Abstract
Mulberry (Morus alba) leaf has been used in Chinese medicine as the remedy for hyperlipidemia and metabolic disorders. Recent report indicated Mulberry leaf extract (MLE) attenuated dyslipidemia and lipid accumulation in high fat diet (HFD)-fed mice. Non-alcoholic fatty liver (NAFLD) is generally considered as the liver component of metabolic syndrome. The hepatic lipid infiltration induces oxidative stress, and is associated with interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) which are regulated by the leptin and adiponectin. MLE could prevent obesity-related NAFLD via downregulating the lipogenesis enzymes while upregulating the lipolysis markers. Treatment of MLE, especially at 2%, enhanced the expression of superoxide dismutase (SOD) and clenched the oxidative stress of liver. MLE decreased the plasma level of leptin but increased adiponectin. The advantage of MLE is supposed mainly attributed to chlorogenic acid derivative. We suggest MLE, with promising outcome of research, could be nutraceutical to prevent obesity and related NAFLD.
Keywords: Mulberry leaf, Non-alcoholic fatty liver, Adiponectin, Oxidative stress, Inflammation
1. Introduction
Overweight and obesity is associated with a great diversity of diseases involving cardiovascular and metabolic systems [1]. There is a metabolic link between the expanded body fat, high triacylglycerol (TG), high low density lipoprotein cholesterol (LDL-C), and low high-density lipoprotein cholesterol (HDL-C), which leads to impaired metabolic regulation of adipose and flux of free fatty acids (FFA) [2].
Liver plays an essential role of modulating plasma lipid level through LDL clearance and HDL recruitment. However, the lipid uptake must affect the hepatic fat composition and burden the liver function. Hence the regulation of hepatic lipid metabolism should be emphasized to prevent dyslipidemia and accompanying illness. Expressions of fatty acid synthase (FAS) and 3-hydroxy-3-methylglutaryl-coenzyme A (HMGCoA) reductase, the important enzymes regulating TG and cholesterol synthesis, are indicated markers of lipogenesis [3]. As well, 1-acylglycerol-3-phosphate acyltransferase (AGPAT) is involved in the synthesis of glycerophospholipid [4]. On the other hand, the expressions of carnitine palmitoyltransferase-1 (CPT-1) and peroxisome proliferator-activated receptor α (PPARα) are critically associated with the process of lipolysis [5].
Non-alcoholic fatty liver (NAFLD) is generally considered to be the liver component of metabolic syndrome, which is frequently accompanied with obesity, dyslipidemia, and insulin resistance [6]. The degree of fat infiltration of liver is related to the subsequent development of necrosis, inflammation, cirrhosis, and the propensity to progress to hepatocellular carcinoma [7]. Increased fat mass and associated fat gene expression enhance lipogenesis and oxidative stress [8]. The oxidative stress, disrupted nitric oxide (NO) signaling, and mitochondrial dysfunction are proposed to be pivotal events that accelerate steatosis and initiate the progression to hepatitis and fibrosis [9]. Oxidative stress could be eliminated by endogenous antioxidative enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx), or the exogenous application of antioxidants [10].
The fatty liver index often accompanied with elevation of aspartate transaminase (AST) and alanine transaminase (ALT) [11], is independently associated with inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) [12]. Leptin and adiponectin are adipokines produced by white adipose tissue. Leptin upregulates TNF-α and IL-6, and is associated with insulin resistance and type 2 diabetes mellitus. In contrast, adiponectin has anti-inflammatory properties and downregulates the expression and release of proinflammatory immune mediators [13].
Mulberry leaf (the leaf of Morus alba), commonly used as the silkworm diet, has been used in Chinese medicine for antidiabetes, antihyperlipidemics, and prevention of coronary artery disease. It contains a lot of components including flavonoid, which is known as a powerful polyphenol and antioxidant [14]. Our previous study suggested mulberry leaf extract (MLE) and its polyphenols possessed antiatherogenesis effect via Inhibiting LDL oxidation and foam cell formation [15]. A recent report indicated that MLE attenuated the dyslipidemia and lipid accumulation in high fat diet (HFD)-fed mice; the mulberry leaf polyphenols induced adipocyte apoptosis and inhibited preadipocyte differentiation [16].
In the present study, we aim to investigate if MLE improves the oxidative stress, inflammation, and ratio of adipokines, thus prevents obesity-induced metabolic disturbance and NAFLD.
2. Material and methods
2.1. Preparation of MLE and chemical analysis
Fresh mulberry leaves (100 g) were harvested and immediately dried at 50 °C. The dried leaves were heated in 1500 mL of deionized water. After filtration, the residue was removed, and the suspension was stored at −80 °C overnight and then evaporated with a freeze-dryer. The dried powder remained was an aqueous fraction of mulberry leaves (MLE), which was used in the following animal experiment. The polyphenols of mulberry leaf was extracted as in the previous report (Yang et al., 2011) [15], and then analyzed for its chemical composition. In our previous report, the composition analysis revealed that it contains neochlorogenic acid (35.5%), cryptochlorogenic (31.7%), chlorogenic (23.8%), rutin (9.2%), isoquercitrin (5.6%), astragalin acid (5.3%), nicotiflorin (3.5%), and protocatechuic acid (1.3%) [17].
