33 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 ; and 12 studies reported data on the percentage scoring ≥13. 73 , 76 , 78 , 82 , 83 , 86 , 87 , 88 , 89 , 90 , 91 , 92 The other studies reported neither outcome.
3.1. Quality assessment
The outcomes of the risk of bias assessment for both the IPD and the aggregate data analyses are presented in Appendix B. For the studies that provided IPD, there were 14 differences (0.94 agreement) in risk of bias ratings between reviewers. All were easily resolved through discussion.
For the studies that did not select participants on FCR severity, the overall risk of bias was low (Appendix B, Figure 1). There were some concerns about the sampling method (domain 2) and the response rate (domain 5). Risk of bias on domain 2 was mostly due to studies' main topic being FCR, which could lead to selection bias. People who experience FCR may be more likely to participate in studies on FCR than people who do not experience FCR, because the topic interests them, though it is also possible that patients with high FCR may be reluctant to join the study as they may want to avoid the topic. The risk of bias assessment did not lead to exclusion of any studies.
3.2. Prevalence of fear of cancer recurrence
Overall, in the IPD analysis (n = 9311), 58.8% of participants scored ≥13, 45.1% scored ≥16 and 19.2% scored ≥22 on the FCRI‐SF. The distributions were similar for survivors and patients (see Table 1).
TABLE 1.
The prevalence of fear of cancer recurrence (FCR) for survivors and patients according to cut‐offs on the Fear of Cancer Recurrence Inventory (FCRI‐SF), using imputed data
| <13 | 13–15 | 16–21 | ≥22 | |
|---|---|---|---|---|
| Cancer survivors n (%) | 2960 (41.1) | 946 (13.2) | 1867 (26.0) | 1417 (19.7) |
| Cancer patients n (%) | 878 (41.4) | 325 (15.3) | 547 (25.8) | 371 (17.5) |
| Total | 3838 (41.2) | 1271 (13.7) | 2414 (25.9) | 1788 (19.2) |
The percentages of the subgroups that scored below, between and above the different FCRI‐SF cut‐off scores are presented in Table 2. Survivors and patients follow a similar pattern. For survivors, 46% of men scored ≥13 and 12% scored ≥22, compared with 64% and 28% of women. In the youngest age category (18–29 years) 88% of survivors scored ≥13 and 48% scored ≥22, compared with 37% and 9% in the highest age category (≥75 years), respectively. Some differences between cancer types were observed. For example, for prostate cancer 37% of survivors scored ≥13, for endometrial cancer 39% and for colorectal cancer 50% compared with 82% for thyroid cancer and 80% for leukemia & non‐Hodgkin lymphoma. For time since cancer diagnosis, in all categories approximately 60% of survivors scored ≥13 and approximately 20% scored ≥22. There were also no major differences between the continents, though respondents from studies conducted in Asia scored somewhat lower.
TABLE 2.
The prevalence of fear of cancer recurrence (FCR) according to Fear of Cancer Recurrence Inventory (FCRI‐SF) cut‐offs, stratified by clinical and demographic characteristics
| Survivors n (%) | Patients n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| <13 | 13–15 | 16–21 | ≥22 | <13 | 13–15 | 16–21 | ≥22 | |
| Sex | ||||||||
| Men | 1133 (54) | 271 (13) | 446 (21) | 259 (12) | 343 (51) | 103 (15) | 153 (23) | 79 (12) |
| Women | 1828 (36) | 675 (13) | 1421 (28) | 1158 (23) | 535 (37) | 222 (15) | 394 (27) | 291 (20) |
| Age groups | ||||||||
| 18–29 years | 22 (12) | 12 (6) | 54 (29) | 95 (52) | 5 (20) | 3 (13) | 4 (16) | 13 (51) |
| 30–44 years | 160 (17) | 106 (11) | 269 (29) | 398 (43) | 68 (26) | 36 (14) | 75 (28) | 85 (32) |
| 45–59 years | 770 (33) | 349 (15) | 735 (32) | 475 (20) | 288 (36) | 136 (17) | 231 (29) | 152 (19) |
| 60–74 years | 1522 (51) | 383 (13) | 684 (23) | 382 (13) | 419 (48) | 133 (15) | 207 (24) | 106 (12) |
| ≥75 years | 486 (63) | 96 (12) | 125 (16) | 67 (9) | 98 (61) | 17 (11) | 30 (19) | 15 (10) |
| Cancer type | ||||||||
| Melanoma | 89 (31) | 42 (15) | 90 (31) | 66 (23) | ||||
| Lung cancer | 56 (32) | 16 (9) | 35 (20) | 67 (38) | 35 (31) | 18 (16) | 38 (34) | 22 (20) |
| Breast cancer | 1332 (37) | 497 (14) | 1005 (28) | 778 (22) | 351 (40) | 143 (16) | 245 (28) | 140 (16) |
| Thyroid cancer | 3 (8) | 6 (15) | 8 (19) | 23 (59) | ||||
| Colorectal cancer | 335 (50) | 88 (13) | 148 (22) | 105 (16) | 203 (52) | 49 (13) | 91 (23) | 48 (12) |
| Endometrial cancer | 123 (61) | 24 (12) | 34 (17) | 22 (11) | 15 (38) | 9 (23) | 8 (21) | 8 (19) |
| Leukemia & non‐hodgkin lymphoma | 15 (20) | 10 (13) | 25 (33) | 27 (35) | ||||
| Prostate cancer | 745 (63) | 145 (12) | 201 (17) | 83 (7) | 158 (54) | 49 (17) | 57 (20) | 27 (9) |
| Other cancer types | 115 (27) | 43 (10) | 138 (32) | 133 (31) | 111 (29) | 55 (14) | 100 (26) | 114 (30) |
| Time since cancer diagnosis | ||||||||
| 0–1 year | 1053 (41) | 355 (14) | 669 (26) | 501 (19) | 487 (44) | 162 (15) | 285 (26) | 171 (15) |
| 2–5 years | 1287 (41) | 409 (13) | 817 (26) | 617 (20) | 274 (39) | 120 (17) | 175 (25) | 141 (20) |
| 6–10 years | 426 (41) | 131 (13) | 273 (26) | 204 (20) | 83 (38) | 29 (13) | 59 (27) | 46 (21) |
| >10 years | 194 (44) | 51 (11) | 109 (24) | 91 (21) | 33 (37) | 14 (15) | 28 (30) | 17 (18) |
| Continent where study was conducted | ||||||||
| Asia | 451 (49) | 116 (13) | 235 (26) | 112 (12) | 251 (40) | 87 (14) | 166 (26) | 127 (20) |
| Australia | 174 (34) | 78 (15) | 156 (30) | 111 (21) | ||||
| Europe | 1115 (41) | 380 (14) | 758 (28) | 480 (18) | 96 (46) | 27 (13) | 55 (26) | 29 (14) |
| North America | 1221 (40) | 372 (12) | 718 (24) | 713 (24) | 531 (41) | 212 (16) | 326 (25) | 215 (17) |
Note: Groups with less than 10 participants were omitted. All data were imputed, except the cancer type variable, since its imputation did not converge.
