Abstract
The effectiveness of l-carnitine in chronic liver disease remains controversial. We conducted this meta-analysis to assess the efficacy of various forms of l-carnitine in the treatment of chronic liver disease. Methods: We searched the Cochrane Library, EMBASE, KMBASE, and Medline databases for all relevant studies published until April 2022 that examined the ability of l-carnitine or its derivatives to normalize liver enzymes in patients with chronic liver disease. We performed meta-analyses of the proportion of patients with alanine aminotransferase (ALT) normalization and post-treatment serum aspartate aminotransferase (AST) and ALT levels. A random effects model was used for meta-analyses. Results: Fourteen randomized controlled trials (1217 patients) were included in this meta-analysis. The proportion of patients in whom ALT normalized was higher in the carnitine-orotate treatment group than in the control group (pooled odds ratio (OR), 95% confidence interval (CI) = 4.61 (1.48–14.39)). The proportion of patients in whom ALT normalized was also higher among those who received the carnitine-orotate complex, a combination of carnitine-orotate, biphenyl dimethyl dicarboxylate, and other minor supplementary compounds than in those who did not without significant heterogeneity (pooled OR (95% CI) = 18.88 (7.70–46.27); df = 1; p = 0.51; I2 = 0%). l-carnitine supplementation effectively lowered serum ALT levels compared to controls (pooled mean difference (95% CI) = −11.99 (−22.48 to −1.49)). Conclusions: l-carnitine supplementation significantly lowered ALT and AST levels and normalized ALT levels in patients with chronic liver disease.
Keywords: carnitine, acetylcarnitine, carnitine-orotate, chronic liver disease
1. Introduction
l-carnitine (3-hydroxy-4-N-trimethylammonium butyrate) is an endogenous compound that plays a pivotal role in fatty acid metabolism [1]. l-carnitine supplementation is marketed in the form of acetyl-l-carnitine (analogs of l-carnitine) [2] and carnitine-orotate (l-carnitine and an ion complex salt of orotic acid) [3,4]. Commercially, the carnitine-orotate complex, a combination of carnitine-orotate, biphenyl dimethyl dicarboxylate, and other minor supplementary compounds, is also available [5].
l-carnitine is mainly derived from foods, such as meat and dairy products. It is also synthesized in the human liver and kidneys; in other words, l-carnitine deficiency occurs more frequently in patients with chronic liver disease or liver cirrhosis [2,6]. Carnitine acts as a carrier for fatty acids across the mitochondrial membrane for subsequent β-oxidation and reduces hepatic free fatty acids, causing decreased triglyceride accumulation in the cytoplasm of hepatocytes [7]. Moreover, it plays an important role in reducing oxidative stress and increasing proinflammatory cytokine expression [8]. l-carnitine, acetyl-l-carnitine, and carnitine-orotate supplements have been used as adjuvant therapies in chronic liver disease, including non-alcoholic steatohepatitis, alcoholic fatty liver disease, viral hepatitis, and cirrhosis [9,10,11].
Accordingly, many studies have investigated the effect of l-carnitine on liver enzymes and liver disease, but the results are inconclusive. In a previous meta-analysis, a pooled analysis suggested that l-carnitine supplementation significantly decreased serum liver enzyme levels [12,13]. However, the previous meta-analysis included healthy volunteers as well as patients with chronic liver disease. In addition, previous studies analyzed only l-carnitine, and many randomized controlled trials (RCTs) on various forms of carnitine were omitted. Therefore, this meta-analysis investigated the effects of l-carnitine supplementation, including acetyl-l-carnitine and carnitine-orotate, in patients with chronic liver disease.
2. Materials and Methods
2.1. Search Strategy
We searched the Cochrane Library, EMBASE, KMBASE and Medline databases for all relevant studies published between January 1990 and April 2022 that examined the ability of carnitine to normalize liver enzymes in patients with chronic liver disease. The following search string was used: ((carnitine) OR (l-carnitine) OR (carnitine-orotate) OR (carnitine-orotate) OR (carnitine complex) OR (godex) OR (hepadif) OR (biphenyl dimethyl dicarboxylate) OR (BDD) OR (DDB)) AND ((liver) OR (hepatic) OR (hepatitis) OR (cirrhosis)). The detailed search strategies used for each database are presented below.
Cochrane library
#1: carnitine or L-carnitine or ‘carnitine-orotate’ or ‘carnitine orotate’ or ‘carnitine complex’ or godex or hepadif or ‘biphenyl dimethyl dicarboxylate’ or BDD or DDB;
#2: liver or hepatic or hepatitis or cirrhosis;
#3: #1 and #2 (with publication year from 1990 to 2022, in Trials).
EMBASE (search interface: Ovid)
1: ((carnitine or L-carnitine or carnitine-orotate or carnitine orotate or carnitine complex or godex or hepadif or biphenyl dimethyl dicarboxylate or BDD or DDB) and (liver or hepatic or hepatitis or cirrhosis)).ab,ti;
2: Limit 1 to (embase and yr=“1990–Current”).
KMBASE
(((((((((([ALL=carnitine] OR [ALL=L-carnitine]) OR [ALL=carnitine-orotate]) OR [ALL=carnitine orotate]) OR [ALL=carnitine complex]) OR [ALL=godex]) OR [ALL=hepadif]) OR [ALL=biphenyl dimethyl dicarboxylate]) OR [ALL=BDD]) OR [ALL=DBB]) AND ((([ALL=liver] OR [ALL=hepatic]) OR [ALL=hepatitis]) OR [ALL=cirrhosis])).
MEDLINE (Search interface: PubMed)
(carnitine[tw] OR L-carnitine[tw] OR (carnitine-orotate[tw]) OR (carnitine orotate[tw]) OR (carnitine complex[tw]) OR godex[tw] OR hepadif[tw] OR (biphenyl dimethyl dicarboxylate[tw]) OR BDD[tw] OR DDB[tw]) AND (liver[tw] OR hepatic[tw] OR hepatitis[tw] OR cirrhosis[tw]) AND (“1990/01/01”[Date—Publication]: “3000”[Date—Publication]).
