Summary
This single‐arm, multicentre, phase I study is the first study of zanubrutinib, a potent, specific, irreversible Bruton tyrosine kinase (BTK) inhibitor, in Chinese patients with relapsed/refractory B‐cell malignancies. The objectives were to evaluate safety and preliminary anti‐tumour activity. Forty‐four patients received zanubrutinib 320 mg once daily (QD) (n = 10) or 160 mg twice daily (BID) (n = 34) until disease progression or unacceptable toxicity. 29.5% of patients received zanubrutinib for at least two years. The most common adverse event (AE) and the most common grade 3 or higher AE was neutrophil count decreased (54.5% and 25.0% respectively). Two patients (4.5%) discontinued treatment due to AEs and one treatment‐emergent AE led to death. All haemorrhagic events were grade 1–2 (except for one non‐serious grade 3 purpura). No second primary malignancies, tumour lysis syndrome, or atrial fibrillation/flutter occurred. The overall response rate was 52.3% (complete response rate, 18.2%). Patients with all cancer subtypes benefited from treatment. BTK C481S/R or L528W mutations were found in zanubrutinib‐progressive patients. The safety/efficacy profiles of patients treated with 320 mg QD and 160 mg BID were comparable and similar daily area under the curve (AUC) was achieved. Overall, zanubrutinib was well tolerated and either of these two regimens is clinically practical. Registered at ClinicalTrials.gov (NCT03189524, on 16 June 2017, https://clinicaltrials.gov/ct2/show/NCT03189524).
Keywords: B‐cell malignancies, efficacy, phase I, safety, zanubrutinib
INTRODUCTION
Bruton tyrosine kinase (BTK) is a critical component of the B‐cell receptor signalling cascade. Inhibition of BTK has emerged as a promising strategy for the treatment of B‐cell malignancies. Zanubrutinib is a second‐generation BTK inhibitor designed to be more selective, minimizing binding with off‐target kinases. 1
This is the first study of zanubrutinib in Chinese patients with B‐cell malignancies and the only study that compares the 320 mg once‐daily (QD) and the 160 mg twice‐daily (BID) dosing regimen in Asian patients, with a long follow‐up time (i.e., >4 years in some patients). Here, we report the final results.
METHODS
Study design
This was a single‐arm, open‐label, multicentre, phase I study (NCT03189524) of zanubrutinib in Chinese patients with B‐cell malignancies. The study had two parts: part I was an assessment of dose based on safety [dose‐limiting toxicity (DLT)] and pharmacokinetics (PK), and part II was a dose expansion phase to obtain additional data at the recommended phase 2 dose (Figure 1). The starting doses were based on data from the first‐in‐human (FIH) study (BGB‐3111‐AU‐003; NCT02343120). 2 Patients could continue treatment until intolerable toxicity, disease progression, withdrawal of informed consent, or death; or until treatment discontinuation per investigator decision. The study protocol and statistical analysis plan are posted at clinicaltrials.gov.
FIGURE 1.
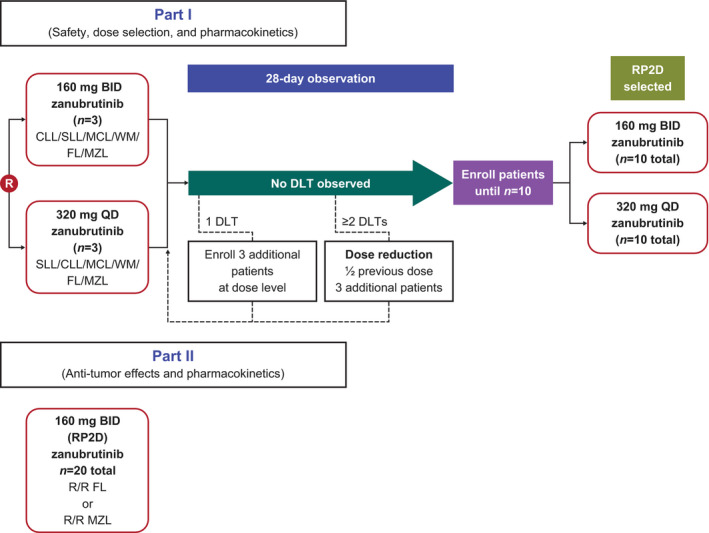
Study design. In part I, three patients (or six patients if one of the first three patients experienced a DLT) were randomly assigned to one of the two treatment arms. If two or more patients experienced a DLT, the dosage was to be reduced by half and three patients were then enrolled at that dose. If zero or one patient experienced a DLT, 10 patients in total were to be enrolled in the arm to further assess the safety, tolerability, PK, and preliminary PD of the study drug and to determine the RP2D. DLT, dose‐limiting toxicity; RP2D, recommended phase II dose
The study was conducted in accordance with the principles of the International Conference on Harmonization Good Clinical Practice Guidelines and the Declaration of Helsinki. The protocol was approved by the institutional review boards and independent ethics committees at each study site, and all patients provided written informed consent prior to taking part. The investigators and their research teams collected the data, all authors had full access to the data, and all authors were responsible for analysing/interpreting the data.
Patients and eligibility criteria
Part I enrolled patients with chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), Waldenström's macroglobulinaemia (WM), follicular lymphoma (FL), and marginal zone lymphoma (MZL), and part II enrolled patients with FL and MZL. Key inclusion criteria were: relapsed/refractory (R/R) disease following one or more lines of therapy, judged by the investigator as requiring treatment; and adequate haematological and organ function. Patients with central nervous system involvement of the disease or disease transformation were excluded.
Procedures
Safety was monitored and AEs were assessed per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. 3 An anti‐tumour activity assessment was conducted every 12 weeks for one year after the first dose and every 24 weeks thereafter based on appropriate disease‐specific tumour response criteria (International Workshop Group on Chronic Lymphocytic Leukaemia 4 for CLL; National Cancer Institute Working Group NHL 5 for non‐Hodgkin lymphoma (NHL) including MZL, MCL, FL and SLL; International Workshops on Waldenström's macroglobulinaemia 6 , 7 for WM).
Study end‐points and statistical analysis
The primary objective was to evaluate the safety and tolerability of zanubrutinib in Chinese patients with B‐cell malignancies. The secondary objective was to assess the PK of single and multiple oral doses. Efficacy and mechanisms of tumour resistance were also explored.
