Abstract
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) rich 2-monoacylglycerols (2-MAG), omega-3 polyunsaturated free fatty acids (ω-3 PUFFAs) concentrate, and PUFA enriched acylglycerols were prepared from salmon frame bone oil (SFBO) by enzymatic alcoholysis, urea complexation, and enzymatic esterification, respectively. The yields of 2-MAG, ω-3 PUFFAs concentrate, and PUFA enriched acylglycerols were 40.25, 16.52, and 15.65%, respectively. ω-3 PUFFAs concentrate and PUFA enriched acylglycerols showed darker red color than SFBO and 2-MAG due to aggregation of astaxanthin pigment in ω-3 PUFFAs concentrate during urea complexation. The viscosity and specific gravity of SFBO and PUFA enriched acylglycerols showed similar values whereas 2-MAG and ω-3 PUFFAs showed significantly (p < 0.05) lower values. Stability parameters like acid value, peroxide value, free fatty acid value, and p-anisidine value of SFBO and ω-3 PUFAs concentrates were within acceptable limits except extreme high acid value and free fatty acid value of ω-3 PUFFAs concentrate. Thermogravimetric analysis showed similar and higher thermal stability of SFBO and PUFA enriched acylglycerols than 2-MAG and ω-3 PUFFAs concentrate. The ω-3 PUFAs content in 2-MAG, ω-3 PUFFAs concentrate, and PUFA enriched acylglycerols was increased to 20.81, 52.96, and 51.74% respectively from 13.54% in SFBO. ω-3 PUFFAs concentrate and PUFA enriched acylglycerols showed higher DPPH and ABTS radical scavenging activity than SFBO and 2-MAG. The results obtained from this study suggest the production of PUFA enriched acylglycerols rich in ω-3 PUFAs supplements from fish oil for human and pet animals.
Keywords: Comparative analysis, ω-3 PUFFAs concentrate, Salmon frame bone oil, PUFA enriched acylglycerols, 2-MAG
1. Introduction
At present, modified oils and fats rich in ω-3 PUFAs are drawing great attention among the health cautious people for their functional or pharmaceutical roles. The market demand of ω-3 PUFAs rich fish oil is increasing due to its positive effects on human health and awareness of the nutritional value [1]. A number of studies confirmed the positive health effects of fish consumption in reducing coronary heart disease among the diverse populations [2]. Many of the researches worked on concentrating ω-3 and ω-6 PUFAs as human body is unable to synthesize those fatty acids sufficiently and needs to intake from external source with diet [3]. Fish oils typically contain α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) among the ω-3 PUFAs of which EPA (5 double bonds) and DHA (6 double bonds) are the common and widely researched [4]. The approaches for producing modified oils and fats rich in ω-3 PUFAs includes esterification, chemical hydrolysis, physical fractionation or chemical interesterification. But the chemical form of the final product is important to take into account, as the bioavailability of the ω-3 PUFAs vary depending on the existing forms such as in free fatty acids (FFAs), acylglycerols (AG), ethyl esters (EE), or phospholipids. Absorption of ω-3 PUFAs in EE formis poor in human body as pancreatic lipases are weak for EEs.
Among the various methods for ω-3 PUFAs concentrate production, distillation requires high temperature which cause oxidation, polymerization, and isomerization of double bonds [5,6]. Low temperature crystallization cannot produce highly concentrated ω-3 PUFAs [7]. Use of high pressure in supercritical fluid extraction process cause high capital cost, which limit the use in processing companies [5]. This study was based on the efficient, easy, and cheap methods for ω-3 PUFAs concentrate production to compare various parameters of the final products. Production of 2-MAG enriched in EPA and DHA from enzymatic alcoholysis of fish oil catalyzed by sn-1,3 specific lipase is a simple method [8]. The fatty acids of 1,3 positions are converted to ethyl esters and 2 positioned fatty acids remained in the acylglycerol fraction. The extent of ω-3 PUFAs in the 2-MAG depends on their extent in 2 position of glycerol backbone. The final product is 2-MAG, which is considered as good but the problem of its applicability during low content of ω-3 PUFAs in the original oil and interchange of fatty acids from 2 position of glycerol, resulting production of ethyl esters. Urea complexation is considered as the most efficient and simplest method where ω-3 PUFAs are obtained in the form of free fatty acids (FFAs). It is a simple, inexpensive, robust, quick, and environmental friendly technique [9,10]. PUFAs in FFAs form are absorbed more efficiently than PUFAs in triacylglycerols (TAG) or EE form, but they may have irritant effects and are highly prone to auto-oxidation [11]. Preparation of triacylglycerols using ω-3 PUFAs and glycerol by enzymatic esterification can solve these obstacles. Acylglycerols, especially TAG, are natural, highly bioavailable, and easy to use in industrial formulations [12]. A recent study concluded that supplementation of omega-3 fatty acids in the form of TAG for 6 months increased EPA and DHA in red blood cells significantly compared with providing FA–EE form [13]. A numbers of physico-chemical, catalytic/bio-catalytic techniques can be used for TAG production [5,14]. Isomerization and oxidation reactions cause losses of ω-3 PUFAs in traditional physico-chemical methods of TAG formation [15]. On the other hand, there is a number of advantages of using lipases as those ensure mild reaction conditions, low energy consumptions, limited undesirable side products which reduces purification and separation steps [16].
Atlantic salmon (Salmo salar) is rich of lipids, health beneficial omega-3 fatty acids, and high quality proteins. The characteristic pink color of Atlantic salmon is due to the carotenoid pigment astaxanthin, which is lipid soluble and has numerous health benefits including antioxidant, anti-inflammation, anti-cancer, anti-tumor, and anti-diabetic effects [17–21]. Salmon by-products are the materials left after removal of directly consumptionable main products (e.g., fillets) in the salmon processing/filleting industries. By-products are the secondary products not suitable for direct human consumption and need additional processing steps to use. Fish processing may generate up to 50% of the whole fish weight as by-products including heads, frame bones, skin, and viscera [22] which are generally discarded as processing leftovers or used for the production of animals feed or fertilizers. Utilization of fish by-products for fish oil production has many advantages; primarily it increases the overall value of the catch, reduce waste disposal/treatment cost, and ultimately lowers environmental pollution.
Although some researchers worked to produce ω-3 PUFAs concentrate from fish oil by various methods, there is very limited information of the final ω-3 PUFAs concentrate regarding physio-chemical, thermal, and bio-potential analyses. For the direct consumption and utilization of ω-3 PUFAs concentrate as food ingredients, it is important to know those above mentioned properties. In the present study, ω-3 PUFAs rich 2-MAG, ω-3 PUFFAs concentrate, and PUFA enriched acylglycerols were produced from SFBO by different methods such as lipase catalytic alcoholysis, urea complexation, and lipase mediated esterification, respectively. The main objective of the study was to evaluate the yield, physico-chemical properties, thermogravimetric analysis, fatty acids composition, and biological (radical scavenging) activities of the final ω-3 PUFAs concentrates obtained by different methods.
2. Materials and methods
2.1. Materials and reagents
Salmon frame bone oil (SFBO) was collected from Seawell Co., Ltd. (frozen salmon was imported from Norway), Haeundaegu, Busan, Republic of Korea. Lipase Novozymes-435 (Candida antarctica) and Lipozyme RMIM (Rhizopus miehei) immobilized in macroporous anion exchange resin was bought from Novozymes A/S, Denmark. Fatty acid methyl esters (FAME), astaxanthin standard, p-anisidine, DPPH, ABTS, ascorbic acid, trolox, and butylated hydroxytoluene (BHT) were obtained from Sigma–Aldrich Co., St. Louis, Missouri, USA. Urea purity >99.00% was purchased from Junsei Chemical Co., Ltd., Tokyo, Japan. Analytical or HPLC graded solvents only were used in this study.
2.2. Alcoholysis reaction for 2-MAG production
Alcoholysis reaction of SFBO was catalyzed by Novozymes-435 following the method of Rodriguez et al. [23] with slight modifications. In the reaction mixture, 50 g of SFBO, 200 g of ethanol (94%), and 25 g of lipase was taken in a 500 mL Erlenmeyer flask, filled the space inside with inert nitrogen gas, and closed the mouth using silicone rubber stoppers. The main reaction equation is as follows:
where, TAGs: Triacylglycerols, MAG: Monoacylglycerols, DAGs: Diacylglycerols, FFA: Free fatty acids, FAEEs: Fatty acid ethyl esters.