2.2. Inclusion/Exclusion Criteria
The inclusion criteria were as follows: (a) patients: diagnosis of chronic liver disease; (b) intervention: taking the target drugs, including carnitine-orotate, carnitine-orotate complex, and l-carnitine; (c) comparator: no target drugs; (d) outcome: proportion of patients with alanine aminotransferase (ALT) normalization or difference in post-treatment serum aspartate aminotransferase (AST) and ALT levels between the treatment and control groups; and (e) study design: RCT (Table 1). Non-human studies or abstract-only publications were excluded.
Table 1.
Inclusion criteria of the present meta-analysis. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
| Inclusion Criteria | Description |
|---|---|
| Population | Patients with chronic liver disease |
| Intervention | Taking the target drugs, including carnitine-orotate, carnitine-orotate complex, and L-carnitine |
| Comparison | No target drugs |
| Outcomes | Proportion of patients with ALT normalization or difference in post-treatment serum AST and ALT levels |
| Study design | Randomized controlled trial |
2.3. Study Selection
Duplicates from multiple search engines were removed from the literature search results. After the titles and abstracts were reviewed, irrelevant studies were excluded based on the inclusion and exclusion criteria. Thereafter, we reviewed the full text of all the remaining studies. Two investigators (H.O. and C.H.P.) independently assessed the eligibility of each study. Any disagreements were resolved by discussion and consensus. If no agreement was reached, a third investigator (D.W.J.) determined the final eligibility.
2.4. Quality Assessment
Two investigators (H.O. and C.H.P.) independently assessed the methodological quality of the individual studies using the Cochrane Risk of Bias assessment tool for RCTs [14].
2.5. Data Extraction
The data were extracted using a form developed in advance. Two investigators (H.O. and C.H.P.) independently extracted the following information: first author, year of publication, study design, study period, country, baseline characteristics of the study participants, dosage of target drugs, proportion of participants with ALT normalization, post-treatment serum AST and ALT levels, and other major findings of each study. If values for the meta-analysis were not sufficiently reported by the individual studies, we asked the corresponding author for the data.
2.6. Study Endpoints
The primary endpoint of this meta-analysis was the proportion of patients with post-treatment ALT normalization. The secondary endpoint was the mean difference in the post-treatment serum AST and ALT levels between the treatment and control groups.
2.7. Statistical Analysis
As most studies of carnitine-orotate (or the carnitine-orotate complex) investigated the proportion of patients with ALT normalization, pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. A random-effects model was used. For a meta-analysis of studies that reported continuous variable outcomes (e.g., post-treatment serum AST and ALT levels), mean differences (MDs) and 95% CIs between the treatment and control groups were calculated.
We assessed heterogeneity using two methods: Cochran’s Q test, wherein p values < 0.1 were considered statistically significant for heterogeneity, and I2 statistics, wherein values > 50% were suggestive of significant heterogeneity [15]. Based on Cochrane recommendations, publication bias was assessed when ≥10 studies were included [14]. Publication bias was assessed using a funnel plot [16], a radial plot [17], Egger’s test [18], and the AS-Thompson test [19]. We performed sensitivity analyses after excluding studies (1) involving decompensated cirrhosis; and (2) with a high risk of bias. Additionally, we performed a jackknife sensitivity analysis after excluding one different study each time.
All P values were two-tailed, and those <0.05, except on the heterogeneity test, were considered statistically significant. Analyses and reporting were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [20]. All statistical analyses were conducted using Review Manager 5.3 (version 5.3.5; Cochrane Collaboration, Copenhagen, Denmark) and R (version 4.4.4; R Foundation for Statistical Computing, Vienna, Austria).
Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022332856 (accessed on 28 May 2022)[21].
3. Results
3.1. Study Selection and Characteristics
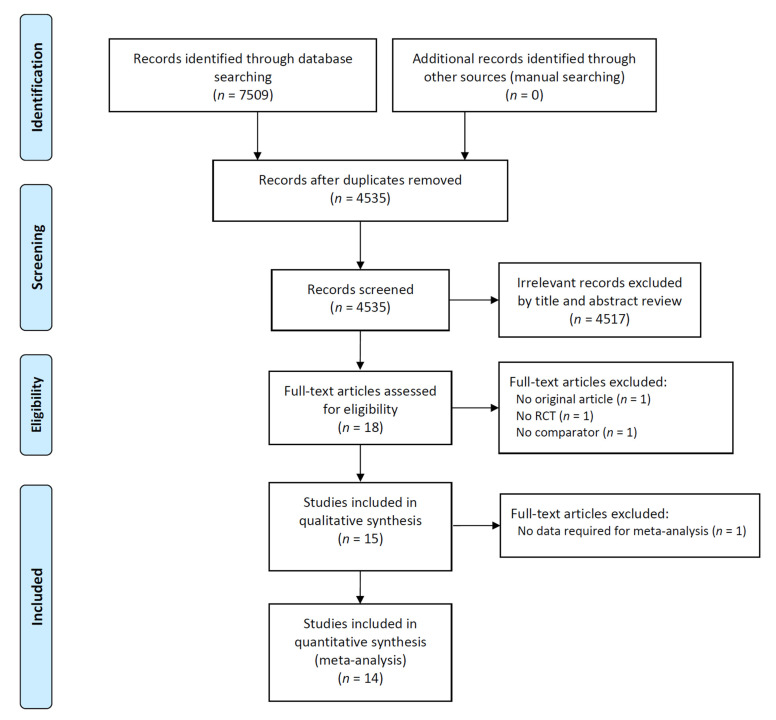
Fourteen studies including 1217 patients were included in this meta-analysis (Figure 1). The characteristics of the included studies are summarized in Table 2. The studies were published during 2000–2016 and included enrollment periods during 2000–2014 [22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Among the 14 studies included, four investigated the efficacy of carnitine-orotate or carnitine-orotate complex and reported the proportion of patients with post-treatment ALT normalization [22,23,24,25]. The other 10 studies assessed the impact of l-carnitine on serum AST and ALT levels [26,27,28,29,30,31,32,33,34,35]. Of the 14 included studies, one involved patients with decompensated cirrhosis [31].
