Abstract
Background: Extracts of medicinal plant like lemongrass offer a new choice for optional antimicrobial therapy against various oral microorganisms. The objective of this study was to assess, verify, and compare the antimicrobial effectiveness of locally administered 2% lemongrass gel and 10% doxycycline hyclate gel as an adjunct to scaling and root planing (SRP) in treating chronic periodontitis. Method: This is a double-blind parallel arm randomized controlled study. Forty subjects were randomly divided into Group A and B for 2% lemongrass gel and 10% doxycycline hyclate gel, respectively. The clinical assessments of Gingival Index (GI), Plaque Index (PI), Probing Pocket Depth (PPD), and Clinical Attachment Level (CAL) together with microbial colony counts for Porphyromonas gingivalis, Actinomyces naeslundii, and Prevotella intermedia were done at baseline, 1st month, and 3rd month follow-ups. Results: The results showed there was a significant reduction in the mean scores of GI, PPD, and CAL clinical indices from baseline to the 1st and 3rd month follow-ups in both the 2% lemongrass gel and 10% doxycycline gel groups (p < 0.05). Similarly, there was significant reduction in mean CFU scores for all periodontal pathogens from baseline to 1st and 3rd month follow-ups in both the 2% lemongrass gel and 10% doxycycline gel groups (p < 0.05). Conclusions: It could be concluded that the local delivery of 2% lemongrass gel as an adjunct to scaling and root planing is effective and comparable to 10% doxycycline gel in the treatment of chronic periodontitis.
Keywords: cymbopogon citratus, local drug delivery, non-surgical therapy, periodontal pathogens
1. Introduction
The applications of polymers in dentistry are increasing day by day. The irreplaceable merits of different classes of natural polymers over synthetic polymers as drug carriers still urge researchers to depend solely on biomaterials. These advantages include hydrophilicity, biocompatibility, non-immunogenicity, non-toxicity, non-toxic degradation products, antimicrobial and antioxidant capacities, and high stability for tissue engineering [1,2,3]. Also, from an environmental perspective, the worldwide demand for eco-friendly products has witnessed sustainable growth for accommodating green technology advancements. Hence, industrial channels are searching for more reliable products to gain patients’ satisfaction and ease their commercialization [4,5,6,7,8].
One such field is pain relief in dentistry. The use of polymers in dentistry has evolved from simple reinforcement for dental materials to application in healing and pain relief. Periodontitis is defined as ‘an inflammatory disease of the supporting tissues of the teeth caused by specific microorganisms or groups of specific microorganisms, resulting in progressive destruction of the periodontal ligament and alveolar bone with increased probing depth formation, recession, or both’ [1]. The most prevalent periodontal disease is chronic periodontitis, which is caused by the deposition of bacterial plaque over time. The therapy for gingivitis and periodontitis has evolved quite differently in these two areas, depending on the advancements made in the area of drug and polymer development and changes in patient populations and needs. The advancements that are most pertinent to polymers dealt with (1) their use as carriers for the controlled delivery of bioactive agents, particularly antimicrobials (2) their use in conjunction with tissue regeneration and healing and (3) factors pertinent to the effectiveness of implemented therapies [2,9,10,11,12,13,14].
Cymbopogon citratus, Stapf, or lemongrass, is a naturally available flavonoid and contains cellulose as a natural polymer. This lemongrass polymer is working towards the quest for the development of a biodegradable natural polymer that has anti-bacterial, anti-filarial, anti-fungal, anti-inflammatory, and antioxidant properties [15]. Lemongrass has active phenol and flavonoid substances which, at concentrations below 2%, have shown to have bacteriostatic properties against several microorganisms [16]. In an in vitro study, lemongrass essential oil was effective against periodontal pathogens like Actinomyces naeslundii and Porphyromonas gingivalis and also a majority of clinical-isolate groups, including tetracycline hydrochloride-resistant strains [17]. Mouth rinse with active Cymbopogon citratus essential oil as an adjunct to SRP has shown to be effective in reducing the severity of gingivitis [18,19]. The use of lemongrass essential oil as a local drug delivery (LDD) in chronic periodontitis is limited.
Non-surgical mechanical periodontal therapies such as scaling and root planing (SRP), and in some cases surgical periodontal therapy with access flaps, have been archived widely in the literature to hamper the progression of tissue destruction in periodontal disease [2]. Mechanical therapy, however, may not always reduce or eliminate the anaerobic infection at the base of the pocket, within the gingival tissues, and in areas inaccessible to periodontal instruments [3]. The reduction or elimination of the pathogenic microorganisms in the subgingival microenvironment is indicative of a successful periodontal therapy [4,5].
The adjunctive use of antimicrobial agents in addition to non-surgical therapy has shown to provide additional benefits [6]. The use of systemic antimicrobial agents in the treatment of periodontal disease was widespread in the past. The main drawback of systemic antimicrobials is achieving and maintaining a therapeutic concentration at the infected site. Antimicrobial agents locally applied into the periodontal pockets may further suppress periodontal pathogens. Various local drug delivery (LDD) systems have been introduced to overcome the disadvantages of the systemic route of antimicrobial administration. LDD systems in periodontal therapy can provide up to 100-fold higher drug doses at the target site compared to systemic administration [7]. To date, doxycycline, and metronidazole are the most widely used antibiotics for LDD in the treatment of periodontal disease. The concentration of tetracycline in gingival crevicular fluid (GCF) is 5–10-fold higher than serum levels due to their anti-collagenase property [8,9]. Such antimicrobial therapy as an adjunct requires reduced dosage and fewer applications and also has high patient compliance.