3.3. Mean fear of cancer recurrence severity scores
The mean FCR severity score for all participants (n = 9311) was 14.8 (95% CI 13.7–16.0). Mean FCR scores stratified by clinical and demographic characteristics are presented in Table 3. The FCRI‐SF scores in this table may be considered normative scores. Mean FCR severity scores and main characteristics per study are presented in appendix C. On average, patients scored two points higher than survivors, and women scored approximately two points higher than men. Fear of cancer recurrence severity scores were lower for higher age groups, with the youngest group 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 scoring 16.9 and 17.0 and the oldest group (≥75) scoring 10.9 and 12.6 for survivors and patients respectively. Looking at cancer types, all mean scores ranged between 11.2 and 16.8, with the highest mean scores for lung cancer and melanoma. Fear of cancer recurrence severity scores were similar across different time periods since cancer diagnosis. For patients, the mean FCR severity scores were slightly higher (1.1 points) for respondents with longer times since cancer diagnosis, while for survivors, FCR severity scores were slightly lower (1.3 points) for respondents with longer times since cancer diagnosis. Comparing the continents, respondents from studies carried out in Australia scored highest, followed by respondents from studies in North America, Europe and finally Asia.
TABLE 3.
Mean fear of cancer recurrence (FCR) severity scores stratified by clinical and demographic characteristics
| Survivors | Patients | |||
|---|---|---|---|---|
| n | Mean (CI) | n | Mean (CI) | |
| Total | 7190 | 14.3 (13.0–15.5) | 2121 | 16.2 (15.6–16.8) |
| Sex | ||||
| Men | 2108 | 13.0 (11.8–14.1) | 678 | 14.6 (13.8–15.4) |
| Women | 5082 | 15.1 (14.6–15.5) | 1443 | 16.3 (14.2–18.5) |
| Age groups | ||||
| 18–29 years | 183 | 16.9 (15.2–18.7) | 25 | 17.0 (13.6–20.4) |
| 30–44 years | 933 | 16.8 (15.4–18.3) | 264 | 17.9 (9.6–26.3) |
| 45–59 years | 2329 | 15.5 (13.9–17) | 807 | 16.9 (8.6–25.3) |
| 60–74 years | 2970 | 13.2 (11.6–14.7) | 865 | 14.8 (6.5–23.1) |
| ≥75 years | 775 | 10.9 (9.3–12.6) | 161 | 12.6 (4–21.2) |
| Cancer type | ||||
| Melanoma | 302 | 16.2 (13.5–18.9) | ||
| Lung cancer | 175 | 15.5 (14.4–16.7) | 114 | 16.8 (13–20.5) |
| Breast cancer | 3675 | 15.0 (13.8–16.2) | 883 | 15.5 (14.7–16.2) |
| Thyroid cancer | 40 | 14.2 (11.8–16.6) | ||
| Colorectal cancer | 697 | 14.1 (13.4–14.9) | 395 | 15.2 (12.2–18.3) |
| Endometrial cancer | 247 | 12.0 (9.8–14.3) | 40 | 16.3 (7.1–25.5) |
| Leukemia & non‐hodgkin lymphoma | 77 | 11.4 (9.6–13.1) | ||
| Prostate cancer | 1191 | 11.2 (10.6–11.9) | 293 | 12.6 (10–15.2) |
| Other cancer types | 452 | 13.9 (13–14.9) | 381 | 16.7 (13.1–20.3) |
| Time since cancer diagnosis | ||||
| 0–1 year since diagnosis | 2577 | 14.7 (13.1–16.4) | 1105 | 15.8 (14.5–17.1) |
| 2–5 years since diagnosis | 3130 | 14.1 (12.2–16) | 710 | 16.3 (11.5–21) |
| 6–10 years since diagnosis | 1034 | 14.2 (11.8–16.5) | 218 | 16 (10.4–21.7) |
| >10 years since diagnosis | 445 | 13.4 (9.9–16.9) | 92 | 16.9 (9.3–24.4) |
| Continent where study was conducted | ||||
| Asia | 915 | 13.0 (8.3–17.8) | 631 | 14.3 (3.4–25.2) |
| Australia | 519 | 15.4 (11.4–19.4) | ||
| Europe | 2733 | 14.0 (10.7–17.3) | 206 | 15.7 (6.1–25.2) |
| North America | 3023 | 15.0 (12.8–17.3) | 1284 | 17.0 (16.4–17.6) |
Note: All data was imputed, except the Cancer type variable, since its imputation did not converge.
3.4. Associations with fear of cancer recurrence severity
We assessed the statistical significance of the associations between FCR severity and the included variables using multilevel regression analyses, whereby all variables were analyzed in the same model. The reference categories were men, breast cancer and North America. Separate models were made for survivors and patients.
For survivors, statistically significant associations were found between FCR severity and age (β = −0.16, p < 0.001), sex (β = 1.18, p < 0.01), endometrial cancer (β = −3.02, p < 0.01), leukemia and non‐Hodgkin lymphoma (β = −2.77, p < 0.05) and prostate cancer (β = −1.36, p < 0.05). For continent where the study was conducted, there was only a significant association with Asia (β = −2.78, p < 0.05). There were no significant associations with time since cancer diagnosis. The explained variance (R 2) of the model with all the factors was 0.19.
