Abstract
Rhizoma corydalis and Radix Angelicae Dahurica (Yuanhu–Baizhi) herbal medicine pair has been used for thousands of years and has been reported to be potentially active in recent cancer therapy. But the exact active components or fractions remain unclear. In this study, a new comprehensive two-dimensional (2D) 3-aminopropyltriethoxysilane (APTES)-decorated MCF7-cell membrane chromatography (CMC)/capcell-C18 column/time-of-flight mass spectrometry system was established for screening potential active components and clarifying the active fraction of Yuanhu–Baizhi pair. APTES was modified on the surface of silica, which can provide an amino group to covalently link cell membrane fragments with the help of glutaraldehyde in order to improve the stability and column life span of the MCF7 CMC column. The comprehensive 2D MCF7-CMC system showed good separation and identification abilities. Our screen results showed that the retention components are mainly from the alkaloids in Yuanhu (12 compounds) and the coumarins (10 compounds) in Baizhi, revealing the active fractions of Yuanhu–Baizhi herbal medicine pair. Oxoglaucine, protopine, berberine, osthole, isopimpinellin and palmitic acid were selected as typical components to test the effects on cell proliferation and their IC50 were calculated as 38.17 μM, 29.45 μM, 45.42 μM, 132.7 μM, 156.8 μM and 90.5 μM respectively. Cell apoptosis assay showed that the drug efficacy was obtained mainly through inducing cell apoptosis. Furthermore, a synergistic assay results demonstrated that oxoglaucine (representative of alkaloids from Yuanhu) and isopimpinellin (representative of coumarins from Baizhi) showed significant synergistic efficacy with GFT, indicating that these components may act on other membrane receptors. The proposed 2D CMC system could also be equipped with other cells for further applications. Besides, the follow-up in-vitro experimental strategy using cell proliferation assay, cell apoptosis assay and synergistic assay proved to be a practical way to confirm the active fractions of herbal medicine.
Keywords: Cell membrane chromatography, Comprehensive two-dimensional chromatography, Herbal medicine, Rhizoma corydalis and Radix Angelicae Dahurica, Anti-breast-cancer components
1. Introduction
Traditional Chinese Medicines (TCMs) has aroused more and more attention [1,2], especially in targeting potential active components or leading compounds in modern drug research in cancer therapy [3,4], and some TCMs in combination with chemo- or radio-therapy are capable of enhancing the efficacy and diminishing the side effects and complications [5,6]. Statistics indicate that, 75% of anti-cancer drugs are other than synthetic, with 49% of them from natural products from 1940s to 2014 [7]. Rhizoma corydalis and Radix Angelicae Dahurica (Yuanhu–Baizhi) herbal medicine pair has been used together for thousands of years in China for analgesia and anti-inflammatory. It was reported that Rhizoma corydalis (Yuanhu) extracts showed chemo-preventive activity and anti-metastasis effect against human breast cancer cells [8]. Radix Angelicae Dahuricae (Baizhi) extracts showed anti-cancer activity against colon cancer cells [9]. Nowadays, this herbal medicine pair has been used as an anti-breast-cancer synergist in clinical [10]. However, the potential anti-breast cancer components or fractions in Yuanhu–Baizhi pair are still ambiguous.
As a widely used method to screen potential active components from herbal medicine, cell membrane chromatography (CMC) shows a promising prospect [11,12]. To date, the mechanism of CMC has been fully investigated [13–16]. It is used to screen potential active components by the immobilized bioactive cell membranes on stationary phase [17–20]. At the same time, by deploying chromatography, CMC also possesses the ability to screen potential active components online according to retention behavior [15,21–24]. CMC not only shows advantages in bioactive ability, but also in high through-put out of the nature of chromatography, which make it a practical tool to screen potential active components from herbal medicine [25].
As known that CMC shows poor plate number and resolution ability like other frontal chromatography. According to our previously researches [14,15,21,23,24] and other reports [26], the comprehensive 2D system is a powerful tool to solve this problem. The 2D system not only optimizes the resolution but also improves the sensitivity of CMC model. Thus a comprehensive 2D system is applied in our research to solve the resolution problem. Besides resolution, CMC also faces another problem, that is, column life span. As a biological active chromatography, CMC usually shows short life span during usage due to the falling off or decay of cell membranes on the silica. Therefore, to solve the column life span problem, a newly-designed APTES decorated silica gel is adopted to the MCF7-CMC model [27]. This strategy further facilitated the immobilization of cell membranes on stationary phase by forming covalent bond between them. As a result, CMC columns demonstrate longer life span and better stability. In all, these modifications make CMC more applicable and convenient to use for a longer time. And the components screened by the proposed method would be ideal sources of leading compound for further research.
In this study, we devised a comprehensive two dimensional (2D) 3-aminopropyltriethoxysilane (APTES)-decorated MCF7-CMC/capcell-C18 column/time-of-flight mass spectrometry (TOFMS) system to clarify the potential anti-cancer active components and fractions in Yuanhu–Baizhi herbal pair. This established model could also be equipped with other cells and applied to other herbal medicines. Furthermore, the follow-up in-vitro experimental strategy using cell proliferation assay, cell apoptosis assay and synergistic assay is a credible way to confirm the active fractions of herbal medicine.
2. Experimental
2.1. Reagents and instruments
Silica gel (5 μm, 200 Å) was purchased from Qingdao Meigao Chemical Co., Ltd. (Qingdao, China). (3-aminopropyl) triethoxysilane (APTES, purity > 99.5%), glutaric dialdehyde (GDD, purity > 99.5%) and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Co. (Missouri, USA). Fetal bovine serum (FBS) was purchased from Gibco Life Technology Co., (Australia). Roswell park memorial institute medium (RPMI-1640), Phosphate buffer saline (PBS), trypsin and penicillin streptomycin (PS) were purchased from Corning Cellgro (Shanghai, China). The Milli-Q Academic A10 water purification system (Millipore, Bedford, MA, USA) provides ultrapure water for all experiments. Acetonitrile and formic acid of HPLC grade were purchased from Merck Co., (Darmstadt, Germany). While all the other reagents were of analytical grade. The cell counting kit-8 (CCK-8) and cell apoptosis kit were purchased from Dojindo China CO., Ltd (Shanghai, China).