The reaction mixture was placed in a shaking incubator (LS1-4018 A, Daihan Labtech Co., Ltd, Kyonggi-do, Korea) maintaining 250 rpm and 37 °C for 3 h. Then the enzyme was filtered to stop the reaction using a Buchner funnel and vacuum pump. 2-MAG produced in the alcoholysis reaction was separated from other reaction products by solvent extraction procedure used by Munio et al. [24]. In the first step, ethanol was removed from the reaction mixture by using a rotary evaporator up to 20 mm Hg at 45 °C. 9 volume of 90% ethanol and 27 volume of hexane were added and mixed with 2-MAG ethyl esters mixture. Then the mixture solution was stirred at 300 rpm for 5min, taken in a separator funnel, and 2-MAG was collected with hydro-ethanolic fraction. The hexanic fraction was mixed with 90% ethanol to adjust the volume and the residual 2-MAG in hexane was separated repeatedly 2 more times with hydro-ethanolic fraction. The 3 hydro-ethanolic fractions were mixed together and the solvents were removed at 45 °C under vacuum.
2.3. Urea complexation for ω-3 PUFA concentrate production
2.3.1. Preparation of free fatty acids of SFBO
Free fatty acids from SFBO for urea complexation were prepared according to Wanasundara and Shahidi [10]. 250 g SFBO was taken in a 2 L size conical flask and added with 50 mg of BHT. Then potassium hydroxide (KOH) 57.50 g, distilled water 110 mL, and 660 mL aqueous ethanol (94%, v/v) was mixed with the oil. The oil mixture was heated at 62 °C using a hot water bath for 1 h under nitrogen pressure. After completing saponification, 500 mL of distilled water was added and unsaponifiable matters were washed with hexane (1 L). The saponified materials in the aqueous fraction was acidified to pH 1.0 adding 3 N HCl. 500 mL hexane was added with the mixture, mixed well by stirring and taken in a separatory funnel. The fatty acids in the mixture were then extracted with hexanic fraction, dried over anhydrous Na2SO4, and the solvent was evaporated at 42 °C under vacuum pressure to recover free fatty acids. Free fatty acids were preserved at −60 °C until use.
2.3.2. Preparation of ω-3 PUFFAs concentrate by urea complexation
Exactly, 100 g of free fatty acids were taken in a 2 L size beaker. Urea solution 20% (w/w) in 95% aqueous ethanol was added to maintain urea to fatty acid ratio 2.5:1 (w/w) and stirred at 65 °C using an electro-magnetic stirrer until the whole mixture converted transparent uniform solution. The urea–fatty acid mixture solution was kept at room temperature for an hour and later placed inside the refrigerator at −16 °C for 8 h to complete crystal formation. The urea–fatty acid crystal was separated from the ethanol–fatty acid solution by filtering immediately under suction using a Buchner funnel and filter paper (size-110 mm, F1113 grade, Chmlab group, Barcelona, Spain). Equal volume of distilled water was added with the filtrate and 6 N HCl was added slowly to transform pH 4–5. After addition of hexane of equal volume, the mixture was stirred at 250 rpm for 1 h keeping the beaker closed by aluminumfoil paper to reduce solvent evaporation. The fatty acid mixtures were taken in a separatory funnel to fractionate the hexane layer (containing fatty acids) from residual urea containing aqueous layer. The hexanic fraction was washed repeatedly with distilled water in order to remove remained urea residues and dried over anhydrous Na2SO4. A rotary vacuum evaporator was used to remove the solvent at 42 °C. Recovery of ω-3 PUFAs in ω-3 PUFFAs concentrates from initial SFBO was calculated using the following equation:
2.4. Production of PUFA enriched acylglycerols from ω-3 PUFFAs concentrate
PUFA enriched acylglycerols from ω-3 PUFFAs concentrate was prepared according to Bispo et al. [25]. At stoichiometric proportion, 250 g of ω-3 PUFFAs concentrate produced by urea complexation and 30.54 g glycerol (added 5 g surplus glycerol) was taken in an open mouth 500 mL bottle. Then, 25 g of Lipozyme RM IM was added and the reactants were esterified at 55 °C over a water bath and gentle stirring by nitrogen bubble for 48 h. The enzyme from the produced acylglycerol was separated by filtration.
2.5. Confirmation of SFBO and ω-3 PUFAs concentrates by TLC
Different ω-3 PUFAs concentrates were confirmed by TLC analysis followed the procedure described by Shin et al. [26] after slight modifications. Aluminum foil backed silica gel plates (20 cm × 20 cm), pre-coated with 0.2 mm layer of a silica gel 60 (ALUGRAM® SIL G/UV254; Macherey-Najel, Germany) was activated by heating at 75 °C for 30 min. The samples were prepared by diluting 4000 ppm with isopropanol/hexane (5:4, v/v). The mixture solution 5 μL was spotted onto the TLC plate, air dried for 10 min, and placed in a development tank containing ethyl acetate:cyclohexane (2:3, v/v). Iodine vapor in a glass tank was used for the detection of the spot. The lipid species were identified by comparing with the standards (Oleic acid and Mono, Di, Triglyceride Mix, Supelco, Bellefonte, Pennsylvania, USA). Different acylglycerols were quantified by GelQuant. NET software.
2.6. Physical parameters analysis
A Portable Reflectance Spectrophotometer, Lovibond RT Series (Solstice Park, Amesbury, UK) was used for color measurement of the samples. Black and white color were used for standardizing the instrument at each time of measurement. The viscosity of the samples was measured using a viscometer (DVII-Brookfield, Middleboro, USA) attached with a small sample adapter and spindle 62 at 100 rpm. The temperature was maintained at 25 ± 2 °C using a hot water bath (Lab Partner, PDWB-11, Taeshin Bio Science, Itewon, Korea). Specific gravity of the samples was analyzed according to AOCS Official Method, To 1b-64 [27].
2.7. Astaxanthin content determination by HPLC
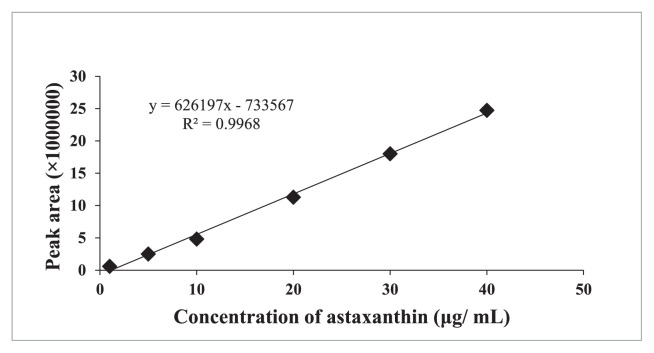
Astaxanthin content of SFBO and ω-3 PUFAs concentrates was determined according to Ali-Nehari et al. [28]. A waters model 600E system controller (Milford, USA) HPLC equipped with a model 484 UV/VIS detector and Eclipse Plus C18 column (5 μm, 4.6 × 250 mm, Agilent, USA) was used for astaxanthin content determination. Mobile isocratic phase consisted of ethanol, dichloromethane, and acetonitrile at volume ratio of 85:10:5 and flowrate was maintained at 1 mL/min. Wavelength of 470 nm was fixed for detecting astaxanthin and quantification was done based on the obtained peak area comparing with astaxanthin standard. Acetonitrile was used to dissolve standard astaxanthin at different concentration (1–40 μg/mL) for preparing a standard curve (Fig. 1).
Fig. 1.
Standard curve used for the determination of astaxanthin content.
2.8. Chemical parameters analysis
Acid value (AV), Peroxide value (POV), Free fatty acid value (FFA value), and p-anisidine value (PAV) of the samples were determined according to AOCS Official Method Cd 3d-63 [29], Modified IDF method [30], AOCS Official Method Ca 5a-40 [31], and AOCS Official Method Cd 18-90 [32], respectively. Oxidative stability Index (OSI) was measured by a Metrohm Rancimat (Model 743, Herisau, Switzerland) following the method of Farhoosh [33] with slight modifications. In brief, 5 g of sample was taken in a reaction vessel, a stream of filtered, cleaned, and dried air was bubbled (20 L h−1) into oil heated at 121.6 °C by an electric heating block. A measuring vessel containing 40 mL of distilled water was used for collecting the effluent air containing volatile organic acids from the oil samples. The conductivity of the water was measured automatically and recorded as oxidation proceeded. Eight samples were analyzed at a time simultaneously. Samples were randomized on the position of heating blocks for all determinations.