For patients, there were significant associations between FCR severity and age (β = −0.10, p < 0.001), sex (β = 1.38, p = 0.01), colorectal cancer (β = 1.58, p < 0.05), lung cancer (β = 3.02, p < 0.001), and the group of “other cancer types” (β = 4.06, p < 0.001). There were no significant associations with time since cancer diagnosis and continent where the study was conducted. The explained variance (R 2) of the model with all the factors was 0.14.
3.5. Characteristics of groups according to Fear of Cancer Recurrence Inventory cut‐off scores
To inform those who wish to address a specific FCR severity group – for example, when designing an intervention for the group scoring above one of the cut‐offs – we present the characteristics of each FCR severity group in Appendix D. For this analysis, 12 additional studies were included, namely those who selected respondents based on the severity of their FCR.
The two highest FCR severity groups (scoring 16–21 and ≥22 on the FCRI‐SF) had the following characteristics: approximately three‐quarters of respondents were women; approximately three‐quarters were aged between 45 and 74 years; approximately 60% of survivors and 45% of patients had breast cancer; and about 90% of patients and 80% of survivors had been diagnosed with cancer within the past 5 years.
3.6. Aggregate data analysis
To compare the results of the data we collected in our IPD analysis with the studies that did not provide data, we conducted an aggregate data analysis (see Table 4). In the aggregate data analysis, we included all studies that did not provide data, that did not select participants based on their level of FCR and that reported data on a) mean FCR severity score, and/or b) percentage of participants scoring ≥13. The combined mean FCR score was 16.1 (14.4–17.7), compared with 14.3 for survivors and 16.2 for patients in the IPD analysis. The percentage of participants scoring ≥13 was 50.6% in the aggregate data analysis, which was 8.2% lower than the percentage in the IPD analysis.
TABLE 4.
Aggregate data analysis of a) mean fear of cancer recurrence (FCR) severity scores 93 and b) percentage of respondents scoring ≥13
| a) | ||
|---|---|---|
|
| b) | ||
|---|---|---|
| Author (publication year) | n | % Scoring ≥13 |
| Costa, D. S. J., et al. (2016) | 286 | 72 |
| Dieng, M., et al. (2016) | 164 | 68 |
| Galica, J., et al. (2020) | 15 | 93 |
| Herman, S., et al. (2014) | 242 | 85 |
| Kasparian, N. A., et al. (2016) | 19 | 32 |
| Peng, L., et al. (2019) | 207 | 77 |
| Petzel, M. Q. B., et al. (2012) | 224 | 34 |
| Shun, S. C., et al. (2018) | 97 | 55 |
| Smith, T. G., et al. (2019) | 2107 | 39 |
| Thewes, B., et al. (2012) | 218 | 70 |
| Van Liew, J. R., et al. (2014) | 138 | 60 |
| Walburg, V., et al. (2019) | 108 | 44 |
4. DISCUSSION
4.1. Main findings
In this sizeable international IPD meta‐analysis, we found that more than half (59%) of cancer survivors and patients report at least a moderate level of FCR (FCRI‐SF ≥13) and that about 1 in 5 (19%) experience a high level of FCR (FCRI‐SF ≥22), indicative of a need for specialized intervention. There were no major differences between survivors and patients in the prevalence of FCR. Fear of cancer recurrence was consistently more prevalent among women and younger respondents. While FCR affects survivors and patients across cancer types, on average, participants with lung cancer and melanoma reported the highest scores and participants with prostate cancer reported the lowest scores; although it is important to note that not all cancer types were represented. Fear of cancer recurrence is also experienced across continents and at all time points since cancer diagnosis. Our IPD results are comparable to the results of our aggregate data analysis and to a recent meta‐analysis, which found 53.9% scored ≥13, 43.3% ≥16 and 30% ≥22. 23 The higher percentage scoring ≥22 in the meta‐analysis is most likely due to a difference in inclusion criteria. In the present study, studies that selected patients based on their level of FCR were excluded, while in the recent meta‐analysis these studies were included.
In the regression analyses, significant associations were found between FCR severity and age and sex for both survivors and patients, with younger respondents and women reporting higher FCR levels. This is consistent with earlier findings. 3 , 9 , 20 , 21 Regarding cancer types, with breast cancer as the reference category, patients with lung cancer and colorectal cancer reported significantly higher levels of FCR, and survivors with endometrial cancer, prostate cancer, and leukemia and non‐Hodgkin lymphoma reported significantly lower levels of FCR. Thus, some observed differences in prevalence between cancer types are not reflected by significant associations. In these cases, the difference in prevalence may be explained by other variables (e.g. age). Also, for some cancer types, the number of participants was relatively low, and there could be sampling bias. No significant associations were found for time since cancer diagnosis, which is in line with previous research, 7 suggesting that without intervention or treatment, FCR likely persists over time. For survivors, FCR was somewhat lower for respondents from Asia. While we have no clear explanation for this, it could be due to cultural differences in the experience or self‐reporting of FCR.
We also explored the characteristics of respondents within each FCR severity group, to inform people who aim to target a specific group. The two highest FCR severity groups (16–21 and ≥22) had the following characteristics: most respondents were aged between 45 and 74 years, most were women, most were within five years since diagnosis, and about half had breast cancer. Notably, these results are affected by the characteristics of the participants in the included studies.
4.2. Study limitations
A major strength of the present study is the large amount of data included in the analyses; 46 datasets including data from 11,226 respondents from 13 countries. There were also 41 studies with 14,381 respondents that did not provide data. Twenty‐four of these studies could be included in the aggregate data analysis, which found similar results to the IPD analysis.
Some limitations should also be noted, for instance the underrepresentation of some groups. There were no studies from South America or Africa and very few from low and middle‐income countries (LMICs). Also, survivors and patients aged ≥70 years were underrepresented. In our sample, 23% of survivors were aged ≥70 years and only 3% were aged ≥80 years, while for example, in the USA, 49% of cancer survivors are aged ≥70 years and 21% are aged ≥80 years. 94 Underrepresentation of the elderly is a common issue in cancer research. 95 Considering that the prevalence of FCR is low in this age group, caution needs to be taken when extrapolating our findings on prevalence of FCR to the cancer population as a whole.