Yuanhu and Baizhi were purchased from Shanghai Tonghanchuntang Pharmaceutical Co. Ltd. (Shanghai, China). Oxoglaucine, protopine, osthole, isopimpinellin and palmitic acid (purity > 98%) were obtained from Efebio Co., Ltd. (Shanghai, China). Gefitinib (GFT), berberine and dexamethasone (DXM) (purity > 98%) were purchased from Dalian Meilun Biotech Co., Ltd. (Dalian, China).
The electronic balance was HA-202M from A&D Company, Limited. (Tokyo, Japan). The centrifuge was Heraeus multifuge X1R (230 V) from Thermo Co., Ltd. (USA). The ultrasonic processor was JY92-IIN from Scientz Biotechnology (Ningbo, China). The HPLC system was Agilent-1200 from Agilent Technologies Co., Ltd. (California, America) coupled with an Agilent-6200 TOF/MS spectrometer and all of them were controlled by a Mass Hunter workstation. An electronically controlled MXP9960 10-port dual-position valve (Rheodyne, USA) was used, equipped with a CMC column and a Capcell-C18 column (100 mm × 3.0 mm I.D.) from Shiseido, Japan.
2.2. Preparation of samples and standard solutions
Firstly, Yuanhu and Baizhi were disposed into powder by a universal pulverizer. Then the powder was refluxed in 80% ethanol at 90 °C in electric jacket for 2 h and condensed with rotary evaporator to 2 g mL−1 at last. For the Yuanhu–Baizhi pair (YD) was extracted in the same manner, according to the Chinese Pharmacopoeia [28](2015), (Yuanhu: Baizhi, 2: 1, W/W).
The samples were diluted to 10 mg ml−1 and filtered by 0.2 μm filter membrane to remove large particles of suspended impurities before analysis. Standard solutions of oxoglaucine, protopine, berberine, osthole, isopimpinellin and palmitic acid (200 mM each) were prepared by dissolving in DMSO with assistance of supersonic wave. The stock solutions were subsequently stored at 4 °C in the dark before being used, with fresh stock solution being prepared on a weekly basis. For cell proliferation and apoptosis assays, the stock solutions were diluted to different concentrations with supplemented RPMI-1640.
2.3. Cell culture
MCF7 was selected as a representative breast cancer cell series in this paper, which was kindly provided by prof. Hao Zou from School of Pharmacy, Second Military Medical University. MCF7 cells were cultured in RPMI-1640 supplemented with 10% FBS and 100 μg mL−1 PS at 37 °C in a humidified atmosphere with 5% CO2, and were harvest during exponential phase for all experiments. Cells were stored in liquid-nitrogen before resuscitation.
2.4. Yuanhu–Baizhi herbal medicine pair in vitro efficacy assay
Cell proliferation assay was carried out to investigate the in vitro efficacy of Yuanhu–Baizhi herbal medicine pair. CCK-8 kits were used according to the manufacturer’s instructions. After cell dissociation and cell counting, 5 × 103 MCF7 cells were implemented into each well of 96-well plate. After 24 h, MCF7 cells were treated with Yuanhu–Baizhi extract at various concentrations from 1.5625 mg mL−1 to 200 mg mL−1 for 48 h. Then the solution was removed and a mixed solution of CCK-8 and complete RPMI-1640 (1:10, V/V) was added into each well. After 1 h incubation, the 96-well plate was determined at 450 nm on a micro-plate absorbance reader (Bio-RAD instruments, USA).
2.5. Preparation of APTES-decorated MCF7-CMC columns
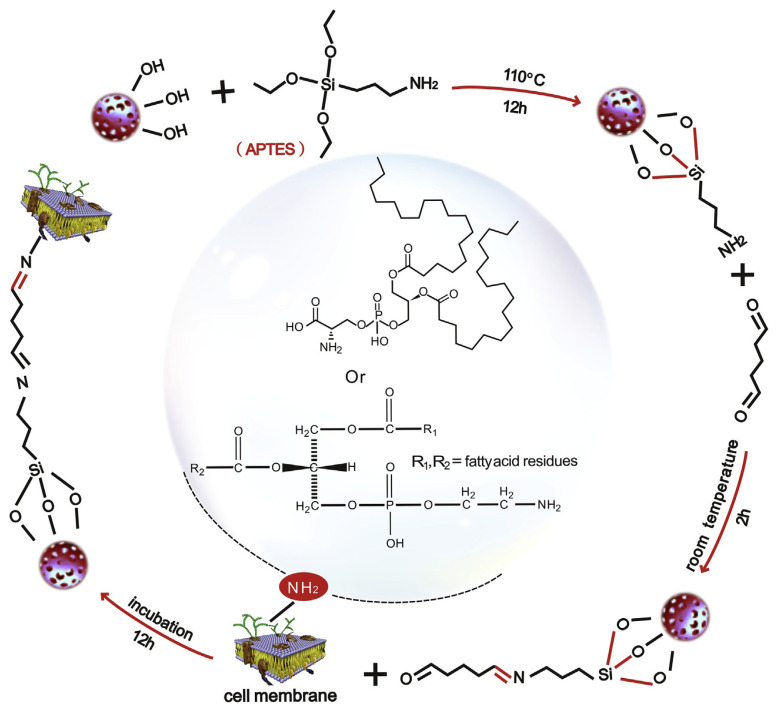
APTES-decorated MCF7-CMC columns were prepared according to our previous operating procedures [27]. Firstly, the APTES-decorated stationary phase was prepared as reported previously [27], and the reaction scheme is shown in Fig. 1. Then, 3.5 × 107 cells were harvested and washed with PBS (10 mM, pH 7.4) for three times under 1000 × g centrifuge. The cells deposited on the bottom of the centrifuge tube were reconstituted with 5 mL of PBS. Then the suspension was placed into an ultrasonic processor to proceed 7 cycles of disruption (2 s at 400 W per cycle with 20 s interval). After the disruption, the suspension was centrifuged at 3000 × g for 10 min. Then the precipitate was discarded and the supernatant was collected and centrifuged at 14,000 × g for 20 min. The precipitation was then reconstituted in 5 mL PBS. This suspension was mixed with 0.05 g APTES-decorated silica gel under vacuum and agitation conditions to react for 5 min to form APTES-decorated cell membrane solid phase (APTES-CMSP). All of the previous operations were carried out under the condition of 4 °C, mainly to prevent the collapse of cell membrane proteins after the release of the degradation enzyme. After incubation at 4 °C for 12 h, the APTES-CMSP was washed there times with PBS by centrifuging at 1000 × g for 5 min. In the end, the pellet was dissolved in 4 mL PBS and packed into the column (10 mm × 2 mm I.D.) with PBS by an LC pump (Waters 996). The packing program was set as follows: 0–5 min, from 0.2 mL min−1 to 1.0 mL min−1; and 5–6 min, stay at 1.0 mL min−1. Then ammonium acetate (10 mM) was used as mobile phase. The column is equilibrated at 0.2 mL min−1 under 37 °C for 1 h until stable baseline is obtained with stable column pressure. The CMC column was stored in ammonium acetate (10 mM) at 4 °C before or after use.
