Abstract
A single-use screen-printed carbon electrode strip was designed and fabricated. Nano-hybrids, prepared by deposition of platinum (Pt) nanoparticles on multi-wall carbon nanotube (MWCNT), was modified on the surface of screen-printed carbon electrode for the development of a fast, sensitive and cost-effective hydrogen peroxide (H2O2) detection amperometric sensor strip. With Pt-MWCNT nanohybrids surface modification, current generated in response to H2O2 by the screen-printed carbon electrode strip was enhanced 100 fold with an applied potential of 300 mV. Quality of as-prepared electrode strip was assured by the low coefficient of variation (CV) (<5%) of currents measured at 5 s. Three linear detection ranges with sensitivity of 75.2, 120.7, and 142.8 μA mM−1 cm−2 were observed for H2O2 concentration in the range of 1–15 mM, 0.1–1 mM, and 10–100 μM, respectively. The lowest H2O2 concentration could be measured by the as-prepared strip was 10 μM. H2O2 levels in green tea infusion and pressed Tofu could be rapidly detected with results comparable to that measured by ferrous oxidation xylenol orange (FOX) assay and peroxidase colorimetric method.
Keywords: Platinum-multi-wall carbon, nanotube (Pt-MWCNT), Disposable carbon electrode, Hydrogen peroxide (H2O2), Amperometric sensor
1. Introduction
Hydrogen peroxide (H2O2) as a chemical compound is often used as an oxidizer, bleaching agent, and disinfectant. H2O2 as a product generated from human body or enzymatic reaction is a valuable biomarker of inflammation [1,2], some chronic diseases [3–5], and an essential mediator in food [6], industry, medical, and environmental analysis [7,8]. Therefore, developing a reliable, sensitive, rapid and low-cost sensor for detecting H2O2 has become an area of increased importance in recent years [9–15]. There already have various colorimetric, fluorescence, and electrochemical methods been developed to detect the H2O2 concentration. Among these methods, the electrochemical is the most preferred one due to its simplicity, high sensitivity, and rapid detection [16–18]. Most of electro-chemical sensors developed for H2O2 detection are amperometric in which the surface of electrodes are modified with redox enzymes to catalyze the redox reaction of H2O2 to generate electron current when a potential is applied. However, the high cost and low stability of the employed enzymes usually limit their long-term applications. Thus, there is a critical need to develop high stability, low cost and high sensitivity electrochemical nonenzymatic sensors for the detection of H2O2.
It has been reported that platinum, rhodium, ruthenium, palladium and several other noble metals are very good catalysts for redox reaction with H2O2 [19], among which platinum and palladium were considered as better catalysts then other metals [20]. In recent time single-wall, multi-wall carbon nanotube (MWCNT), and several carbon nanomaterials have been used as a support for catalysts to promote electron transfer reactions in the development of electrochemical sensors due to their superior electrical conductivity [21–25]. However, only few studies were reported on employing MWCNT in single-use screen-printed carbon-based electrodes for the development of low-cost disposable biosensor strips [26,27]. In this study, nanohybrid of MWCNT and Pt nanoparticles was prepared to modify the surface of a screen-printed disposable carbon electrode for the detection of H2O2 by amperometric method. With significantly augmented conductivity and catalytic surface area, the Pt-MWCNT nanohybrid surface-modified carbon (Pt-MWCNT/C) electrode is expected to generate a higher current signal with a lower applied potential, as a consequence, a cost-effective and fast response electrochemical sensor with high sensitivity can be obtained [28–33]. This nanohybrid modified carbon electrode strip is also expected to find success to assay various biochemical compounds by detecting the H2O2 produced by the corresponding oxidative enzymatic reactions, such as glucose oxidase, cholesterol oxidase, alcohol oxidase, and l-amino acid oxidase. In this work, the Pt-MWCNT nanohybrid was prepared and characterized by SEM, TEM and cyclic voltammetry. The optimal amount of Pt-MWCNT modified on screen-printed carbon electrode surface, applied potential, and detection time employed in the amperometric detection of H2O2 were investigated. Beside, the quality of as-prepared disposable Pt-MWCNT/C electrode strip was also studied by measuring the coefficient of variation of currents detected at different time with fixed applied potential.
In addition, H2O2 due to its ready availability and affordable price is often used as bleaching and antimicrobial agent in many processed products available in the local food markets of many developing countries. Consuming these H2O2 containing processed food products can lead to headaches, nausea, and the potential risk of cancer, therefore, well-monitoring the residual level of H2O2 in the processed food by an inexpensive and rapid method is an important task. Green tea is known to have rich content of polyphenols such as catechins and the presence of these polyphenols has already been proved will generate H2O2 in green tea infusion [34–36]. In this work, as-prepared Pt-MWCNT/C electrode strip was employed to detect H2O2 residual level in the pressed Tofu obtained from local market and H2O2 level generated in green tea infusion. Two other colorimetric methods, ferrous oxidation xylenol orange (FOX) assay and peroxidase method were also employed to demonstrate the accuracy of the amperometric method developed based on this disposable H2O2 electrochemical sensor strip.
