Abstract
Six samples of red thyme (Thymus zygis) and two samples of winter thyme (Thymus hyemalis) essential oils (EOs) were obtained from plants cultivated in south-eastern Spain and extracted by steam distillation. Analysis by gas chromatography coupled with mass spectrometry detection provided the relative (%) and absolute (mM) concentrations. Thymol (30–54%), p-cymene (14–27%) and γ-terpinene (8–28%) were the most abundant components of T. zygis EO, while 1,8-Cineole (3–37%), p-cymene (1–29%), linalool (8–13%) and thymol (0–19%) were the most abundant components in the case of T. hyemalis EO. Enantioselective gas chromatography identified (−)-linalool, (−)-borneol and (+)-limonene as the main enantiomers. Several methods to evaluate antioxidant capacities were applied to the EOs, concluding that their activities were mainly due to thymol and linalool. The inhibition of lipoxygenase activity, mainly due to thymol, p-cymene and linalool, suggested their possible use as anti-inflammatories. The high antibacterial and antifungal activities determined for the EOs means that they can be used as natural preservatives. The results support the potential use of Thymus sp. EOs as natural food, cosmetic and pharmaceutical ingredients.
Keywords: Antioxidants, Lipoxygenase inhibitors, Microbial sensitivity tests, Oils, Volatile, Thymus plant
1. Introduction
The genus Thymus, predominantly found in the Mediterranean region, Asia, Southern Europe and North Africa, is constituted by more than three hundred species [1]. There are several ecotypes, which differ in their morphological characteristics and in the composition of their essential oils (EOs), although all of them are characterized by amoderate odor and sometimes a very pronounced balsamic and spicy flavor [2].
Thymus zygis Loefl. ex L., also known as red thyme, is a widespread endemic plant in the Iberian Peninsula [3]. Thymus hyemalis Lange, winter thyme, is mainly found in the southeast of Spain (Alicante, Murcia and Almeria provinces) [4]. The EOs obtained from both Thymus species show a high degree of variability, depending on seasonal, phenological or edaphoclimatic conditions [5–10].
Essential oils are increasingly studied for use in the chemical, cosmetic, food, fragrance and pharmaceutical industries due to their potential bioactivities [11]. This is particularly the case with the EOs from Thymus species due to the presence of bioactive compounds [12,13]. Indeed, thyme EO is among the world’s ten most commonly used EOs as a food preservative [14].
Gas chromatography, coupled with mass spectrometry or flame ionization detection, provides a detailed description of the compounds of EOs, expressed as percentages of total area, and is a useful technique for comparing the composition of EOs – for example, those studied here with those obtained from plants growing in different areas or conditions [8,15]. Moreover, using calibration curves of commercially available terpenes, the absolute concentration of each compound can be determined, which is useful for determining the quality of EOs and also whether they have been adulterated by dilution with volatile solvents.
Furthermore, analysis of the chiral distribution of EO components provides information about the origin and quality of EOs by differentiating between natural and adulterated EOs. Also, the prevalence of different enantiomers could show differences in bioactivity and organoleptic properties [16,17]. There are few chiral studies of some biomolecules on the EOs of Thymus sp. [18,19].
Several antioxidant assays serve as models for the preliminary evaluation of potential preservative and pharmacological activities. EOs may diminish oxidative processes in food and cosmetic products and so be used to replace synthetic antioxidants, increasing consumer acceptance of the products [20]. In the same way, they have a potential for use in human health care since they may reduce the oxidative stress that often enhances disease development [21,22].
Lipoxygenase (LOX) catalyzes the biosynthesis of leukotrienes from arachidonic acid and its hyperactivity has been related with inflammatory, tumoral, ischemic, skin and Alzheimer’s diseases and also diabetes [23,24]. The inhibition of soybean lipoxygenase can be used as an in vitro model to assay human lipoxygenase bioactivities [25].
The use of natural ingredients to prevent the growth of microorganisms is gaining interest [26]. Particularly, organic food cannot include chemical additives [2]. For this reason, several Thymus EO species have been proposed as natural antimicrobial alternative [15,27–31].
