Abstract
Antibiotics have been widely used in the treatment of livestock diseases. However, the emergence of issues related to drug resistance prompted governments to enact a series of laws regulating the use of antibiotics in livestock. Following control of the problem of drug resistant bacteria, public attention has shifted to the recurring incidence of human health and safety issues caused by residual veterinary drugs in livestock products. To guarantee the safety and hygiene of meat, milk, and eggs from food-producing animals, governments and relevant agencies established laws and regulations for the use of veterinary drugs. It is, therefore, necessary to monitor the content of residual drugs in livestock products at regular intervals to assess whether the regulations have resulted in the effective management of food product safety, and to prevent and manage sudden problems related to this issue. A 2011–2015 livestock product post-marketing monitoring program launched by the Taiwan Food and Drug Administration (TFDA) inspected 1487 livestock products. Over the past 5 years, there were 34 samples identified that did not conform to the regulations; these samples included residue drugs such as β-agonists, chloramphenicols, β-lactam antibiotics, sulfa drugs, enrofloxacin, and lincomycin. Inspections of commercial livestock products with the consistent cooperation of agricultural authorities did not detect the drugs that were banned by the government, whereas the detection of other drugs decreased annually with an increase in the post-market monitoring sample size. In the future, the TFDA will continue to monitor the status of residual veterinary drugs in commercial livestock products, adjust the sampling of food products annually according to monitoring results, and closely cooperate with agricultural authorities on source management.
Keywords: Agricultural authorities, Livestock products, Post-market monitoring, Veterinary drug residues
1. Introduction
With a gradual increase in the global demand for proteins, which has necessitated vigorous development of the livestock industry, antibiotics are being increasingly used for the treatment of livestock diseases. Furthermore, because animal feed containing antibiotics results in improved growth and feed conversion rates in food-producing animals, feeding model wherein small amounts of antibiotics are added to animal feed was instituted in the livestock industry. Unfortunately, the presence of drug-resistant bacteria in food-producing animals has emerged [1]. Evidences had shown that there was a survey to estimate the prevalence and antimicrobial resistance of Salmonella from pigs at slaughter in Taiwan. The rates of resistance to the following drugs were observed: tetracycline (88.2%), gentamycin (82.7%), chloramphenicol (54.3%), amoxicillin (34.6%), nalidixic acid (30.7%), ampicillin (26.8%), kanamycin (18.1%), cephalothin (7.1%), nitrofurantoin (6.3%), ciprofloxacin (0.8%). Among 127 Salmonella strains, 119 strains (93.7%) were resistant to 2 or more antibiotics [2]. Some studies have reported that animal feed containing antibiotics results in the emergence of antibiotic resistance, which severely affects medical treatment methods in human and veterinary medicine, and can lead to situations in which drugs are completely ineffective [3]. This problem has encouraged national governments to increase their focus on the use of antibiotics in animal feed within the livestock industry and to implement regulations restricting these types of animal feed [4].
Successful outcomes of these regulatory actions have been reported [5,6]. For example, in Taiwan, the Veterinary Drugs Control Act was enacted to improve the quality of veterinary drugs, enhance animal health, and foster a robust livestock industry. To prevent or reduce the development of drug-resistant bacteria in animals, the Bureau of Animal and Plant Health Inspection and Quarantine (BAPHIQ), Council of Agriculture, Executive Yuan, has monitored domestic pig and chicken farms since 1990, testing for drug resistance in pathogens that affect humans, such as Salmonella, Campylobacter, Escherichia coli, and Enterococcus, within livestock manure. The results of this monitoring have been used as a basis for halting the use of certain antibiotics. For example, after discontinuing the use of avoparcin in chicken farms, the resistance of enterococci in chickens towards vancomycin significantly decreased to the point of no resistance [7]. The competent authority BAPHIQ, the Council of Agriculture continuously announces relevant information on its website [8].
