Abstract
A liquid chromatography tandem mass spectrometric method was developed for the determination of two β-agonists (ractopamine and salbutamol) in pig hair samples. An isotope of ractopamine-d5 or salbutamol-d6 as an internal standard was used to carry out quantitative analysis. Concentrated sodium hydroxide was used to pretreat hair samples and then purified by the solid phase extraction (SPE) procedure. The extracted solution was evaporated and reconstituted for injection in the instrument with electrospray ionization (ESI) operating in a positive multiple-reaction-monitoring (MRM) mode. Ractopamine and salbutamol separation were performed on C18 analytical column under gradient condition. The internal standard calibration curve was linear in the range of concentration from 0.5 to 100 ng mL−1 (R2 > 0.995). Recoveries of this method estimated at three spiked concentrations of 100, 250 and 500 ng mL−1 in pig hair samples, were 79–82% for ractopamine and 77–96% for salbutamol. The corresponding inter-day and intra-day precisions expressed as relative standard deviation (RSD %) were 3.8–6.4% and 3.8–8.6%, respectively. The analytical time for one sample was 8 min. The detection limit of this method was 0.6 and 8.3 ng mL−1 for ractopamine and salbutamol, respectively. This developed method can be applied for monitoring the use of the β-agonists salbutamol and ractopamine in swine feed incurred pig hair.
Keywords: Salbutamol, Ractopamine, Hair, Tandem mass
1. Introduction
Animal feedstuffs are important components of the food production chain of animal origin as it has an impact on animal health and efficiency [1]. The presence of feed additives residue is a major contributor to the concerns of meat safety [2]. Several β-agonists are used as additives for growth and lean meat promotion in animals including salbutamol and ractopamine. β-agonists or -adrenergic agonists are synthetic phenethanolamine compounds used as bronchodilatory and tocolytic agents for therapeutic purposes [3]. However in many countries, β-agonists as feed additives are prohibited for animals [4] including Taiwan [5]. Consumer demand leaner and healthier pork products [6] and most β-agonists can increase muscle mass and decrease fat tissue [1]. Salbutamol is primarily used as a clinical treatment for asthma in humans [7] and is approved for human use in Taiwan [5]. Ractopamine has a repartitioning effect [8] and is a phenethanolamine with beta-adrenergic agonist properties [9] originally used as tocolytics, bronchodilators, and heart tonics in human and veterinary medicine [10]. The required withdrawal period of ractopamine residue in edible tissue has been studied for many years. β-agonists generally have high clearance rates in animal tissue, especially in the serum [11]. The excretory intervals of various animals when following different routes of administration have different times, ranging from 5 to 192 h [2]. Fortunately, when the concentration of β-agonists metabolized in the tissue under the analytical method detection limit, the residue in retina and hair was still detectable. Although the retina has the longest residue period of 20 weeks after treatment, it can only be sampled in the slaughterhouse [5] which highlights the importance of this presented method. Several studies have used pig hair for residue analysis as pig hair samples are more suitable for β-agonists inspection for reasons such as easy and multiple sampling times throughout the production period.
Residue can be observed in hair one hour after administration [6] and is more sensitive compared to serum or plasma [6]. The analytical methods for β-agonists in animal tissues demand more efficient and sensitive test with the advancement in technology. Many methods were developed with a limit of detection (LOD) in the range of ppb level. These frequently used methods includes enzyme-link immunosorbent assay (ELISA) [5,7,8], gas chromatography-mass spectrometry (GC/MS) [1,9–12], high performance liquid chromatography (HPLC) coupled fluorescence detector (FL) [3] or tandem mass spectrometry (MS/MS) [13–16]. Each method has unique extraction technology, clean-up step or derivative procedure for increasing the efficiency and sensitivity of β-agonists detection in different food producing species. However, analytical methods for β-agonists in hair samples are few [19] but other studies have used pig hair samples to determine drug residues [15,19].
Common β-agonist additives in pig feed in Taiwan are ractopamine and salbutamol (Fig. 1). Ractopamine has been the subject of research in different food producing animals, while less research has been published on Salbutamol. For various compounds reliable analytical methods are needed to discover and confirm the presence of veterinary drug residues. Even though β-agonist compounds are banned they are still added in animal feed to contribute to the productivity of livestock which pose a health risk through meat consumption with residual feed additives [12]. Therefore, the objective of this study was to develop a reliable analytical method for ractopamine and salbutamol residues in pig hair and methodology confirmation by using real samples.