In this study we describe the composition of Thymus EO (relative and absolute concentrations) grown in the province of Murcia (S.E. Spain) and the enantiomeric distribution of some of its compounds. Moreover, antioxidant activity, LOX inhibition and antimicrobial activity are determined. The study will serve to characterize thyme EOs from Murcia, in depth for the first time in the literature, to enhance their potential biotechnological applications.
2. Materials and methods
2.1. Plant material
Six samples of T. zygis and two samples of T. hyemalis were taken from plants grown in Murcia (Spain). Their EOs were obtained by steam distillation in a Clevenger-type apparatus for 3 h, dried over anhydrous sodium sulfate and stored at 4 °C until use. Samples Tzt1, Tzt2, Tzt3 and Tzt4 are EOs of T. zygis thymol chemotype and samples Tzl1, Tzl2, Th3 and Th4 are EOs of T. zygis linalool chemotype and T. hyemalis, respectively. The plants yielding Tzt1 and Tzt4 were grown in a Lower Meso-Mediterranean bioclimatic zone, Tzt3, Tzl1 and Th2 were grown in an Upper Meso-Mediterranean bioclimatic zone and Tzt2, Tzl2 and Th1 were grown in a Supra-Mediterranean bioclimatic zone. The characteristics of the bioclimatic zones of Spain have been described previously [32]. Plant species were identified in the Plant Biology Department of Murcia University by Dr. Pedro Sanchez-Gomez. The Department of Biochemistry and Molecular Biology-A storage the voucher specimens.
2.2. Chemicals
All the compounds used in this work were of analytical grade, with a purity higher than 95%. The standard substances for GC identification and determination, the chemicals for the antioxidant capacity assays, and the reagents for soybean lipoxygenase inhibition were purchased from Sigma–Aldrich, Spain. The following culture media for bacteria and yeasts were provided by VWR Chemicals, Spain: Mueller Hinton Agar (MHA), Mueller Hinton Broth (MHB), Roswell Park Memorial Institute (RPMI-1640), Sabouraud Dextrose Agar (SDA), tryptic soy broth (TSB) and yeast peptone dextrose (YPD).
Solvents of analytic grade and buffers were purchased from Merck (Madrid, Spain). Type I (18 MΩ cm) deionized water (MilliQ-Reference, Millipore, Madrid, Spain) was used throughout in this work.
2.3. Microorganisms and culture conditions
The following microorganisms from the American Type Culture Collection (ATCC) were tested: Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9027 and Candida albicans ATCC 10231. All microorganisms were acquired from Sigma–Aldrich. The stock cultures were preserved in screw-capped tubes containing TSB or YPD with 15% glycerol, for bacteria and yeast cells, respectively. Isolated colonies, selected from an 18- to 24-h agar plate, were transferred to MHB in the case of bacteria and RPMI-1640 in the case of C. albicans to obtain the necessary cultures for the tests.
2.4. Fast gas chromatography–mass spectrometry (FGC/MS)
Analyses of the EOs using FGC/MS were conducted using an Agilent GC7890 chromatograph coupled to an Agilent MS5975 mass spectrometry detector, with electronic impact ionization and single quadrupole analyzer. To enhance repeatability, a Gerstel automatic multi-purpose sampler MPS-2XT was used, with triplicate sandwich injection (plunger to needle): 0.2 μL air, 0.2 μL isooctane, 0.2 μL air, 0.3 μL sample and 0.2 μL air.
The analysis was performed on a non-polar, low bleed capillary fused-silica column (SLB-5ms from Supelco; 15 m length × 0.1 mm internal diameter × 0.1 μm film thickness). The carrier gas used was hydrogen (constant flow of 0.8 mL/min, 46.345 psi starting column head pressure), produced with an electrolytic Parker–Domnik–Hunter generator, fed with type I laboratory water.