Figure 1.
Study flow diagram.
Table 2.
Baseline characteristics of the included studies.
| Publication Year | First Author | Study Design | Study Period | Country | Study Population | Inclusion of Patients with Decompensated Cirrhosis | Treatment | Control | Other Medication (Both Treatment and Control Groups) | Number of Participants | Age, Years, Mean ± SD | Male Sex, % | BMI, kg/m2, Mean ± SD | Baseline Laboratory Findings | Risk of Bias | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AST, IU/mL,Mean ± SD | ALT, IU/mL,Mean ± SD | |||||||||||||||
| 2001 | Kang JS [22] |
Double-blinded RCT | 2000 | Korea | Chronic hepatitis | No | Treatment A: Carnitine-orotate (900 mg/day) Treatment B: Carnitine-orotate (600 mg/day) |
Placebo | BDD 150 mg/day | 95 | Treatment A: 49.0 ± 9.7; Treatment B: 41.6 ± 11.7; Control: 44.0 ± 11.6 |
Treatment A: 63.6; Treatment B: 63.3; Control: 71.9 |
N/A | N/A | N/A | Unclear |
| 2001 | Park MS [23] |
Double-blinded RCT | N/A | Korea | Chronic hepatitis | No | Treatment A: Carnitine-orotate (900 mg/day) Treatment B: Carnitine-orotate (600 mg/day) |
Placebo | BDD 150 mg/day | 154 | Treatment A: 44.6 ± 11.5; Treatment B: 43.2 ± 13.0; Control: 45.6 ± 13.8 |
Treatment A: 92.5; Treatment B: 77.6; Control: 82.7 |
N/A | NA | N/A | Unclear |
| 2013 | Jun DW [24] |
Open-label RCT | N/A | Korea | Chronic hepatitis B | No | Carnitine-orotate complex (900 mg/day as carnitine-orotate) | No carnitine-orotate complex | Entecavir 0.5 mg/day | 130 | Treatment: 43.0 ± 9.8; Control: 44.9 ± 10.0 |
Treatment: 63.5 Control: 66.1 |
N/A | Treatment: 118.8 ± 70.3; Control: 111.3 ± 70.2 |
Treatment: 159.4 ± 67.5; Control: 160.3 ± 74.9 |
Unclear |
| 2015 | Bae JC [25] |
Double-blinded RCT | 2011–2012 | Korea | NAFLD with type 2 DM | No | Carnitine-orotate complex (900 mg/day as carnitine-orotate) | Placebo | 78 | Treatment: 50.6 ± 9.3; Control: 52.0 ± 9.4 |
Treatment: 64.1; Control: 74.4 |
Treatment: 28.2 ± 2.6; Control: 26.7 ± 3.7 |
Treatment: 61.8 ± 25.5; Control: 51.7 ±22.1 |
Treatment: 94.9 ± 36.4; Control: 79.2 ± 27.2 |
Low | |
| 2000 | Uygun A [26] |
Open-label RCT | N/A | Turkey | NAFLD | N/A | Treatment A: l-carnitine 1 g/day; Treatment B: 2 g/day; Treatment C: 3 g/day |
No l-carnitine | 101 | Treatment A: 43.4 ± 10.8; Treatment B: 42.5 ± 9.7; Treatment C: 40.0 ± 9.1; Control: 42.6 ± 7.3 |
Treatment A: 79.2; Treatment B: 61.5; Treatment C: 75.0; Control: 69.6 |
Treatment A: 4.4 ± 2.3; Treatment B: 26.2 ± 3.1; Treatment C: 25.4 ± 2.7; Control: 25.8 ± 2.5 |
Treatment A: 57.5 ± 3.9; Treatment B: 47.8 ± 2.1; Treatment C: 51.0 ± 6.3; Control: 46.3 ± 6.0 |
Treatment A: 72.3 ± 5.4; Treatment B: 71.5 ± 4.6; Treatment C: 75.1 ± 7.0; Control: 70.5 ± 7.3 |
High | |
| 2002 | Malaguarnera M [27] |
Open-label RCT | N/A | Italy | Chronic hepatitis C | No | L-carnitine 2 g/day | No L-carnitine | IFN-α 3 million IU three times a week | 70 | Treatment: 56.8 ± 7.2; Control: 57.7 ± 6.1 |
Treatment: 62.9; Control: 60.0 |
Treatment: 26 ± 1.9; Control: 26 ± 2.4 |
Treatment: 110 ± 86; Control: 114 ± 79 |
Treatment: 186 ± 99; Control: 163 ± 108 |
Unclear |
| 2008 | Malaguarnera M [28] |
Double-blinded RCT | 2000–2003 | Italy | Minimal hepatic encephalopathy | No | Acetyl-L-carnitine 4 g/day | Placebo | 115 | Treatment: 48 ± 10; Control: 45 ± 11 |
Treatment: 55.0; Control: 63.6 |
Treatment: 24.8 ± 3.1; Control: 25.1 ± 3.3 |
Treatment: 68 ± 31; Control: 67 ± 32 |
Treatment: 71 ± 40; Control: 68 ± 44 |
Unclear | |
| 2008 | Romano M [29] |
Open-label RCT | 2000–2003 | Italy | Chronic hepatitis C | No | L-carnitine 2 g/day | No -carnitine | IFN-α 3 million IU three times a week + ribavirin 1000 mg | 70 | Treatment: 50.1 ± 6.1; Control: 50.4 ± 5.6 |
Treatment: 56.7; Control: 53.3 |
Treatment: 25.8 ± 3.1; Control: 25.7 ± 3.2 |
Treatment: 125.0 ± 46.2; Control: 116.0 ± 49.3 |
Treatment: 162.0 ± 49.2; Control: 156.0 ± 47.4 |
Unclear |
| 2010 | Malaguarnera M [30] |
Double-blinded RCT | 2004–2006 | Italy | NASH | N/A | L-carnitine 2 g/day | Placebo | 74 | Treatment: 47.9 ± 5.4; Control: 47.8 ± 5.8 |
Treatment: 55.6; Control: 52.6 |
Treatment: 26.6 ± 3.7; Control: 26.5 ± 3.8 |
