Abstract
Volatile compounds in ‘Sweetheart’ lychee were examined using gas chromatography-olfactometry/mass spectrometry (GC-O/MS). Solvent assisted flavor evaporation (SAFE) technique was used to identify the aroma-active compounds in lychee. Further characterization of the most important odorants in ‘Sweetheart’ lychee was achieved using aroma extract dilution analysis (AEDA). Thirty-one key aroma-active odorants were identified in the flavor dilution (FD) factor range of 2–1024. Methional (cooked potato) and geraniol (sweet, floral) exhibited the highest FD factors of 1024 and 512, respectively, these were followed by furaneol (sweet, caramel), nerol (floral, sweet), dimethyl trisulfide (DMTS) (preserved vegetable, sulfury), linalool (floral), (E,Z)-2,6 nonadienal (cucumber) and nerolidol (metalic, sesame oil). Furthermore, the flavor profile of ‘Sweetheart’ lychee was described by sensory analysis. Floral, tropical fruit, peach/apricot and honey were scored with relatively high scores for each aroma attribute. The sweetness rating was the highest score among all the attributes.
Keywords: AEDA, Aroma-active compounds, GC-MS/O, SAFE, Sensory analysis
1. Introduction
Lychee (Litchi chinensis Sonn.), also known as litchi, originates from the northern tropical and southern sub-tropical regions of south China with records of cultivation dating back to 111 B.C. [1]. It is a commercially important member of the Sapindaceae family, which also includes longan (Dimocarpus longan Lour.) and rambutan (Nephelium lappaceum L.). Asian countries such as China, India, Thailand and Vietnam play leading roles in worldwide lychee production, while in the United States, Florida makes the biggest contribution, followed by Hawaii and California [1,2]. Although native to Asia, lychee has increased in popularity in other countries outside of Asia and has attracted much attention from the mainstream markets.
At full maturity, lychee flesh consists of a white, juicy aril that surrounds a large brown seed and is covered with a red leathery skin [3]. Lychee has a distinctive flavor and is usually described as rose-floral and fruity-floral in aroma with a desirable, sweet taste [4]. An early study identified 42 volatiles from lychee fruit grown in Florida [5]. Using these results, a basic portrayal describing volatile constituents of lychee fruit was compiled. For the next twenty years, few investigations into lychee aroma were conducted until free and glycosidically bound aroma compounds in lychee were studied [6], which contributed additional information to the understanding of volatile constituents in lychee fruit. More recently, gas chromatography-olfactometry (GC-O) has been used to examine odor-active compounds in lychee cultivars [3]. Similarly, volatile profiles of nine lychee cultivars with a high commercial value in southern China were studied and interpreted using odor active value (OAV) [7]. It has become increasingly clear that in order to understand lychee aroma, the study of volatile compositions alone is not enough. It is also necessary to closely examine the odor-active compounds as well as understand their roles in the overall aroma profile of lychee fruit.
As previously mentioned, despite existing reports of volatile constituents of various lychee cultivars, a detailed aroma profile of lychee cultivars, which could demonstrate the diverse importance of volatile components to the overall aroma profile, has not yet been investigated. Therefore, the characteristic aroma-active compounds in lychee cultivars are still not clear. ‘Sweetheart’, a relatively recent introduction is hearty and thrives in South Florida's sub-tropical climate. Therefore, it is considered a rising star that exhibits superior quality. This high quality lychee cultivar is characterized by fresh, large, luscious lychee fruits with very small seeds. The demand of ‘Sweetheart’ has grown far greater than its supply since released. Therefore, ‘Sweetheart’ shows great competitive potential in the lychee market, which has been dominated by ‘Mauritius’ and ‘Brewster’. The goal of this study was to identify which major odor components contribute to the aroma profile of ‘Sweetheart’ lychee by using solvent assisted flavor evaporation (SAFE) coupled with gas chromatography-olfactometry/ mass spectrometry (GC-O/MS). The aroma extract dilution analysis (AEDA) was applied to further elucidate the importance of volatile components in lychee fruit. Information collected has the potential to further benefit studies into the lychee breeding program and lychee related products, such as dried lychee, lychee juice, lychee wine, and canned lychee.
2. Materials and methods
2.1. Lychee samples
Lychee cultivar (Sweetheart) was obtained from the Tropical Research and Education Center, University of Florida (Homestead, FL). The Brix/acid ratio of freshly made lychee juice was measured as 65.4. Fresh lychee fruits were used for sensory evaluation upon arrival while the rest were stored at −20 °C before analytical analysis.