Increasing concern over the unwanted side effects and emergence of highly resistant microbes with increased pathogenicity at the treated sites has altered the general perception of the capabilities of these antimicrobial agents [10,11]. In light of this, there is a need to look for alternate options that are effective, relatively safe, and economical [12]. Research in phytosciences, an emerging multidisciplinary science, has revealed various medicinal plants possessing antimicrobial activity with fewer side effects, reduced toxicity, and cost-effectiveness. Extracts of these medicinal plants offer a new choice for optional antimicrobial therapy against various oral microorganisms [13,14].
Keeping the above factors in mind, the present study was planned with the aim to assess and compare the effectiveness of locally administered 2% gel made from lemongrass polymer and 10% doxycycline hyclate gel as an adjunct to SRP in treating chronic periodontitis and also to verify and compare the antimicrobial effect of 2% gel made from lemongrass polymer and 10% doxycycline hyclate gel in chronic periodontitis.
2. Materials and Methods
2.1. Study Design
A double-blind parallel arm randomized controlled study was planned to evaluate the effectiveness of 2% lemongrass gel as an adjunct to SRP therapy in chronic periodontitis with 10% doxycycline hyclate gel as active control. This study also aimed to evaluate in vivo antimicrobial effect of both LDD agents. The study protocol was developed, and all subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Institute of dental science, Bareilly, India. “(IDS/ETHCC/14/10).” The study was registered with the Clinical Trial Registry of India (CTRI REF/2021/03/042330 AU).
Study subjects and Sample size
Sample size (n) was calculated by using formula
| (1) |
where Zα/2 = 1.96, Z1−β = 0.842 are respectively the 95% confidence value obtained from the standard normal distribution with power of the study at 80%. At least seventeen subjects were needed to detect a significant difference in PPD after intervention with an effect size of 0.50 and a standard deviation of 0.45 from the pilot study using ten subjects. To compensate for the dropouts, 10% of n is added to get the final sample size. Hence, the final minimum sample size in each group was n = 20.
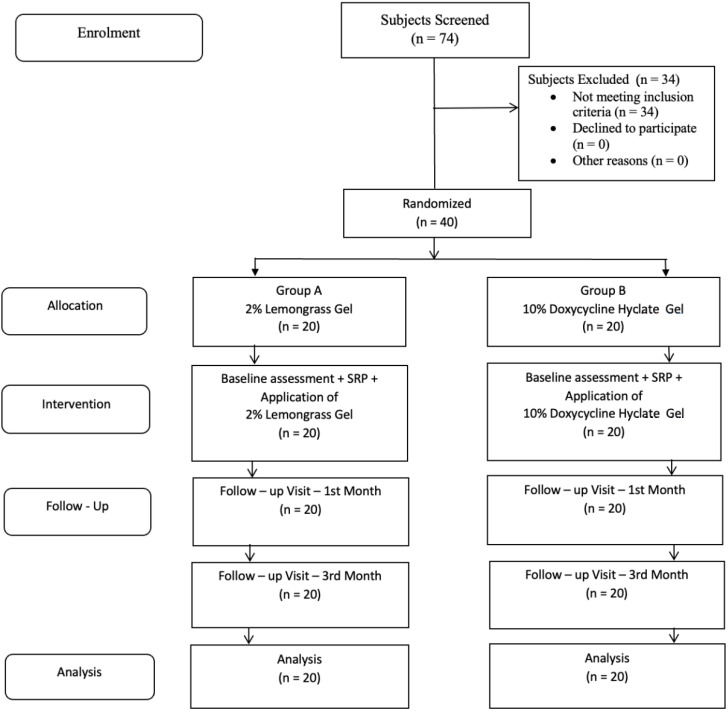
A total of 74 subjects were screened for the study, of which 34 subjects did not satisfy the inclusion criteria. The inclusion criteria for the patient selection were: (a) patients in the age group of 18–60 years, (b) untreated chronic periodontitis with a minimum of four periodontal pockets per quadrant, and (c) periodontal pockets with probing depth 4 to 6 mm. Patients with systemic diseases, who had received antibiotics during the previous three months, or who had received antiseptic/antiplaque agents in the last three months, pregnant women, and patients who had undergone periodontal treatment in the last six months were excluded from the study. The details of study procedure were explained to all eligible subjects (n = 40) and consent was obtained.
2.2. Formulation of 2% Lemongrass Gel and 10% Doxycycline Hyclate Gel
For this study, a stable bio-absorbable controlled-release formulation of 2% lemongrass gel and 10% doxycycline hyclate for the treatment of periodontal pockets was prepared by Department of Pharmacy, Mahatma Jyotiba Phule Rohilkhand University, Bareilly, India [20].
2.2.1. Preparation of 2% Lemongrass Gel
Appropriate quantity of Carbopol 934 was soaked in water for a period of 2 h. Carbopol was then neutralized with Triethanolamine (TEA) by stirring. Then, 2% lemongrass essential oil was dissolved in appropriate and pre-weighted amounts of propylene glycol and ethanol. The solvent blend was transferred to the Carbopol container and agitated for an additional 20 min. The dispersion was then allowed to hydrate and swell for 60 mins, then the pH was adjusted with 98% TEA until the desired pH value approximately reached (6.8–7). During pH adjustment, the mixture was stirred gently with a spatula until a homogeneous gel was formed.