Treatment: 132.8 ± 14.7; Control: 135.4 ± 15.1 |
Treatment: 125.7 ± 12.9; Control: 120.2 ± 12.8 |
Unclear | |
| 2011 | Malaguarnera M(Metab Brain Dis)a [31] |
Double-blinded RCT | 2002–2006 | Italy | Severe hepatic encephalopathy | Yes | Acetyl-L-carnitine 4 g/day | Placebo | 60 | Treatment: range, 37–64; Control: range, 35–65 |
Treatment: 46.7; Control: 50.0 |
N/A | Treatment: 119.2 ± 13.1; Control: 114.2 ± 24.5 |
Treatment: 106.7 ± 15.7 b; Control: 136.3 ± 31.0 |
High | |
| 2011 | Malaguarnera M(Scand J Gastroenterol)a [32] | Double-blinded RCT | 2002–2005 | Italy | Minimal hepatic encephalopathy | No | Acetyl-L-carnitine 4 g/day | Placebo | 67 | Treatment: range, 37–65; Control: range, 34–67 |
Treatment: 60.6; Control: 55.9 |
N/A | Treatment: 102.1 ± 15.2 b; Control: 80.4 ± 19.8 |
Treatment: 117.4 ± 16.0 b; Control: 90.2 ± 14.3 |
Unclear | |
| 2011 | Malaguarnera M(World J Gastroenterol)a [33] | Open-label RCT | 2004–2007 | Italy | Chronic hepatitis C | No | L-carnitine 4 g/day | Placebo | Peg-IFN-α 2b 1.5 μg/kg/week + rivavirin 800–1200 mg/day (adjusted to body weight) | 69 | Treatment: 47.6 ± 4.9; Control: 47.1 ± 5.4 |
Treatment: 62.9; Control: 58.8 |
Treatment: 27.1 ± 3.1; Control: 27.4 ± 2.9 |
Treatment: 145.0 ± 44.2; Control: 136.0 ± 41.4 |
Treatment: 182.1 ± 46.2; Control: 174.1 ± 42.2 |
Unclear |
| 2014 | Somi MH [34] |
Open-label RCT | 2012–2014 | Iran | NAFLD | No | L-carnitine 1 g/day | No L-carnitine | 80 | Treatment: 40.3 ± 7.8; Control: 41.1 ± 8.3 |
Treament: 82.5; Control: 82.5 |
Treatment: 29.4 ± 3.9; Control: 28.6 ± 3.2 |
Treatment: 60.5 ± 28.3; Control: 52.6 ± 24.4 |
Treatment: 81.7 ± 40.1 b; Control: 54.1 ± 17.6 |
High | |
| 2016 | Alavinejad P [35] |
Double-blinded RCT | N/A | Iran | NAFLD with type 2 DM | No | L-carnitine 2.25 g/day | Placebo | 54 | Treatment: 60 ± 5; Control: 59 ± 9 |
Treatment: 75.0; Control: 65.4 |
Treatment: 28.6 ± 4.6; Control: 29.5 ± 3.6 |
Treatment: 122.7 ± 13.6; Control: 125.3 ± 14.0 |
Treatment: 124.0 ± 11.3; Control: 120.0 ± 10.8 |
Unclear | |
a Parentheses indicate the journal in which the study was published. b There was a significant difference in baseline values between treatment and control groups. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BDD, biphenyl dimethyl dicarboxylate; BMI, body mass index; DM, diabetes mellitus; DNA, deoxyribonucleic acid; IFN, interferon; IU, international unit; N/A, not available; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; RCT, randomized controlled trial; SD, standard deviation.
The risk of bias assessment is shown in Figure S1. Five (36%) patients had an unclear risk of bias in the domain of random sequence generation because random sequence generation methods were not clearly described in those studies. The other nine studies (64%) had a low risk of bias for random sequence generation. The risk of allocation concealment was unclear in 13 (93%) studies. All studies were assessed as having a low risk of performance and detection bias because the current study outcomes were less likely to be affected by participant and investigator blinding. Incomplete outcome data and selective reporting were not identified in any RCT. Four studies (27%) were assessed as having a high risk of other biases because baseline AST or ALT levels differed between the treatment and control groups despite randomization [26,31,32,34].
3.2. Impact of Carnitine-Orotate (or Carnitine-Orotate Complex) on ALT Normalization
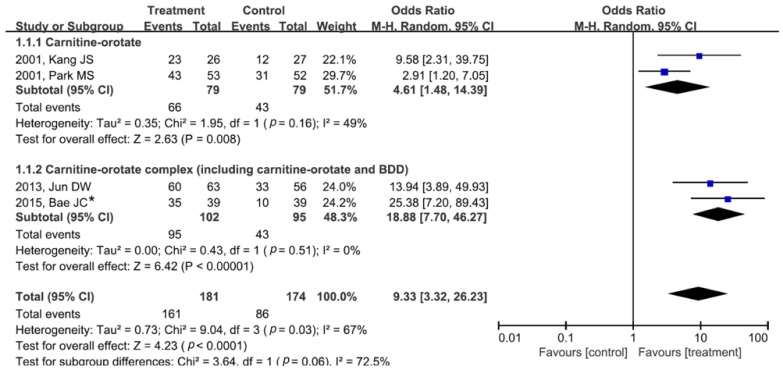
The impact of carnitine-orotate (or carnitine-orotate complex) on ALT normalization is shown in Figure 2. In studies on carnitine-orotate, the proportion of patients with ALT normalization was higher in the treatment group versus that in the control group (pooled OR (95% CI) = 4.61 (1.48–14.39)). Heterogeneity was not identified (degrees of freedom (df) = 1, p = 0.16, I2 = 49%). In studies of the carnitine-orotate complex, the proportion of patients with ALT normalization was also higher in the treatment versus control group without significant heterogeneity (pooled OR (95% CI) = 18.88 (7.70–46.27); df = 1, p = 0.51, I2 = 0%).
