Abstract
The timing of seaward migration is a key life‐history trait for many anadromous fish species, with growth and survival at sea depending on a match/mismatch scenario between the timing of the sea entry and optimal conditions. Based on a 25‐year study with 15,226 individually tagged brown trout (Salmo trutta) in a Norwegian river, we analysed how the within‐season timing of sea migration impacted growth and survival. In both first‐time and veteran migrants, marine growth was highest for early migrating individuals, large individuals, and those with a low condition factor when entering the sea. Survival was highest for individuals entering the sea early in the season. In first‐time migrants, survival increased with body length. Survival also increased with the number of other smolts migrating simultaneously. As the early smolts were the most successful, it may seem strange that many smolts migrate later in the season. We suggest that late‐migrating smolts may not be of a size and/or physiological state suitable for early marine conditions, and may make the best of a bad situation.
Keywords: anadromous, growth, match/mismatch, migration, survival
1. INTRODUCTION
Seasonal migration is a strategy utilized by many animals, including salmonids and other fishes, and may evolve when the use of multiple habitats results in increased lifetime fitness (e.g., Chapman cknoa, 2012; Dingle & Drake, 2007). Salmonids spawn in fresh water, but individuals of many species perform feeding migrations to the sea, which is a strategy termed anadromy (Gross, 1987). Anadromous life histories may evolve through natural selection when migration between fresh water and salt water results in an increased lifetime fitness compared to remaining in freshwater habitats (Gross, 1987). The duration of the marine stay varies among species and populations, from a few weeks to several years (Behnke, 2010; Klemetsen et al., 2003).
The seasonal timing of seaward migration is often a key life‐history trait for anadromous fish species. Migrations must be properly timed to avoid unfavourable conditions and utilize resource availability to maximize fitness (Dingle & Drake, 2007). The mortality of anadromous salmonids is high immediately after they enter salt water for the first time, mainly because of osmotic challenges (Finstad & Ugedal, 1998; Ugedal et al., 1998) and predation (Parker, 1971; Thorstad et al., 2016; Ward & Hvidsten, 2011). Size‐selective mortality at this life stage is common (Jensen et al., 2018a; Parker, 1971).
The seaward migration is likely initiated by in‐river environmental cues because these may be a signal that predicts favourable conditions in the sea (Thorstad et al., 2012). In‐river environmental cues that stimulate seaward migration of anadromous salmonids may differ among rivers, likely reflecting different local adaptations that ensure optimal conditions and high survival on entrance in the marine environment (Hvidsten et al., 1995, 1998; Kallio‐Nyberg et al., 2006; McCormick et al., 1998). However, the duration of the migration period within a population in a given year may span several weeks or months (Byrne et al., 2004; Jonsson & Jonsson, 2009; Pemberton, 1976). The causes and consequences of individual variation in the within‐year migration timing have received little attention (but see, e.g., del Villar‐Guerra et al., 2014, 2019). Understanding the fitness consequences of the different migration strategies may give new insights into how this variation is maintained both within and among populations.
In this study, the effects of the within‐season timing of migration to sea on marine growth, duration of the stay at sea, and survival rates in anadromous brown trout (Salmo trutta) from the River Halselva (northern Norway) were studied for 25 years. S. trutta is native to Europe and western Asia, and anadromous forms are found from Portugal in the south to the White Sea in northern Russia in the north (Elliott, 1989). S. trutta typically migrate to sea in spring, but timing differs between rivers and geographic areas (Jensen et al., 2012; Klemetsen et al., 2003), and in some watersheds autumn migrants may represent a significant contribution (Birnie‐Gauvin et al., 2019). In the River Halselva, the median date for first‐time migrants to leave the river is 4 July, and the time period during which 25%–75% of the S. trutta smolts have left the river averages 28 days (Jensen et al., 2012). Veteran migrants migrate to sea 4 weeks earlier than smolts.
S. trutta perform marine migrations of variable durations, with migrations being generally shorter in the north than in the south (Byrne et al., 2004; Jonsson & Jonsson, 2009; Pemberton, 1976). Some fish return to fresh water in autumn following only a few summer months at sea and continue to spend their subsequent summers at sea and winters in fresh water. Some individuals may remain at sea also during the following winter and stay at sea until they mature and return to fresh water to spawn (Thorstad et al., 2016). In northern Norway, S. trutta usually spend one to three summer months at sea to feed (Nevoux et al., 2019; Thorstad et al., 2016). In the River Halselva, this period averages 56 days for first‐time migrants (Jensen et al., 2018b). Thereafter, they return to fresh water to overwinter, and continue to migrate between fresh water and the sea each summer (Jensen et al., 2015). Some individuals may overwinter in other large watersheds before returning to the River Halselva on maturation (Jensen et al., 2015). Maturity usually occurs after two to four marine migrations (Jensen et al., 2019).
The effects of migration timing, fish size, condition factor, and duration of the stay at sea on growth rate, total marine growth, and survival were investigated using generalized linear or additive mixed models. We also studied the impact of shoaling on the survival of first‐time migrants by comparing survival with the daily number of individuals leaving the river. This was tested on S. trutta alone, but also on all first‐time migrating salmonids, including Atlantic salmon (Salmo salar) and Arctic char (Salvelinus alpinus). All fish were trapped and individually tagged 200 m upstream from the sea. Thereafter, tagged fish were recorded on their annual migrations between the river and the sea each time they migrated up‐ and downstream for the rest of their lives. This long‐term study is based on the behaviour of 15,226 tagged S. trutta individuals.
2. MATERIALS AND METHODS
2.1. Study area
The Halselva watershed has a catchment area of 143 km2 and drains into the Alta Fjord at 70°2'N, 22°57'E (Figure 1). Anadromous salmonids can migrate 20 km upstream, including to a 1.2 km2 lake located 2 km inland, 30 m above sea level (Figure 1). S. trutta and S. alpinus are the most abundant fish species. S. salar and European eel (Anguilla anguilla) occur in low numbers. The watershed is ice‐covered from December to March/April, which is usually a period with low water discharge. The water discharge increases during the snowmelt in May–June, decreases again in July–August, is rather stable during September–October, before it drops again in November (Jensen et al., 2012). The mean annual water discharge is 4.3 m3 s−1. The river temperature is 0 °C during winter and increases to a maximum of 13 °C in August. Sea temperatures at 3 m depth vary from 2.5 °C in late March to 11 °C in late July–early August (Jensen et al., 2012).
FIGURE 1.