Fig. 1.
Scheme of APTES-decorated silica gel synthesis and its reaction with cell membranes.
2.6. Comprehensive 2D APTES-decorated MCF7-CMC/capcell-C18 column/TOFMS system
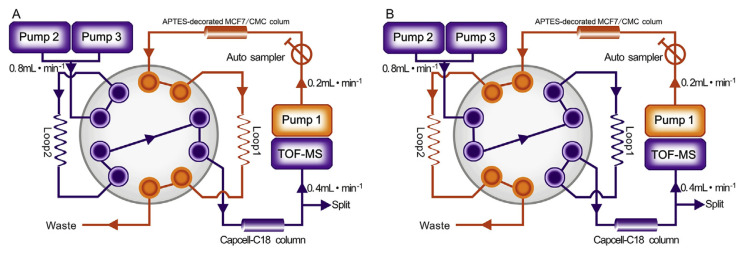
As our group reported before, the comprehensive 2D APTES-decorated CMC/capcell-C18 column/TOFMS system was performed on an Agilent 1200 series HPLC system controlled by Agilent Mass Hunter Workstation (Agilent Technologies, Palo Alto, CA, USA) [27]. As shown in Fig. 2, for the first dimension, CMC column (10 × 2 mm I.D., 5 μm) was used and the mobile phase was 10 mM ammonia acetate delivered at 0.2 mL min−1. A Capcell-C18 column (100 mm × 3.0 mm I.D., Shiseido, Japan) was used as the second dimensional column. The mobile phase of the second dimension was composed of solvent A (acetonitrile) and solvent B (0.1% formic acid) and delivered at 0.8 mL min−1 by a linear gradient elution program. The second dimension elution program for Yuanhu was set as follows: 0–8 min, from 10% A to 25% A; 8–10 min, 25% A; 10–10.01 min, from 25% A to 10% A; 10.01–13 min, 10% A. While the program for Baizhi was set as follows: 0–8 min, from 10% A to 60% A; 8–10 min, 60% A; 10–10.01 min, from 60% A to 10% A; 10.01–13 min, 10% A. Further detailed operations of the 2D system were published in our group’s previous researches [14,15,21,23,24,29].
Fig. 2.
Block scheme of 2D APTES-decorated MCF7-CMC/Capcell-C18 column/TOFMS system. (A) Position 1 and (B) position 2.
2.7. In vitro efficacy assay of potential active components
To validate in vitro efficacy of the components screened out by CMC, cell proliferation assay and cell apoptosis assay were carried out. Cell proliferation assay kits for CCK-8 were used according to the manufacturer’s instructions. According to reported researches, MCF7 cells were rich in epidermal growth factor receptors (EGFR) [30,31], thus GFT, an EGFR antagonist, was used as a positive control. After cell dissociation and cell counting, 5 × 103 cells were plated on each well of 96-well plate. After incubation for 24 h, MCF7 cells were treated with drugs at various concentrations for 48 h. Then the solution was removed and a mixed solution of CCK-8 and RPMI-1640 (1:10, V/V) was added into each well. Bubbles were removed with a hair dryer prior to detection at 450 nm on a micro-plate absorbance reader (Bio-RAD instruments, USA).
Cell apoptosis was assayed using Annexin V-FITC Apoptosis Detection kit according to the manufacturer’s instructions. In brief, 3 × 105 cells were implemented into each well of six-well plate. After incubation for 24 h, MCF7 cells were treated with drugs at the dose of 50 μM or 100 μM for 48 h. Then, cells in each well were washed and harvested with PBS and Annexin V/FITC was subsequently added respectively. After incubation for 15 min, cells were washed and resuspended with PBS. Propidium iodide (PI) was then added. At last, stained cells were identified by flow cytometer (Becton Dickinson) and analyzed by WinMDI 2.8 software (Scripps Institute, La Jolla, CA, USA).
All the experiments were performed at least three times in a parallel manner. Data were expressed as means ± standard deviation and statistical differences were estimated with Student’s t-test.
2.8. Synergistic efficacy assay
To investigate the synergistic efficacy of Yuanhu–Baizhi herbal medicine pair with GFT, a synergistic efficacy assay was carried out. In brief, oxoglaucine (representative of alkaloids in Yuanhu) and isopimpinellin (representative of coumarins in Baizhi) were selected and serially diluted to the concentrations of 100, 50, 25, 12.5, 6.25 μM and 200, 100, 50, 25, 12.5 μM respectively. Yuanhu–Baizhi pair extract was serially diluted to 50, 25, 12.5, 6.25 and 3.125 mg mL−1, while GFT was serially diluted to 100, 50, 25, 12.5 and 6.25 μM. Then 5 × 103 cells were implemented into each well of 96-well plate. After 24 h, various concentrations of oxoglaucine, isopimpinellin or Yuanhu–Baizhi pair extract were mixed with various concentrations of GFT respectively to incubate with MCF7 cells. After incubation for 48 h, drug synergistic results were determined by CCK8 kits according to manufacturers’ directions as described in Section 2.7.