Injection conditions were: temperature 300 °C, septum purge 3 mL/min and split valve 1:100. The column temperature gradient was 60–300 °C, at a rate of 20 °C/min to 142 °C, and 40 °C/min to 300 °C, and then held for 0.5 min. Some high-resolution steps were adjusted at a rate of 2 °C/min: 92–94 °C, 121–123 °C, 133–135 °C.
Conditions of MS: temperature of the transfer line 280 °C, electronic impact ionization energy 70 eV, mass range 30–300 atomic mass units, scan rate 21.035 scan/s, electron-multiplier voltage 1129, ion source temperature 230 °C, quadrupole temperature 150 °C.
The above mentioned equipment was controlled by ChemStation software and analyzed using ChemStation, MS-Search, AMDIS and the mass spectral databases NIST 08 and Wiley 7, as well as the in-lab built pure compound spectral database.
The quantitative determination of absolute concentrations was carried out by means of calibration curves of each commercially available component described in the Thymus sp. EOs (Table S1).
2.5. Enantioselective gas chromatography–mass spectrometry (EsGC/MS)
The same analytical device described above was used with an Astec Chiraldex B-DM column (30 m length × 0.25 mm internal diameter × 0.12 μm film thickness) from Supelco and hydrogen as carrier gas (constant flow of 2.5 mL/min, 8 psi starting column head pressure). This capillary column was made of fused silica with a non-bonded 2,3-di-O-methyl-6-t-butyl silyl derivative of β-cyclodextrin. Chiral compounds were identified by retention time of the commercially available pure enantiomers, and double checked with the NIST/Wiley/in-lab spectral databases. The peak areas of the triplicates were integrated and the percentages of levorotatory (−) and dextrorotatory (+) enantiomers were determined.
Conditions: injector temperature 200 °C, transfer line temperature 200 °C, split 1:100, sandwich injection volumes: 0.2 μL air, 0.2 μL acetone, 0.2 μL air, 0.5 μL sample, 0.2 μL air. Temperature program: 35–170 °C at a rate of 4 °C/min.
2.6. Antioxidant capacity
Six assay methods were followed in triplicate. The oxygen radical absorption capacity (ORAC) values account for the ability of the samples to scavenge the peroxyl radical (ROO•) and were determined following the method described by Ou et al. [33]. The ABTS radical cation (ABTS•+) bleaching assay was carried out as detailed by Re et al. [34]. The scavenging capacity of 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radical was obtained following the method of Brandwilliams et al. [35]. These results for the three methods were expressed in Trolox equivalent antioxidant capacity (TEAC) units per volume of EO.
The degree of chelation of ferrous ions, or chelating power (ChP), of the essential oils and their major components was also evaluated [36], expressing the results in ethylenediaminetetraacetic acid equivalents (EDTAE) per volume of EO.
The reducing power (RdP) of samples on potassium ferricyanide [37] was reported in ascorbic acid equivalents (AAE) per volume of EO.
Soybean lecithin homogenate was used as lipid-rich medium in the thiobarbituric acid reactive substances (TBARS) assay [38], expressing the results in butylhydroxytoluene equivalents (BHTE) per volume of EO.
2.7. Lipoxygenase inhibition
The lipoxygenase (LOX) (linoleate:oxygen oxidoreductase, EC 1.13.11.12) assay [39,40], was based on absorption at 234 nm of the hydroperoxyde conjugated dienes (ɛ234 = 25,000 M−1 cm−1), which are formed when linoleic acid (18:2, used as substrate) is oxidized in the presence of oxygen and LOX. Nordihydroguaiaretic acid (NDGA) was used as positive inhibitor control.
The lipoxidase preparation from Glycine max (soybean), certified as a homodimer of 108 kDa with a pI of 5.65, was purchased from Sigma.
Triplicate assays were carried out on a double beam PerkinElmer Lambda 35 spectrophotometer with the UV-Winlab software, at 25 °C. The reference contained all components with the exception of substrate and inhibitors. Non-enzymatic assays were carried out, and their rates were subtracted from the steady state rates of the respective enzymatic reactions.