Map of the study area, with the location of the fish traps in the River Halselva for catching all ascending and descending fish
2.2. Fish sampling
During 1987–2012, migrating fish were sampled in traps in the river, 200 m upstream from the sea. All descending fish larger than 10 cm were captured in a Wolf trap (apertures 10 mm, inclination 1:10; Wolf, 1951). The ascending fish were captured in a fixed box trap (Jensen et al., 2018b). The traps were operated during the ice‐free period of the year and were emptied twice a day, at 08:00 AM and 08:00 PM. All fish were anaesthetized with Benzoak or AQUI‐S before body length (L, in mm) and mass (M, in g) were recorded and the fish tagged. The fish were externally inspected to determine sexual maturity and the sex of individuals classified as mature was determined. After that, they were kept for observation for 12 h before they were released at the traps. Fish larger than 14 cm (since 1993, S. alpinus and S. trutta larger than 18 cm) were tagged with individually numbered external Carlin tags (Carlin, 1955). Smaller fish were tagged by removing a flap on one or both maxillary bones (Gjerde & Refstie, 1988) in a systematic manner to enable future identification of year of descent. Observed mortality due to handling, anaesthetization and tagging was very low (less than 0.1%), although increased mortality later in life due to tagging with Carlin tags has been documented elsewhere (Hansen, 1988). During the first study year (1987), first‐time migrants could not be distinguished with certainty from veteran migrants when they were captured in the traps. Beginning in the second study year (from 1988 and onwards), first‐time migrants could be recognized in the downstream trap because they were untagged, whereas the veteran migrants had been tagged during a previous migration. First‐time migrants of S. trutta that left the river before 1 August each year during 1988–2012, with body length between 180 and 280 mm, were used in this study, in total 15,226 individuals. Tagged fish were later recorded whenever they passed the fish traps for the rest of their lives. We use the term veteran migrant when we refer to S. trutta that had been to sea one or several times earlier in life. Up to 11 subsequent sea migrations were observed (Jensen et al., 2019).
2.3. Data analyses
We first calculated the growth rate (standardized mass‐specific growth rate, Ω, % d−1), total growth increment (increase in body mass during the marine migration, g) and survival (i.e., return rate, percentage) during the marine migration for S. trutta entering the sea in different weeks of the year. Return to the trap or recaptures at sea or in other watersheds was used as a proxy for survival until the point of return or recapture.
The standardized mass‐specific growth rate (Ω, % d−1) was estimated as follows (Ostrovsky, 1995):
where M 0 is the body mass of the individual fish at river descent, M 1 is the body mass of the same individual when returning to the river later in the year, t 0 is the date when the fish descended, t 1 is the date when the fish ascended again, t 1 – t 0 is the time the fish stayed at sea and b is the allometric mass exponent for the relationship between specific growth rate and body mass. The parameter b may vary across S. trutta populations (Forseth et al., 2009), but we chose the value b = 0.31, as estimated by Elliott et al. (1995). Ω eliminates the effects of differences in initial body size (Sigourney et al., 2008).
The Fulton's condition factor (CF) was estimated from the mass (M) and body length (L) of the fish as:
When analysing the effects of within‐season timing on growth and survival at sea, we fitted mixed models to account for variation in individual responses not accounted for by the fixed factors and inducing correlation in the model among repeated measurements on the same individuals. For all models, the year of first marine migration was defined as a random factor. For models with repeated observations from the same individual, i.e., models pooling subsequent marine migrations, individual fish tag number was also used as a random factor.
Generalized linear mixed models (GLMMs) were used when the response variable was assumed to have a non‐normal distribution, otherwise ordinary linear mixed models (LMMs) were fitted. Generalized additive mixed models (GAMMs, implemented in the R package gamm4) by Wood and Scheipl (2020) were investigated when nonlinear associations were considered relevant. For each model, we tested the residual distributions for appropriateness, as well as for overdispersion when the response variable was binomially distributed.
Model selection was based on the explained deviance, effect sizes and relevance of the model terms. Several of the predictors were correlated so individual term coefficients and P values cannot be evaluated independently and must be interpreted with caution. The correlation structure of the predictor variables was investigated by pair‐wise correlations and principal component analysis to aid the selection procedure.
Initially, separate models for the first and each subsequent marine migration were fitted because the magnitude and importance of different predictor variables may vary depending on size, experience and behaviour. When models for subsequent marine migrations did not show any significant differences, they were pooled. This procedure resulted in separate models for first‐time migrants for all response variables and pooled models for second and later summers at sea for all but one response variable. When modelling the duration of the marine migration, separate models for the second summer at sea and third summer and later are also presented.
In addition to the list of predictors specified below for each model, sea temperatures during marine migration were also evaluated during the initial phase of the modelling, but no reliable systematic effects of sea temperature were found. When sea temperature was included as a smoothed nonlinear term, it showed a fluctuating pattern for some models, indicating that it was a proxy for year‐to‐year variation not accounted for by other fixed explanatory variables.
As both growth and survival at sea are expected to be affected by the duration of the marine migration (days) in addition to within‐season timing of migration, we first evaluated the associations between the duration of migration and the predictor capture date during downstream migration (day of the year), body length (mm) and condition factor at downstream migration. An ordinary LMM was used, with year of first marine migration and individual fish tag number as random factors.
The model for standardized mass‐specific growth rate had capture date during downstream migration (day of the year), body length (mm) at downstream migration, condition factor at downstream migration and duration of marine migration as predictor candidates. We fitted a GAMM as we found a nonlinear association with duration of marine migration and approximately normally distributed residuals.
Total marine growth was also modelled by a GAMM, with capture date during downstream migration (day of the year), body length (mm) at downstream migration, condition factor at downstream migration and duration of marine migration as potential predictors.
Marine survival rates were modelled by a GLMM with a binomially distributed response and a logit link function, with year of first marine migration and individual fish tag number as random factors. Capture date during downstream migration (day of the year), body length (mm) at downstream migration, condition factor at downstream migration, duration of marine migration and number of smolts migrating the same day were tested as potential predictors. The daily number of migrating smolts included those that were too small to be individually tagged and we tested both the number of S. trutta smolts only, as well as the total number of salmonid smolts (S. trutta, S. alpinus and S. salar). No overdispersion was detected. Data from the last 2 years of the time series (2011 and 2012) were excluded from the data set used to model smolt survival because in the first two or three marine migrations it is possible that smolts may overwinter elsewhere and their survival could not be recorded within the remaining study period (Jensen et al., 2015).
All regression modelling and analyses were performed using the statistical software R, version 4.0.3 (R‐Core‐Team, 2020).
2.4. Ethics statement
The use and care of experimental fish complied with Norwegian animal welfare laws and policies.
3. RESULTS
3.1. Migration timing
First‐time migrants (smolts) migrated to the sea over a long time period, but most frequently during the weeks 25–28 (Figure 2), with a median date of 4 July. In most years, the median migration took place during weeks 26 (24%) and 27 (60%) (range 25–28), with no significant trend during the study period (r = 0.303, P > 0.05). For first‐time migrants that entered the sea during these 4 weeks, the standardized mass‐specific growth rate during the marine migration (Figure 1) was high (mean 9.00 ± 0.12% d−1), but was lower for individuals entering the sea earlier or later than weeks 25–28 (Figure 2). The total marine growth was the highest for first‐time migrants that entered the sea early in the season, in weeks 21–23, and decreased successively for later‐migrating individuals (Figure 2). Survival was the highest for first‐time migrants that entered the sea during week 23 (Figure 2).
FIGURE 2.