2.2.2. Preparation of 10% Doxycycline Hyclate Gel
A total of 5% sesame oil was added to 95% of the melted Glyceryl monooleate (GMO) at 60–70 °C with continuous stirring. After the above solution was cooled to room temperature, 10% of doxycycline hyclate was added to it until a homogenous gel was obtained.
2.3. Study Procedure
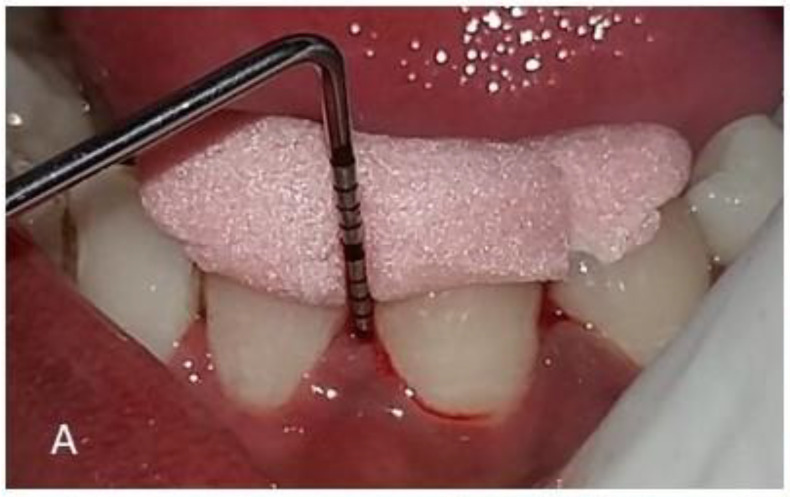
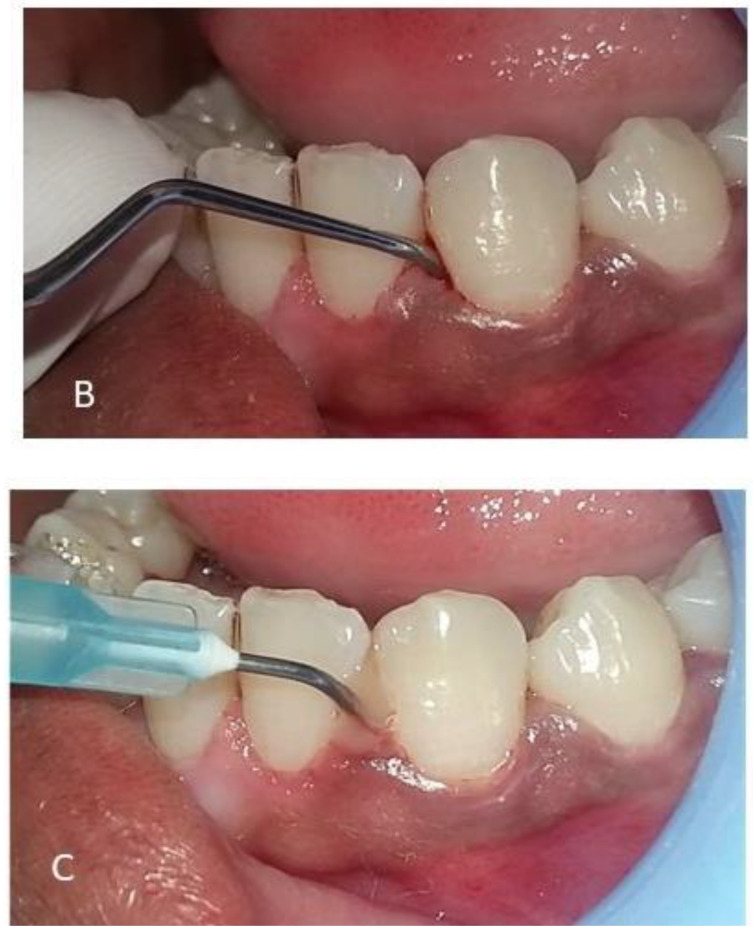
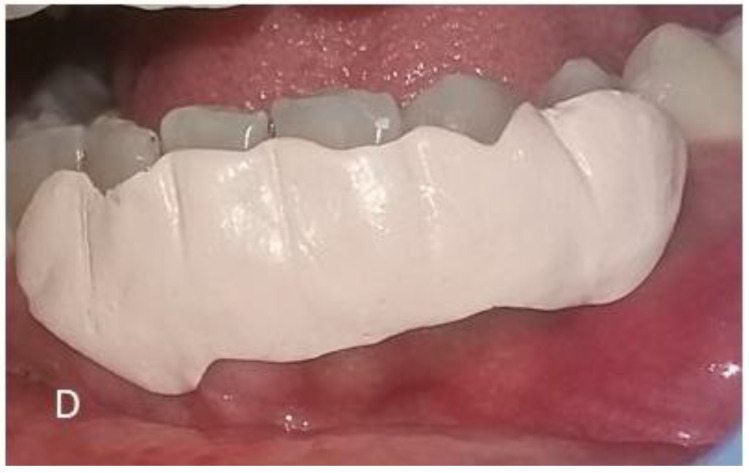
Forty subjects were randomly allocated into two equal intervention groups using a lottery method with Group A subjects (n = 20) for 2% lemongrass gel and Group B (n = 20) for 10% doxycycline hyclate gel as interventions, respectively. Only one investigator was aware of the intervention groups, and the subjects were coded for identification. The same investigator carried out the placement of local drug delivery for each study subject. The clinical periodontal status of each subject was recorded at baseline, i.e., before SRP therapy and application of local drug delivery and on 1st and 3rd month follow-up assessments. The clinical steps involved are shown in Figure 1.