2. Materials and methods
2.1. Materials
Hydrogen hexachloroplatinate hexahydrate H2PtCl6·6H2O (38–40% Pt) and hydrogen peroxide (35%) were obtained from ACROS. The MWCNTs (95% purity, 20–40 nm diameter) were purchased from Scientech Co. (Taiwan). Carbon ink (E3451) and Ag/AgCl ink (E2430) were obtained from Ercon Co. (MA, USA) for screen-printing the disposable electrodes. o-Dianisidine and horseradish peroxidase (HRP) were obtained from Sigma. The other chemicals employed were of analytic grade.
2.2. Fabrication of electrode sensor strip
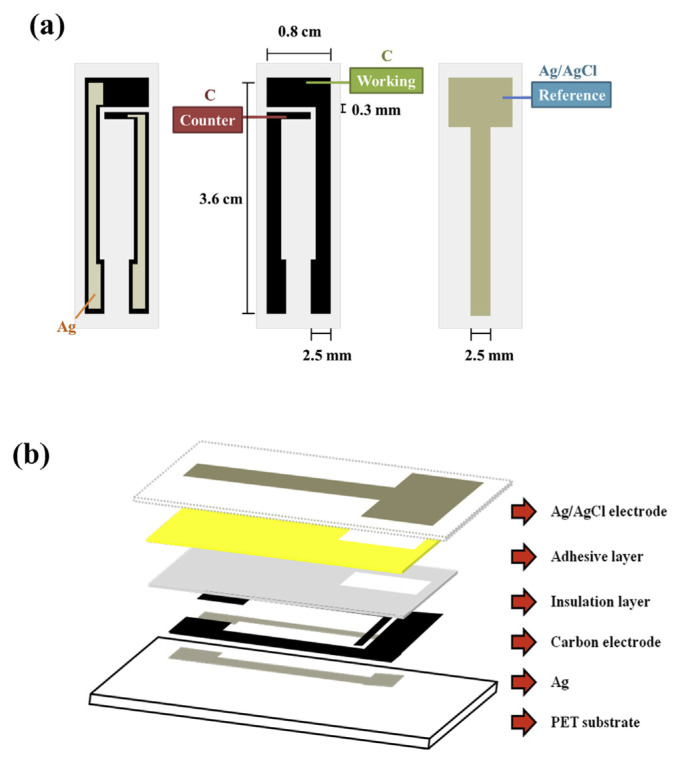
The disposable sensor strip was prepared by screen-printing carbon black ink onto an Ag coated PET plastic film as working and counter electrodes. The Ag/AgCl reference electrode was screen-printed on the other side of PET film using Ag/AgCl ink. The dimension and assembly of the electrode strip is shown in Fig. 1. The reaction area of each electrode is 5.60 mm2, 1.60 mm2 and 8.96 mm2 for working, counter, and reference electrode, respectively. Approximately, 3 μl of sample solution will be sucked into the reaction area by the capillary action.
Fig. 1.
Schematic design of single-use electrochemical H2O2 sensor strip with carbon ink screen-printed on the front and Ag/AgCl ink screen-printed on the back of PET film. (a) 2-D design and dimension, (b) 3-D assembly of the strip.
2.3. Pt-MWCNT nanohybrid preparation
Pt-MWCNT nanohybrid with Pt:MWCNT weight ratio of 1:4 was prepared based on Watanabe method [37]. Briefly, MWCNT was first treated with a strong acid mixture containing 1:1 ratio of 65% HNO3 and 98% H2SO4 at 100 °C for 8 h. Chloroplatinic acid solution (5.13 mM) of 7 ml was adjusted to pH 7.0 by 0.1 M NaCO3 solution then 0.15 g of NaHSO3 powder was added. The Pt precursor solution was then well-mixed with 25 ml 0.64% (w/w) well-dispersed MWCNT solution. H2O2 (35%) of 6 ml was then slowly added into the mixture and the pH was adjusted to 6.0 by using 1 M NaOH. After heated at 100 °C for 3 h, the precipitate collected by centrifugation was washed with ethanol and dried at 80 °C. The dried precipitate was ground into powder then reduced at 300 °C under 10% H2:90% Ar atmosphere.