Growth and survival of Salmo trutta during their first marine migration, sorted by the week of the year when they migrated to the sea. (a) Standardized mass‐specific growth rate (Ω, % d−1, solid line) and total marine growth increment (g, broken line), including 95% confidence intervals. (b) Number of fish descending to the sea (bars), divided between individuals that were still alive after the first marine migration (Alive), those that died during this period (Dead) and the proportion of surviving individuals (percentage, solid line). Survival rates during weeks 19–20 are uncertain because of low sample sizes
Veteran migrants entered the sea earlier than smolts. The median dates for second‐, third‐, fourth‐ and fifth‐time migrants were 25, 26, 29 and 29 days earlier than for first‐time migrants, respectively. All these groups migrated most frequently during weeks 21–25 (Figure 3) and 50% of the individuals of all groups had passed the trap during week 23. The standardized mass‐specific growth rate was high (8–10% d−1) during these weeks. As for first‐time migrants, the total mass increment during the marine migration was the highest for early migrating individuals (Figure 3). Among second‐time migrants, survival rates during the marine migration were the highest (62–63%) for individuals that entered the sea during weeks 22–23, whereas for third‐, fourth‐ and fifth‐time migrants survival rates did not vary much between weeks (variation between 61% and 75%, 59% and 62% and 49% and 57% survival for third‐, fourth‐ and fifth‐time migrants, respectively, Figure 3).
FIGURE 3.
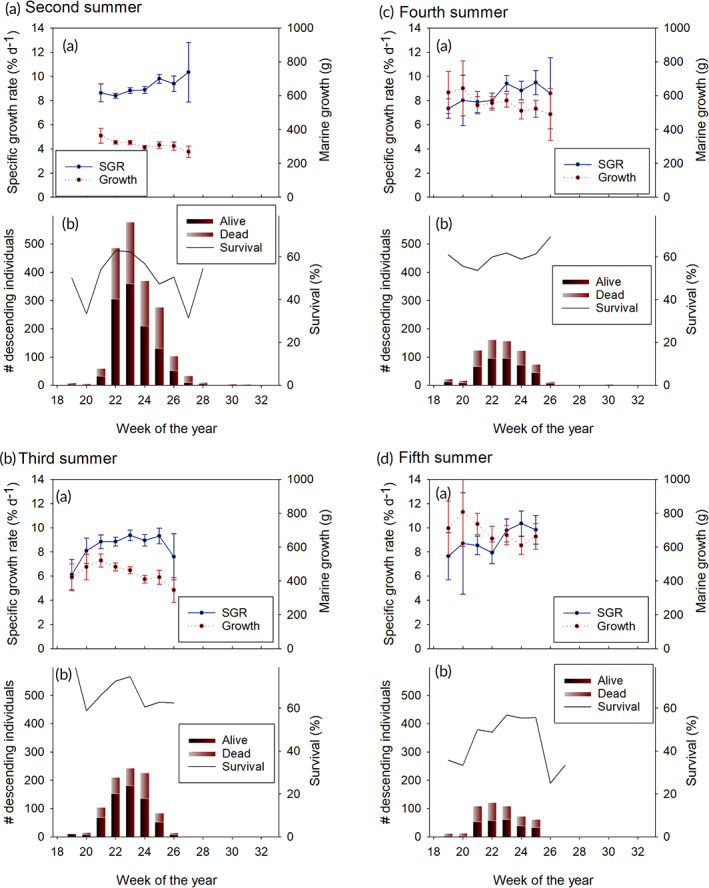
Growth and survival of Salmo trutta during their (a) second, (b) third, (c) fourth and (d) fifth marine migration, sorted by the week of the year when they migrated to the sea. The notations are the same as those in Figure 2
3.2. Duration of the marine migration
There was strong evidence of an association between the duration of marine migration and date at downstream migration for both first‐time and veteran migrants, with a longer time spent at sea for early than late migrating individuals (LMM, Table 1). In addition, for veteran migrants there was strong evidence of an association between the duration of the marine migration and the date at downstream migration, with a longer time spent at sea for early‐migrating than for later‐migrating individuals (Table 1). Body length was less important for the duration of marine migration after the second summer at sea than for first‐time and second‐time migrants. For all three stage‐specific models (Table 1), migrating 1 day later reduced the expected duration of marine migration by at least 0.6 days.
TABLE 1.
Results from a linear mixed‐effects model used to identify variables associated with the duration of marine migration for the first, second, third and later summers at sea
| Fixed effects | Estimates | Standard errors | t value |
|---|---|---|---|
| First summer at sea | |||
| (Intercept) | 196 | 3.59 | 54.7*** |
| Date at downstream migration | −0.67 | 0.014 | −48.8*** |
| Body length at downstream migration | −0.091 | 0.0088 | −10.4*** |
| Second summer at sea | |||
| (Intercept) | 179 | 7.79 | 23.0*** |
| Date at downstream migration | −0.62 | 0.043 | −14.6*** |
| Body length at downstream migration | −0.059 | 0.0104 | −5.7*** |
| Third summer and later | |||
| (Intercept) | 164 | 6.09 | 27.0*** |
| Date at downstream migration | −0.68 | 0.038 | −18.0*** |
Note: Explanatory variables in the model were capture date (i.e., day of the year) during downstream migration at the trap (date at downstream migration) and body length at downstream migration. Smolt year and Fish‐ID (for >2 summers at sea) were used as random factors. *** P < 0.001.
3.3. Standardized mass‐specific growth rate
For first‐time migrants, the GAMM describing the standardized mass‐specific growth rate during marine migration included linear terms for the date at downstream migration and condition factor at migration to sea, and a nonlinear smoothed term for the duration of marine migration (Table 2). First‐time migrants that entered the sea later and had a higher condition factor tended to have a lower mass‐specific growth rate than first‐time migrants that entered the sea earlier and had a lower condition factor. First‐time migrants with intermediate‐duration marine migrations (around 30–60 days) had a larger mass‐specific growth rate than those with a shorter or longer marine migration (Figure 4a). For veteran migrants, the mass‐specific growth rate was not associated with the timing of downstream migration. However, there were linear and negative associations between mass‐specific growth rate and duration of sea migration, body length at downstream migration and condition factor at downstream migration for the veteran migrants (Table 2).
TABLE 2.
Results from an additive mixed‐effects model used to identify variables associated with the standardized mass‐specific growth rate (Ω) during the first summer at sea, and during the second and later summers at sea
| Fixed effects | Estimates | Standard errors | t value |
|---|---|---|---|
| First summer at sea | |||
| (Intercept) | 19.1 | 0.80 | 24.0*** |
| Date at downstream migration | −0.024 | 0.0036 | −6.7*** |
| Condition factor at downstream migration | −7.80 | 0.57 | −13.7*** |
| Smoothed terms | Ref.df | F‐value | |
| Duration of marine migration | 7.47 | 122.9*** | |
| Second and later summers at sea | |||
| (Intercept) | 23.9 | 0.48 | 50.1*** |
| Duration of marine migration | −0.11 | 0.0028 | −40.4*** |
| Body length at downstream migration | −0.0058 | 0.00046 | −12.7*** |
| Condition factor at downstream migration | −7.6 | 0.48 | −16.0*** |
Note: Explanatory variables in the model were capture date (i.e., day of the year) during downstream migration at the trap (date at downstream migration), body length and condition factor at downstream migration, and a smoothed nonlinear term for the duration of the marine migration (Figure 4a). Smolt year and Fish‐ID (for >2 summers at sea) were used as random factors. *** P < 0.001.
FIGURE 4.

