Abstract
Staphylococcal enterotoxins cause food poisoning of various degrees of severity. For milk and meat products, there is a high probability of contamination with staphylococcal enterotoxin H (SEH). In this regard specific and sensitive methods are required to be developed for its detection and monitoring. In this work, the gene seh was expressed and a preparation of recombinant toxin was obtained. Using hybridoma technology, a panel of high-affinity monoclonal antibodies (mAbs) to SEH was produced. The antibodies were characterized and shown to have no cross-reactivity towards the main staphylococcal enterotoxins (A, B, C1, D, E, G and I). Based on these mAbs, a method for specific and quantitative detection of SEH was developed in the format of sandwich enzyme immunoassay (linear range, 0.2–3 ng/ml). All the mAbs produced revealed SEH by immunoblotting. Immunochemical analysis of the culture fluids of staphylococcal isolates obtained from the milk of mastitis-infected cows by immunoblotting and sandwich enzyme immunoassay demonstrated the conformity of these methods. Using the developed method, the toxin was revealed in blood serum and liquid food products practically to 100%. From non-liquid foods, it was shown to be extracted to a maximum with a buffer of pH 4.0–4.5.
Keywords: Staphylococcus aureus, Staphylococcal enterotoxins, Staphylococcal enterotoxin H, Monoclonal antibodies, Sandwich enzyme immunoassay
1. Introduction
Staphylococci are widespread in nature: about 30% of the planet’s population are carriers of Staphylococcus aureus [1]. The pathogenic strains of S. aureus produce toxins (staphylococcal enterotoxins, SEs) and cause diseases of varying degree of severity, from mild ailments to septic shock [2,3]. According to the estimates of the US Centers for Disease Control and Prevention, 240,000 cases of staphylococcal food poisoning (SFP) occur each year in the US, leading to hospitalization in 1000 cases and to six deaths. In the European Union, the number of SFP outbreaks is rising, with 386 SFP outbreaks reported in 2013 [4]. Enterotoxins, the main factors of staphylococcal pathogenicity, are globular proteins with a molecular mass of 27–30 kDa, soluble in water and saline solutions. They are rich in lysine, aspartic acid, glutamic acid and tyro-sine residues. Most of them possess a cystine loop required for proper conformation and, probably, involved in emetic activity. They are highly stable, are persistent to most proteolytic enzymes, such as pepsin or trypsin, thus preserving their activity in the digestive tract after ingestion, and are resistant to low pH values. Staphylococcal enterotoxins are also highly heat stable; they are thought to be more heat stable in foodstuffs than in a laboratory culture medium [5,6].
The toxic effect of SEs in primates manifests itself in enteric disorders and vomiting. In the infected organism, they act as superantigens, interacting simultaneously with T-cell receptors (TCR) and molecules of MHC class II (MHC-II) antigens on antigen presenting cells, which leads to the suppression of adaptive immune response and nonspecific activation of T-lymphocytes that massively release cytokines; this, in turn, results in a systemic shock and immune system imbalance. Superantigenic and emetic activities of SEs are two separate functions localized on separate domains of the protein [5].
SEH was found relatively recently. Structurally SEH is close to other SEs; it has about a 30% homology of the amino acid sequence with each of them. In contrast with other SEs (of pI 7.0–8.6), it is an acidic protein with an isoelectric point of 5.7 [7]. Most SEs stimulate the Vβ region of the T-cell receptor; SEH interacts with the T-cell receptor Vα region [8].
There is a sufficient amount of data on its participation in staphylococcal food poisoning. Shown that SEH-producing S. aureus isolates are of high prevalence in staphylococcal food poisoning cases [9]. S. aureus is the main cause of mastitis [10]. The milk of mastitis-infected animals and the products made from this milk can contain staphylococci and SEs. The seh gene was found in S. aureus isolates from milk and dairy products in South Italy [11], Japan [12] and Norway [13]. The seh gene was found in isolates S. aureus not only of cattle, but also of small ruminants. Studies conducted in Italy showed that the seh gene was the most frequent – 33.3% [14]. PCR technology showed the presence of the seh gene in dried fat-free milk during the large-scale milk-product poisoning of more than 13,000 people in Japan in July 2000. Wherein seh was detected more often than other SEs genes in S. aureus isolates from milk of cows with mastitis [12]. SEH is capable of inducing the apoptosis of epithelial cells of staphylococcus-infected cow mammary glands, which may be an important factor of staphylococcal pathogenicity of mastitis [15]. Facts that indicate the risk of contamination of milk and milk products SEH make necessary a highly sensitive detection of toxin.
SEH can be assayed by molecular biological methods (by detection of the seh gene [16] and by immunochemical methods (by detection of the protein as a gene product evaluation), using polyclonal antibodies specific for SEH itself [17]. However, the use of polyclonal antibodies has a number of disadvantages (such as cross-reactivity), and the reproducibility of results often depends on the antibody batch. While PCR is one of the techniques commonly used to screen for SE genes, due to its simplicity and low cost, variations in observed SEH concentrations [18] suggest that genotyping alone is insufficient to assess the risk associated with a particular contamination. The spread of cases of SEH-caused food poisoning makes it necessary to develop selective and highly sensitive methods of detecting this enterotoxin in food products. Currently, many different formats for the immunochemical determination of the analyte can be developed on the basis of monoclonal antibodies: various versions of Enzyme-Linked Immuno Sorbent Assay, the use of fluorescent and fluorescent labels, including fluorescence-polarization and fluorescence resonance energy transfer. Monoclonal antibodies are also successfully used in the creation of biosensors that detect the sought-for substances in situ in real time, in which the binding of the antibody to the ligand in the biosensor leads to a detectable signal [19]. However, the success of any immunochemical platform is primarily determined by the quality of the antibodies used. The objective of this work was to obtain selective reagents – monoclonal antibodies to SEH, and to use them for developing methods of immunochemical assay SEH in biological fluids and food products.