Figure 1.
Clinical steps involved in the study. (A) Recording clinical parameters at baseline. (B) Subgingival plaque sample collection. (C) LDD at the site of Periodontal Pocket. (D) Placement of periodontal dressing.
In this study both clinical and microbiological periodontal parameters were used for outcomes assessment.
2.4. Clinical Assessments
Clinical assessments for periodontitis were done as per American Academy of Periodontology (AAP) 1999 classification [21]. The indices used were: Gingival index (GI), Plaque Index (PI) Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL) [1,22,23]. All clinical assessments were done with the University of North Carolina-15 probe (Hu Friedy®). A single blinded investigator was trained and calibrated for recording clinical indices with ten independent subjects on an interval of 24 h. The intra-class correlation coefficient was found to be 0.82 which indicate good reliability.
2.5. Microbiological Analysis
Before recording clinical parameters, subgingival plaque samples were collected from the selected sites at baseline, 1st month and 3rd month visits to evaluate the changes in colony forming units (CFU) of primary periodontal pathogens, i.e., Porphyromonas gingivalis, Actinomyces naeslundii and Prevotella intermedia [24]. The teeth were isolated using cotton rolls and a plaque sample was obtained using sterile area specific Gracey curettes (Hu Friedy®) in a previously fumigated minor operation theatre. The samples were then transferred in a vial containing 10 mL Robertson Cooked Meat Broth Medium and were incubated at 37 °C for 24–96 h. The total number of CFU’s was determined based on serial dilution from 10–1 to 10–3 on selective media. Finally, each bacteria’s count was determined based on typical colony and bacterial morphology in 102 CFU/milliliter on Muller Hinton Agar.
2.6. Study Intervention
Immediately after baseline assessment, all subjects received SRP therapy followed by application of local drug delivery. The investigator assigned for application of LDD gels applied 2% lemongrass gel and 10% doxycycline hyclate gel into periodontal pockets as per patient codes. The LDD gel was inserted into the bases of the pockets using a special syringe with a blunt cannula. The end of the blunt cannula was moved coronally to fill the pocket, and the excess gel was removed using a curette or wet cotton pellet. The site was covered with a periodontal dressing (Coe-Pak®) to prevent the medication from being flushed out of the pocket. All subjects were advised to use 0.2% of chlorhexidine rinses, and standard oral hygiene instructions were given. After one week, the subjects were reviewed for any discomfort such as transient discomfort, erythema, transient resistance, allergy following treatment, and visual examination to record any soft tissue changes after removal of periodontal dressing. The subjects were recalled for follow-up assessments on 1st and 3rd months, and oral hygiene maintenance was reinforced. The study design flow chart is given in Figure 2. All subjects who participated completed the study, and none of the subjects had any complications during recall visits.
Figure 2.
Flow chart of the study.
2.7. Statistical Analysis
The collected data was first entered into MS-Excel spreadsheet and further subjected to analysis using SPSS for Windows, Version 16.0. Chicago, SPSS Inc. (Chicago, IL, USA) Descriptive results were presented as frequency, mean, and standard deviation. Inter-group comparisons were made by independent Student’s t-test and intra-group comparisons to test changes during follow-up visits were obtained by using repeated measures of ANOVA test and Least Significant Difference post hoc test. The level of significance was set at 5%.
3. Results
At baseline, there was no statistically significant difference between the groups in their mean clinical and microbiological scores (p > 0.05) (Table 1). The repeated measures ANOVA test showed that there was a significant reduction in all clinical mean scores from baseline to the 1st and 3rd month follow-ups in both 2% lemongrass gel and 10% doxycycline gel groups (p < 0.05) (Table 2). Similarly, there was significant reduction in all microbiological mean CFU scores from baseline to the 1st and 3rd month follow-ups in both 2% lemongrass gel and 10% doxycycline gel groups (p < 0.05) (Table 3). A pairwise Least Significant Difference (LSD) post hoc test showed that there was a significant reduction of GI, PPD, CAL, and all three microbiological mean CFU count scores in both the 2% lemongrass gel and 10% doxycycline gel groups from baseline to the 1st and 3rd month follow-up scores (p < 0.05). There was no statistically significant difference in the mean PI scores from baseline to the 1st and 3rd month follow-up scores in 2% lemongrass gel group (p > 0.05) (Table 2 and Table 3).
Table 1.