Smoothed, nonlinear relationships between the duration of the sea migration for first‐time migrants (in days) and the (a) standardized mass‐specific growth rate (Ω) and (b) growth (in g). The scale of the vertical axes shows the contribution from the smooth components of the fitted generalized additive mixed models described in Tables 2 and 3, respectively. The shaded area indicates two standard errors above and below the smoothed curve. The rug plot at the foot of each panel shows the jittered values for individual period lengths
3.4. Marine growth
The GAMM for marine growth of first‐time migrants showed strong evidence of associations with date, body length and condition factor at downstream migration (Table 3). Marine growth tended to be greater among early migrants, large individuals and those with a low condition factor than among late migrants, small individuals and those with a high condition factor. Among first‐time migrants, we also found strong evidence of a nonlinear association between marine growth and duration of marine migration, where marine growth increased with the duration of marine migration, but only among individuals staying for up to approximately 60 days at sea (Figure 4b). Individuals staying longer at sea did not tend to have a larger growth than those staying approximately 60 days at sea (Figure 4b). Among veteran migrants, marine growth was mainly associated with body length and condition factor when they left the river, with a larger marine growth in larger individuals and individuals with a low condition factor than in smaller individuals and individuals with a high condition factor (Table 3). There was less evidence for an association between marine growth and date at downstream migration or the duration of marine migration among veteran migrants.
TABLE 3.
Results from an additive mixed‐effects model used to identify variables associated with the marine growth (in grams) during the first summer at sea, and the second and later summers at sea
| Fixed effects | Estimates | Standard errors | t value |
|---|---|---|---|
| First summer at sea | |||
| (Intercept) | 110 | 21.4 | 5.1*** |
| Date at downstream migration | −0.84 | 0.079 | −10.7*** |
| Body length at downstream migration | 1.08 | 0.037 | 29.2*** |
| Condition factor at downstream migration | −46 | 12.0 | −3.9*** |
| Smoothed terms | Ref. d.f. | F value | |
| Duration of marine migration | 5.74 | 150*** | |
| Second and later summers at sea | |||
| (Intercept) | 9.4 | 31.1 | 0.30 |
| Body length at downstream migration | 1.66 | 0.033 | 50*** |
| Condition factor at downstream migration | −257 | 35.1 | −7.3*** |
Note: Explanatory variables in the model were capture date (i.e., day of the year) during downward migration at the trap (date at downstream migration), and body length and condition factor at downstream migration. In addition, a non‐linear effect of the duration of marine migration was included (Figure 4b). Smolt year and Fish‐ID (for >2 summers at sea) were used as random factors. *** P < 0.001.
3.5. Marine survival
The marine survival of first‐time migrants was strongly associated with date and body length at downstream migration (GLMM, Table 4). Except for some very early migrants, early‐migrating smolts survived better than late‐migrating smolts (Figure 5a). Large smolts survived better than small smolts, independent of the timing of downstream migration (Figure 5b). In addition, early migrating veterans experienced higher survival rates than later migrating veterans. Furthermore, veterans with a high condition factor at downstream migration survived better than those with a lower condition factor (Table 4). The survival of veterans decreased with increasing body length at downstream migration.
TABLE 4.
Results from a generalized linear mixed model used to identify variables associated with survival during the first summer at sea, and the second and later summers at sea
| Fixed effects | Estimates | Standard errors | z value |
|---|---|---|---|
| First summer at sea | |||
| (Intercept) | −0.71 | 0.32 | −2.2* |
| Date at downstream migration | −0.0097 | 0.0012 | −8.0*** |
| Body length at downstream migration | 0.0073 | 0.00089 | 8.2*** |
| Second summer at sea and older | |||
| (Intercept) | 1.66 | 0.64 | 2.6** |
| Date at downstream migration | −0.012 | 0.0031 | −3.8*** |
| Body length at downstream migration | −0.0025 | 0.00045 | −5.5*** |
| Condition factor at downstream migration | 1.85 | 0.43 | 4.3*** |
Note: Explanatory variables associated with sea survival were date (i.e., day of the year) during downstream migration at the trap (date at downstream migration), and body length and condition factor at downstream migration. Smolt year and Fish‐ID (for >2 summers at sea) were used as random factors. * P < 0.05, ** P < 0.01, *** P < 0.001.
FIGURE 5.

Observed (open circles) and predicted (filled circles) survival rates for first‐time migrant Salmo trutta (smolts), averaged over (a) 7 days and (b) 10 mm body length intervals, respectively. Model predictions are calculated from the generalized linear mixed model for the first summer at sea (Table 4). Dashed lines show the overall observed survival rate for smolts (0.295). Red lines indicate the approximate 95% confidence intervals for the survival rates for each date of the year or body length interval. Interval estimates without confidence intervals indicated have sample sizes (<20 fish) that are too small for uncertainty calculations to be reliable
A positive relationship was found between the survival of smolts and the number of S. trutta smolts that migrated to the sea during the same day (r 2 = 0.006, F 1,1279 = 7.73, P = 0.006). The survival of S. trutta smolts was also related to the sum of all salmonid smolts migrating during the same day (S. trutta, S. alpinus and S. salar), but was less significant (r 2 = 0.004, F 1,1279 = 5.53, P = 0.019) than for S. trutta.
4. DISCUSSION
Early migration seems to be a beneficial strategy in the River Halselva S. trutta population in terms of growth and survival, for both first‐time and veteran migrants. First‐time migrants that entered the sea earlier and had a lower condition factor tended to have a higher mass‐specific growth rate than those entering the sea later and with a higher condition factor, while for veteran migrants the mass‐specific growth rate was not associated with the timing of downstream migration. The duration of the period at sea, however, and hence the total growth during the marine migration, was the highest for the early migrants, and early migrants had the highest survival rates. The low variation in mass‐specific growth rate with migration timing, especially for veteran migrants, indicates stable food availability for S. trutta in the Alta Fjord throughout the whole summer. Hence, the higher survival among early migrants may mainly be because of larger body size than that of later migrants.
The ability to survive, reproduce and contribute genetically to the next generation determines individual fitness (Fisher, 1958). Hence, survival to first spawning and survival to spawn multiple times in iteroparous species such as S. trutta are key to increased fitness (Hendry et al., 2018). The highest marine survival rates among first‐time migrants were recorded for smolts migrating to the sea in June and early July (in weeks 22–26). The environmental conditions during this period in terms of high‐water discharge may have provided some protection against predators and contributed to a higher survival rate. A large number of predators such as saithe (Pollachius virens) and cod (Gadus morhua) may assemble at the estuary foraging on descending smolts, and mortality rates of S. salar smolts from predation have been reported to exceed 20% in the first few days after leaving fresh water (Hvidsten & Lund, 1988; Hvidsten & Møkkelgjerd, 1987; Ward & Hvidsten, 2011). For smolts in the River Halselva, it has been shown that the sea is a dangerous place, with daily mortality rates estimated to be 14–15 times higher at sea than in fresh water (Jensen et al., 2019), which is likely, at least in part, owing to higher predation rates at sea. In spite of high daily mortality rates at sea, early migrants, which spent more time at sea than later migrants, had the highest survival rates. This may appear contradictory, but size‐selective mortality during the sea stay is common in the Hals population of S. trutta (Jensen et al., 2018a) and this may explain some of this variation.