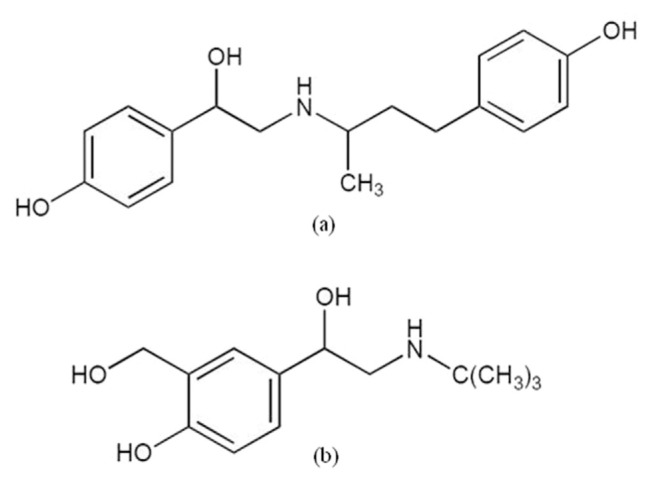
Fig. 1.
Chemical structure for (a) ractopamine and (b) salbutamol.
2. Materials and methods
2.1. Reagents and chemicals
Reference standard ractopamine and salbutamol were purchased from Sigma–Aldrich (St. Louis, MO, USA). Internal standard ractopamine-d5 and salbutamol-d6 were purchased from RIVM (Bilthoven, Netherlands). Methanol, acetonitrile, ethyl acetate and dichloromethane, all of LC grade, were purchased from Merck (Darmstadt, Germany). Sodium hydroxide, hydrochloric acid (37%) and formic acid (98–100%), all of the reagent grade, were purchased from Merck (Darmstadt, Germany). Ammonium formate solution (10M) was purchased from Sigma–Aldrich (St. Louis, MO, USA). Alcohol (95%) was purchased from ECHO chemical (Miaoli, Taiwan). De-ionized water (18.2 MΩ) was purified by the Millipore Synergy 185 ultrapure water system (Billerica, MA).
Formate buffer (pH = 3.0) was prepared according to a standard protocol used in the lab by adding formic acid 97%–0.01% at 5 mM ammonium formate in a de-ionized solution.
2.2. Instrument and condition
Chromatography was performed on an Agilent 1200 series HPLC and tandem mass spectrometry system (Santa Clara, CA), consisting of a binary pump (G1312B), a degasser (G1379B), auto-sampler (G1367C), column oven (G1316B), and a 2-μm filter disc attached to the analytical column (Zorbax SB-C18 50 × 2.1 mm i.d. particle size 1.8 μm) combined with electrospray ionization (ESI) tandem mass (6410 Triple Quad).
2.2.1. HPLC conditions
The mobile phase contained formate buffer (A) and acetonitrile (B) at the initial step. The gradient profile started at 1% B, increased to 90% in 2 min and then at constant proportions from 2 to 5 min. The stop time was 5 min and 3 min post time column conditions with initial state, formate buffer (A) acetonitrile (B) (99:1 v/v). Separation was achieved at a flow rate of 0.2 mL min−1 and the column was maintained at 40 °C. A volume of 10 μL was used for injection into the HPLC system.
2.2.2. Mass spectrometry condition
Nitrogen gas pressure of the nebulizer was set at 30 psi, dry nitrogen gas flow was 8 L min−1 at 325 °C, and the capillary voltage was 4000 V. The ESI source was operated in positive mode and mass data was performed in multiple-reaction-monitoring (MRM) mode. The precursor ion and related productions of analytes are summarized in Table 1.
Table 1.
Main ion fragments of ractopamine and salbutamol.