Treatment‐emergent AEs (TEAEs) were summarized. AEs of special interest (AESI) were: haemorrhage (any haemorrhage and major haemorrhage defined as subdural haematoma and subdural haemorrhage, and serious or grade 3 or higher bleeding or central nervous system bleeding of any grade); atrial fibrillation and/or flutter; hypertension; second primary malignancies; tumour lysis syndrome; infections and cytopenias.
The overall response rate (ORR), complete response rate (CRR), and median progression‐free survival (PFS) and 95% confidence intervals (CIs) were estimated. The distribution of PFS and duration of response (DOR) were summarized using the Kaplan–Meier method.
No hypothesis testing was planned. The sample size for part II was determined based on the assumption that the ORR was 35%. The 95% CI of the ORR would be 15.4% to 59.2% with 20 patients enrolled, which is sufficient to demonstrate anti‐tumour activity of zanubrutinib.
Pharmacokinetics analysis
Serial blood samples were collected to measure zanubrutinib plasma concentrations following single and multiple‐dose administration. Plasma concentrations of zanubrutinib were determined using a validated high‐performance liquid chromatography with tandem mass spectrometric procedure (WuXi AppTec, Shanghai, China) with a calibration range of 1.00–1000 ng/ml, and a lower limit of quantification of 1.00 ng/ml. Non‐compartmental PK analysis was conducted using Phoenix® WinNolin® software (version 7.0, Certara, Princeton, NJ, USA).
Mutational analysis
Biomarker samples were collected from patients who had disease progression after zanubrutinib treatment. Formalin‐fixed paraffin‐embedded tumour tissue samples from seven FL and two MZL patients, and bone marrow aspirate from one WM patient, along with peripheral blood from four CLL patients were used to analyse the mutational status of the BTK gene and other haematological malignancy‐related genes using validated next‐generation sequencing (NGS) panels. Three samples were assessed by a NGS panel that included 175 haematological malignancy‐related genes, and 11 samples were assessed by a NGS panel containing 475 lymphoma‐related genes. The whole exons and partial introns of these genes were captured by probe hybridization and sequencing reads were mapped to the hg19 genome; mutations with clinical significance or biological function were filtered through the oncology knowledge base (OncoKB; https://www.oncokb.org) 8 or the literature for downstream analysis. Both panels covered the whole exons of the BTK and PLCG2 genes.
RESULTS
The first patient was dosed on 5 July 2016. After the final analysis, all patients who were still on zanubrutinib were transferred to the long‐term extension study (NCT04170283). As of the database lock for this final analysis (15 October 2020), 44 patients had been enrolled. The overall median follow‐up time was 31.5 months (range: 2.3–49.5 months). In part I, the median follow‐up time was 46.1 months (range: 4.6–48.2 months) for the 160 mg BID dose group and 45.8 months (range: 3.0–49.5 months) for the 320 mg QD dose group. The median follow‐up time for patients in part II was 30.0 months (range: 2.3–36.9 months).
Twenty‐one patients were in part I: CLL/SLL (n = 9), WM (n = 2), and NHL [FL (n = 6), MZL (n = 2), MCL (n = 2)]. Of these, 11 patients were treated with zanubrutinib 160 mg BID and 10 were treated with zanubrutinib 320 mg QD. Twenty‐three patients (FL: n = 20; MZL: n = 3) were in part II. The primary reason for study discontinuation in all patients (n = 44) was transfer to the long‐term extension study (n = 23); other reasons were loss to follow‐up (n = 9), death (n = 6), withdrawal by patient (n = 5), and disease progression (n = 1).
No DLT events occurred in part I. Therefore, 160 mg BID was chosen as the recommended phase II dose (RP2D) dose based on the totality of safety, efficacy, PK, and progressive disease (PD) data from the FIH study. 2 In particular, while nearly full occupancy of BTK in peripheral blood mononuclear cells was achieved in patients with either BID or QD doses, the median BTK occupancy in lymph node tissue was 100% with the 160 mg BID dose versus 94% with the 320 mg QD dose at steady‐state trough. 2
Baseline characteristics (Table 1) and prior anti‐cancer drug therapies (Table 2) were generally similar across dosing subgroups, with the exception of patients in the 160 mg BID group in part I who had received more prior therapy (median number of lines was four) than the 320 mg QD group (median of three lines) and the part II patients (median of three lines).
TABLE 1.
Demographics and disease characteristics of Chinese patients with relapsed/refractory B‐cell malignancies receiving zanubrutinib
| Part I | Part II | Total, (N = 44) | ||
|---|---|---|---|---|
| 160 mg BID, (n = 11) | 320 mg QD, (n = 10) | 160 mg BID, (n = 23) | ||
| Age, years | ||||
| Median | 54.0 | 55.0 | 46.0 | 52.0 |
| Min–max | 35–67 | 34–66 | 28–67 | 28–67 |
| Age group, n (%) | ||||
| <65years | 10 (90.9) | 9 (90.0) | 21 (91.3) | 40 (90.9) |
| ≥65years | 1 (9.1) | 1 (10.0) | 2 (8.7) | 4 (9.1) |
| Sex, n (%) | ||||
| Male | 9 (81.8) | 7 (70.0) | 8 (34.8) | 24 (54.5) |
| Female | 2 (18.2) | 3 (30.0) | 15 (65.2) | 20 (45.5) |
| ECOG performance status, n (%) | ||||
| 0 | 4 (36.4) | 5 (50.0) | 17 (73.9) | 26 (59.1) |
| 1 | 7 (63.6) | 5 (50.0) | 5 (21.7) | 17 (38.6) |
| 2 a | 0 (0.0) | 0 (0.0) | 1 (4.3) | 1 (2.3) |
| BMI, kg/m2 | ||||
| Median | 25.8 | 22.2 | 24.6 | 24.6 |
| Min–max | 21.5–30.7 | 20.6–32.8 | 17.7–31.6 | 17.7–32.8 |
| Diagnosis, n (%) | ||||
| CLL | 4 (36.4) | 3 (30.0) | 0 (0.0) | 7 (15.9) |
| SLL | 1(9.1) | 1(10.0) | 0 (0.0) | 2 (4.5) |
| WM | 1 (9.1) | 1 (10.0) | 0 (0.0) | 2 (4.5) |
| NHL | 5 (54.5) | 5 (60.0) | 23 (100.0) | 33 (79.5) |
| FL | 3 | 3 | 20 | 26 |
| MZL | 2 | 0 | 3 | 5 |
| MCL | 0 | 2 | 0 | 2 |
| Time from initial diagnosis to study entry, years | ||||
| Median | 4.7 | 2.4 | 2.3 | 2.8 |
| Min–max | 1.1–8.2 | 0.5–10.1 | 0.8–20.1 | 0.5–20.1 |
| Bone marrow biopsy infiltration of abnormal lymphocytes, n (%) | ||||
| Yes | 5 (45.5) | 6 (60.0) | 9 (39.1) | 20 (45.5) |
| No | 6 (54.5) | 4 (40.0) | 14 (60.9) | 24 (54.5) |
Note: Percentages are based on n, number of patients who received ≥1 dose of zanubrutinib.