2.2. Animal experiment
The animal experimental project was approved by the Animal Model Experimental Ethics Committee of Chung-Shan Medical University, and was in accordance with the recommendation in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Briefly, male Wistar rats (weight 220 ± 10 g) were obtained from LuxBiotech Co., Taiwan. The rats were acclimated in laboratory conditions (23 ± 2 °C, 60 ± 5% relative humidity, and 12 h light/dark cycle), and fed a basic chow consisting of 12% fat. After one week-adaptation, the Wistar rats were randomly grouped. Each group (n = 8) was fed a unique diet for 14 wks and weighed every 2 wks. The groups and their corresponding meals were (1) control, normal diet; (2) HFD, normal diet supplemented 2% cholesterol and 20% lard oil; (3) HFD + 0.5% MLE; (4) HFD + 1% MLE; (5) HFD + 2% MLE. All the formulas of MLE were added from 4 wk. In the end of the experiment, blood and livers were collected from rats fasted for 12–14 h and then sacrificed. The blood was collected by EDTA tubes and centrifuged at 3000 rpm or 10 min at 4 °C. The supernatant plasma was transferred into new tubes for determination of serum biomarkers. The livers were quickly frozen in liquid nitrogen for the extraction of liver lipids or freshly cut into pieces for H-E stain.
2.3. Serum biochemical markers
The serum sample was collected using ethylenediaminetetraacetic acid (EDTA) tubes and centrifuged at 3000 rpm (1400 g) for 10 min at 4 °C. Concentrations of glucose, TGs, total cholesterol, LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), FFA, aspartate aminotransferase (AST), alanine aminotransferase (ALT) were measured by enzymatic colorimetric methods using commercial kits (Randox Laboratories, Ltd., Antrim, UK). The analysis of serum was carried out by an automatic analyzer (Olympus AU2700, Olympus Co., Tokyo, Japan).
2.4. Determinations of total cholesterol and TG in liver
Livers were extracted from the animals and used for analyzing their lipid content. Briefly, liver (1.25 g) was homogenized with 10 mL of chloroform/methanol (1:2, v/v), and then thoroughly mixed with chloroform (1.25 mL) and distilled water (1.25 mL). After centrifugation (1500 g for 10 min), the lower clear organic phase solution was transferred into a new glass tube and then lyophilized. A total of 0.1 g of lyophilized powder was dissolved in 1 mL of chloroform/methanol (1:2, v/v) as the liver lipid extract and stored at 20 °C for less than 3 days. The liver cholesterol and TGs in the lipid extracts were measured by enzymatic colorimetric methods using commercial kits (Randox Laboratories, Ltd., Antrim, UK).
2.5. Preparation of the protein extract of liver
The liver pieces (0.5 g) were added with radioimmunoprecipitation assay (RIPA) buffer (5 mL) and protein inhibitors, and homogenized at 4 °C. The tissue homogenates were centrifuged (10,000 g for 20 min at 4 °C), and the resulting supernatants (whole-tissue extracts) were used for Western blot analyses. The total protein concentrations of the whole tissue extracts were determined through the Bradford protein assay kit (Bio-Rad, Hercules, CA, USA).
2.6. Western blot analysis
Equal amounts of protein samples (50 μg) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% nonfat milk powder with 0.1% Tween-20 in Tris-buffered saline (TBS) and then incubated with the primary antibody at 4 °C overnight. Afterward, membranes were washed three times with 0.1% Tween-20 in TBS and incubated with the secondary antibody conjugated to horseradish peroxidase (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Antibodies of FAS and HMG-CoA reductase were obtained from Transduction Laboratory (Lexington, KY, USA) and Upstate Biotechnology (Lake Placid, NY, USA), respectively. AGPAT antibody used was Abcam ab76018. Antibodies of PPARα and CPT-1 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Band detection was thereafter revealed by enhanced chemiluminescence using electrochemiluminescence (ECL) Western blotting detection reagents and exposed in Fujifilm Las-3000 (Tokyo, Japan). Protein quantitative was determined by densitometry using Fujifilm-Multi Gauge, version 2.2, software.
2.7. H–E stain of liver
Liver tissues were fixed in 4% paraformaldehyde overnight and embedded in paraffin by routine procedure. Consecutive 4 μm paraffin sections were made, and processed for histological examination by conventional methods with hematoxylin and eosin (H&E) stain.
2.8. TBARS
0.2 mL liver homogenate was mixed with 0.25 mL of 25% (w/v) trichloroacetic acid, and then centrifuged (10,000 rpm for 30 min at 10 °C). 0.3 mL supernatant reacted with 0.3 mL of 1% thiobarbituric acid (TBA) for 40 min at 95 °C in the dark. Samples were then analyzed by a Hitachi F2000 fluorescence spectrophotometer with excitation at 532 nm, for measuring malondialdehyde (MDA), the lipid peroxidation product. The preparation curve was prepared with 0–50 nm of standards (r2 = 0.9927).
2.9. Activity of antioxidative enzymes
The analysis of antioxidative enzymes was performed according to the previous report [18]. SOD was determined with a modified Marklund method. Catalase activity was measured with the modified method proposed by Abei. The activity of GSH-Px was determined by the method of Lawrence and Burk.