2.9. Thermogravimetric analysis
A Thermogravimetric Analyzer (Perkin–Elmer Model, USA, TGA 7) was used for analyzing the thermal stability of samples. The aluminum pan containing approximately 5 mg of sample was placed in the furnace and the exact sample weight was measured. The sample was programmed to heat from 50 to 700 °C at an increasing rate of 10 °C/min under air atmosphere. To facilitate a suitable environment for oxidation process, O2 was used as sample purge gas and flow was maintained at 50 cm3/min. The reduction of the sample weight was automatically recorded every 6 s on a plotter (ColorPro, Hewlett-Packard).
2.10. Fatty acid analysis
Fatty acid composition of SFBO and prepared ω-3 PUFAs concentrates were determined using a 6890 Agilent (Agilent Technologies, Wilmington, USA) gas chromatograph facilitated with a fused silica capillary column (100 m length × 0.25 mm internal diameter, 0.2 μm of film) (Supelco, Bellefonte, USA). Fatty acid methyl esters for SFBO, 2-MAG, PUFA enriched acylglycerols, and ω-3 PUFFAs concentrate were prepared according to the methods of American Oil Chemists’ Society (AOCS), Ce 2-66 (2) and Ce 2-66 (3) [34], respectively. Oven temperature was programmed to start with a constant temperature of 130 °C for 3 min, then increased to 240 °C at a rate of 4 °C/min, and then held at 240 °C for 10 min. The injector and detector temperature were maintained at 250 °C. Standard fatty acid methyl esters were used for identifying the fatty acid methyl esters and quantification was done by obtained peak area (%).
2.11. Antioxidant activity measurements
2.11.1. DPPH radical scavenging activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity of SFBO and ω-3 PUFAs concentrates was measured following the method of Gulcin et al. [35] with modifications. In brief, 4.0 mL of 0.2 mM DPPH solution (in ethanol) was measured and taken in a test tube with 1.0 mL of sample solutions (10–100 mg/mL ethanol). The DPPH-sample solution was mixed thoroughly by vortexing 4 s and incubated in dark at room temperature for 30 min. The absorbance of the sample solution was measured at 517 nm using a microplate reader (Synergy HT, Bio Tek Instruments, Inc., Winooski, VT, USA). A sample blank and control of trolox (10–100 μg/mL) were also measured using the same method.
As is the absorbance of sample, Ab is the absorbance of blank, and A0 is the absorbance of sample and ethanol.
2.11.2. ABTS+ radical scavenging activity
2,2′-azino-di, 3-ethylbenzthiazoline-6-sulphonic acid (ABTS+) scavenging activity of SFBO and ω-3 PUFAs concentrates was measured following the procedure of Zheleva-Dimitrova et al. [36] with modifications. ABTS+ stock solution was prepared by mixing equal volume of 7 mM ABTS+ with 2.45 mM/L K2S2O8 and keeping the mixture in dark at room temperature for 16 h. The ABTS+ stock solution was diluted to get absorbance value 0.70 ± 0.02 at 734 nm with ethanol (94%) that was ABTS+ working solution. Then 4.0 mL of ABTS+ working solution was mixed with 1.0 mL of sample solution (10–100 mg/mL ethanol). The ABTS-sample solution was mixed thoroughly by vortexing 4 s, incubated in dark at room temperature for 6min and the absorbance was measured at 734 nm using a microplate reader (Synergy HT, Bio Tek Instruments, Inc., Winooski, VT, USA). Absorbance of the sample blank and control of ascorbic acid (10–100 μg/mL distilled water) was also measured using the above procedure.
As is the absorbance of sample, Ac is the absorbance of blank, and A0 is the absorbance of sample and ethanol.
2.12. Statistical analysis
Means ± standard deviations (SD) of the triplicates are presented. Analysis of variance (ANOVA) was performed by SPSS software (version 20.0, SPSS Inc., Chicago, IL, USA) for statistical analysis. P < 0.05 was regarded as significant and the differences among the treatment were performed by Duncan’s Multiple Range Tests.
3. Results and discussion
3.1. Confirmation of lipid species by TLC
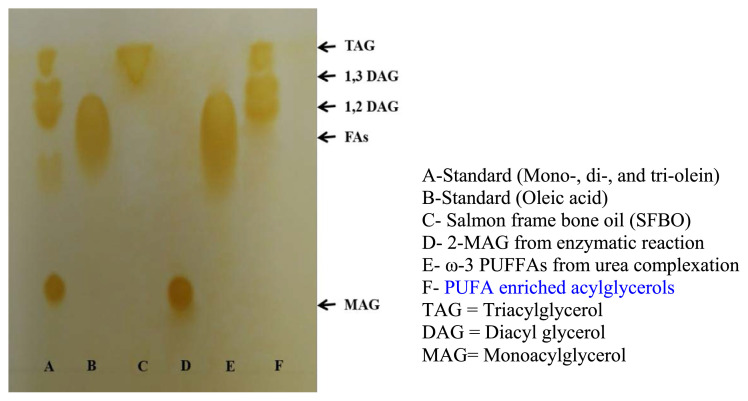
The TLC analysis of SFBO and ω-3 PUFAs concentrates is placed in Fig. 2. The retention factors (Rf) values of TAG, 1,3 DAG, 2,3-DAG, fatty acids, and MAG were found 0.95, 0.85, 0.79, 0.71, and 0.24 respectively. Lane C contained SFBO, which gave single TAG band and Lane D contained 2-MAG purified from enzymatic reacted products gave only single band of MAG, proved presence of no other compounds. Lane E contained PUFFAs from urea complexation, which showed similar Rf value of 0.71 as we found in oleic acid standard. PUFFAs contained mostly longer chain fatty acids of similar polarity which traveled close distance and gave a large single band. PUFA enriched acylglycerols was plotted in Lane F which contained 20% TAG, 40% 1,3 DAG and 40% 1,2 DAG. During esterification reaction, surplus glycerol was used to facilitate the reaction which may render the depletion of PUFFAs and formation of DAG. Mainly DAG and MAG were obtained when higher proportion of glycerol was used in direct esterification reaction, Halldorsson et al. [14]. The formation of TAG by esterification reaction by 1,3 specific lipases is due to the spontaneous acylmigration of the fatty acids in the reaction medium from 1(3) MAG to 2-MAG and 1,3-DAG to 1(3), 2-DAG [37,38].
Fig. 2.
TLC analysis of SFBO and ω-3 PUFAs concentrates.