In salmonids, growth is an important contributor to reproductive success, particularly in females. The fecundity in terms of the number of eggs increases linearly with body size (Elliott, 1995; Jonsson & Jonsson, 1999). In males, the relationship between body size and reproductive success is less clear because males may use different tactics to gain access to females during spawning, either by being small and cryptic or by being big and able to fight and chase away other males, and perhaps also impress the females (Fleming, 1998). However, growth and large body size are, to some extent, important also for males, particularly for males who do not rely on cryptic tactics during spawning. The difference between females and males in the advantages of a large body size also results in more females than males of S. trutta adopting the marine migration strategy to utilize better growth opportunities at sea than in fresh water, both in the River Halselva (63% females among first‐time migrants; Jensen et al., 2012) and in other rivers (Thorstad et al., 2016).
If early migration is the most successful strategy, the large variation in migration timing among individuals is perhaps not intuitive. The migration period within a river in a given year may last several weeks (Byrne et al., 2004; Jonsson & Jonsson, 2009; Pemberton, 1976). This may be because individuals possess different qualities in terms of body size and physiological state that may make them more or less suitable for an early sea entry. The later‐migrating smolts may have a size and/or physiological condition that makes them more vulnerable to cold temperatures, osmoregulatory problems, and predation early in the season. Hence, the costs of early migration for these individuals may exceed the benefits. The timing of the marine migration in the spring varies among populations and regions, likely as an adaptation to ensure optimal conditions and high survival when they enter the sea (Hvidsten et al., 1998; Kallio‐Nyberg et al., 2006; McCormick et al., 1998). Early sea entry may be beneficial because it is usually a period with a rich prey fauna, which is important for feeding and growth (Berg & Berg, 1987; Knutsen et al., 2001, 2004; Olsen et al., 2006). In contrast, early spring is also a period with a high mortality risk due to osmoregulatory problems and predation from other fishes, birds and mammals (Greenstreet et al., 1993; Hedger et al., 2011; Hvidsten & Møkkelgjerd, 1987; Sigholt & Finstad, 1990). The transition of salmonids from fresh water to sea water involves challenging physiological adaptations and radical habitat alteration (Hoar, 1988; Thorstad et al., 2012). Osmoregulatory problems in salmonids, including S. trutta, may increase at sea temperatures lower than 6–7 °C (Arnesen et al., 1998; Finstad et al., 1988; Sigholt & Finstad, 1990; Thomsen et al., 2007) and migration to the sea too early, at temperatures lower than this, might be risky. In the River Halselva, sea temperatures usually exceed 6 °C around 1 June (week 22) (Jensen et al., 2012). We found that the survival of individuals entering the sea before that was lower than average, which might have been partly related to osmoregulatory challenges at these low temperatures.
The large body size among early first‐time migrants in this and many other rivers (Bohlin et al., 1993; Ewing et al., 1984; Jensen et al., 2012; Jutila & Jokikokko, 2008) may be beneficial for meeting the challenges of early sea entry. In general, we found that large individuals among first‐time migrants survived better than small ones. The poorer swimming capacity of small compared to large individuals may result in a higher predation risk and thereby lower sea survival, particularly at low water temperatures early in the season (Jensen et al., 2018a; Parker, 1971; Ugedal et al., 1998). Hence, large individuals may be fit to migrate early and benefit from the feeding opportunities at sea at a lower risk and mortality than small individuals. Large individuals may therefore be favoured because they migrate early and stay longer at sea, and because they are large. These benefits related to large body size may also be the reason that large veterans migrated to the sea earlier than first‐time migrants. Another reason for the smaller smolts migrating late in the season may be that some of these were too small for smoltification in the spring, but reached a length and physiological state suitable for smoltification in the summer, and therefore migrated to the sea later. Therefore, smolts migrating late in the season may simply be making the best of a bad situation.
Poor body condition at the time of migration was associated with a higher growth rate and larger mass increment during marine migration. Energetic status influences life‐history decisions and behaviour of S. trutta during several life stages. Individuals with poor body condition are more likely to migrate to the sea than those with higher condition (Davidsen et al., 2014; Wysujack et al., 2009). Furthermore, individuals with low triglyceride levels have been associated with early sea entry (Eldøy et al., 2021). The high growth of S. trutta with poor body condition in the present study might be a result of energetic status influencing their migration and feeding strategies while at sea. The S. trutta from River Halselva usually stay no deeper than 3 m from the surface, preferably in the inner Alta Fjord (Atencio et al., 2021; Rikardsen et al., 2007a), where they feed mainly on fish, insects and crustaceans (Rikardsen et al., 2007b). They become virtually all piscivorous at body lengths of approximately 250 mm, that is, usually already during the first marine migration, and when available, herring (Clupea harengus) seems to be the preferred prey (Rikardsen et al., 2007b). Fast‐growing S. trutta switch to a more piscivorous diet at a younger age and smaller size than slow‐growing S. trutta (Klemetsen et al., 2003). Eldøy et al. (2015) showed that long‐distance migrating individuals had poorer body condition in advance of migration and used pelagic areas more often than short‐distance migrants. They suggested that long‐distance migrants found more energy‐rich prey in the outer fjord areas and therefore could gain mass faster and return earlier to fresh water because they had utilized their compensatory growth potential. Hence, they showed that body condition may influence the feeding and migration behaviour of the fish. There were no signs that first‐time migrants adopted a risky behavioural strategy, although poor body condition was not associated with increased mortality. However, for veteran migrants poor body condition is associated with reduced survival. In our study, individuals with an intermediate duration of marine migration, approximately 30–60 days, had a higher growth rate and total growth than those staying longer at sea. This is consistent with the suggestion by Eldøy et al. (2015) that individuals that feed on more energy‐efficient prey may gain mass faster and be able to return to a safer freshwater environment earlier.
Survival increased when many smolts migrated to the sea on the same day, although such shoaling only explained a small proportion of the variation in survival. Synchronous migration may be expected when the optimal period for seawater entry is short; however, such synchronicity may also reflect an antipredator behaviour that increases survival through predator swamping and confusion effects (Finstad & Jonsson, 2001). As flow is the main proximate factor explaining day‐to‐day variation in downstream migration of S. trutta in the River Halselva (Jensen et al., 2012), this positive effect of shoaling on survival was expected. Shoaling is a common antipredator adaptation in several fish species (Krause & Ruxton, 2002) and has also been observed in salmonids migrating from fresh water to the sea. An increased chance of survival by shoaling behaviour was demonstrated by Hvidsten and Johnsen (1993), who observed increased survival of hatchery‐produced S. salar smolts when they were released into shoals of migrating wild smolts. In the present study, survival of S. trutta smolts was also significantly correlated with the sum of all salmonid smolts migrating during the same day; however, it was less significant than that for S. trutta alone. The opposite was expected, but this could be because of the different behaviour between the three species when arriving at sea.
The results of this study were relatively similar between first‐time and veteran migrants. Early veteran migrants had higher survival rates, similar to first‐time migrants, and growth rate and growth were associated with body size and condition factor. In contrast to first‐time migrants, growth and growth rates were not associated with migration timing. A smaller impact of migration timing may be as expected, as the veteran migrants entered the sea earlier and over a shorter time period than the first‐time migrants.