2.10. Enzyme-linked Immunosorbent Assay (ELISA)
Serum concentrations of TNF-α, IL-6, leptin, and adiponectin were determined in triplicate using a commercial enzyme-linked immunosorbent assay (TNF-α and IL-6 from RayBiotech; leptin from RayBiotech and adiponectin from Assay-Max™). Briefly, after two-fold dilution, 100 μL diluted serum sample was added to the monoclonal antibody-coated 96-well plate at 4 °C overnight. After washed, samples were subsequently incubated with the secondary antibody for 1 h at room temperature. Samples were then treated with 100 μL streptavidin (45 min), 100 μL TMB One-Stop Substrate Reagent (30 min), and 50 μL stop solution, respectively. The OD values were detected under 450 nm.
2.11. Statistical analysis
The statistical software SPSS v.12.0 was used to analyze the data. One-way ANOVA was performed (p < 0.05), while Holm–Sidak method was used for post test.
3. Results
3.1. MLE lowered the body weight and body fat
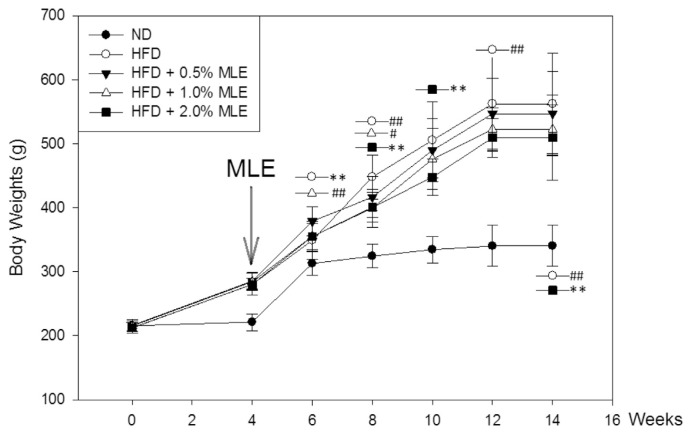
Fig. 1 shows that MLE significantly lowered the body weight from 6 wk. In the end of experiments, 2% of MLE exhibited a good effect to decrease the body weight of rats fed with HFD (500 vs. 560 g). As well, HFD increased the body fat, but 0.2% of MLE effectively reduced the fat mass (Table 1).
Fig. 1.
Effect of MLE on the body weight of HFD-fed rats. Rats were fed with ND, normal diet; HFD, high fat diet; and HFD supplemented with different doses of MLE. Data was presented as the mean ± SD. #p < 0.05, ##p < 0.01, compared with the ND group. *p < 0.05, **p < 0.01 compared with the HFD group.
Table 1.
Effects of MLE on the body fat of HFD-fed rats.
| Adipose tissue weight (mg/rat) | ND | HFD | HFD + 0.5% MLE | HFD + 1% MLE | HFD + 2% MLE |
|---|---|---|---|---|---|
| Gonads | 1824.27 ± 473.00 | 11232.63 ± 3615.64## | 8987.56 ± 2166.31 | 10146.67 ± 2236.22 | 7481.67 ± 3110.53* |
| Perirenal fat | 1054.18 ± 677.41 | 13990.38 ± 3825.63## | 12514.44 ± 1934.32 | 13935.20 ± 3633.34 | 8642.70 ± 3828.58** |
| Mesenteric fat | 1088.09 ± 680.76 | 9796.22 ± 3520.17## | 10463.70 ± 2964.70 | 10385.40 ± 2676.27 | 6474.10 ± 3375.58* |
| Subcutaneous | 1546.18 ± 715.72 | 9087.13 ± 3057.13## | 8610.56 ± 2771.96 | 9046.00 ± 3243.30 | 5893.67 ± 2277.51* |
| Groin fat | 587.55 ± 433.93 | 3455.38 ± 2823.03## | 2397.60 ± 1543.88 | 2397.00 ± 1043.03 | 1414.10 ± 608.42* |
| Total Peripheral fat | 6100.27 ± 478.65 | 47561.72 ± 3871.71## | 42973.86 ± 3688.67** | 45910.27 ± 4213.86** | 29906.23 ± 2758.80** |
| Total body crude fat | 0.05 ± 0.04 | 0.21 ± 0.05## | 0.16 ± 0.04** | 0.16 ± 0.03* | 0.15 ± 0.05* |
ND, normal diet; HFD, high fat diet; HFD + 0.5% MLE, HFD supplemented with 0.5% MLE; HFD + 1% MLE, HFD supplemented with 1% MLE; HFD + 2% MLE, HFD supplemented with 2% MLE. Data was presented as the mean ± SD.
p < 0.05,
p < 0.01, compared with the ND group.
p < 0.05,
p < 0.01 compared with the HFD group.
3.2. MLE lowered the serum biochemical parameters
Compared with the control, HFD increased serum TG (90.33 vs. 48.27 mg/dL) and cholesterol (69.89 vs. 59.00 mg/dL). Treatment of MLE, especially at 2%, significantly decreased 24% of TG and 26% of cholesterol in HFD-fed rats. While HDL-C was not altered, LDL-C was slightly elevated in HFD group (no significance). Treatment of MLE significantly decreased LDL-C to 14.00 and 13.67 mg/dL, respectively (Table 2).
Table 2.
