Abstract
Quantitative investigation and systematic studies of quercetin, total phenolics, flavonoids, antioxidants, antibacterial and antibiofilm or antibiofouling properties of methanolic extracts of onions obtained from six different varieties have been carried out to explore their relative merits in terms of biological activities of fresh and aging onions. Total phenolic content in the extracts was examined spectrophotometrically using Folin–Ciocalteau’s phenol reagent and total antioxidant activity was studied by FRAP and DPPH methods. In vitro antibacterial activity of the extracts was investigated on Gram-negative (Escherichia coli and Pseudomonas aeruginosa) and Gram-positive (Staphylococcus aureus and Bacillus cereus) respectively, by using a modified Kirby–Bauer disc diffusion method. Antibiofilm activity was tested by crystal violet assay. The best results against biofilm formation were observed for the extracts obtained from onions stored for three months. The total phenolic and antioxidant content found to be increased upon aging in all the six varieties; red skinned onion (Happyhong) showed the highest level of total phenolics (5110.07 ± 196.56 μg GAEg−1 FW) and total flavonoids (2254.00 ± 154.82 μg QEg−1 FW) after three months. The results showed that in all the varieties, quercetin content as well as biological activity increases with aging in the stored onions compared with the fresh ones.
Keywords: Antioxidant activity, Antibacterial activity, Deteriorated onion, Quercetin, Total phenolics
1. Introduction
Onion (Allium cepa L.) is one of the most important vegetable crops grown worldwide. During the last 10 years its production has increased by more than 25% of the total production [1]. In most countries, approximately 10% of the total annual harvest is either discarded or inadequately treated in the market as worthless onions because they fail to meet the quality standards required for marketing to the customers [2]. The current quality demand by customers and search for high quality levels lead to the rejection of even more numbers of onions as worthless during selection and calibration stages (irregular shape, injured parts, non-commercial sizes). Dealing with onion wastes from storage and processing industries has become a real and huge problem for the market suppliers. Firstly, it is neither suitable for cattle fodder because of its strong characteristic pungent smell, nor can be used as an organic fertilizer because of rapid development of phytopathogenic agents, such as Sclerotium cepivorum [3]. Therefore, the only option remains with their disposal is the landfill. And, the landfill disposal of onion waste results in high economic costs, as well as a bad impact on the environment. Furthermore, their removal by incineration does not seem appropriate because of high water content, rendering it expensive with additional fuel consumption, and environmental pollution with the release of carbon dioxide and other gases. Therefore, utilization of the worthless onions effectively becomes a challenging problem and a burning topic of waste reuse and environmental research. Moreover, if the recovery of valuable phytochemicals and production of novel compounds of nutraceutical and pharmaceutical importance from onion wastes are fruitful, the environmental problems could be successfully solved. Thus, the onion producers and breeders are interested in developing the alternative means for the valorization of onion wastes to promote its profitable usage, and its conversion to food grade products. Nowadays researchers are aiming to manage onion wastes in such a way that the undesirable leftover plant materials are eliminated and used for the production/isolation of some useful phytochemicals that may otherwise be lost with the waste. Ramos et al. (2006) carried out oxidation of onion waste consisting mainly dried onions and observed good antibacterial and antioxidant property by oxidized products [4]. Furthermore, high antimicrobial activity of the onion skin waste extracts has also been observed against bacteria Escherichia coli, Pseudomonas fluorescens and Bacillus cereus, and fungi Aspergillus niger, Trichoderma viride and Penicillium cyclopium [5]. Onion waste is reported to contain high content of phenolics and flavonoids, mainly quercetin which shows high antibacterial activity [6]. Recovering antioxidants from onion waste is very appealing in terms of low processing costs which allow its extensive use in the food industry [7]. Overall processing scheme for exploiting onion wastes has been proposed by Waldron [8].
Two flavonoid subgroups present in onions are anthocyanins, which impart a red/purple color to some varieties, and flavonols, such as quercetin and its derivatives, which play an important role in the production of yellow and brown compounds in the skins of many varieties [9]. In onions, quercetin aglycone accounts up to 10% of the total flavonoids, and the remaining is in the form of its glucosides. In addition, phenolics and polyphenols from skin and extracts from the edible part of onion (A. cepa L.) are also known for its antimicrobial and antibiofilm properties [10,11]. These compounds have increased the interest of the food and pharmaceutical industries in order to improve food stability against microbiological spoilage agents. The flavonol composition in deteriorated or aging onions which are either discarded in the landfills or stored under intermediate facilities (before final disposal) changes due to natural physiological processes as well as activities of parasitic microorganisms. Culled onions (A. cepa) are commonly fed to cattle. The safety of feeding depends upon acceptability, species susceptibility and toxic potential of the onions [11] as these might result in secondary organ damage or even death when onions are consumed in large quantities. Onion toxicities are consistently noted in animals that ingest onions more than 0.5% of their body weight at one time [12]. Spontaneous ingestion of onions (A. cepa) causes hemolytic anemia and methemoglobinemia, leading to cyanosis hemolytic anemia with the formation of Heinz bodies, and fatal consequences has been previously reported in cattle, sheep, dogs, cats, and horses [13]. The epidemiology of onion toxicosis differs among species. The clinical symptoms of onion toxicosis observed in the cattles include in appetence, staggering, abortion, onion odor of the breath and feces, elevated heart and respiratory rates, pale mucous membranes, jaundice, and elimination of brown urine [14]. Onion toxicosis in water buffalo causes pale mucous membranes, lethargy, constant vocalization, tenesmus, weakness, dyspnea, production of dark urine, and the presence of an onion odor in their breath [15]. It also causes gallbladder enlargement and secretion of clotted bile, and excretion of onion fragments with ruminal contents. Onions also have fungicidal and bactericidal properties that alter rumen microbial populations [16]. These activities, in conjunction with high water content, may alter patterns of ruminal digestion. One of the effective treatment recommendations for onion toxicosis is restriction of intake. Blood transfusion may be given to severely affected animals which may promote clinical recovery [17]. Administration of antibiotics to be taken orally may be beneficial in reducing ruminal anaerobic bacteria that promote formation of some oxidative substances [18]. Proper and systematic utilization of onion wastes will also help in protecting the small randomly roaming domestic animals, such as dogs, cats and other cattles from the toxicities arising from changes in the bioactive components which may bring fatal consequences. Therefore, it is important to utilize and study the content of onion waste produced during and after its storage period.