Comparison of mean scores of both clinical and microbiological parameters at baseline.
| Groups | N | Mean | SD | p-Value | |
|---|---|---|---|---|---|
| GI | 2% Lemongrass Gel | 20 | 1.78 | 0.26 | 0.868 |
| 10% Doxycycline Gel | 20 | 1.76 | 0.24 | ||
| PI | 2% Lemongrass Gel | 20 | 1.82 | 0.20 | 0.814 |
| 10% Doxycycline Gel | 20 | 1.84 | 0.19 | ||
| PPD | 2% Lemongrass Gel | 20 | 5.30 | 0.47 | 0.744 |
| 10% Doxycycline Gel | 20 | 5.35 | 0.49 | ||
| CAL | 2% Lemongrass Gel | 20 | 5.50 | 0.51 | 0.759 |
| 10% Doxycycline Gel | 20 | 5.55 | 0.51 | ||
| Porphyromonas Gingivalis (103 CFU) | 2% Lemongrass Gel | 20 | 1.06 | 0.54 | 0.757 |
| 10% Doxycycline Gel | 20 | 1.00 | 0.60 | ||
| Actinomyces Naeslundi (102 CFU) | 2% Lemongrass Gel | 20 | 0.69 | 0.43 | 0.683 |
| 10% Doxycycline Gel | 20 | 0.75 | 0.51 | ||
| Prevotella Intermedia (103 CFU) | 2% Lemongrass Gel | 20 | 1.12 | 0.48 | 0.274 |
| 10% Doxycycline Gel | 20 | 0.94 | 0.52 |
Statistically significant at 5% level of significance.
Table 2.
Comparison of mean clinical parameters scores within groups at baseline, 1 month, and 3 month follow-ups.
| Groups | Assessment Period | Mean | SD | p-Value | |
|---|---|---|---|---|---|
| GI | 2% Lemongrass Gel | Baseline | 1.78 | 0.26 | <0.0001 * Ф |
| 1 Month | 1.34 | 0.27 | |||
| 3 Months | 1.22 | 0.28 | |||
| 10% Doxycycline Gel | Baseline | 1.76 | 0.24 | <0.0001 * Ф | |
| 1 Month | 1.34 | 0.23 | |||
| 3 Months | 1.16 | 0.28 | |||
| PI | 2% Lemongrass Gel | Baseline | 1.82 | 0.20 | 0.021 * |
| 1 Month | 1.78 | 0.25 | |||
| 3 Months | 1.73 | 0.23 | |||
| 10% Doxycycline Gel | Baseline | 1.84 | 0.19 | 0.015 * Ф | |
| 1 Month | 1.72 | 0.26 | |||
| 3 Months | 1.70 | 0.28 | |||
| PPD | 2% Lemongrass Gel | Baseline | 5.30 | 0.47 | <0.0001 * Ф |
| 1 Month | 3.30 | 0.47 | |||
| 3 Months | 3.25 | 0.44 | |||
| 10% Doxycycline Gel | Baseline | 5.35 | 0.49 | <0.0001 * Ф | |
| 1 Month | 3.40 | 0.50 | |||
| 3 Months | 3.15 | 0.37 | |||
| CAL | 2% Lemongrass Gel | Baseline | 5.50 | 0.51 | <0.0001 * Ф |
| 1 Month | 3.50 | 0.61 | |||
| 3 Months | 3.45 | 0.60 | |||
| 10% Doxycycline Gel | Baseline | 5.55 | 0.51 | <0.0001 * Ф | |
| 1 Month | 3.60 | 0.50 | |||
| 3 Months | 3.35 | 0.49 |
* Statistically significant at 5% level of significance by repeated measures ANOVA. Ф Statistically significant at 5% level of significance by pairwise Least Significant Difference post hoc test.
Table 3.
Comparison of mean microbiological parameter scores within groups at baseline, 1 month, and 3 month follow-ups.
| Groups | Assessment Period | Mean | SD | p-Value | |
|---|---|---|---|---|---|
| Porphyromonas Gingivalis (103 CFU) | 2% Lemongrass Gel | Baseline | 1.06 | 0.54 | <0.0001 * Ф |
| 1 Month | 0.31 | 0.34 | |||
| 3 Months | 0.28 | 0.31 | |||
| 10% Doxycycline Gel | Baseline | 1.00 | 0.60 | <0.0001 * Ф | |
| 1 Month | 0.37 | 0.33 | |||
| 3 Months | 0.38 | 0.35 | |||
| Actinomyces Naeslundi (102 CFU) | 2% Lemongrass Gel | Baseline | 0.69 | 0.43 | <0.0001 * Ф |
| 1 Month | 0.33 | 0.34 | |||
| 3 Months | 0.31 | 0.30 | |||
| 10% Doxycycline Gel | Baseline | 0.75 | 0.51 | <0.0001 * Ф | |
| 1 Month | 0.33 | 0.31 | |||
| 3 Months | 0.32 | 0.31 | |||
| Prevotella Intermedia (103 CFU) | 2% Lemongrass Gel | Baseline | 1.12 | 0.48 | <0.0001 * Ф |
| 1 Month | 0.70 | 0.36 | |||
| 3 Months | 0.67 | 0.35 | |||
| 10% Doxycycline Gel | Baseline | 0.94 | 0.52 | <0.0001 * Ф | |
| 1 Month | 0.51 | 0.41 | |||
| 3 Months | 0.52 | 0.41 |
* Statistically significant at 5% level of significance by repeated measures ANOVA. Ф Statistically significant at 5% level of significance by pairwise Least Significant Difference post hoc test.
The mean difference was highest in PPD and CAL assessments from baseline to the follow-up visits in both groups. The mean differences of microbial CFUs from baseline to the 1st and 3rd month follow-ups in both groups were comparable (Table 2 and Table 3).