The degree of inhibition (DI) was calculated using Eq. (1):
| (1) |
where v0 and vi are the steady state rates in the absence and presence of inhibitor, respectively. The main compounds of the EOs were studied to determine their half maximal inhibitory concentration (IC50) values. Data of DI (%) against 8 different inhibitor concentrations were plotted, and fitted by non-linear regression (Eq. (2)):
| (2) |
using the Sigma Plot software (systatsoftware.com).
2.8. Antimicrobial activity
Minimum inhibitory concentrations (MIC) were determined in triplicate assays using the microdilution method in 96-well microtiter plates, according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI), M07-A10 [41] standard for bacteria and M27-A3 [42] for Candida, with minor modifications. Briefly, the EOs were prepared at a stock concentration of 20 μL/mL with 1% Tween 80 and 5% DMSO. Then, two-fold dilutions were prepared to obtain a final concentration range of 0.08–10 μL/mL with 0.5% Tween 80 and 2.5% DMSO. In addition, the antimicrobial activity was tested for each individual compound of the EOs with a concentration above 1% dissolved in the same solvent of EOs and with a range of assay concentrations 15–0.12 mM. All assays were carried out as follows: 100 μL of each strain, with a concentration of 106 CFU/mL in MHB for bacteria or 1 × 103 to 5 × 103 in RPMI for yeast, were added to 100 μL of each EO or individual compound. These plates were incubated for 24 h for bacteria and 48 h for the yeast, at 35 ± 1 °C, under aerobic conditions and mixed on a plate shaker at 100 rpm. Streptomycin (0.06–8 μg/mL) and fluconazole (0.13–16 μg/mL) were used as reference antibacterial and antifungal compounds, respectively. The negative control contained 200 μL MHB with 0.5% Tween 80 and 2.5% DMSO. The positive control (without the antimicrobial agent) consisted of 100 μL of working culture bacteria and 100 μL of the solvent of the EOs (with 1% Tween 80 and 5% DMSO included). It was checked that the 0.5% Tween 80 and 2.5% DMSO mixture, used for emulsifying the EOs, did not have any antimicrobial activity. The lowest concentration of EO with no visible growth of microorganisms after the incubation period was defined as the MIC.
For the triplicate determination of the minimum bactericidal concentration (MBC) and the minimum fungicidal concentration (MFC), 100 μL from wells showing no growth in the MIC assay were spread on MHA (bacteria) or SDA (yeast) and incubated for 24 h at 35 ± 1 °C. The MBC was defined as the lowest EO concentration at which the microorganisms failed to grow in broth and then on agar.
2.9. Statistical analysis
The experimental data recorded as mean ± standard deviation (SD) of at least triplicate determinations, were analyzed by univariate and multivariate statistical methods [43]. Each error value was magnified using the corresponding error propagation rules for arithmetic operations. 0.0 data values in tables mean values lower than 0.05 units. Data quality was analyzed by ANOVA, and means were tested using Tukey’s (HSD) test, considering differences to be significant at p < 0.05 (represented by different letters next to numerical values in text and tables). Principal component analysis (PCA) and agglomerative hierarchical clustering (AHC) based on Euclidean distance were performed to determine the similarity between them. Multivariate statistical analyses were carried out using Statistica software (software.dell.com).
3. Results and discussion
3.1. FGC/MS study
3.1.1. Experimental data
The essential oils were obtained in yields ranging from 0.4 to 0.8% (w/w), and FGC/MS was used, as described in Section 2.4, to determine their respective components. The composition of EOs is expressed in percentage of total area (>99% of the total area was identified) and in absolute concentration of the commercially available compounds (>90% of the total area). The data of the first column reveal when an EO has been diluted with any kind of solvent.
The four samples of T. zygis chem. thymol (Table 1A) have the same 11 main molecules, i.e., α-thujene, α-pinene, myrcene, α-terpinene, p-cymene, γ-terpinene, linalool, terpinen-4-ol, thymol, carvacrol and E-β-caryophyllene.