Here, in the present research work, our aim is to analyse the variations in chemical composition of onions during aging process under storage of certain period ranging between one to six months. The visible symptoms of chemical composition variation upon aging and deterioration begin to appear on the onion skin or surface which continue to progress until it becomes unsuitable for any domestic purpose. We found major differences in the patterns of contents of quercetin and its glucosides during aging. There was observed a major reduction in total flavonoids (Q + QMG + QDG), however, a drastic increase in the quercetin content was also observed. Further, we also evaluated the antibiofilm and antibacterial potential. This study hints for a plausible economic exploitation of aging and deteriorated otherwise worthless or waste onions in pharmaceutical and food industries due to its high quercetin content.
2. Methods and materials
2.1. Chemicals and microorganisms
All solvents used in this study were of high-performance liquid chromatography (HPLC) grade. Trifluoroacetic acid (extra-pure grade) was supplied by Alfa Aesar (Ward Hill, MA, USA). Quercetin-3,4′-O-diglucoside and quercetin-4′-O-monoglucoside were supplied by Polyphenols Laboratories AS (Sandnes, Norway). Gallic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,4,6-Tris (1-pyridyl)-5-triazine (TPTZ), ferric chloride, Folin Ciocalteu reagent and ampicillin used were purchased fromSigma–Aldrich (St. Louis, MO, USA), and 2,2-diphenyl-1-picrylhydrazyl from Wako Pure Chemicals (Osaka, Japan) were used for antioxidant assays. Stock solutions of quercetin (1 mg mL−1) and quercetin glucosides (4 mg mL−1) were prepared in methanol. All the solutions were stored at −20 °C and calibration standards were obtained by appropriate dilution of the stock solutions. The standard bacterial strains of the Gram negative bacteria E. coli (KCTC-1924), Pseudomonas aeruginosa (KCTC-2004), and of the Gram positive bacteria B. cereus (KCTC-1012) and Staphylococcus aureus (KCTC-1916) were obtained from the Korean Collection for Type Cultures (KCTC).
2.2. Sample storage and preparation
Onions were grown at the Bioenergy Crop Research Center, National Institute of Crop Science, Rural Development Administration (Muan, Republic of Korea). Six onion varieties selected for this study were two red skinned (Happyhong, Unijinara), two white skinned (Mokpo24ho, Mokpo21ho), and two yellow skinned (Marusino330, Sinsunhwang). Bulbs were harvested during April to May 2013 and dried in the field for 1 week, trimmed of leaves and roots, and subsequently stored at 4 ± 1 °C with relative humidity between 70 and 75% with proper ventilations. For each sampling eight onion bulbs were selected from storage and sampled according to its storage capacity and till get deteriorate.
2.3. Extraction of flavonoids
Flavonoids were extracted in triplicate according to the method of Bonaccorsi [19] with slight modifications. Eight onion bulbs were chopped into small pieces and mixed. Approximately 10 g of the chopped sample was left overnight in 100 mL of methanol at 4 °C. Then the methanol extract was separated and the residue was homogenized with a blender in 100 mL of methanol for 3 min, followed by stirring on a magnetic stirrer for 30 min. The slurry was centrifuged at 10,000 rpm for 30 min at 4 °C. The supernatant was removed, the residue was mixed with a new portion of methanol, and centrifugation was repeated. The combined methanolic fractions were evaporated on a rotary evaporator at 40 °C to approximately 8 mL and made up to 10 mL with methanol. The extracts were stored at −20 °C before analysis.
2.4. HPLC analysis
HPLC analysis of all extracts was carried out using an Agilent 1100 chromatograph (Agilent, Palo Alto, CA, USA) equipped with a solvent delivery system, an auto-sampler, a DAD detector set at 360 nm, and a ChemStation data acquisition system. Flavonoids were separated on a Zorbax Eclipse XDB C-18 column (250 mm × 4.6 mm) with particle size of 5 μm (Agilent, Santa Clara CA, USA) protected with a Phenomenex (USA) C18-type guard column. The column was maintained at 25 °C. The mobile phase consisted of 0.1% TFA in water (solvent A) and methanol (solvent B). A gradient elution program was set as follows: 0–10 min, 20% B; 10–15 min, 20–80% B; 15–22 min, 80–20% B. The flow rate was 0.8 mL min−1, and the injected volume was 10 μL. Quercetin flavonols were quantified through comparison with respective calibration curves.
2.5. Analysis of total flavonoids
The total flavonoids were determined by using a colorimetric method [20]. Methanolic extract (0.5 mL) was mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL of distilled water. The reaction mixture was kept for 30 min at room temperature and the absorbance was measured against blank at 415 nm with Shimadzu UV-1700 spectrophotometer. The calibration curve was made from quercetin solutions and results were expressed in terms of μg of quercetin equivalents per gram fresh weight (μg QEg−1 FW).
2.6. Analysis of total phenolics
The total phenolics were analysed spectrophotometrically, using Folin Ciocalteu calorimetric method [21] with some modification. Onion extract (100 μL) mixed with 2.9 mL distilled water in a test tube followed by the addition of 0.5 mL dilute Folin Ciocalteu reagent. Samples were mixed properly and allowed to stand for 15 min. Then 2 mL of 20% sodium carbonate aqueous solution was added. The reaction mixture was incubated at room temperature for 90 min and the absorbance was measured at 790 nm against the bank by using Shimadzu UV-1700 spectrophotometer. All results were expressed in terms of μg of gallic acid equivalents per gram fresh weight (GAEg−1 FW).
2.7. Analysis of total antioxidant activity
The total antioxidant activity of onion samples was estimated by using two standard assays of the Ferric Reducing Ability of Plasma (FRAP) and 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) suggested by Benzie and Strain [22] and Brand-Williams [23], respectively. Trolox was used as standard for both experiments. Results are expressed in terms of μmol Trolox equivalent per gram fresh weight (μmol TEg−1 FW).
2.7.1. FRAP assay
The FRAP assay was performed as described by Benzie and Strain [22] and was carried out with Shimadzu UV-1700 spectrophotometer. The experiment was conducted at 37 °C under low pH (3.6) with a blank sample in parallel. At low pH, complex of Fe3+ – TPTZ is reduced to Fe2+ – TPTZ form, with development of intense blue color at the wavelength maxima of 593 nm. The FRAP working regent was prepared freshly by mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ in 40 mM HCl, and 20mMFeCl3·H2O in the ratio of 10:1:1. For each assay, 2.90 mL of FRAP reagent and 100 μL of methanol extract were mixed. Absorbance of the reaction (incubated at 37 °C) was measured at 593 nm after 30 min.