With the gradual increase in control over the problem of drug resistance, other situations in which residual antibiotics in products from food-producing animals may affect human health have emerged. For example, in Spain, in 1990, more than 100 people who consumed the liver that was contaminated with clenbuterol, experienced increased heart rates, muscular tremors, headaches, nausea, fever, chills, and other symptoms, and required emergency medical treatment [9]. Furthermore, there have been reports of rash outbreaks in individuals who drank milk containing residual penicillin [10].
To ensure the safety and hygiene of meat, milk, and egg products, regulatory laws have been established for veterinary drugs used in food-producing animals, which allow regular monitoring for residual veterinary drugs in livestock products. This can help assess the efficacy of government policies in managing food safety, prevention, and control of sudden food safety incidents [11,12]. Taiwan considers the safety and hygiene of commercial agricultural, livestock, and aquatic products as a serious public health management responsibility [13–21], and uses these monitoring results as a reference to assess risks to food product safety and as a primary source for information on risk management policies and risk communication strategies [22]. Therefore, the Taiwan Food and Drug Administration (TFDA) together with regional health bureaus implemented a post-market monitoring program for livestock products between 2011 and 2015. This program was used to monitor and develop an understanding of whether levels of residual veterinary drugs in commercial livestock products complied with the veterinary drug residue standards in Taiwan.
2. Methods
2.1. Sample sources
Samples were taken by the TFDA and regional health bureaus that assessed the seasonality and regional specialty of products, and then acquired samples including those of pork, pig organs, mutton, beef, processed meats, cow milk, and sheep milk, from retail markets, traditional markets, supermarkets, restaurants, and wholesale markets. The strategy is considered according to the intensive feeding status in Taiwan food producing animal farm where are mostly located in the mid-southern Taiwan. And the seasonal changes might be a factor for leading animal sickness, what follows the medication. The quantity of such samples has increased annually.
2.2. Testing methods
Tests were conducted according to the directions of the TFDA. The analyzed items and the analytical instruments used are described in Table 1. All antibiotics listed in Table 1 are the commonly used in Taiwan.
Table 1.
Analysis types, instruments and items of veterinary drug residues in foods in this study.
| Analysis types and instrumentsa | Analysis items | |
|---|---|---|
| β-Agonists | LC/MS/MS | brombuterol, cimaterol, cimbuterol, clenbuterol, clencyclohexerol, clenisopenterol, clenpenterol, clenproperol, fenoterol, formoterol, isoxsuprine, mabuterol, mapenterol, 3-o-methyl-colterol, ractopamine, salbutamol, salmeterol, terbutaline, tulobuterol, zilpaterol |
| Chloramphenicols | LC/MS/MS | chloramphenicol, florfenicol, florfenicol amine, thiamphenicol |
| β-Lactam antibiotics | LC/MS/MS | ampicillin, amoxicillin, benzylpenicillin, cephalexin, cefapirin, cefuroxime, cloxacillin, oxacillin |