Figure 2.
Forest plot of alanine aminotransferase normalization after carnitine-orotate (or carnitine-orotate complex) versus control treatment. The carnitine–orotate complex includes carnitine-orotate, BDD, and other minor supplementary compounds. The dosage of carnitine-orotate in the included studies in this analysis was 900 mg per day, which is the usual dosage for clinical purposes. * The values in this study were provided by the corresponding author. BDD, biphenyl dimethyl dicarboxylate; M-H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom.
Since two studies on carnitine-orotate analyzed the efficacy of different drug dosages (carnitine-orotate 900 mg vs. 600 mg), we further performed dosage subgroup analyses (Figure S2). When the usual dosage for clinical purposes (carnitine-orotate 900 mg) was used, it effectively normalized ALT levels, as described in the main results (Figure 2). However, the efficacy of lower-dosage carnitine-orotate (600 mg) was not identified in the subgroup analysis (pooled OR (95% CI) = 1.27 (0.66–2.44); df = 1, p = 0.72, I2 = 0%).
3.3. Impact of l-Carnitine (or Acetyl-l-Carnitine) Supplementation on Serum AST and ALT Levels
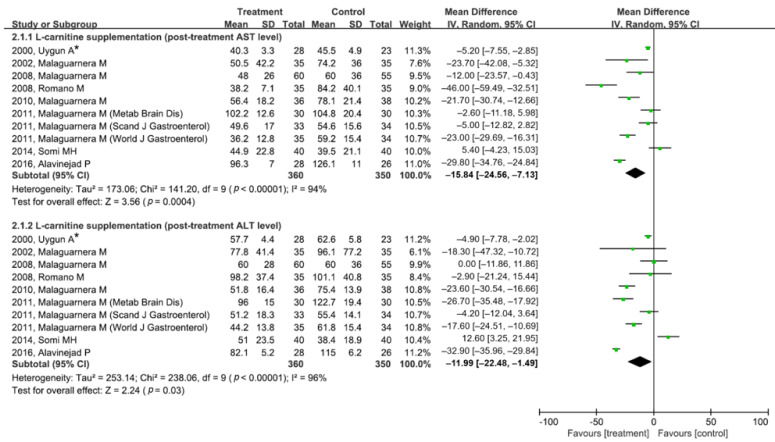
Since 10 studies on l-carnitine or acetyl-l-carnitine supplementation assessed and reported the main outcomes as continuous variables (i.e., serum AST and ALT levels), the mean difference in post-treatment AST or ALT levels between the treatment and control groups was analyzed (Figure 3). In a meta-analysis of post-treatment AST levels, l-carnitine or acetyl-l-carnitine supplementation effectively lowered serum AST levels compared to the control (pooled MD (95% CI) = −15.84 (−24.56 to −7.13)). The post-treatment serum ALT level was lower in the treatment group than in the control group (pooled MD (95% CI) = −11.99 (−22.48 to −1.49)). Significant heterogeneity was identified in both meta-analyses (AST level: df = 9, p < 0.01, I2 = 94%; ALT level: df = 9, p < 0.01, I2 = 96%). Egger’s test and the AS-Thompson test for publication bias found no significant asymmetry in the funnel plot and radial plot (p > 0.1) (Figure S3).
Figure 3.
Forest plot of post-treatment serum AST and ALT levels after l-carnitine (or acetyl-l-carnitine) supplementation versus control treatment. Parentheses following the first author’s name indicate the journal in which the study was published. * Multiple doses were investigated in this study. Data from the maximal dosage group (3 g/day) were used in the analysis. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; df, degrees of freedom; IV, inverse variance; SD, standard deviation.
3.4. Sensitivity Analysis
Two sensitivity analyses were performed. First, we performed a sensitivity analysis of l-carnitine or acetyl-l-carnitine supplementation on serum AST and ALT levels after excluding a study of patients with decompensated cirrhosis [31] (Figure S4). Post-treatment AST levels were also lower in the l-carnitine or acetyl-l-carnitine supplementation group than in the control group (pooled MD (95% CI) = −17.39 (26.90 to −7.88)). The post-treatment ALT levels tended to be lower in the treatment group than in the control group despite no significant difference (pooled MD (95% CI) = −10.25 (−21.60 to 1.10)). Second, in the sensitivity analysis, after the exclusion of four studies showing different baseline characteristics in AST or ALT levels [26,31,32,34] (Figure S5), post-treatment AST and ALT levels were also lower in the l-carnitine or acetyl-l-carnitine supplementation group than in the control group (pooled MD (95% CI): AST level, −25.77 (−32.92 to −18.62); ALT level, −17.14 (–27.89 to −6.38)). In jackknife sensitivity analyses (Figure S6), a significant study that affected the pooled meta-analysis results was not identified. These sensitivity analyses showed the robustness of our meta-analyses.