2. Materials and methods
2.1. Isolation and cloning of the seh gene
S. aureus strain MRSA2308 provided by N.F. Gamaleya Federal Research Center for Epidemiology & Microbiology was used as a bacterial strain for the PCR amplification of the whole staphylococcal seh gene. The PCR was performed using the specific primers P1 (5′-TTTCCATGGAAGATTTACACGA-TAAAAGTGAGTTAAC-3′) and P2 (5′-TTTGCGGCCGCTACTTT-TTTCTTAGTATATAGATTTAC-3′). The primers were designed according to the seh sequence (GenBank Accession Number AY345144.1). The forward primer (P1) contained an engineered NcoI site (underlined), and the reverse primer (P2) incorporated an engineered NotI site (underlined). The optimized PCR conditions were as follows: initial denaturation at 95 °C for 2 min followed by 30 cycles of denaturation (95 °C, 1 min), annealing (50 °C, 30 s), extension (72 °C, 1 min), and final extension at 72 °C for 5 min. The amplified product was isolated from the agarose gel with a Cleanup Mini kit (Eurogen, Moscow) and cloned into the expression vector pET28b containing 6 × His tag as a NcoI–NotI fragment. The ligated product was transformed in the expression host Rosetta-gami Escherichia coli (DE3). Recombinant clones were screened by the PCR for the presence of toxin gene. The sequence of the plasmid pET28b-seh was confirmed by DNA sequencing in Eurogen (Moscow).
2.2. Expression and purification of recombinant SEH
To isolate SEH, E. coli cells transformed with the plasmid containing the seh gene were cultivated in the LB medium with kanamycin at 30 °C to a density of A680 = 0.6 and then induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a concentration of 1 mM. The bacterial mass was separated by centrifugation at 4000 g for 30 min; the mass was lysed with 8 M urea in 20 mM phosphate buffer (pH 8.0) for 2 h. SEH was isolated by metal-chelate chromatography; the cell lysate was centrifuged at 4000 g, applied on a Ni-NTA agarose column (Quiagen USA) and incubated for 2 h at room temperature. After that, the column was washed with 8 M urea in 20 mM phosphate buffer (pH 8.0) containing 20 mM imidazole. SEH was eluted with the same solution containing 200 mM imidazole. The SEH preparation obtained was purified by gel filtration on a Sephacryl S-100 HR column (0.9 × 90 cm) equilibrated with 20 mM HEPES-Na (pH 7.4) containing 150 mM NaCl. The first peak containing the aggregated forms of the protein was discarded. Last peak containing the monomeric form of the protein was collected and used in further experiments.
2.3. Protein concentration assay
The concentration of SEH was measured by the Bradford method [20]; the concentration of immunoglobulins, by absorbance at 280 nm [21].
2.4. Preparation of hybridoma cell lines
BALB/c mice (2–3 months of age) were subcutaneously immunized with a preparation of SEH (10 μg per mouse). The first immunization was performed with Freund’s complete adjuvant and the next (in 2 weeks) with incomplete adjuvant. Prior to injection, the antigen solution in phosphate-buffered saline (PBS) was emulsified with an equal volume of adjuvant. After 3 days, popliteal lymph nodes were isolated from mice under sterile conditions. Lymphocytes of the immunized animal were mixed with cells of the myeloma line SP2/0 and treated with a polyethylene glycol solution (50%, w/v) to obtain hybrid cells [22]. The cells were transferred to 96-well culture plates in a DMEM (Dulbecco’s Modified Eagle Medium) containing 20% (v/v) FCS (fetal cow serum) and HAT (0.1 mM hypoxanthine, 16 μM thymidine and 0.4 μM aminopterin). A day before cell fusion, macrophages from mouse peritoneal cavity were placed into culture plates to create a feeder layer. Hybrid clones were grown in a humid atmosphere with 5% CO2 at 37 °C. The presence of specific antibodies against SEH in the supracellular supernatants was tested by indirect solid-phase EIA (enzyme-linked immuno-assay) by the reaction with plastic-immobilized toxin (1 μg/ml) in 0.05 M Na-carbonate buffer (pH 9.6). In the case of a positive response, cells were selected, cultivated and cloned twice by limiting dilutions in a DMEM medium containing 20% FCS.
2.5. Production and isolation of monoclonal antibodies against SEH
Monoclonal antibodies were produced in the ascitic fluid of BALB/c mice, which were injected with pristane (0.2 ml per mouse) 7–10 days before the introduction of hybridoma cells. The antibodies were isolated by affinity chromatography on a protein A Sepharose column [23]. The antigen-binding activity of the mAb preparations was evaluated using indirect solid-phase EIA by the reaction with the plastic-immobilized SEH antigen.
2.6. Determination of the types of heavy and light chains of monoclonal antibodies
The types of heavy and light chains of the obtained antibodies were determined by indirect solid-phase EIA, using a commercial kit (Rapid ELISA mouse mAB isotyping kit) according to producer’s recommendation.
2.7. Conjugation of antibodies with biotin
A solution of antibodies (1 mg/ml) in 0.1 M bicarbonate buffer (pH 9.0) was supplemented with biotin N-hydroxysuccinimide ester in dimethylsulfoxide (1 mg/ml) at a molar ratio of 1:20. After incubation for 4 h at room temperature, the solution was dialyzed against PBS.
2.8. Indirect solid-phase enzyme immunoassay
SEH at a concentration of 1 μg/ml was introduced into the wells of EIA plates, into 0.05 M carbonate buffer (pH 9.6). The sorption was continued overnight at 4 °C. In the case of the mAb cross-reactivity analysis, SEs (A, B, C1, D, E, G and I) were introduced and sorbed. The free binding sites of plastic were blocked by incubation with a 1% (w/v) solution of gelatin in PBS for 30 min, and the samples to be examined (supracellular supernatants, immune sera, ascitic fluids, purified mAbs) were introduced into the wells of the plate. If necessary, the samples were prediluted with PBS containing 0.1% Tween 20 (PBST). The incubation with antigen was carried out for 1 h at 20 °C. Then the plates were washed with PBST and – after addition of a conjugate of rabbit antibodies against mouse immunoglobulins mixed with horseradish peroxidase and diluted with PBST according to producer’s instruction – were incubated for 40 min. For detection, a 4 mM solution of o-phenylenediamine in citrate-phosphate buffer (26 mM citric acid, 50 mM Na2HPO4, pH 5.0) containing 0.003% (v/v) H2O2 was used. After development of color, the reaction was stopped by addition of an equal volume of 10% (v/v) sulfuric acid, and absorbance was measured at 490 nm using an Anthos 2020 multi-plate reader.
2.9. Determination of antibodies’ affinity constants
The affinity constants of antibodies were determined by indirect solid-phase EIA according to the protocol in Beatty et al. (1987).
2.10. Sandwich EIA for SEH detection
Sandwich EIA was carried out as described earlier [24]. Capture mAbs were immobilized into wells of EIA plates, then biotin-conjugated detection antibodies (-bio) and a SEH preparation were added. Control wells were only filled with detection antibodies. The immune complexes formed were treated with streptavidin-conjugated peroxidase; after addition of o-phenylenediamine, the complexes were detected by measuring the absorbance at 490 nm. When the expression of SEH by S. aureus isolates was analyzed, the corresponding culture fluids supplemented with 0.1% Tween 20 and 10% normal rabbit serum were added.