| Nitrofuran metabolites | LC/MS/MS | 1-aminohydantoin, 3-amino-2-oxazolidinone, 5-methylmorpholino-3-amino-2-oxazolidinone, semicarbazide hydrochloride |
| Carbadox and its metabolites | LC/MS/MS | carbadox, desoxycarbadox, quinoxaline-2-carboxylic acid |
| Aminoglycosides | LC/MS/MS | apramycin, dihydrostreptomycin, gentamicin, kanamycin, neomycin, spectinomycin, streptomycin |
| Tetracyclines | LC/MS/MS | 4-epimer-chlortetracycline, 4-epimer-oxytetracycline, 4-epimer-tetracycline, chlortetracycline, oxytetracycline, tetracycline, doxycycline |
| Antiprotozoal drugs | LC/MS/MS | diclazuril, dimetridazole, halofuginone, metronidazole, narasin, nicarbazin, robenidine hydrochloride |
| Multi-residue analysis | LC/MS/MS |
Sulphonamides: sulfabenzamide, sulfacetamide, sulfachlorpyridazine, sulfadiazine, sulfadimethoxine, sulfadoxine, sulfaethoxypyridazine, sulfaguanidine, sulfamerazine, sulfameter, sulfamethazine, sulfamethizole, sulfamethoxazole, sulfamethoxypyridazine, sulfamonomethoxine, sulfapyridine, sulfaquinoxaline, sulfathiazole, sulfatroxazole, succinylsulfathiazole, trimethoprim. Fluoroquinolone: ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, lomefloxacin, marbofloxacin, sarafloxacin, flumequine Quinolone: fleroxacin, norfloxacin, pefloxacin, nalidixic acid, oxolinic acid, piromidic acid Tranquilizer: azaperol, azaperone Antiparasitics: dicyclanil, eprinomectin, fluazuron, morantel, tetramisole, trichlorfon Others: carazolol, clopidol, ethopabate, ormetoprim, pipemidic acid |
| Antibiotics and their metabolites | LC/MS/MS |
Macrolide antibiotic: clarithromycin, erythromycin, josamycin, kitasamycin, oleandomycin, tilmicosin, tylosin, virginiamycin M1 Lincosamide antibiotic: clindamycin, lincomycin β-Lactam antibiotic: mecillinam Cephalosporin antibiotic: cefoperazone Others: natamycin, neospiramycin I, orbifloxacin, spiramycin I |
| Diethylstilbestrol and hexestrol | HPLC/PDA | diethylstilbestrol, hexestrol |
| 17α-estradiol, 17β-estradiol, zeranol | HPLC/PDA | 17α-estradiol, 17β-estradiol, zeranol |
| 4-Androstene-3,17-dione, 17α-hydroxyprogesterone, progesterone, testosterone | HPLC/PDA | 4-androstene-3,17-dione, 17α-hydroxyprogesterone, progesterone, testosterone |
The methods of analysis are announced by the TFDA.
3. Results and discussion
3.1. Commercial livestock product veterinary drug residue monitoring results
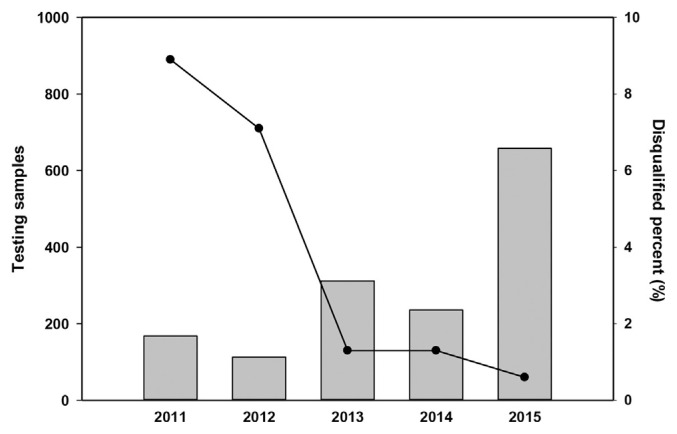
The sampling of commercial livestock products between 2011 and 2015 accumulated 1487 samples; monitoring requirements were more comprehensive in 2015. In addition to the routine sampling of commercial livestock products, we sampled high-risk items including those with high failure rates between 2011 and 2014, and items that the public commonly consumed or consumed in large volumes (such as pork, chicken, and milk). The analyses showed that noncompliance rates exhibited a gradually decreasing annual trend (Fig. 1). Only a small portion of samples contained veterinary drug residue levels not conforming to regulations (Table 2). Those samples were primarily comprised a variety of antibiotic residues including residues of chloramphenicol, nitrofuran metabolites, β-agonists, tetracycline, sulfas and quinolone drugs, carbadox and its metabolites, beta-lactams, aminoglycosides, various antibiotic metabolites, insecticides, diethylstilbestrol and hexestrol, zeranol, 17α-estradiol and 17β-estradiol, luteal hormone progesterone, and 17α-hydroxyprogestin.