4. Discussion
The current meta-analysis found that carnitine-orotate and carnitine-orotate complexes taken at the usual dosage significantly affected ALT normalization in patients with chronic liver disease. We also showed that l-carnitine (or acetyl-l-carnitine) supplementation significantly decreased serum AST and ALT levels. Our findings suggest that l-carnitine and its derivatives have beneficial effects in normalizing liver enzymes in patients with chronic liver disease. The novelty of this study lies in the fact that we extracted and analyzed data from studies of patients with chronic liver disease and elevated liver enzyme levels. Previous meta-analyses suggested the efficacy of l-carnitine supplementation for reducing serum liver enzyme levels, similar to our study findings; however, some individual studies in the previous meta-analyses involved healthy volunteers or patients with liver enzyme elevations caused by reasons other than chronic liver disease [12,13]. In addition, clinically useful information can be provided through the analysis of carnitine-orotate complex containing other minor supplements that have not been previously analyzed.
AST and ALT are enzymes that transport the amino groups of aspartate and alanine to ketoglutaric acid. AST is present in the liver and other organs, including the muscle, while ALT is primarily present in the liver; thus, ALT is a more specific marker of hepatocellular injury than AST [36]. We adopted the odds ratio of the ALT normalization rate as an important analysis target. In real-world practice, clinicians aim to normalize ALT in patients with various chronic liver diseases to ensure a satisfactory prognosis [37,38,39,40,41]. In recent studies, elevated liver enzymes were associated with significantly increased liver-related mortality, and normal-range AST and ALT levels were associated with a lower risk of death [42,43]. Therefore, l-carnitine and its derivatives may help improve the prognosis of patients with chronic liver disease and elevated liver enzyme levels.
There are considerable limitations to the present study. First, there was substantial heterogeneity in the included studies, although the current meta-analysis had a limited study population of patients with chronic liver disease. Interstudy heterogeneity was observed due to various disease etiologies, including chronic viral hepatitis B, chronic viral hepatitis C, and non-alcoholic fatty liver disease, as well as different dosages and durations of l-carnitine supplementation. Therefore, further studies are needed to examine the time- and dose-dependent effects of l-carnitine supplementation. Second, it is also important to note that our study findings should not be generalized to patients worldwide since studies on carnitine-orotate were conducted only in Korea and studies on l-carnitine or acetyl-l-carnitine were conducted only in Italy and other countries. In Italy, the majority of studies were conducted by the same research group. In the future, it will be necessary to assess the efficacy of l-carnitine supplementation in various regions and races worldwide. Third, we should be careful when interpreting the effect size since medications other than l-carnitine (e.g., antiviral agents) were administered to the treatment and control groups in 6 of the 14 enrolled studies. Finally, the patient follow-up period of the studies included in this paper is relatively short, within one year. As mentioned above, the relationship between the improvement of ALT and the prognosis of the disease is emphasized, so long-term follow-up studies are needed in the future.
5. Conclusions
In conclusion, the pooled data from RCTs provide evidence of the beneficial effects of l-carnitine supplementation for lowering serum ALT and AST levels. Our findings also showed that carnitine-orotate can be effective in patients with chronic liver diseases with intervention doses of 900 mg/day for ALT normalization.
Supplementary Materials
The following supporting information can be downloaded from https://www.mdpi.com/article/10.3390/jpm12071053/s1,: Figure S1. Traffic light plot for risk of bias in individual studies; Figure S2. Forest plot for ALT normalization between carnitine-orotate and control groups according to the dosage of carnitine-orotate (900 mg vs. 600 mg); Figure S3. Analysis of publication bias. Using Egger’s test (post-treatment AST level: p-value 0.2498, post-treatment ALT level: p-value 0.5642) and AS-Thompson’s test (post-treatment AST level: p-value 0.3925, post-treatment ALT level: p-value 0.675) for publication bias, no significant asymmetry is seen in the funnel plot and radial plot (p > 0.1) for pooled analysis of mean difference; Figure S4. Sensitivity analysis of l-carnitine (or acetyl-l-carnitine) supplementation for post-treatment ALT level after excluding a study involving patients with decompensated cirrhosis [31]; Figure S5. Sensitivity analysis of l-carnitine (or acetyl-l-carnitine) supplementation for post-treatment ALT level after the exclusion of four studies showing different baseline AST or ALT levels between treatment and control groups [26,31,32,34]; Figure S6. Sensitivity analysis of the effect of l-carnitine (or acetyl-l-carnitine) supplementation on post-treatment AST and ALT levels with jackknife sensitivity analysis showing the robustness of meta-analysis.