Another limitation relates to the use of FCRI‐SF scores as a measure of FCR: FCRI‐SF scores do not reflect all key characteristics of clinical FCR, 13 since hyper‐alertness to bodily symptoms is not included. 23
Finally, the severity of one's FCR may affect interest in participating in studies on FCR. In one FCR intervention study that did not select on FCR levels, it was found that older patients and patients with less FCR were less likely to participate. 65 On the other hand, patients who use avoidance to cope with high FCR may be less likely to participate.
4.3. Clinical implications
As we have shown, FCR is a highly prevalent concern, affecting more than half of cancer survivors and patients. Consequently, this is an issue that needs to be addressed by healthcare providers and policy makers. We recommend providing brief psycho‐education about FCR to all cancer survivors and patients, to normalize FCR and help individuals seek support when they need it, even if they are no longer undergoing hospital‐based treatment or surveillance. Due to the high prevalence of FCR, psycho‐education for all may be more effective than screening. An example of a brief psycho‐educational program is a recently piloted intervention including normalization, prognostic information, recurrence symptoms education, advice on managing worry and if FCR was high, referral to a psycho‐oncologist. 96 Since FCR exists at all times since cancer diagnosis, we also recommend discussing FCR on multiple occasions.
Also, the best way to address FCR still needs to be investigated. Additional research is needed to identify which patients desire support and how to tailor interventions to different levels of FCR and to individual needs and preferences. 17 While current interventions are often face to face and specialist led, 17 accessible, low‐resource programs (e.g. online or group therapy) may be fitting for the group with moderate FCR (FCRI‐SF scores between 13 and 22) and can be more easily scaled.
4.4. Implications for future research
We have identified several medical and demographic factors that are associated with fear, but in agreement with previous research, these factors only explain a limited proportion of the variance in FCR severity. 97 Therefore, there may be other important factors. We recommend investigating the role of other factors, such as cancer stage, type of treatment and psychosocial factors, including prior and current psychiatric disorders. Also, we recommend investigating the prevalence of FCR in understudied cancer types, such as thyroid cancer and hematological cancers, understudied regions of the world, including South America, Africa and LMICs, and understudied groups, such as racial and ethnic minority groups. Furthermore, to increase comparability between studies, we recommend for researchers to report proportions above both the 13 and 22 cut‐offs, when reporting FCRI‐SF data.
Finally, since FCR is a multidimensional construct and since these dimensions are captured by the FCRI, future research could explore more deeply what the characteristics of this fear are and how different aspects of the fear relate to each other, including the role of triggers, coping styles and social circumstances. Differences between patient groups or even individual patients could be explored, in order to target interventions and help people suffering from FCR better.
CONFLICTS OF INTEREST
None declared.
ETHICAL STATEMENT
This study was assessed by the Medical Research Ethics Committee at the Utrecht University Medical Center, who judged that the Medical Research Involving Human Subjects Act (WMO) does not apply that to this study and that therefore no official approval was required.
AUTHOR CONTRIBUTIONS
Luigjes‐Huizer, Y.L. and van der Lee, M.L. are members of the steering committee. Humphris, G., Kasparian, N.A., Lam, W.W.T., Lebel, S., Simard, S., Smith, A.B., Zachariae, R., are members of the international advisory board. Previously mentioned authors, Helsper, C.W. and de Wit, N.J. contributed to the concept and design of the study. Luigjes‐Huizer, Y.L. and Tauber, N.M. conducted the systematic review and the risk of bias assessment. Luigjes‐Huizer, Y.L. collected the data and performed the analyses, including the aggregate data analysis. Van Vugt, B.B. was the second reviewer for the aggregate data analysis. Luigjes‐Huizer, Y.L., Helsper, C.W., van der Lee, M.L. and Monninkhof, E.M. drafted the manuscript. All authors critically reviewed the manuscript and approved the final version.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Buffart, L.M. provided example formats for the data sharing agreement and access and publication policy. Afiyanti, Y., Ahn, Y., Bell, K.J.L., Cilessen, L., Corter, A.L., Custers, J.A.E., Dirkse, D., Dixon, C.L., Eyrenci, A., Fisher, P.L., Galica, J., Garland, S.N., Hebert, J., Jakobsen, I., Jeppesen, M.M., Johns, S.A., Jun, S. Kang, D.W., Lam, W.W.T., Lebel, S., Luigjes‐Huizer, Y.L., Maheu, C., Liu, J., Mititelu, R., Murphy, M.J., Otto, A.K., Russell, L., Savard, J., Simard, S., Speckens, A., Sukyati, I., Tauber, N.M., van der Gucht, K., van Helmondt, S.J., Vatandoust, S., Wijayanti, T. and Zdenkowski, N. shared their data and contributed to the study concept, design, and conduct of the study that they were responsible for. This study was supported by the Dutch Cancer Society (KWF) grant number 10936. KWF is not involved in study design, collection, management, analysis and interpretation of data, writing of the report and decision to submit the report for publication, nor does it have authority over the publications.
