Abstract
Gastric cancer is a heterogeneous and prevalent disease. The traditional environmental exposures associated with an elevated risk of gastric cancer are less prevalent in the United States today. Genetic risks and risks associated with inflammation remain. Most cases are sporadic and familial clustering is observed in about 10% of the cases. Hereditary gastric cancer accounts for a very low percentage of cases. Here we review the genetic and environmental risk factors associated with the disease.
Keywords: adenocarcinoma, diet, H. pylori, stomach
1. INTRODUCTION
The incidence of gastric cancer has decreased worldwide since the 1970s, likely due to multiple factors including effective H. pylori treatment 1 and improved refrigeration. 2 Despite this progress, it continues to be a major global health concern, particularly in Eastern Asian countries, with over 1 million cases diagnosed in 2020. 3 Prognosis for the treatment of this disease remains poor, and it is currently the fourth most common cause of cancer mortality worldwide. In the United States, an estimated 26 560 new cases were diagnosed in 2021 with 11 180 patients dying from the disease, making it the 16th most diagnosed cancer and 17th leading cause of cancer death. 4 The traditional environmental exposures (dietary risks) associated with an elevated risk of gastric cancer are less prevalent in the United States today. Genetic risks and risks associated with inflammation of the stomach remain. Most cases are sporadic and familial clustering is observed in about 10% of cases. 5 , 6 Defining populations at the highest risk is important to the consideration of novel screening protocols. Here, we review genetic and environmental risk factors that contribute to the formation of gastric cancer.
2. GENETIC RISK FACTORS AND FAMILIAL SYNDROMES
Gastric cancer risk is likely modulated by multiple factors. Genetic susceptibility may modify the effect of environmental and dietary exposures, resulting in the high variation of gastric cancer incidence seen around the world. Here, we review some genetic factors associated with this increased risk. We also review familial cancer syndromes that confer a higher risk of gastric cancer (summarized in Table 1).
Table 1.
Cumulative risk of developing gastric cancer in various genetic syndromes
| Genetic syndromes | Lifetime risk | References |
|---|---|---|
| Hereditary diffuse gastric cancer | 67%–70% for Male | Hansford et al. 7 ; National Comprehensive Cancer Network 8 |
| 56%–83% for Female | ||
| Hereditary nonpolyposis colorectal cancer | ||
| MLH1 | 2%–11% | Barrow et al. 9 ; Bonadona 10 ; Capelle et al. 11 ; Vasen et al. 12 |
| MSH2 | 0.2%–9% | Barrow et al. 9 ; Bonadona 10 ; Capelle et al. 11 ; Vasen et al. 12 |
| MSH6 | 0%–10% | Barrow et al. 9 ; Capelle et al. 11 ; Moller et al. 13 |
| Familial adenomatous polyposis | 0.6%–2% | National Comprehensive Cancer Network 8 ; Syngal et al. 14 |
| Gastric adenocarcinoma and proximal polyposis of the stomach | 12%–20% | Kim et al. 15 ; Worthley et al. 16 ; Setia et al. 17 ; Foretova et al. 18 |
| MUTYH‐associated polyposis | 1%–5% | Vogt et al. 19 ; Win et al. 20 |
| Juvenile polyposis syndrome | 5%–21% | National Comprehensive Cancer Network 8 ; Attard and Young 21 |
| Peutz–Jegher syndrome | 29% | National Comprehensive Cancer Network 8 ; Giardiello et al. 22 |
| Li–Fraumeni syndrome | 2%–5% | Kim et al. 15 ; Masciari et al. 23 |
2.1. Age and sex
2.1.1.