2.11. Detection of SEH in food products
The toxin was added to samples of food products (milk with fat content of 3.2, 2.5 and 1.5%; meat broth; cheese; minced chicken; minced beef; cottage cheese with fat content 9%) in terms of 2 μg per 10 g product; the samples were stirred thoroughly for 30 min. Non-liquid products were mixed and subjected to further extraction in a Philips blender. Extraction was performed by (1) the addition of an equal volume of PBST; (2) addition of two volumes of PBST; (3) addition of NaCl to a concentration of 0.5 M and (4) decreasing pH to 4.0–4.5, addition HCl to a concentration of 12 mM. Upon addition of the extraction buffer, the mixture was stirred for 30 min. In the case of an acidic extraction after incubation the mixture was neutralized by addition of NaOH to a concentration of 12 mM. PH was monitored with indicator paper. Then the mixture was centrifuged at 12,000 g for 10 min, and the toxin content in the supernatant was determined by sandwich EIA. Solutions of SEH in PBST were used as positive controls; extracts of food products without the toxin, as negative controls. All the measurements were repeated at least 3 times.
2.12. Electrophoresis and western blot
Electrophoretic analysis of recombinant SEH and mAb preparations was carried out in a 12% polyacrylamide gel by the Laemmli method [25] in a Bio-Rad Mini Protean Tetra System chamber (Bio-Rad, USA). Proteins from the culture fluids were precipitated with acetone (1:9, v/v; 1-h incubation and 20-min centrifugation at 12,000 g). The gels were stained with a 0.04% (w/v) solution of Coomassie G-250 in 3.4% (v/v) perchloric acid.
Immunoblotting was carried out in a Bio-Rad apparatus (Bio-Rad, USA) according to manufacturer’s instruction. The proteins were transferred onto a nitrocellulose membrane (VladiSart (Russia); the membrane was treated with a blocking solution containing 1% (w/v) gelatin in PBST. When investigating the ability of antibodies to interact with SEH, the membrane was cut into fragments, which were treated with each of the biotinylated antibodies (1 μm/ml). For the analysis of culture fluids, the membrane was treated with biotin-conjugated mAb SEH-14 (1 μg/ml). After treating with streptavidin-conjugated alkaline phosphatase (Termo Scientific, USA), the blot was stained according to the instruction of the conjugate producer.
2.13. Isolation and identification of S. aureus strains from milk
To accumulate Staphylococcus microorganisms, an aliquot of milk (1 ml) was added to 9 ml of mannitol salt broth (HiMedia Laboratories Pvt. Ltd., India). Reinoculation of this culture into Baird Parker Agar (HiMedia Laboratories Pvt. Ltd., India) and Azide Blood Agar Pronadisa (Conda, Spain) differential-diagnostic media yielded 30–100 colonies. For further study, isolated colonies with characteristic features (causing β-hemolysis and creating a zone of lecithinase activity) were selected. To interpret biochemical features, a system for identification of staphylococci, micrococci and related genera (bioMérieux, Australia) was used. Attribution of the strains to coagulase-positive staphylococci was done using a preparation of freeze-dried plasma (freeze-dried citrate rabbit plasma; ECOlab, Russia).
2.14. Toxigenicity analysis of staphylococcal isolates
Single identified colonies of S. aureus isolates were cultivated in 10 ml of beef-extract and salt broths on a shaker at 37 °C and intensive aeration for 18 h. Cells were separated from the medium by centrifugation at 4000 g for 10 min. The supernatant was sterilized by filtration through a 0.2-μm syringe filter (Corning, USA). The content of SEH in the filtrates of overnight cultures was determined by enzyme immunoassay and immunoblotting. The bacterial pellet was used to isolate total DNA and to perform the PCR analysis for the presence of the seh gene. The genomic DNA of S. aureus was purified with a QIAamp DNA purification kit (Qiagen GmbH, Hilden, Germany). The presence of the seh gene in the strains of S. aureus was determined by the PCR method. The primers used to detect the seh were designed according to its published nucleotide sequence (GenBank Accession Number AY345144.1). The nucleotide sequences of the forward and reverse primers were 5′-CACATCATATGCGAAAGCAGA-3′ (21 NT) and 5′-CCTTTTAAATCATAAATGTCGAATGA-3′ (26 NT). The optimized PCR conditions were as follows: initial denaturation at 95 °C for 2 min followed by 30 cycles of denaturation (95 °C, 25 s), annealing (50 °C, 15 s), extension (72 °C, 40 s) and final extension at 72 °C for 7 min. The presence of the amplified seh gene was analyzed using 1% agarose gel electrophoresis. The expected size of the PCR product must be 566 bp.
3. Results and discussion
3.1. Gene cloning and production of recombinant SEH
The specific primers P1 and P2 were used to amplify the nucleotide sequence of the full length seh. Primers corresponded to the terminal regions of the gene and contained sites for the restriction enzymes NcoI and NotI. The region of the gene from 1 to 72 nucleotides corresponds to a signal peptide that is necessary for protein transfer through the plasma membrane and is peeled off by signal endopeptidase. This construction allowed during gene expression the accumulation of a protein product in the cytoplasm of cells. The seh gene of S. aureus MRSA2308 was amplified by the PCR to yield a 671-bp amplicon (Fig. 1). The amplified gene was ligated into the pET28b vector having 6 × His tag. The 6 × His affinity tag facilitates purification using the Ni–NTA matrix, it is poorly immunogenic and, in a general case, does not affect secretion, compartmentalization or folding of the fusion protein. The DNA of the cloned fragment of the recombinant pET28b-seh was sequenced and the nucleotide code was translated into the amino acid one; the sequence was compared with the SEH sequence from the PubMed Protein database (http://www.ncbi.nlm.nih.gov/protein/AAA19777.1). The obtained sequence revealed a 100% homology with the region 25–241 of the whole SEH. For objectives this work, the recombinant toxin should not contain an N-terminal signal sequence of SEH since the target protein was intended to be purified from the cytoplasmic fraction of bacterial biomass. Moreover, monoclonal antibodies that can be obtained to that signal sequence would be unsuitable for the analysis of the mature toxin in various samples.
Fig. 1.
An electrophoregram of the seh gene in 1% agarose gel. M, GeneRuler 100 bp Plus DNA ladder; 1, seh gene.