3. Results and discussion
3.1. Selectivity of the comprehensive 2D APTES-decorated MCF7-CMC/capcell-C18 column/TOFMS system
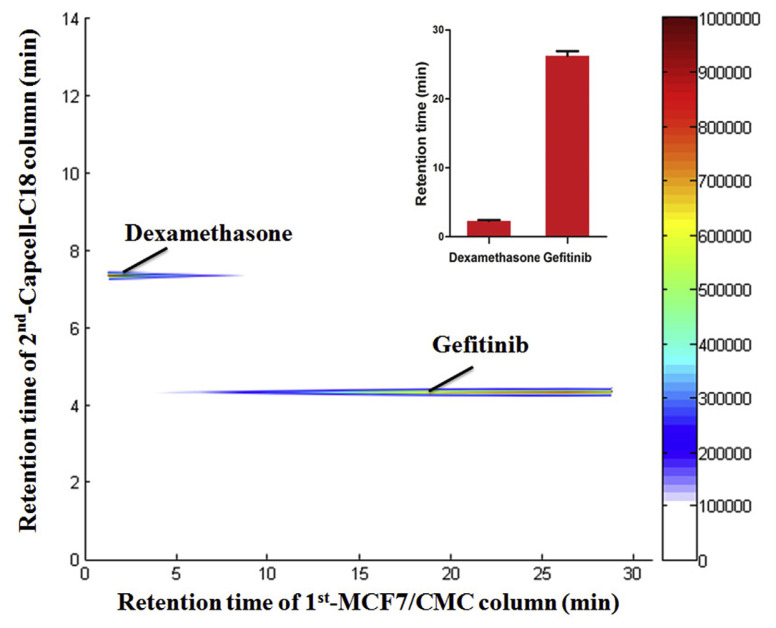
Firstly, the selectivity of the 2D APTES-decorated MCF7-CMC system was evaluated. As known, MCF7 cells are rich in the expression of epidermal growth factor receptor on cell membranes [32]. Thus GFT (epidermal growth factor receptor antagonist) and DXM (hormones, not targeted on membranes), were selected as the positive and negative control drugs respectively to confirm the selectivity of the APTES-decorated 2D MCF7-CMC system [14,15,21,23,24,29]. As shown in Fig. 3, GFT showed strong retention behavior, reaching peak at about 25 min. While DXM barely retained on this CMC model, reaching peak at about 3 min. This indicates satisfactory selectivity of this system. Injections were repeated for 3 times and the result showed satisfying intraday precision as shown in Fig. 4. APTES-decorated CMC column life span was extended from 3 days to at least 14 days and the reproducibility (RSD %) was raised to less than 10% within days (n = 5) as reported [27].
Fig. 3.
Selectivity evaluation of the 2D APTES-decorated MCF7-CMC system (DXM as a negative control ligand and GFT as a positive ligand).
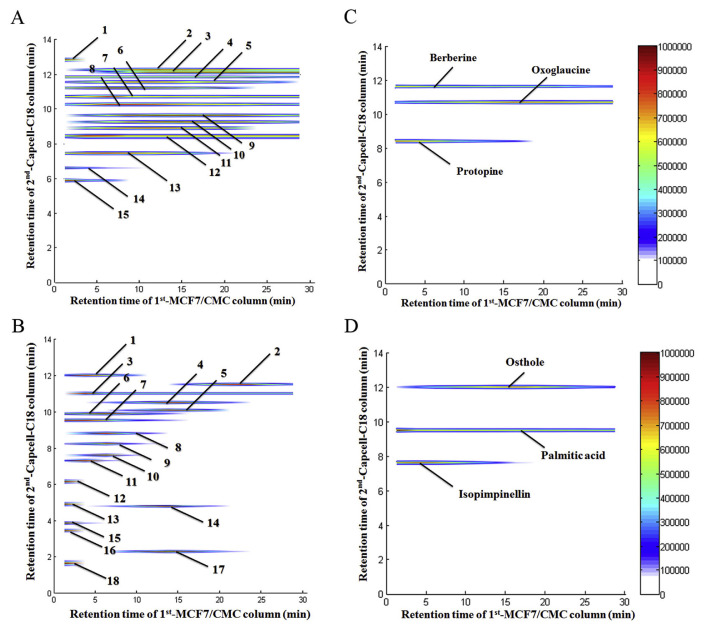
Fig. 4.
Typical 2D chromatography plots of (A) Yuanhu extract (information of components 1–15 were listed in Table 1), (B) Baizhi extract (information of components 1–18 were listed in Table 2), (C) mixed standard solutions (berberine, oxoglaucine and protopine) from Yuanhu and (D) mixed standard solutions (osthole, isopimpinellin and palmitic acid) from Baizhi retaining results of 2D MCF7-CMC system.
3.2. Application to Yuanhu–Baizhi herbal medicine pair
The comprehensive 2D APTES-decorated MCF7-CMC/capcell-C18 column/TOFMS system was applied into the screening of potential active compounds from Yuanhu and Baizhi. Data were obtained through Agilent MassHunter software and stored as csv files. Then the csv files were transferred into MATLAB 2010a to plot a comprehensive 2D contour map by programed command lines written by ourselves [14,15,21,23,24,29]. As shown in Fig. 4A and B, 12 components from Yuanhu (No. 2 to No. 13; all of them were alkaloids) and 13 from Baizhi (No. 1 to No. 11, No. 14 and No. 17; 10 of them were coumarins) showed strong retaining behavior at the first dimensional chromatography of CMC. Besides, those components have been tentatively identified by referring to high-resolution MS data obtained from TOF/MS and the compound library we established for Yuanhu and Baizhi using our previous reported protocols [33]. The identification results are shown in Tables 1 and 2 and compounds with possible isomers listed together at the same line. Thus those components are likely to bind targets on MCF7 cell membranes and could be potential active to inhibit or kill breast cancer cells. Six of them, oxoglaucine, protopine and berberine from Yuanhu; osthole, isopimpinellin and palmitic acid from Baizhi were selected out and confirmed with authentic standards as shown in Fig. 4C and D. Their retention behaviors were in accordance with the screen results, indicating that the good recognition capability of the comprehensive 2D CMC system.
Table 1.