Abbreviations: BID, twice daily; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; CLL, chronic lymphocytic leukaemia; FL, follicular lymphoma; max, maximum; MCL, mantle cell lymphoma; min, minimum; MZL, marginal zone lymphoma; NHL, non‐Hodgkin lymphoma; QD, once daily; SLL, small lymphocytic lymphoma; WM, Waldenström's macroglobulinaemia.
One patient had an ECOG performance status score of 1 during screening but it declines to a score of 2 on cycle 1 day 1.
TABLE 2.
Prior therapy in Chinese patients with relapsed/refractory B‐cell malignancies receiving zanubrutinib
| Part I | Part II | Total (N = 44) | ||
|---|---|---|---|---|
| 160 mg BID, (n = 11) | 320 mg QD, (n = 10) | 160 mg BID, (n = 23) | ||
| Number of prior lines of therapy | ||||
| Median | 4.0 | 3.0 | 3.0 | 3.0 |
| Min–max | 1–8 | 1–9 | 1–6 | 1–9 |
| Number of prior lines of therapy, n (%) | ||||
| 1 | 3 (27.3) | 1 (10.0) | 4 (17.4) | 8 (18.2) |
| 2 | 1 (9.1) | 2 (20.0) | 3 (13.0) | 6 (13.6) |
| 3 | 1 (9.1) | 4 (40.0) | 7 (30.4) | 12 (27.3) |
| 4 | 1 (9.1) | 1 (10.0) | 4 (17.4) | 6 (13.6) |
| 5 | 2 (18.2) | 1 (10.0) | 3 (13.0) | 6 (13.6) |
| ≥6 | 3 (27.3) | 1 (10.0) | 2 (8.7) | 6 (13.6) |
| Best response for last therapy, n (%) | ||||
| Complete response | 2 (18.2) | 0 (0.0) | 2 (8.7) | 4 (9.1) |
| Partial response | 2 (18.2) | 2 (20.0) | 6 (26.1) | 10 (22.7) |
| Stable disease | 2 (18.2) | 3 (30.0) | 3 (13.0) | 8 (18.2) |
| Progressive disease | 1 (9.1) | 3 (30.0) | 5 (21.7) | 9 (20.5) |
| Not applicable | 4 (36.4) | 2 (20.0) | 7 (30.4) | 13 (29.5) |
| Time from end of last therapy to study entry, months | ||||
| Median | 4.5 | 3.9 | 6.0 | 4.6 |
| Min–max | 0.0–51.4 | 0.0–38.2 | 0.0–87.4 | 0.0–87.4 |
Note: Zanubrutinib dose regimen is based on the first dose received. Percentages are based on number of patients who received ≥1 dose of zanubrutinib.
Abbreviations: BID, twice daily; min, minimum; max, maximum; QD, once daily.
Safety
All patients received at least one dose of zanubrutinib. The median average daily dose and relative dose intensity was 320 mg/day and 100%, respectively, overall and in both 160 mg BID and 320 mg QD dosing groups. Overall, 29.5% of patients received zanubrutinib for two or more years and 42.9% of patients in part I were on zanubrutinib for three or more years.
The most commonly reported TEAEs were neutrophil count decreased, anaemia, and upper respiratory tract infection (Table 3). The most frequently reported grade 3 or higher TEAEs were similar (Table 4).
TABLE 3.
Most common a TEAEs (any grade) in Chinese patients with relapsed/refractory B‐cell malignancies receiving zanubrutinib
| Preferred term, n (%) | 160 mg BID, (n = 34) | 320 mg QD, (n = 10) | Total (N = 44) |
|---|---|---|---|
| Patients with ≥1 any‐grade TEAE | 33 (97.1) | 10 (100.0) | 43 (97.7) |
| Neutrophil count decreased | 17 (50.0) | 7 (70.0) | 24 (54.5) |
| Anaemia | 12 (35.3) | 4 (40.0) | 16 (36.4) |
| Upper respiratory tract infection | 10 (29.4) | 5 (50.0) | 15 (34.1) |
| White blood cell count decreased | 11 (32.4) | 3 (30.0) | 14 (31.8) |
| Platelet count decreased | 8 (23.5) | 2 (20.0) | 10 (22.7) |
| Rash | 8 (23.5) | 2 (20.0) | 10 (22.7) |
| Hematuria | 6 (17.6) | 3 (30.0) | 9 (20.5) |
| Hyperuricemia | 6 (17.6) | 3 (30.0) | 9 (20.5) |
| Pneumonia | 6 (17.6) | 3 (30.0) | 9 (20.5) |
| Cough | 5 (14.7) | 3 (30.0) | 8 (18.2) |
| Weight increased | 5 (14.7) | 3 (30.0) | 8 (18.2) |
Abbreviations: BID, twice daily; QD, once daily; TEAE, treatment‐emergent adverse event.
Occurring in ≥15% of patients overall.
TABLE 4.