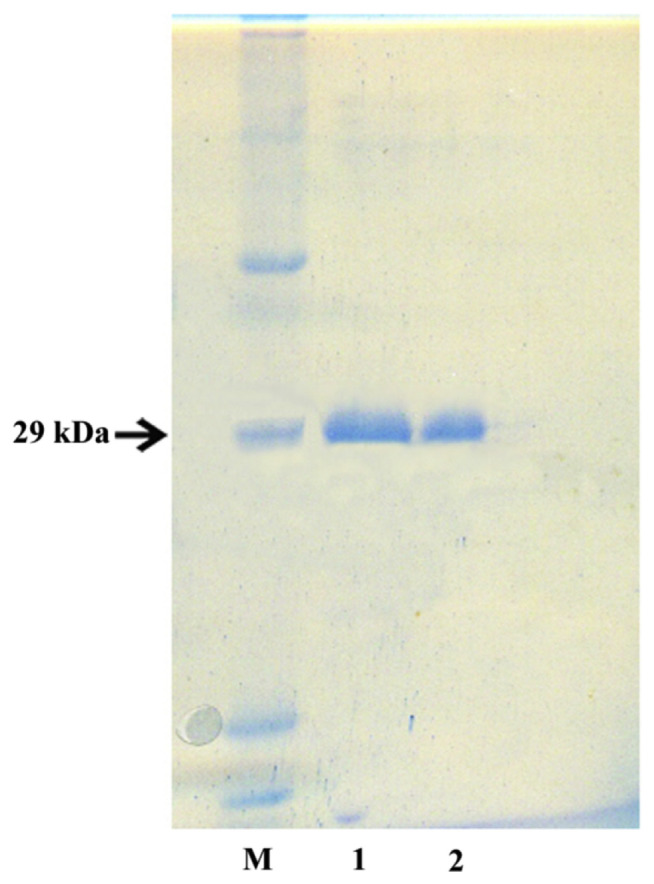
The protein product was produced in Rosetta-gami (DE3) E. coli cells transformed with the constructed plasmid pET28b-seh. A schematic map of the pET28b-seh is shown in Fig. 2. Purification of SEH from the bacterial lysate was performed using metal-chelate chromatography with subsequent gel filtration. As the result, the produced preparation of SEH was virtually homogeneous (Fig. 3). Intracellular production of SEH was made up 7 mg of pure protein per 1 l of bacterial culture.
Fig. 2.
The map of the vector pET28-seh with the cloned seh gene.
Fig. 3.
SDS electrophoresis of purified SEH in 12% polyacrylamide gel. M, protein marker; 1, SEH after Ni-NTA; 2, SEH after gel filtration on a Sephacryl S-100 HR column.
3.2. Production and characterization of monoclonal antibodies against SEH
The recombinant preparation of SEH was used to produced hybridomas to the toxin. For hybridization by the Kohler–Milstein method, lymphocytes from popliteal lymph nodes and cells of the myeloma line SP2/0 were used. Hybridomas secreting specific antibodies were selected by indirect EIA. Twenty four hybridomas were selected and recloned; 18 stable clones, secreting monoclonal antibodies against SEH, were singled out. Production of monoclonal antibodies was conducted in the ascitic fluids of BALB/c mice preinjected with pristane.
To characterize monoclonal antibodies, their isotyping was performed (Table 1). The antibodies were isolated using affinity chromatography on a protein A Sepharose column. The purity of the isolated antibodies was not less than 95%. Table 1 shows the mAb affinity constants determined by the Beatty method [26]. The values of Kaff varied within the range of 0.013–2.1 × 109 M−1; the number of mAbs with Kaff of 109 M−1 and higher was more than a half, which indicated their high specificity to SEH (Table 1).
Table 1.
Immunochemical characteristics of monoclonal antibodies against SEH.
| No. | Antibody | Types of heavy and light chain | Affinity constant × 109 |
|---|---|---|---|
| 1 | SEH-1 | IgG1, κ | 0.69 |
| 2 | SEH-2 | IgG2a, κ | 0.23 |
| 3 | SEH-3 | IgG2a, κ | 3.2 |
| 4 | SEH-4 | IgG2a, κ | 0.93 |
| 5 | SEH-7 | IgG2b, κ | 1.2 |
| 6 | SEH-8 | IgG1, κ | 1.0 |
| 7 | SEH-11 | IgG1, κ | 1.7 |
| 8 | SEH-12 | IgG2a, κ | 1.04 |
| 9 | SEH-13 | IgG1, κ | 2 |
| 10 | SEH-14 | IgG2a, κ | 1.56 |
| 11 | SEH-15 | IgG1, κ | 1.9 |
| 12 | SEH-16 | IgG2a, κ | 1.54 |
| 13 | SEH-17 | IgG2a, κ | 1.7 |
| 14 | SEH-18 | IgG1, κ | 1.8 |
| 15 | SEH-19 | IgG1, κ | 2.1 |
| 16 | SEH-21 | IgG2b, κ | 0.013 |
| 17 | SEH-22 | IgG1, κ | 0.96 |
| 18 | SEH-24 | IgG1, κ | 1 |
The use of immunoblotting for SEH detection is described in the literature [27]; all the mAbs obtained reacted with SEH in this test and can be used in immunoblotting assays for the toxin.
Staphylococcus can contain several enterotoxin genes and, correspondingly, secrete several toxins simultaneously. Herewith, food products get contaminated by several SEs, so it is important that the antibodies used in the tests are specific to the analyzed toxin and do not react with other toxins. Structurally, SEH is attributed to the first group of SEs, which is comprized of enterotoxins O, N, D, J, P, A and E [28]. Inside the group, SEs can have significant differences in their amino acid sequences and a substantial homology in the organization of the secondary structures, and contain homologous, functionally similar domains capable of cross-reactive interaction with antibodies [29]. Taking into account that each SE interacts with its own set of TCR and MHC-II variants, the occurrence of more than one toxin in nutrition significantly complicates the intoxication pattern. The presence of all toxins in contaminated food products should be determined; their detection and quantitative assessment requires highly specific tools, antibodies. For this reason, an important stage of the work was to analyze the cross-reactivity of the produced mAbs with the main staphylococcal enterotoxins.
Table 2 shows their cross-reactivity towards frequently occurring representatives of SE families: A, B, C1, D, E, G and I. Among those mAbs, only SEH-21 possessed a significant cross-reactivity; it reacted with SEB and SEC1 more efficiently than with SEH. Six mAbs (SEH-1, SEH-15, SEH-17, SEH-18, SEH-19 and SEH-22) showed an insignificant reactivity with SEC1; SEH-1 slightly reacted with SEA; SEH-1 and SEH-15 with SEB; SEH-21 with SED.