2.2. Chemicals
2,3-Butanedione, 3-methyl-3-buten-2-one, 2-methyl-2-butanol, myrcene, α-phellandrene, 3-methyl-3-buten-1-ol, p-cymene, octanal, 1-octen-3-one, 6-methyl-5-heptene-2-one, (Z)-rose oxide, dimethyl trisulfide, (E)-2-octenal, methional, linalool, (E,Z)-2,6-nonadienal, isovaleric acid, (E,E)-2,4-nonadienal, citronellol, nerol, geraniol, guaiacol, nerolidol, 4-hydroxy-2,5-dimethyl-3(2H)-furanone (furaneol) and 2-ethyl-4-hydroxy-5-methyl-3(2H)-furanone (homofuraneol) were purchased from Sigma–Aldrich Chemicals Co. (St. Louis, MO). A mixture of n-alkanes (C7–C30) was used for the retention index (RI) analyses and was purchased from Sigma–Aldrich Chemicals Co. The extraction solvent, dichloromethane, was also purchased from Sigma–Aldrich Chemicals Co. and freshly distilled before use. Hexane was purchased from EMD Millipore Corporation (Darmstadt, Germany). Anhydrous sodium sulfate and sodium chloride were purchased from Fisher Scientific Co. (Hampton, NH).
2.3. Sensory analysis
Freshly peeled lychee fruits were randomly mixed and served in plastic cups at room temperature. Eleven pre-identified flavor attributes including cabbage, honey, tropical fruit, garlic/onion, floral, sweetness, astringency, sourness, citrus, green/woody, and peach/apricot were determined by 7 trained panelists and then used to evaluate lychee samples by 60 untrained panelists in the age range of 23–55. Samples were scored using a nine-point scale ranging from 1.0 (very slight) to 9.0 (very intense), at intervals of 1.0. All of the sensory tests were carried out within 48 h after the fruits were received.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol and consent procedure received ethical approval from the Institutional Review Board (IRB) of the University of Florida. Informed consent was obtained from all individual participants included in the study.
2.4. Solvent assisted flavor evaporation (SAFE)
Fifteen lychee fruits were peeled, pitted, and subsequently mixed with liquid nitrogen. The mixture was then pulverized in a Waring blender (Model 51BL31, Waring Co., Torrington, CT, USA) for 1 min. One hundred and ninety-seven grams of lychee powder were obtained, transferred into a centrifuge bottle, and mixed with 400 mL of distilled dichloromethane and 100 ppm of cyclohexyl butyrate (internal standard). The mixture was then placed on an Excella E5 platform shaker (New Brunswick Scientific Co, Inc., Enfield, CT) at 200 rpm for 1 h. The solvent extract was separated from the crude-extract through centrifugation at 5000 rpm for 5 min followed by separation through a separatory funnel. The final extract was collected and subsequently introduced into the SAFE apparatus [8]. A balanced thermostat throughout the SAFE system was maintained at 40 °C using a pre-warmed, circulating water bath, while the condensation chamber was kept frozen using liquid nitrogen. The final distillate condensed in a liquid nitrogen cooled round-bottom flask. It was then thawed at room temperature before being dehydrated using anhydrous sodium sulfate. Finally, the resulting distillate was concentrated using a Vigreux column (ST/NS 24/40, Length: 300 mm, OD: 42 mm) and micro-distilled to a final volume of ~150 μL. The final volume was placed into a GC vial containing a 150 μL glass insert and run for analysis.
2.5. Gas chromatography-mass spectrometry/ olfactometry (GC-MS/O)
The GC-MS/O system consisted of a Clarus 680 GC (Perki-nElmer, Inc., Waltham, MA) equipped with a Clarus SQ 8T MS and a SNFR olfactory port. A TR-FFAP column (30 m × 0.25 mm, 0.25 μm film thickness; Thermo Scientific, Waltham, MA) was used for separation. The column temperature was programmed to increase from 40 °C (after a 1 min hold) to 230 °C at a rate of 5 °C/min with a final hold time of 10 min. The carrier gas was helium maintained at a constant pressure. The GC injector and transfer lines were both maintained isothermally at 250 °C. Mass spectra in the electron impact mode (MS-EI) were applied at 70 eV ionization energy. The MS was set to scan from m/z 50 to 300 in the positive mode with a 2.5 min solvent delay. A Swafer™ S2 mode was used to split the sample into the MS and the olfactory port (240 °C).