Fig. 1.
Testing samples and disqualified percent of veterinary drug residues in foods between 2011 and 2015. Bars represent the numbers of testing samples. The line graph represents the disqualified percentages.
Table 2.
Summary of veterinary drug residues in foods from 2011 to 2015.
| Year | Testing samples | Sample list | No. of disqualified/No. of total samples (Disqualified percentage) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Beef | Pork | Lamb | Pork visceral | Dairy | Goat milk | Meat ball | Sausage | Burger meat | |||
| 2011 | 168 | 59 | 55 | 10 | 27 | 10 | 7 | – | – | – | 15/168 (8.9%) |
| 2012 | 113 | 20 | 21 | 10 | 10 | 10 | 12 | 30 | – | – | 8/113 (7.1%) |
| 2013 | 312 | 30 | 88 | 10 | 10 | 22 | 11 | 121 | 10 | 10 | 4/312 (1.3%) |
| 2014 | 236 | 40 | 81 | 10 | 10 | 20 | 10 | 44 | 10 | 11 | 3/236 (1.3%) |
| 2015 | 658 | 123 | 335 | 23 | 13 | 46 | 8 | 90 | – | – | 4/658 (0.6%) |
| Sum | 1487 | 272 | 580 | 63 | 70 | 108 | 47 | 285 | 20 | 21 | 34/1487 (2.3%) |
3.2. Disqualified samples and possible effects of veterinary drug residue on human health
We further analyzed drugs that were disqualified during testing. Residues of β-agonists were found in 10 samples of beef and 2 samples of pork; residue of chloramphenicols were found in 12 samples of pork and processed pork products; residues of β-lactams were found in 5 samples of processed pork products; residue of azaperone was found in 4 samples of pork and processed pork products; residues of sulfa drugs were found in 1 sample of processed pork products, the residue of enrofloxacin was found in 1 sample of processed pork products. Sheep milk tested positive for the residue of lincomycin one time. The numbers of noncompliant samples and drug residues found in these samples are presented in Table 3.
Table 3.
Summary of veterinary drug residue items in foods from 2011 to 2015.
| Year | Disqualified | Detection residues | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Product | No. | β-Agonist | Chloramphenicols | β-Lactams | Azaperone | Enrofloxacin | Lincomycin Sulphonamides | |
| 2011 | Beef | 10 | ● | |||||
| Pork | 5 | ● | ● | |||||
| 2012 | Meat ball | 8 | ● | ● | ||||
| 2013 | Goat milk | 1 | ● | |||||
| Meat ball | 3 | ● | ● | ● | ||||
| 2014 | Meat ball | 3 | ● | ● | ● | |||
| 2015 | Meat ball | 2 | ● | ● | ||||
| Pork | 2 | ● | ||||||
The meat ball products are made from pork.
3.3. Management and prevention of livestock products failing to comply with veterinary medicine residue standards
For products that failed to comply with the regulatory standards, the TFDA encouraged local health bureaus to collaborate with agricultural authorities to trace the source of these products. Among the 34 disqualified samples, only 10 of imported beef were found in 2011. The other 24 disqualified samples were domestic commercial livestock products. To address non-compliance in imported livestock products, the government will strengthen border inspections. Taking the above sample of beef as an example, the government responded by implementing the “Three Controls and Five Verifications” measure. For domestically produced noncompliant livestock products, relevant authorities will act in accordance with the law and request that agricultural authorities strengthen the management of livestock production sources and guide farmers in the proper use of veterinary medicines. In the following paragraphs, we discuss those drug residues found in disqualified products, and how the government will manage this non-compliance.