Table 2.
Cross-reactivity of monoclonal antibodies against SEH with S. aureus enterotoxins. For all mAbs except SEH-21, the maximal reaction with toxin H was taken as the 100% reactivity; for SEH-21, the 100% reactivity was defined as the maximal reaction with toxins B and C1.
| No. | Antibody | Reaction with S. aureus enterotoxins | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| A | B | C1 | D | E | G | I | H | ||
| 1 | SEH-1 | 2% | 5% | 3% | 7% | – | 2% | 100% | |
| 2 | SEH-2 | – | – | – | – | – | 100% | ||
| 3 | SEH-3 | – | – | – | – | – | – | – | 100% |
| 4 | SEH-4 | – | – | – | – | – | – | – | 100% |
| 5 | SEH-7 | – | – | – | – | – | – | – | 100% |
| 6 | SEH-8 | – | – | – | – | – | – | – | 100% |
| 7 | SEH-11 | – | – | – | – | – | – | – | 100% |
| 8 | SEH-12 | – | – | – | – | – | – | – | 100% |
| 9 | SEH-13 | – | – | – | – | – | – | – | 100% |
| 10 | SEH-14 | – | – | – | – | – | – | – | 100% |
| 11 | SEH-15 | – | 2% | 7% | – | – | – | – | 100% |
| 12 | SEH-16 | – | – | – | – | – | – | – | 100% |
| 13 | SEH-17 | – | – | 2% | – | – | – | – | 100% |
| 14 | SEH-18 | – | – | 3% | – | – | – | – | 100% |
| 15 | SEH-19 | – | – | 4% | – | – | – | – | 100% |
| 16 | SEH-21 | – | 100% | 100% | – | – | – | – | 50% |
| 17 | SEH-22 | – | – | – | – | – | – | – | 100% |
| 18 | SEH-24 | – | – | – | – | – | – | – | 100% |
3.3. Development of test systems for quantitative SEH assays
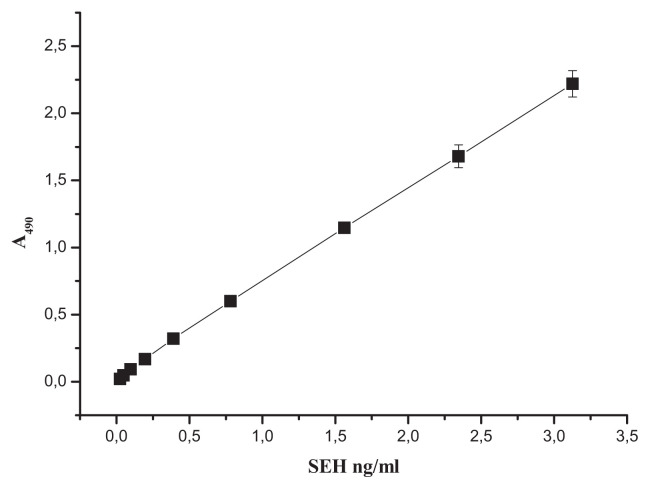
Various ELISA variants based on monoclonal antibodies are widely used for food analysis [30]. The sandwich EIA was chosen as the most reliable, sensitive and specific detection technique that has been well proven in SE assays [31–35]. The antibodies obtained were used for development of a test system quantifying SEH in the format of solid-phase sandwich enzyme immunoassay as described earlier (Peters and Baumgarten 1992). The antibodies were matched in pairs in such a way that they would react with the toxin simultaneously, not interfering with each other, and would be suitable for use in the format of solid-phase sandwich enzyme immunoassay. To reveal detection pairs of mAbs, all possible matches were analyzed, with each antibody tested as both the capture (bottom) one and the detection (top) one. The mAbs for detection (hereinafter referred to as “bio”) were labeled with biotin using biotin N-hydroxysuccinimide ester. The use of the biotin label not only allowed identifying the signal with streptavidin linked to horseradish peroxidase, but also significantly amplifying it. In total, 306 antibody pairs were tested with respect to their signal/background ratios (values of absorbance of chromogenic peroxidase substrate in the experimental wells with SEH against control wells without the toxin). As the result, 134 mAb pairs were found to have their signal/background ratios above 20. For 5 pairs with the maximal ratio values (about 50), the minimal limit of SEH detection was determined. The minimal detection limit was defined as the concentration of the tested agent, at which the signal exceeded the background by a margin of two standard deviations. The mAbs pairs SEH-22 + SEH-4bio, SEH-22 + SEH-7bio, SEH-16 + SEH-4bio, SEH-12 + SEH-16bio revealed antigen up to a concentration of 0.4 ng/ml. The mAbs pair SEH-24 + SEH-18bio detected SEH up to 0.2 ng/ml. For this pair, a calibration curve for measuring SEH was built (Fig. 4). The test system based on mAbs SEH-24 and SEH-18 reliably detected SEH within the linear range of 0.2–3 ng/ml with the measurement error of 6%, not reacting with SEs of types A, B, C1, D, E, G and I.
Fig. 4.
A reference curve for calculating the SEH concentration from sandwich EIA data. Capture antibodies, SEH-24; detection antibodies, SEH-18bio.
3.4. SEH assay for blood serum and food products
The test system developed for the detection of SEH was tried out on food products chosen based on statistical data about their contamination with SEs. S. aureus can grow within a broad range of temperatures (7–48.5 °C) and pH (4.2–9.3) and sodium chloride concentrations (up to 15%). For this reason, there is a high probability of detecting SEs in most diverse food products [36].
A certain amount of SEH was added to samples of products; the contents were incubated for 30 min. When non-liquid food products were used, extraction was made by four different methods. The extracts were analyzed by the developed method of sandwich EIA. As a positive control, the same preparation of SEH in PBST was used. The results of screening tests are presented in Table 3.
Table 3.
Detection of SEH in food products.
| Food product | Detected toxina, % of toxin added | |||
|---|---|---|---|---|
| Milk, 3.2% | 100 ± 0.5 | |||
| Milk, 2.5% | 100 ± 0.5 | |||
| Milk, 1.5% | 100 ± 0.5 | |||
| Meat broth | 100 ± 0.5 | |||
| Toxin extracted in non-liquid productsa, % of toxin added | ||||
|
| ||||
| With an equal volume of PBST | With two volumes of PBST | 0.5 M NaCl | pH 4.0–4.5 | |
|
|
|
|
|
|
| Cheese | 100 ± 1 | 100 ± 1 | 100 ± 1 | 100 ± 1 |
| Minced chicken | 86 ± 2 | 100 ± 1 | 86 ± 2 | 91 ± 1 |
| Minced beef | 85 ± 2 | 100 ± 1 | 85 ± 2 | 91 ± 1 |
| Cottage cheese, 9% | 32 ± 1.5 | 70 ± 1 | 70 ± 1 | 80 ± 1 |
| Minced beef after thermal treatment (100 °C, 30 min) | 5 ± 0.5 | |||
Mean values (n = 3) taken from the linear range of titration curves.