The risk of gastric cancer increases with age. Between 2014 and 2018, 2% of gastric cancer cases were in those less than 34 years in age, 38% of cases were in those between 35 and 64, and 60% occurred in those above the age of 65. 4 The median age of diagnosis in this timeframe was 68 years. 4 Risk with increased age is likely secondary to longer exposure to potential carcinogens, increased susceptibility to mucosal damage, delayed healing of the gastric mucosa, increased incidence of mucosal cancer stem cell markers, increased prevalence of chronic active gastritis, intestinal metaplasia, and mucosal atrophy, especially in those infected with H. Pylori. 24 , 25 , 26
Men are twice as likely as women to be affected by gastric cancer. 3 , 4 Studies evaluating sociodemographic characteristics, environmental factors, sex hormones, hormonal interventions, and smoking habits to explain the difference in incidence between men and women have been inconclusive. 27 , 28 , 29 However, the prevalence of H. Pylori infection appears to be higher in men and likely contributes to the higher incidence of gastric cancer seen in the gender. 30 , 31
2.2. Blood type A
The relationship between group A blood and gastric cancer was first described by Aird et al. in 1953. 32 Since then, many studies, including several recent meta‐analyses, have confirmed their findings that those with group A blood carry a higher relative risk (1.11–1.21) of gastric cancer compared with other blood types. 33 , 34 , 35 , 36 There have been several proposed mechanisms behind this association, including alterations in gastric secretory function, intracellular adhesion receptors, membrane signaling, immune surveillance, inflammatory response to H. Pylori and malignant cells, and increased susceptibility to pernicious anemia. 37 , 38 , 39
2.3. Hereditary diffuse gastric cancer
Hereditary diffuse gastric cancer (HDGC) is an autosomal dominant genetic pre‐disposition syndrome characterized by early onset diffuse gastric cancer affecting multiple generations, and lobular breast cancer. Forty percent of HDGC families have germline mutations in CDH1 and over 100 different pathogenic germline mutations have been reported. 7 Guilford et al. 40 first identified CDH1 as the responsible gene for early onset diffuse gastric cancer in a study of several Māori families with the disease. Human CDH1 is located at chromosome 16q22 and encodes for E‐cadherin, a transmembrane protein with five tandemly repeated extracellular domains and a cytoplasmic domain that connects to the actin cytoskeleton through a complex with α‐, β‐ and γ‐catenin. This cell adhesion molecule is important for establishing cell polarity and maintaining normal tissue morphology and cellular differentiation. 41 Downregulation of E‐cadherin expression often correlates with strong invasive potential and poor prognosis of human carcinomas. In a recent study of 183 patients with HDGC who underwent CDH1 testing, the cumulative incidence of gastric cancer was found to be 70% in males and 56% in females. 7
Up to 60% of families meeting clinical criteria for HDGC do not have a detectable CDH1 mutation. 7 In 2013, Majewski et al. 42 identified a germline mutation in CTNNA1, resulting in loss of α‐E‐catenin expression. Since then, four additional families with HDGC have been shown to express the mutation. 7 , 43 Not much is currently known regarding the penetrance of CTNNA1 variants. However, there is evidence suggesting that it carries a similar risk of diffuse gastric cancer as CDH1 mutations. 44 The International Gastric Cancer Linkage Consortium (IGCLC) guidelines now recommend genetic testing for CTNNA1 mutations, in addition to CDH1 mutations, in those meeting genetic testing criteria for HDGC. 45 However, the incidence of CTNNA1 mutation in CDH1‐negative HDGC‐like disease remains low and most of the genetic susceptibility remains unknown with further research needed to characterize additional mutations leading to increased susceptibility.
2.4. Hereditary nonpolyposis colorectal cancer (HNPCC)
HNPCC, also known as Lynch syndrome, is an autosomal dominant disorder, affecting DNA mismatch repair genes. It is characterized by a mutation in 1 of 4 DNA mismatch repair genes: MLH1, MSH2, MSH6, and PMS2. 46 Affected patients have a significantly increased risk for colorectal and endometrial cancer, however mutations in MLH1, MSH2, and MSH6 have been shown to increase lifetime risk of gastric cancer as well. MLH1 mutations have been shown to confer a lifetime risk up to 10.9%, MSH2 mutations confer a risk up to 9.0%, and MSH6 mutations up to 10.4%. 8 , 9 , 10 , 11 , 12 , 13 Most gastric cancers in patients with HNPCC are intestinal‐type and have the same natural history as sporadic intestinal‐type gastric cancer. 47
2.5. Familial adenomatous polyposis (FAP)
FAP is an autosomal dominant disorder arising from a mutation in the APC tumor‐suppressor gene. It is characterized by the formation of >100 synchronous colorectal adenomas and a near 100% risk of colon cancer. FAP also increases the risk of gastric polyps with rates ranging from 23% to 100%, and these polyps tend to be more numerous and occur at a younger age, although these are typically benign. 14 The risk of gastric cancer in those with FAP is comparable to the general population risk, however, in countries with a higher prevalence of gastric cancer, such as Japan and Korea, higher risk has been reported. 14 , 15 , 48