2.7.2. DPPH assay
The DPPH assay was carried out according to method of Brand-Williams [23] with some modifications. The DPPH solution was prepared by dissolving 24 mg DPPH with 100 mL methanol and stored at −20 °C until used. The working solution was prepared by diluting the DPPH solution with methanol to obtain the absorbance in the range of 1.1 ± 0.02 units at 515 nm. The above diluted DPPH solution (950 μL) was mixed with 50 μL blank, standards or sample extract and diluted with methanol (1:1, v/v) and incubated for 20 min. The absorbance was measured at 515 nm against the bank by using Shimadzu UV-1700 spectrophotometer. All results were expressed in terms of μmol TEg−1 FW.
2.8. Antibacterial and antibiofilm activity
The antibacterial activities have been checked against Gram-negative E. coli and Gram-positive S. aureus by using a modified Kirby–Bauer disk diffusion method [24]. Aliquots of bacterial suspension (100 μL) were spread on Difco™ nutrient broth containing testing microorganism with optical density of 0.7 at 595 nm. Filter paper disks (8 mm) saturated with 20 μL of the onion extract and were placed in on the agar plate. The plates were incubated for 20–36 h at 37 °C, and then the zones of inhibition were measured.
2.8.1. Biofilm assay
To evaluate the biofilm formation, Microtiter Dish Biofilm Formation Assay has been used with some modification [25]. Overnight grown cultures were diluted 1:100 in fresh LB medium. Different concentrations of onion extract were added to the 96-well micro-titer plate and incubated at 37 °C for 24 h. Thereafter, the medium was removed and the wells were thoroughly washed with 1 × PBS and 100 μL of 0.1% (w/v) crystal violet was added and incubated for 20 min. The crystal violet was removed and washed thoroughly with 1 × PBS. For quantification of attached cells the crystal violet was solubilized in 30% acetic acid, and the absorbance was measured at 570 nm. Reduction of the biofilm was correlated with control, in the control samples (100% of biofilm formation and bacterial growth), the extract was replaced by methanol.
2.9. Statistical analysis
Results presented in graphs are the means ± standard deviations for 3 replicate samples. In chromatographic assays, each replicate solution was injected 2 times, and the average peak areas were used to calculate analyte concentrations. Differences between mean values were assessed by Student’s t test with a significance level of p < 0.05. All statistical calculations were made using OriginPro 8.1 software (OriginLab; Northampton, MA, USA).
3. Results and discussion
3.1. Quercetin and its glucosides
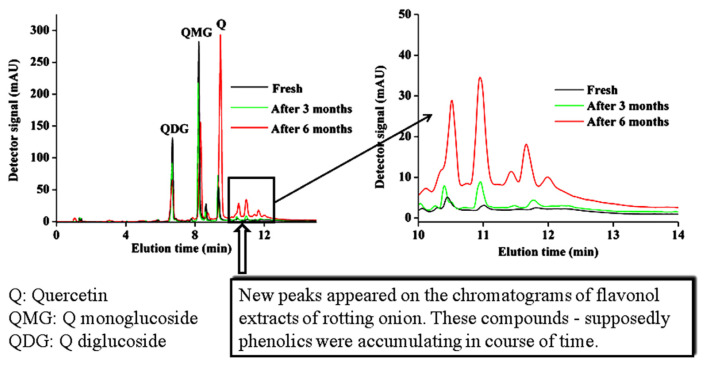
Depending upon varieties, the four major flavonoids in onions are quercetin aglycone (Q), quercetin-4′-O-monoglucoside (QMG), quercetin-3,4′-O-diglucoside (QDG), and anthocyanin. The corresponding changes in the quercetin and its glucosides before and after a certain storage period have been determined by HPLC. A significant difference in total quercetin estimated and its components between fresh and deteriorated onion samples (after six months) was observed. Total flavonoids (Q + QMG + QDG) content was found to be less in the fresh onions compared with the onions stored for three months. While, onions aged and deteriorated for six months, contain less total flavonoids. In general, the total flavonoids increased in all the six varieties aged for three months, except for the white varieties which contain components below the detection level (Table 1). For instance, total flavonoids content in the red variety (Happyhong) increases from 0.610 μmol g−1 FW to 0.689 μmol g−1 FW, and thereafter, declines to 0.530 μmol g−1 FW. A similar trend was observed in the case of yellow variety. QMG and QDG levels were found to decrease ~92% and 98% after six months, whereas quercetin level was found to increase ~97% in red and yellow varieties (Table 1). Biochemistry for the deteriorations of yellow ‘Marusino330’ variety has been shown in Fig. 1. It has been reported that the metabolic processes are affected by external factors, such as temperature, humidity, light, etc., and result in the change in chemical composition of onions [26,27]. Earlier, it has been reported that the biological pathogens play important role in the chemical composition of onions [28,29]. Decomposition of onion tissues has two consequences: decrease of the number of quercetin producing cells and release of enzymes, such as peroxidase and hydrolase from onion tissues. The presence of free enzymes explains the drop in quercetin conjugates, QMG found in trace amounts and QDG found to be nil after six months. The deterioration process of onions can be characterized by progressive hydrolysis of quercetin glucosides and subsequent intensification of catabolic reactions.
Table 1.
Change in flavonoid content (μmol g−1 FW) of fresh, three months and six months stored onion from six different varieties, all the values are mean ± SD, n = 3; SD: Standard deviation, FW: Fresh weight.
| Onion varieties | Storage months | Q | QMG | QDG | Total |
|---|---|---|---|---|---|
| Marusino330 | Fresh | 0.017 ± 0.00ab | 0.258 ± 0.02bc | 0.457 ± 0.03ac | 0.732 |
| After 3 months | 0.013 ± 0.00a | 0.338 ± 0.03ab | 0.573 ± 0.04bc | 0.924 | |
| After 6 months | 0.641 ± 0.02ac | 0.016 ± 0.00ac | 0.009 ± 0.00ac | 0.666 | |
| Sinsunhwang | Fresh | 0.012 ± 0.00ab | 0.158 ± 0.02bc | 0.357 ± 0.03ab | 0.527 |
| After 3 months | 0.014 ± 0.00ac | 0.238 ± 0.03ab | 0.463 ± 0.04ac | 0.715 | |
| After 6 months | 0.500 ± 0.02ab | 0.012 ± 0.00bc | 0.007 ± 0.00c | 0.519 | |
| Happyhong | Fresh | 0.015 ± 0.00c | 0.188 ± 0.02a | 0.407 ± 0.03ab | 0.610 |
| After 3 months | 0.013 ± 0.00bc | 0.233 ± 0.03c | 0.443 ± 0.04ab | 0.689 | |
| After 6 months | 0.509 ± 0.02c | 0.014 ± 0.00ac | 0.007 ± 0.00bc | 0.530 | |
| Unijinara | Fresh | 0.017 ± 0.00a | 0.208 ± 0.02ab | 0.392 ± 0.03ac | 0.657 |
| After 3 months | 0.013 ± 0.00b | 0.229 ± 0.03ac | 0.413 ± 0.04ab | 0.655 | |
| After 6 months | 0.488 ± 0.02ab | 0.011 ± 0.00ab | 0.008 ± 0.00c | 0.507 | |
| Mokpo24ho | Fresh | 0.012 ± 0.00ac | 0.020 ± 0.00ac | nd | 0.032 |
| After 3 months | 0.013 ± 0.00ab | 0.012 ± 0.00ab | nd | 0.025 | |
| After 6 months | 0.024 ± 0.00a | 0.003 ± 0.00a | nd | 0.027 | |
| Mokpo21ho | Fresh | 0.016 ± 0.00ab | 0.030 ± 0.00ab | nd | 0.046 |
| After 3 months | 0.012 ± 0.00ab | 0.024 ± 0.00bc | nd | 0.036 | |
| After 6 months | 0.028 ± 0.00c | 0.004 ± 0.00bc | nd | 0.032 |
Abbreviations: Q = Quercetin; QMG = Quercetin-4′-O-monoglucoside; QDG = Quercetin-3,4′-O-diglucoside; Total = Q + QMG + QDG, nd = not detected.