3.2. Yields of ω-3 PUFAs concentrates
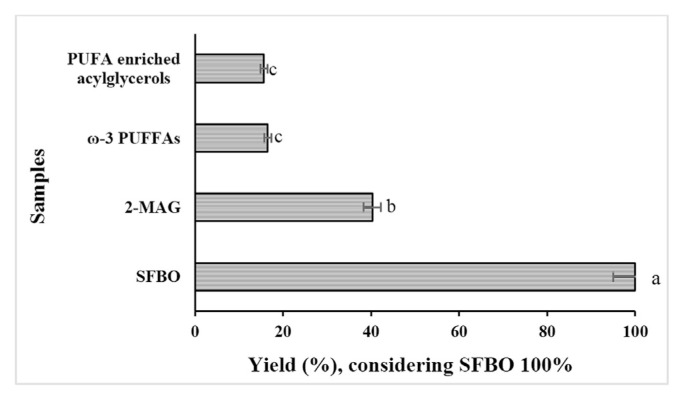
Fig. 3 shows the yields of different ω-3 PUFAs concentrates (SFBO considered as 100%). 2-MAG is produced by discarding the fatty acids in sn-1,3 position and the yield was 40.25%. The yield of 2-MAG in enzymatic alcoholysis of fish oil mostly depends on the contents and position of PUFAs in 2 position. Esteban et al. [39] obtained the yield of 2-MAG by enzymatic alcoholysis of fish oil from 30 to 60% depending on the different reaction conditions. ω-3 PUFFAs concentrate was produced by urea complexation which gave an yield of 16.52%. The yield and ω-3 fatty acids concentration mostly depends on the initial ω-3 fatty acids content of initial oil and influencing parameters of urea complexation, mostly urea to fatty acids ratio. The initial ω-3 fatty acids content of oil is important for determining the ω-3 fatty acids content of final product and yield. The recovery of ω-3 PUFAs in the ω-3 PUFFAs concentrate was found 64.62% whereas the recovery of EPA–DHA was found 86.17%. Urea complexation procedure concentrates fatty acids based on the degree of unsaturation. EPA–DHA contain high number of double bonds (5 and 6, respectively) which prevented to abduct with urea. On the contrary, α-Linolenic acid with only 3 double bonds attributed tendency to bind with urea rendering lower recovery of ω-3 PUFAs in the ω-3 PUFFAs concentrate. EPA and DHA are mostly considered as health care food ingredients [40]. The yield of ω-3 fatty acids from the urea complexation depends on the relative percentage of the different fatty acids present in the starting oil [25]. Esterified PUFA enriched acylglycerols produced from ω-3 PUFFAs concentrate and glycerol gave a yield of 15.65%. In the esterification reaction, 3 molecules of H2O is produced with the formation of 1 molecule of TAG which is evaporated and released from the reaction mixture with nitrogen flow. The loss of H2O molecule rendering the reduction of yield of PUFA enriched acylglycerols than PUFAs concentrate though the reduction of PUFA enriched acylglycerols yield is compensated by addition of glycerol as a reactant. Esterification of sardine oil containing 39.5% ω-3 PUFAs gave a yield of 29.04% PUFA enriched acylglycerols containing 77.40% ω-3 PUFAs [25]. The ω-3 PUFAs content of seal blubber oil was increased from 32.16% to 88.20% by urea complexation where the yield of ω-3 PUFFAs was 21.50%. The yield of ω-3 PUFFAs concentrate and PUFA enriched acylglycerols were inferior due to low ω-3 PUFAs content of the initial SFBO.
Fig. 3.
Yield of different ω-3 PUFAs concentrates. Values are the mean of 3 determinations. Different letters with the bar indicate significant differences (p < 0.05).
3.3. Physical properties and astaxanthin content of SFBO and ω-3 PUFAs concentrates
Oil color is important for acceptance by consumers relating presence or absence of impurities. Salmon oil color indicates the extent of astaxanthin, a crimson red colored carotenoid pigment, contributing various health beneficial issues. L*, a*, b* system is a most popular instrumental method for color measurement based on the primary parameters of lightness (L*), redness (a*), and yellowness (b*). The lower L* value indicates darkness and higher content of astaxanthin pigment. The a* value generally exhibits the best correlation to increase carotenoid pigments [41]. The L* value of ω-3 PUFFAs (30.52) and PUFA enriched acylglycerols (29.86) were significantly (p < 0.05) lower whereas a* values 12.51 and 11.98 were higher than SFBO and 2-MAG (Table 1) indicating aggregation of astaxanthin in PUFAs. The b* values of SFBO and 2-MAG were higher 10.46 and 8.68 respectively than ω-3 PUFFAs concentrate and PUFA enriched acylglycerols 4.3 and 3.33 respectively, indicating more yellowness of SFBO and 2-MAG than ω-3 PUFFAs concentrate and PUFA enriched acylglycerols. The L*, a*, and b* values of red and pink salmon head oils were 32.1 and 40.3, 4.9 and 2.7, 14.6 and 16.6, respectively [42]. The astaxanthin content of ω-3 PUFFAs concentrate and PUFA enriched acylglycerols was found almost double than SFBO and 2-MAG. The processing activity of SFBO during urea complexation rendered the incretion of PUFA concentration and aggregation of astaxanthin in ω-3 PUFFAs concentrate. In the urea complexation process, the saturated and monounsaturated fatty acids are easily complexed with urea, crystallize at cooling, and are removed by filtration. Urea molecules remain straight chain form at hot (62–65°) ethanolic solution and during cooling subsequently transformed to hexagonal crystal form enclosing fatty acid molecules depending on the degree of saturation [43]. The formation of the complex depends on the size, shape, and geometry of the fatty acids and Van der Waals forces, London dispersion forces, and induced electrostatic attractions [44]. At crystallization condition, the long and straight chain saturated and monounsaturated fatty acids are abducted with urea while the PUFAs remain in the alcoholic solution, from there PUFAs are recovered with hexane. Astaxanthin forms astaxanthin esters in fish oil which were hydrolyzed and liberated from the fatty acids during free fatty acid formation for urea complexation. Astaxanthin is highly soluble in organic solvents like acetone, ethanol, hexane and acetic acid but not in water [45]. During collection of ω-3 PUFFAs concentrate with hexanic fraction, astaxanthin was solubilized and aggregated.
Table 1.
Physical properties and astaxanthin content of SFBO and different ω-3 PUFAs concentrates.
| Parameters | SFBO | 2-MAG | ω-3 PUFFAs | PUFA enriched acylglycerols |
|---|---|---|---|---|
| Color | ||||
| L* | 36.08a ± 0.66 | 36.02a ± 0.56 | 30.52b ± 0.11 | 29.86b ± 0.39 |
| a* | 9.06b ± 0.19 | 9.46b ± 0.22 | 12.51a ± 0.38 | 11.98a ± 0.51 |
| b* | 10.46a ± 0.17 | 8.68b ± 0.29 | 4.3c ± 0.21 | 3.33d ± 0.18 |
| Astaxanthin (μg/g) | 21.81b ± 0.70 | 21.94b ± 0.41 | 44.23a ± 0.42 | 43.66a ± 0.51 |
| Viscosity (cP) | 49.09a ± 0.67 | 39.57b ± 0.63 | 18.86c ± 0.69 | 48.88a ± 0.66 |
| Sp. gravity | 0.91a ± 0.01 | 0.90a ± 0.01 | 0.87b ± 0.01 | 0.90a ± 0.01 |
Values are means ± standard deviations (n = 3). In each row, the different superscript letters differ significantly (P < 0.05).
The L* value is the “lightness” of a sample from 0 to 100 with 100 being pure white; the a* value describes red (+) to green (−); the b* value represents yellow (+) to blue (−). cP = centipoise, 1 cP = 0.01 g per centimeter-second.
The viscosity of oils depends on the nature of the triacylglycerols (TAGs), i.e., viscosity changes with the arrangements of fatty acids in the glycerol backbone. Chemical properties such as fatty acids chain length and degree of saturation or unsaturation affects viscosity of oils. Table 1 shows that the highest value of viscosity is exhibited by SFBO (49.09) followed by PUFA enriched acylglycerols, 2-MAG, and ω-3 PUFFAs concentrate 48.88, 39.57, and 18.86, respectively. Wiedermann [46] reported that impurities like Mg, Fe, Ca, P, free fatty acids, insoluble impurities, moistures, and peroxides etc. contained in fish oils, may be highly interactive with the SFBO. Viscosity property of SFBO and PUFA enriched acylglycerols did not varied significantly (p < 0.05), which indicate similar natural viscous property of PUFA enriched acylglycerols. 2-MAG consisted of single fatty acids in glycerol backbone, which rendering lower viscosity and ω-3 PUFFAs concentrate consisted of majority of long chain unsaturated free fatty acids rendering lowest viscous property. Sp. gravity of the lipid species showed positive linear relation with viscosity (Table 1). The standard value for Sp. gravity of fish oil is 0.928 according to Food and Agriculture Organization (FAO) [47] which is very close to the values obtained in SFBO and PUFA enriched acylglycerols. Viscosity and Sp. gravity depends on various factors of oils of which degree of saturation, impurities, and polymerization is important [48].
3.4. Stability properties of SFBO and different ω-3 PUFAs concentrates
Free fatty acids and some non-lipid acidic compounds are responsible for determining the acid value, an indicator of fish oil acidity. Hydrolysis of triacylglycerol renders to generation of free fatty acids whereas improper storage/spoilage of raw materials may cause the production of non-lipid acidic compounds e.g. acetic acid [49]. Acid value is the amount of KOH (mg) necessary for neutralizing one gram of oil/fat and higher free fatty acid content is directly proportional to an increase of the acid value [50,51]. The acid value of ω-3 PUFFAs concentrate was extremely higher (140.45) followed by 2-MAG, PUFA enriched acylglycerols, and SFBO (12.01, 8.34, and 7.10, respectively). ω-3 PUFFAs consist of only fatty acids without combining glycerol, so that the carboxylic group (−COOH) of fatty acid is not neutralized, rendering high acid value. 2-MAG showed higher acid value due to oxidation of oils and production of acidic compounds at enzymatic reaction conditions. PUFA enriched acylglycerols showed the acid value in the marginal range of acceptable limit 7–8 mg KOH/g [52].