Components of Yuanhu on 2D APTES-decorated MCF7/CMC system identified by TOFMS.
| Peak number | Identification | tR (CMC, min) | tR (C18, min) | m/z | Abundance match (%) | Formula | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Expected | Detected | Error (ppm) | ||||||
| 1 | Noroxyhydrastinine | 0–2.5 | 12.8 | 192.0655 ([M + H]+) | 192.0654 ([M + H]+) | −0.5 | 99.14 | C10H9NO3 |
| 2 | Dehydroglaucineb | 2.5–30 | 12.2 | 352.1543 ([M + H]+) | 352.1542 ([M + H]+) | −0.3 | 99.64 | C21H21NO4 |
| 3 | Dehydrocorydalineb | 5–30 | 12.1 | 367.1778 ([M + H]+) | 367.1739 ([M + H]+) | −10.6 | 92.29 | C22H24NO4 |
| 4 | Berberinea,b | 1–30 | 11.8 | 337.1309 ([M + H]+) | 337.1297 ([M + H]+) | −3.6 | 99.63 | C20H18NO4 |
| 5 | Tetrahydroprotopapaverine | 1–27.5 | 11.5 | 330.1700 ([M + H]+) | 330.1707 ([M + H]+) | 2.1 | 91.51 | C19H23NO4 |
| 6 | d-Corydaline | 1–22.5 | 11.2 | 370.2013 ([M + H]+) | 370.2014 ([M + H]+) | 0.3 | 99.88 | C22H27NO4 |
| 7 | Oxoglaucinea,b | 1–30 | 10.7 | 352.1179 ([M + H]+) | 352.1184 ([M + H]+) | 1.4 | 91.79 | C20H17NO5 |
| 8 | Tetrahydrocorysamineb,c/dehydronantenineb,c/dihydrochelerythrineb,c | 1–30 | 10.2 | 338.1388 ([M + H]+) | 338.1387 ([M + H]+) | −0.3 | 99.87 | C20H19NO4 |
| 9 | Stepharanineb | 2.5–30 | 9.6 | 325.1309 ([M + H]+) | 325.1284 ([M + H]+) | −7.7 | 82.28 | C19H18NO4 |
| 10 | dl-Tetrahydrocoptisineb | 2.5–30 | 9.2 | 324.1230 ([M + H]+) | 324.1232 ([M + H]+) | 0.6 | 99.85 | C19H17NO4 |
| 11 | dl-Tetrahydropalmatinec/d-corybulbinec/yanhuninec | 2.5–22.5 | 8.9 | 356.1854 ([M + H]+) | 356.1856 ([M + H]+) | 0.6 | 99.59 | C21H25NO4 |
| 12 | Protopinea,b | 1–30 | 8.4 | 354.1336 ([M + H]+) | 354.1338 ([M + H]+) | 0.6 | 97.94 | C20H19NO5 |
| 13 | Tetrahydrocolumbaminec/tetrahydrojatrorrhizinec/isocorypalminec/corydalminec | 1–17.5 | 7.4 | 342.1700 ([M + H]+) | 342.1699 ([M + H]+) | −0.3 | 99.68 | C20H23NO4 |
| 14 | Pontevedrine | 1–10 | 6.6 | 384.1442 ([M + H]+) | 384.1450 ([M + H]+) | 2.1 | 97.79 | C21H21NO6 |
| 15 | Scoulerine | 1–7.5 | 5.9 | 328.1543 ([M + H]+) | 328.1544 ([M + H]+) | 0.3 | 99.89 | C19H21NO4 |
Confirmed by authentic standard compounds.
Peak that was not completely flushed out by 1st-CMC column within 30 min.
Possible isomers that cannot be separated by 2nd-C18 column and TOFMS using m/z.
Table 2.
Components of Baizhi on 2D APTES-decorated MCF7/CMC system identified by TOFMS.
| Peak number | Identification | tR (CMC, min) | tR (C18, min) | m/z | Abundance match (%) | Formula | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Expected | Detected | Error (ppm) | ||||||
| 1 | Suberosinc/ostholea,c | 1–10 | 12.0 | 245.1172 ([M + H]+) | 245.1172 ([M + H]+) | 0.00 | 85.97 | C15H16O3 |
| 2 | Phellopterinb,c/cnidilinbb,c | 17.5–30 | 11.5 | 301.1071 ([M + H]+) | 301.1072 ([M + H]+) | 0.33 | 99.88 | C17H16O5 |
| 3 | Dibutylphthalateb | 2.5–30 | 11.0 | 279.1591 ([M + H]+) | 279.1592 ([M + H]+) | 0.36 | 99.90 | C16H22O4 |
| 4 | Xanthotoxolc/bergaptolc | 7.5–22.5 | 10.5 | 203.0339 ([M + H]+) | 203.0339 ([M + H]+) | 0.00 | 99.91 | C11H6O4 |
| 5 | Imperatorinc/isoimperationc | 7.5–20 | 10.1 | 271.0965 ([M + H]+) | 271.0966 ([M + H]+) | 0.37 | 99.91 | C16H14O4 |
| 6 | Dahuribirin B | 1–15 | 9.9 | 651.2072 ([M + H]+) | 651.2072 ([M + H]+) | 0.00 | 98.73 | C34H34O13 |
| 7 | Palmitic acida | 1–15 | 9.5 | 257.2475 ([M + H]+) | 257.2490 ([M + H]+) | 1.70 | 98.92 | C16H32O2 |
| 8 | Alloimperatorinc/oxypeucedaninc/isooxypeucedaninc | 2.5–12.5 | 8.8 | 287.0914 ([M + H]+) | 287.0915 ([M + H]+) | 0.35 | 99.89 | C16H14O5 |
| 9 | Xanthotoxinc/bergaptenc/sphondinc | 1–12.5 | 8.2 | 217.0495 ([M + H]+) | 217.0496 ([M + H]+) | 0.46 | 99.96 | C12H8O4 |
| 10 | Isopimpinellina,c/pimpinellinc | 2.5–10 | 7.6 | 247.0601 ([M + H]+) | 247.0602 ([M + H]+) | 0.40 | 99.94 | C13H10O5 |
| 11 | Psoralenc/isopsoralenc | 1–7.5 | 7.3 | 187.0390 ([M + H]+) | 187.0392 ([M + H]+) | 1.07 | 99.92 | C11H6O3 |
| 12 | Marmesinc/smyrindiolc | 1–2.5 | 6.1 | 247.0965 ([M + H]+) | 247.0963 ([M + H]+) | −0.81 | 99.60 | C14H14O4 |
| 13 | Marmesininc/nodakeninc | 1–2.5 | 4.9 | 409.1493 ([M + H]+) | 409.1494 ([M + H]+) | 0.24 | 98.56 | C20H24O9 |
| 14 | Scopoletin | 5–20 | 4.8 | 193.0495 ([M + H]+) | 193.0495 ([M + H]+) | 0.00 | 96.71 | C10H8O4 |
| 15 | Scopolin | 1–5 | 3.9 | 355.1024 ([M + H]+) | 355.1028 ([M + H]+) | 1.13 | 99.10 | C16H18O9 |
| 16 | Umbelliferone | 1–2.5 | 3.4 | 163.0390 ([M + H]+) | 163.039 ([M + H]+) | 0.00 | 99.73 | C9H6O3 |
| 17 | Desmodimine | 7.5–22.5 | 2.3 | 238.1074 ([M + H]+) | 238.1074 ([M + H]+) | 0.00 | 94.99 | C12H15NO4 |
| 18 | Adenosine | 1–2.5 | 1.6 | 268.1040 ([M + H]+) | 268.1044 ([M + H]+) | 1.49 | 98.64 | C10H13N5O4 |
Confirmed by authentic standard compounds.