Results represent mean ± SD of three independent experiments.
Different letters in the same column indicate significant differences (ANOVA followed by the Dunn–Sidak’s multiple range tests at p = 0.05).
Fig. 1.
HPLC chromatograms represent the biochemistry for the deteriorations of yellow onion variety.
3.2. Total flavonoid content
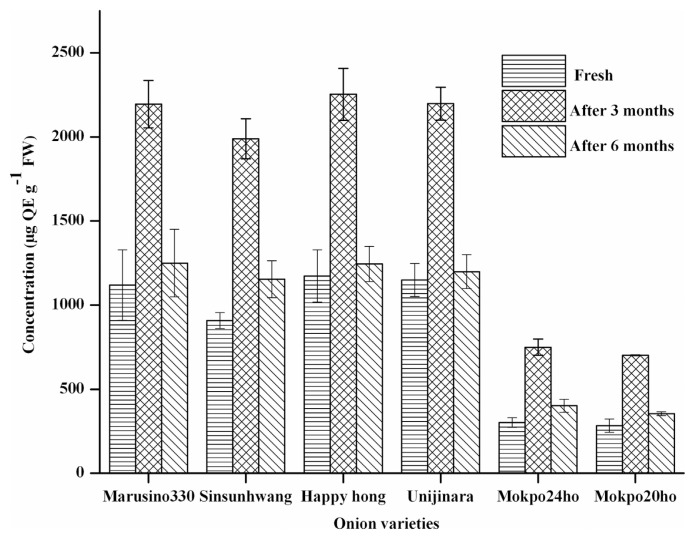
The total flavonoids content examined in six onion varieties was found to vary in the range of 283.69 ± 40.09 to 2274.67 ± 155.21 μg QEg−1 FW, and results are shown in Fig. 2. The occurrence of total flavonoid content was in the following order: red skinned (Happyhong, Unijinara) > yellow-skinned (Marusino330, Sinsunhwang) > white-skinned (Mokpo24ho, Mokpo21ho). After aging for three months under storage, the total flavonoids content was observed to increase, and later, in aged and deteriorated onions under storage for six months, was observed to decrease. This indicates that some flavonoids were destroyed probably. One of the most probable reasons for the decrease in total flavonoids content could be due to degradation of flavonoids which depends on the structure of particular flavonoids. In most of the fruits and vegetables, flavonoids contain C-glycosidic bonds and exist as dimers, oligomers, and results in the formation of monomers by the hydrolysis of the same [30]. Olsson [31] reported that neither storage nor heat treatment causes any significant difference in the total flavonol content in sweet as well as in red onion cultivars.
Fig. 2.
Change in total flavonoids content of fresh, three months and six months stored onion from six different varieties (mean ± SD, n = 3), abcvalues with same superscripts within the same variety indicate no significant difference (P = 0.05).
3.3. Total phenolic content
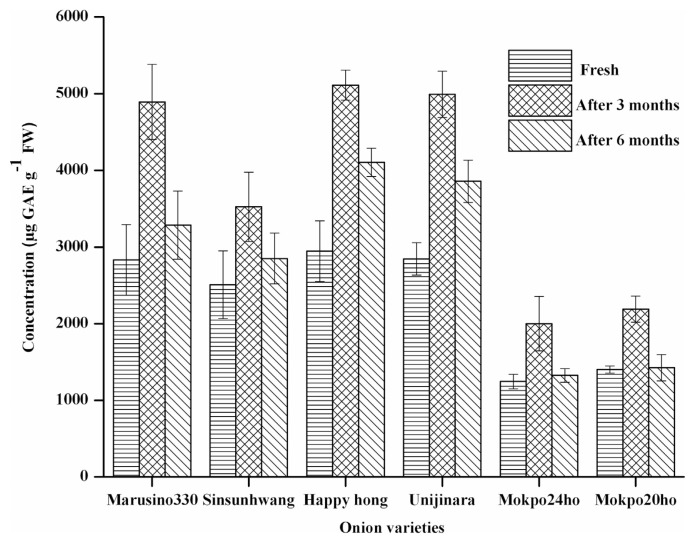
The total phenolic content in the six onion varieties during different time intervals of storage for six months is presented in Fig. 3. Among the different varieties, the overall concentration of total phenolics vary from 1245.22 ± 95.44 to 2945.58 ± 394.54 μg GAE g−1 FW in fresh samples. In each variety, the total phenolic content was found to increase significantly in the first three months of storage. In this period, the total phenolic content in red variety (Happyhong) was found to increase from 2945.58 ± 394.54 to 5510.02 ± 196.55 μg GAE g−1 FW; in white variety (Mokpo24ho), it increased from 1245.22 ± 95.44 to 2000.33 ± 356.47 μgGAE g−1 FW; and in yellow variety (Marusino330) it increased from 2830.54 ± 461.21 to 4892.22 ± 490.03 μgGAE g−1 FW. For all onion varieties, the total phenolic content in aged and deteriorated onions under storage for six months was found to decrease. Onions under storage is accompanied by many physiological and chemical changes which include change in the amount of reducing sugars, rate of respiration, water loss, and change in glycoalkaloid content [32]. The following changes were observed not only detrimental to the quality of onions, but also, resulting in the formation of new compounds [33]. It can be seen in Fig. 1 that new peaks appeared in the chromatograms of flavonol extracts of aged and deteriorated onions. These compounds could be phenolics which were accumulating during the course of time under storage.
Fig. 3.