The extent of toxin detection (%) was defined as a ratio of the absorbance for food extracts or toxin-containing liquid food products to the absorbance of toxin in PBST, which were both in the linear range. The results showed that in milk and meat broth practically all the toxin was detected. The milk samples tested differed by their fat content: 3.2, 2.5 and 1.5%. The titration curves obtained were almost identical to the control curve of toxin in PBST and had the same minimal detection limit of 0.2 ng/ml. To extract the toxin in non-liquid food products, use was made of methods that enabled preservation of protein in maximally native state; no detergents or chemical denaturing agents such as guanidine chloride or urea were used. It is also important that samples of food products could be used in immunoassay with a minimum of manipulations. In extraction with an equal volume of PBST, the toxin was revealed fully only in cheese. In minced meat, it was detected by 86% only, and in cottage cheese, by 32%. A two-fold increase of the extraction buffer increased the toxin extraction efficiency to 100% from minced meat; to 70%, from cottage cheese. Dilution of the extract decreased the assay sensitivity by 33.3% in general. Addition of NaCl to a concentration of 0.5 M during the extraction did not increase its detection value in minced meat, and in cottage cheese increased to 70%. A decrease of pH of the extraction buffer to 4.0–4.5 made it possible to increase the toxin detection in minced meat to 91%, and in cottage cheese, to 80% (Table 3). For this reason, extraction in weakly acidic conditions appears to be optimal. The different levels of detection in cheese and cottage cheese are difficult to interpret as these products are sufficiently close by their chemical nature and differ by the content of fat, water and salt. A thermal treatment of beef (30-min boiling with toxin added) lowered the SEH detection to 5%. This decrease could be explained by an incomplete extraction of toxin due to its nonspecific adsorption by food components at high temperature, as well as by the data on the possible inactivation of SEs in low concentrations in preserved products at their sterilization [36], which corresponded to the conditions of the experiment.
The detection mAb pair SEH-24 + SEH-18bio was also tested for the ability to reveal SEH in a complex biological medium, blood plasma. The toxin was fully revealed.
3.5. Analysis of culture fluids of S. aureus isolates
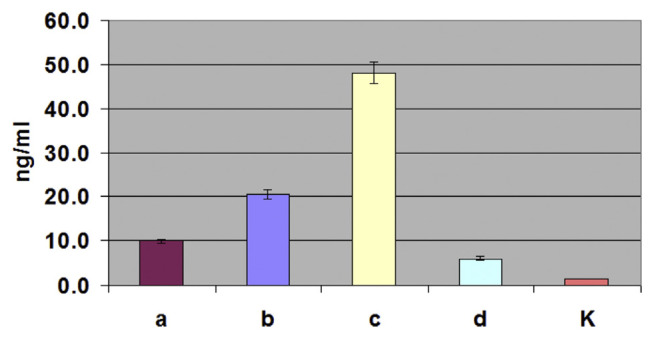
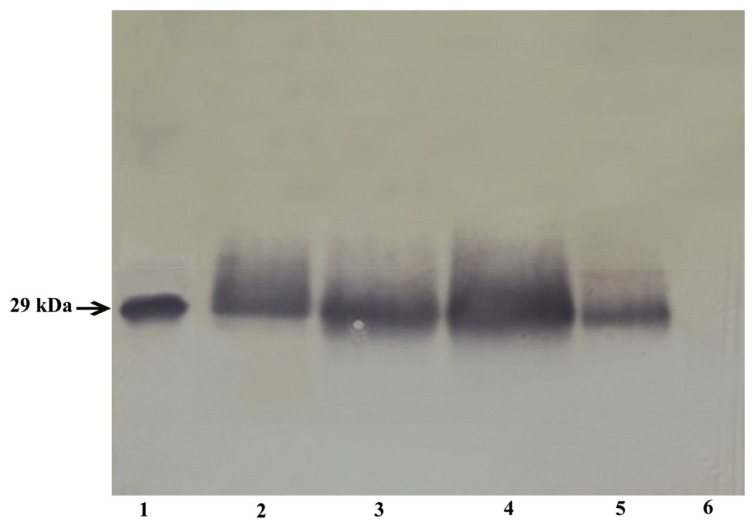
S. aureus was isolated from the milk of cows suffering from mastitis. To identify the seh-gene-containing isolates, primers other than the primers used to clone and express the gene were used. These primers provided high specificity of the analysis and were developed using on line service Primer-POWER. These primers were unsuitable for the cloning and expression of the seh gene, since they did not provide expression of the full-length product. The isolates were analyzed by the PCR for the presence of the seh gene; the seh-containing strains were cultivated, and the production of the protein in the culture was assessed by sandwich EIA and immunoblotting. Fig. 5 shows the results of sandwich EIA assays of seh-containing isolates using the diagnostic mAb pair SEH-24 + SEH-18bio. The maximal level of SEH expression under the conditions used was 45 ng/ml. Taking into account a possibility of protein A being in the samples, the sandwich EIA measurements were conducted in the presence of normal rabbit serum, as described by De Boer et al. (1999). The analysis of culture fluids of the same seh-containing isolates by immunoblotting using mAb SEH-14 is shown in Fig. 6. The immunoblotting data confirmed the presence of SEH in the examined samples and showed a concordance between the methods of analysis.
Fig. 5.
Detection of SEH in the culture fluid of S. aureus isolates by sandwich EIA. a, b, c and d, culture fluids of isolates carrying the seh gene; K, culture fluid of an isolate without the seh gene (negative control).
Fig. 6.
Detection of SEH in the culture fluid of isolates by immunoblotting. 1, recombinant SEH (100 ng); 2–5, samples obtained from 2-ml culture fluid of isolates a, b, c and d containing the seh gene; 6, sample obtained from 2-ml culture fluid of an isolate without the seh gene.