In conclusion, the within‐season timing of migration to the sea influenced both the growth and survival of S. trutta. Body condition and size at the time of migration also influenced growth and survival. Marine growth and survival were the highest for early migrating individuals, although a too‐early migration seemed to be risky, likely because of unfavourable environmental conditions. Although not discussed here, variation among years in environmental conditions may also contribute to maintaining variation in migration timing. As the early first‐time migrants were the most successful, one may ask why a large proportion of the population migrates later in the season. We suggest that this is linked to individual differences in body size and physiological state. A large proportion of the individuals may not be in a state suitable for early marine conditions, and late‐migrating smolts may make the best of a bad situation. Hence, there is likely a trade‐off between the potential for energy gain and survival, which maintains the variation in migration timing. This trade‐off can be further explored using individual‐based state‐dependent models that can make us better able to understand both variation among individuals and among populations in migration behaviour. This study provides important input data for models that can be used to further improve our understanding of migration between different habitats.
FUNDING INFORMATION
This study was funded by Statkraft Energi AS, the Miljødirektoratet and the Norwegian Institute for Nature Research (NINA). B.F. acknowledges financial support obtained from DNV.
CONFLICT OF INTEREST
The authors declare there are no competing interests.
AUTHOR CONTRIBUTIONS
A.J.J. designed the study. A.J.J. and B.F. collected the data. O.H.D. and A.J.J. analysed the data. A.J.J. and O.H.D. wrote the first draft of the manuscript, with all authors contributing to subsequent revisions. All authors gave final approval for publication.
ACKNOWLEDGMENTS
We thank the staff at the Talvik Research Station for assistance with the fish traps.
Jensen, A. J. , Diserud, O. H. , Finstad, B. , Fiske, P. , & Thorstad, E. B. (2022). Early‐season brown trout (Salmo trutta) migrants grow and survive better at sea. Journal of Fish Biology, 100(6), 1419–1431. 10.1111/jfb.15052
Funding information Miljødirektoratet
REFERENCES
- Arnesen, A. M. , Johnsen, H. K. , Mortensen, A. , & Jobling, M. (1998). Acclimation of Atlantic salmon (Salmo salar L.) smolts to “cold” sea water following direct transfer from fresh water. Aquaculture, 168, 351–367. [Google Scholar]
- Atencio, B. J. , Thorstad, E. B. , Rikardsen, A. H. , & Jensen, J. L. A. (2021). Keeping close to the river, shore, and surface: The first marine migration of brown trout (Salmo trutta) and Arctic charr (Salvelinus alpinus) post‐smolts. Journal of Fish Biology., 99, 462–471. 10.1111/jfb.14737. [DOI] [PubMed] [Google Scholar]
- Behnke, R. J. (2010). Trout and salmon of North America. New York: Simon and Schuster. [Google Scholar]
- Berg, O. K. , & Berg, M. (1987). The seasonal pattern of growth of the sea trout (Salmo trutta L.) from the Vardnes river in northern Norway. Aquaculture, 62, 143–152. [Google Scholar]
- Birnie‐Gauvin, K. , Thorstad, E. B. , & Aarestrup, K. (2019). Overlooked aspects of the Salmo salar and Salmo trutta lifecycles. Reviews in Fish Biology and Fisheries, 29, 749–766. 10.1007/s11160-019-09575-x. [DOI] [Google Scholar]
- Bohlin, T. , Dellefors, C. , & Faremo, U. (1993). Optimal time and size for smolt migration in wild sea trout (Salmo trutta). Canadian Journal of Fisheries and Aquatic Sciences, 50, 224–232. 10.1139/f93-025. [DOI] [Google Scholar]
- Byrne, C. J. , Poole, R. , Dillane, A. , Rogan, G. , & Whelan, K. F. (2004). Temporal and environmental influences on the variation in sea trout (Salmo trutta L.) smolt migration in the Burrishoole system in the west of Ireland from 1971 to 2000. Fisheries Research, 66, 85–94. 10.1016/S0165-7836(03)00146-2. [DOI] [Google Scholar]
- Carlin, B. (1955). Tagging of salmon smolts in the river lagan. Report of the Institue of Freshwater Research Drottningholm, 36, 57–74. [Google Scholar]
- Chapman, B. B. , Skov, C. , Hulthén, K. , Brodersen, J. , Nilsson, P. A. , Hansson, L.‐A. , & Brönmark, C. (2012). Partial migration in fishes: Definitions, methodologies and taxonomic distribution. Journal of Fish Biology, 81, 479–499. 10.1111/j.1095-8649.2012.03349.x. [DOI] [PubMed] [Google Scholar]
- Davidsen, J. G. , Daverdin, M. , Sjursen, A. D. , Rønning, L. , Arnekleiv, J. V. , & Koksvik, J. I. (2014). Does reduced feeding prior to release improve the marine migration of hatchery brown trout Salmo trutta smolts? Journal of Fish Biology, 85, 1992–2002. 10.1111/jfb.12485. [DOI] [PubMed] [Google Scholar]
- del Villar‐Guerra, D. , Aarestrup, K. , Skov, C. , & Koed, A. (2014). Marine migrations in anadromous brown trout (Salmo trutta). Fjord residency as a possible alternative in the continuum of migration to the open sea. Ecology of Freshwater Fish, 23, 594–603. 10.1111/eff.12110. [DOI] [Google Scholar]
- del Villar‐Guerra, D. , Larsen, M. H. , Baktoft, H. , Koed, A. , & Aarestrup, K. (2019). The influence of initial developmental status on the life‐history of sea trout (Salmo trutta). Scientific Reports, 9, 13468. 10.1038/s41598-019-49175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle, H. , & Drake, V. A. (2007). What is migration? Bioscience, 57, 113–121. 10.1641/B570206. [DOI] [Google Scholar]
- Eldøy, S. H. , Bordeleau, X. , Lawrence, M. J. , Thorstad, E. B. , Finstad, A. G. , Whoriskey, F. G. , … Davidsen, J. G. (2021). The effects of nutritional state, sex and body size on the marine migration behaviour of sea trout. Marine Ecology Progress Series, 665, 185–200. 10.3354/meps13670. [DOI] [Google Scholar]
- Eldøy, S. H. , Davidsen, J. G. , Thorstad, E. B. , Whoriskey, F. , Aarestrup, K. , Næsje, T. F. , … Arnekleiv, J. V. (2015). Marine migration and habitat use of anadromous brown trout (Salmo trutta). Canadian Journal of Fisheries and Aquatic Sciences, 72, 1366–1378. 10.1139/cjfas-2014-0560. [DOI] [Google Scholar]
- Elliott, J. M. (1989). Wild brown trout Salmo trutta: An important national and international resource. Freshwater Biology, 21, 1–5. 10.1111/j.1365-2427.1989.tb01343.x. [DOI] [Google Scholar]