| Compound | Precursor ion (m/z) | Product ion (m/z) | Dwell | Collision energy | Cone voltage |
|---|---|---|---|---|---|
| Ractopamine-d5 | 307 | 121 | 100 | 92 | 20 |
| 307 | 107 | 100 | 92 | 32 | |
| Ractopamine | 302 | 284 | 100 | 100 | 10 |
| 302 | 164 | 100 | 100 | 15 | |
| Salbutamol-d6 | 246 | 148 | 100 | 92 | 16 |
| 246 | 121 | 100 | 92 | 32 | |
| Salbutamol | 240 | 222 | 100 | 100 | 5 |
| 240 | 148 | 100 | 100 | 15 |
2.3. Calibration curve
Dissolved 10 mg of the standard using methanol in a 10 mL calibrated flask with a concentration of 1000 μg mL−1 and stored at 2–8 °C. The internal standard was diluted to 50 ng mL−1 using methanol. The stock solution was diluted to 100, 50, 10, 5, 1 and 0.5 ng mL-1 using methanol. A 100 μL calibration standard and 100 μL of 50 ng mL−1 internal standard solutions were added into 2 mL Eppendorfs and vortexed. The solution was evaporated to dryness under a stream of nitrogen gas. The residue was then dissolved by adding formate buffer (100 μL) for the internal calibration curve. Formate buffer (pH = 3.0) was prepared by adding formic acid (97%) to 0.01% to 5 mM ammonium formate in a de-ionized solution.
2.4. Sample preparation
The collected hair samples from the treatment groups RAC and SAL from the back of the pigs were placed into a glass beaker, washed with de-ionized water (500 mL), alcohol (500 mL) and dichloromethane (500 mL) sequentially and dried at 60 °C in an oven for 30 min. The dried hair samples were then kept at −20 °C until further use. 100 mg of dry hair were placed in a 15 mL polypropylene centrifuge tube. Ractopamine-d5 and salbutamol-d6 of 100 ng mL−1 as internal standards were added to 50 μL and then placed into a centrifuge tube. A volume of 5 mL of 2 N sodium hydroxide solution was added to the samples, capped and placed in an oven set at 80 °C for 30 min and vortexed after the first 15 min then placed back into the oven. After the reaction, the samples were cooled to room temperature; then 1 mL of 37% hydrochloric acid was added. The samples were then centrifuged at 4000 rpm for 10 min. The supernatants were then transferred into a 50 mL polypropylene centrifuge tube and adjusted to pH 8–9 using 5 N sodium hydroxide solution. Afterwards, the samples were centrifuged at 4000 rpm for 5 min. The extracted samples were purified using the solid phase extraction procedure. Agilent C18 SPE cartridge (500 mg) was conditioned using 5 mL of methanol and 5 mL of de-ionized water. Samples were decanted into an SPE cartridge then the cartridge was washed with 5 mL of deionized water followed by 2 mL of 50% acetonitrile and dried by suction for 10 min and 5 min, respectively. Finally, the analytes were eluted with 2 mL of methanol and collected into a 5 mL centrifuge tube. The elution was evaporated at 40 °C under a stream of nitrogen gas. 100 μL of de-ionized water was added to dissolve the residue and then mixed with 1 mL of ethyl acetate for 1 min. The mixtures were then centrifuged at 4000 rpm for 5 min. The supernatants were collected in 2 mL Eppendorfs, evaporated to dryness under a stream of nitrogen gas, and 100 μL of formate buffer was added and vortexed. The samples were then centrifuged at 12,500 rpm at 4 °C for 5 min. The collected supernatants were filtered through a 0.22 μm filter and 10 μL of each sample was injected into the LC-MS/MS system.
2.5. Validation assay
The concentrations of β–agonists used for assessment of limit of detection (LOD) and limit of quantification (LOQ) were: 10, 8, 6, 4 and 2 ng mL−1 and the blank solution with 50 ng mL−1 internal standards. The equation was constructed by plotting the relative peak area against concentration, using the linear least-square regression method. The procedure involved the calculation of the calibration curve deviation (sy/x) and then multiplying by 3 or 10, respectively. The result was divided by the slope of the calibration curve to find the corresponding LOD and the LOQ [13].
The accuracy of the analytical method developed for ractopamine and salbutamol in pig hair was estimated by the recovery of the spiked samples at concentrations of 100, 250 and 500 ng mL−1. The analysis precision was estimated by the relative standard deviation (RSD%) of freshly prepared standard samples at the same concentration levels. For the repeatability (intraday assay) study, three series were analysed (five samples for each spiking level) and RSD% was calculated for each level. Additionally, the intermediate precision (inter-day assay) was determined at the same concentrations levels (three samples for each spiking level) and the analysis was performed for five days.
2.6. Animal treatment
Six Landrace × Yorkshire × Duroc (LYD) pigs, weighing 80 kg were used in this experiment and divided into two groups, RAC and SAL. The pigs in the RAC group were orally administered with 20 ppm of ractopamine medical feed and the pigs in the SAL group were orally administered with 5 ppm salbutamol medical feed for 28 days followed by a withdrawal of 14 days. During the first two weeks, the experimental animals were fed blank feed without any β-agonists to ensure no preexisting residue. All pigs were reared in the same experimental farm for two months.