Peak that was not completely flushed out by 1st-CMC column within 30 min.
Possible isomers that cannot be separated by 2nd-C18 column and TOFMS using m/z.
3.3. Effects of the potentially active components on MCF7 cell proliferation and apoptosis
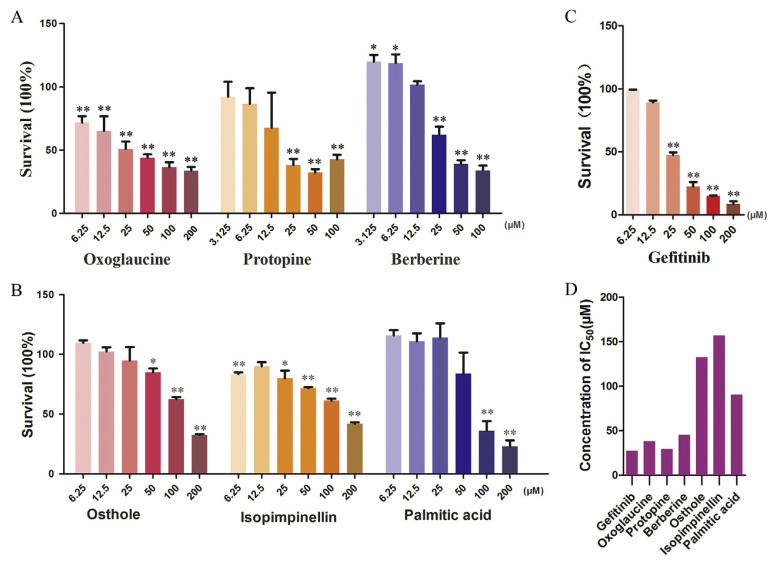
As shown in Supplementary Fig. S1, by investigating the in vitro efficacy of Yuanhu–Baizhi extract on MCF7 cells at different concentrations by CCK-8 assay, we can see that Yuanhu–Baizhi extract can inhibit the growth of MCF7 cells in a dose dependent way. The IC50 was calculated to be 11.6 mg mL−1. Yuanhu–Baizhi herbal medicine pair was proved to be potentially effective in inhibiting MCF7 cells and could be an ideal source to screen potential anti-breast-cancer components. To validate the efficacy of the six potential active components, cell proliferation assay was carried out. Having incubated each potential compound with MCF7 cells for 48 h, we found that oxoglaucine, protopine, berberine, osthole, isopimpinellin and palmitic acid, showed good inhibition ability on MCF7 cells with IC50 values of 38.17 μM, 29.45 μM, 45.42 μM, 132.7 μM, 156.8 μM and 90.5 μM respectively in a dose dependent manner as shown in Fig. 5. GFT is selected as positive drug and IC50 is determined to be 27.3 μM. Thus oxoglaucine, protopine, berberine, osthole, isopimpinellin and palmitic acid can be identified as potential active anti-breast-cancer components from Yuanhu–Baizhi herbal medicine pair. Their effects were roughly consistent with their retention behaviors in the MCF7 CMC model.
Fig. 5.
(A) MCF-7 cell proliferation inhibitory effects of (A) oxoglaucine, protopine and berberine in Yuanhu; (B) osthole, isopimpinellin and palmitic acid in Baizhi; (C) positive control drug GFT. (D) IC50 comparison of GFT, oxoglaucine, protopine, berberine, osthole, isopimpinellin and palmitic acid. Statistical differences were estimated with Student’s t-test (*p < 0.05 was taken as statistically significant and **p < 0.01 was considered as dramatically significant vs. the negative control, n = 3).
To further investigate whether their efficacy was obtained through inducing cell death or apoptosis, Annexin V-FITC/PI staining was carried out to test the MCF7 cell apoptosis rates. As shown in Supplementary Fig. S2, having been incubated with oxoglaucine, protopine, berberine at 50 μM and osthole, isopimpinellin, palmitic acid at 100 μM, cell apoptosis rate was significantly improved compared with control group. This indicates efficacy of these compounds is mainly obtained through inducing cell apoptosis. These results confirm that our comprehensive 2D APTES-decorated MCF7-CMC system is a reliable method for identifying anti-breast cancer components in herbal medicine extracts.
3.4. Synergistic efficacy assay
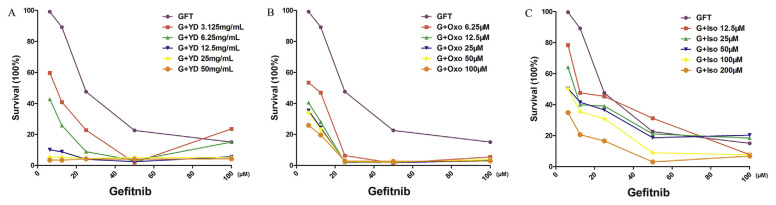
To investigate whether the active fractions of Yuanhu–Baizhi have synergistic efficacy with GFT, a synergistic efficacy assay was carried out using CCK-8 approach. Oxoglaucine is representative component of alkaloids in Yuanhu and isopimpinellin is representative coumarins component of in Baizhi. Various concentrations of Yuanhu–Baizhi extract, oxoglaucine and isopimpinellin were incubated with GFT respectively. As shown in Fig. 6, when incubated with the same level of GFT, Yuanhu–Baizhi extract, oxoglaucine and isopimpinellin all showed stronger efficacy in a dose dependent way. And drug efficacy also gets stronger with the increasing of GFT concentration. This result demonstrates that Yuanhu–Baizhi extract, oxoglaucine and isopimpinellin all show synergistic efficacy with GFT. Thus we can see that Yuanhu–Baizhi, oxoglaucine and isopimpinellin are potential sensitizer for GFT in the therapy of breast cancer and their targets or mechanisms are different from positive drug GFT. Furthermore, oxoglaucine and isopimpinellin are the representative components for Yuanhu and Baizhi respectively to play a part in synergistic efficacy with GFT. Thus this result also reveals that alkaloids in Yuanhu and coumarins in Baizhi are the active fractions of Yuanhu–Baizhi pair to play the key role in its anti-breast-cancer efficacy. These active components or fractions could be ideal leading compound sources for further research.