4. Conclusions
The sensitivity of the developed test system is at least 5 times higher than the previously described similar methods [9,17]. In regard to sensitivity, the developed test system for SEH assay is not inferior to the most popular Ridascreen (R-Biopharm) test systems for the detection of staphylococcal enterotoxins A, B, C, D, and E. The test system Ridascreen is also made in the sandwich-IEA format and has a minimum detection limit of 0.25 ng/ml (http://www.r-biopharm.com/products/food-feed-analysis/microbiology-hygiene/staphylococcal-enteroxin-set/item/ridascreen-set-abcde). The detecting pair MA developed in this paper can be used to detect SEH in various modern platforms, such as surface-enhanced Raman scattering [37] nanotechnology, for the synthesis of nanoparticles – biosensors with immobilized antibodies for the analysis of food products [38]. Also, the developed detection pair can be used to create a gold-nanoparticle-based immunochromatographic strip in combination with antibodies directed against other toxins [39–44] and using immunomagnetic bead technology [45].
Thus, a method of producing a highly purified recombinant SEH was developed; 18 hybridomas, secreting highly affine monoclonal antibodies to SEH, were obtained. The hybridomas maintained a high stability when cultivated both in vitro and in vivo. On their basis, specific test systems were designed in the format of sandwich enzyme immunoassay. The minimal concentration of SEH detected with one of the developed test systems is 0.2 ng/ml. Conditions of sample preparation and SEH assay in liquid and solid food products were selected. The test system was demonstrated to be suitable for detection and quantitative analysis of SEH in meat and milk products and in blood serum. The designed method was used to measure SEH production by S. aureus isolates obtained from the milk of mastitis-infected cows.
Funding Statement
This study was supported by the Russian Science Foundation project “Molecular aspects of the pathogenicity of staphylococcal mastitis”, No 15 16 00020.
Footnotes
Funding
This study was supported by the Russian Science Foundation project “Molecular aspects of the pathogenicity of staphylococcal mastitis”, No 15 16 00020.
Conflicts of interest
The manuscript has not been published previously (partly or in full). The manuscript has not been submitted to more than one journal for simultaneous consideration. The authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results. N.V. Rudenko, A.P. Karatovskaya, A.N. Noskov, A.O. Shepelya-kovskaya, M.P. Shchannikova, I.V. Loskutova, O.A. Artye-mieva, D.A. Nikanova, E.A. Gladyr, F.A. Brovko declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Not applicable.
References
- 1. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;3:603–61. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huvenne W, Hellings PW, Bachert C. Role of staphylococcal superantigens in airway disease. Int Arch Allergy Immunol. 2013;161(4):304–14. doi: 10.1159/000350329. [DOI] [PubMed] [Google Scholar]
- 3. Macias ES, Pereira FA, Rietkerk W, Safai B. Superantigens in dermatology. J Am Acad Dermatol. 2011;64(3):455–72. doi: 10.1016/j.jaad.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 4. Johler S, Giannini P, Jermini M, Hummerjohann J, Baumgartner A, Stephan R. Further evidence for staphylococcal food poisoning outbreaks caused by egc-encoded enterotoxins. Toxins. 2015;7:997–1004. doi: 10.3390/toxins7030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genet Mol Res. 2003;2:63–76. [PubMed] [Google Scholar]
- 6. Hsieh CM, Huang Y-H, Chen C-P, Hsieh B-C, Tsai T. 5-Aminolevulinic acid induced photodynamic inactivation on Staphylococcus aureus and Pseudomonas aeruginosa. J Food Drug Anal. 2014;22:350–5. doi: 10.1016/j.jfda.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su YC, Wong AC. Identification and purification of a new staphylococcal enterotoxin H. Appl Environ Microbiol. 1995;61:1438–43. doi: 10.1128/aem.61.4.1438-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petersson K, Petersson H, Skartved NJ, Walse B, Forsberg G. Staphylococcal enterotoxin H induces V alpha-specific expansion of T cells. J Immunol. 2003;170:4148–54. doi: 10.4049/jimmunol.170.8.4148. [DOI] [PubMed] [Google Scholar]
- 9. Sakai F, Ihara H, Aoyama K, Igarashi H, Yanahira S, Ohkubo T, et al. Characteristics of enterotoxin H-producing Staphylococcus aureus isolated from clinical cases and properties of the enterotoxin productivity. J Food Prot. 2008;71(9):1855–60. doi: 10.4315/0362-028x-71.9.1855. [DOI] [PubMed] [Google Scholar]
- 10. Deb R, Kumar A, Chakraborty S, Verma AK, Tiwari R, Dhama K, et al. Trends in diagnosis and control of bovine mastitis: a review. Pak J Biol Sci. 2013;16(23):1653–61. doi: 10.3923/pjbs.2013.1653.1661. [DOI] [PubMed] [Google Scholar]
- 11. Basanisi MG, La Bella G, Nobili G, Franconieri I, La Salandra G. Genotyping of methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk and dairy products in South Italy. Food Microbiol. 2017;62:141–6. doi: 10.1016/j.fm.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 12. Ikeda T, Tamate N, Yamaguchi K, Makino S. Mass outbreak of food poisoning disease caused by small amounts of staphylococcal enterotoxins A and H. Appl Environ Microbiol. 2005;71:2793–5. doi: 10.1128/AEM.71.5.2793-2795.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jørgensen HJ, Mathisen T, Løvseth A, Omoe K, Qvale KS, Loncarevic S. An outbreak of staphylococcal food poisoning caused by enterotoxin H in mashed potato made with raw milk. FEMS Microbiol Lett. 2005;252:267–72. doi: 10.1016/j.femsle.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 14. Macori G, Giacinti G, Bellio A, Gallina S, Bianchi DM, Sagrafoli D, et al. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in the ovine dairy chain and in farm-related humans. Toxins. 2017;9:161. doi: 10.3390/toxins9050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Chen W, Ali T, Alkasir R, Yin J, Liu G, et al. Staphylococcal enterotoxin H induced apoptosis of bovine mammary epithelial cells in vitro. Toxins. 2014;6:3552–67. doi: 10.3390/toxins6123552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Derzelle S, Dilasser F, Duquenne M, Deperrois V. Differential temporal expression of the staphylococcal enterotoxins genes during cell growth. Food Microbiol. 2009;8:896–904. doi: 10.1016/j.fm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 17. Su YC, Wong AC. Detection of staphylococcal enterotoxin H by an enzyme-linked immunosorbent assay. J Food Prot. 1996;59(3):327–30. doi: 10.4315/0362-028x-59.3.327. [DOI] [PubMed] [Google Scholar]