2.6. Aroma extract dilution analysis (AEDA)
The flavor dilution factor (FD) of each of the odorants was determined using an AEDA method [9]. The flavor extract was injected into the GC column. Odor-active compounds were detected by GC-O, while two experienced panelists determined the sensory description of the volatiles. The extract was then diluted stepwise 1:1 (by volume) through the addition of hexane. Each sample of the dilution series was then reanalyzed until there was no perceivable odor detected at the olfactory port.
2.7. Qualification of odorants
Identification of odorants was based on suggestions by the National Institute of Standards and Technology (NIST) and confirmed using a linear retention index (RI), reference standards, and odor characteristics. RI of each identified compound was calculated using an n-alkanes (C7–C30) standard. With regard to any absent reference standards, tentative identification was based on the suggestions from the NIST library and a comparison of RI as reported in the Flavornet database or previous literature.
3. Results and discussion
3.1. Sensory evaluation
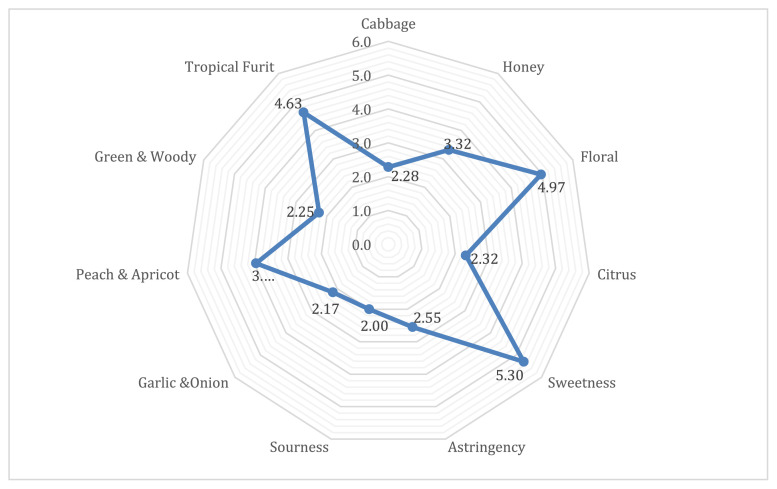
The individual intensity of eleven flavor characteristics is shown in Fig. 1. Among all examined aroma attributes, floral was the most intense, followed by tropical fruit, peach/apricot and honey. By contrast, the lychee samples showed relatively low odor intensities for citrus, cabbage, green/woody and garlic/onion. Other flavor attributes include sweetness, which was the most intense among all the flavor attributes. Additionally, astringency showed a greater intensity when compared to sourness. Further discussion correlating sensory evaluation with analytical analysis is presented in the next section.
Fig. 1.
Sensory panel descriptive analysis average scores for Sweetheart cultivar.
3.2. Identification and characterization of key odorants in sweetheart lychee
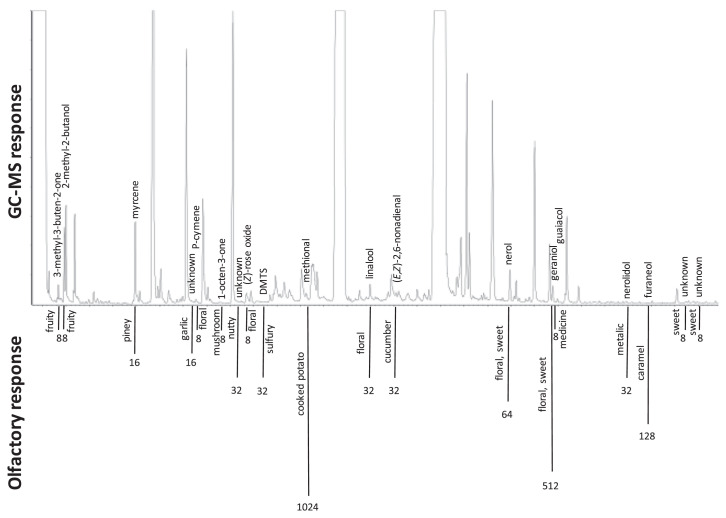
Thirty-one key aroma-active odorants were detected in the solvent extract for the FD factor range of 2–1024 (Fig. 2). Methional (cooked potato) and geraniol (sweet, floral) showed the highest FD factors of 1024 and 512, respectively. These were followed by furaneol (sweet, caramel), nerol (floral, sweet), dimethyl trisulfide (DMTS) (pickled vegetable, sulfury), linalool (floral), (E,Z)-2,6-nonadienal (cucumber) and nerolidol (metallic, sesame oil) (Table 1). Similar results were obtained from previous lychee studies, in which geraniol, furaneol and linalool were illustrated as most odor-active compounds in different Chinese lychee cultivars [7,10] while methional, DMTS, linalool and (E,Z)-2,6-nonadienal were characterized in three major Florida lychee cultivars as odor volatiles [3]. However, other odorants with high odor activities reported in previous investigations, such as trans-rose oxide, 1-octen-3-ol, vanillin, 2-acetyl-2-thiazoline, 2-phenylethanol, (Z)-2-nonenal and β-damascenone were not located in this study.