- Elliott, J. M. (1995). Fecundity and egg density in the redd for sea trout. Journal of Fish Biology, 47, 893–901. [Google Scholar]
- Elliott, J. M. , Hurley, M. A. , & Fryer, R. J. (1995). A new, improved growth model for brown trout, Salmo trutta . Functional Ecology, 9, 290–298. 10.2307/2390576. [DOI] [Google Scholar]
- Ewing, R. D. , Hart, C. E. , Furtish, C. A. , & Concannon, G. (1984). Effects of size and time of release on seaward migration of spring Chinook salmon, Oncorhynchus tshawytscha . Fisheries Bulletin, 82, 157–164. [Google Scholar]
- Finstad, B. , & Jonsson, N. (2001). Factors influencing the yield of smolt releases in Norway. Nordic J. Freshw. Res., 75, 37–55. [Google Scholar]
- Finstad, B. , Staurnes, M. , & Reite, O. B. (1988). Effect of low temperature on sea‐water tolerance in rainbow trout (Salmo gairdneri). Aquaculture, 72, 319–328. [Google Scholar]
- Finstad, B. , & Ugedal, O. (1998). Smolting of sea trout (Salmo trutta L.) in northern Norway. Aquaculture, 168, 341–349. 10.1016/S0044-8486(98)00360-3. [DOI] [Google Scholar]
- Fisher, R. A. (1958). The genetical theory of natural selection. New York: Dover. [Google Scholar]
- Fleming, I. A. (1998). Pattern and variability in the breeding system of Atlantic salmon (Salmo salar), with comparisons to other salmonids. Canadian Journal of Fisheries and Aquatic Sciences, 55(Suppl. 1), 59–76. 10.1139/d98-009. [DOI] [Google Scholar]
- Forseth, T. , Larsson, S. , Jensen, A. J. , Jonsson, B. , Näslund, I. , & Berglund, I. (2009). Thermal growth performance of juvenile brown trout Salmo trutta: No support for thermal adaptation hypotheses. Journal of Fish Biology, 74, 133–149. [DOI] [PubMed] [Google Scholar]
- Gjerde, B. , & Refstie, T. (1988). The effect of fin‐clipping on growth rate, survival and sexual maturity of rainbow trout. Aquaculture, 73, 383–389. 10.1016/0044-8486(88)90071-3. [DOI] [Google Scholar]
- Greenstreet, S. P. R. , Morgan, R. I. G. , Barnett, S. , & Redhead, P. (1993). Variation in numbers of shags Phalacrocorax aristotelis and common seals Phoca vitulina near the mouth of an Atlantic salmon Salmo salar river at the time of the smolt run. Journal of Animal Ecology, 62, 565–576. 10.2307/5205. [DOI] [Google Scholar]
- Gross, M. R. (1987). Evolution of diadromy in fishes. American Fisheries Society Symposium, 1, 14–25. [Google Scholar]
- Hansen, L. P. (1988). Effects of Carlin tagging and fin clipping on survival of Atlantic salmon (Salmo Salar L.) released as smolts. Aquaculture, 70, 391–394. 10.1016/0044-8486(88)90122-6. [DOI] [Google Scholar]
- Hedger, R. D. , Uglem, I. , Thorstad, E. B. , Finstad, B. , Chittenden, C. M. , Arechavala‐Lopez, P. , … Økland, F. (2011). Behaviour of Atlantic cod, a marine fish predator, during Atlantic salmon post‐smolt migration. ICES Journal of Marine Science, 68, 2152–2162. 10.1093/icesjms/fsr143. [DOI] [Google Scholar]
- Hendry, A. P. , Schoen, D. J. , Wolak, M. E. , & Reid, J. M. (2018). The temporary evolution of fitness. Annual Review of Ecology, Evolution and Systematics, 49, 456–476. 10.1146/annurev-ecolsys-110617-062358. [DOI] [Google Scholar]
- Hoar, W. S. (1988). The physiology of smolting salmonids. In Hoar W. S. & Randall D. J. (Eds.), Fish physiology, volume II, part B (pp. 257–343). New York: Academic Press. [Google Scholar]
- Hvidsten, N. A. , Heggberget, T. G. , & Jensen, A. J. (1998). Sea water temperature at Atlantic salmon smolt enterance. Nordic Journal of Freshwater Research, 74, 79–86. [Google Scholar]
- Hvidsten, N. A. , Jensen, A. J. , Vivås, H. , Bakke, Ø. , & Heggberget, T. G. (1995). Downstream migration of Atlantic salmon smolts in relation to water flow, water temperature, moon phase and social interaction. Nordic Journal of Freshwater Research, 70, 38–48. [Google Scholar]
- Hvidsten, N. A. , & Johnsen, B. O. (1993). Increased recapture rate of adult Atlantic salmon released as smolts into large shoals of wild smolts in the river Orkla, Norway. North American Journal of Fisheries Management, 13, 272–276. [Google Scholar]
- Hvidsten, N. A. , & Lund, R. A. (1988). Predation on hatchery‐reared and wild smolts of Atlantic salmon, Salmo salar L., in the estuary of river Orkla. Norway. Journal of Fish Biology, 33, 121–126. 10.1111/j.1095-8649.1988.tb05453.x. [DOI] [Google Scholar]
- Hvidsten, N. A. , & Møkkelgjerd, P. I. (1987). Predation on salmon smolts, Salmo salar L., in the estuary of the river Surna. Norway. Journal of Fish Biology, 30, 273–280. 10.1111/j.1095-8649.1987.tb05752.x. [DOI] [Google Scholar]
- Jensen, A. J. , Diserud, O. H. , Finstad, B. , Fiske, P. , & Rikardsen, A. H. (2015). Between‐watershed movements of two anadromous salmonids in the Arctic. Canadian Journal of Fisheries and Aquatic Sciences, 72, 855–863. 10.1139/cjfas-2015-0015. [DOI] [Google Scholar]
- Jensen, A. J. , Finstad, B. , & Fiske, P. (2018a). Evidence for the linkage of survival of anadromous Arctic char and brown trout during winter to marine growth during the previous summer. Canadian Journal of Fisheries and Aquatic Sciences, 75, 663–672. 10.1139/cjfas-2017-0077. [DOI] [Google Scholar]
- Jensen, A. J. , Finstad, B. , & Fiske, P. (2019). The cost of anadromy: Marine and freshwater mortality rates in anadromous Arctic char and brown trout in the Arctic region of Norway. Canadian Journal of Fisheries and Aquatic Sciences, 76, 2408–2417. 10.1139/cjfas-2018-0428. [DOI] [Google Scholar]
- Jensen, A. J. , Finstad, B. , Fiske, P. , Forseth, T. , Rikardsen, A. , & Ugedal, O. (2018b). Relationship between marine growth and sea survival of two anadromous salmonid fish species. Canadian Journal of Fisheries and Aquatic Sciences, 75, 621–628. 10.1139/cjfas-2016-0408. [DOI] [Google Scholar]
- Jensen, A. J. , Finstad, B. , Fiske, P. , Hvidsten, N. A. , Rikardsen, A. H. , & Saksgård, L. (2012). Timing of smolt migration in sympatric populations of Atlantic salmon (Salmo salar), brown trout (Salmo trutta), and Arctic charr (Salvelinus alpinus). Canadian Journal of Fisheries and Aquatic Sciences, 69, 711–723. 10.1139/f2012-005. [DOI] [Google Scholar]