2.4. Electrode modification with Pt-MWCNT and characterization
Pt-MWCNT nanohybrid slurry with concentration of 2 mg/ml was prepared by dispersing the dried Pt-MWCNT nanohybrid in 0.1% (w/v) Triton X-100 under ultrasonication for 60 min. Various amount of Pt-MWCNT were deposited by spreading 0.5 μL of Pt-MWCNT slurry of different concentration onto the surface of electrode. After drying, 1.6 μL of 0.05% (w/v) Triton X-100 solution was again loaded onto the surface of Pt-MWCNT nanohybrid modified electrode. The electrocatalytic performance of bare carbon electrode and Pt-MWCNT modified electrode sensor strip in 0.1 M PBS buffer with and without 3 mM H2O2 were studied by cyclic voltammetry (CV) with applied potential in the range of −0.8 to 0.8 V at a scan rate of 100 mV/s using a CHI 6600A electrochemical workstation (CH instrument, Austin, TX, USA). The quality of as-prepared disposable Pt-MWCNT/C electrode strip was assured by studying the coefficient of variation of currents measured from 10 randomly selected electrodes under same applied potential. The morphology of the Pt nanoparticles along with MWCNT was characterized using a Philips Tecnai F20 G2 FEI-TEM operated at 80 kV. The surface of carbon and Pt-MWCNT/ C electrode were observed using JEOL JSM-6500 FE-SEM.
2.5. Detection of H2O2
H2O2 of various concentrations were prepared by diluting 35% H2O2 solution obtained from ACROS in pH 7.4, 0.1 M PBS buffer. The proper diluted standard H2O2 solution was employed to establish a linear calibration curve based on the currents generated at an applied potential of 300 mV using the as-prepared Pt-MWCNT/C electrode strip. For detecting H2O2 level in green tea infusion and leaching solution of processed food products, green tea powder and pressed Tofu were employed as model compounds. Green tea powder from local market of 0.2 g was mixed in 20 ml distilled water at 50 °C for 10 min. After centrifugation (13,000 rpm, 5 min), the supernatant was diluted with equal volume of pH 7.4 0.1 M PBS buffer and considered as an infusion. The infusion solution was shaken for various time at 37 °C before poly-vinylpolypyrrolidone (PVPP) powder was added to 5% (w/v) for polyphenolic compounds removal. After mixing with PVPP for 30 min at room temperature, the solution was subjected to centrifugation to obtain the clarified green tea infusion for H2O2 detection. To measure residual H2O2 level in pressed Tofu obtained from a local market in Taipei city (Taiwan), pressed Tofu of 1 g was added into 10 ml pH 7.4, 0.1 M PBS buffer and stirred for 1 h at room temperature to leach H2O2. H2O2 concentration in the leaching solution was then measured after proper dilution. In addition to the amperometric detection using Pt-MWCNT/C electrode strip, ferrous oxidation xylenol orange (FOX) method [38] and the enzymatic method using horseradish peroxidase (HRP) with o-dianisidine (ODA) dye [39] were also employed for H2O2 detection as references. Briefly, sample solution of 20 μl was mixed with 180 μl of ferrous oxidation xylenol orange reagent (0.45 mM xylenol orange, 0.45 mM Fe(NH4)(SO4)2 in 0.11 M HClO4) for 30 min at room temperature. The optical density of solution was measured at 550 nm using UV–Vis spectrometer. For enzymatic method, 150 μl sample solution was well-mixed with 720 μl ODA solution (66 μg/ml in 0.1 M pH 7.0 phosphate buffer) and 30 μl HRP solution (1 mg/ml) at room temperature then the optical density of the solution was measured at 727 nm.
3. Results and discussion
3.1. Characterization of Pt-MWCNT/C electrode strip