Table 1A.
Fast gas chromatography determination of T. zygis chem. thymol EO compounds.
| N a | LRIb | LRIc | Compound | Qualifying and quantitation ionsd (m/z) | Tzt1 | Tzt2 | Tzt3 | Tzt4 | IM | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| Concentration (mM ± SD) | Area (% ± SD) | Concentration (mM ± SD) | Area (% ± SD) | Concentration (mM ± SD) | Area (% ± SD) | Concentration (mM ± SD) | Area (% ± SD) | ||||||
| 1 | 928 | 929 | α-Thujene | 39, 77, 93, 136 | 1.3e ± 0.0 | 1.3e ± 0.0 | 0.1g ± 0.0 | 0.7f ± 0.0 | 1,2 | ||||
| 2 | 930 | 938 | α-Pinene | 77, 93, 105, 121 | 76.3b ± 6.4 | 1.5f ± 0.1 | 93.9a ± 4.8 | 1.7e ± 0.1 | 36.8c ± 1.7 | 0.7h ± 0.0 | 38.3c ± 0.5 | 0.8g ± 0.0 | 1,2,3 |
| 3 | 943 | 955 | Camphene | 79, 93, 107, 121 | 51.7b ± 3.7 | 0.7f ± 0.0 | 170.3a ± 1.7 | 2.1e ± 0.1 | 29.2c ± 0.8 | 0.4f ± 0.0 | 1,2,3 | ||
| 5 | 970 | 982 | β-Pinene | 69, 77, 93, 121 | 12.1c ± 0.7 | 0.3f,g ± 0.0 | 17.3b ± 0.8 | 0.4f ± 0.1 | 81.7a ± 3.8 | 1.7e ± 0.0 | 7.0d ± 0.1 | 0.2g ± 0.0 | 1,2,3 |
| 6 | 963 | 985 | 3-Octanone | 43, 57, 71, 99 | 0.3e ± 0.1 | 0.1f ± 0.0 | 1,2 | ||||||
| 7 | 979 | 989 | Myrcene | 41, 69, 79, 93 | 145.6a ± 6.4 | 1.8e ± 0.1 | 139.9a ± 5.2 | 1.6f ± 0.1 | 32.7c ± 0.6 | 0.4h ± 0.0 | 90.7b ± 1.3 | 1.3g ± 0.0 | 1,2,3 |
| 8 | 985 | 1002 | 3-Octanol | 41, 59, 83, 101 | 0.1 ± 0.0 | tr | 1,2 | ||||||
| 9 | 999 | 1010 | α-Phellandrene | 77, 93, 119, 136 | 17.3a ± 0.9 | 0.2e ± 0.0 | 18.0a ± 0.6 | 0.2e ± 0.0 | 5.4c ± 0.1 | 0.1g ± 0.0 | 12.0b ± 0.1 | 0.1f ± 0.0 | 1,2,3 |
| 10 | 1005 | 1012 | 3-Carene | 77, 79, 91, 93 | 0.1e ± 0.0 | 0.1e ± 0.0 | 0.1f ± 0.0 | 1,2 | |||||
| 11 | 1008 | 1019 | α-Terpinene | 77, 93, 121, 136 | 85.9b ± 4.2 | 1.7f ± 0.0 | 102.1a ± 5.5 | 1.9e ± 0.1 | 14.6d ± 0.5 | 0.2h ± 0.0 | 56.3c ± 1.3 | 1.2g ± 0.0 | 1,2,3 |
| 12 | 1011 | 1027 | p-Cymene | 91, 117, 119, 134 | 862.1b ± 29.0 | 19.4f ± 0.6 | 1212.8a ± 13.0 | 27.2e ± 0.7 | 756.6c ± 12.5 | 16.1g ± 0.2 | 705.7c ± 22.9 | 14.3h ± 0.1 | 1,2,3 |
| 13 | 1020 | 1029 | Limonene | 68, 79, 93, 121 | 47.1b ± 3.0 | 0.6f ± 0.0 | 50.8a,b ± 2.3 | 0.6f ± 0.0 | 22.1c ± 0.3 | 0.3g ± 0.0 | 53.5a ± 0.8 | 0.8e ± 0.0 | 1,2,3 |