Peroxide value is measured for quantifying total hydro peroxides produced in oil due to primary oxidation and is an indicator of quality and stability [53]. Lower molecular weight compounds like free fatty acids, aldehydes, ketones, alcohols etc. are generated from the breakdown of oxidative products [54]. Storage period, exposure to temperature during processing and contact with atmospheric oxygen affects peroxide value. In the present study, 2-MAG showed the highest peroxide value whereas SFBO showed the lowest peroxide value. The enzymatic breakdown, incubation temperature, and contact of alcohol might be responsible for higher peroxide values in 2-MAG. The peroxide value of PUFA enriched acylglycerols was very close to peroxide value of ω-3 PUFFAs concentrate due to removal of produced H2O by nitrogen gas bubble in esterification reaction that resisted oxidation. The peroxide values of all samples of the present study were in the range of acceptable limit (≤5 meq/kg). The peroxide value of ω-3 PUFAs produced from tuna by-product oil by urea complexation was 2.77 meq/kg [55,56].
FFAs are generated due to the hydrolysis of esters bonds of TAG, an important quality parameters of edible fish oils. Fatty acids are essential for the building blocks of fat/lipids binding with glycerol backbone. But when they are floating freely, are referred to as free fatty acids and an indicator of oil rancidity/hydrolysis. The free fatty acid values of SFBO, 2-MAG, ω-3 PUFFAs concentrate, and PUFA enriched acylglycerols varied significantly (p < 0.05) (Table 2). ω-3 PUFFAs concentrate contained free fatty acids rather than triacylglycerol and showed the excessive free fatty acid value of 81.88. SFBO and PUFA enriched acylglycerols showed close and lower free fatty acid value indicating higher extent of esterification reaction during PUFA enriched acylglycerols formation. Addition of surplus glycerol during esterification conveniently utilized majority of the fatty acids for PUFA enriched acylglycerols formation.
Table 2.
Stability properties of normal SFBO and different ω-3 PUFAs concentrates.
| Parameters | SFBO | 2-MAG | ω-3 PUFFAs | PUFA enriched acylglycerols |
|---|---|---|---|---|
| Acid value (mg KOH/g) | 7.10d ± 0.12 | 12.01b ± 0.32 | 140.45a ± 0.64 | 8.34c ± 0.32 |
| Peroxide value (meq/kg) | 1.26d ± 0.05 | 3.70a ± 0.07 | 2.08c ± 0.12 | 2.37b ± 0.10 |
| Free fatty acid value (%) | 2.37d ± 0.16 | 4.52b ± 0.09 | 81.88a ± 0.33 | 3.80c ± 0.32 |
| p-Anisidine value | 6.89b ± 0.14 | 5.48c ± 0.35 | 9.06a ± 0.27 | 6.81b ± 0.16 |
| OSI value (h) | 1.62a ± 0.10 | 1.54b ± 0.01 | – | 1.63a ± 0.1 |
Values are means ± standard deviations (n = 3). In each row, the different superscript letters differ significantly (P < 0.05).
The p-anisidine value is the measurement of the secondary oxidation products, especially aldehydes contents of oil. Thus, p-anisidine value indicates the oxidative status and flavor quality of lipids. Hydro peroxides generated in primary oxidation are very unstable which rapidly breakdown to various volatile and non-volatile secondary oxidation products resulting rancidity [57]. The p-anisidine value of the samples varied between 5.48 and 9.06 (Table 2), which were within the acceptable limit. Global Organization of EPA and DHA (GOED) and Food and Agricultural Organization (FAO) set the acceptable limit of p-anisidine value of fish oil ≤20 [58]. ω-3 PUFFAs concentrate was in unstable form and highly prone to secondary oxidation, showed the highest p-anisidine value amongst the samples. PUFA enriched acylglycerols was prepared from the ω-3 PUFFAs concentrate showing decease of the p-anisidine value due to removal of volatile secondary oxidative products with nitrogen bubble during the esterification process. The secondary oxidatives in SFBO may be due to the extraction process and storage conditions. Estiasih et al. [55] reported p-anisidine value 9.88 in ω-3 PUFAs produced from tuna by-products oil by urea complexation. The oxidative stability index (OSI) period of different oils are presented in Table 2. SFBO and PUFA enriched acylglycerols were similar in tolerating oxidative stress indicate good stability of esterified SAG. 2-MAG showed lower induction time as it contained unsaturated long chain single fatty acids which was highly prone to oxidize at adverse conditions.
3.5. Thermogravimetric properties
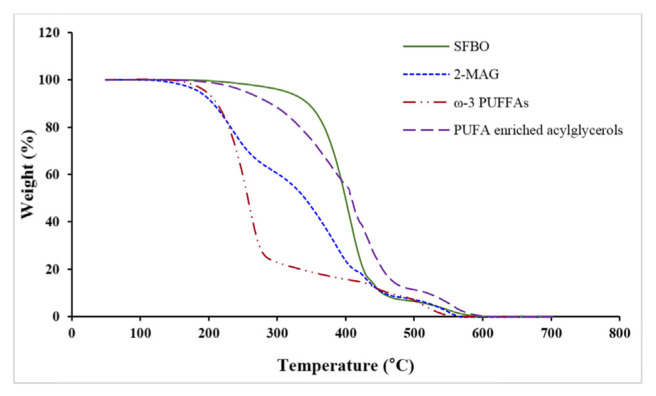
The thermal behavior of SFBO and different ω-3 PUFAs concentrates is presented in the thermogravimetric curves (Fig. 4). From 200 to 450 °C, the weight of the samples reduced drastically. At the presence of atmospheric oxygen, the oils are oxidized leading to the formation of peroxides, a well-known oxidation product involving the increment of sample mass [59]. In this study, no increment of weight was observed in the thermogravimetric curves for all the samples analyzed at presence of oxygen indicating the thermal decomposition of lipids was not related to oxygen absorption. Among the four different samples, the thermal stability was as follows: SFBO ≥ PUFA enriched acylglycerols > 2-MAG > ω-3 PUFFAs concentrate. Higher weight loss was observed in ω-3 PUFFAs concentrate and 2-MAG. Between 200 and 300 °C, the weight loss of PUFAs and 2-MAG were 72.42 and 33.39% where as it was only 4.30 and 12.69% for SFBO and PUFA enriched acylglycerols, respectively. ω-3 PUFFAs concentrate was not in stable form and was highly prone to decomposition than SFBO and PUFA enriched acylglycerols. 2-MAG contained only −OH group at 1,3 position of glycerol backbone, responsible for the higher tendency of decomposition than SFBO and PUFA enriched acylglycerols. PUFA enriched acylglycerols rich in ω-3 PUFAs showed similarity in degradation pattern with SFBO indicating good prospects in thermal application based use. Sathivel [42] reported that there was drastic thermal decomposition of red and pink salmon oils between 200 and 450 °C, and the weight loss was linearly correlated with increasing heating. He also mentioned that, the thermal decomposition of the oils was not correlated with oxygen absorption as now eight increment was observed in the thermogravimetric curves.
Fig. 4.
The pattern of the thermal degradation of SFBO and different ω-3 PUFAs concentrates.