2.6. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS)
GAPPS is an autosomal‐dominant disorder characterized by multiple (>100) fundic gland polyposis with areas of multifocal dysplasia. Recently discovered, the syndrome was first described by Worthey et al. in 2012 in three families from Australia, the USA, and Canada. 16 The gene mutations responsible were described by Li et al. in 2016 as various point mutations in Exon 1B of the APC gene. 49 Given its recent discovery, data concerning lifetime risk of gastric cancer is sparse, however, in the available literature, risks of 12%–20% have been described. 15 , 16 , 17 , 18
2.7. MUTYH‐associated polyposis (MAP)
MAP is an autosomal recessive disorder caused by mutations in the base‐excision repair gene MUTYH. MAP is characterized by multiple adenomas in the colon and rectum and typically becomes symptomatic between the fourth and seventh decade of life, with a cumulative lifetime risk of colon cancer approaching 100%. Patients affected with MAP have up to a 5% lifetime risk of gastric cancer. 19 , 20
2.8. Juvenile polyposis syndrome (JPS)
JPS is an autosomal dominant disorder caused by mutations in BMPR1A or SMAD4 ‐ tumor suppressor genes in the TGF‐B signaling family that regulate cell growth inhibition and apoptosis. 14 JPS is characterized by multiple hamartomatous polyps throughout the GI tract. SMAD4 mutations carry an increased risk of gastric polyposis and gastric cancer compared to BMPR1A mutations, with lifetime gastric cancer risk approaching 21%. 21
2.9. Peutz–Jegher syndrome (PJS)
PJS is an autosomal dominant disorder caused by mutations in the STK11 gene, which encodes a threonine kinase that functions as a tumor suppressor. 50 Clinically, it is characterized by hamartomatous polyps in the GI tract and mucocutaneous pigmentation. Patients with PJS are at increased risk of a variety of common GI and non‐GI tumors, with a lifetime gastric cancer risk of 29%. 22
2.10. Li–Fraumeni syndrome (LFS)
LFS is an autosomal dominant disorder caused by germline mutations in the TP53 tumor promotor gene. Classically associated with sarcomas of the soft tissue and bone, adrenal cortical carcinomas, breast cancer, leukemia, and brain tumors, LFS is also associated with a broad range of other neoplasms. Patients affected by LFS carry a 2%–5% lifetime risk of being diagnosed with gastric cancer. 15 , 23
3. ENVIRONMENTAL FACTORS
Given the high variation in incidence seen around the world, environmental factors likely play a major role in modulating the risk of gastric cancer. Here, we review several environmental risk factors that affect gastric cancer risk. Table 2 provides a summary of these risk factors and their associated relative risk.
Table 2.
Risk factors for gastric cancer
| Factor | Relative risk (RR) | References |
|---|---|---|
| Male sex | 1.95 | Sung et al. 3 |
| Family history | 1.5–3.5 | La Vecchia et al. 6 ; Yaghoobi et al. 51 ;Yaghoobi et al. 52 |
| H. Pylori infection | 3.8–5.8 | Vohlonen et al. 53 ; Helicobacter, Cancer Collaborative Group 54 ; Kikuchi et al. 55 |
| Smoking | 1.53–1.84 | Koizumi et al. 56 ; Nishino et al. 57 ; Ladeiras‐Lopes et al. 58 ; Nomura et al. 59 |
| Heavy alcohol intake | 1.20–1.65 | Wang et al. 60 ; Tramacere et al. 61 ; Duell et al. 62 ; Rota et al. 63 |
| Blood group A | 1.11–1.21 | Yu et al. 33 ; Wang et al. 34 ; Edgren et al. 35 ; Vasan et al. 36 |
| Moderate‐high salt intake | 1.41–1.68 | D'Elia et al. 64 |
3.1. Geographic variation
3.1.1.
Gastric cancer incidence varies markedly across the globe with almost a 40‐fold difference between the lowest and highest incidence countries. Incidence rates are highest in Eastern Asian and Eastern European countries, with lower rates in North American, North Europe, and Africa 3 , 65 (Figure 1). The reason behind geographical variations is likely multifactorial, with contributions from environmental, genetic, and infectious factors. Diet is an important factor in the development of gastric cancer, and variations in diet across the globe are likely a major contributor. Differences in H. pylori genotypes may also explain why certain populations with high rates of H. pylori infection experience increased incidence of gastric cancer, while other populations with similarly high infection rates do not. 66 Variations in cigarette and alcohol use likely contribute to a variable risk profile as well. Studies evaluating the risk of gastric cancer in migrants from high‐incidence countries to low‐incidence countries have found a decrease in cancer risk in successive generations, with the risk approaching the overall risk of the host country, further supporting the idea that environmental factors play a large role in carcinogenesis and contribute to the variation seen around the world. 67 , 68
Figure 1.
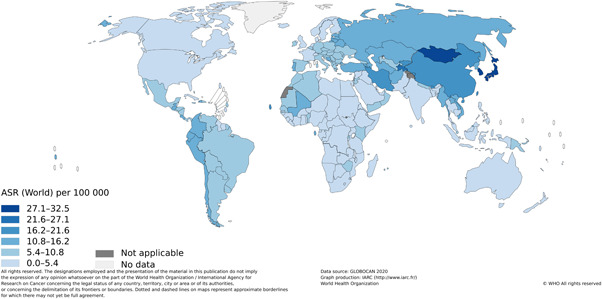
Global age‐standardized incidence rates of gastric cancer. Copyright 2022 International Agency Research on Cancer (IARC). Reprinted with permission.