3.3.1. β-Agonists
Ractopamine and salbutamol are β-agonists. Since 2011, ractopamine had not been used in human medical treatments, either in Taiwan or internationally, although it is still used as an additive in animal feed in the United States, Canada, and Japan. Ractopamine promotes the transfer of nutrients in animals from adipose to muscle tissue, causing an increase in the metabolism of fat, increasing the formation of proteins, and significantly increasing lean meat ratios and feed conversion ratios [23]. Salbutamol is primarily used as a clinical treatment for asthma in humans; it is approved for human use in Taiwan. However, the use of salbutamol can cause side effects, such as slight skeletal muscle tremors in the hands, an increase in heart rate because of peripheral blood vessel dilation, high blood sugar, headaches, and stress.
Council of Agriculture, Executive Yuan, announced on October 11, 2006 that “The manufacture, prescription, import, export, sale, display of, or administration to food producing animals of β-agonists including salbutamol, terbutaline, clenbuterol, and ractopamine is prohibited.” To prevent the use of forbidden β-agonists by pig producers, the agricultural authorities established annual plans for the implementation of monitoring for the illegal use of β-agonists and checks for the illegal sale of such drugs. Furthermore, from March 2012, pig producers were required to provide affidavits stating, “No β-Agonists were administered to marketed pigs.” Since 2013, of 47,038 hair samples collected from pigs at the time of production, only four did not conform to regulations; additionally, test results of post-market samples of livestock products have not revealed any β-Agonist residue. These finding confirmed that the government’s method of managing this class of drugs was sufficiently effective (Tables 2 and 3).
3.3.2. Chloramphenicols
Chloramphenicols, including chloramphenicol, thiamphenicol, and florfenicol, are broad-spectrum antibiotics. Because of reports indicating that the use of chloramphenicol by humans increases the incidence of aplastic anemia, the use of this drug in food-producing animals was banned in 1994 in the United States and Australia [24]. Both florfenicol and thiamphenicol are artificially synthesized antibiotics; their composition and reactivity are similar to those of chloramphenicol. Furthermore, as they do not cause a risk for aplastic anemia, they have replaced chloramphenicol in the treatment of animals. They are commonly used to treat and prevent Actinobacillus pleuropneumonia, E. coli, Pasteurella multocida, and Salmonella infections in pigs [25]. In cows and sheep, these drugs are used to treat bacterial respiratory diseases, mastitis, and corneal conjunctivitis [26].
The Council of Agriculture, Executive Yuan, announced on December 26, 2002 a ban on the use of chloramphenicol in food-producing animals. However, our test results of post-market livestock product inspections revealed that in 2012, 2013, and 2014, some processed pork products tested positive for chloramphenicol (Table 3). To combat this issue, agricultural authorities have invited county and municipal governments, pig-raising organizations, and pig slaughterhouses to cooperatively conduct research into response measures. An important consensus was reached that slaughtered pigs should be accompanied by a mark indicating their source. Thus, it was decided that the legislation should include the following statement: “From February 10, 2013, reared pigs should be accompanied by proof of origin when entering slaughterhouses.” In addition, on August 9, 2013, the Council of Agriculture, Executive Yuan, released a revised notice stating, “Chloramphenicol may not be manufactured, prescribed, imported, exported, sold, or displayed.” Nevertheless, in September 2015, agricultural authorities detected residual chloramphenicol in a sample of pig blood collected from a pig farm. This result indicated that during production there remain instances in which veterinary drugs are used in violation of government regulations; hence, we have suggested that the government should continue to strengthen its “Drug Quality Inspection of Livestock and Poultry in Livestock Farms,” to guide livestock farms and farmers in the correct concepts of veterinary drug administration.