Author Contributions
Conceptualization, D.W.J.; methodology, C.H.P.; formal analysis, C.H.P.; investigation, H.O. and C.H.P.; data curation, H.O. and C.H.P.; writing—original draft preparation, H.O. and C.H.P.; writing—review and editing, H.O., C.H.P. and D.W.J.; visualization, C.H.P.; supervision, D.W.J.; project administration, D.W.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it was a meta-analysis based on previously published studies.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are included in the study and supplementary information.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by a research grant from Celltrion Pharm Inc., Incheon 21975, Korea (Grant number CTP-GOD 4.10).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Virmani M.A., Cirulli M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022;23:2717. doi: 10.3390/ijms23052717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Y., Wang Y.X., Liu C.J., Wang L.X., Han Z.W., Wang C.B. Comparison of pharmacokinetics of L-carnitine, acetyl-L-carnitine and propionyl-L-carnitine after single oral administration of L-carnitine in healthy volunteers. Clin. Investig. Med. 2009;32:E13–E19. doi: 10.25011/cim.v32i1.5082. [DOI] [PubMed] [Google Scholar]
- 3.An J.-H., Youn W., Kiyonga A.N., Lim C., Park M., Suh Y.-G., Ryu H.C., Kim J.S., Park C.-W., Jung K. Kinetics of the Solution-Mediated Polymorphic Transformation of the Novel l-Carnitine Orotate Polymorph, Form-II. Pharmaceutics. 2018;10:171. doi: 10.3390/pharmaceutics10040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J.S.R., Joung J.G., Kim H.M. Crystalline Polymorph of l-Carnitine Orotate, Production Method Therefor, or Use Thereof. WO 2017105090 A1. 2016 December 14;
- 5.Hong J.H., Lee M.K. Carnitine Orotate Complex Ameliorates Insulin Resistance and Hepatic Steatosis Through Carnitine Acetyltransferase Pathway. Diabetes Metab. J. 2021;45:933–947. doi: 10.4093/dmj.2020.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N., Zhao H. Role of Carnitine in Non-alcoholic Fatty Liver Disease and Other Related Diseases: An Update. Front. Med. 2021;8:689042. doi: 10.3389/fmed.2021.689042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringseis R., Keller J., Eder K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: Evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur. J. Nutr. 2012;51:1–18. doi: 10.1007/s00394-011-0284-2. [DOI] [PubMed] [Google Scholar]
- 8.Ribas G.S., Vargas C.R., Wajner M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene. 2014;533:469–476. doi: 10.1016/j.gene.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Hanai T., Shiraki M., Imai K., Suetugu A., Takai K., Shimizu M. Usefulness of Carnitine Supplementation for the Complications of Liver Cirrhosis. Nutrients. 2020;12:1915. doi: 10.3390/nu12071915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savic D., Hodson L., Neubauer S., Pavlides M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD) Nutrients. 2020;12:2178. doi: 10.3390/nu12082178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y., Jiang W., Chen G., Zhu W., Ding W., Ge Z., Tan Y., Ma T., Cui G. L-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2017;26:333–338. doi: 10.17219/acem/61609. [DOI] [PubMed] [Google Scholar]
- 12.Askarpour M., Djafarian K., Ghaedi E., Sadeghi O., Sheikhi A., Shab-Bidar S. Effect of L-Carnitine Supplementation on Liver Enzymes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Arch. Med. Res. 2020;51:82–94. doi: 10.1016/j.arcmed.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Pirmadah F., Ramezani-Jolfaie N., Mohammadi M., Talenezhad N., Clark C.C.T., Salehi-Abargouei A. Does L-carnitine supplementation affect serum levels of enzymes mainly produced by liver? A systematic review and meta-analysis of randomized controlled clinical trials. Eur. J. Nutr. 2020;59:1767–1783. doi: 10.1007/s00394-019-02068-4. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2; Updated February 2021; Cochrane (London SW1Y 4QX, United Kingdom): 2021. [(accessed on 22 April 2022)]. Available online: www.training.cochrane.org/handbook.
- 15.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Easterbrook P.J., Berlin J.A., Gopalan R., Matthews D.R. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 17.Copas J., Lozada-Can C. The radial plot in meta-analysis: Approximations and applications. J. R. Stat. Soc. : Ser. C (Appl. Stat.) 2009;58:329–344. doi: 10.1111/j.1467-9876.2008.00650.x. [DOI] [Google Scholar]
- 18.Rücker G., Schwarzer G., Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat. Med. 2008;27:746–763. doi: 10.1002/sim.2971. [DOI] [PubMed] [Google Scholar]
- 19.Thompson S.G., Sharp S.J. Explaining heterogeneity in meta-analysis: A comparison of methods. Stat. Med. 1999;18:2693–2708. doi: 10.1002/(SICI)1097-0258(19991030)18:20<2693::AID-SIM235>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Oh H., Park C.H., Jun D.W. Systematic Review with Meta-analysis of Randomized Trials : Impact of L-carnitine Supplementation on Liver Enzyme in Chronic Liver Disease. [(accessed on 28 May 2022)];PROSPERO 2022 CRD42022332856. 2022 Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022332856. [Google Scholar]
- 22.Kang J.-S., Hong J.-H., Park M.-S., Lee M.-H., Lee J.-B., Lee J.-W. A Randomized Controlled, Double-blind Evaluation of the Short-Term(8 weeks) Efficacy and Safety of Godex in Korean Chronic Hepatitis Patients. J. Korean Soc. Clin. Pharmacol. Ther. 2001;9:81–96. doi: 10.12793/jkscpt.2001.9.1.81. [DOI] [Google Scholar]
- 23.Park M.-S., Kang J.-S., Chon C.-Y., Paik S.-W., Rim K.-S., Kwak M.-J., Jeon Y.-C., Lee M.-H. Oral Godex Capsule for Chronic Liver Disease:A Double-blind, Randomized, Multicenter Controlled Trial. J. Korean Soc. Clin. Pharmacol. Ther. 2001;9:151–162. doi: 10.12793/jkscpt.2001.9.2.151. [DOI] [Google Scholar]