- 18. Schubert J, Podkowik M, Bystron J, Bania J. Production of staphylococcal enterotoxins in microbial broth and milk by Staphylococcus aureus strains harboring seh gene. Int J Food Microbiol. 2016;235:36–45. doi: 10.1016/j.ijfoodmicro.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 19. Meulenberg EP. Immunochemical methods for ochratoxin A detection: a review. Toxins. 2012;4:244–66. doi: 10.3390/toxins4040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Hay FC, Westwood OMR. Practical immunology. 4th ed. 1–39. Blackwell Science; 2002. Isolation and structure of immunoglobulins Antibodies as probes; pp. 133–134. [Google Scholar]
- 22. Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 23.Peters JH, Baumgarten H. Monoclonal antibodies. Springer; 1992. Mass production of monoclonal antibodies Purifying monoclonal antibodies and producing antibody fragments; pp. 223–33.pp. 264–271. [Google Scholar]
- 24. Rudenko NV, Abbasova SG, Grishin EV. Production and characterization of the monoclonal antibodies to Bacillus anthracis protective antigen. Bioorg Chem. 2011;37:344–53. doi: 10.1134/s1068162011030162. (in Russian) [DOI] [PubMed] [Google Scholar]
- 25. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26. Beatty JD, Beatty BG, Vlahos WG. Measurement of monoclonal antibody affinity by non-competitive immunoassay. J Immunol Methods. 1987;100:173–9. doi: 10.1016/0022-1759(87)90187-6. [DOI] [PubMed] [Google Scholar]
- 27. Salgado-Pabón W, Case-Cook LC, Schlievert PM. Molecular analysis of staphylococcal superantigens. Methods Mol Biol. 2014;1085:169–85. doi: 10.1007/978-1-62703-664-1_10. [DOI] [PubMed] [Google Scholar]
- 28. Thomas D, Chou S, Dauwalder O, Lina G. Diversity in Staphylococcus aureus enterotoxins. Chem Immunol Allergy. 2007;93:24–41. doi: 10.1159/000100856. [DOI] [PubMed] [Google Scholar]
- 29. Günther S, Varma AK, Moza B, Kasper KJ, Wyatt AW, Zhu P, et al. A novel loop domain in superantigens extends their T cell receptor recognition site. J Mol Biol. 2007;371(1):210–21. doi: 10.1016/j.jmb.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chadseesuwan U, Sangdokmai A, Pimpitak U, Puthong S, Palaga T, Komolpis K. Production of a monoclonal antibody against aflatoxin M1 and its application for detection of aflatoxin M1 in fortified milk. J Food Drug Anal. 2016;24:780–7. doi: 10.1016/j.jfda.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bennett RW. Staphylococcal enterotoxin and its rapid identification in foods by enzyme-linked immunosorbent assay-based methodology. J Food Prot. 2005;68(6):1264–70. doi: 10.4315/0362-028x-68.6.1264. [DOI] [PubMed] [Google Scholar]
- 32. Wang W, Liu L, Xu L, Ma W, Kuang H, Xu C. Detection of β-lactamase residues in milk by sandwich ELISA. Int J Environ Res Public Health. 2013;10(7):2688–98. doi: 10.3390/ijerph10072688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuang H, Wang W, Xu L, Ma W, Liu L, Wang L, et al. Monoclonal antibody-based sandwich ELISA for the detection of staphylococcal enterotoxin A. Int J Environ Res Public Health. 2013;10(4):1598–608. doi: 10.3390/ijerph10041598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feng M, Yong Q, Wang W, Kuang H, Wang L, Xu C. Development of a monoclonal antibody-based ELISA to detect Escherichia coli O157:H7. Food Agric Immunol. 2013;24(4):481–7. [Google Scholar]
- 35. Wu X, Wang W, Liu L, Kuang H, Xu C. Monoclonal antibody-based cross-reactive sandwich ELISA for the detection of Salmonella spp. in milk samples. Anal Methods. 2015;7(21):9047–53. [Google Scholar]
- 36. Chardash RA, Potter NN. Stability of staphylococcal enterotoxin A to selected conditions encountered in foods. J Food Sci. 1976;41:906–9. [Google Scholar]
- 37. Wang W, Liu L, Xu L, Kuang H, Zhu J, Xu C. Nanoshell-enhanced Raman spectroscopy on a microplate for staphylococcal enterotoxin B sensing. ACS Appl. Mater. Interfaces. 2016;8:15591–7. doi: 10.1021/acsami.6b02905. [DOI] [PubMed] [Google Scholar]
- 38. Inbaraj BS, Chen BH. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J Food Drug Anal. 2016;24:15–28. doi: 10.1016/j.jfda.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang W, Liu L, Xu L, Kuang H, Zhu J, Xu C. Gold-nanoparticle-based multiplexed immunochromatographic strip for simultaneous detection of staphylococcal enterotoxin A, B, C, D, and E. Part Part Syst Charact. 2016;33(7):388–95. [Google Scholar]
- 40. Wang W, Liu L, Song S, Xu L, Kuang H, Zhu J, et al. Gold nanoparticle-based strip sensor for multiple detection of twelve Salmonella strains with a genus-specific lipopolysaccharide antibody. Sci China Mater. 2016;59(8):665–74. [Google Scholar]
- 41. Wang W, Liu L, Song S, Xu L, Zhu J, Kuang H. Gold nanoparticle-based paper sensor for multiple detection of 12 Listeria spp. by P60-mediated monoclonal antibody. Food Agric Immunol. 2017;28(2):274–87. [Google Scholar]
- 42. Wang W, Liu L, Song S, Tang L, Kuang H, Xu C. A highly sensitive ELISA and immunochromatographic strip for the detection of Salmonella typhimurium in milk samples. Sensors. 2015;15(3):5281–92. doi: 10.3390/s150305281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang W, Feng M, Kong D, Liu L, Song S, Xu C. Development of an immunochromatographic strip for the rapid detection of Pseudomonas syringae pv. maculicola in broccoli and radish seeds. Food Agric Immunol. 2015;26(5):738–45. [Google Scholar]
- 44. Wang W, Liu L, Song S, Xu L, Kuang H, Zhu J, et al. Identification and quantification of eight Listeria monocytogene serotypes from Listeria spp. using a gold nanoparticle-based lateral flow assay. Microchim Acta. 2017;184(3):715–24. [Google Scholar]
- 45. Deng Q, Qiua M, Wang Y, Lv P, Wu C, Sun L, et al. A sensitive and validated immunomagnetic-bead based enzyme-linked immunosorbent assay for analyzing total T-2 (free and modified) toxins in shrimp tissues. Ecotoxicol Environ Saf. 2017;142:441–7. doi: 10.1016/j.ecoenv.2017.04.037. [DOI] [PubMed] [Google Scholar]