Effects of MLE on the serum biochemical parameters of HFD fed rats.
| ND | HFD | HFD + 0.5% MLE | HFD + 1% MLE | HFD + 2% MLE | |
|---|---|---|---|---|---|
| AST (U/L) | 170.43 ± 22 | 190.75 ± 29.25 | 188.63 ± 42.15 | 188.13 ± 38.60 | 158.38 ± 42.65 |
| ALT (U/L) | 72.57 ± 8.62 | 84.43 ± 8.73# | 66.57 ± 22.10 | 65.00 ± 9.35** | 65.56 ± 10.6** |
| TG (mg/dL) | 48.27 ± 10.28 | 90.33 ± 23.49## | 79.10 ± 11.44 | 72.82 ± 13.67* | 68.91 ± 13.63** |
| CHO (mg/dL) | 59.00 ± 10.48 | 69.89 ± 10.94# | 63.00 ± 9.87 | 59.00 ± 11.41* | 51.64 ± 6.99** |
| HDL-C (mg/dL) | 33.90 ± 6.10 | 35.38 ± 7.27 | 36.90 ± 5.09 | 35.10 ± 6.44 | 33.80 ± 3.10 |
| LDL-C (mg/dL) | 14.11 ± 3.62 | 20.22 ± 6.51 | 18.00 ± 4.21 | 14.00 ± 3.71* | 13.67 ± 4.82* |
| GLU (mg/dL) | 101.30 ± 6.29 | 110.50 ± 7.17## | 102.33 ± 7.45 | 105.89 ± 7.04 | 105.78 ± 6.00 |
ND, normal diet; HFD, high fat diet; HFD + 0.5% MLE, HFD supplemented with 0.5% MLE; HFD + 1% MLE, HFD supplemented with 1% MLE; HFD + 2% MLE, HFD supplemented with 2% MLE. Data was presented as the mean ± SD.
p < 0.05,
p < 0.01, compared with the ND group.
p < 0.05,
p < 0.01 compared with the HFD group.
The level of AST was not altered, while ALT was significantly increased by HFD. Treatment of MLE significantly lowered the level of ALT. These results implicate MLE is effective to counter liver dysfunction induced by HFD (Table 3).
Table 3.
Effect of MLE on the hepatic lipid content of HFD fed rats.
| ND | HFD | HFD + 0.5% MLE | HFD + 1% MLE | HFD + 2% MLE | |
|---|---|---|---|---|---|
| Liver-cholesterol (g/g protein) | 0.05 ± 0.02 | 0.30 ± 0.14# | 0.24 ± 0.16 | 0.17 ± 0.17* | 0.06 ± 0.03* |
| Liver-triglyceride (g/g protein) | 0.16 ± 0.05 | 0.67 ± 0.11# | 0.55 ± 0.21 | 0.44 ± 0.34* | 0.34 ± 0.17* |
ND, normal diet; HFD, high fat diet; HFD + 0.5% MLE, HFD supplemented with 0.5% MLE; HFD + 1% MLE, HFD supplemented with 1% MLE; HFD + 2% MLE, HFD supplemented with 2% MLE. Data was presented as the mean ± SD.
p < 0.05,
p < 0.01, compared with the ND group.
p < 0.05,
p < 0.01 compared with the HFD group.
3.3. MLE lowered the liver lipid
The contents of liver TG and cholesterol was elevated 4 folds and 6 folds compared with the control. Treatment of MLE significantly reduced hepatic lipid. 2% MLE diminished half of the TG, and reduced cholesterol to the level of the control (Table 3). Histological examination revealed the consistent finding (Fig. 2). HFD induced the occurrence of fatty liver, but MLE attenuated the pathogenesis.
Fig. 2.
Effect of MLE on the prevention of fatty liver. Rats were fed with different diets. After sacrifice, the livers were fixed, embedded, sectioned, and then stained with hematoxylin and eosin (original magnification: 200×).
3.4. MLE reduced hepatic lipogenesis but enhanced lipolysis
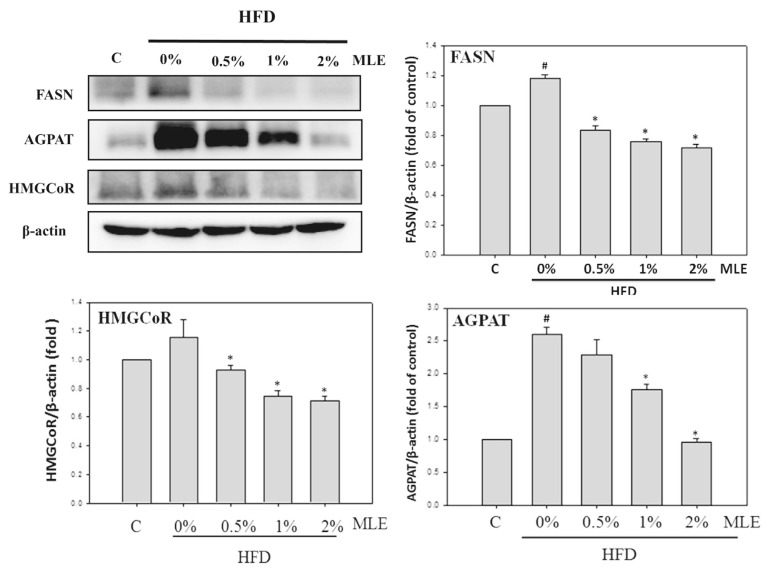
HFD increased both the expression of FASN and HMGCoAR about 1.2 folds. Treatment of MLE dose-dependently decreased these enzymes even below the level of the control. HFD increased AGPAT about 2.5 folds. 2% of MLE completely diminished the elevation induced by HFD (Fig. 3).