3.3.3. Other veterinary drugs
Ten other livestock products were found that contained antibiotic and sedative drugs with residual values that exceeded established standards (Table 3). Benzylpenicillin and procaine benzylpenicillin, members of the β-lactam class of antibiotics, are widely used for the treatment of bacterial infections [27]. They are used for the treatment of Haemophilus influenza infections in food-producing animals, erysipelas in pigs, bovine bacterial pneumonia, mastitis, arthritis, and other diseases [28]. Enrofloxacin is a veterinary medicine, that is widely used in the treatment of both gram-negative and gram-positive bacterial infections [29]. It is used primarily to prevent bacterial infections in pigs, such as E. coli A. pleuropneumonia, porcine mycorrhizal pneumonia, P. multocida, and Salmonella infections. In addition, it is used as a treatment of bovine respiratory tract infections. Lincomycin is a lincosamide class antibiotic used for the treatment of bacterial pneumonia, bronchitis, bacterial meningitis, bacterial sepsis, and actinomycetes. In pigs, it is used primarily to prevent and treat Mycoplasma pneumonia and swine dysentery [25]. Sulfa drugs are commonly used to prevent and treat atrophic rhinitis, E. coli infections, and pasteurellosis in pigs [25]. In cows, they are used to treat neosporosis and toxoplasmosis (protozoan diseases). Azaperone is a butyrophenone class drug and used as a tranquilizer, primarily in pigs. It acts as a norepinephrine antagonist, inhibiting the brainstem and cerebral cortex, including calm and reducing activity [30]. Clinically, it can be used in pig transportation, shipping, or during penning to prevent fighting, induce calmness, and reduce body temperature.
The issues related to veterinary drug residues described above originate from food-producing animals that received drug treatment during the feeding process or as a result of illness. After slaughtering, the detection of residual amounts of these drugs or toxicologically active metabolites within the edible tissues (meat, organs) or other products (milk, eggs) of these food-producing animals is called veterinary drug residue [31]. The causes of infectious diseases in food-producing animals are comparatively complex, and improper feed management methods can lead directly to animal death or reduced immunity, which increases the susceptibility of animals to pathogens in infectious environments. Thus, optimal prevention and treatment strategies for reducing the use of veterinary medicines involves making improvements in raising management and environmental hygiene, using vaccines for disease prevention, and avoiding intensive feeding and animal distress. Furthermore, when farmers use veterinary drugs, they should administer and halt administration in accordance with usage guidelines, and be aware of any notices related to their use. Regulations for residues of relevant veterinary drugs are shown in Table 4.
Table 4.
Summary of the regulation of other veterinary drug residues in Taiwan.
| Name | Animals | Residues parts | Regulation (ppm) |
|---|---|---|---|
| Thiamphenicol | Cattle, Pig, Goat, Sheep | Muscle, Liver, Kidney, Fat | 0.05 |
| Cattle, Goat, Sheep | Milk | ||
| Florfenicol | Cattle | Muscle | 0.2 |
| Pig | 0.3 | ||
| Cattle | Liver | 3 | |
| Pig | 2 | ||
| Cattle | Kidney | 0.3 | |
| Pig | 0.5 | ||
| Cattle | Fat (with skin tissue) | 0.3 | |
| Pig | 0.5 | ||
| Benzylpenicillin | Livestock | Muscle, Liver, Kidney, Fat | 0.05 |
| Procaine benzylpenicillin | Milk | 0.004 | |
| Azaperone | Pig | Muscle, Fat | 0.06 |
| Liver, Kidney | 0.1 | ||
| Enrofloxacin | Cattle, Pig | Muscle, Fat | 0.1 |
| Cattle | Liver | 0.3 | |
| Pig | 0.2 | ||
| Cattle | Kidney | 0.2 | |
| Pig | 0.3 | ||
| Cattle | Milk | 0.1 | |
| Lincomycin | Livestock | Muscle, Fat | 0.1 |
| Liver | 0.5 | ||
| Kidney | 1.5 | ||
| Milk | 0.15 | ||
| Sulfa drugs | Livestock | Muscle, Liver, Kidney, Fat, Milk | 0.1 |
The proper and safe use of veterinary drugs and medicated animal feed can promote the growth of food-producing animals and treat diseases in these animals. It is also helpful in promoting the development of the animal husbandry industry, although improper use can result in food safety concerns. To guarantee the safety of livestock products consumed by consumers, the FDA conducts rolling adjustments of product sampling according to monitoring results, and supports its testing of commercial food products by investigating livestock products not conforming to national regulations. In addition, such products are referred to agricultural authorities for management in accordance with the law. We further suggest that “Drug Quality Inspection of Livestock and Poultry in Livestock Farms” be strengthened, and that continuous counseling be provided to livestock producers or farmers on the proper concepts of drug administration. For livestock product importers, customs and border inspections should be strengthened in order to safeguard food safety and maintain the interests of consumers.