Luigjes‐Huizer YL, Tauber NM, Humphris G, et al. What is the prevalence of fear of cancer recurrence in cancer survivors and patients? A systematic review and individual participant data meta‐analysis. Psychooncology. 2022;31(6):879‐892. 10.1002/pon.5921
ENDNOTE
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Canceratlas.cancer.org . Cancer Survivorship [Internet]. [cited 2022 Jan 21]. Available from: https://canceratlas.cancer.org/the‐burden/cancer‐survivorship/
- 3. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7:300‐322. [DOI] [PubMed] [Google Scholar]
- 4. Ness S, Kokal J, Fee‐Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 5. Harrison SE, Watson EK, Ward AM, et al. Primary health and supportive care needs of long‐term cancer survivors: a questionnaire survey. J Clin Oncol. 2011;29(15):2091‐2098. [DOI] [PubMed] [Google Scholar]
- 6. Lebel S, Ozakinci G, Humphris G, et al. From normal response to clinical problem: definition and clinical features of fear of cancer recurrence. Support Care Cancer. 2016;24(8):3265‐3268. 10.1007/s00520-016-3272-5 [DOI] [PubMed] [Google Scholar]
- 7. Götze H, Taubenheim S, Dietz A, Lordick F, Mehnert‐Theuerkauf A. Fear of cancer recurrence across the survivorship trajectory: results from a survey of adult long‐term cancer survivors. Psycho‐Oncol. 2019;28(10):2033‐2041. [DOI] [PubMed] [Google Scholar]
- 8. Koch L, Bertram H, Eberle A, et al. Fear of recurrence in long‐term breast cancer survivors—still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the Cancer Survivorship—a multi‐regional population‐based study. Psycho‐Oncol. 2014;23:547‐554. [DOI] [PubMed] [Google Scholar]
- 9. Crist JV, Grunfeld EA. Factors reported to influence fear of recurrence in cancer patients: a systematic review. Psycho‐Oncol. 2013;22(5):978‐986. [DOI] [PubMed] [Google Scholar]
- 10. Simard S, Savard J. Screening and comorbidity of clinical levels of fear of cancer recurrence. J Cancer Surviv. 2015;9(3):481‐491. [DOI] [PubMed] [Google Scholar]
- 11. Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long‐term (≥5 years) cancer survivors ‐ a systematic review of quantitative studies. Psycho‐Oncol. 2013;22(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 12. Costa DSJ. Screening for clinical levels of fear of cancer recurrence. Psycho‐Oncol. 2017;26(11):2002‐2003. 10.1002/pon.4390 [DOI] [PubMed] [Google Scholar]
- 13. Mutsaers B, Maheu C, Smith ABen, et al. Identifying the key characteristics of clinical fear of cancer recurrence: an international Delphi study. Psycho‐Oncol. 2020(406):437. [DOI] [PubMed] [Google Scholar]
- 14. Williams JTW, Pearce A, Smith AB. A Systematic Review of Fear of Cancer Recurrence Related Healthcare Use and Intervention Cost‐Effectiveness. Psycho‐Oncol. 2021;30(8):1185–1195. [DOI] [PubMed] [Google Scholar]
- 15. Ng DWL, Foo CC, Ng SSM, et al. The Role of Metacognition and its Indirect Effect through Cognitive Attentional Syndrome on Fear of Cancer Recurrence Trajectories: A Longitudinal Study. Psycho‐Oncol. 2020;29:271‐279. 10.1002/pon.5234 [DOI] [PubMed] [Google Scholar]
- 16. Savard J, Ivers H. The evolution of fear of cancer recurrence during the cancer care trajectory and its relationship with cancer characteristics. J Psychosom Res. 2013;74(4):354‐360. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L52412009 [DOI] [PubMed] [Google Scholar]
- 17. Tauber NM, O’Toole MS, Dinkel A, et al. Effect of psychological intervention on fear of cancer recurrence: a systematic review and meta‐analysis. J Clin Oncol. 2019;37(31):2899‐2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simard S, Savard J. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer. 2009;17(3):241‐251. [DOI] [PubMed] [Google Scholar]
- 19. Fardell JE, Jones G, Smith ABen, et al. Exploring the screening capacity of the Fear of Cancer Recurrence Inventory‐Short Form for clinical levels of fear of cancer recurrence. Psycho‐Oncol. 2018;27(2):492‐499. [DOI] [PubMed] [Google Scholar]
- 20. Pang C, Humphris G. The relationship between fears of cancer recurrence and patient gender: a systematic review and meta‐analysis. Front Psychol. 2021;12(February):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galica J, Maheu C, Brennenstuhl S, Townsley C, Metcalfe K. Examining predictors of fear of cancer recurrence using leventhal’s commonsense model: distinct implications for oncology nurses. Cancer Nurs. 2021;44(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 22. Lim E, Humphris G. The relationship between fears of cancer recurrence and patient age: a systematic review and meta‐analysis. Cancer Rep. 2020;3(3):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith AB, Costa D, Galica J, et al. Spotlight on the fear of cancer recurrence inventory (Fcri). Psychol Res Behav Manag. 2020;13:1257‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thewes B, Butow P, Zachariae R, Christensen S, Simard S, Gotay C. Fear of cancer recurrence: a systematic literature review of self‐report measures. Psycho‐Oncol. 2012;21(6):571‐587. [DOI] [PubMed] [Google Scholar]
- 25. van Helmondt SJ, van der Lee ML, de Vries J. Translation and validation of the Dutch version of the fear of cancer recurrence inventory (FCRI‐NL). J Psychosom Res. 2017;102(August):21‐28. 10.1016/j.jpsychores.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 26. Jakobsen IH, Jeppesen MM, Simard S, Thaysen HV, Laurberg S, Juul T. Initial validation of the Danish version of the Fear of Cancer Recurrence Inventory (FCRI) in colorectal cancer patients. J Cancer Surviv. 2018;12(6):723‐732. [DOI] [PubMed] [Google Scholar]
- 27. Eyrenci A, Sertel Berk HÖ. Validity and reliability of the Turkish version of fear of cancer recurrence inventory. Turk Onkol Derg. 2018;33(2):54‐64. [Google Scholar]