Fig. 3.
Effects of MLE on the lipogenesis protein of liver. Rats were fed with different diets. After sacrifice, the liver protein was extracted. FASN, SREBP-1 and AGPAT were analyzed with immunoblotting. Data was presented as the mean ± SD. #p < 0.05, ##p < 0.01, compared with the ND group. *p < 0.05, **p < 0.01 compared with the HFD group.
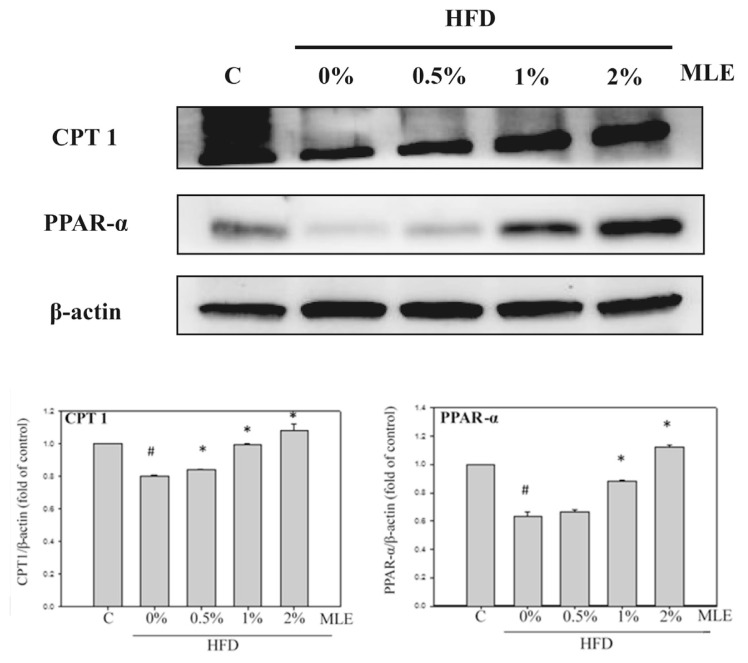
On the contrary, HFD decreased CPT1 and PPARα, but MLE recovered both of the lipolysis markers dose-dependently. 2% of MLE even increased the level of CPT1 and PPARα slightly above the control (Fig. 4).
Fig. 4.
Effects of MLE on the lipolysis protein of liver. Rats were fed with different diets. After sacrifice, the liver protein was extracted. CPT-1, PPARα were analyzed with immunoblotting. Data was presented as the mean ± SD. #p < 0.05, ##p < 0.01, compared with the ND group. *p < 0.05, **p < 0.01 compared with the HFD group.
3.5. MLE decreased hepatic oxidative stress but increased anti-oxidative enzymes
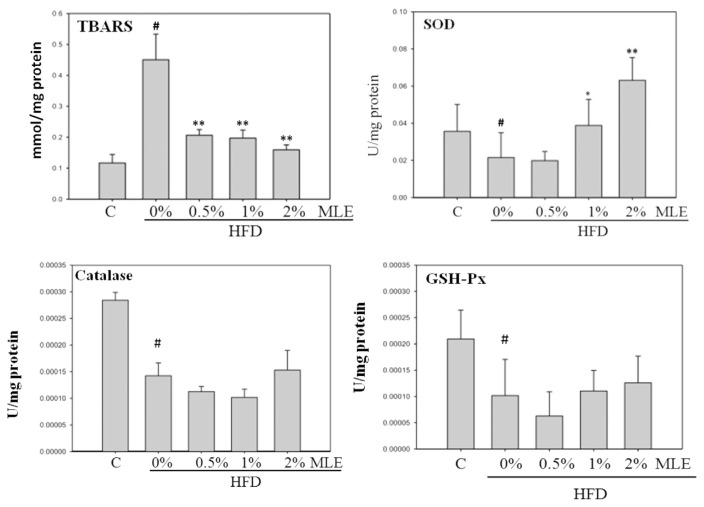
TBARS analysis revealed that HFD increased the oxidative stress almost 5 folds, which was diminished by MLE. On the contrary, HFD decreased the level of SOD. Treatment of MLE, especially at 2%, enhanced the expression of SOD substantially. However, the HFD reduced-expression of catalase and GSH-Px could not be altered by MLE (Fig. 5).
Fig. 5.
Effects of MLE on the oxidative stress and antioxidant enzymes of liver. Rats were fed with different diets. After sacrifice, the liver chops were collected and processed. Data was presented as the mean ± SD. #p < 0.05, ##p < 0.01, compared with the ND group. *p < 0.05, **p < 0.01 compared with the HFD group.