Fig. 2.
GC chromatogram of Sweetheart lychee (SAFE extract) with corresponding identified aroma-active compounds (FD ≥ 8).
Table 1.
Key aroma-active compounds identified in Sweetheart lychee.
| Odorant | RI (FFAP) | Odor quality | FD factor | Detection/identificationa |
|---|---|---|---|---|
| Diacetyl | 963 | Stale, yogurt | 4 | O, S |
| 3-Methyl-3-buten-2-one | 976 | Fruity, sweet | 8 | O, MS, S |
| 2-Methyl-2-butanol | 990 | Fruity | 8 | O, MS, S |
| α-Phellandrene | 1122 | Grassy, green | 4 | O, S |
| Myrcene | 1128 | Grassy, piney | 16 | O, MS, S |
| 3-Methyl-3-buten-1-ol | 1223 | Pine | 4 | O, MS, S |
| Unknown | 1241 | Garlic | 16 | O |
| p-Cymene | 1244 | Floral, grassy | 8 | O, S |
| Octanal | 1271 | Citrus-like | 2 | O, MS, S |
| 1-Octen-3-one | 1283 | Mushroom | 8 | O, S |
| Unknown | 1313 | Nutty, steamed rice | 32 | O |
| 6-Methyl-5-heptene-2-one | 1319 | Cooked rice, fruity | 2 | O, MS, S |
| (Z)-Rose oxide | 1329 | Floral | 8 | O, MS, S |
| DMTS | 1354 | Pickled vegetable, sulfury | 32 | O, MS, S |
| (E)-2-Octenal | 1408 | Peanut, green | 4 | O, S |
| Methional | 1432 | Cooked potato | 1024 | O, S |
| Linalool | 1526 | Floral | 32 | O, MS, S |
| (E,Z)-2,6-Nonadienal | 1573 | Cucumber | 32 | O, S |
| Unknown | 1603 | Paper board | 2 | O |
| Unknown | 1643 | Sulfury, tropical fruit | 4 | O |
| Isovaleric acid | 1657 | Smelly plant | 8 | O, S |
| (E,E)-2,4-Nonadienal | 1719 | Stale grain, metalic | 2 | O, S |
| Citronellol | 1742 | Floral, steamed rice | 4 | O, MS, S |
| Nerol | 1754 | Floral, sweet | 64 | O, MS, S |
| Geraniol | 1839 | Floral, sweet | 512 | O, MS, S |
| Nerolidol | 2013 | Metalic, sesame oil | 32 | O, MS, S |
| Guaiacol | 1866 | Medicine, bitter | 8 | O, S |
| Furaneol | 2044 | Sweet, caramel | 128 | O, S |
| Homofuraneol | 2095 | Floral, caramel | 2 | O, S |
| Unknown | 2145 | Sweet, caramel | 8 | O |
| Unknown | 2170 | Sweet, fruity | 8 | O |
The odorant was detected/identified by matching the standard compounds (S), mass spectrum (MS), and odor quality (O) with the reference odorant.
Methional is a sulfur-containing compound perceived with a cooked-potato note. Due to its high odor intensity and low threshold value, methional is considered a key food odorant and is detected in more than 25% of a wide range of 227 food samples [11]. As the primary flavor compound in potato [12], methional has also been identified as a key odor-active compound in tropical fruit such as mango [13]. Furthermore, it has been demonstrated to be one of the key odorants of cooked cauliflower's “sulfur” odors [14]. Typically, methional has been associated with an undesirable flavor. It has been reported as responsible for the possible off-flavor of stored orange juice [15]. Moreover, methional has been reported that to show higher odor units in the less preferred tomato cultivars when compared to five fresh tomato cultivars. Also, the biogenesis of methional has been confirmed from methionine [16]. Methional was identified in a previous lychee study using sulfur selective detector, pulsed flame photometric detector (PFPD) [3]. However, its role in the perception of lychee flavor has not been closely studied. Interestingly, in this study, methional was identified as the most important odorant of this new lychee variety. This was consistent with the sensory results showing the “tropical fruit” note received the second highest score among all the olfactory attributes. Moreover, methional has been reported to be the biosynthetic precursor of ethylene, a natural plant hormone [17].