Blank hair samples were taken from six pigs before treatment for the control standard. Hair samples were collected 14 days during treatment and at withdrawal 14 days withdrawal period from the pigs back. All animal experimental procedures including the care and handling of the animals were approved by the Institutional Animal Care and Use Committee.
3. Results
3.1. Optimization of LC-MS/MS
Tuning of the LC-MS/MS system was performed by Agilent optimizer software with individual standards (1 μg mL−1 in formate buffer), targeting the most abundant fragment ions. In the MS scan mode, the precursor ion for ractopamine-d5, ractopamine, salbutamol-d6 and salbutamol was chosen as the precursor ions [M+H]+ at m/z 307, 302, 246 and 240, respectively. In the MRM mode, the first two highest responses of product ions were used as quantitative assay (Table 1). The MRM chromatographs of internal standard spiked and real samples for ractopamine and salbutamol are shown respectively in Fig. 2. The precursor ions of ractopamine, salbutamol, and their isotopes are the same as previous studies [14–17], but the two characteristic product ions were different. Nevertheless, the relative ion intensities were in the range 20–25% of product ion to precursor ion which depended on mass intensity. The qualitative analysis was done according to the European Commission Decision 2002/657/EC [18].
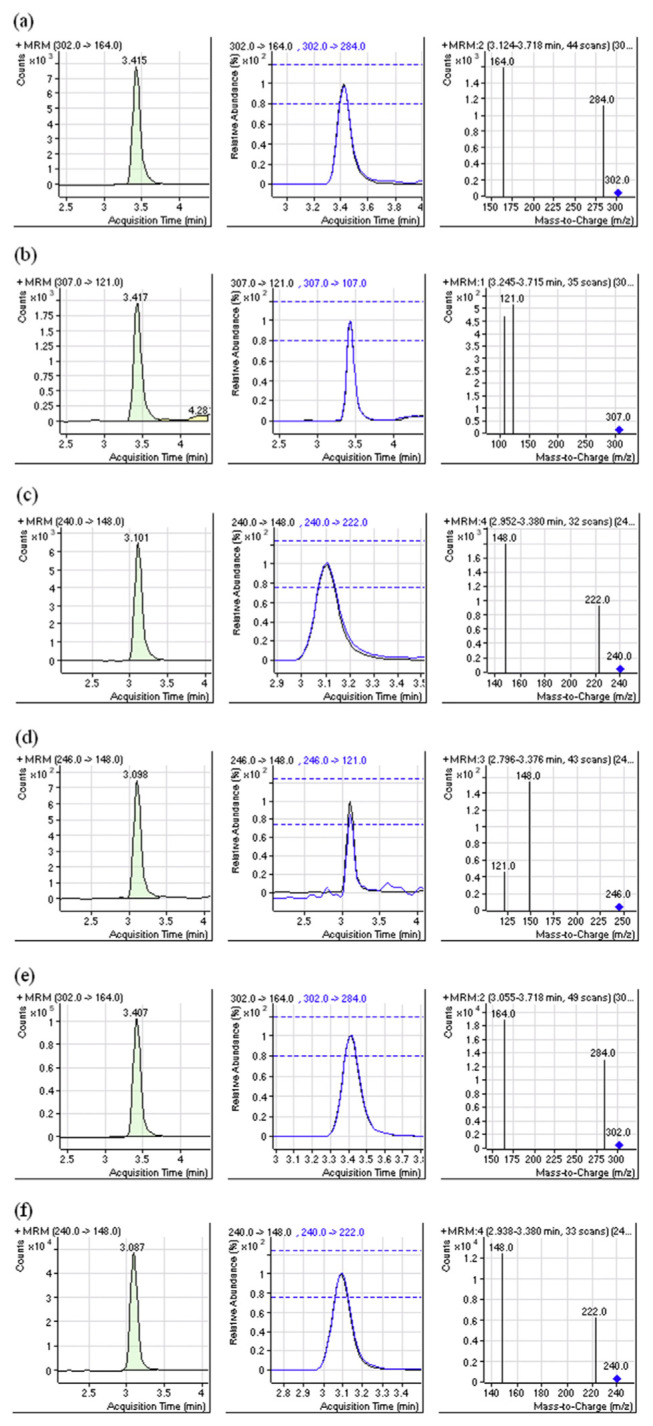
Fig. 2.