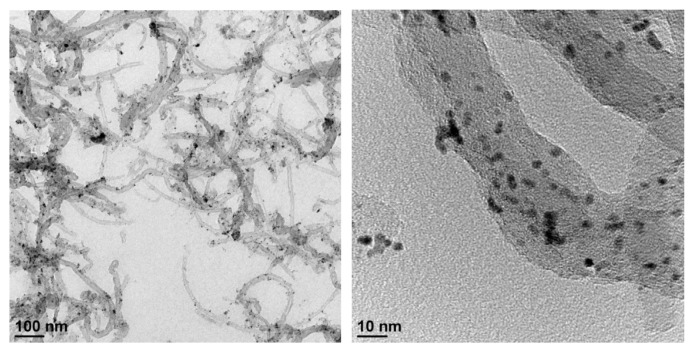
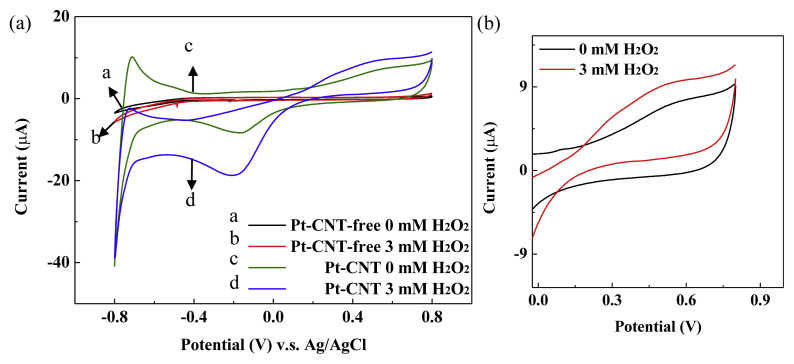
TEM images shown in Fig. 2 show that most of the nano-sized Pt particles are spherical and highly dispersed on the MWCNTs with no significant particle aggregation. The average size of Pt particles was evaluated statistically by ImageJ software measuring the diameter of 50 nanoparticles in selected TEM images to be 3.37 ± 0.54 nm. The MWCNTs on the other hand, has a diameter approximately 30 nm with length much longer than 100 nm. More than 50% of MWCNTs are free of Pt nanoparticles probably because the ratio of Pt to MWCNTs was designed to be 20% in the preparation of Pt-MWCNTs nanohybrid. Fig. S1 (supplementary figure) shows the SEM image of Pt-MWCNTs modified carbon electrode (Pt-MWCNTs/C). In addition to the widely distributed carbon nanoparticles, the entrapped MWCNTs wires could also be observed. The electrochemical performances of bare carbon and Pt-MWCNTs/C electrodes toward H2O2 (3 mM H2O2 in pH 7.4, 0.1 M PBS containing 10 mM KCl) were investigated using cyclic voltammetry (CV, apply potential from −800 mV to 800 mV with scan rate of 100 mV/s). As shown in Fig. 3a, no obvious redox peaks and quite low currents were observed on the bare carbon electrode regardless of H2O2 addition. In contrast, the presence of Pt-MWCNT resulted in a significantly enhanced current on Pt-MWCNTs/C electrode that could be ascribed to the large reactive surface provided by Pt-MWCNT and the catalytic activity of Pt nanoparticles. A redox peak between −0.2 and 0.0 V on Pt-MWCNTs/C electrode could be observed in 0.1 M PBS regardless of H2O2 addition. It is not clear to us what is the exact redox process generated this peak. However, as the applied potential higher than 185 mV, it can be observed that H2O2 started to be oxidized and generated a current exceed that obtained in PBS without H2O2 (Fig. 3b). In comparison with Pt-MWCNT/C electrode, bare carbon electrode needs a much higher potential (>460 mV) to oxidize H2O2 but generate a less sensible current. Evidently, Pt-MWCNT nanohybrid is a very effective nanocatalyst for amperometric detection of H2O2 at a lower applied potential.
Fig. 2.
TEM images of as-prepared Pt-MWCNT catalytic nanohybrid.
Fig. 3.
Effect of H2O2 and Pt-MWCNT modification on cyclic voltammograms of single-use carbon electrode sensor strips. Pt-MWCNT of 0.179 ng/cm2 was modified on the surface of carbon electrode sensor strip (Scan rate: 100 mV/s). (a) CV of bare carbon and Pt-MWCNT/C electrode; (b) Magnified CV of Pt-MWCNT/C electrode.
3.2. Optimization of Pt-MWCNT/carbon electrode
In developing an amperometric method, usually the applied potential employed to oxidize the target analyte should be kept at a low level to minimize the interference caused by the oxidation of interferents. As observed in the CV of H2O2 using Pt-MWCNT/C electrode (Fig. 3b), apparent oxidation current could be detected at applied potential higher than 185 mV. As a consequence, the potential applied was increased from 200 mV to study the effect of applied potential on H2O2 detection. Fig. S2 shows that the current obtained at 5 s increased linearly with the potential applied but leveled-off after 400 mV. Although the higher current generated at higher potential will benefit the H2O2 detection sensitivity, but the higher potential will also result in more electric active compounds to be oxidized to generate interference current. Therefore, 300 mV was employed in the rest of experiments for H2O2 detection using the as-prepared Pt-MWCNT/C electrode sensor strip.