4. Discussion
Various polymers have been explained by researchers for drug delivery. Different polymers exhibit different mucoadhesive properties depending on their physical and chemical strength. For example, a more flexible polymer exhibits a higher degree of mucoadhesive property [25]. Mucoadhesive polymers possessing hydrophilic functional groups such as COOH, OH, NH2, and SO4H are more favorable candidates for the formulation of targeted drug delivery. These polymers bearing the desired functional group interact with mucus through physical entanglement as well as through chemical bonds resulting in the formation of a cross-linked network. For example, urea is a well-accepted hydrogen-bonding disruptor that decreases the mucoadhesion of mucin/pectin samples. Other properties that may affect the mucoadhesive nature of the polymer include chain length, degree of hydration, degree of cross-linking, polymer concentration, charge, etc.
In the last three decades, periodontal therapy has seen significant progress in various aspects. There has been a shift from surgical treatment procedures to techniques and methods aimed at delivering the drug locally along with scaling and root planing to the affected sites by targeting the specific periodontopathic microorganisms in bringing improvements in clinical parameters of the periodontium. The adjunctive use of antimicrobial agents in addition to non-surgical therapy has been shown to provide additional benefits. Such antimicrobial therapy as an adjunct needs a reduced dosage and fewer applications and should also have high patient compliance. Various LDD systems have been introduced to overcome the disadvantages of the systemic route of antimicrobial administration. A number of LDD systems have been used in different clinical trials with different degrees of success. Upon analysis of the various clinical reports, it is seen that most of the LDD systems have resulted in significant improvement in the clinical parameters. However, the kind of improvement in the clinical parameters has not been consistent. Many of the published research on LDD systems in the literature has not evaluated the change in the microbial count of periodontopathic bacteria.
In the present research, we have used Carbopol 934 for the formulation of 2% lemongrass gel, which eventually yielded many therapeutic benefits by releasing the drug in a sustained manner. Carbopol or carbomer are high molecular weight polymers of acrylic acid cross-linked with either allyl sucrose or allyl ethers of penta erythritol. These contain 56% and 68% of carboxylic acid groups calculated on the dry bases [26]. These are used as suspending agents or viscosity-increasing agents, dry and wet binders, as well as rate-controlling agents in tablets, enzyme inhibitors of intestinal protease in peptide-containing dosage forms, etc. Carbomer is a pH-dependent polymer that stays in solution form at acidic pH but forms a low viscosity gel at alkaline pH. Carbopol offers the advantage of exhibiting excellent mucoadhesive properties in comparison with other polymers (e.g., cellulose derivatives and polyvinyl alcohol) [27]. Different mucoadhesive formulations containing carbopol have been developed and it was found that these demonstrated excellent mucoadhesive properties and release the drug in a controlled manner for a longer period of time. Tan et al. [28] developed a bioadhesive gel incorporating lidocaine using carbopol and Polyvinylpyrrolidone (PVP). The results indicated that an increase in carbopol concentration significantly increased gel compressibility, hardness, and adhesiveness, that is, the factors that affect the ease of gel removal from the container, ease of gel application onto the mucosal membrane, and gel bioadhesion, respectively. Moreover, the resulting formulation provided a sustained release as compared with the conventional dosage forms. Similar results were obtained by Bilensoy et al. [29]. They developed 5-FU containing thermosensitive, mucoadhesive gel based on carbopol 934 and pluronic F12 for the treatment of Human Papillomavirus (HPV)-induced cervical cancer. The resulting formulation demonstrated better anticancer activity at lower doses, avoiding unwanted side effects of the drug. In another study, Patel and Chavda [30] prepared amoxicillin-loaded gastroretentive microspheres using carbopol-934 providing sustained release.
In the present study, there was significant reduction in all clinical mean scores from baseline to 1st and 3rd month follow-up in both 2% lemongrass gel and 10% Doxycycline Gel groups. Similarly, there was significant reduction in all microbiological mean CFU scores from baseline to 1st and 3rd month follow-up in both 2% Lemongrass Gel and 10% Doxycycline Gel groups. The mean differences for both groups from baseline to 1st and 3rd month follow-up scores showed that there was significant reduction of GI, PPD, CAL and all three microbiological mean CFU count scores in both 2% Lemongrass Gel and 10% Doxycycline Gel groups. The mean difference was highest in PPD and CAL assessments from baseline to follow-up visits in both groups. The mean differences of microbial CFU’s from baseline to 1st and 3rd month follow-up in both groups were comparable.
This is attributable to the antibacterial property of doxycycline and lemongrass. Studies have shown that the beneficial effects of doxycycline in periodontal diseases are due to the antibacterial property of doxycycline against periodontopathogens [6] and the inhibitory action of the pathologically elevated tissue degrading activities of matrix metalloproteinases (MMPs) in the inflamed gingival tissues of adult periodontitis [9]. The control group-associated results of the present study are in accordance with the findings of the previous studies [6,24].
Lemongrass has several beneficial properties that can be of use in periodontal therapy. Previous studies have demonstrated the anti-inflammatory and antimicrobial properties of lemongrass in terms of inhibition of the production of interleukine-1β (IL-1β) and IL-6 [31]. Citral, the main component of the lemongrass, is responsible for its anti-microbial property by causing extensive leakage of critical molecules and ions from the bacterial cell and permeabilization of the bacterial cytoplasmic membrane leading to their death [32] and in addition to this, the anti-inflammatory action of lemongrass is due to the blockage of the LPS-induced activation of Nuclear Factor kappa-B (NF-ĸB) [33,34]. The observations of the test group in the present study are in accordance with the findings of previous studies [17,35,36]. In addition to this, lemongrass oil has also been shown to decrease the volatile sulfur compounds, hence inhibiting halitosis [36].