3.6. Fatty acid composition
The ω-3 fatty acids content of SFBO was 13.64% whereas it was 20.81, 52.96, and 51.74% in 2-MAG, ω-3 PUFFAs concentrate, and PUFA enriched acylglycerols, respectively (Table 3). This study was conducted to increase the ω-3 fatty acids content using low ω-3 fatty acids containing salmon by-product oil for valorization. Enzymatic alcoholysis was conducted for the removal of fatty acids at sn-1,3 position, thus increasing ω-3 PUFAs content in 2-MAG. ω-3 polyunsaturated fatty acids in fish oil usually exists in the sn-2 position [26]. The ω-3 PUFAs content in sn-2 position varies on the oil types and the ω-3 PUFAs in the 2-MAG is determined by total ω-3 PUFAs content in the TAG and extent in 2-position. Munio et al. [24] reported 90% of total DHA in cod liver oil and 54% of total DHA in tuna oil were in the position-2. SFBO contained low amount of ω-3 PUFAs which was increased to 20.81% in 2-MAG. Rodriguez et al. [23] conducted alcoholysis reaction of cod liver oil catalyzed by Novozymes 435 and found EPA and DHA content increased from 16.10 to 26.40% when ethanol (96%, v/v) was used as reaction media. The cod liver oil and tuna oil contained EPA–DHA 20.60% and 29.60%, which were increased to 40.80 and 44.90% respectively by sn-1,3 lipase catalyzed alcoholysis reaction [39]. ω-3 PUFFAs concentrate was produced by urea complexation, a highly efficient procedure to decrease saturated fatty acids and increase ω-3 PUFAs. Saturated fatty acids content were decreased after urea complexation whereas polyunsaturated fatty acids content were increased, especially ω-3 PUFAs. Saturated fatty acids content after urea complexation were decreased to 5.87% from 21.89% in SFBO. The ω-3 PUFAs after urea complexation rose dramatically to 52.96% from initial 13.54% in SFBO. The ω-3 PUFAs of salmon oil were increased from 17.64% in crude normal oil to 50.97% by urea complexation [56]. Urea to fatty acid ratio and crystallization temperature are the two key factors for concentrating ω-3 PUFAs [56,60]. The esterification reaction process did not affect the fatty acid composition, i.e., the fatty acid composition of PUFA enriched acylglycerols was similar of ω-3 PUFFAs concentrate. The reaction was conducted at mild temperature of 55 °C, without solvent and continuous nitrogen flow in the reaction media kept the ω-3 PUFFAs concentrate and PUFA enriched acylglycerols withstand against oxidation. Bispo et al. [25] also reported similar results of urea complexed PUFAs esterification which was supportive of the present study.
Table 3.
Fatty acid composition of SFBO and different ω-3 PUFAs concentrates.
| Sl No. | Name of fatty acids | SFBO | 2-MAG | ω-3 PUFFAs | PUFA enriched acylglycerols |
|---|---|---|---|---|---|
| 1. | Caprylic Acid (C8:0) | 1.23 | ND | ND | ND |
| 2. | Myristic Acid (C14:0) | 3.29 | ND | 0.47 | 0.45 |
| 3. | Palmitic Acid (C16:0) | 12.22 | 4.62 | ND | ND |
| 4. | Palmitoleic Acid (C16:1) | 4.13 | 3.48 | 1.35 | 1.36 |
| 5. | Stearic Acid (C18:0) | 3.53 | 0.94 | 1.35 | 1.36 |
| 6. | Oleic Acid (C18:1n9C) | 34.34 | 38.59 | 3.07 | 3.15 |
| 7. | Elaidic Acid (C18:1n9t) | 3.38 | 1.73 | 0.61 | 0.56 |
| 8. | Linoleic Acid (C18:2n6c) | 16.01 | 23.84 | 25.73 | 24.65 |
| 9. | Eicosenoic Acid (C20:1) | 3.30 | ND | 1.26 | 1.20 |
| 10. | α-Linolenic Acid (C18:3n3)/ALA | 4.00 | 8.53 | 7.41 | 7.35 |
| 11. | Eicosadienoic Acid (C20:2) | 1.66 | 1.43 | 4.06 | 4.12 |
| 12. | Behenic Acid (C22:0) | 0.43 | ND | 2.22 | 2.20 |
| 13. | Eicosatrienoic Acid (C20:3n6) | 0.36 | ND | 1.25 | 1.23 |
| 14. | Euric Acid (C22:1n9) | 0.39 | ND | 0.12 | 0.10 |
| 15. | Eicosatrienoic Acid (C20:3n3) | 0.88 | ND | 0.38 | 0.32 |
| 16. | Tricosanoic Acid (C23:0) | 0.49 | ND | 1.66 | 1.60 |
| 17. | Docosadienoic Acid (C22:2n6) | 0.91 | 0.92 | 3.34 | 3.35 |
| 18. | Eicosapentaenoic Acid (C20:5n3)/EPA | 3.93 | 2.17 | 20.51 | 20.25 |
| 19. | Nervonic Acid (C24:1) | 0.37 | ND | ND | ND |
| 20. | Docosahexaenoic Acid (C22:6n3)/DHA | 4.73 | 10.11 | 24.66 | 24.14 |
| ∑EPA + DHA | 8.66 | 12.28 | 45.17 | 44.39 | |
| ∑ω-3 PUFAs | 13.54 | 20.81 | 52.96 | 51.74 | |
| ∑ω-6 PUFAs | 17.28 | 24.76 | 30.32 | 29.23 | |
| ∑SFA | 21.19 | 5.56 | 5.70 | 5.61 |
N.B.: EPA: Eicosapentaenoic acid, DHA: Docosahexaenoic acid, PUFA: Polyunsaturated fatty acid, SFA: Saturated fatty acid, ND: Not detected.
3.7. Antioxidant activity
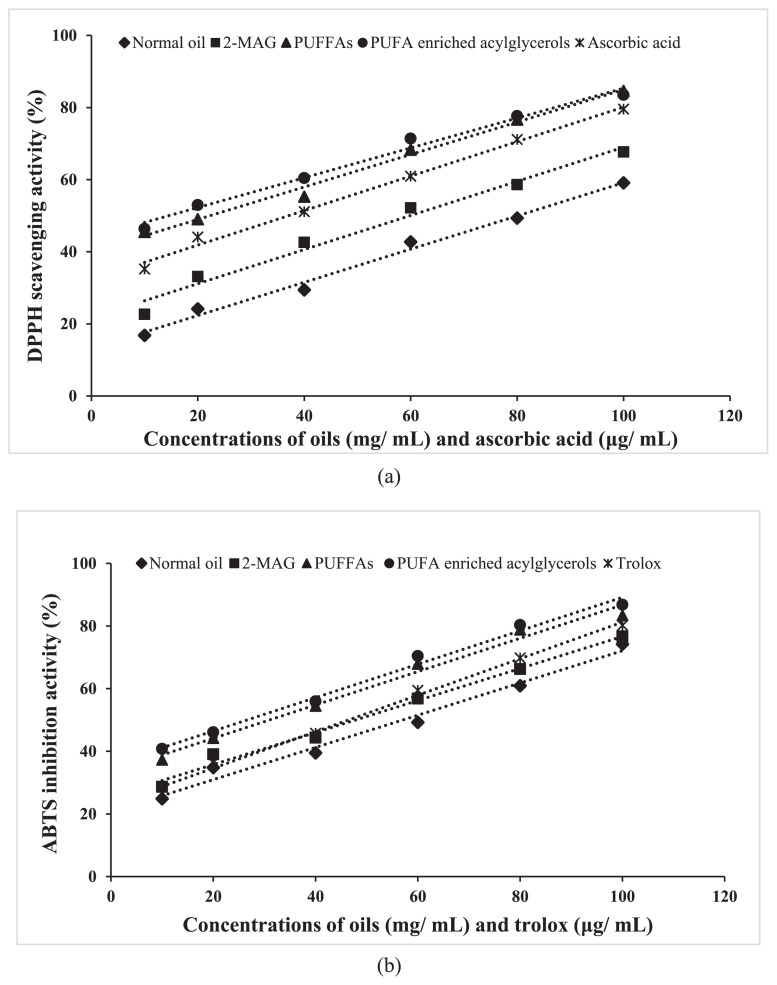
SFBO and ω-3 PUFAs concentrates showed in vitro DPPH and ABTS radical scavenging inhibition in a concentration dependent manner (Fig. 5). DPPH and ABTS radicals based assays are considered as the most popular spectrophotometric methods for antioxidant activity determination [61]. For antioxidant activity evaluation, DPPH and ABTS scavenging methods have been used due to simple, sensitive, rapid, and reproducibility [62]. The antioxidant compounds in salmon oil includes carotenoids (astaxanthin), tocopherols, phospholipids and phenolic compounds [18,63–65]. DPPH, a free radical becomes a stable diamagnetic molecule by accepting an electron or hydrogen radical. In the alcoholic solution, DPPH shows a strong absorption band at 517 nm due to odd electron. When the DPPH solution get opportunity to react with suitable reducing agent, producing new bond and change the color of solution [66]. SFBO and different ω-3 PUFAs concentrates showed similarity in DPPH radical scavenging and ABTS inhibition capacity. The maximum antioxidant activity was obtained in PUFA enriched acylglycerols and ω-3 PUFFAs concentrate and the lowest activity was obtained from the SFBO. PUFA enriched acylglycerols and ω-3 PUFFAs concentrate contained higher astaxanthin pigment that contributed for higher antioxidant activity. Modifications and concentrating ω-3 fatty acids in PUFA enriched acylglycerols and ω-3 PUFFAs concentrate from SFBO may also aggregated some other compounds attributing higher antioxidant activity.