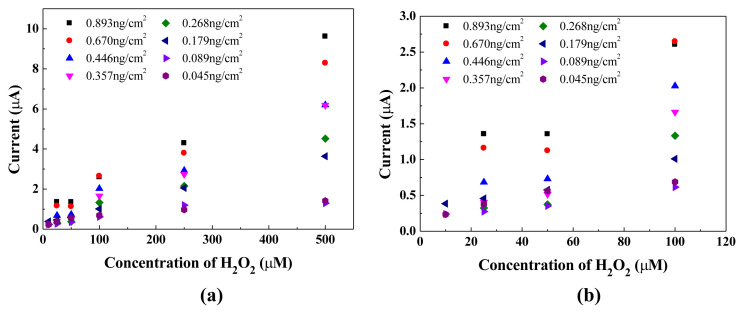
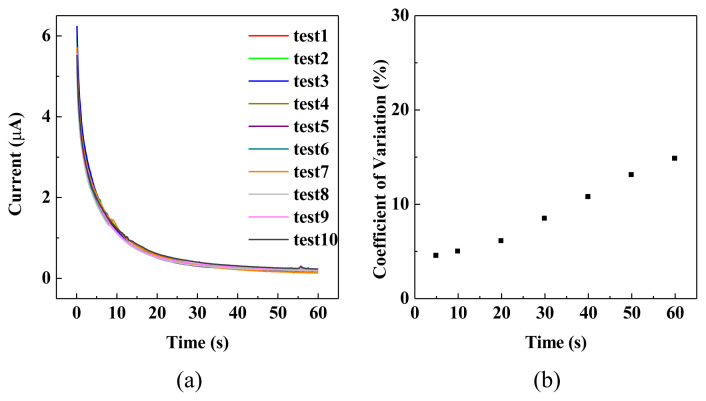
The effect of Pt-MWCNT nanohybrid loading amount on the current generated from detecting H2O2 concentration was also studied. As shown in Fig. 4, the response current at 5 s increased with the concentration of H2O2 at Pt-MWCNT concentration higher than 0.179 ng/cm2. No significant difference of response currents could be observed at concentration lower than 0.179 ng/cm2 for H2O2 concentration up to 500 μM. Evidently, nanohybrid concentration lower than 0.179 ng/cm2 is not enough to catalyze the redox reaction involved with the H2O2 concentration applied. The performance variation of the as-prepared Pt-MWCNT/carbon electrode strips was also studied by testing 10 randomly selected strips on the current generated at applied potential of 300 mV in 250 μM H2O2 solution. As shown in Fig. 5a, all the strips demonstrated very similar behaviors that current decreased sharply within 10 s and leveled-off after 30 s. The current variations with respect to time are shown in Fig. 5b that coefficient of variation increased linearly with detection time after 20 s. However, at detection time less than 10 s the coefficient of variation was less than 5%. Due to the higher current and smaller current variation, the current detected at 5 s using Pt-MWCNT/C electrode strip was employed to establish a calibration curve for H2O2 concentration determination.
Fig. 4.
Effect of Pt-MWCNT amount on current generated at 5 s by the Pt-MWCNT/C electrode strip in H2O2 concentration (a) 0–500 μM; (b) 0–100 μM.
Fig. 5.
(a) i–t curves of 10 different Pt-MWCNT/C electrode strips measuring 250 μM H2O2 at an applied potential of 300 mV. (b) Coefficient of variation of current measured at different time from 10 i–t curves.
3.3. H2O2 detection
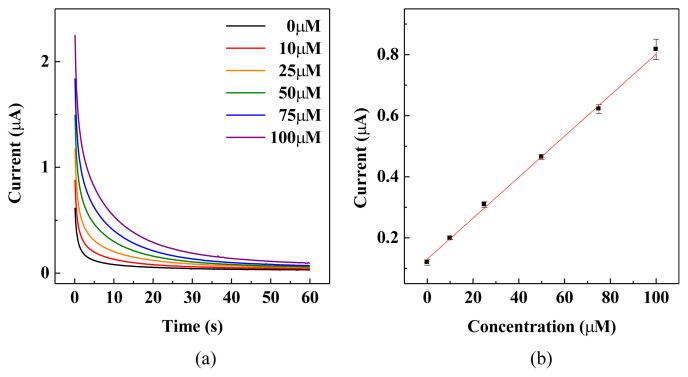
Amperometric method was employed to detect H2O2 concentration with applied potential of 300 mV using Pt-MWCNT/ C electrode strips. As shown in Fig. S3, a linear detection range could be established for H2O2 concentration from 1 to 15 mM. The sensitivity was determined to be 75.2 μA mM−1 cm−2 with coefficient of variation of 2.24%. A more sensitive linear detection range with sensitivity of 120.7 μA mM−1 cm−2 could also be obtained for H2O2 concentration lower than 1 mM. The limit of detection (LOD) of Pt-MWCNT/C electrode strip was studied by employing H2O2 concentration in the range of 0–100 μM. As shown in Fig. 6, a more sensitive linear detection range with sensitivity of 142.8 μA mM−1 cm−2 could be obtained using currents measured at 5 s. H2O2 concentration as low as 10 μM could be detected with a current of 0.2 μA.
Fig. 6.
H2O2 detection limit of Pt-MWCNT/C electrode strip. (a) i–t curve for concentration in the range of 0–100 μM. (b) calibration curve of H2O2 concentration 0–100 μM based on currents measured at 5 s.