3.6. MLE reduced inflammatory cytokines
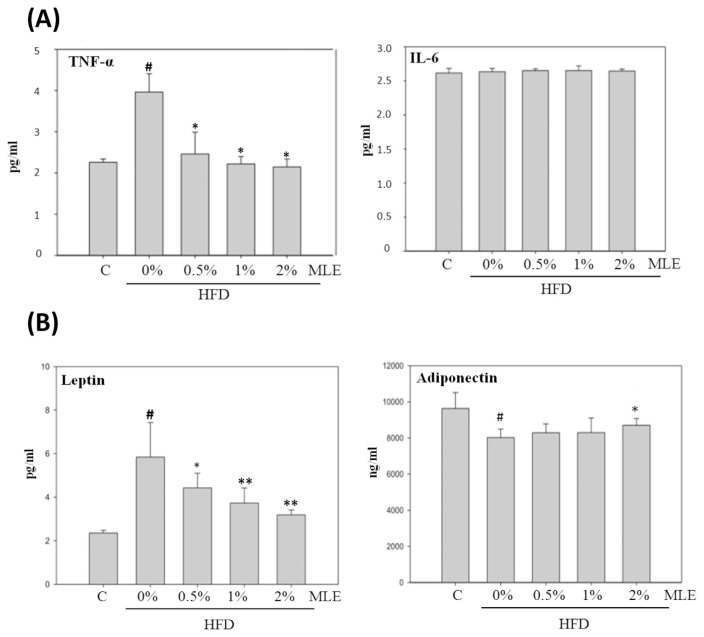
The level of serum TNF-α was increased 2-folds in HFD-fed diet, which was completely inhibited by the supplement of MLE. However, neither HFD nor MLE affected the level of IL-6 (Fig. 6A).
Fig. 6.
Effect of MLE on serum inflammatory cytokines (A) and adipokines (B). Rats were fed with different diets. After sacrifice, the serum was collected and analyzed with ELISA. Data was presented as the mean ± SD. #p < 0.05, ##p < 0.01, compared with the ND group. *p < 0.05, **p < 0.01 compared with the HFD group.
3.7. MLE decreased the level leptin but increased the level of adiponectin
HFD increased plasma leptin about 2 folds, and MLE returned the level dose-dependently. Adiponectin was decreased by HFD, but slightly increased by 2% of MLE (Fig. 6B).
4. Discussion
In the present study, we demonstrated MLE possessed a good ability to prevent obesity-related NAFLD. MLE regulated the hepatic lipogenesis and lipolysis enzymes, reduced the liver lipid, and clenched the oxidative stress of liver. Moreover, MLE decreased the plasma level of leptin but increased adiponectin, thus could ameliorate the inflammation accompanied with NAFLD.
In view of the distribution of body fat, there existed the controversies of whether visceral or subcutaneous fat contributed to the related metabolic disorder. Kim et al. measured the fasting levels of serum insulin, FFA, glucose disappearance rate, and hepatic glucose production after surgical removal of visceral or subcutaneous fat tissue in monosodium glutamate-obese rats. They suggested visceral fat affect FFA level and insulin sensitivity more than subcutaneous fat [19]. Hence the redistribution of body fat by reducing visceral fat and increasing the subcutaneous fat could be more protective from metabolic syndrome [20]. However, because visceral fat only accounts for a small amount of total body fat, there is other report indicating subcutaneous fat as the dominant contributor to the excess of circulating FFA [21]. Our data showed that treatment of MLE reduced not only the visceral but also the subcutaneous fat. The reduction of total body fat must contribute to decrease the hepatic influx of FFA, thus prevents the liver steatosis.
Moreover, the hepatic lipid reduction and hypolipidemic effect of MLE must be attributed to the regulation of hepatic enzymes inhibiting lipid synthesis and promoting lipid degradation, thus decrease TG and cholesterol. The hepatic TG deposition and secretion of TG-rich lipoproteins, affect the lipoprotein lipase activity and the distribution of lipoprotein subtypes [2]. In our study, although it is not significant, the slight alteration of plasma LDL could be resulted from the lowering of TG.
There is growing evidence that overnutrition negatively interfere with immune system. In the regulation of inflammatory processes, white adipose tissue plays a critical role as an endocrine organ which produces number of adipokines. Among them leptin and adiponectin represent a critical link among metabolism and immunity. Leptin, whose serum level strongly correlates with proportion of body fat stores, is primarily known as a satiety factor suppressing appetite and stimulating energy expenditure, while also a pro-inflammatory adipokine inducing T helper 1 cells [22]. Conversely, adiponectin negatively correlates with body fat mass and is therefore more abundant in lean subjects. Adiponectin, decreased in obesity and conditions associated with insulin resistance, plays an important role in insulin-sensitizing, and acts as an anti-inflammatory factor especially with regard to atherosclerosis [22]. In the present study, accompanying with the reduction of body fat, we showed that MLE modulated the serum levels of such adipokines. Therefore, MLE could attenuate the liver inflammation associated with NAFLD.
In fact, the increased oxidative stress in accumulated fat plays a critical role in the pathogenesis of obesity-associated metabolic syndrome. Production of ROS increased selectively in adipose tissue of obese subjects, accompanied by the increase of NADPH oxidase and decrease of antioxidative enzymes. The oxidative stress caused dysregulated production of adipokines [23]. We demonstrated HFD-induced fat deposition and SOD reduction were simultaneously recovered by MLE. The anti-oxidative ability of MLE could attenuate the dysregulation of adipocytokines, thus improved hyperlipidemia and hepatic steatosis. According to Vincent HK et al., inflammatory pathways induce oxidative stress in obesity. Adipokine levels increase, indirectly causing ROS formation via several intracellular signaling pathways and insulin receptor impairment. Leukocyte infiltration causes enzymatic formation of ROS. Both pathways generate ROS and oxidative damage [24].