Fig. 5.
Antioxidant activity of SFBO and different ω-3 PUFAs concentrates (a) DPPH radical scavenging activity (b) ABTS+ inhibition activity.
4. Conclusion
Alcoholysis reaction catalyzed by sn-1,3 specific lipase, urea complexation, and enzymatic esterification of ω-3 PUFFAs was applied for concentrating ω-3 PUFAs in 2-MAG, PUFFAs concentrate, and PUFA enriched acylglycerols from SFBO. Enzymatic alcoholysis increased ω-3 PUFAs content only 20.81% due to lower ω-3 PUFAs content in SFBO whereas urea complexation efficiently increased ω-3 PUFAs content 52.96%. The astaxanthin content after urea complexation was almost doubled in ω-3 PUFFAs concentrate. Esterification reaction was proficient keeping the ω-3 PUFAs and astaxanthin content withstand and forming PUFA enriched acylglycerols including triacylglycerol. The acid value and free fatty acid value of ω-3 PUFFAs concentrate produced by urea complexation were very high, as this was in free fatty acid form. So, further investigation is recommended to determine the compatibility of ω-3 PUFFAs for direct consumption. PUFA enriched acylglycerols produced from esterified reaction showed the physico-chemical and thermal properties similar to SFBO. So, preparation of PUFA enriched acylglycerols may be an efficient approach for providing ω-3 PUFAs to the consumers.
Acknowledgments
The authors gratefully acknowledge the financial support for the research work provided by Business for Cooperative R&D (Grant No. C0350298) between Industry, Academy, and Research Institute funded Korea Small and Medium Business Administration in 2015.
Funding Statement
The authors gratefully acknowledge the financial support for the research work provided by Business for Cooperative R&D (Grant No. C0350298) between Industry, Academy, and Research Institute funded Korea Small and Medium Business Administration in 2015.
REFERENCES
- 1. Tengku-Rozina TM, Birch EJ. Enrichment of omega-3 fatty acids of refined Hoki oil. J Am Oil Chem Soc. 2013;90:1111–9. [Google Scholar]
- 2. Mozaffarian D, Bryson CL, Lemaitre RN, Siscovick DS, Burke GL. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–21. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 3. Chin YF, Salimon J, Said M. Optimization of urea complexation by Box-Behnken Design. Sains Malays. 2010;39:795–803. [Google Scholar]
- 4. Karim FT, Ghafoor K, Ferdosh S, Al-Juhaimi F, Ali E, Yunus KB, et al. Microencapsulation of fish oil using supercritical antisolvent process. J Food Drug Anal. 2017 doi: 10.1016/j.jfda.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shahidi F, Wanasundara UN. Omega-3 fatty acid concentrates: nutritional aspects and production technologies. Trends Food Sci Technol. 1998;9:230–40. [Google Scholar]
- 6. Kapoor R, Patil UK. Importance and production of omega-3 fatty acids from natural sources. Int Food Res J. 2011;18:493–9. [Google Scholar]
- 7. Latyshev NA, Ermolenko EV, Kasyanov SP. Concentration and purification of polyunsaturated fatty acids from squid liver processing wastes. Eur J Lipid Sci Technol. 2014;116:1608–13. [Google Scholar]
- 8. Zuyi L, Ward OP. Lipase-catalyzed alcoholysis to concentrate the n-3 polyunsaturated fatty acid of cod liver oil. Enzyme Microb Technol. 1993;15:601–6. [Google Scholar]
- 9. Hayes DG, Bengtsson YC, Van Alstine JM, Setterwall F. Urea complexation for the rapid, ecologically responsible fractionation of fatty acids from seed oil. J Am Oil Chem Soc. 1998;75:1403–9. [Google Scholar]
- 10. Wanasundara UN, Shahidi F. Concentration of omega 3-polyunsaturated fatty acids of seal blubber oil by urea complexation: optimization of reaction conditions. Food Chem. 1999;65:41–9. [Google Scholar]
- 11. Hernandez EM. Issues in fortification and analysis of omega-3 fatty acids in foods. Lipid Technol. 2014;26:103–6. [Google Scholar]
- 12. Iwasaki Y, Yamane T. Enzymatic synthesis of structured lipids. J Mol Catal B Enzym. 2000;10:129–40. [Google Scholar]
- 13. Neubronner J, Schuchardt JP, Kressel G, Merkel M, von Schacky C, Hahn A. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur J Clin Nutr. 2011;65:247–54. doi: 10.1038/ejcn.2010.239. [DOI] [PubMed] [Google Scholar]
- 14. Halldorsson A, Magnusson CD, Gudmundur G, Haraldsson GG. Chemoenzymatic synthesis of structured triacylglycerols by highly regioselective acylation. Tetrahedron. 2003;59:9101–9. [Google Scholar]
- 15. Haraldsson GG, Gudmundsson BO, Almarsson O. The synthesis of homogenous triglycerides of eicosapentaenoic acid and docosahexaenoic acid by lipase. Tetrahedron. 1995;51:941–52. [Google Scholar]
- 16. Gandhi NN. Applications of lipase. J Am Oil Chem Soc. 1997;74:621–34. [Google Scholar]
- 17. Kobayashi M. Invivo antioxidant role of astaxanthin under oxidative stress in the green alga Hematococcus pluvialis. Appl Microbiol Biotechnol. 2000;54:550–5. doi: 10.1007/s002530000416. [DOI] [PubMed] [Google Scholar]
- 18. Naguib YM. Antioxidant activities of astaxanthin and other carotenoids. J Agric Food Chem. 2000;48:1150–4. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- 19. Chew BP, Park JS, Wong MW, Wong TS. A comparison of the anticancer activities of dietary beta carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999;19:1848–53. [PubMed] [Google Scholar]
- 20. Naito T, Uchiyama K, Aoi W, Hasegawa G, Nakamura N, Yoshida N, et al. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. Biofactors. 2004;20:49–59. doi: 10.1002/biof.5520200105. [DOI] [PubMed] [Google Scholar]
- 21. Bennedsen M, Wang X, Willen R, Wadstroem T, Andersen LP. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulated cytokine release by splenocytes. Immunol Lett. 1999;70:185–9. doi: 10.1016/s0165-2478(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 22. Ferdosh S, Sarker ZI, Norulaini N, Oliveira A, Yunus K, Chowdury AJ, et al. Quality of tuna fish oils extracted from processing the by-products of three species of Neritic Tuna using supercritical carbon dioxide. J Food Process Preserv. 2015;39:432–41. [Google Scholar]
- 23. Rodriguez A, Esteban L, Martin L, Jimenez MJ, Hita E, Castillo B, et al. Synthesis of 2-monoacylglycerols and structured triacylglycerols rich in polyunsaturated fatty acids by enzyme catalyzed reactions. Enzyme Microb Technol. 2012;51:148–55. doi: 10.1016/j.enzmictec.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 24. Munio MM, Esteban L, Robles A, Hita E, Jimenez MJ, Gonzalez PA, et al. Synthesis of 2-monoacylglycerols rich in polyunsaturated fatty acids by ethanolysis of fish oil catalyzed by 1,3 specific lipases. Process Biochem. 2008;43:1033–9. [Google Scholar]
- 25. Bispo P, Batista I, Bernardino RJ, Bandarra NM. Preparation of triacylglycerols rich in omega-3 fatty acids from sardine oil using a Rhizomucor miehei lipase: focus in the EPA/DHA ratio. Appl Biochem Biotechnol. 2014;172:1866–81. doi: 10.1007/s12010-013-0616-1. [DOI] [PubMed] [Google Scholar]
- 26. Shin SK, Sim JE, Kishimura H, Chun BS. Characteristics of menhaden oil ethanolysis by immobilized lipase in supercritical carbon dioxide. J Ind Eng Chem. 2012;18:546–50. [Google Scholar]
- 27.AOCS. Official method To 1b-64. Sampling and analysis of commercial fats and oils: specific gravity. Champaign-Urbana. Illinois, USA: American Oil Chemists’ Society; 2006. [Google Scholar]