There was no statistically significant difference in mean PI scores from baseline to the 1st and 3rd month follow-up scores in the 2% lemongrass gel group. This could be because of a lack of oral hygiene maintenance.
The results of this study suggested that 2% lemongrass gel as an LDD system is one of the nonsurgical treatment modalities in bringing improvement in clinical & microbiological parameters. However, further investigation with a larger sample size on a prolonged post-operative follow-up is required to conclusively establish the effectiveness of this gel.
5. Conclusions
It could be concluded that the local delivery of 2% lemongrass gel as an adjunct to scaling and root planing is effective and comparable to 10% doxycycline gel in the treatment of chronic periodontitis, and 2% lemongrass gel is an effective antimicrobial agent against primary periodontal pathogens, i.e., Porphyromonas gingivalis, Actinomyces naeslundii, and Prevotella intermedia. Further studies targeting other periodontal pathogens associated with specific periodontal conditions need to be conducted.
Author Contributions
Conceptualization, P.M., S.T.G., S.M., S.M.A.-Q., M.A.M., R.R.N., S.T., S.C., A.A. and V.V. methodology, P.M., S.T.G., S.M., M.A.M., R.R.N. and S.C.; software, P.M., S.T.G. and S.M.; validation, P.M., S.T.G., S.M., R.R.N., S.T. and V.V.; formal analysis, P.M., S.T.G. and S.M.; investigation, P.M., S.T.G., S.M. and S.M.A.-Q.; resources, P.M., S.T.G., S.M. and A.A.; data curation, P.M., S.T.G. and S.M.; writing—original draft preparation, P.M., S.T.G. and S.M.; writing—review and editing, P.M., S.T.G., S.M., S.M.A.-Q., M.A.M., R.R.N., S.T., S.C., A.A. and V.V.; visualization, P.M., S.T.G. and S.M.; supervision, P.M., S.T.G. and S.M.; project administration, P.M., S.T.G., S.M. and S.C.; funding acquisition, P.M., S.T.G., S.M., S.M.A.-Q., M.A.M., R.R.N., S.C., A.A. and V.V.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Institute of dental science, Bareilly, India. “(IDS/ETHCC/14/10).” The study was registered with the Clinical Trial Registry of India (CTRI REF/2021/03/042330 AU).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data can be made available on demand by the chief researcher for academic purposes by email.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
The authors extend their appreciation to the Deanship of Scientific research at King Khalid University, Saudi Arabia for funding this work through Small Group Project under the grant number (RGP.1/204/43).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Newman M.G., Takei H., Klokkevold P.R., Carranza F.A. Newman and Carranza’s Clinical Periodontology E-Book. Elsevier Health Sciences; IOWA City, IA, USA: 2018. pp. 50–370. [Google Scholar]
- 2.Salvi G.E., Mombelli A., Mayfield L., Rutar A., Suvan J., Garrett S., Lang N.P. Local antimicrobial therapy after initial periodontal treatment. J. Clin. Periodontol. 2002;29:540–550. doi: 10.1034/j.1600-051X.2002.290611.x. [DOI] [PubMed] [Google Scholar]
- 3.Rams T.E., Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontology 2000. 1996;10:139–159. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 4.Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 5.Zambon J.J. Periodontal diseases: Microbial factors. Ann. Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 6.Walker C.B., Godowski K.C., Borden L., Lennon J., Nangó S., Stone C., Garrett S. The effects of sustained release doxycycline on the anaerobic flora and antibiotic-resistant patterns in subgingival plaque and saliva. J. Periodontol. 2000;71:768–774. doi: 10.1902/jop.2000.71.5.768. [DOI] [PubMed] [Google Scholar]
- 7.Soskolne W.A. Subgingival delivery of therapeutic agents in the treatment of periodontal diseases. Crit. Rev. Oral Biol. Med. 1997;8:164–174. doi: 10.1177/10454411970080020501. [DOI] [PubMed] [Google Scholar]
- 8.Slots J., Rosling B.G. Suppression of the periodontopathic microflora in localized juvenile periodontitis by systemic tetracycline. J. Clin Periodontol. 1983;10:465–486. doi: 10.1111/j.1600-051X.1983.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 9.Golub L.M., Sorsa T., Lee H.M., Ciancio S., Sorbi D., Ramamurthy N.S., Gruber B., Salo T., Konttinen Y.T. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J. Clin. Periodontol. 1995;22:100–109. doi: 10.1111/j.1600-051X.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen M.G., Slots J. Responsible use of antimicrobials in periodontics. J. Calif. Dent. Assoc. 2000;28:185–193. [PubMed] [Google Scholar]
- 11.Slots J., Pallasch T.J. Dentists’ role in halting antimicrobial resistance. J. Dent. Res. 1996;75:1338–1341. doi: 10.1177/00220345960750060201. [DOI] [PubMed] [Google Scholar]