Multiple-reaction-monitoring chromatography from left hand to right hand is extracted ion chromatogram, tolerance for relative ion intensities and mass spectrum. (a) spiked 100 ng g−1 ractopamine of pig hair (b) internal standard of 50 ng g−1 ractopamine (c) spiked 100 ng g−1 salbutamol of pig hair (d) internal standard of 50 ng g−1 salbutamol (e) real sample of ractopamine (f) real sample of salbutamol.
3.2. Limit of detection and limit of quantification
To estimate the LOD of ractopamine and salbutamol, the linear internal standard calibration curve was prepared using a 5 point range 10, 8, 6, 4 and, 2 ng/mL−1 respectively and a blank solution with a neat solution of formate buffer and 50 ng mL−1 as internal standards of isotopes. The linear equation of the internal standard calibration curve of ractopamine was y = 0.032 x + 0.662 (R2 = 0.996). The LOD was then calculated by dividing 3 times the standard deviation (sy/x) of the linear calibration curve with the slope (0.032), and the LOQ was calculated by dividing 10 times of sy/x with the slope of the linear calibration equation. The LOD and LOQ calibrations of salbutamol were the same as ractopamine. The LOD of this study was 0.6 ng mL−1 for ractopamine and 8.3 ng mL−1 for salbutamol; otherwise, the LOQ was 1.9 ng mL−1 for ractopamine and 27.8 ng mL−1 for salbutamol.
3.3. Validation and real sample test
Recoveries were 79.0%–84.5% for ractopamine and 88.2%–95.8% for salbutamol, respectively (Table 2). Repeatability as intra-day assay was 2.4%–4.7% and 4.3%–6.6% for ractopamine and salbutamol, respectively. Reproducibility as inter-day assay was 3.4%–7.3% and 5.5%–8.2% for ractopamine and salbutamol, respectively. The obtained results are summarized in Table 3. As for the results, the method was deemed acceptable, repeatable and reproducible for the control of ractopamine and salbutamol use as feed additives. The monitoring of beta-agonists residues in pig hair was done every year (at least 8000 samples) by government in Taiwan. This developed method was referenced to make a national method by Taiwan government.
Table 2.
Analysis accuracy for ractopamine and salbutamol in pig hair.
| Spiked concentration (ppb) | Recovery of ractopamine (%) | Recovery of salbutamol (%) |
|---|---|---|
| 100 | 84.6a | 92.2a |
| 250 | 79.0 | 95.8 |
| 500 | 80.2 | 88.2 |
The recovery rate was tested for five repeated measurements (n = 5).
Table 3.
Analysis precision for ractopamine and salbutamol in pig hair.
| Spiked concentration (ppb) | Intra-day assaya RSD (%) |
Inter-day assayb RSD (%) |
||
|---|---|---|---|---|
|
|
|
|||
| Ractopamine | Salbutamol | Ractopamine | Salbutamol | |
| 100 | 4.1 | 5.4 | 7.3 | 5.5 |
| 250 | 2.4 | 6.6 | 6.0 | 6.5 |
| 500 | 4.7 | 4.3 | 3.4 | 8.2 |
Each sample was measured 5 times in the same day (n = 5).
Each sample was measured 3 times in 5 different days (n = 15).
4. Discussion
There are three kinds of calibration curves that were efficient, matrix matched and matrix fortified for quantitative analysis. In order to reduce the matrix effect of quantitative analysis, the matrix match standard calibration curve is usually used for mass spectrometry analysis. However, since pig hair is very light 100 mg of hair was chosen for the analytical procedure in an effort to avoid concentrating analytes. The blank sample matrix was insufficient to prepare the standard curve so the internal standard for sample extraction was used.
In Table 3, the precision of the analytical method was estimated by relative standard deviation of intra–day and inter–day assay. The data show good repeatability because of the maximum RSD 6.6% for intra-day assay. For inter–day assay, the RSD were 3.4–7.3 and 5.5–8.2 for ractopamine and salbutamol, respectively. As the RSD values were less than 10%, the measurement results were similar and this proved good reproducibility.