Acknowledgments
This study was funded by the Ministry of Health and Welfare, Taiwan, under the following grants: DOH103-FDA-82001 and MOHW104-FDA-B-113-104801. We wish to acknowledge Ms. Lin YY and Ms. TL Chen for their help in enhancing the quality of the work.
Funding Statement
This study was funded by the Ministry of Health and Welfare, Taiwan, under the following grants: DOH103-FDA-82001 and MOHW104-FDA-B-113-104801.
Footnotes
Conflicts of interest statement
Authors declare no competing interests.
REFERENCES
- 1. Aarestrup FM, Seyfarth AM, Emborg HD, Pedersen K, Hendriksen RS, Bager F. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob Agents Chemother. 2001;45:2054–9. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin CH. Unpublished master’s theses. Institute of Veterinary Medicine, National Taiwan University; Prevalence and antimicrobial resistance of Salmonella from pigs at slaughter in Taiwan. [Google Scholar]
- 3. Wegener HC, Aarestrup FM, Jensen LB, Hammerum AM, Bager F. Use of antimicrobial growth promoters in food animals and Enterococcus faecium resistance to therapeutic antimicrobial drugs in Europe. Emerg Infect Dis. 1999;5:329–35. doi: 10.3201/eid0503.990303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castanon JI. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. 2007;86:2466–71. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- 5. Boerlin P, Wissing A, Aarestrup FM, Frey J, Nicolet J. Antimicrobial growth promoter ban and resistance to macrolides and vancomycin in Enterococci from pigs. J Clin Microbiol. 2001;39:4193–5. doi: 10.1128/JCM.39.11.4193-4195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lauderdale TL, Shiau YR, Wang HY, Lai JF, Huang IW, Chen PC, et al. Effect of banning vancomycin analogue avoparcin on vancomycin-resistant Enterococci in chicken farms in Taiwan. Environ Microbiol. 2007;9:819–23. doi: 10.1111/j.1462-2920.2006.01189.x. [DOI] [PubMed] [Google Scholar]
- 7. Sung HT. The occurrence of antimicrobial resistance and how to prevent. Agric Poli Rev. 2004;144:49–52. [Google Scholar]
- 8.Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture. Executive Yuan. Available at: External link https://amdrug.baphiq.gov.tw/Animal/AMItem1.aspx#.