- 28. Liu J, Mahendran R, Chua SM, et al. Validation of the English and Mandarin versions of the fear of cancer recurrence inventory in an Asian population. J Health Psychol. 2017(October). [DOI] [PubMed] [Google Scholar]
- 29. Shin J, Goo A, Ko H, et al. Validation study for the Korean version of fear of cancer recurrence inventory. J Kor Med Sci. 2017;32(11):1792‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ciotti R, Simard S, Ardizzoia A, et al. Quality of life and quality of care in cancer survivors: fear of cancer recurrence ‐ preliminary results of a transcultural availability of validated tools. J Clin Oncol. 2010;28(15). [Google Scholar]
- 31. Ng DWL, Kwong A, Suen S, et al. Fear of cancer recurrence among Chinese cancer survivors: prevalence and associations with metacognition and neuroticism. Psycho‐Oncol. 2019;28(6):1243‐1251. 10.1002/pon.5073 [DOI] [PubMed] [Google Scholar]
- 32. Wijayanti T, Afiyanti Y, Rahmah H, Milanti A. Fear of cancer recurrence and social support among Indonesian gynecological cancer survivors. Arch Oncol. 2018;24(2):12‐19. 10.2298/AOO180201004W [DOI] [Google Scholar]
- 33. Bateni FS, Rahmatian M, Kaviani A, Simard S, Soleimani M, Nejatisafa A‐A. The Persian version of the fear of cancer recurrence inventory (FCRI): translation and evaluation of its psychometric properties. Arch Breast Cancer. 2019;6(4):174‐180. [Google Scholar]
- 34. Buffart LM, Kalter J, Chinapaw MJM, et al. Predicting OptimaL cAncer RehabIlitation and Supportive care (POLARIS): rationale and design for meta‐analyses of individual patient data of randomized controlled trials that evaluate the effect of physical activity and psychosocial interventions on healt. Syst Rev. 2013;2:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. RStudio Team . RStudio: Integrated Development for R. RStudio, PBC; 2020. Available from: http://www.rstudio.com/ [Google Scholar]
- 36. Abo‐Zaid G, Guo B, Deeks JJ, et al. Individual participant data meta‐analyses should not ignore clustering. J Clin Epidemiol. 2013;66(8):865‐873. e4. 10.1016/j.jclinepi.2012.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jolani S, Debray TPA, Koffijberg H, van Buuren S, Moons KGM. Imputation of systematically missing predictors in an individual participant data meta‐analysis: a generalized approach using MICE. Stat Med. 2015;34(11):1841‐1863. [DOI] [PubMed] [Google Scholar]
- 38. Bell KJL, Mehta Y, Turner RM, et al. Fear of new or recurrent melanoma after treatment for localised melanoma. Psycho‐Oncol. 2017;26(11):1784‐1791. [DOI] [PubMed] [Google Scholar]
- 39. Compen F, Bisseling E, Schellekens M, et al. Face‐to‐face and internet‐based mindfulness‐based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol. 2018;36(23):2413‐2421. [DOI] [PubMed] [Google Scholar]
- 40. Corter AL, Broom R, Porter D, Harvey V, Findlay M. Predicting nonadherence to adjuvant endocrine therapy in women with early stage breast cancer. Psycho‐Oncol. 2018;27(9):2096‐2103. [DOI] [PubMed] [Google Scholar]
- 41. Custers JAE, Tielen R, Prins JB, De Wilt JHW, Gielissen MFM, Van Der Graaf WTA. Fear of progression in patients with gastrointestinal stromal tumors (GIST): is extended lifetime related to the Sword of Damocles? Acta Oncol Madr. 2015;54(8):1202‐1208. [DOI] [PubMed] [Google Scholar]
- 42. Custers JAE, Gielissen MFM, Janssen SHV, de Wilt JHW, Prins JB. Fear of cancer recurrence in colorectal cancer survivors. Support Care Cancer. 2016;24(2):555‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Custers JAE, Gielissen MFM, de Wilt JHW, et al. Towards an evidence‐based model of fear of cancer recurrence for breast cancer survivors. J Cancer Surviv. 2017;11(1):41‐47. 10.1007/s11764-016-0558-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Custers JAE, Kwakkenbos L, van de Wal M, Prins JB, Thewes B. Re‐validation and screening capacity of the 6‐item version of the Cancer Worry Scale. Psycho‐Oncol. 2018;27(11):2609‐2615. [DOI] [PubMed] [Google Scholar]
- 45. Dirkse D, Hadjistavropoulos HD, Alberts NA, et al. Making Internet‐delivered cognitive behaviour therapy scalable for cancer survivors: a randomized non‐inferiority trial of self‐guided and technician‐guided therapy. J Cancer Surviv. 2020;14(2):211‐225. [DOI] [PubMed] [Google Scholar]
- 46. Dixon CL. Examining fear of recurrence in cancer survivors. Diss Abstr Int Sect B Sci Eng [Internet]. 2019;80(7‐B(E)). [Google Scholar]
- 47. Fisher PL, Byrne A, Salmon P. Metacognitive therapy for emotional distress in adult cancer survivors: a case series. Cogn Ther Res. 2017;41(6):891‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fisher PL, Byrne A, Fairburn L, Ullmer H, Abbey G, Salmon P. Brief metacognitive therapy for emotional distress in adult cancer survivors. Front Psychol. 2019;10(JAN):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guimond AJ, Ivers H, Savard J. Is emotion regulation associated with cancer‐related psychological symptoms? Psychol Heal. 2019;34(1):44‐63. 10.1080/08870446.2018.1514462 [DOI] [PubMed] [Google Scholar]
- 50. Hébert J, Fillion L. Assessment of the feasibility and acceptability, and pre‐test of the utility of an individualized survivorship care plan (ISCP) for women with endometrial cancers during the transition of the end of active treatment to cancer survivorship. Can Oncol Nurs J. 2017;27(2):153‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jakobsen IH, Juul T, Thaysen HV, Johansen C, Laurberg S. Differences in baseline characteristics and 1‐year psychological factors between participants and non‐participants in the randomized, controlled trial regarding patient‐led follow‐up after rectal cancer (FURCA). Acta Oncol (Madr). 2019;58(5):627‐633. 10.1080/0284186X.2019.1581948 [DOI] [PubMed] [Google Scholar]