Our data showed that ALT was significantly lowered in MLE-treated groups, suggesting MLE is beneficial to prevent liver dysfunction. As a marker of hepatic disorders, ALT is associated with the pathogenesis of metabolic syndrome, type 2 diabetes mellitus and subsequent cardiovascular disease [25]. According to the report of Chang et al., in the absence of a detectable ultrasonic change, ALT and AST might be able to reflect a mild stage of hepatic steatosis, which is sufficient to mediate the association with insulin resistance and related complications [26]. Considering the sub-cellular distribution and biochemical properties of hepatic enzymes, ALT is especially superior for monitoring early or mild liver changes [27].
The chemical analysis showed that several polyphenols are contained in MLE, among them chlorogenic acid derivative is the most abundant. In addition, rutin and quercetin are also existent. Chlorogenic acid supplement rescued obesity and insulin resistance caused by high-fat feeding of male C57BL/6 J mice. This effect is at least partly, by increasing energy expenditure and spontaneous locomoter activity [28]. In Sprague–Dawley rats fed with a high-cholesterol diet, chlorogenic acid markedly altered the increased plasma total cholesterol and low-density lipoprotein, while decreased high-density lipoprotein induced by the diet. The hepatic lipid deposition was significantly attenuated in animals supplemented with chlorogenic acid, probably via the up-regulation of PPAR [29]. Chlorogenic acid inhibited FAS and HMGCoA reductase, but promoted fatty acid oxidation and PPARα expression. As well, chlorogenic acid lowered serum leptin and insulin, while increased serum adiponectin induced by HFD [30]. Rutin activated brown fat [31], suppressed inflammation in macrophages, and blocked the HFD-induced obesity and fatty liver [32]. In the mouse model of NAFLD, quercetin enhanced mitochondria oxidative metabolism [33], and ameliorated the dysregulation of lipid metabolism genes via PI3K/AKT pathway [34]. Hence in the present study, the effect of MLE could be attributed to the synergistic effects of these components.
It was estimated that over 20% of the adult population in developed countries have NAFLD, and the occurrence increased even among children [6]. We suggest MLE, rich of various flavonoids and polyphenols, and with promising outcome of research, could be a nutraceutical to prevent obesity and related NAFLD.
Acknowledgment
This work was supported by a grant from the Ministry of Science and Technology, Taiwan (NSC 104-2811-B-040-007).
Funding Statement
This work was supported by a grant from the Ministry of Science and Technology, Taiwan (NSC 104-2811-B-040-007).
Footnotes
Conflict of interests
The authors declare no conflict of interests.
References
- 1. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 2. Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 3. Yang M-Y, Peng C-H, Chan K-C, Yang Y-S, Huang C-N, Wang C-J. The hypolipidemic effect of Hibiscus sabdariffa polyphenols via inhibiting lipogenesis and promoting hepatic lipid clearance. J Agric Food Chem. 2009;58:850–9. doi: 10.1021/jf903209w. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal AK, Barnes RI, Garg A. Functional characterization of human 1-acylglycerol-3-phosphate acyltransferase isoform 8: cloning, tissue distribution, gene structure, and enzymatic activity. Arch Biochem Biophys. 2006;449:64–76. doi: 10.1016/j.abb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 5. Peng C-H, Liu L-K, Chuang C-M, Chyau C-C, Huang C-N, Wang C-J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J Agric Food Chem. 2011;59:2663–71. doi: 10.1021/jf1043508. [DOI] [PubMed] [Google Scholar]
- 6. Friis-Liby I, Aldenborg F, Jerlstad P, Rundstrom K, Bjornsson E. High prevalence of metabolic complications in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2004;39:864–9. doi: 10.1080/00365520410006431. [DOI] [PubMed] [Google Scholar]
- 7. Amarapurkar D, Kamani P, Patel N, Gupte P, Kumar P, Agal S, et al. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–3. [PubMed] [Google Scholar]
- 8. Guo J, Ren W, Li A, Ding Y, Guo W, Su D, et al. Fat mass and obesity-associated gene enhances oxidative stress and lipogenesis in nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58:1004–9. doi: 10.1007/s10620-012-2516-6. [DOI] [PubMed] [Google Scholar]
- 9. Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol-and obesity-induced fatty liver diseases. Free Radic Biol Med. 2008;44:1259–72. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuhrman B, Oiknine J, Aviram M. Iron induces lipid peroxidation in cultured macrophages, increases their ability to oxidatively modify LDL, and affects their secretory properties. Atherosclerosis. 1994;111:65–78. doi: 10.1016/0021-9150(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 11. Gasbarrini G, Vero V, Miele L, Forgione A, Hernandez A, Greco A, et al. Nonalcoholic fatty liver disease: defining a common problem. Eur Rev Med Pharmacol Sci. 2005;9:253. [PubMed] [Google Scholar]