3.2. Salt intake
Diet is an important, modifiable risk factor for gastric cancer. The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and the World Health Organization/Food and Agricultural Organization (WHO/FAO) expert panel have both stated that salt is a probable cause of gastric cancer. 69 , 70 Early ecological studies first established the link between salt and gastric cancer. In a 1996 study evaluating 39 populations from 24 countries, Joossens et al. showed a link between urinary salt excretion and cancer. 71 Subsequent case‐control and prospective cohort studies have confirmed the increased risk of gastric cancer with higher salt intake. A meta‐analysis of prospective studies by D'Elia et al. 64 found a 40%–70% increased risk of cancer in those with high and moderate salt intake compared to low salt intake. In the same study, foods with high salt content, such as pickled foods, salted fish, and processed meat were all significantly associated with an increased risk of cancer.
There are several mechanisms via which salt may contribute to carcinogenesis. High salt concentration has been shown to disrupt the mucosal barrier of the stomach and lead to inflammation and atrophy. Experimental studies in mice and gerbils have shown increased rates of H. Pylori colonization with a high salt diet due to alternations in the protective mucin layer. 72 , 73 High salt intake has also been found to increase CagA expression, a potent virulence factor in H. Pylori and a known risk factor for gastric cancer development in those infected. 74 Studies also suggest that salt intake may enhance the effect of other carcinogens such as N‐nitroso compounds. 75 , 76
3.3. Fruit and vegetables
Fruits and vegetables may have a protective effect against gastric cancer, possibly related to their antioxidant capacity. A meta‐analysis by Riboli and Norat 77 found a pooled risk of 0.81 and 0.74 for each 100 g increase in vegetable and fruit, respectively. 77 However, when only more reliable prospective studies were considered, the benefit was no longer significant. Indeed, two large prospective studies also found minimal to no risk reduction with increased consumption of fruits and vegetables. 78 , 79
3.4. Smoking
Cigarette smoking has been implicated as a risk factor in many cancers, and gastric cancer is no exception. The risk of gastric cancer in smokers is 1.5–1.8 times higher compared to those that do not smoke. 56 , 57 , 58 , 59 The relationship appears to be dose‐dependent, with a higher number of cigarettes per day and a longer duration of smoking conveying a higher risk; however, evidence of this effect has not been consistent in the literature. 59 , 80 Smoking cessation attenuates the risk, with a longer duration of cessation associated with an increased risk reduction. 59 , 80
Cigarette smoke contains a variety of carcinogenic components, and the mechanism behind the effects of smoking on tumorigenesis in the stomach is an area of active research. Possible mechanisms include activation of nicotinic acetylcholine receipts, formation of DNA adducts, stimulation of tumor angiogenesis, and induction of cell proliferation. 81 , 82 Additionally, smoking is a risk factor for induction of chronic inflammation in the GI tract via alteration of mucosal cell proliferation, induction of immune dysfunction, and increased risk of bacterial or viral infection, which may further contribute to carcinogenesis. 81
3.5. Alcohol
Heavy alcohol intake increases the risk of gastric cancer by up to 65% when compared to low‐moderate intake, specifically increasing the risk for noncardia cancer. 60 , 61 , 62 , 63 While the mechanism behind the increased risk is unclear, it is likely multifactorial. One mechanism may be the presence of N‐nitrosodimethylamine (NDMA), a carcinogen, in certain alcoholic beverages, particularly beer. 62 Additionally, alcohol consumption results in the formation of acetaldehyde, a known carcinogen. 60 Ethanol may also directly damage the gastric mucosa, allowing other carcinogenic substances to penetrate into the mucosa and allowing tumorigenesis. 60 Finally, heavy alcohol consumption likely induces a chronic inflammatory state in the stomach, thus predisposing the organ to cancer. 61 Interestingly, some reports indicate that low to moderate alcohol consumption may have a protective effect against gastric cancer. 61 , 62 However, these findings are not consistent across all studies and may be a result of confounding. Possible mechanisms of the protective effect may be a possible bacteriocidic effect of ethanol on H. Pylori or favorable dietary patterns of light‐moderate alcohol drinkers compared to non‐drinkers and heavy drinkers. While there is good evidence that heavy drinking does increase the risk of gastric cancer, further studies are needed to understand the mechanisms at play.