- 9. Mitchell GA, Dunnavan G. Illegal use of beta-adrenergic agonists in the United States. J Anim Sci. 1998;76:208–11. doi: 10.2527/1998.761208x. [DOI] [PubMed] [Google Scholar]
- 10. Ormerod AD, Reid TM, Main RA. Penicillin in milk – its importance in urticaria. Clin Allergy. 1987;17:229–34. doi: 10.1111/j.1365-2222.1987.tb02007.x. [DOI] [PubMed] [Google Scholar]
- 11. Canadian Food Inspection Agency. National chemical residue monitoring program 2013–2014 Report. 2015 [Google Scholar]
- 12. United States Department of Agriculture Food Safety and Inspection Service Office of Public Health Science. United States national residue program for meat, poultry, and egg products FY 2014 residue sample results. 2015 [Google Scholar]
- 13. Lee KS, Lin AY. Survey of sulfa drug residue in meat products. Ann Rept NLFD Taiwan ROC. 1992;10:133–5. [Google Scholar]
- 14. Kao YM, Cheng CL, Chang PC, Liu CH. Investigation of sulfa drugs residues in porcine and chicken livers. Ann Rept NLFD Taiwan ROC. 1996;14:325–8. [Google Scholar]
- 15. Chou SG, Tsai YD, Tsai YY. Survey of nine anabolic hormone residues in fresh meat. Ann Rept NLFD Taiwan ROC. 1997;15:184–9. [Google Scholar]
- 16. Chen SS, Tsai Whu MC, Yang SS, Chou SS. Method for the analysis of residual thiamphenicol in beef, pork and chicken. Ann Rept NLFD Taiwan ROC. 1998;16:228–37. [Google Scholar]
- 17. Jea DM, Tsai YD, Chen TH, Lee WC, Lee HF, Lee KS, et al. Investigation on sulfa drug residue in marketed poultry and meat. Ann Rept NLFD Taiwan ROC. 2002;20:272–8. [Google Scholar]
- 18. Tseng SH, Chou PJ, Chen SY, Su SC, Chou SS. Survey on β-agonists residues in marketed meat and poultry. Ann Rept NLFD Taiwan ROC. 2007;25:255–60. [Google Scholar]
- 19. Jea DM, Wang HP, Chen HC, Chou HK. Residues of sulfa drugs and clopidol in marketed pork poultry and aquatic products. Ann Rept NLFD Taiwan ROC. 2007;25:261–6. [Google Scholar]
- 20. Wang HP, Lai CY, Chen HC, Chou HK. A survey of quinolone residues in livestock and marine products. Ann Rept NLFD Taiwan ROC. 2008;26:295–305. [Google Scholar]
- 21. Li WC, Hsieh CW, Chou PJ, Ku YF, Su SC, Shih YCD. Survey on β-agonists residues in marketed meat and poultry. Ann Rept NLFD Taiwan ROC. 2009;27:188–96. [Google Scholar]
- 22. Food and Drug Administration. White paper on food safety and nutrition 2008–2012. 2008:31. [Google Scholar]
- 23.Hanrahan JP. β-Agonists and their effects on animal growth and carcass quality. Barking, Amsterdam: Elsevier Applied Science; 1987. [Google Scholar]
- 24. Stolker AA, Brinkman UA. Analytical strategies for residue analysis of veterinary drugs and growth-promoting agents in food-producing animals-a review. J Chromatogr A. 2005;1067:15–53. doi: 10.1016/j.chroma.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 25.Liu CK, Hsieh WY, Hsu CB, Lin CH, Yu P. Raising management and safety medication handbook of pig. Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Executive Yuan; 2015. [Google Scholar]
- 26.Liu CK, Chen CM, Chang TC, Zhang WF, Chang SK, Chou CC, et al. Raising management and safety medication handbook of ruminants. Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Executive Yuan; 2010. [Google Scholar]
- 27. Goto T, Ito Y, Yamada S, Matsumoto H, Oka H. High-throughput analysis of tetracycline and penicillin antibiotics in animal tissues using electrospray tandem mass spectrometry with selected reaction monitoring transition. J Chromatogr A. 2005;1100:193–9. doi: 10.1016/j.chroma.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 28.Rossi S. Australian medicines handbook. Adelaide: Australian Medicines Handbook; 2004. [Google Scholar]
- 29. Idowu OR, Peggins JO, Cullison R, Bredow JV. Comparative pharmacokinetics of enrofloxacin and ciprofloxacin in lactating dairy cows and beef steers following intravenous administration of enrofloxacin. Res Vet Sci. 2010;89:230–5. doi: 10.1016/j.rvsc.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 30. European Medicines Agency. Committee for veterinary medicinal products azaperone summary report (2) 1997 [Google Scholar]
- 31.Liu CK. Caution with animal drugs to prevent food safety. Veterinarian Newsletter (2) Animal Health Research Institute, Council of Agriculture; 2011. pp. 15–7. [Google Scholar]