Following methional, geraniol, furaneol, nerol, DMTS, linalool, (E,Z)-2,6-nonadienal and nerolidol also showed important influences on the aroma profile of ‘Sweetheart’ lychee. Geraniol, nerol and linalool are the most aroma-active terpenes due to their low sensory threshold. They are widespread in nature, primarily in plants as components of essential oils. Geraniol is the major constituent of rose oil and palmarosa oil, and describes as a rose-like scent. Nerol is an isomeric form for geraniol, which also gives a fresh, sweet rose scent. Linalool is produced by many flowers and spice plants with a pleasant floral scent. The strong combination of these three volatiles explained the most intense floral note of lychee fruit perceived in the sensory evaluation. Besides giving the impression of a floral note, linalool can also contribute sweet and citrus scents to the overall aroma profile of lychee. It is commonly found in fruits such as citrus fruits [18–21], peach and apricot [22,23]. (E,Z)-2,6-nonadienal is one of the key odorants in cucumbers [24], responsible for the diffuse fresh, green odor. It has also been identified as the main component in violet leaves [25]. Furaneol was first reported as a product of the Maillard reaction in 1960 and then has been intensively studied due to its key role in the flavor of many fruits and baked foods [26]. It is perceived as favorable caramel-like note at a very low odor threshold. Furaneol has been previously isolated as natural product from various fresh fruits such as strawberry [27], pineapple [28], tomato [29] and kiwi [30]. The biosynthesis of furaneol has been largely investigated in strawberry fruit [31,32]. According to the current knowledge, d-fructose-1,6-diphosphate is considered as the natural progenitor of furaneol in fruit [33]. Similar to furaneol, homofuraneol is also a well-known Maillard product and was identified in this study although with a relatively low FD factor. It has been identified as one of the key odorants in soy sauce [34]. Besides, it has been reported that homofuraneol was found in fresh fruits such as Cucumis melo L [35]. and mandarin [36]. In this study, it is the first time that homofuraneol is identified in lychee fruit as aroma-active odorant. In a recent paper, the reactivity and stability of furaneol and homofuraneol has been reviewed due to their importance in the flavor profiles of a variety of food [37]. All these previously discussed volatiles may largely contribute desirable aroma notes including floral, sweet and citrus, and when combined together, form the overall perception of lychee aroma in accordance with the attributes perceived and evaluated in sensory analysis.
Additionally, DMTS detected in the lychee samples is a sulfur-containing compound described with sulfury, pickled vegetable notes. The detection threshold of DMTS was determined to be as low as 0.0099 ppb in a water [38]. The high FD factor of DMTS could be at least partially responsible for the tropical fruit attribute of ‘Sweetheart’ lychee indicated during the sensory evaluation. Nerolidol was identified for the first time in lychee in this study. It has been found in the essential oils of various of plants [39] and is used as a flavoring agent [40]. Our data indicates that nerolidol also makes an important contribution to ‘Sweetheart’ lychee aroma.
In our study, six unknown compounds were also identified as aroma-active components in Sweetheart lychee using olfactometry. These were volatiles with low odor thresholds and in low quantities, and therefore, were not detected by GC-MS. However, they were perceived as sulfur, nutty and sweet notes which might be associated with sulfur-containing compounds, pyrazines and lactones. Among them, the sulfury note could be correlated with cabbage and garlic/onion attributes evaluated in the sensory analysis. Further identification by specific techniques such as pulsed flame photometric detector (PFPD) and/or two-dimensional gas chromatography (2D-GC) will be conducted to confirm the conjecture in our future study.
Besides the potent aroma-active compounds listed in Table 1, a variety of potential odorants were also detected from ‘Sweetheart’ lychee (Table 2). They were tentatively identified from SAFE extract but were not identified as odor-active components by olfactometry. Many of them have also been identified in previous lychee studies [3,5–7,10,41]. This may be due to the low concentrations of these compounds in ‘Sweetheart’ lychee as well as their relatively high sensory thresholds. It also implied that volatile components in lychee were not necessarily odor-active and might not contribute to the overall aroma profile of lychee fruit. The quantitation of odor-active compounds identified in this paper will be conducted in future study.
Table 2.