| 14 | 1024 | 1031 | Z-β-Ocimene | 41, 79, 93, 105 | 30.3a ± 2.0 | 0.2e ± 0.0 | 32.5a ± 1.3 | 0.2e ± 0.0 | 23.9b ± 0.6 | 0.1f ± 0.0 | 20.0c ± 0.3 | 0.1f ± 0.0 | 1,2,3 |
| 15 | 1023 | 1034 | 1,8-Cineole | 43, 81, 93, 108 | 26.2b ± 1.5 | 0.6f ± 0.0 | 41.0a ± 2.7 | 0.9e ± 0.0 | 16.9c ± 0.3 | 0.4g ± 0.0 | 1,2,3 | ||
| 16 | 1036 | 1041 | E-β-Ocimene | 41, 79, 93, 105 | 0.1e ± 0.0 | 0.1e ± 0.0 | 0.1f ± 0.0 | 1,2 | |||||
| 17 | 1053 | 1059 | γ-Terpinene | 77, 93, 121, 136 | 448.5c ± 22.4 | 8.3h ± 0.3 | 711.1b ± 11.3 | 13.3f ± 0.4 | 1462.8a ± 38.2 | 28.0e ± 0.4 | 710.8b ± 16.5 | 12.1g ± 0.1 | 1,2,3 |
| 18 | 1070 | 1072 | E-Sabinene hydrate | 77, 93, 121, 136 | 36.2a ± 1.0 | 0.5e ± 0.0 | 22.2b ± 0.5 | 0.3f ± 0.0 | 0.1g ± 0.0 | 19.6c ± 0.8 | 0.3f ± 0.0 | 1,2,3 | |
| 19 | 1080 | 1088 | Terpinolene | 93, 105, 121, 136 | 0.2e ± 0.0 | 0.1f ± 0.0 | 0.2e ± 0.0 | 0.1g ± 0.0 | 1,2 | ||||
| 20 | 1089 | 1090 | Z-Lilalool oxide | 43, 59, 68, 111 | 0.1f,g ± 0.0 | 0.1f ± 0.0 | 0.1e ± 0.0 | 0.1g ± 0.0 | 1,2 | ||||
| 21 | 1081 | 1105 | Linalool | 41, 69, 93, 121 | 223.6c ± 2.8 | 4.1g ± 0.0 | 361.1b ± 5.6 | 5.8e ± 0.1 | 0.1h ± 0.0 | 386.8a ± 13.6 | 5.2f ± 0.0 | 1,2,3 | |
| 23 | 1071 | 1110 | β-Terpinene | 77, 93, 121, 136 | 0.1e ± 0.0 | 0.1e,f ± 0.0 | 0.1f ± 0.0 | 1,2 | |||||
| 26 | 1148 | 1157 | Camphor | 81, 95, 108, 152 | 13.3c ± 0.5 | 0.1g ± 0.0 | 170.4a ± 3.0 | 2.2e ± 0.1 | 0.1g ± 0.0 | 15.7b ± 0.5 | 0.3f ± 0.0 | 1,2,3 | |
| 28 | 1174 | 1181 | Borneol | 41, 95, 110, 121 | 75.8b ± 0.4 | 1.0f ± 0.0 | 440.1a ± 20.2 | 3.0e ± 0.1 | 54.9b ± 0.4 | 0.8g ± 0.0 | 1,2,3 | ||
| 29 | 1161 | 1187 | Terpinen-4-ol | 71, 93, 121, 136 | 43.5b ± 0.9 | 1.5f ± 0.0 | 35.0c ± 0.5 | 1.1g ± 0.0 | 8.9d ± 0.3 | 0.3h ± 0.0 | 45.7a ± 0.3 | 1.8e ± 0.0 | 1,2,3 |
| 30 | 1172 | 1197 | p-Cymene-8-ol | 65, 91, 117, 132 | 0.1 ± 0.0 | 1,2 | |||||||
| 31 | 1192 | 1202 | α-Terpineol | 68, 93, 121, 136 | 13.4c ± 0.9 | 0.2e ± 0.0 | 21.6b ± 0.2 | 0.2e ± 0.1 | 0.1f ± 0.0 | 33.4a ± 0.3 | 0.2e ± 0.0 | 1,2,3 | |
| 35 | 1208 | 1230 | Citronellol | 41, 69, 81, 95 | 8.5a ± 0.3 | 0.3e ± 0.0 | 7.4b ± 0.2 | 0.1f ± 0.0 | 1,2,3 | ||||
| 36 | 1241 | 1239 | Methyl ether of carvacrol | 77, 91, 117, 134 | 14.3b ± 0.3 | 0.2g ± 0.0 | 30.1a ± 0.5 | 0.5e ± 0.0 | 14.7b ± 0.3 | 0.2f ± 0.0 | 1,2,3 | ||