Change in total phenolic content of fresh, three months and six months stored onion from six different varieties (mean ± SD, n = 3), abcvalues with same superscripts within the same variety indicate no significant difference (P = 0.05).
3.4. Total antioxidant activities
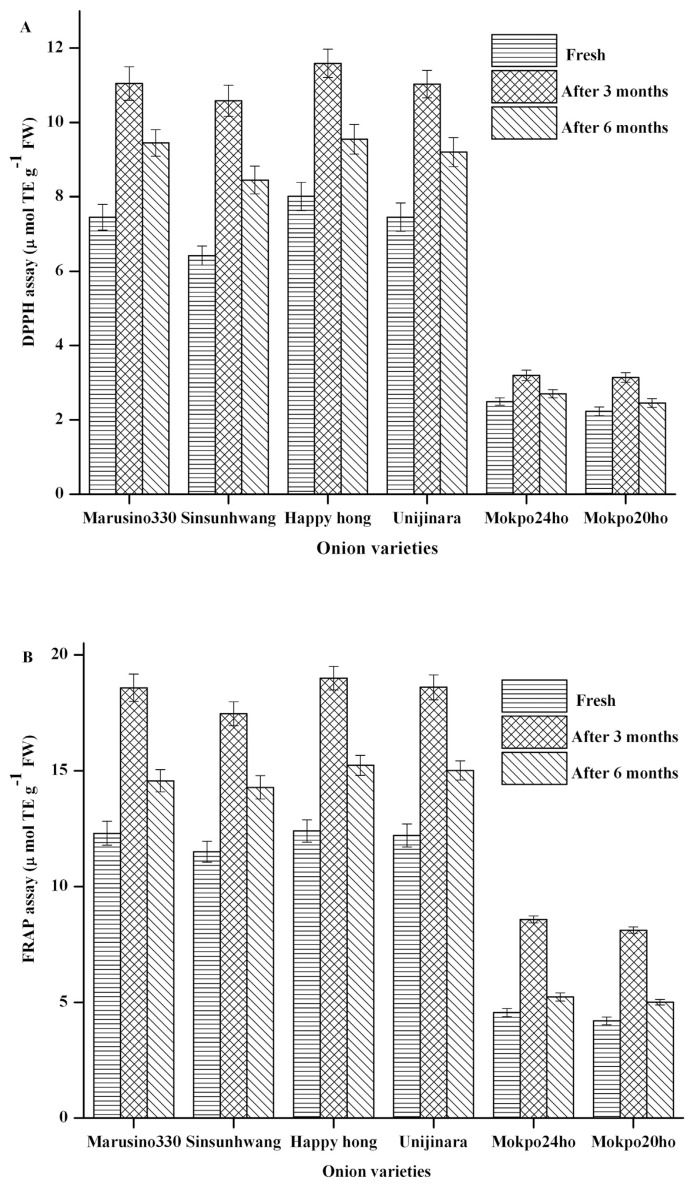
The antioxidant activities of the six onion varieties in the present study were evaluated by DPPH and FRAP assay methods, and the results are presented in Fig. 4A and B. The DPPH and FRAP values for all the onion varieties were found to increase after three months. Red onion (Happyhong) shows the highest antioxidant activity followed by yellow (Marusino330) and white onion (Mokpo24ho). The antioxidant activity of red onion (Happyhong) increased from 8.01 ± 0.38 to 11.59 ± 0.38 μmol TE g−1 FW for DPPH, and 12.40 ± 0.48 to 18.99 ± 0.51 μmol TE g−1 FW for FRAP in the onions stored for three months. Similarly, for white onion (Mokpo24ho), the increase was found to be varying between 2.49 ± 0.10 to 3.2 ± 0.14 μmol TEg−1 FW for DPPH and 4.56 ± 0.18 to 8.58 ± 0.15 μmol TEg−1 FW for FRAP. The FRAP values for the white onion were found to be consistent with the results obtained for Calcot and white onion [34]. The total antioxidant activity for all the six varieties was found to be continuously declining during the entire storage period of last three months. The decrease in the antioxidant activity is believed to be either due to the loss of antioxidants or formation of compounds possessing pro oxidant activity. Furthermore, the alterations in the structure of the existing antioxidants, as well as formation of novel antioxidant components might have enhanced the initial antioxidant status. Similar results have been reported from other studies [35,36].
Fig. 4.
Change in antioxidant activity of fresh, three months and six months stored onion from six different varieties A) DPPH, B) FRAP (mean ± SD, n = 3), abcvalues with same superscripts within the same variety indicate no significant difference (P = 0.05).
3.5. Antibacterial and antibiofilm activity
The antimicrobial and antibiofilm assays of extracts obtained from fresh onion varieties showed moderate activities compared with the extracts from aged onions stored for three months. The results of antibacterial and antibiofilm activity tests are summarized in Table 2. The antibacterial activity of red variety of A. cepa extract was found to be higher compared with yellow and white varieties. Extract of red onion (Happyhong) obtained after three month of storage showed significantly stronger inhibitory effects against four tested bacterial strains compared to yellow variety. However, extracts obtained from white variety showed negligible activity. Onion extract obtained from the red variety also showed potential antibiofilm activity along with antimicrobial activity. Important factors regulating antimicrobial and antibiofilm activities are believed to be due to the presence of different levels of bioactive compounds. Previous reports suggested that the red onion contains higher amounts of total phenolic acids which are responsible for antioxidant activity [37]. Jeong et al. (2009) reported that the high antioxidant and anticancer activities of the red onion extracts were due to greater amounts of total phenolics and quercetin compared with white and yellow varieties [38]. Furthermore, it was also reported that the unutilized outer layers of the red variety contain high amounts of quercetin and other bioactive compounds exhibiting high AOA and FRSA along with substantial protection against DNA damage caused by free radicals [39]. Gram positive bacteria were found to be relatively sensitive to the onion extracts and flavonol standards while gram negative bacteria were observed to be moderately resistant. The highest zone of inhibition for gram positive bacteria was observed to be 13.5 ± 0.9mm for the red onion extract (Happyhong), whereas for the yellow onion extract (Sinsunhwang) was 11.3 ± 0.7 mm. On the other hand, for gram negative bacteria, the inhibition zone was found to be relatively smaller. White onion extracts exhibited weak or no inhibition activities against both, gram positive as well as gram negative bacteria. The zone of inhibition was observed to be decreased for the aged and deteriorated onions investigated after six months of storage. Except for the red onions, the extracts obtained from all other onion varieties including the inner edible part, showed low or no inhibition activities. Apparently, after six month of storage the bioactive compounds were found to be decreased in onions, which resulted in a relatively smaller inhibition zone for all the tested bacterial strains. For comparison, antibacterial activity tests of ampicillin with 100 μg mL−1 were performed. Ampicillin at this concentration showed inhibition against E. coli, S. aureus and B. cereus with formation of inhibition zones, viz., 20.0 ± 1.2, 22.0 ± 0.9 and 21.0 ± 0.8, respectively. Onions are known as a good natural source of flavonoids mainly occurring in their glycosidic form. Red and yellow onions are considered as one of the best natural sources of quercetin, a bioflavonoid, best known for its scavenging activity against free radicals. Besides antioxidant properties, it also possesses anti-fungal, antibacterial, and anti-inflammatory properties. In addition to quercetin, onion produces allicin, which is sulfur containing volatile compound and exists in intact onions. The antibacterial activity of onion is mostly due to the allicin (thiosulfinates) [40]. However, these volatile compounds have limited use regarding food preservation because of their strong flavor and biochemical instability [41]. The antimicrobial activity of onion extracts against the spoilage and pathogenic microbes causing food poisoning has been studied by Ref. [42]. Quercetin exhibits higher inhibition activity than kaempferol. In the disc diffusion assay, quercetin exhibits strong inhibitory action against all the bacterial strains involved in this study, whereas kaempferol was found to be effective only against the gram positive bacteria, viz., S. aureus and Micrococcus luteus. The study of Santas et al. (2010) confirmed the presence of flavonoids in crude onion extracts and observed to be efficient inhibitors of gram positive bacteria, while the gram negative bacteria were relatively more resistant [42]. The flavonoids in the onions not increase the organoleptic quality of food, but also enhance the stability and preservation of food systems.