Additional potential odorants detected in Sweetheart lychee.
| Compound | RI (FFAP) | Odor quality | Identificationa |
|---|---|---|---|
| Ethyl acetate | 875 | Fruity | MS, RI |
| DMDS | 1049 | Onion, cabbage | MS, RI |
| Hexanal | 1059 | Grassy | MS, RI |
| Heptanal | 1163 | Fat, citrus | MS, RI |
| Limonene | 1168 | Lemon, orange | MS, RI |
| (E)-2-Hexenal | 1200 | Green | MS, RI |
| γ-Terpinene | 1217 | Turpentine | MS, RI |
| 1-Hexanol | 1334 | Toasted, nutty | MS, RI |
| (E)-Rose oxide | 1341 | Sweet, rose | MS, RI |
| (Z)-3-Hexen-1-ol | 1364 | Green, fresh | MS, RI |
| (E)-2-Hexen-1-ol | 1387 | Green, leaf | MS, RI |
| (Z)-Linalool oxide | 1418 | Floral | MS, RI |
| 1-Octen-3-ol | 1431 | Mushroom | MS, RI |
| (E)-Linalool oxide | 1447 | Floral | MS, RI |
| 2-Ethyl-1-hexanol | 1469 | Rose, green | MS, RI |
| Decanal | 1476 | Soapy, citrus-like | MS, RI |
| Benzaldehyde | 1503 | Almond | MS, RI |
| 1-Octanol | 1537 | Chemical, metal | MS, RI |
| Terpinen-4-ol | 1578 | Turpentine | MS, RI |
| Neral | 1662 | Lemon | MS, RI |
| α-Terpineol | 1674 | Oil, anise | MS, RI |
| Geranial | 1711 | Lemon | MS, RI |
| Isogeraniol | 1790 | Sweet, floral | MS, RI |
| Benzyl alcohol | 1857 | Sweet, floral | MS, RI |
| Phenylethyl alcohol | 1890 | Floral, herb | MS, RI |
Compounds were identified by matching the mass spectrum (MS) and reference retention index (RI).
4. Conclusion
This study provides the first comprehensive characterization of the key odor-active components contributing to the aroma profile of ‘Sweetheart’ lychee. Methional, geraniol, furaneol, nerol, DMTS, linalool, (E,Z)-2,6 nonadienal and nerolidol were demonstrated as the most important aroma-active compounds in ‘Sweetheart’ lychee. Moreover, for the first time, nerolidol and homofuraneol were identified as odor-active compounds in lychee fruit in this study. The sensory evaluation of fresh lychee fruit using predetermined flavor attributes was demonstrated to be in accordance with the result of the characterized aroma profile of ‘Sweetheart’ lychee. The information collected in this study can offer useful information for understanding the popularity of ‘Sweetheart’ lychee. It is possible to help with the further development of new lychee varieties by tracing back the biosynthesis of key aroma-active volatiles in lychee fruits to assist with the improvement of the sensory quality of lychee related products through investigating the possible influence of industrial processing on certain products.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest to this work.
References
- 1. Mitra S, Menzel C, Siddiqui SAB, Huang X, Singh H, Babita S, et al. 3. Overview of lychee production in the 5 Asia-Pacific region. 2002 [Google Scholar]
- 2.Evans E, Degner R, Crane J, Rafie R, Balerdi C. Is it still profitable to grow lychee in Florida Food and resource economics department document FE496 Florida cooperative extension service Institute of Food and Agricultural Sciences. University of Florida; 2008. [Google Scholar]
- 3. Mahattanatawee K, Perez-Cacho PR, Davenport T, Rouseff R. Comparison of three lychee cultivar odor profiles using gas chromatography-olfactometry and gas chromatography-sulfur detection. J Agric Food Chem. 2007;55:1939–44. doi: 10.1021/jf062925p. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann K. Exotische Lebensmittel: inhaltsstoffe und Verwendung; für Biologen, Chemiker und Ernährungswissenschaftler. Springer-Verlag; 2013. [Google Scholar]
- 5. Johnston JC, Welch RC, Hunter G. Volatile constituents of litchi (Litchi chinesis Sonn.) J Agric Food Chem. 1980;28:859–61. [Google Scholar]
- 6. Chyau C-C, Ko P-T, Chang C-H, Mau J-L. Free and glycosidically bound aroma compounds in lychee (Litchi chinensis Sonn.) Food Chem. 2003;80:387–92. [Google Scholar]