- Jonsson, B. , & Jonsson, N. (2009). Migratory timing, marine survival and growth of anadromous brown trout Salmo trutta in the river Imsa, Norway. Journal of Fish Biology, 74, 621–638. 10.1111/j.1095-8649.2008.02152.x. [DOI] [PubMed] [Google Scholar]
- Jonsson, N. , & Jonsson, B. (1999). Trade‐off between egg mass and egg number in brown trout. Journal of Fish Biology, 55, 767–783. 10.1111/j.1095-8649.1999.tb00716.x. [DOI] [Google Scholar]
- Jutila, E. , & Jokikokko, E. (2008). Seasonal differences in smolt traits and post‐smolt survival of wild Atlantic salmon, Salmo salar, migrating from a northern boreal river. Fisheries Managemant and Ecology, 15, 1–9. [Google Scholar]
- Kallio‐Nyberg, I. , Jutila, E. , Jokikokko, E. , & Saloniemi, I. (2006). Survival of reared Atlantic salmon and sea trout in relation to marine conditions of smolt year in the Baltic Sea. Fisheries Research, 80, 295–304. [Google Scholar]
- Klemetsen, A. , Amundsen, P.‐A. , Dempson, J. B. , Jonsson, B. , Jonsson, N. , O'Connell, M. F. , & Mortensen, E. (2003). Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecology of Freshwater Fish, 12, 1–59. 10.1034/j.1600-0633.2003.00010.x. [DOI] [Google Scholar]
- Knutsen, J. A. , Knutsen, H. , Gjøsæter, J. , & Jonsson, B. (2001). Food of anadromous brown trout at sea. Journal of Fish Biology, 59, 533–543. 10.1111/j.1095-8649.2001.tb02359.x. [DOI] [Google Scholar]
- Knutsen, J. A. , Knutsen, H. , Olsen, E. M. , & Jonsson, B. (2004). Marine feeding of anadromous Salmo trutta during winter. Journal of Fish Biology, 64, 89–99. 10.1111/j.1095-8649.2004.00285.x. [DOI] [Google Scholar]
- Krause, J. , & Ruxton, G. D. (2002). Living in groups. Oxford: Oxford University Press. [Google Scholar]
- McCormick, S. D. , Hansen, L. P. , Quinn, T. P. , & Saunders, R. L. (1998). Movement, migration, and smolting of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences, 55(Suppl. 1), 77–92. [Google Scholar]
- Nevoux, M. , Finstad, B. , Davidsen, J. G. , Finlay, R. , Josset, Q. , Poole, R. , … Jonsson, B. (2019). Environmental influences of life history strategies in partial anadromous brown trout (Salmo trutta, Salmonidea). Fish and Fisheries, 20, 1051–1082. 10.1111/faf.12396. [DOI] [Google Scholar]
- Olsen, E. M. , Knutsen, H. , Simonsen, J. H. , Jonsson, B. , & Knutsen, J. A. (2006). Seasonal variation in marine growth of sea trout, Salmo trutta, in coastal Skagerrak. Ecology of Freshwater Fish, 15, 446–452. 10.1111/j.1600-0633.2006.00176.x. [DOI] [Google Scholar]
- Ostrovsky, I. (1995). The parabolic pattern of animal growth: Determination of equation parameters and their temperature dependencies. Freshwater Biology, 33, 357–371. 10.1111/j.1365-2427.1995.tb00398.x. [DOI] [Google Scholar]
- Parker, R. R. (1971). Size selective predation among juvenile salmonid fishes in a British Columbia inlet. Journal of Fisheries Research Board of Canada, 28, 1503–1510. 10.1139/f71-231. [DOI] [Google Scholar]
- Pemberton, R. (1976). Sea trout in North Argyll Sea lochs: II. Diet. Journal of Fish Biology, 9, 195–208. 10.1111/j.1095-8649.1976.tb04673.x. [DOI] [Google Scholar]
- R Core Team . (2020). R: A language and environment for statistical computing, version 4.0.3. R foundation for statistical computing. Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- Rikardsen, A. H. , Dempson, J. B. , Amundsen, P.‐A. , Bjørn, P. A. , Finstad, B. , & Jensen, A. J. (2007b). Temporal variability in marine feeding of sympatric Arctic charr and sea trout. Journal of Fish Biology, 70, 837–852. 10.1111/j.1095-8649.2007.01345.x. [DOI] [Google Scholar]
- Rikardsen, A. H. , Diserud, O. H. , Elliott, J. M. , Dempson, J. B. , Sturlaugsson, J. , & Jensen, A. J. (2007a). The marine temperature and depth preferences of Arctic charr (Salvelinus alpinus) and sea trout (Salmo trutta), as recorded by data storage tags. Fisheries Oceanography, 16, 436–447. 10.1111/j.1365-2419.2007.00445.x. [DOI] [Google Scholar]
- Sigholt, T. , & Finstad, B. (1990). Effect of low temperature on seawater tolerance in Atlantic salmon (Salmo salar) smolts. Aquaculture, 84, 167–172. 10.1016/0044-8486(90)90346-O. [DOI] [Google Scholar]
- Sigourney, D. B. , Letcher, B. H. , Obedzinsky, M. , & Cunjak, R. A. (2008). Size‐independent growth in fishes: Patterns, models and metrics. Journal of Fish Biology, 72, 2435–2455. 10.1111/j.1095-8649.2008.01830.x. [DOI] [Google Scholar]
- Thomsen, D. S. , Koed, A. , Nielsen, C. , & Madsen, S. S. (2007). Overwintering of sea trout (Salmo trutta) in freshwater: Escaping salt and low temperature or an alternative life strategy? Canadian Journal of Fisheries and Aquatic Sciences, 64, 793–802. 10.1139/F07-059. [DOI] [Google Scholar]
- Thorstad, E. B. , Todd, C. D. , Uglem, I. , Bjørn, P. A. , Gargan, P. G. , Vollset, K. W. , … Finstad, B. (2016). Marine life of the sea trout. Marine Biology, 163, 47. 10.1007/s00227-016-2820-3. [DOI] [Google Scholar]
- Thorstad, E. B. , Whoriskey, F. , Uglem, I. , Moore, A. , Rikardsen, A. H. , & Finstad, B. (2012). A critical life stage of the Atlantic salmon Salmo salar: Behaviour and survival during the smolt and initial post‐smolt migration. Journal of Fish Biology, 81, 500–542. 10.1111/j.1095-8649.2012.03370.x. [DOI] [PubMed] [Google Scholar]
- Ugedal, O. , Finstad, B. , Damsgård, B. , & Mortensen, A. (1998). Seawater tolerance and downstream migration in hatchery‐reared and wild brown trout. Aquaculture, 168, 395–405. [Google Scholar]
- Ward, D. M. , & Hvidsten, N. A. (2011). Predation: Compensation and context dependence. In Aas Ø., Einum S., Klemetsen A., & Skurdal J. (Eds.), Atlantic salmon ecology (pp. 199–220). Oxford, UK: Blackwell Publishing, Ltd. [Google Scholar]
- Wolf, P. A. (1951). A trap for the capture of fish and other organisms moving downstream. Transaction of the American Fisheries Society, 80, 41–45. 10.1577/1548-8659(1950)80(41:ATFTCO)2.0.CO;2. [DOI] [Google Scholar]
- Wood, S. & Scheipl, F. (2020). gamm4: Generalized additive mixed models using “mgcv” and “lme4.” R package version 0.2–6. https://CRAN.R-project.org/package=gamm4.
- Wysujack, K. , Greenberg, L. A. , Bergman, E. , & Olsson, I. C. (2009). The role of the environment in partial migration: Food availability affects the adoption of a migratory tactic in brown trout Salmo trutta . Ecology of Freshwater Fish, 18, 52–59. 10.1111/j.1600-0633.2008.00322.x. [DOI] [Google Scholar]