- 52. Jeppesen MM, Jensen PT, Hansen DG, Christensen RD, Mogensen O. Patient‐initiated follow up affects fear of recurrence and healthcare use: a randomised trial in early‐stage endometrial cancer. BJOG. 2018;125:1705‐1714. [DOI] [PubMed] [Google Scholar]
- 53. Johns SA, Stutz PV, Talib TL, et al. Acceptance and commitment therapy for breast cancer survivors with fear of cancer recurrence: a 3‐arm pilot randomized controlled trial. Cancer. 2020;126(1):211‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kang DW, Fairey AS, Boulé NG, Field CJ, Courneya KS. Exercise duRing active surveillance for prostate cancer‐the ERASE trial:A study protocol of a phase II randomised controlled trial. BMJ Open. 2019;9(7):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lane BE, Garland SN, Chalifour K, et al. Prevalence and factors associated with fear of recurrence in a mixed sample of young adults with cancer. J Cancer Surviv. 2019;13(6):842‐851. https://doi‐org.proxy.library.uu.nl/10.1007/s11764‐019‐00802‐9 [DOI] [PubMed] [Google Scholar]
- 56. Luigjes‐Huizer YL, Van Der Lee ML, De Wit NJ, Helsper CW. Study protocol of the BLANKET trial: a cluster randomised controlled trial on the (cost‐) effectiveness of a primary care intervention for fear of cancer recurrence in cancer survivors. BMJ Open. 2019;9(12):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maheu C, Lebel S, Courbasson C, et al. Protocol of a randomized controlled trial of the fear of recurrence therapy (FORT) intervention for women with breast or gynecological cancer. BMC Cancer. 2016;16(1):1‐12. 10.1186/s12885-016-2326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Murphy MJ, Newby JM, Butow P, et al. Randomised controlled trial of internet‐delivered cognitive behaviour therapy for clinical depression and/or anxiety in cancer survivors (iCanADAPT Early). Psycho‐Oncol. 2020;29(1):76‐85. [DOI] [PubMed] [Google Scholar]
- 59. Otto AK, Soriano EC, Siegel SD, LoSavio ST, Laurenceau JP. Assessing the relationship between fear of cancer recurrence and health care utilization in early‐stage breast cancer survivors. J Cancer Surviv. 2018;12(6):775‐785. [DOI] [PubMed] [Google Scholar]
- 60. Russell L, Ugalde A, Orellana L, et al. A pilot randomised controlled trial of an online mindfulness‐based program for people diagnosed with melanoma. Support Care Cancer. 2019;27(7):2735‐2746. [DOI] [PubMed] [Google Scholar]
- 61. Simard S, Savard J, Ivers H. Fear of cancer recurrence: specific profiles and nature of intrusive thoughts. J Cancer Surviv. 2010;4(4):361‐371. [DOI] [PubMed] [Google Scholar]
- 62. van de Wal M, Thewes B, Gielissen M, Speckens A, Prins J. Efficacy of blended cognitive behavior therapy for high fear of recurrence in breast, prostate, and colorectal cancer survivors: the SWORD study, a randomized controlled trial. J Clin Oncol. 2017;35(19):2173‐2183. https://www.scopus.com/inward/record.uri?eid=2‐s2.0‐85021649029&doi=10.1200%2FJCO.2016.70.5301&partnerID=40&md5=f159be668de252f5b14f60fe08799680%0Ahttp://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/658/CN‐01395658/frame.html [DOI] [PubMed] [Google Scholar]
- 63. Sukyati I, Afiyanti Y, Rahmah H, Milanti A. Fear of recurrence predictors among Indonesian gynecological cancer survivors. J Int Dent Med Res. 2019;11(3):1463‐1467. [Google Scholar]
- 64. Van Der Gucht K, Takano K, Labarque V, et al. A mindfulness‐based intervention for adolescents and young adults after cancer treatment: effects on quality of life, emotional distress, and cognitive vulnerability. J Adolesc Young Adult Oncol. 2017;6(2):307‐317. [DOI] [PubMed] [Google Scholar]
- 65. van Helmondt SJ, van der Lee ML, van Woezik RAM, Lodder P, de Vries J. No effect of CBT‐based online self‐help training to reduce fear of cancer recurrence: first results of the CAREST multicenter randomized controlled trial. Psycho‐Oncol. 2020;29(1):86‐97. [DOI] [PubMed] [Google Scholar]
- 66. Lebel S, Simard S, Harris C, et al. Empirical validation of the English version of the fear of cancer recurrence inventory. Qual Life Res. 2016;25(2):311‐321. [DOI] [PubMed] [Google Scholar]
- 67. Savard J, Savard MH, Caplette‐Gingras A, Casault L, Camateros C. Development and feasibility of a group cognitive‐behavioral therapy for fear of cancer recurrence. Cognit Behav Pract. 2018;25(2):275‐285. [Google Scholar]
- 68. van de Wal M, van Oort I, Schouten J, Thewes B, Gielissen M, Prins J. Fear of cancer recurrence in prostate cancer survivors. Acta Oncol (Madr). 2016;55(7):821‐827. [DOI] [PubMed] [Google Scholar]
- 69. Vatandoust S, Chen G, Sposato L, et al. Patient reported outcome measures (PROMs) in patients (pts) with locally advanced rectal cancer (LARC) managed with a watch and wait (W & W) approach after a clinical complete response to chemoradiotherapy (CRT). J Clin Oncol. 2019. 37:15. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L629337117 [Google Scholar]
- 70. Zdenkowski N, Butow P, Spillane A, et al. Single‐arm longitudinal study to evaluate a decision aid for women offered Neoadjuvant systemic therapy for operable breast cancer. JNCCN J Natl Compr Cancer Netw. 2018;16(4):378‐385. [DOI] [PubMed] [Google Scholar]
- 71. Mititelu R, Aruljothy A, Fitzpatrick TR. Physical activity, locus of control, and fear of recurrence among cancer survivors from a community‐based cancer support program. Treating Vulnerable Populations of Cancer Survivors: A Biopsychosocial Approach; 2016:1‐160. [Google Scholar]
- 72. Lim SO, Jun S. Factors influencing the improvement in lifestyle among patients with colorectal cancer. Korean J Adult Nurs. 2019;31(3):325‐336. [Google Scholar]
- 73. Dieng M, Butow PN, Costa DSJ, et al. Psychoeducational intervention to reduce fear of cancer recurrence in people at high risk of developing another primary melanoma: results of a randomized controlled trial. J Clin Oncol. 2016. [DOI] [PubMed] [Google Scholar]