3.6. H. pylori
H. pylori is a spiral‐shaped, gram‐negative, microaerophilic bacterium thought to be transferred via oral‐oral or fecal‐oral transmission. 83 , 84 The pathogen was first described by Warren and Marshall in 1983 as the possible mechanism behind chronic active gastritis. 85 Since then, H. Pylori has been established as one of the primary risk factors for gastric cancer, accounting for up 89% of noncardia gastric cancer and 18% of cardia gastric cancer. 86 In 1994, the Internal Agency for Research on Cancer (IARC) an entity of the World Health Organization declared H. Pylori a class I carcinogen. 87 H. Pylori infection has been shown to increase the risk of gastric cancer three‐ to sixfold. 53 , 54 , 55 The mechanism behind how H. Pylori increases this risk is unclear, but two possible pathways have been considered: direct modulation of gastric mucosa through virulence factors such as CagA and VacA, and indirect action of H. Pylori on gastric epithelial cells. What is clear is that H. pylori infection induces a state of chronic active inflammation that can last decades. This chronic, active gastritis can subsequently promote gastric carcinogenesis, typically via the Correa model which suggests that chronic gastric inflammation leads to a cascade of mucosal atrophy, metaplasia, dysplasia, and eventually carcinoma. 88 Host genetic factors likely play a role as well – specific gene polymorphisms in genes encoding for tumor necrosis factor‐α, interleukin (IL)‐1, IL‐8, and IL‐10 have been associated with increased risk of gastric cancer in the setting of H. Pylori infection. 89 H. Pylori is a very common pathogen, infecting approximately half of the world's population, however, only a small percentage of that infected progress to gastric cancer, suggesting that progression is modulated by various elements including bacterial, environmental, and host genetic factors.
4. CONCLUSION
Gastric cancer remains one of the most common causes of cancer mortality worldwide. Various modifiable and nonmodifiable factors modulate the risk of gastric cancer, and it is important to consider these risks when defining populations for novel screening methods. H. Pylori, diet, and smoking are important modifiable risk factors. Continued studies are needed to further delineate the genetic susceptibilities in those with a family history of the disease.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
SYNOPSIS
Gastric adenocarcinoma is an important cause of worldwide cancer mortality with a significant geographic variation. This review highlights various factors that modify the risk of gastric cancer.
ACKNOWLEDGMENT
Dr. Shah is a Postdoctoral Fellow under an Institutional Award from the NCI (1R38CA245095‐01A1).
Shah D, Bentrem D. Environmental and genetic risk factors for gastric cancer. J Surg Oncol. 2022;125:1096‐1103. 10.1002/jso.26869
DATA AVAILABILITY STATEMENT
NA.
REFERENCES
- 1. Parsonnet J, Vandersteen D, Goates J, Sibley RK, Pritikin J, Chang Y. Helicobacter pylori infection in intestinal‐ and diffuse‐type gastric adenocarcinomas. J Natl Cancer Inst. 1991;83(9):640‐643. [DOI] [PubMed] [Google Scholar]
- 2. Coggon D, Barker DJ, Cole RB, Nelson M. Stomach cancer and food storage. J Natl Cancer Inst. 1989;81(15):1178‐1182. [DOI] [PubMed] [Google Scholar]
- 3. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 4. National Cancer Institute : Surveillance E, and End Results Program. Cancer Stat Facts: Stomach Cancer. https://seer.cancer.gov/statfacts/html/stomach.html.
- 5. Zanghieri G, Di Gregorio C, Sacchetti C, et al. Familial occurrence of gastric cancer in the 2‐year experience of a population‐based registry. Cancer. 1990;66(9):2047‐2051. [DOI] [PubMed] [Google Scholar]
- 6. La Vecchia C, Negri E, Franceschi S, Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer. 1992;70(1):50‐55. [DOI] [PubMed] [Google Scholar]
- 7. Hansford S, Kaurah P, Li‐Chang H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1(1):23‐32. [DOI] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network . Genetic/Familial High‐Risk Assessment: Colorectal (Version 1.2021); 2021. [Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. [DOI] [PubMed]
- 9. Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet. 2009;75(2):141‐149. [DOI] [PubMed] [Google Scholar]
- 10. Bonadona V. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305(22):2304‐2310. [DOI] [PubMed] [Google Scholar]
- 11. Capelle LG, Van Grieken NCT, Lingsma HF, et al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010;138(2):487‐492. [DOI] [PubMed] [Google Scholar]