Table 2.
Change in antibacterial activity of fresh, three months and six months stored onion extract (1 mg mL−1) from six different varieties.
| Onion varieties | Storage months | Diameter of growth inhibition zone (mm) | |||
|---|---|---|---|---|---|
|
| |||||
| Gram-negative | Gram-positive | ||||
|
|
|
||||
| E. coli | P. aeruginosa | S. aureus | B. cereus | ||
| Marusino330 | Fresh | 8.0 ± 0.6bc | nd | 10.0 ± 0.8bc | 10.0 ± 0.8ac |
| After 3 months | 9.0 ± 0.8ab | nd | 11.2 ± 0.7ac | 11.2 ± 0.7ab | |
| After 6 months | 6.0 ± 0.3ac | nd | 8.0 ± 0.6ab | 8.0 ± 0.6abc | |
| Sinsunhwang | Fresh | 7.0 ± 0.6a | nd | 10.2 ± 0.6b | 10.0 ± 0.8ab |
| After 3 months | 7.8 ± 0.5bc | nd | 11.3 ± 0.7ab | 11.2 ± 0.7b | |
| After 6 months | 5.6 ± 0.4c | nd | nd | 8.0 ± 0.6ac | |
| Happyhong | Fresh | 10.2 ± 0.6ab | 10.2 ± 0.6ab | 12.2 ± 0.9c | 10.0 ± 0.8bc |
| After 3 months | 11.5 ± 0.7ac | 11.5 ± 0.7ac | 13.3 ± 0.8ab | 11.2 ± 0.7ab | |
| After 6 months | 8.0 ± 0.5ab | 8.0 ± 0.5a | 10.0 ± 0.8ac | 8.0 ± 0.6ab | |
| Unijinara | Fresh | 11.5 ± 0.8ac | 10.2 ± 0.6bc | 13.2 ± 0.6ab | 10.0 ± 0.6ab |
| After 3 months | 11.8 ± 0.9c | 11.5 ± 0.7ac | 13.5 ± 0.9ac | 11.2 ± 0.4bc | |
| After 6 months | 9.0 ± 0.8ab | 8.0 ± 0.5ac | 10.0 ± 0.6c | 8.0 ± 0.6ac | |
| Mokpo24ho | Fresh | nd | nd | nd | nd |
| After 3 months | nd | nd | nd | nd | |
| After 6 months | nd | nd | nd | nd | |
| Mokpo21ho | Fresh | nd | nd | nd | nd |
| After 3 months | nd | nd | nd | nd | |
| After 6 months | nd | nd | nd | nd | |
| Standard | Ampicillin | 20.0 ± 1.2 ab | nd | 22.0 ± 0.9 bc | 21.0 ± 0.8 bc |
Results represent mean ± SD of three independent experiments.
Different letters in the same column indicate significant differences (ANOVA followed by the Dunn–Sidak’s multiple range tests at p = 0.05).
The potential use of onion wastes as a source of bioactive phenolic compounds that also show antimicrobial activity is therefore, promising. And, this should be further studied in connection with exploring the detailed chemical compositions of the extracts. At present, the data regarding detailed profiling of chemical composition of fresh and aged onions complementing with the corresponding antimicrobial activities are scanty or insufficient.
3.5.1. Antibiofilm activity
Extracts from different medicinal plants have been reported to target the formation of biofilms formed by S. aureus and P. aeruginosa with antimicrobial compounds, such as quercetin, tannic acid, kaempferol, and luteolin [7], ellagic acid [43]. Onion extracts were screened for their ability to inhibit the formation of biofilms formed by S. aureus and P. aeruginosa without affecting the cell growth. The onion extracts showed marked activity against S. aureus and P. aeruginosa biofilms. At a concentration of 50 μg mL−1, onion extracts obtained from red (Happyhong) and yellow (Marusino330) varieties were observed to inhibit the biofilm formation by S. aureus and P. aeruginosa without inhibiting bacterial growth. At the concentration of 3.1 μg mL−1, no inhibitory activity was observed. To our knowledge, this study reports for the first time the inhibitory activity of onion extracts by S. aureus and P. aeruginosa biofilm formation. The percentage inhibition of biofilm formation against the tested bacteria is shown in Table 3. The highest antibiofilm activity was observed in the case of extracts obtained from aged onions stored for three months. At the concentration of 50 μg/mL, extract of red onion showed the reduction rate of 59.5% and 61.5% against S. aureus and P. aeruginosa. Activity of the yellow onion extract was found to be quite promising compared with white onion. The aged and deteriorated yellow and white onions did not show any inhibition against the biofilm formation. In this study, standard quercetin exhibited inhibition of 80% of the biofilm of this bacterium at a concentration of 50 μg mL−1. In the present study, it can be thus concluded that the mechanism behind biofilm inhibition did not involve bacterial death, but instead, stimulated bacterial cell growth. However, in few cases, such as standard quercetin, the inhibitory effect is due to bacterial growth inhibition. Except quercetin, onion extract contains many phenolic compounds that affect the biofilm formation of this bacterium.
Table 3.