- 74. Dodds SE, Pace TWW, Bell ML, et al. Feasibility of Cognitively‐Based Compassion Training (CBCT) for breast cancer survivors: a randomized, wait list controlled pilot study. Support Care Cancer. 2015;23(12):3599‐3608. [DOI] [PubMed] [Google Scholar]
- 75. Galica J, Brennenstuhl S, Maheu C, Townsley C, Metcalfe K. Examining the dimensionality of the fear of cancer recurrence inventory. Psycho‐Oncol. 2018;27(11):2602‐2608. [DOI] [PubMed] [Google Scholar]
- 76. Galica J, Giroux J, Francis JA, Maheu C. Coping with fear of cancer recurrence among ovarian cancer survivors living in small urban and rural settings: a qualitative descriptive study. Eur J Oncol Nurs [Internet]. 2020;44(November 2019):101705. 10.1016/j.ejon.2019.101705 [DOI] [PubMed] [Google Scholar]
- 77. Hong SJ, Shin NM, Jung S. A predictive model of fear of cancer recurrence for patients undergoing chemotherapy. Support Care Cancer. 2020;28(9):4173‐4181. [DOI] [PubMed] [Google Scholar]
- 78. Kasparian NA, Mireskandari S, Butow PN, et al. “Melanoma: questions and Answers.” Development and evaluation of a psycho‐educational resource for people with a history of melanoma. Support Care Cancer. 2016;24(12):4849‐4859. 10.1007/s00520-016-3339-3 [DOI] [PubMed] [Google Scholar]
- 79. Seguin Leclair C, Lebel S, Westmaas JL. The relationship between fear of cancer recurrence and health behaviors: a nationwide longitudinal study of cancer survivors. Heal Psychol. 2019;38(7):596‐605. [DOI] [PubMed] [Google Scholar]
- 80. Merckaert I, Lewis F, Delevallez F, et al. Improving anxiety regulation in patients with breast cancer at the beginning of the survivorship period: a randomized clinical trial comparing the benefits of single‐component and multiple‐component group interventions. Psycho‐Oncol. 2017;26(8):1147‐1154. [DOI] [PubMed] [Google Scholar]
- 81. Nelson AM, Jim HSL, Small BJ, et al. Sleep disruption among cancer patients following autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53(3):307‐314. 10.1038/s41409-017-0022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Peng L, Huang W, Zhang W, et al. Psychometric properties of the short form of the fear of cancer recurrence inventory (FCRI) in Chinese breast cancer survivors. Front Psychiatr. 2019;10(August):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Petzel MQB, Parker NH, Valentine AD, et al. Fear of cancer recurrence after curative pancreatectomy: a cross‐sectional study in survivors of pancreatic and periampullary tumors. Ann Surg Oncol. 2012;19(13):4078‐4084. [DOI] [PubMed] [Google Scholar]
- 84. Shin J, Shin DW, Lee J, et al. Association between perception of care coordination and health outcomes in Korean cancer survivors. Health Qual Life Outcome. 2020;18(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tesson S, Richards I, Porter D, et al. Women’s preferences for contralateral prophylactic mastectomy following unilateral breast cancer: what risk‐reduction makes it worthwhile? Breast. 2017;31:233‐240. 10.1016/j.breast.2016.11.025 [DOI] [PubMed] [Google Scholar]
- 86. Walburg V, Rueter M, Lamy S, et al. Fear of cancer recurrence in Non‐ and Hodgkin lymphoma survivors during their first three years of survivorship among French patients. Psychol Health Med. 2019;24(7):781‐787. [DOI] [PubMed] [Google Scholar]
- 87. Costa DSJ, Dieng M, Cust AE, Butow PN, Kasparian NA. Psychometric properties of the Fear of Cancer Recurrence Inventory: an item response theory approach. Psycho‐Oncol. 2016;838(October 2015):832‐838. [DOI] [PubMed] [Google Scholar]
- 88. Herman S, Razavi D, Lewis F, et al. Detecting dysfunctional fear of cancer recurrence in non‐metastatic breast cancer patients wishing psychological help: Usefulness of a “fear of cancer recurrence inhibition task”. Psycho‐Oncol. 2014;23:346–347. [Google Scholar]
- 89. Shun SC, Chou YJ, Kuo HJ. Was level of fear of recurrence associated with level of fatigue in colorectal cancer patients? Support Care Cancer. 2018;26(2):S134. [Google Scholar]
- 90. Smith TG, Strollo S, Hu X, Earle CC, Leach CR, Nekhlyudov L. Understanding long‐term cancer survivors’ preferences for ongoing medical care. J Gen Intern Med. 2019;34(10):2091‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thewes B, Bell ML, Butow P. Fear of cancer recurrence in young breast cancer survivors: the role of meta‐cognitive style and disease‐related factors. Asia Pac J Clin Oncol. 2012;8:253‐254. [DOI] [PubMed] [Google Scholar]
- 92. Van Liew JR, Christensen AJ, Howren MB, Karnell LH, Funk GF. Fear of recurrence impacts health‐related quality of life and continued tobacco use in head and neck cancer survivors. Heal Psychol. 2014;33(4):373‐381. [DOI] [PubMed] [Google Scholar]
- 93. Review Manager (RevMan). The Cochrane Collaboration; 2020.
- 94. American Cancer Society . Cancer Treatment and Survivorship Facts and Figures 2019‐2021. Am Cancer Soc. 2019:1‐48. [Internet] Available from: https://www.cancer.org/research/cancer‐facts‐statistics/survivor‐facts‐figures.html [Google Scholar]
- 95. van Marum RJ. Underrepresentation of the elderly in clinical trials, time for action. Br J Clin Pharmacol. 2020;86(10):2014‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liu J, Butow P, Bui KT, et al. Novel clinician‐lead intervention to address fear of cancer recurrence in breast cancer survivors. JCO Oncol Pract. 2021;17(6):e774‐e784. [DOI] [PubMed] [Google Scholar]
- 97. Smith AB, Sharpe L, Thewes B, et al. Medical, demographic and psychological correlates of fear of cancer recurrence (FCR) morbidity in breast, colorectal and melanoma cancer survivors with probable clinically significant FCR seeking psychological treatment through the ConquerFear study. Support Care Cancer. 2018;26(12):4207‐4216. 10.1007/s00520-018-4294-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