| 40 | 1232 | 1256 | Geraniol | 41, 69, 79, 93 | 13.6 ± 0.5 | 0.2 ± 0.0 | 1,2,3 | ||||||
| 43 | 1298 | vic-Thymol | 91, 115, 135, 150 | 1.9 ± 0.0 | 1,2 | ||||||||
| 44 | 1266 | 1309 | Thymol | 91, 115, 135, 150 | 3147.8b ± 17.8 | 50.3f ± 0.9 | 1923.2c ± 27.5 | 29.9h ± 1.0 | 3090.8b ± 98.6 | 48.2g ± 0.5 | 3636.2a ± 15.2 | 53.8e ± 0.2 | 1,2,3 |
| 45 | 1275 | 1315 | Carvacrol | 77, 91, 135, 150 | 112.9a ± 2.5 | 2.9e ± 0.1 | 62.3c ± 2.9 | 1.4g ± 0.1 | 34.3d ± 1.3 | 0.4h ± 0.0 | 80.7b ± 3.6 | 2.2f ± 0.0 | 1,2,3 |
| 46 | 1305 | 1328 | 6-Ethyl-3,4-dimethylphenol | 91, 121, 135, 150 | 0.1 ± 0.0 | 1,2 | |||||||
| 49 | 1419 | 1427 | E-β-Caryophyllene | 41, 79, 93, 133 | 31.3b ± 0.7 | 1.4f ± 0.0 | 50.4a ± 1.0 | 2.2e ± 0.0 | 0.7h ± 0.0 | 24.1c ± 0.3 | 1.2g ± 0.0 | 1,2,3 | |
| 50 | 1455 | 1447 | Aromadendrene | 91, 133, 161, 204 | 0.1e ± 0.0 | 0.2e ± 0.0 | 0.2f ± 0.0 | 0.1f ± 0.0 | 1,2 | ||||
| 51 | 1454 | 1465 | α-Humulene | 80, 93, 121, 147 | 0.1e ± 0.0 | 0.1e ± 0.0 | 0.1e ± 0.0 | 1,2, | |||||
| 54 | 1473 | 1496 | Ledene | 107, 135, 171, 204 | 0.1f ± 0.0 | 0.1f ± 0.0 | 0.3e ± 0.0 | 0.1f ± 0.0 | 1,2 | ||||
| 55 | 1514 | 1527 | δ-Cadinene | 134, 161, 189, 204 | 0.1f ± 0.0 | 0.1e ± 0.0 | 0.1g ± 0.0 | 1,2 | |||||
| 57 | 1575 | 1594 | Caryophyllene oxide | 41, 79, 91, 105 | 16.1b ± 0.7 | 0.1f ± 0.0 | 24.4a ± 1.5 | 0.2e ± 0.0 | 14.3b ± 0.5 | 0.1f ± 0.0 | 1,2,3 | ||
| Oxygenated terpenes: | |||||||||||||
| Alcohol | 60.9 | 42.5 | 51.6 | 65.1 | |||||||||
| Ketone | 0.1 | 2.3 | 0.1 | 0.2 | |||||||||
| Aldehyde | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||
| Ester | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||
| Ether | 1.0 | 1.6 | 0.2 | 0.8 | |||||||||
| Monoterpene hydrocarbons | 36.4 | 50.4 | 47.3 | 32.1 | |||||||||
| Oxygenated monoterpenes | 61.8 | 46.2 | 51.7 | 66.0 | |||||||||
| Sesquiterpene hydrocarbons | 1.8 | 2.7 | 1.0 | 1.5 | |||||||||
| Oxygenated sesquiterpenes | 0.1 | 0.2 | 0.0 | 0.1 | |||||||||
| Total terpene hydrocarbons | 38.1 | 53.1 | 48.3 | 33.6 | |||||||||
| Total oxygenated terpenes | 61.9 | 46.3 | 51.7 | 66.1 | |||||||||
| Non isoprenoid components | 0.0 | 0.6 | 0.0 | 0.3 | |||||||||
IM = Identification method: 1 = by LRI, 2 = by NIST 08 & Wiley 7, 3 = by comparison with pure compounds. tr = Traces (<0.1%).