Most common a grade ≥ 3 TEAEs in Chinese patients with relapsed/refractory B‐cell malignancies receiving zanubrutinib
| Preferred term, n (%) | 160 mg BID, (n = 34) | 320 mg QD, (n = 10) | Total, (N = 44) |
|---|---|---|---|
| Patients with ≥1 grade 3 or higher TEAE | 18 (52.9) | 6 (60.0) | 24 (54.5) |
| Neutrophil count decreased | 6 (17.6) | 5 (50.0) | 11 (25.0) |
| Anaemia | 4 (11.8) | 1 (10.0) | 5 (11.4) |
| Neutropenia | 4 (11.8) | 0 (0.0) | 4 (9.1) |
| Upper respiratory tract infection | 2 (5.9) | 2 (20.0) | 4 (9.1) |
| Pneumonia | 3 (8.8) | 0 (0.0) | 3 (6.8) |
| White blood cell count increased | 2 (5.9) | 1 (10.0) | 3 (6.8) |
| Platelet count decreased | 1 (2.9) | 1 (10.0) | 2 (4.5) |
| Thrombocytopenia | 1 (2.9) | 1 (10.0) | 2 (4.5) |
| White blood cell count decreased | 1 (2.9) | 1 (10.0) | 2 (4.5) |
Abbreviations: BID, twice daily; QD, once daily; TEAE, treatment‐emergent adverse event.
Occurring in ≥2 patients overall.
Serious AEs were reported in nine (20.5%) patients. All events were resolved/resolving or stable, except for toxic epidermal necrolysis (TEN) in a 63‐year‐old male patient with MZL in the 160 mg BID cohort in part I. He experienced the event 45 days after treatment started and zanubrutinib was terminated as a result. An enlarged lymph node was found one month later in this patient, which was judged by the investigator to be a signal of disease progression. The patient died 88 days after the last dose of study drug; cause of death was indicated as TEN and disease progression. Notably, the patient had received several drugs that are associated with TEN, including febuxostat, furosemide, and torsemide.
Infections and neutropenia were the most frequently reported AESIs (Table 5). Most AESIs of infection were reported as recovered/resolved, and none led to dose interruptions or study drug discontinuation. All haemorrhagic events were grade 1 or 2, except for one non‐serious grade 3 purpura, which was considered to be a major haemorrhage. There were no reports of second primary malignancies, tumour lysis syndrome, atrial fibrillation/flutter, or grade 3 or higher hypertension.
TABLE 5.
Incidence of TEAEs (any grade) of special interest in Chinese patients with relapsed/refractory B‐cell malignancies receiving zanubrutinib
| Any grade TEAEs of special interest grouping term a , n (%) | 160 mg BID, (n = 34) | 320 mg QD, (n = 10) | Total, (N = 44) |
|---|---|---|---|
| Patients with ≥1 TEAE of special interest | 30 (88.2) | 10 (100.0) | 40 (90.9) |
| Haemorrhage | 11 (32.4) | 4 (40.0) | 15 (34.1) |
| Major haemorrhage | 1 (2.9) | 0 (0.0) | 1 (2.3) |
| Hypertension | 0 (0.0) | 1 (10.0) | 1 (2.3) |
| Infections | 19 (55.9) | 7 (70.0) | 26 (59.1) |
| Cytopenias | |||
| Anaemia | 14 (41.2) | 4 (40.0) | 18 (40.9) |
| Neutropenia | 18 (52.9) | 7 (70.0) | 25 (56.8) |
| Thrombocytopenia | 9 (26.5) | 3 (30.0) | 12 (27.3) |
| Atrial fibrillation/flutter | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Second primary malignancies | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Tumour lysis syndrome | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: BID, twice daily; MedDRA, Medical Dictionary for Regulatory Activities; QD, once daily; TEAE, treatment‐emergent adverse event.
According to MedDRA Version 23.0.
Adverse events leading to treatment discontinuation were reported in two patients (4.5%; one TEN, noted above, and the other ‘abortion induced’ which was assessed as unlikely to be zanubrutinib‐related). AEs leading to dose interruption occurred in eight (18.2%) patients. Almost all dose interruptions lasted for at most eight days and most events resolved or were resolving after the dose interruption (Table S2). There were no AEs leading to dose reduction.
Six deaths were reported with causes reported as disease progression (n = 3), unknown reason (n = 2; both patients had disease progression with one dying 57 days and one 299 days after the last dose of zanubrutinib), and an AE (n = 1; the aforementioned patient with TEN).
The safety profiles of the zanubrutinib 160 mg BID and 320 mg QD cohorts were comparable. The most common TEAEs overall were cytopenias and infection. The only all‐grade TEAE that differed in incidence by more than 20% was upper respiratory tract infection (Table 3). The only grade 3 or higher TEAE that differed in incidence by more than 20% was neutrophil count decreased (Table 4). No AESI had a difference in incidence rate between the two dose groups of more than 20% (Table 5).
Efficacy
The ORR was 52.3% (95% CI: 36.7%–67.5%), with a CRR of 18.2% (95% CI: 8.2%–32.7%, Table 6). Within each cancer subtype, all patients showed clinical benefit. The response was most pronounced in patients with CLL/SLL in whom the ORR was 100% (95% CI: 66.4%–100%) and CRR was 33.3% (95% CI: 7.5%–70.1%).
TABLE 6.
Treatment response by disease type in Chinese patients with relapsed/refractory B‐cell malignancies receiving zanubrutinib
| Response category, n (%) | CLL/SLL, (n = 9) | MCL, (n = 2) | WM, (n = 2) | FL, (n = 26) | MZL, (n = 5) | Total, (N = 44) |
|---|---|---|---|---|---|---|
| Best overall response | ||||||
| CR | 3 (33.3) | 1 (50.0) | 0 (0.0) | 4 (15.4) | 0 (0.0) | 8 (18.2) |
| CRi | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| VGPR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PR | 5 (55.6) | 0 (0.0) | 1 (50.0) | 8 (30.8) | 0 (0.0) | 14 (31.8) |
| PR‐L | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) |
| MR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stable disease | 0 (0.0) | 1 (50.0) | 1 (50.0) | 7 (26.9) | 3 (60.0) | 12 (27.3) |
| PD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (15.4) | 0 (0.0) | 4 (9.1) |
| Discontinued prior to first assessment | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (11.5) | 2 (40.0) | 5 (11.4) |
| Overall response rate a | 9 (100.0) | 1 (50.0) | 1 (50.0) | 12 (46.2) | 0 (0.0) | 23 (52.3) |
| 95% CI | (66.4–100.0) | (1.3–98.7) | (1.3–98.7) | (26.6–66.6) | (0.0–52.2) | (36.7–67.5) |
| Partial response rate b | 8 (88.9) | 1 (50.0) | 1 (50.0) | 12 (46.2) | 0 (0.0) | 22 (50.0) |
| 95% CI | (51.8–99.7) | (1.3–98.7) | (1.3–98.7) | (26.6–66.6) | (0.0–52.2) | (34.6–65.4) |
| Complete response rate c | 3 (33.3) | 1 (50.0) | 0 (0.0) | 4 (15.4) | 0 (0.0) | 8 (18.2) |
| 95% CI | (7.5–70.1) | (1.3–98.7) | (0.0–84.2) | (4.4–34.9) | (0.0–52.2) | (8.2–32.7) |
Note: 95% CIs were calculated using the Clopper–Pearson method.