Biofilm formations and bacterial growth of P. aeruginosa and S. aureus for fresh, three months and six months stored onion extract (50 μg mL−1) from six different varieties.
| Onion varieties | Storage months | Gram-negative | Gram-positive | ||
|---|---|---|---|---|---|
|
| |||||
| P. aeruginosa | S. aureus | ||||
|
|
|
||||
| Biofilm formation (%) | Bacterial growth (%) | Biofilm formation (%) | Bacterial growth (%) | ||
| Marusino330 | Fresh | 50.1 ± 2.6ab | 240.1 ± 12.6abc | 50.1 ± 2.6ab | 231.0 ± 17.2ac |
| After 3 months | 45.5 ± 4.8bc | 242.5 ± 14.2ab | 45.5 ± 3.2ac | 211.2 ± 13.2ad | |
| After 6 months | 70.2 ± 8.3cb | 270.2 ± 18.4bc | 60.2 ± 5.3ab | 225.0 ± 16.8ab | |
| Sinsunhwang | Fresh | 55.2 ± 1.8ab | 281.1 ± 14.3cd | 52.1 ± 5.3c | 230.0 ± 14.5b |
| After 3 months | 50.8 ± 4.8bc | 270.5 ± 14.9ad | 48.5 ± 2.9ac | 250.2 ± 16.8bc | |
| After 6 months | 72.7 ± 8.3acd | 290.2 ± 18.3ab | 70.2 ± 8.3ad | 238.0 ± 20.1cd | |
| Happyhong | Fresh | 40.1 ± 3.6d | 210.1 ± 15.3bc | 42.1 ± 4.2ab | 226.3 ± 15.3ab |
| After 3 months | 38.5 ± 2.2cb | 232.5 ± 14.8a | 40.5 ± 3.2b | 253.2 ± 16.3ac | |
| After 6 months | 60.2 ± 3.1ac | 255.2 ± 16.5b | 60.8 ± 8.4b | 273.2 ± 13.2ab | |
| Unijinara | Fresh | 45.5 ± 2.4ad | 230.1 ± 15.4ac | 41.1 ± 2.1ac | 235.0 ± 15.2b |
| After 3 months | 42.6 ± 1.8ab | 242.5 ± 18.8bc | 41.5 ± 6.2ad | 301.2 ± 14.3ab | |
| After 6 months | 62.4 ± 1.6ac | 260.2 ± 15.3ad | 70.2 ± 5.7ab | 241.6 ± 16.3ab | |
| Standard | Quercetin (50 μg mL−1) | 20.0 ± 1.0 ac | 59.0 ± 3.9 ad | 23.0 ± 1.2 c | 61.0 ± 4.8 a |
Results represent mean ± SD of three independent experiments.
Different letters in the same column indicate significant differences (ANOVA followed by the Dunn–Sidak’s multiple range tests at p = 0.05).
4. Conclusions
In the present research work, we have studied six onion varieties, namely red skinned (Happyhong, Unijinara), yellow skinned (Marusino330, Sinsunhwang) and white skinned (Mokpo24ho, Mokpo21ho) exploring the various aspects of bioactive compounds in terms of total flavonoids, phenolics, antioxidants and their antimicrobial activity with respect to duration of storage under specified conditions (fresh, after three and six months of storage). In all the onion varieties, the total phenolics, total flavonoids and antioxidant activities were found to increase after three months of storage. In addition, the change in the quantity of individual flavonoids, such as quercetin and its glucoside form was found to be noticeably significant. The total flavonoids (Q + QMG + QDG) were estimated to be highest after three months of storage. However, aged and deteriorated onions after prolonged storage contain high quercetin and negligible amount of glucosides. Red onion extract showed potential antimicrobial and antibiofilm activity, while the yellow onions exhibited moderate effect and the white one showed highly diminished or negligible effects. Our findings reveal that the opportunities associated with the utilization of deteriorated onions not only explores an economic source of quercetin, but also, a good source for isolating value added bioactive compounds including pure quercetin for developing nutraceutical/pharmaceutical products.
Acknowledgment
This research was supported by the Nano Material Technology Development Program of the Korean National Research Foundation (NRF) funded by Korean Ministry of Education, Science, and Technology (2012M3A7B4049675). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by Priority Research Centers Program (2014R1A6A1031189).
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2017.06.009.
Funding Statement
This research was supported by the Nano Material Technology Development Program of the Korean National Research Foundation (NRF) funded by Korean Ministry of Education, Science, and Technology (2012M3A7B4049675). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by Priority Research Centers Program (2014R1A6A1031189).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.FAO, Food and Agricultural Organization. Area and production data. [access date 01 Dec 2009]. www.fao.org .
- 2.MAFF, The Ministry of Agriculture, Forestry and Fishery of Japan. Planted area, production and shipment of vegetables. 2001. [Google Scholar]
- 3. Schieber A, Stintzing FC, Carle R. By-products of plant food processing as a source of functional compounds – recent developments. Trends Food Sci Technol. 2001;12:401–13. [Google Scholar]
- 4. Ramos Freddy A, Takaishi Y, Shirotori M, Kawaguchi Y, Tsuchiya K, Shibata H, et al. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) skin. J Agric Food Chem. 2006;54(10):3551–7. doi: 10.1021/jf060251c. [DOI] [PubMed] [Google Scholar]
- 5. Ahiabor C, Gordon A, Ayittey K, Agyare R. In vitro assessment of antibacterial activity of crude extracts of onion (Allium cepa L.) and shallot (Allium aescalonicum L.) on isolates of Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and Salmonella typhi (ATCC 19430) IJAR. 2016;2(5):1029–32. [Google Scholar]
- 6. Jonathan Santas, Almajano MP, Carbó R. Antimicrobial and antioxidant activity of crude onion (Allium cepa L.) extracts. Inter J Food Sci Technol. 2010;45(2):403–9. [Google Scholar]
- 7. Singh V, Krishan P, Shri R. Extraction of antioxidant phytoconstituents from onion waste. J Pharmacogn Phytochem. 2017;6(1):502–5. [Google Scholar]
- 8. Waldron K. Useful ingredients from onion waste. Food Sci Technol. 2001;15:38–41. [Google Scholar]
- 9. Downes K, Chope GA, Terry LA. Postharvest application of ethylene and 1-methylcyclopropene either before or after curing affects onion (Allium cepa L.) bulb quality during long term cold storage. Postharvest Biol Technol. 2010;55:36–44. [Google Scholar]
- 10. Škerget M, Majhenič L, Bezjak M, Knez Ž. Antioxidant, radical scavenging and antimicrobial activities of red onion (Allium cepa L.) skin and edible part extracts. Chem Biochem Eng Q. 2009;23(4):435–44. [Google Scholar]