Based on the established linear detection range, the as-prepared electrode strip was applied for measuring H2O2 level in green tea and pressed Tofu samples. Green tea has been recognized as a healthy drink because of its rich content of polyphenol chemical compounds, mainly the catechins. It has been reported that catechins will react with the dissolved oxygen to generate H2O2 and its production increases with pH. The presence of H2O2 also explains the observed bactericidal effect of green tea infusion [34]. Therefore, green tea was employed as a model solution for detecting its H2O2 level by the as-prepared electrode strip. However, the presence of rich polyphenolic compounds in green tea will also participate the redox reaction between H2O2 and Pt-MWCNT nanohybrid, as a consequence, significant interferences were observed in the obtained i–t curves (data not shown). Polyvinylpolypyrrolidone (PVPP) is well-known for its high polyphenolic compounds removal capacity [40]. Thus, all the green tea samples were pre-treated with PVPP powder to remove the polyphenolic compounds before H2O2 detection were carried out by amperometric method. As observed in the i–t curves of green tea solutions (Fig. S4), the current increased significantly after 1 h incubation. The result is consistent with previous observation that H2O2 will be generated with time in green tea solution [36]. As shown in Table 1, H2O2 level in green tea solution after 1 h incubation was determined to be about 703 μM. Two other colorimetric assay methods were also employed to assure the accuracy of H2O2 level detected by the as-prepared single-use electrode strip. H2O2 concentration of 530 μM was determined by HRP enzymatic method, while 749 μM was detected by FOX assay. These results show that the facile and fast amperometric method using as-prepared electrode strip can determine H2O2 level in green tea infusion with acceptable accuracy. Finally, the fast amperometric H2O2 detection method was also applied for detecting H2O2 residual level in pressed Tofu obtained from a local market. As shown in Table 2, the colorimetric methods (HRP and FOX assay) generate approximately 10% higher H2O2 level than our fast electrochemical method. The higher H2O2 level detected by the colorimetric methods could be ascribed to the fact that H2O2 level in the leaching solution of pressed Tofu was too high to be directly detected by the sensitive colorimetric method, as a consequence, several folds of dilution was employed before colorimetric tests were carried out. In contrast, sample solution could be directly employed without dilution for amperometric Pt-MWCNT/C sensor detection because the amperometric sensor demonstrated a linear detection range from 1 to 15 mM of H2O2 as shown in Fig. S3a. The sample dilution employed in colorimetric method may possibly resulted in overestimated H2O2 level as compared with that detected from undiluted sample by amperometric method. In addition to the advantage of facile and fast H2O2 detection, no tedious dilution is required for using the as-prepared disposable electrode strip for detection because a quite broad linear detection range could be established (up to 15 mM) as shown in Fig. S3.
Table 1.
H2O2 concentration in green tea solution detected by amperometric single-use electrode sensor strip and colorimetric methods.
| Test 1 | Test 2 | Test 3 | Mean | |||
|---|---|---|---|---|---|---|
| Methods | amperometric sensor strip | colorimetric FOX | methods ODA/HRP | |||
| μM | 724.2 | 700.1 | 687.6 | 703.9 | 530.2 | 749.1 |
Table 2.
H2O2 concentration detected in pressed Tofu sample in pH 7.4 PBS by amperometric single-use Pt-MWCNT/C electrode sensor strip and colorimetric methods.
| Test 1 (μM) | Test 2 (μM) | Test 3 (μM) | Mean (μM) | CV (%) | |
|---|---|---|---|---|---|
| Pt-MWCNT/C sensor strip | 862.3 | 901.2 | 893.1 | 885.5 | 2.31 |
| FOX method | 1168.7 | 1208.7 | 1161.7 | 1179.7 | 2.15 |
| ODA + HRP | 1015.6 | 950 | 1005.5 | 990.4 | 3.57 |
4. Conclusions
Pt-MWCNT nanohybrid was prepared to modify the surface of a disposable screen-printed carbon electrode for fast and sensitive detection of H2O2 by amperometric method. Pt-MWCNT modification not only lowered the applied potential to 300 mV but also generated a current approximately 100 fold higher than that of bare carbon electrode. The quality of as-prepared electrode strips was assured by its low coefficient of variation (<5%) on current measured at 5 s. The fast current response to H2O2 with applied 300 mV could generate 3 linear detection ranges with sensitivity decreased with H2O2 concentration. The sensitivity of linear detection range was 75.2, 120.7, and 142.8 μA mM−1 cm−2 for H2O2 concentration in the range of 1–15 mM, 0.1–1 mM, and 10–100 μM, respectively. The lowest H2O2 concentration could be measured by the as-prepared strip was 10 μM. This fast response, single-use H2O2 electrode strip was successfully applied to detect the H2O2 level in green tea infusion and pressed Tofu with results comparable to that obtained from FOX and HRP enzymatic colorimetric methods.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2017.08.005.