Abbreviations: CI, confidence interval; CLL/SLL, chronic lymphocytic leukaemia/small lymphocytic lymphoma; CR, complete response; CRi, CR with incomplete marrow recovery; FL, follicular lymphoma; MCL, mantle cell lymphoma; MR, minor response; MZL, marginal zone lymphoma; PR, partial response; PR‐L, partial response with lymphocytosis; PD, progressive disease; NE, not estimable; VGPR, very good partial response; WM, Waldenström's macroglobulinaemia.
Overall response includes best overall response (of non‐stable disease, non‐PD, and non‐NE).
Partial response includes best overall response of PR or better (i.e., excluding PR‐L and MR).
Percentages are based on numbers of patients with best overall response of CR.
The overall median time to response in all subtypes was 2.8 months (Table S1). Median PFS was 16.4 months (95% CI: 8.1–21.9; Figure 2). The longest median PFS was 44.3 months in CLL/SLL patients (Figure 3). The median DOR was 13.9 months (Figure 4), and median overall survival in the total population was not reached.
FIGURE 2.
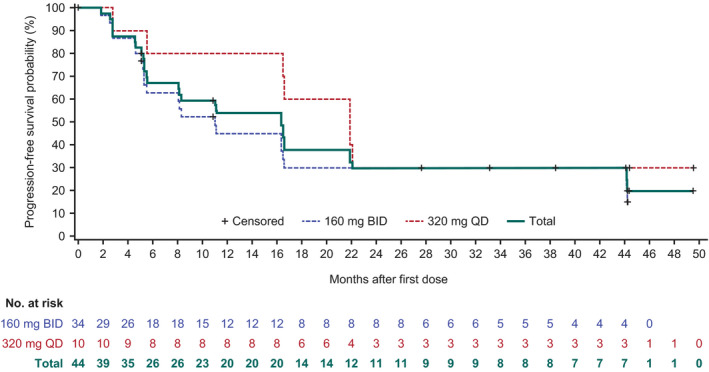
Progression‐free survival in Chinese patients with relapsed/refractory (R/R) B‐cell malignancies receiving zanubrutinib by dose regimen. There was no significant difference between the two dose groups as indicated by the log‐rank test (p = 0.25)
FIGURE 3.
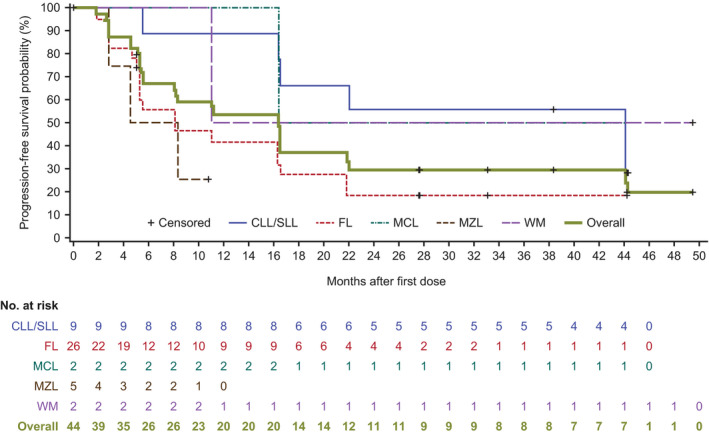
Progression‐free survival in Chinese patients with relapsed/refractory (R/R) B‐cell malignancies receiving zanubrutinib by disease type
FIGURE 4.
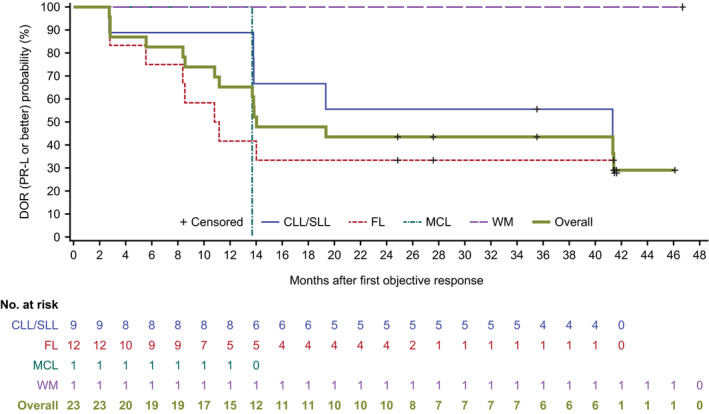
Duration of response [partial response with lymphocytosis (PR‐L) or better] in Chinese patients with relapsed/refractory (R/R) B‐cell malignancies receiving zanubrutinib. Response was defined as PR‐L or better
The efficacy of zanubrutinib 160 mg BID and 320 mg QD was compared in part I, taking into account the similarity of the tumour types and comparable follow‐up durations (Table 7). Response rates (ORR, partial response rate, and CRR), were similar between the dose groups. Numerically, patients on 320 mg QD zanubrutinib achieved higher overall and partial response rates; however, those in the 160 mg BID cohort reached a higher CRR.
TABLE 7.