- 12. Vasen HF, Stormorken A, Menko FH, et al. MSH2 mutation carriers are at higher risk of cancer than MLH1 mutation carriers: a study of hereditary nonpolyposis colorectal cancer families. J Clin Oncol. 2001;19(20):4074‐4080. [DOI] [PubMed] [Google Scholar]
- 13. Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the prospective Lynch syndrome database. Gut. 2018;67(7):1306‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim W, Kidambi T, Lin J, Idos G. Genetic syndromes associated with gastric cancer. Gastrointest Endosc Clin N Am. 2022;32(1):147‐162. [DOI] [PubMed] [Google Scholar]
- 16. Worthley DL, Phillips KD, Wayte N, et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut. 2012;61(5):774‐779. [DOI] [PubMed] [Google Scholar]
- 17. Setia N, Clark JW, Duda DG, et al. Familial gastric cancers. Oncologist. 2015;20(12):1365‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foretová L, Navrátilová M, Svoboda M, et al. GAPPS ‐ gastric adenocarcinoma and proximal polyposis of the stomach syndrome in 8 families tested at Masaryk Memorial Cancer Institute ‐ prevention and prophylactic gastrectomies. Klin Onkol. 2019;32(Suppl2):S109‐S117. [DOI] [PubMed] [Google Scholar]
- 19. Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH‐associated polyposis. Gastroenterology. 2009;137(6):1976‐1985. [DOI] [PubMed] [Google Scholar]
- 20. Win AK, Reece JC, Dowty JG, et al. Risk of extracolonic cancers for people with biallelic and monoallelic mutations in MUTYH. Int J Cancer. 2016;139(7):1557‐1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Attard TM, Young RJ. Diagnosis and management of gastrointestinal polyps: pediatric considerations. Gastroenterol Nurs. 2006;29(1):16‐22. [DOI] [PubMed] [Google Scholar]
- 22. Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz‐Jeghers syndrome. Gastroenterology 2000;119(6):1447‐1453. [DOI] [PubMed] [Google Scholar]
- 23. Masciari S, Dewanwala A, Stoffel EM, et al. Gastric cancer in individuals with Li‐Fraumeni syndrome. Genet Med. 2011;13(7):651‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levi E, Sochacki P, Khoury N, Patel BB, Majumdar AP. Cancer stem cells in Helicobacter pylori infection and aging: implications for gastric carcinogenesis. World J Gastrointest Pathophysiol. 2014;5(3):366‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sonnenberg A, Genta RM. Changes in the gastric mucosa with aging. Clin Gastroenterol Hepatol. 2015;13(13):2276‐2281. [DOI] [PubMed] [Google Scholar]
- 26. Tarnawski AS, Ahluwalia A, Jones MK. Angiogenesis in gastric mucosa: an important component of gastric erosion and ulcer healing and its impairment in aging. J Gastroenterol Hepatol. 2014;29(Suppl 4):112‐123. [DOI] [PubMed] [Google Scholar]
- 27. Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta‐analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(1):20‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lindblad M, Rodriguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non‐cardia adenocarcinoma among men and women in a nested case‐control study. Cancer Causes Control. 2005;16(3):285‐294. [DOI] [PubMed] [Google Scholar]
- 29. Freedman ND, Derakhshan MH, Abnet CC, Schatzkin A., Hollenbeck AR, McColl KE. Male predominance of upper gastrointestinal adenocarcinoma cannot be explained by differences in tobacco smoking in men versus women. Eur J Cancer. 2010;46(13):2473‐2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ibrahim A, Morais S, Ferro A, Lunet N, Peleteiro B. Sex‐differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: systematic review and meta‐analysis of 244 studies. Dig Liver Dis. 2017;49(7):742‐749. [DOI] [PubMed] [Google Scholar]
- 31. Replogle ML, Glaser SL, Hiatt RA, Parsonnet J. Biologic sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am J Epidemiol. 1995;142(8):856‐863. [DOI] [PubMed] [Google Scholar]
- 32. Aird I, Bentall HH, Roberts JA. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1(4814):799‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu H, Xu N, Li ZK, et al. Association of ABO blood groups and risk of gastric cancer. Scand J Surg. 2020;109(4):309‐313. [DOI] [PubMed] [Google Scholar]
- 34. Wang Z, Liu L, Ji J, et al. ABO blood group system and gastric cancer: a case‐control study and meta‐analysis. Int J Mol Sci. 2012;13(10):13308‐13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edgren G, Hjalgrim H, Rostgaard K, et al. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010;172(11):1280‐1285. [DOI] [PubMed] [Google Scholar]
- 36. Vasan SK, Hwang J, Rostgaard K, et al. ABO blood group and risk of cancer: a register‐based cohort study of 1.6 million blood donors. Cancer Epidemiol. 2016;44:40‐43. [DOI] [PubMed] [Google Scholar]