Fig. 6.
(A) Combination inhibitory effects of (A) GFT and Yuanhu–Baizhi extract (YD); (B) GFT and oxoglaucine (Oxo); (C) GFT and isopimpinellin (Iso) towards MCF7 cells.
4. Conclusions
An APTES-decorated MCF7-CMC/capcell-C18 column/TOFMS system was developed in our research to investigate the potential anti-breast-cancer components and fractions in Yuan-hu–Baizhi herbal pair with some modifications we made according to our previous CMC models [14,15,21,23,24,29]. This system combined the advantages of biological activity and online high-throughput, which is very suitable for the screening of potential active components from complex system, especially from herbal medicine. The screen results showed that 12 alkaloids in Yuanhu and 10 coumarins in Baizhi retained, revealing the active fractions of Yuanhu–Baizhi herbal medicine pair. We have identified oxoglaucine, proto-pine, berberine from Yuanhu and osthole, isopimpinellin, palmitic acid from Baizhi as potential active components with the help of a comprehensive 2D ATPES-decorated MCF7-CMC/capcell-C18 column/TOFMS system. Besides, cell apoptosis assays were also carried out to prove that the efficacy of these components was obtained mainly through inducing the apoptosis of MCF7 cells in a dose dependent manner. Additionally, a synergistic experiment was carried out and showed that Yuanhu–Baizhi pair extract, oxoglaucine and isopimpinellin all showed synergistic efficacy with GFT. This result indicates that alkaloids in Yuanhu and coumarins in Baizhi are potential active fractions of Yuanhu–Baizhi herbal pair, whose targets or mechanisms are different from positive drug GFT. These active components or fractions could be ideal leading compound sources for further research. This application has further broadened the range of use for CMC and could be a promising way to screen potential anti-breast cancer components or fractions from herbal medicine.
Acknowledgments
Financial support is gratefully acknowledged from National Natural Sciences Foundation of China (No. 81503039, 81573396 and 81673386) and “Yang-Fan project” of Science and Technology Commission of Shanghai Municipality (No. 15YF1400200).
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2017.11.010.
Funding Statement
Financial support is gratefully acknowledged from National Natural Sciences Foundation of China (No. 81503039, 81573396 and 81673386) and “Yang-Fan project” of Science and Technology Commission of Shanghai Municipality (No. 15YF1400200).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Hung HY, Wu TS. Recent progress on the traditional Chinese medicines that regulate the blood. J Food Drug Anal. 2016;24(2):221–38. doi: 10.1016/j.jfda.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feng LY, Battulga A, Han E, Chung H, Li JH. New psychoactive substances of natural origin: a brief review. J Food Drug Anal. 2017;25(3):461–71. doi: 10.1016/j.jfda.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy – from TCM theory to mechanistic insights. Planta Med. 2010;76(11):1118–31. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 4. Wang S, Wu X, Tan M, Gong J, Tan W, Bian B, et al. Fighting fire with fire: poisonous Chinese herbal medicine for cancer therapy. J Ethnopharmacol. 2012;140(1):33–45. doi: 10.1016/j.jep.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 5. Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang Z, et al. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci Technol. 2015;9(1):16–34. doi: 10.5582/bst.2015.01019. [DOI] [PubMed] [Google Scholar]
- 6. Chen ZF, Liang H. Progresses in TCM metal-based antitumour agents. Anti Cancer Agents Med Chem. 2010;10(5):412–23. doi: 10.2174/1871520611009050412. [DOI] [PubMed] [Google Scholar]
- 7. Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–61. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 8. He K, Gao JL. Protopine inhibits heterotypic cell adhesion in Mda-Mb-231 cells through down-regulation of multi-adhesive factors. Afr J Tradit Complement Altern Med. 2014;11(2):415. doi: 10.4314/ajtcam.v11i2.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng YM, Shen JZ, Wang Y, Lu AX, Ho WS. Anti-oxidant and anti-cancer activities of Angelica dahurica extract via induction of apoptosis in colon cancer cells. Phytomedicine – Int J Phytother Phytopharmacol. 2016;23(11):1267–74. doi: 10.1016/j.phymed.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 10. Xu H, Tao Y, Lu P, Wang P, Zhang F, Yuan Y, et al. A computational drug-target network for yuanhu zhitong prescription. Evid Base Complement Alternative Med – eCAM. 2013;2013:658531. doi: 10.1155/2013/658531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh NS, Habicht KL, Dossou KS, Shimmo R, Wainer IW, Moaddel R. Multiple protein stationary phases: a review. J Chromatogr B Anal Technol Biomed Life Sci. 2014;968:64–8. doi: 10.1016/j.jchromb.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou X, Wang S, Zhang T, Ma J, Zhang J, Zhang Y, et al. Recent advances in cell membrane chromatography for traditional Chinese medicines analysis. J Pharmaceut Biomed Anal. 2014;101:141–50. doi: 10.1016/j.jpba.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 13. Li Y, He L. Establishment of the model of vascular endothelial cell membrane chromatography and its preliminary application. Chin Sci Bull. 2007;52(7):922–8. [Google Scholar]