- 7. Wu Y, Pan Q, Qu W, Duan C. Comparison of volatile profiles of nine litchi (Litchi chinensis Sonn.) cultivars from Southern China. J Agric Food Chem. 2009;57:9676–81. doi: 10.1021/jf902144c. [DOI] [PubMed] [Google Scholar]
- 8. Engel W, Bahr W, Schieberle P. Solvent assisted flavour evaporation – a new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur Food Res Technol. 1999;209:237–41. [In English] [Google Scholar]
- 9. Grosch W. Detection of potent odorants in foods by aroma extract dilution analysis. Trends Food Sci Tech. 1993;4:68–73. [Google Scholar]
- 10. Ong PK, Acree TE. Gas chromatography/olfactory analysis of lychee (Litchi chinesis Sonn.) J Agric Food Chem. 1998;46:2282–6. [Google Scholar]
- 11. Dunkel A, Steinhaus M, Kotthoff M, Nowak B, Krautwurst D, Schieberle P, et al. Nature's chemical signatures in human olfaction: a foodborne perspective for future biotechnology. Angew Chem Int Ed. 2014;53:7124–43. doi: 10.1002/anie.201309508. [DOI] [PubMed] [Google Scholar]
- 12. Di R, Kim J, Martin MN, Leustek T, Jhoo J, Ho C-T, et al. Enhancement of the primary flavor compound methional in potato by increasing the level of soluble methionine. J Agric Food Chem. 2003;51:5695–702. doi: 10.1021/jf030148c. [DOI] [PubMed] [Google Scholar]
- 13. Munafo JP, Jr, Didzbalis J, Schnell RJ, Schieberle P, Steinhaus M. Characterization of the major aroma-active compounds in mango (Mangifera indica L.) cultivars Haden, White Alfonso, Praya Sowoy, Royal Special, and Malindi by application of a comparative aroma extract dilution analysis. J Agric Food Chem. 2014;62:4544–51. doi: 10.1021/jf5008743. [DOI] [PubMed] [Google Scholar]
- 14. Engel E, Baty C, le Corre D, Souchon I, Martin N. Flavor-active compounds potentially implicated in cooked cauliflower acceptance. J Agric Food Chem. 2002;50:6459–67. doi: 10.1021/jf025579u. [DOI] [PubMed] [Google Scholar]
- 15. Bezman Y, Rouseff RL, Naim M. 2-Methyl-3-furanthiol and methional are possible off-flavors in stored orange juice: aroma-similarity, NIF/SNIF GC-O, and GC analyses. J Agric Food Chem. 2001;49:5425–32. doi: 10.1021/jf010724+. [DOI] [PubMed] [Google Scholar]
- 16. Mayer F, Takeoka GR, Buttery RG, Whitehand LC, Naim M, Rabinowitch HD. Studies on the aroma of five fresh tomato cultivars and the precursors of cis-and trans-4, 5-epoxy-(E)-2-decenals and methional. J Agric Food Chem. 2008;56:3749–57. doi: 10.1021/jf0732915. [DOI] [PubMed] [Google Scholar]
- 17. Pryor WA, Tang RH. Ethylene formation from methional. Biochem Biophys Res Commun. 1978;81:498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- 18. Elmaci Y, Altug T. Flavor characterization of three mandarin cultivars (Satsuma, Bodrum, Clemantine) by using GC/MS and flavor profile analysis techniques. J Food Qual. 2005;28:163–70. [Google Scholar]
- 19. Buettner A, Schieberle P. Evaluation of aroma differences between hand-squeezed juices from Valencia late and Navel oranges by quantitation of key odorants and flavor reconstitution experiments. J Agric Food Chem. 2001;49:2387–94. doi: 10.1021/jf001363l. [DOI] [PubMed] [Google Scholar]
- 20. Buettner A, Schieberle P. Evaluation of key aroma compounds in hand-squeezed grapefruit juice (Citrus paradisi Macfayden) by quantitation and flavor reconstitution experiments. J Agric Food Chem. 2001;49:1358–63. doi: 10.1021/jf001235x. [DOI] [PubMed] [Google Scholar]
- 21. Qiao Y, Xie BJ, Zhang Y, Zhang Y, Fan G, Yao XL, et al. Characterization of aroma active compounds in fruit juice and peel oil of Jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules. 2008;13:1333–44. doi: 10.3390/molecules13061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horvat R, Chapman G, Jr, Robertson J, Meredith F, Scorza R, Callahan A, et al. Comparison of the volatile compounds from several commercial peach cultivars. J Agric Food Chem. 1990;38:234–7. [Google Scholar]