- 28. Ali-Nehari A, Kim SB, Lee YB, Lee HY, Chun BS. Characterization of oil including astaxanthin extracted from krill (Euphausia superba) using supercritical carbon dioxide and organic solvent as comparative method. Korean J Chem Eng. 2012;29:329–36. [Google Scholar]
- 29.AOCS. Sampling and analysis of commercial fats and oils: acid value. Champaign-Urbana. Illinois, USA: American Oil Chemists’ Society; 2006. Official method Cd 3d-63. [Google Scholar]
- 30. Hornero-Méndez D, Pérez-Gálvez A, Mínguez-Mosquera MI. A rapid spectrophotometric method for the determination of peroxide value in food lipids with high carotenoid content. J Am Oil Chem Soc. 2001;78:1151–5. [Google Scholar]
- 31.AOCS. Sampling and analysis of commercial fats and oils: free fatty acids. Champaign-Urbana. Illinois, USA: American Oil Chemists’ Society; 2006. Official method Ca 5a-40. [Google Scholar]
- 32.AOCS. Official method Cd 18-90. Sampling and analysis of commercial fats and oils: p-anisidine value. Champaign-Urbana Illinois, USA: American Oil Chemists’ Society; 2006. [Google Scholar]
- 33. Farhoosh R. The Effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J Am Oil Chem Soc. 2007;84:205–9. [Google Scholar]
- 34.AOCS. Official Method Ce 2-66. Fatty acid composition by gas chromatography. Champaign-Urbana. Illinois, USA: American Oil Chemists’ Society; 2006. [Google Scholar]
- 35. Gulcin I, Elmastas M, Aboul-Enein HY. Antioxidant activity of clove oil – a powerful antioxidant source. Arab J Chem. 2012;5:489–99. [Google Scholar]
- 36. Zheleva-Dimitrova D, Nedialkov P, Kitanov G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn Mag. 2010:74–8. doi: 10.4103/0973-1296.62889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu X, Skands ARH, Hoy CE, Mu H, Balchen S, Adler-Nissen J. Production of specific-structured lipids by enzymatic interesterification: elucidation of acyl migration by response surface design. J Am Oil Chem Soc. 1998;75:1179–86. [Google Scholar]
- 38. Rosu R, Yasui M, Iwasaki Y, Yamane T. Enzymatic synthesis of symmetrical 1,3-diacylglycerols by direct esterification of glycerol in solvent-free system. J Am Oil Chem Soc. 1999;76:839–43. [Google Scholar]
- 39. Esteban L, Munio MM, Robles A, Hita E, Jimenez MJ, Gonzalez PA, et al. Synthesis of 2-monoacylglycerols (2-MAG) by enzymatic alcoholysis of fish oils using different reactor types. Biochem Eng J. 2009;44:271–9. [Google Scholar]
- 40. Wu PW. A review on the analysis of ingredients with health care effects in health food in Taiwan. J Food Drug Anal. 2015;23:343–50. doi: 10.1016/j.jfda.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjerking B. Carotenoid pigmentation of salmonid fishes – recent progress. Norway: Nov 19–22, 2000. [Google Scholar]
- 42. Sathivel S. Thermal and flow properties of oils from salmon head. J Am Oil Chem Soc. 2005;82:147–52. [Google Scholar]
- 43. Haq M, Getachew AT, Saravana PS, Cho YJ, Park SK, Kim MJ, et al. Effects of process parameters on EPA and DHA concentrate production from Atlantic salmon by-product oil: optimization and characterization. Korean J Chem Eng. 2016 doi: 10.1007/s11814-016-0362-5. [DOI] [Google Scholar]
- 44.Swern D. Fatty acids. Their chemistry, properties, production, and uses. New York, USA: 1964. [Google Scholar]
- 45. Lee YR, Baokun T, Kyung HR. Extraction and separation of astaxanthin from marine products: a Review. Asian J Chem. 2014;26:4543–9. [Google Scholar]
- 46. Wiedermann LH. Degumming, refining and beaching soybean oil. J Am Oil Chem Soc. 1981;58:159–66. [Google Scholar]
- 47.FAO. FAO/INFOODS database. Densities and specific gravities in foods. Oils and fats. Density database version 2.0. Rome, Italy: 2012. [Google Scholar]
- 48. Kim J, Kim DN, Lee SH, Yoo SH, Lee S. Correlation of fatty acid composition of vegetable oils with rheological behavior and oil uptake. Food Chem. 2010;118:398–402. [Google Scholar]
- 49. Rubio-Rodriguez N, de Diego SM, Beltran S, Jaime I, Sanz MT. Supercritical fluid extraction of fish oil from fish byproducts: a comparison with other extraction methods. J Food Eng. 2012;109:238–48. [Google Scholar]
- 50. Rubio-Rodriguez N, de Diego SM, Beltran S, Jaime I, Sanz MT. Supercritical fluid extraction of the omega-3 rich oil contained in hake (Merluccius capensis–Merluccius paradoxus) by-products: study of the influence of process parameters on the extraction yield and oil quality. J Supercrit Fluid. 2008;47:215–26. [Google Scholar]
- 51. Barthet VJ, Gordon V, Daun JK. Evaluation of a colorimetric method for measuring the content of FFA in marine and vegetable oils. Food Chem. 2008;111:1064–8. [Google Scholar]
- 52. Deepika D, Vegneshwaran VR, Julia P, Sukhinder KC, Sheila T, Heather M, et al. Investigation on oil extraction methods and its influence on omega-3 content from cultured salmon. J Food Process Technol. 2014;5:1–13. [Google Scholar]
- 53. Ekwu FC, Nwagu A. Effect of processing on the quality of cashew nut oils. J Sci Agric Food Tech Environ. 2004;4:105–10. [Google Scholar]
- 54. Boran G, Karacam H, Boran M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006;98:693–8. [Google Scholar]
- 55. Estiasih T, Ahmadi K, Nisa FC. Optimizing conditions for the purification of omega-3 fatty acids from the by-product of tuna canning processing. J Food Sci Technol. 2013;5:522–9. [Google Scholar]
- 56. Pando ME, Bravo B, Berrios M, Galdame A, Rojas C, Romero N, et al. Concentrating n-3 fatty acids from crude and refined commercial salmon oil. Czech J Food Sci. 2014;32:169–76. [Google Scholar]
- 57. Aidos I, van der Padt A, Boom RM, Luten JB. Upgrading of Maatjes herring by-products: production of crude fish oil. J Agric Food Chem. 2001;49:3697–704. doi: 10.1021/jf001513s. [DOI] [PubMed] [Google Scholar]
- 58.GOED. GOED voluntary monograph. Salt Lake City, Utah, United States of America: Global Organization for EPA and DHA; 2014. [Google Scholar]
- 59. Sathivel S, Yin H, Prinyawiwatkul W, King JM. Comparisons of chemical and physical properties of catfish oils prepared from different extracting processes. J Food Sci. 2009;74:70–6. doi: 10.1111/j.1750-3841.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- 60. Liu S, Zhang C, Hong P, Ji H. Concentration of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) of tuna oil by urea complexation: optimization of process parameters. J Food Eng. 2006;73:203–9. [Google Scholar]
- 61. Bendini A, Cerretani L, Pizzolante L, Toschi TG, Guzzo F, Ceoldo S, et al. Phenol content related to antioxidant and antimicrobial activities of Passiflora spp. extracts. Eur Food Res Technol. 2006;223:102–9. [Google Scholar]
- 62. Ozcelik B, Lee JH, Min DB. Effects of light, oxygen and pH on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method to evaluate antioxidants. J Food Sci. 2003;68:487–90. [Google Scholar]
- 63. Haq M, Ahmed R, Cho YJ, Chun BS. Quality properties and bio-potentiality of edible oils from Atlantic salmon byproducts extracted by supercritical carbon dioxide and conventional methods. Waste Biomass Valor. 2016 doi: 10.1007/s12649-016-9710-2. [DOI] [Google Scholar]
- 64. Bandarra NM, Campos RM, Batista I, Nunes ML, Empis JM. Antioxidant synergy of α-tocopherol and phospholipids. J Am Oil Chem Soc. 1999;76:905–13. [Google Scholar]
- 65. Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–8006. [Google Scholar]