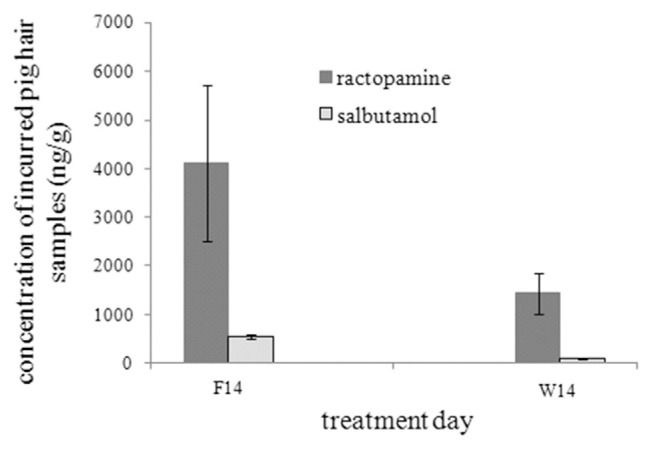
Hair was taken from two sides over a two days period. As shown in Fig. 3, the concentrations of ractopamine and salbutamol detected in acquired pig hairs were 4129 ± 38.9 and 561 ± 7.4 ng mL−1 at feeding 14 days and 1441 ± 28.7 and 102 ± 6.9 ng mL−1 at 14 days withdrawal period, respectively. These results demonstrated that pigs fed medical feed with 20 ppm ractopamine incorporation had an average of 4 ppm accumulation in their hair, while 5 ppm integration of salbutamol into the feed had a concentration of 0.5 ppm accumulation in their hair. The analytical time of each sample was 8 min which is considered rapid compared to other screening methods mentioned earlier in the introduction. However, this study was done to develop a method for the determination of the β-agonists RAC and SAL in pig hair. The results show that is method is applicable, however, the metabolites in the hair of the two β-agonists still needs further study. The overall findings of this study are the capability of this LC-tandem mass method to separate the β-agonists ractopamine and salbutamol simultaneously if used together or separately. The use ractopamine–d5 or salbutamol–d6 as internal standards to conduct a quantitative study. Finally the proficiency of this method to detect ractopamine and salbutamol accumulated residue in pig hair samples before slaughter. Therefore, this developed method may be applied for the control of the illegal use of ractopamine and salbutamol as feed additives in Taiwan and countries where their use is prohibited.
Fig. 3.
The accumulation concentration in pig hair samples after feeding ractopamine and salbutamol of 3 pigs at 14 day (F14) and withdrawal 14 day (W14).
5. Conclusions
Veterinary drug residues have become a matter of public concern because of possible adverse effects which means continuous monitoring for prohibited drug use is important. A simple, fast and reliable method using LC-MS has been developed for the determination of ractopamine and salbutamol in pig hairs. This method can be used by companies and government agencies for monitoring the use of RAC and SAL throughout the growth period of swine without slaughtering the animal.
Acknowledgements
The authors greatly thank the Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, for financially supporting this research (98AS-9.2.2-BQ-B1 (1)).
Funding Statement
The authors greatly thank the Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, for financially supporting this research (98AS-9.2.2-BQ-B1 (1)).
Footnotes
Conflicts of interest
We declare no conflict of interest involved in this study.