- 24.Jun D.W., Kim B.I., Cho Y.K., Kim H.J., Kwon Y.O., Park S.Y., Han S.Y., Baek Y.H., Jung Y.J., Kim H.Y., et al. Efficacy and safety of entecavir plus carnitine complex (GODEX®) compared to entecavir monotherapy in patient with ALT elevated chronic hepatitis B: Randomized, multicenter open-label trials. The GOAL study. Clin. Mol. Hepatol. 2013;19:165–172. doi: 10.3350/cmh.2013.19.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae J.C., Lee W.Y., Yoon K.H., Park J.Y., Son H.S., Han K.A., Lee K.W., Woo J.T., Ju Y.C., Lee W.J., et al. Improvement of Nonalcoholic Fatty Liver Disease With Carnitine-Orotate Complex in Type 2 Diabetes (CORONA): A Randomized Controlled Trial. Diabetes Care. 2015;38:1245–1252. doi: 10.2337/dc14-2852. [DOI] [PubMed] [Google Scholar]
- 26.Uygun A., Kadayifçi A., Bağci S., Erdil A., Deveci S., Saka M., Ateş Y., Bulucu F., Karaeren N., Dağalp K. L-Carnitine therapy in non-alcoholic steatohepatitis. Turk. J. Gastroenterol. 2000;11:196–201. [Google Scholar]
- 27.Malaguarnera M., Maugeri D., Saraceno B., Romano M., Neri S., Rapisarda R., Pistone G. Effects of carnitine on biochemical responses in patients with chronic hepatitis C treated with interferon-α. Clin. Drug Investig. 2002;22:443–448. doi: 10.2165/00044011-200222070-00004. [DOI] [Google Scholar]
- 28.Malaguarnera M., Gargante M.P., Cristaldi E., Vacante M., Risino C., Cammalleri L., Pennisi G., Rampello L. Acetyl-L-carnitine treatment in minimal hepatic encephalopathy. Dig. Dis. Sci. 2008;53:3018–3025. doi: 10.1007/s10620-008-0238-6. [DOI] [PubMed] [Google Scholar]
- 29.Romano M., Vacante M., Cristaldi E., Colonna V., Gargante M.P., Cammalleri L., Malaguarnera M. L-carnitine treatment reduces steatosis in patients with chronic hepatitis C treated with alpha-interferon and ribavirin. Dig. Dis. Sci. 2008;53:1114–1121. doi: 10.1007/s10620-007-9983-1. [DOI] [PubMed] [Google Scholar]
- 30.Malaguarnera M., Gargante M.P., Russo C., Antic T., Vacante M., Malaguarnera M., Avitabile T., Li Volti G., Galvano F. L-carnitine supplementation to diet: A new tool in treatment of nonalcoholic steatohepatitis—A randomized and controlled clinical trial. Am. J. Gastroenterol. 2010;105:1338–1345. doi: 10.1038/ajg.2009.719. [DOI] [PubMed] [Google Scholar]
- 31.Malaguarnera M., Vacante M., Motta M., Giordano M., Malaguarnera G., Bella R., Nunnari G., Rampello L., Pennisi G. Acetyl-L-carnitine improves cognitive functions in severe hepatic encephalopathy: A randomized and controlled clinical trial. Metab. Brain Dis. 2011;26:281–289. doi: 10.1007/s11011-011-9260-z. [DOI] [PubMed] [Google Scholar]
- 32.Malaguarnera M., Bella R., Vacante M., Giordano M., Malaguarnera G., Gargante M.P., Motta M., Mistretta A., Rampello L., Pennisi G. Acetyl-L-carnitine reduces depression and improves quality of life in patients with minimal hepatic encephalopathy. Scand. J. Gastroenterol. 2011;46:750–759. doi: 10.3109/00365521.2011.565067. [DOI] [PubMed] [Google Scholar]
- 33.Malaguarnera M., Vacante M., Giordano M., Motta M., Bertino G., Pennisi M., Neri S., Malaguarnera M., Li Volti G., Galvano F. L-carnitine supplementation improves hematological pattern in patients affected by HCV treated with Peg interferon-α 2b plus ribavirin. World J. Gastroenterol. 2011;17:4414–4420. doi: 10.3748/wjg.v17.i39.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somi M.H., Fatahi E., Panahi J., Havasian M.R., Judaki A. Data from a randomized and controlled trial of LCarnitine prescription for the treatment for Non- Alcoholic Fatty Liver Disease. Bioinformation. 2014;10:575–579. doi: 10.6026/97320630010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alavinejad P., Zakerkish M., Hajiani E., Hashemi S.J., Chobineh M., Moghaddam E.K. Evaluation of L-carnitine efficacy in the treatment of non-alcoholic fatty liver disease among diabetic patients: A randomized double blind pilot study. J. Gastroenterol. Hepatol. Res. 2016;5:2191–2195. doi: 10.17554/j.issn.2224-3992.2016.05.662. [DOI] [Google Scholar]
- 36.Kwo P.Y., Cohen S.M., Lim J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Off. J. Am. Coll. Gastroenterol. 2017;112:18–35. doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 37.Inoue J., Kobayashi T., Akahane T., Kimura O., Sato K., Ninomiya M., Iwata T., Takai S., Kisara N., Sato T., et al. Non-Achievement of Alanine Aminotransferase Normalization Associated with the Risk of Hepatocellular Carcinoma during Nucleos(t)ide Analogue Therapies: A Multicenter Retrospective Study. J. Clin. Med. 2022;11:2354. doi: 10.3390/jcm11092354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S., Lee Y., Bang S.M., Bak H., Yim S.Y., Lee Y.S., Yoo Y.J., Jung Y.K., Kim J.H., Seo Y.S., et al. Early Normalization of Alanine Aminotransferase during Antiviral Therapy Reduces Risk of Hepatocellular Carcinoma in HBV Patients. J. Clin. Med. 2021;10:1840. doi: 10.3390/jcm10091840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y.J., Jang B.K., Kim E.S., Park K.S., Cho K.B., Chung W.J., Hwang J.S. Rapid normalization of alanine aminotransferase predicts viral response during combined peginterferon and ribavirin treatment in chronic hepatitis C patients. Korean J. Hepatol. 2012;18:41–47. doi: 10.3350/kjhep.2012.18.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sogabe M., Okahisa T., Kurihara T., Takehara M., Kagemoto K., Okazaki J., Kida Y., Hirao A., Tanaka H., Tomonari T., et al. Differences among patients with and without nonalcoholic fatty liver disease having elevated alanine aminotransferase levels at various stages of metabolic syndrome. PLoS ONE. 2020;15:e0238388. doi: 10.1371/journal.pone.0238388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verma S., Jensen D., Hart J., Mohanty S.R. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD) Liver. Int. 2013;33:1398–1405. doi: 10.1111/liv.12226. [DOI] [PubMed] [Google Scholar]
- 42.Hyeon C.K., Chung M.N., Sun H.J., Kwang H.H., Kyu Oh D., Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: Prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee T.H., Kim W.R., Benson J.T., Therneau T.M., Melton L.J. 3rd. Serum aminotransferase activity and mortality risk in a United States community. Hepatology. 2008;47:880–887. doi: 10.1002/hep.22090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the study and supplementary information.