- 37. Paré G, Chasman DI, Kellogg M, et al. Novel association of ABO histo‐blood group antigen with soluble ICAM‐1: results of a genome‐wide association study of 6,578 women. PLoS Genet. 2008;4(7):e1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts JA. Blood groups and susceptibility to disease: a review. Br J Prev Soc Med. 1957;11(3):107‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sievers ML. Hereditary aspects of gastric secretory function; race and ABO blood groups in relationship to acid and pepsin production. Am J Med. 1959;27:246‐255. [DOI] [PubMed] [Google Scholar]
- 40. Guilford P, Hopkins J, Harraway J, et al. E‐cadherin germline mutations in familial gastric cancer. Nature 1998;392(6674):402‐405. [DOI] [PubMed] [Google Scholar]
- 41. Grunwald GB. The structural and functional analysis of cadherin calcium‐dependent cell adhesion molecules. Curr Opin Cell Biol. 1993;5(5):797‐805. [DOI] [PubMed] [Google Scholar]
- 42. Majewski IJ, Kluijt I, Cats A, et al. An alpha‐E‐catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J Pathol. 2013;229(4):621‐629. [DOI] [PubMed] [Google Scholar]
- 43. Weren RDA, van der Post RS, Vogelaar IP, et al. Role of germline aberrations affecting CTNNA1, MAP3K6 and MYD88 in gastric cancer susceptibility. J Med Genet. 2018;55(10):669‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Benusiglio PR, Colas C, Guillerm E, et al. Clinical implications of CTNNA1 germline mutations in asymptomatic carriers. Gastric Cancer. 2019;22(4):899‐903. [DOI] [PubMed] [Google Scholar]
- 45. Blair VR, McLeod M, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol. 2020;21(8):e386‐e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vasen HF. Review article: the Lynch syndrome (hereditary nonpolyposis colorectal cancer). Aliment Pharmacol Ther. 2007;26(Suppl 2):113‐126. [DOI] [PubMed] [Google Scholar]
- 47. Aarnio M, Salovaara R, Aaltonen LA, Mecklin JP, Järvinen HJ. Features of gastric cancer in hereditary non‐polyposis colorectal cancer syndrome. Int J Cancer. 1997;74(5):551‐555. [DOI] [PubMed] [Google Scholar]
- 48. Lynch HT, Grady W, Suriano G, Huntsman D. Gastric cancer: new genetic developments. J Surg Oncol. 2005;90(3):114‐133. [DOI] [PubMed] [Google Scholar]
- 49. Li J, Woods SL, Healey S, et al. Point Mutations in Exon 1B of APC reveal gastric adenocarcinoma and proximal polyposis of the stomach as a familial adenomatous polyposis variant. Am J Hum Genet. 2016;98(5):830‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vaahtomeri K, Makela TP. Molecular mechanisms of tumor suppression by LKB1. FEBS Lett. 2011;585(7):944‐951. [DOI] [PubMed] [Google Scholar]
- 51. Yaghoobi M, Bijarchi R, Narod SA. Family history and the risk of gastric cancer. Br J Cancer. 2010;102(2):237‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yaghoobi M, McNabb‐Baltar J, Bijarchi R, Hunt RH. What is the quantitative risk of gastric cancer in the first‐degree relatives of patients? A meta‐analysis. World J Gastroenterol. 2017;23(13):2435‐2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vohlonen I, Pukkala E, Malila N, et al. Risk of gastric cancer in Helicobacter pylori infection in a 15‐year follow‐up. Scand J Gastroenterol. 2016;51(10):1159‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Helicobacter, Cancer Collaborative G . Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49(3):347‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kikuchi S, Wada O, Nakajima T, et al. Serum anti‐Helicobacter pylori antibody and gastric carcinoma among young adults. Research Group on Prevention of Gastric Carcinoma among Young Adults. Cancer. 1995;75(12):2789‐2793. [DOI] [PubMed] [Google Scholar]
- 56. Koizumi Y, Tsubono Y, Nakaya N, et al. Cigarette smoking and the risk of gastric cancer: a pooled analysis of two prospective studies in Japan. Int J Cancer. 2004;112(6):1049‐1055. [DOI] [PubMed] [Google Scholar]
- 57. Nishino Y, Inoue M, Tsuji I, et al. Tobacco smoking and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2006;36(12):800‐807. [DOI] [PubMed] [Google Scholar]
- 58. Ladeiras‐Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta‐analysis of cohort studies. Cancer Causes Control. 2008;19(7):689‐701. [DOI] [PubMed] [Google Scholar]
- 59. Nomura AM, Wilkens LR, Henderson BE, Epplein M., Kolonel LN. The association of cigarette smoking with gastric cancer: the multiethnic cohort study. Cancer Causes Control. 2012;23(1):51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang PL, Xiao FT, Gong BC, Liu FN. Alcohol drinking and gastric cancer risk: a meta‐analysis of observational studies. Oncotarget. 2017;8(58):99013‐99023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tramacere I, Negri E, Pelucchi C, et al. A meta‐analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23(1):28‐36. [DOI] [PubMed] [Google Scholar]