Treatment response by zanubrutinib dose and disease type in Chinese patients with relapsed/refractory B‐cell malignancies
| Response category, n (%) | Zanibrutinab 160 mg BID | Zanibrutinib 320 mg QD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CLL/SLL, (n = 5) | NHL d , (n = 5) | WM, (n = 1) | Total, (N = 11) | CLL/SLL, (n = 4) | NHL d , (n = 5) | WM, (n = 1) | Total, (N = 10) | ||
| Overall response rate a | 5 (100.0) | 3 (60.0) | 0 (0.0) | 8 (72.7) | 4 (100.0) | 3 (60.0) | 1 (100.0) | 8 (80.0) | |
| 95% CI | (47.8–100.0) | (14.7–94.7) | (0.0–97.5) | (39.0–94.0) | (39.8–100.0) | (14.7–94.7) | (2.5–100.0) | (44.4–97.5) | |
| Partial response rate b | 4 (80.0) | 3 (60.0) | 0 (0.0) | 7 (63.6) | 4 (100.0) | 3 (60.0) | 1 (100.0) | 8 (80.0) | |
| 95% CI | (28.4–99.5) | (14.7–94.7) | (0.0–97.5) | (30.8–89.1) | (39.8–100.0) | (14.7–94.7) | (2.5–100.0) | (44.4–97.5) | |
| Complete response rate c | 2 (40.0) | 1 (20.0) | 0 (0.0) | 3 (27.3) | 1 (25.0) | 1 (20.0) | 0 (0.0) | 2 (20.0) | |
| 95% CI | (5.3–85.3) | (0.5–71.6) | (0.0–97.5) | (6.0–61.0) | (0.6–80.6) | (0.5–71.6) | (0.0–97.5) | (2.5–55.6) | |
Note: Zanubrutinib dose regimen is based on the first dose received. 95% CIs were calculated using the Clopper–Pearson method.
Abbreviations: BID, twice daily; CI, confidence interval; CLL/SLL, chronic lymphocytic leukaemia/small lymphocytic lymphoma; CR, complete response; FL, follicular lymphoma; MCL, mantle cell lymphoma; MR, minor response; MZL, marginal zone lymphoma; NE, not estimable; NHL, non‐Hodgkin lymphoma; PR, partial response; PR‐L, partial response with lymphocytosis; PD, progressive disease; QD, once daily; WM, Waldenström's macroglobulinaemia.
Overall response includes best overall response (of non‐stable disease, non‐PD, and non‐NE).
Partial response includes best overall response of PR or better (i.e., excluding PR‐L and MR).
Percentages are based on number of patients with best overall response being CR.
NHL includes FL, MZL and MCL.
Pharmacokinetics
Zanubrutinib was rapidly absorbed and eliminated after oral administration of the 160 mg and 320 mg doses, with C max occurring at ~2 h post dose. The representative plasma concentration–time profile at steady state on week 2 day 1 is shown in Figure 5. The steady‐state mean (% CV) AUC0–8 h value was 1148 (55.5%) ng h/ml at 320 mg QD, which was approximately twofold higher than the 631.3 (63.2%) ng h/ml at 160 mg BID. Thus, total daily AUC in the 160 mg BID and 320 mg QD dose groups would be expected to be comparable.
FIGURE 5.
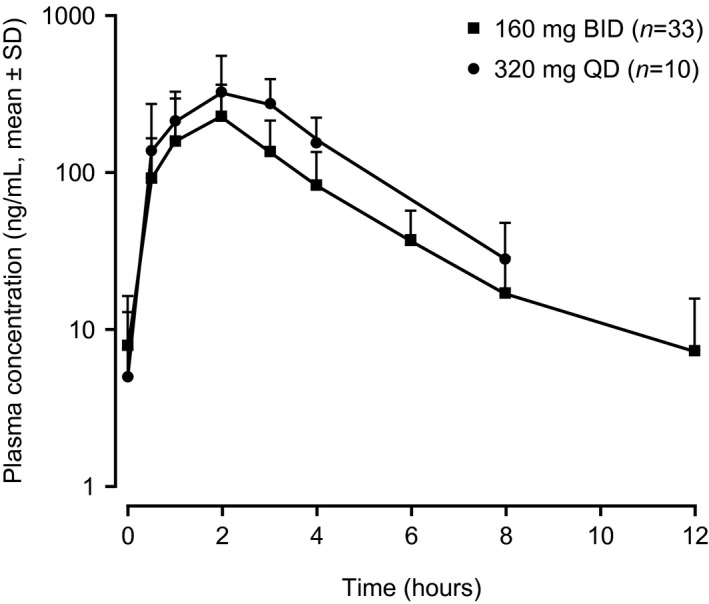
Plasma concentration–time profile of zanubrutinib on day 1 of week 2 after multiple doses in Chinese patients with relapsed/refractory B‐cell malignancies
Mutation biomarker analysis
BTK C481S/R or L528W mutations were detected in two CLL patients, one FL patient, and one WM patient who had disease progression on study days 162, 336, 672, and 1345, respectively (Table 8). Among seven disease‐progressive FL patients, four (57.1%) carried TP53 mutations and three (42.8%) carried BCL10/CARD11 mutations (Figure S1).
TABLE 8.
BTK mutations in four zanubrutinib‐progressive patients
| Patient | Indication | Study day at disease progression | BOR by investigator | BTK mutation (VAF) |
|---|---|---|---|---|
| 101 016 | FL | Day 162 | PR | L528W (5.66%) |
| 102 004 | WM | Day 336 | Stable disease | C481S (9.95%) |
| 102 002 | CLL | Day 672 | PR |
C481S with 1442G > C (44.4%) C481S with 1441 T > A (6.7%) |
| 102 006 | CLL | Day 1345 | PR‐L |
C481S with 1442G > C (30.5%) C481S with 1441 T > A (16.0%) C481R with 1441 T > C (14.8%) L528W with 1583 T > G (8.2%) |
Abbreviations: BOR, best overall response; BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukaemia; FL, follicular lymphoma; PR, partial response; PR‐L, partial response with lymphocytosis; VAF, variant allele frequency; WM, Waldenström's macroglobulinaemia.