- 14. Chen X, Cao Y, Lv D, Zhu Z, Zhang J, Chai Y. Comprehensive two-dimensional HepG2/cell membrane chromatography/monolithic column/time-of-flight mass spectrometry system for screening anti-tumor components from herbal medicines. J Chromatogr A. 2012;1242:67–74. doi: 10.1016/j.chroma.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 15. Ding X, Chen X, Cao Y, Jia D, Wang D, Zhu Z, et al. Quality improvements of cell membrane chromatographic column. J Chromatogr A. 2014;1359:330–5. doi: 10.1016/j.chroma.2014.07.071. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Xu L, Mao R, Zhao X, Xu B, Tang C, et al. An insertion/self-fusion mechanism for cell membrane immobilization on porous silica beads to fabricate biomimic carriers. Biomater Sci. 2017;5(7):1334–41. doi: 10.1039/c7bm00419b. [DOI] [PubMed] [Google Scholar]
- 17. de Moraes MC, Cardoso CL, Seidl C, Moaddel R, Cass QB. Targeting anti-cancer active compounds: affinity-based chromatographic assays. Curr Pharmaceut Des. 2016;22(39):5976–87. doi: 10.2174/1381612822666160614080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Habicht KL, Singh NS, Khadeer MA, Shimmo R, Wainer IW, Moaddel R. Characterization of a multiple endogenously expressed adenosine triphosphate-binding cassette transporters using nuclear and cellular membrane affinity chromatography columns. J Chromatogr A. 2014;1339:80–5. doi: 10.1016/j.chroma.2014.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Zhang N, Ma J, Zhu Y, Wang M, Wang X, et al. A Platelet/CMC coupled with offline UPLC-QTOF-MS/MS for screening antiplatelet activity components from aqueous extract of Danshen. J Pharmaceut Biomed Anal. 2016;117:178–83. doi: 10.1016/j.jpba.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 20. Xue H, Cheng Y, Wang X, Yue Y, Zhang W, Li X. Rutaecarpine and evodiamine selected as beta1-AR inhibitor candidates using beta1-AR/CMC-offline-UPLC/MS prevent cardiac ischemia-reperfusion injury via energy modulation. J Pharmaceut Biomed Anal. 2015;115:307–14. doi: 10.1016/j.jpba.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 21. Chen X, Cao Y, Zhang H, Zhu Z, Liu M, Liu H, et al. Comparative normal/failing rat myocardium cell membrane chromatographic analysis system for screening specific components that counteract doxorubicin-induced heart failure from Acontium carmichaeli. Anal Chem. 2014;86(10):4748–57. doi: 10.1021/ac500287e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Cao Y, Xie Y, Zhang X, Yang Q, Li X, et al. Traditional Chinese medicine for the treatment of primary dysmenorrhea: how do Yuanhu painkillers effectively treat dysmenorrhea? Phytomedicine – Int J Phytother Phytopharmacol. 2013;20(12):1095–104. doi: 10.1016/j.phymed.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 23. Jia D, Chen X, Cao Y, Wu X, Ding X, Zhang H, et al. On-line comprehensive two-dimensional HepG2 cell membrane chromatographic analysis system for charactering anti-hepatoma components from rat serum after oral administration of Radix scutellariae: a strategy for rapid screening active compounds in vivo. J Pharmaceut Biomed Anal. 2016;118:27–33. doi: 10.1016/j.jpba.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 24. Wu X, Chen X, Dan J, Cao Y, Gao S, Guo Z, et al. Characterization of anti-leukemia components from Indigo naturalis using comprehensive two-dimensional K562/cell membrane chromatography and in silico target identification. Sci Rep. 2016;6:25491. doi: 10.1038/srep25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hou X, Zhou M, Jiang Q, Wang S, He L. A vascular smooth muscle/cell membrane chromatography-offline-gas chromatography/mass spectrometry method for recognition, separation and identification of active components from traditional Chinese medicines. J Chromatogr A. 2009;1216(42):7081–7. doi: 10.1016/j.chroma.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 26. Ma W, Zhang D, Zheng L, Zhan Y, Zhang Y. Potential roles of Centipede Scolopendra extracts as a strategy against EGFR-dependent cancers. Am J Tour Res. 2015;7(1):39–52. [PMC free article] [PubMed] [Google Scholar]
- 27. Ding X, Cao Y, Yuan Y, Gong Z, Liu Y, Zhao L, et al. Development of APTES-decorated HepG2 cancer stem cell membrane chromatography for screening active components from Salvia miltiorrhiza. Anal Chem. 2016;88(24):12081–9. doi: 10.1021/acs.analchem.6b02709. [DOI] [PubMed] [Google Scholar]
- 28.Committee NP. Pharmacopoeia of the People’s Republic of China. Beijing, China: Chemical Industry; Press: 2015. [Google Scholar]
- 29. Wang D, Lv D, Chen X, Liu Y, Ding X, Jia D, et al. Activity ranking of synthetic analogs targeting vascular endothelial growth factor receptor 2 by an integrated cell membrane chromatography system. J Sep Sci. 2015;38(24):4159–65. doi: 10.1002/jssc.201500857. [DOI] [PubMed] [Google Scholar]
- 30. Moerkens M, Zhang Y, Wester L, Water B, Meerman J. Epidermal growth factor receptor signalling in human breast cancer cells operates parallel to estrogen receptor α signalling and results in tamoxifen insensitive proliferation. BMC Cancer. 2014;14(283):15. doi: 10.1186/1471-2407-14-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Songa X, Wei Z, Shaikh Z. Requirement of ERα and basal activities of EGFR and Src kinase in Cd-induced activation of MAPK/ERK pathway in human breast cancer MCF-7 cells. Toxicol Appl Pharmacol. 2015;287(1):25. doi: 10.1016/j.taap.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song X, Wei Z, Shaikh ZA. Requirement of ERalpha and basal activities of EGFR and Src kinase in Cd-induced activation of MAPK/ERK pathway in human breast cancer MCF-7 cells. Toxicol Appl Pharmacol. 2015;287(1):26–34. doi: 10.1016/j.taap.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen XF, Lou ZY, Zhang H, Tan GG, Liu ZR, Li WH, et al. Identification of multiple components in Guanxinning injection using hydrophilic interaction liquid chromatography/time-of-flight mass spectrometry and reversed-phase liquid chromatography/time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2011;25(11):1661–74. doi: 10.1002/rcm.5003. [DOI] [PubMed] [Google Scholar]