- 62. Duell EJ, Travier N, Lujan‐Barroso L, et al. Alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am J Clin Nutr. 2011;94(5):1266‐1275. [DOI] [PubMed] [Google Scholar]
- 63. Rota M, Pelucchi C, Bertuccio P, et al. Alcohol consumption and gastric cancer risk‐A pooled analysis within the StoP project consortium. Int J Cancer. 2017;141(10):1950‐1962. [DOI] [PubMed] [Google Scholar]
- 64. D'Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta‐analysis of prospective studies. Clin Nutr. 2012;31(4):489‐498. [DOI] [PubMed] [Google Scholar]
- 65. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today Lyon, France: International Agency for Research on Cancer: Global Cancer Observatory: Cancer Today. 2020. https://gco.iarc.fr/today
- 66. Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47(12):1077‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Haenszel W, Kurihara M, Segi M, Lee RK. Stomach cancer among Japanese in Hawaii. J Natl Cancer Inst. 1972;49(4):969‐988. [PubMed] [Google Scholar]
- 68. Maskarinec G, Noh JJ. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn Dis. 2004;14(3):431‐439. [PubMed] [Google Scholar]
- 69. World Health Organization . Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 916:i‐viii, 1‐149, backcover; 2003. [PubMed]
- 70. Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253‐256. [DOI] [PubMed] [Google Scholar]
- 71. Joossens JV, Hill MJ, Elliott P, et al. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol. 1996;25(3):494‐504. [DOI] [PubMed] [Google Scholar]
- 72. Fox JG, Dangler CA, Taylor NS, King A., Koh TJ, Wang TC. High‐salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59(19):4823‐4828. [PubMed] [Google Scholar]
- 73. Kato S, Tsukamoto T, Mizoshita T, et al. High salt diets dose‐dependently promote gastric chemical carcinogenesis in Helicobacter pylori‐infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119(7):1558‐1566. [DOI] [PubMed] [Google Scholar]
- 74. Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67(10):4709‐4715. [DOI] [PubMed] [Google Scholar]
- 75. Takahashi M, Nishikawa A, Furukawa F, Enami T, Hasegawa T, Hayashi Y. Dose‐dependent promoting effects of sodium chloride (NaCl) on rat glandular stomach carcinogenesis initiated with N‐methyl‐N'‐nitro‐N‐nitrosoguanidine. Carcinogenesis. 1994;15(7):1429‐1432. [DOI] [PubMed] [Google Scholar]
- 76. Tatematsu M, Takahashi M, Fukushima S, Hananouchi M, Shirai T. Effects in rats of sodium chloride on experimental gastric cancers induced by N‐methyl‐N‐nitro‐N‐nitrosoguanidine or 4‐nitroquinoline‐1‐oxide. J Natl Cancer Inst. 1975;55(1):101‐106. [DOI] [PubMed] [Google Scholar]
- 77. Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(3 Suppl):559S‐569SS. [DOI] [PubMed] [Google Scholar]
- 78. González CA, Pera G, Agudo A, et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC‐EURGAST). Int J Cancer. 2006;118(10):2559‐2566. [DOI] [PubMed] [Google Scholar]
- 79. Boffetta P, Couto E, Wichmann J, et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2010;102(8):529‐537. [DOI] [PubMed] [Google Scholar]
- 80. González CA, Pera G, Agudo A, et al. Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Int J Cancer. 2003;107(4):629‐634. [DOI] [PubMed] [Google Scholar]
- 81. Li LF, Chan RL, Lu L, et al. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms (review). Int J Mol Med. 2014;34(2):372‐380. [DOI] [PubMed] [Google Scholar]
- 82. Shin VY, Cho CH. Nicotine and gastric cancer. Alcohol. 2005;35(3):259‐264. [DOI] [PubMed] [Google Scholar]
- 83. Khatoon J, Rai RP, Prasad KN. Role of Helicobacter pylori in gastric cancer: Updates. World J Gastrointest Oncol. 2016;8(2):147‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Everhart JE. Recent developments in the epidemiology of Helicobacter pylori . Gastroenterol Clin North Am. 2000;29(3):559‐578. [DOI] [PubMed] [Google Scholar]
- 85. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273‐1275. [PubMed] [Google Scholar]
- 86. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609‐e616. [DOI] [PubMed] [Google Scholar]
- 87. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 61. International Agency for Research on Cancer; 1994:1‐241.7715068 [Google Scholar]
- 88. Correa P. A model for gastric cancer epidemiology. Lancet. 1975;2(7924):58‐60. [DOI] [PubMed] [Google Scholar]
- 89. Shanks AM, El‐Omar EM. Helicobacter pylori infection, host genetics and gastric cancer. J Dig Dis. 2009;10(3):157‐164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NA.