DISCUSSION
In this first study of zanubrutinib in Chinese patients, the duration of follow‐up was relatively long, with 42.9% of patients in part I receiving treatment for three or more years. The average daily dose and relative dose intensity were given at the planned levels. The long exposure with good tolerability could potentially result in durable responses. Cytopenias and infections were the most common AEs; however, they were manageable and did not lead to treatment discontinuation or dose reduction. The rate of AEs leading to treatment discontinuation was relatively low at 4.5%. Although 18.2% of patients interrupted treatment due to AEs, re‐initiation occurred rapidly because the AEs resolved quickly. Most cases of haemorrhage were grade 1 or 2. There were no reports of second primary malignancies, tumour lysis syndrome, or atrial fibrillation/flutter ― consistent with a previous report that zanubrutinib leads to less atrial fibrillation/flutter events than ibrutinib. 9
This study also demonstrated the anti‐tumour activity of zanubrutinib in relapsed/refractory B‐cell malignancies. The ORR is clinically meaningful because the study population was heavily pretreated and had poor prognostic factors. Further, eight patients attained a complete response, seven of whom had indolent lymphomas ― a subtype which does not typically show a complete response to treatment. The PFS and DOR data also demonstrated the clinical benefit of zanubrutinib treatment. The present study in Chinese patients showed a similar response to zanubrutinib as that observed in the BGB‐3111‐AU‐003 study conducted outside of China, 10 , 11 suggesting that zanubrutinib can benefit all patients regardless of ethnicity.
This is the only study that tested zanubrutinib 320 mg QD in China, and therefore provided the opportunity to compare the safety and efficacy of that dose with the 160 mg BID dose in Asian patients with B‐cell malignancies. Except for a numerically higher frequency of neutrophil count decreased and upper respiratory tract infection in the 320 mg QD group versus the 160 mg BID group in the current study, the safety profile was generally comparable between the two dose regimens, which is consistent with the lack of notable differences in safety end‐points in a global study with a much larger dataset (n = 278 for the BID dose; n = 95 for the QD dose). 12 Possible reasons for the observed differences in our study are more patients with decreased neutrophil counts at baseline in the 320 mg QD group compared with the 160 mg BID group (neutrophil count decreased at screening or at cycle 1 day 1: 40.0% vs 17.6%), more patients with bone marrow infiltration at screening (60.0% in the 320 mg QD group vs 41.2% in the 160 mg BID group), and variability due to the limited size of the 320 mg QD group (n = 10). No marked difference in response rates was observed between the two dose regimens in the current study. This is consistent with results of the global study in which both doses led to clinically meaningful and comparable response rates in patients with MCL, WM and CLL/SLL, and durable responses across tumour types. 12 , 13
The PK profile after single and multiple doses of zanubrutinib (at 160 mg and 320 mg) was also assessed. The mean accumulation ratio based on C max or AUC0–8 h was close to 1, indicating limited accumulation after repeated dosing, which is consistent with the terminal elimination half‐life (2.3–3.4 h). The present study showed that comparable total daily AUC was achieved between 160 mg BID and 320 mg QD regimens. Based on a pooled analysis of phase I/II/III studies 12 and exposure–response analyses, 2 the differences in C max and trough concentration between the two regimens are unlikely to have a meaningful impact on efficacy and safety end‐points. Given the comparable safety, efficacy, and daily AUC between the two regimens, and the convenience of once‐daily dosing, which may improve treatment adherence, 320 mg QD has been approved together with 160 mg BID in the United States, Europe and other countries/regions.
The PK of zanubrutinib in Chinese patients in the present study is generally consistent with those in the global FIH study which enrolled mostly non‐Asian patients, and with previous reports showing lack of an ethnicity impact on zanubrutinib PK. 14 , 15 In healthy volunteers in a single protocol with controlled extrinsic factors, 14 the exposure of zanubrutinib in Asian (first‐generation Chinese) subjects was comparable with that in non‐Asian subjects, as was the magnitude of interaction with rifampin and itraconazole. In addition, a population PK analysis of 632 subjects from nine studies, including PK results from the present study, concluded that race does not significantly affect zanubrutinib exposure. 15
One of the most common resistance mechanisms for ibrutinib is BTK C481S which results in reversible inhibition of BTK. 16 This mutation has also been observed in zanubrutinib‐progressive CLL patients, along with a BTK Leu528Trp mutation, which decreases binding affinity to zanubrutinib and ATP 17 and is rarely reported in ibrutinib‐progressive patients. 18 The current study detected BTK Cys481Ser/Arg or Leu528Trp mutations in four of 14 patients, including two with CLL, one with WM, and one with FL. These findings suggest that BTK mutations could contribute to zanubrutinib resistance across different haematological malignancies. PLCG2 mutations, which have been previously reported in ibrutinib‐progressive patients, 16 were not detected in this study. Mutations in TP53 and CARD11 are associated with worse responses to ibrutinib in FL patients. 19 , 20 In the current study, among zanubrutinib‐progressive FL patients, 57.1% carried TP53 mutations and 42.8% carried BCL10/CARD11 mutations, a rate higher than the 9.5% BCL10, 16% CARD11 and approximately 25% TP53 mutation rates previously reported in recurrent FL patients, 19 , 21 and suggests that TP53 and CARD11/BCL10 mutations may contribute to resistance to BTK inhibitors in FL patients. Five patients had mutational status available in pre‐treatment samples using a different NGS panel. Two of these five patients had TP53 mutations. The same mutation was observed in both the pre‐treatment and the disease progression samples, indicating that, at least in these two patients, the TP53 mutations were present before zanubrutinib treatment, rather than induced during therapy.
In summary, zanubrutinib was well tolerated and the anti‐tumour effect was clinically significant when given as monotherapy at 160 mg BID or 320 mg QD. The totality of data, including PK, safety, and efficacy, support the recommended 320 mg QD and 160 mg BID dose regimens with no modifications needed for Asian patients.
AUTHOR CONTRIBUTION
Chenmu Du, Haiyi Guo, Jane Huang, Zhiyu Tang, Ying Ou, Binghao Wu, and Yiling Yu were responsible for the conception and design of the study. Chenmu Du, Haiyi Guo, Jane Huang, Zhiyu Tang, Ying Ou, Binghao Wu, and Yiling Yu developed the methodology. Yuqin Song, Mingyuan Sun, Junyuan Qi, Wei Xu, Jianfeng Zhou, Dengju Li, Jianyong Li, Lugui Qiu, and Jun Zhu were responsible for the acquisition of data (i.e., acquired and managed patients, provided facilities). Chenmu Du, Haiyi Guo, Zhiyu Tang, Ying Ou, Binghao Wu, and Yiling Yu performed analysis and interpretation of data (i.e., statistical analysis, biostatistics, computational analysis). All authors performed writing, review, and/or revision of the manuscript. Chenmu Du, Haiyi Guo, Zhiyu Tang, Ying Ou, Binghao Wu and Yiling Yu provided administrative, technical, or material support (i.e., reporting or organizing data, constructing databases). All authors read and approved the final manuscript.