References
- 1. Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett. 2000;486:10–3. doi: 10.1016/s0014-5793(00)02197-9. [DOI] [PubMed] [Google Scholar]
- 2. Long LH, Evans PJ, Halliwell B. Hydrogen peroxide in human urine: implications for antioxidant defense and redox regulation. Biochem Biophys Res Commun. 1999;262:605–9. doi: 10.1006/bbrc.1999.1263. [DOI] [PubMed] [Google Scholar]
- 3. Yorek MA. The role of oxidative stress in diabetic vascular and neural disease. Free Radic Res. 2003;37:471–80. doi: 10.1080/1071576031000083161. [DOI] [PubMed] [Google Scholar]
- 4. Zhao B, Yan Y, Zhu L, Li X, Li G. An amperometric biosensor for the detection of hydrogen peroxide released from human breast cancer cells. Biosens Bioelectron. 2013;41:815–9. doi: 10.1016/j.bios.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 5. Faraci FM. Hydrogen peroxide: watery fuel for change in vascular biology. Arterioscler Thromb Vasc Biol. 2006;26:1931–3. doi: 10.1161/01.ATV.0000238355.56172.b3. [DOI] [PubMed] [Google Scholar]
- 6. Marks NE, Grandison AS, Lewis MJ. Use of hydrogen peroxide detection strips to determine the extent of pasteurization in whole milk. Int J Dairy Technol. 2001;54:20–2. [Google Scholar]
- 7. Notsu H, Tatsuma T, Fujishima A. Tyrosinase-modified boron-doped diamond electrodes for the determination of phenol derivatives. J Electroanal Chem. 2002;523:86–92. [Google Scholar]
- 8.Nikolelis DP, Krull U, Wang J, Mascini M, editors. Biosensors for direct monitoring of environmental pollutants in field. Kluwer Academic Publishers; 1998. pp. 220–6. [Google Scholar]
- 9. Zhang Y, Janyasupab M, Liu CW, Lin PY, Wang KW, Xu J, et al. Improvement of amperometric biosensor performance for H2O2 detection based on bimetallic PtM (M = Ru, Au, and Ir) nanoparticles. Int J Electrochem. 2012;2012:1–8. [Google Scholar]
- 10. Chiu MH, Kumar AS, Sornambikai S, Chen PY, Shih Y, Zen JM. Cosmetic hydrogen peroxide detection using nano bismuth species deposited built-in three-in-one screen-printed silver electrode. Int J Electrochem Sci. 2011;7:2352–65. [Google Scholar]
- 11. Demirkol O, Mehmetoglu AC, Qiang Z, Ercal N, Adams C. Impact of food disinfection on beneficial biothiol contents in strawberry. J Agric Food Chem. 2008;56:10414–21. doi: 10.1021/jf802209t. [DOI] [PubMed] [Google Scholar]
- 12. Campanella L, Giancola D, Gregori E, Tomassetti M. Determination of hydroperoxides in nonaqueous solvents or mixed solvents, using a biosensor with two antagonist enzymes operating in parallel. Sens Actuator B Chem. 2003;95:321–7. [Google Scholar]
- 13. Price D, Worsfold PJ, Fauzi R, Mantoura C. Hydrogen peroxide in the marine environment: cycling and methods of analysis. Trends Anal Chem. 1992;11:379–84. [Google Scholar]
- 14. Suzuki K, Namiki H. Proteolysis of fibrinogen deposits enables hydrogen peroxide-stimulated polymorphonuclear leukocytes to spread in an acidified environment. Eur J Pharmacol. 2009;609:140–7. doi: 10.1016/j.ejphar.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 15. Xuan J, Jiang LP, Zhu JJ. Nonenzymatic hydrogen peroxide sensor based on three-dimensional ordered macroporous gold film modified electrode. Chin J Anal Chem. 2010;38:513–6. [Google Scholar]
- 16. Jiang F, Yue R, Du Y, Xu J, Yang P. A one-pot ‘green’ synthesis of Pd decorated PEDOT nanospheres for nonenzymatic hydrogen peroxide sensing. Biosens Bioelectron. 2013;44:127–31. doi: 10.1016/j.bios.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 17. Chen W, Cai S, Ren Q-Q, Wen W, Zhao Y-D. Recent advances in electrochemical sensing for hydrogen peroxide: a review. Analyst. 2012;137:49–58. doi: 10.1039/c1an15738h. [DOI] [PubMed] [Google Scholar]
- 18. Chen S, Yuan R, Chai Y, Hu F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles a review. Microchim Acta. 2013;180:15–32. [Google Scholar]
- 19. Miscoria SA, Barrera GD, Rivas GA. Analytical performance of a glucose biosensor prepared by immobilization of glucose oxidase and different metals into a carbon paste electrode. Electroanalysis. 2002;14:981–7. [Google Scholar]
- 20. Zou Y, Xiang C, Sun LX, Xu F. Glucose biosensor based on electrodeposition of platinum nanoparticles onto carbon nanotubes and immobilizing enzyme with chitosan-SiO2 sol–gel. Biosens Bioelectron. 2008;23:1010–6. doi: 10.1016/j.bios.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 21. Britto PJ, Santhanam KSV, Ajayan PM. Carbon nanotube electrode for oxidation of dopamine. Bioelectrochem Bioenerg. 1996;4:121–5. [Google Scholar]