- 12.Palombo E.A. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid. Based Complement. Altern. Med. 2011;2011:680354. doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan R., Islam B., Akram M., Shakil S., Ahmad A., Ali S.M., Siddiqui M., Khan A.U. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules. 2009;14:586–597. doi: 10.3390/molecules14020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma R., Hebbal M., Ankola A.V., Murugaboopathy V., Shetty S.J. Effect of two herbal mouthwashes on gingival health of school children. J. Tradit. Complement. Med. 2014;4:272–278. doi: 10.4103/2225-4110.131373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah G., Shri R., Panchal V., Sharma N., Singh B., Mann A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass) J. Adv. Pharm. Technol. Res. 2011;2:3–8. doi: 10.4103/2231-4040.79796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammer K.A., Carson C.F., Riley T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 17.Khongkhunthian S., Sookkhee S., Okonogi S. Antimicrobial activities against periodontopathogens of essential oil from lemon grass (Cymbopogon citratus (DC.) Stapf CMU. J. Nat. Sci. 2009;8:11–21. [Google Scholar]
- 18.Susanto S.A., Oktavianti T.A., Wijaya Y., Wira V., Paramitta V.A. Increased glutathione level in saliva of moderate gingivitis patients after lemongrass (cymbopogon citratus) essential oil gargling. Asia Pac. Dent. Stud. J. 2010;1:45–52. [Google Scholar]
- 19.Anand K.M., Goyal R., SubrayaBhat G., Kamath S., Aggarwal M., Meghna A., Bhandarkar B.S., Sukreeth S. A novel anti-oxidant lemon grass oil mouthwash—A clinical trial. Asian J. Exp. Biol. Sci. 2011;2:482–486. [Google Scholar]
- 20.Patel J., Patel B., Banwait H., Parmar K., Patel M. Formulation and Evaluation of Topical Aceclofenac Gel Using Different Gelling Agents. Int. J. Drug Dev. Res. 2011;3:156–164. [Google Scholar]
- 21.Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Loe H., Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 23.Silness J., Loe H. Periodontal disease in pregnancy. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 24.Shaddox L.M., Andia D.C., Casati M.Z., Nociti F.H., Jr., Sallum E.A., Gollwitzer J., Walker C.B. Microbiologic changes following administration of locally delivered doxycycline in smokers: A 15-month follow-up. J. Periodontol. 2007;78:2143–2149. doi: 10.1902/jop.2007.070189. [DOI] [PubMed] [Google Scholar]
- 25.Gu J.M., Robinson J.R., Leung S.H. Binding of acrylic polymers to mucin/epithelial surfaces: Structure-property relationships. Crit. Rev. Ther. Drug Carr. Syst. 1988;5:21–67. [PubMed] [Google Scholar]
- 26.Barry B.W., Meyer M.C. The rheological properties of carbopol gels I. Continuous shear and creep properties of carbopol gels. Int. J. Pharm. 1979;2:1–25. doi: 10.1016/0378-5173(79)90025-5. [DOI] [Google Scholar]
- 27.Davies N.M., Farr S.J., Hadgraft J., Kellaway I.W. Evaluation of mucoadhesive polymers in ocular drug delivery. II. Polymer-coated vesicles. Pharm. Res. 1992;9:1137–1144. doi: 10.1023/A:1015891419676. [DOI] [PubMed] [Google Scholar]
- 28.Tan Y.T., Peh K.K., Hanbali A.O. Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological, and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech. 2000;1:69–78. doi: 10.1208/pt010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilensoy E., Sen M., Dogan A.L., Calis S. Thermosensitive mucoadhesive gel formulation loaded with 5-Fu: Cyclodextrin complex for HPV-induced cervical cancer. J. Incl. Phenom. Macrocycl. Chem. 2007;57:363–370. doi: 10.1007/s10847-006-9259-y. [DOI] [Google Scholar]
- 30.Patel J.K., Chavda J.R. Formulation and evaluation of stomachspecific amoxicillin-loaded carbopol-934P mucoadhesive microspheres for anti-Helicobacter pylori therapy. J. Microencapsul. 2009;2:6365–6376. doi: 10.1080/02652040802373012. [DOI] [PubMed] [Google Scholar]
- 31.Sforcin J., Amaral J., Fernandes A., Jr., Sousa J., Bastos J. Lemongrass effects on IL-1β and IL-6 production by macrophages. Nat. Prod. Res. 2009;23:1151–1159. doi: 10.1080/14786410902800681. [DOI] [PubMed] [Google Scholar]
- 32.Sikkema J., de Bont J.A., Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994;269:8022–8028. doi: 10.1016/S0021-9258(17)37154-5. [DOI] [PubMed] [Google Scholar]
- 33.Lee H.J., Jeong H.S., Kim D.J., Noh Y.H., Yuk D.Y., Hong J. Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-κB activation in RAW264. 7 cells. Arch. Pharm. Res. 2008;31:342–349. doi: 10.1007/s12272-001-1162-0. [DOI] [PubMed] [Google Scholar]
- 34.Bachiega T.F., Sforcin J.M. Lemongrass and citral effect on cytokines production by murine macrophages. J. Ethnopharmacol. 2011;137:909–913. doi: 10.1016/j.jep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Warad S.B., Kolar S.S., Kalburgi V., Kalburgi N.B. Lemongrass essential oil gel as a local drug delivery agent for the treatment of periodontitis. Anc. Sci. Life. 2013;32:205–211. doi: 10.4103/0257-7941.131973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satthanakul P., Taweechaisupapong S., Paphangkorakit J., Pesee M., Timabut P., Khunkitti W. Antimicrobial effect of lemongrass oil against oral malodour micro-organisms and the pilot study of safety and efficacy of lemongrass mouthrinse on oral malodour. J. Appl. Microbiol. 2015;118:11–17. doi: 10.1111/jam.12667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available on demand by the chief researcher for academic purposes by email.