- 23. Guillot S, Peytavi L, Bureau S, Boulanger R, Lepoutre J-P, Crouzet J, et al. Aroma characterization of various apricot varieties using headspace–solid phase microextraction combined with gas chromatography–mass spectrometry and gas chromatography–olfactometry. Food Chem. 2006;96:147–55. [Google Scholar]
- 24. Schieberle P, Ofner S, Grosch W. Evaluation of potent odorants in cucumbers (Cucumis sativus) and muskmelons (Cucumis melo) by aroma extract dilution analysis. J Food Sci. 1990;55:193–5. [Google Scholar]
- 25.Bauer K, Garbe D, Surburg H. Common fragrance and flavor materials: preparation, properties and uses. John Wiley & Sons; 2008. [Google Scholar]
- 26. Schwab W. Natural 4-hydroxy-2,5-dimethyl-3 (2H)-furanone (Furaneol®) Molecules. 2013;18:6936–51. doi: 10.3390/molecules18066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ulrich D, Rapp A, Hoberg E. Analysis of strawberry flavour-quantification of the volatile components of varieties of cultivated and wild strawberries. Z fur Lebensm Unters Forsch. 1995(200):217–217. [Google Scholar]
- 28. Tokitomo Y, Steinhaus M, Büttner A, Schieberle P. Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. Biosci Biotechnol Biochem. 2005;69:1323–30. doi: 10.1271/bbb.69.1323. [DOI] [PubMed] [Google Scholar]
- 29. Buttery RG, Takeoka GR, Naim M, Rabinowitch H, Nam Y. Analysis of furaneol in tomato using dynamic headspace sampling with sodium sulfate. J Agric Food Chem. 2001;49:4349–51. doi: 10.1021/jf0105236. [DOI] [PubMed] [Google Scholar]
- 30. Garcia CV, Quek S-Y, Stevenson RJ, Winz RA. Characterization of the bound volatile extract from baby kiwi (Actinidia arguta) J Agric Food Chem. 2011;59:8358–65. doi: 10.1021/jf201469c. [DOI] [PubMed] [Google Scholar]
- 31. Lavid N, Schwab W, Kafkas E, Koch-Dean M, Bar E, Larkov O, et al. Aroma biosynthesis in strawberry: S-adenosylmethionine: furaneol O-methyltransferase activity in ripening fruits. J Agric Food Chem. 2002;50:4025–30. doi: 10.1021/jf011409q. [DOI] [PubMed] [Google Scholar]
- 32. Pérez AG, Olías R, Olías JM, Sanz C. Biosynthesis of 4-hydroxy-2, 5-dimethyl-3 (2H)-furanone and derivatives in in vitro grown strawberries. J Agric Food Chem. 1999;47:655–8. doi: 10.1021/jf980404z. [DOI] [PubMed] [Google Scholar]
- 33. Roscher R, Bringmann G, Schreier P, Schwab W. Radiotracer studies on the formation of 2, 5-dimethyl-4-hydroxy-3 (2H)-furanone in detached ripening strawberry fruits. J Agric Food Chem. 1998;46:1488–93. [Google Scholar]
- 34. Kaneko S, Kumazawa K, Nishimura O. Comparison of key aroma compounds in five different types of Japanese soy sauces by aroma extract dilution analysis (AEDA) J Agric Food Chem. 2012;60:3831–6. doi: 10.1021/jf300150d. [DOI] [PubMed] [Google Scholar]
- 35.Nussbaumer C, Hostettler B. New flavour compounds of Cucumis melo L, 197 Special Publication- Royal Society of Chemistry; 1996. pp. 70–3. [Google Scholar]
- 36. Evans KC. Characterization of aroma active compounds in mandarin (Citrus reticulata blanco) juice using gas chromatography-olfactometry. 2002 [Google Scholar]
- 37. Weerawatanakorn M, Wu J-C, Pan M-H, Ho C-T. Reactivity and stability of selected flavor compounds. J Food Drug Anal. 2015;23:176–90. doi: 10.1016/j.jfda.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Czerny M, Christlbauer M, Christlbauer M, Fischer A, Granvogl M, Hammer M, et al. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur Food Res Technol. 2008;228:265–73. [Google Scholar]
- 39.O'Neil MJ. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. RSC Publishing; 2013. [Google Scholar]
- 40. Lapczynski A, Bhatia S, Letizia C, Api A. Fragrance material review on nerolidol (isomer unspecified) Food Chem Toxicol. 2008;46:S247–50. doi: 10.1016/j.fct.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 41. Lasekan O, Abbas KA. Distinctive exotic flavor and aroma compounds of some exotic tropical fruits and berries: a review. Crit Rev Food Sci Nutr. 2012;52:726–35. doi: 10.1080/10408398.2010.507910. [DOI] [PubMed] [Google Scholar]