- 22. Liu M, Yangping Wen Y, Li D, Yue R, Xua J, He H. A stable sandwich-type amperometric biosensor based on poly(3,4-ethylenedioxythiophene)–single wall walled carbon nanotubes/ascorbate oxidase/nafion films for detection of L-ascorbic acid. Sens Actuators B. 2011;159:277–85. doi: 10.2116/analsci.27.477. [DOI] [PubMed] [Google Scholar]
- 23. Lu S, Wen Y, Bai L, Liu G, Chen Y, Du H, et al. pH-controlled voltammetric behaviors and detection of phytohormone 6-benzylaminopurine using MWCNT/GCE. J Electroanal Chem. 2015;750:89–99. [Google Scholar]
- 24. Wang J, Yang B, Zhang K, Bin D, Shiraishi Y, Yang P, et al. Highly sensitive electrochemical determination of Sunset Yellow based on the ultrafine Au-Pd and reduced graphene oxide nanocomposites. J Colloid Interface Sci. 2016;481:229–35. doi: 10.1016/j.jcis.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 25. Yao Y, Zhang L, Duan X, Xu J, Zhou W, Wen Y. Differential pulse striping voltammetric determination of molluscicide niclosamide using three different carbon nanomaterials modified electrodes. Electrochim Acta. 2014;127:86–94. [Google Scholar]
- 26. Laschi S, Bulukin E, Palchetti I, Cristea C, Mascini M. Disposable electrodes modified with multi-wall carbon nanotubes for biosensor applications. ITBM-RBM. 2008;29:202–7. [Google Scholar]
- 27. Joshi KA, Tang J, Haddon R, Wang J, Chen W, Mulchandani A. A disposable biosensor for organophosphorus nerve agents based on carbon nanotubes modified thick film strip electrode. Electroanalysis. 2005;17:54–8. [Google Scholar]
- 28. Nakano K, Obuchi E, Nanri M. Thermo-photocatalytic decomposition of acetaldehyde over Pt-TiO2/SiO2. Chem Eng Res Des. 2004;82:297–301. [Google Scholar]
- 29. Xu Y, Lin X. Selectively attaching Pt-nano-clusters to the open ends and defect sites on carbon nanotubes for electrochemical catalysis. Electrochim Acta. 2007;52:5140–9. [Google Scholar]
- 30. Yasumori I, Hirabayashi K. Homogeneous catalysis by Pt(II)-Sn(II) chloride complex. Part 1. – kinetics and mechanisms of the hydrogenations of acetylene and ethylene. Trans Faraday Soc. 1971;67:3283–96. [Google Scholar]
- 31. Wang C, Daimon H, Onodera T, Koda T, Sun S. A general approach to the size- and shape-controlled synthesis of platinum nanoparticles and their catalytic reduction of oxygen. Angew Chem Int Ed Engl. 2008;47:3588–91. doi: 10.1002/anie.200800073. [DOI] [PubMed] [Google Scholar]
- 32. Gontard LC, Chang LY, Hetherington CJD, Kirkland AI, Ozkaya D, Dunin-Borkowski RE. Aberration-corrected imaging of active sites on industrial catalyst nanoparticles. Angew Chem Int Ed Engl. 2007;46:3683–5. doi: 10.1002/anie.200604811. [DOI] [PubMed] [Google Scholar]
- 33. Song H, Kim F, Connor S, Somorjai GA, Yang P. Pt nanocrystals: shape control and Langmuir–Blodgett monolayer formation. J Phys Chem B. 2006;109:188–93. doi: 10.1021/jp0464775. [DOI] [PubMed] [Google Scholar]
- 34. Arakawa H, Maeda M, Okubo S, Shimamura T. Role of hydrogen peroxide in bactericidal action of catechin. Biol Pharm Bull. 2004;27:277–81. doi: 10.1248/bpb.27.277. [DOI] [PubMed] [Google Scholar]
- 35. Nakagawa H, Hasumi K, Woo JT, Nagai K, Wachi M. Generation of hydrogen peroxide primarily contributes to the induction of Fe(II)-dependent apoptosis in Jurkat cells by (−)-epigallocatechin gallate. Carcinogenesis. 2004;25:1567–74. doi: 10.1093/carcin/bgh168. [DOI] [PubMed] [Google Scholar]
- 36. Akagawa M, Shigemitsu T, Suyama K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverage under quasi-physiological conditions. Biosci Biotechnol Biochem. 2003;67:2632–40. doi: 10.1271/bbb.67.2632. [DOI] [PubMed] [Google Scholar]
- 37. Watanabe M, Uchida M, Motoo S. Preparation of highly dispersed Pt + Ru alloy clusters and the activity for the electrooxidation of methanol. J Electroanal Chem. 1987;229:395–406. [Google Scholar]
- 38. Gay CA, Gebicki JM. Perchloric acid enhances sensitivity and reproducibility of the ferric-xylenol orange peroxide assay. Anal Biochem. 2002;304:42–6. doi: 10.1006/abio.2001.5566. [DOI] [PubMed] [Google Scholar]
- 39. Bergmeyer H, Gawehn K, Grassl M. Methods of enzymatic analysis. 1974;1 [Google Scholar]
- 40. Laborde BD, Moine-Ledoux V, Richard T, Saucier CD, Dubourdieu D, Monti JP. PVPP-polyphenol complexes: a molecular approach. J Agric Food Chem. 2006;54:4383–9. doi: 10.1021/jf060427a. [DOI] [PubMed] [Google Scholar]