- 12. Wang P-W, Hsieh C-J, Psang L-C, Cheng Y-F, Liou C-W, Weng S-W, et al. Fatty liver and chronic inflammation in Chinese adults. Diabetes Res Clin Pract. 2008;81:202–8. doi: 10.1016/j.diabres.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 13. López-Jaramillo P, G ómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, et al. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014;18:37–45. doi: 10.1515/hmbci-2013-0053. [DOI] [PubMed] [Google Scholar]
- 14. Kim SY, Gao JJ, Lee W-C, Ryu KS, Lee KR, Kim YC. Antioxidative flavonoids from the leaves of Morus alba. Arch Pharmacal Res. 1999;22:81. doi: 10.1007/BF02976442. [DOI] [PubMed] [Google Scholar]
- 15. Yang M-Y, Huang C-N, Chan K-C, Yang Y-S, Peng C-H, Wang C-J. Mulberry leaf polyphenols possess antiatherogenesis effect via inhibiting LDL oxidation and foam cell formation. J Agric Food Chem. 2011;59:1985–95. doi: 10.1021/jf103661v. [DOI] [PubMed] [Google Scholar]
- 16. Chang Y-C, Yang M-Y, Chen S-C, Wang C-J. Mulberry leaf polyphenol extract improves obesity by inducing adipocyte apoptosis and inhibiting preadipocyte differentiation and hepatic lipogenesis. J Funct Foods. 2016;21:249–62. [Google Scholar]
- 17. Lee Y-J, Hsu J-D, Lin W-L, Kao S-H, Wang CJ. Upregulation of caveolin-1 by mulberry leaf extract and its major components, chlorogenic acid derivatives, attenuates alcoholic steatohepatitis via inhibition of oxidative stress. Food Funct. 2017:397–405. doi: 10.1039/c6fo01539e. [DOI] [PubMed] [Google Scholar]
- 18. Tang C-C, Lin W-L, Lee Y-J, Tang Y-C, Wang C-J. Polyphenol-rich extract of Nelumbo nucifera leaves inhibits alcohol-induced steatohepatitis via reducing hepatic lipid accumulation and anti-inflammation in C57BL/6J mice. Food Funct. 2014;5:678–87. doi: 10.1039/c3fo60478k. [DOI] [PubMed] [Google Scholar]
- 19. Kim YW, Kim JY, Lee SK. Surgical removal of visceral fat decreases plasma free fatty acid and increases insulin sensitivity on liver and peripheral tissue in monosodium glutamate (MSG)-obese rats. J Korean Med Sci. 1999;14:539–45. doi: 10.3346/jkms.1999.14.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–75. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koutsari C, Jensen MD. Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity. J Lipid Res. 2006;47:1643–50. doi: 10.1194/jlr.R600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 22. Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. 2009;43:157–68. [PubMed] [Google Scholar]
- 23. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Investig. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes. 2006;30:400. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 25. Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22:437–43. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- 26. Chang H-C, Huang C-N, Yeh D-M, Wang S-J, Peng C-H, Wang C-J. Oat prevents obesity and abdominal fat distribution, and improves liver function in humans. Plant Foods Hum Nutr. 2013;68:18–23. doi: 10.1007/s11130-013-0336-2. [DOI] [PubMed] [Google Scholar]
- 27. Esteghamati A, Noshad S, Khalilzadeh O, Khalili M, Zandieh A, Nakhjavani M. Insulin resistance is independently associated with liver aminotransferases in diabetic patients without ultrasound signs of nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2011;9:111–7. doi: 10.1089/met.2010.0066. [DOI] [PubMed] [Google Scholar]
- 28. Ghadieh HE, Smiley ZN, Kopfman MW, Najjar MG, Hake MJ, Najjar SM. Chlorogenic acid/chromium supplement rescues diet-induced insulin resistance and obesity in mice. Nutr Metab. 2015;12:19. doi: 10.1186/s12986-015-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wan CW, Wong CNY, Pin WK, Wong MHY, Kwok CY, Chan RYK, et al. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother Res. 2013;27:545–51. doi: 10.1002/ptr.4751. [DOI] [PubMed] [Google Scholar]
- 30. Cho A-S, Jeon S-M, Kim M-J, Yeo J, Seo K-I, Choi M-S, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 2010;48:937–43. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 31. Yuan X, Wei G, You Y, Huang Y, Lee HJ, Dong M, et al. Rutin ameliorates obesity through brown fat activation. FASEB J. 2017;31:333–45. doi: 10.1096/fj.201600459RR. [DOI] [PubMed] [Google Scholar]
- 32. Gao M, Ma Y, Liu D. Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice. Pharm Res. 2013;30:2940–50. doi: 10.1007/s11095-013-1125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim C-S, Kwon Y, Choe S-Y, Hong S-M, Yoo H, Goto T, et al. Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1. Nutr Metab. 2015;12:33. doi: 10.1186/s12986-015-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pisonero-Vaquero S, Martínez-Ferreras Á, García- Mediavilla MV, Martínez-Flórez S, Fernández A, Benet M, et al. Quercetin ameliorates dysregulation of lipid metabolism genes via the PI3K/AKT pathway in a diet-induced mouse model of nonalcoholic fatty liver disease. Mol Nutr Food Res. 2015;59:879–93. doi: 10.1002/mnfr.201400913. [DOI] [PubMed] [Google Scholar]