References
- 1. Gressler V, Franzen ARL, de Lima GJMM, Tavernari FC, Dalla Costa OA, Feddern V. Development of a readily applied method to quantify ractopamine residue in meat and bone meal by QuEChERS-LC–MS/MS. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1015:192–200. doi: 10.1016/j.jchromb.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 2. Xiong L, Gao Y-Q, Li W-H, Yang X-L, Shimo SP. Simple and sensitive monitoring of β2-agonist residues in meat by liquid chromatography–tandem mass spectrometry using a QuEChERS with preconcentration as the sample treatment. Meat Sci. 2015;105:96–107. doi: 10.1016/j.meatsci.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 3. Juan C, Igualada C, Moragues F, León N, Mañes J. Development and validation of a liquid chromatography tandem mass spectrometry method for the analysis of β-agonists in animal feed and drinking water. J Chromatogr A. 2010;1217(39):6061–8. doi: 10.1016/j.chroma.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 4. Liu X, Grandy DK, Janowsky A. Ractopamine, a livestock feed additive, is a full agonist at trace amine–associated receptor 1. J Pharmacol Exp Ther. 2014;350(1):124–9. doi: 10.1124/jpet.114.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee H-C, Chen C-M, Wei J-T, Chiu HY. Analysis of veterinary drug residue monitoring results for commercial livestock products in Taiwan between 2011 and 2015. J Food Drug Anal. 2017 July 7;:1–7. doi: 10.1016/j.jfda.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almeida VVd, Nuñez AJC, Miyada VS. Ractopamine as a metabolic modifier feed additive for finishing pigs: a review. Braz Arch Biol Technol. 2012;55(3):445–56. [Google Scholar]
- 7. Khirani S, Dabaj I, Amaddeo A, Olmo Arroyo J, Ropers J, Tirolien S, et al. Effect of salbutamol on respiratory muscle strength in spinal muscular atrophy. Pediatr Neurol. 2017;73:78–87e1. doi: 10.1016/j.pediatrneurol.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 8. Pleadin J, Perši N, Vulić A, Milić D, Vahčić N. Determination of residual ractopamine concentrations by enzyme immunoassay in treated pig’s tissues on days after withdrawal. Meat Sci. 2012;90(3):755–8. doi: 10.1016/j.meatsci.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 9. Carr SN, Hamilton DN, Miller KD, Schroeder AL, Fernández-Dueñas D, Killefer J, et al. The effect of ractopamine hydrochloride (Paylean®) on lean carcass yields and pork quality characteristics of heavy pigs fed normal and amino acid fortified diets. Meat Sci. 2009;81(3):533–9. doi: 10.1016/j.meatsci.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 10. Lu X, Zheng H, Li X-Q, Yuan X-X, Li H, Deng L-G, et al. Detection of ractopamine residues in pork by surface plasmon resonance-based biosensor inhibition immunoassay. Food Chem. 2012;130(4):1061–5. [Google Scholar]
- 11. Sheu S-Y, Lei Y-C, Tai Y-T, Chang T-H, Kuo T-F. Screening of salbutamol residues in swine meat and animal feed by an enzyme immunoassay in Taiwan. Anal Chim Acta. 2009;654(2):148–53. doi: 10.1016/j.aca.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 12. Ko K, Kurogi K, Davidson G, Liu MY, Sakakibara Y, Suiko M, et al. Sulfation of ractopamine and salbutamol by the human cytosolic sulfotransferases. J Biochem. 2012;152(3):275–83. doi: 10.1093/jb/mvs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JNMJC. Statistics and Chemometrics for Analytical Chemistry. 4th ed. Harlow, England: Person Education Limited; 2000. Chapter 5. Calibration methods in instrumental analysis: regression and correlation; p. 107. [Google Scholar]
- 14. Churchwell MI, Holder CL, Little D, Preece S, Smith DJ, Doerge DR. Liquid chromatography/electrospray tandem mass spectrometric analysis of incurred ractopamine residues in livestock tissues. Rapid Commun Mass Spectrom RCM. 2002;16(13):1261–5. doi: 10.1002/rcm.717. [DOI] [PubMed] [Google Scholar]
- 15. José Blanca, Patricia Muñoz, Miguel Morgado, Nely Méndez, Angela Aranda, Thea Reuvers, et al. Determination of clenbuterol, ractopamine and zilpaterol in liver and urine by liquid chromatography tandem mass spectrometry. Anal Chim Acta. 2005;529:199–205. [Google Scholar]
- 16. Nielen MW, Lasaroms JJ, Essers ML, Oosterink JE, Meijer T, Sanders MB, et al. Multiresidue analysis of beta-agonists in bovine and porcine urine, feed and hair using liquid chromatography electrospray ionisation tandem mass spectrometry. Anal Bioanal Chem. 2008;391(1):199–210. doi: 10.1007/s00216-007-1760-7. [DOI] [PubMed] [Google Scholar]
- 17. Shao B, JX, Zhang J, Meng J, Wu Y, Duan H, et al. Multi-residual analysis of 16 b-agonists in pig liver, kidney and muscle by ultra performance liquid chromatography tandem mass spectrometry. Food Chem. 2009;114:1115-21-21. [Google Scholar]
- 18. Official journal of the European communities. Commission of the European communities, Council directive. 2002/657/EC implementing Council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. 2002:L221. [Google Scholar]
- 19. Wu J, Liu X, Peng Y. Determination of ractopamine in pig hair using liquid chromatography with tandem mass spectrometric detection. J Pharmacol Toxicol Meth. 2014;69(3):211–6. doi: 10.1016/j.vascn.2014.02.001. [DOI] [PubMed] [Google Scholar]