CONFLICT OF INTERESTS
Chenmu Du, Haiyi Guo, Jane Huang, Zhiyu Tang, Ying Ou, Binghao Wu, and Yiling Yu are employees of and own stock in BeiGene. The other authors declare that they have no competing interests.
Supporting information
Table S1
Table S2
Figure S1
ACKNOWLEDGEMENTS
The authors thank the patients who participated in the study, their supporters, and the investigators and clinical research staff from the study centres. This study was supported by research funding from BeiGene (Beijing) Co., Ltd, who confirmed the accuracy of the data and compiled the data for analysis. Medical editing support was funded by BeiGene and provided by Twist Medical, LLC.
Song Y, Sun M, Qi J, Xu W, Zhou J, Li D, et al. A two‐part, single‐arm, multicentre, phase I study of zanubrutinib, a selective Bruton tyrosine kinase inhibitor, in Chinese patients with relapsed/refractory B‐cell malignancies. Br J Haematol. 2022;198:62–72. 10.1111/bjh.18162
Funding information This study was supported by research funding from BeiGene (Beijing) Co., Ltd.
REFERENCES
- 1. Guo Y, Liu Y, Hu N, Yu D, Zhou C, Shi G, et al. Discovery of zanubrutinib (BGB‐3111), a novel, potent, and selective covalent inhibitor of Bruton's tyrosine kinase. J Med Chem. 2019;62:7923–40. [DOI] [PubMed] [Google Scholar]
- 2. Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B‐cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03 [Internet]. National Cancer Institute; c2009. [updated June 14, 2010]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010‐06‐14_QuickReference_8.5x11.pdf [Google Scholar]
- 4. Hallek M, Cheson BD, Catovsky D, Caligaris‐Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the National Cancer Institute‐working group 1996 guidelines. Blood. 2008;111:5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Owen RG, Kyle RA, Stone MJ, Rawstron AC, Leblond V, Merlini G, et al. Response assessment in Waldenström macroglobulinaemia: update from the VIth international workshop. Br J Haematol. 2013;160:171–6. [DOI] [PubMed] [Google Scholar]
- 7. National Comprehensive Cancer Network (NCCN) . NCCN guidelines insights: Waldenström's macroglobulinaemia/lymphoplasmacytic lymphoma, version 2.2013. J Natl Compr Canc Netw. 2012;10:1211–8. [DOI] [PubMed] [Google Scholar]
- 8. Chakravarty D, Gao J, Phillips S, Kundra R, Zhang H, Wang J, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol, published online May 16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tam CS, Opat S, D'Sa S, Jurczak W, Lee H‐P, Cull G, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinaemia: the ASPEN study. Blood. 2020;136:2038–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tam CS, Simpson D, Opat S, Kim WS, Wang M, Cull G, et al. Safety and activity of the highly specific BTK inhibitor BGB‐3111 in patients with indolent and aggressive non Hodgkin's lymphoma. Blood. 2017;130:152. [Google Scholar]
- 11. Cull G, Simpson D, Opat S, Burger JA, Trotman J, Marlton P, et al. Treatment with the Bruton tyrosine kinase inhibitor zanubrutinib (BGB‐3111) demonstrates high overall response rate and durable responses in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): updated results from a phase 1/2 trial. Blood. 2019;134(Supplement_1):500.31395583 [Google Scholar]
- 12. Ou YC, Tang Z, Novotny W, Cohen A, Wang K, Liu L, et al. Rationale for once‐daily or twice‐daily dosing of zanubrutinib in patients with mantle cell lymphoma. Leuk Lymphoma. 2021;62:2612–24. [DOI] [PubMed] [Google Scholar]
- 13. Tam CS, Ou YC, Trotman J, Opat S. Clinical pharmacology and PK/PD translation of the second‐generation Bruton's tyrosine kinase inhibitor, zanubrutinib. Expert Rev Clin Pharmacol. 2021;14:1329–44. [DOI] [PubMed] [Google Scholar]
- 14. Mu S, Tang Z, Novotny W, Tawashi M, Li T‐K, Ou Y, et al. Effect of rifampin and itraconazole on the pharmacokinetics of zanubrutinib (a Bruton's tyrosine kinase inhibitor) in Asian and non‐Asian healthy subjects. Cancer Chemother Pharmacol. 2020;85:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ou YC, Liu L, Tariq B, Wang K, Jindal A, Tang Z, et al. Population pharmacokinetic analysis of the BTK inhibitor zanubrutinib in healthy volunteers and patients with B‐cell malignancies. Clin Transl Sci. 2021;14:764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woyach JA, Furman RR, Liu T‐M, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Handunnetti SM, Tang CPS, Nguyen T, Zhou X, Thompson E, Sun H, et al. BTK Leu528Trp – a potential secondary resistance mechanism specific for patients with chronic lymphocytic leukemia treated with the next generation BTK inhibitor zanubrutinib. Blood. 2019;134(Supplement_1):170. [Google Scholar]
- 18. Maddocks KJ, Ruppert AS, Lozanski G, Heerema NA, Zhao W, Abruzzo L, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bartlett NL, Costello BA, LaPLant BR, Ansell SM, Kuruvilla JG, Reeder CB, et al. Single‐agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood. 2018;131:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruscaggin A, di Bergamo LT, Spina V, Hodkinson B, Forestieri G, Bonfiglio F, et al. Circulating tumor DNA for comprehensive noninvasive monitoring of lymphoma treated with ibrutinib plus nivolumab. Blood Adv. 2021;5:4674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Du MQ, Peng H, Liu H, Hamoudi RA, Diss TC, Willis TG, et al. BCL10 gene mutation in lymphoma. Blood. 2000;95:3885–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Figure S1