- 11. Lee JH, Park JH, Cho HS, Joo SW, Cho MH, Lee J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling. 2013;29(5):491–9. doi: 10.1080/08927014.2013.788692. [DOI] [PubMed] [Google Scholar]
- 12. Cope RB. Allium species poisoning in dogs and cats. Vet Med. 2005;100:562–6. [Google Scholar]
- 13. Aslani MR, Mohri M, Movassaghi AR. Heinz body anaemia associated with onion (Allium cepa) toxicosis in a flock of sheep. Comp Clin Pathol. 2005;14:118–20. [Google Scholar]
- 14. Rae HA. Onion toxicosis in a herd of beef cows. Can Vet J. 1999;40:55–7. [PMC free article] [PubMed] [Google Scholar]
- 15. Vanessa B, Lucioli J, Fernando Henrique F, Patricia Giovana H, Juliano Fleck R, Sandra Davi T, et al. Fatal onion (Allium cepa) toxicosis in water buffalo (Bubalus bubalis) J Vet Diagn Investig. 2009;21(3):402–5. doi: 10.1177/104063870902100321. [DOI] [PubMed] [Google Scholar]
- 16. Mazen MB, Mottelib AA, Amer AA, Abdel-Salam MN. Studies on fungal flora of ruminal juice of sheep gained after fed with certain field stubbles. Assuit Vet Med J. 1984;12:87–93. [Google Scholar]
- 17. Fredrickson EL, Estell RE, Havstad KM. Potential toxicity and feed value of onions for sheep. Livest Prod Sci. 1995;42:45–54. [Google Scholar]
- 18. Selim HM, Yamato O, Tajima M, Maede Y. Rumen bacteria are involved in the onset of onion induced hemolytic anemia in sheep. J Vet Med Sci. 1999;61:369–74. doi: 10.1292/jvms.61.369. [DOI] [PubMed] [Google Scholar]
- 19. Bonaccorsi P, Caristi C, Gargiulli C, Leuzzi U. Flavonol glucoside profile of southern Italian red onion Allium cepa L. J Agric Food Chem. 2005;53:2733–40. doi: 10.1021/jf048152r. [DOI] [PubMed] [Google Scholar]
- 20. Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoids content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 21. Dalamu Kaur C, Singh M, Walia S, Joshi S, Munshi AD. Variations in phenolics and antioxidants in Indian onions (Allium cepa L.) genotype selection for breeding. Nutr Food Sci. 2010;40:6–19. [Google Scholar]
- 22. Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 23. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss U Technol. 1995;28:25–30. [Google Scholar]
- 24. Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 25. George A. Microtiter dish biofilm formation assay. J Vis Exp. 2011;47 doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benkeblia N. Phenylalanine ammonia-lyase, peroxidase, pyruvic acid and total phenolics variation in onion bulbs during long-term storage. Lebensm Wiss U Technol. 2000;33:112–6. [Google Scholar]
- 27. Benkeblia N, Selselet-Attou G. Effects of low temperatures on the changes in oligosaccharides, phenolics and peroxidase in inner bud of onion (Allium cepa L.) during break of dormancy. Acta Agric Scand Sect B Soil Plant Sci. 1999;49:98–102. [Google Scholar]
- 28. Lee JT, Bae DW, Park SH, Shim CK, Kwak YS, Kim HK. Occurrence and biological control of postharvest decay in onion caused by fungi. Plant Pathol J. 2001;17:141–8. [Google Scholar]
- 29.Mark GL, Gitaitis RD, Lorbeer JW. Bacterial diseases of onion. In: Rabinovich HD, Currah L, editors. Allium crop science: recent advances. Wallingford, UK: CAB International; 2002. [Google Scholar]
- 30. Manach C, Scalbert A, Morand C. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 31. Olsson ME, Gustavsson KE, Våsgen IM. Quercetin and isorhamnetin in sweet and red cultivars of onion (Allium cepa L.) at harvest, after field curing, heat treatment, and storage. J Agric Food Chem. 2010;58:2323–30. doi: 10.1021/jf9027014. [DOI] [PubMed] [Google Scholar]
- 32.Burton WG. The potato. 3rd ed. Essex: Longman Scientific and Technical; 1989. pp. 470–504. [Google Scholar]
- 33. Chope GA, Terry LA, White PJ. The effect of the transition between controlled atmosphere and regular atmosphere storage on bulbs of onion cultivars SS1, Carlos and Renate. Postharvest Biol Technol. 1989;44:228–39. [Google Scholar]
- 34. Santas J, Carbó R, Gordon MH, Almajano MP. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008;107:1210–6. [Google Scholar]
- 35. Nicoli MC, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetable. Trends Food Sci Technol. 1999;10:94–100. [Google Scholar]
- 36. Jiménez-Monreal AM, García-Diz L, Martínez-Tomé M. Influence of cooking methods on antioxidant activity of vegetables. J Food Sci. 2009;74:H97–103. doi: 10.1111/j.1750-3841.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- 37. Elhassaneen YA, Sanad MI. Phenolics, selenium, vitamin C, amino acids and pungency levels and antioxidant activities of two Egyptian onion varieties. Am J Food Technol. 2009;4(6):241–54. [Google Scholar]
- 38. Jeong CH, Heo HJ, Choi SG, Shim KH. Antioxidant and anticancer properties of methanolic extracts from different parts of white, yellow, and red onion. Food Sci Biotechnol. 2009;18(1):108–12. [Google Scholar]
- 39. Shela Gorinstein, Leontowicz H, Leontowicz M, Namiesnik J, Najman K, Drzewiecki J, et al. Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols. J Agric Food Chem. 2008;56(12):4418–26. doi: 10.1021/jf800038h. [DOI] [PubMed] [Google Scholar]
- 40. Ahiabor C, Gordon A, Ayittey K, Agyare R. In vitro assessment of antibacterial activity of crude extracts of onion (Allium cepa L.) and shallot (Allium aescalonicum L.) on isolates of Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and Salmonella typhi (ATCC 19430) IJAR. 2016;2(5):1029–32. [Google Scholar]
- 41. Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) LWT Food Sci Technol. 2004;37(2):263–8. [Google Scholar]
- 42. Jonathan Santas, Almajano MP, Carbó R. Antimicrobial and antioxidant activity of crude onion (Allium cepa L.) extracts. Int J Food Sci Technol. 2010;45(2):403–9. [Google Scholar]
- 43. Quave CL, Estévez-Carmona M, Compadre CM, Hobby G, Hendrickson H, Beenken KE, et al. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS One. 2012;7(1):e28737. doi: 10.1371/journal.pone.0028737. [DOI] [PMC free article] [PubMed] [Google Scholar]