N = Reference number for statistical PCA graphs.
LRI = Linear Retention Index from databases NIST 08 & Wiley 7.
LRI = Linear Retention Index calculated from the homologous series of n-alkanes (C7–C30).
Ions used for quantitation are in bold.
However, there are also some differences, such as the high concentration of carvacrol in Tzt1, α-terpinene, p-cymene and E-β-caryophyllene in Tzt2, β-pinene and γ-terpinene in Tzt3, and thymol in Tzt4.
As regard T. zygis chem. linalool and T. hyemalis samples, the differences among species and locations are due to some components (Table 1B). The high concentration of thymol in Tzl1 compared to Tzl2, may be related to the difference in location since both species and chemotype are the same. The high concentrations of p-cymene, linalool and thymol in Th1 and 1,8-cineole and camphor in Th2, correspond to different locations and chemotypes, p-cymene chemotype for Th1 and 1,8-cineole chemotype for Th2 [9]. The global results show two different T. zygis chem. linalool samples with the same 10 principal molecules, i.e., α-pinene, myrcene, α-terpinene, p-cymene, limonene, γ-terpinene, E-sabinene hydrate, linalool, terpinen-4-ol and thymol. Also, the two different samples of T. hyemalis have the same 10 principal molecules, i.e., α-pinene, myrcene, α-terpinene, p-cymene, limonene, 1,8-cineole, γterpinene, linalool, camphor and terpinen-4-ol.
Table 1B.
Fast gas chromatography determination of T. zygis chem. linalool and T. hyemalis EO compounds.
| N a | LRIb | LRIc | Compound | Qualifying and quantitation ionsd (m/z) | Tzl1 | Tzl2 | Th1 | Th2 | IM | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| Concentration (mM ± SD) | Area (% ± SD) | Concentration (mM ± SD) | Area (% ± SD) | Concentration (mM ± SD) | Area (% ± SD) | Concentration (mM ± SD) | Area (% ± SD) | ||||||
| 1 | 928 | 929 | α-Thujene | 39, 77, 93, 136 | 0.3c ± 0.0 | 0.2d ± 0.0 | 0.7c ± 0.0 | 0.5d ± 0.0 | 1,2 | ||||
| 2 | 930 | 938 | α-Pinene | 77, 93, 105, 121 | 170.1a ± 11.8 | 3.0d ± 0.0 | 185.9a ± 17.8 | 3.2c ± 0.1 | 194.8a ± 16.6 | 3.7d ± 0.1 | 187.8a ± 11.6 | 4.0c ± 0.0 | 1,2,3 |
| 3 | 943 | 955 | Camphene | 79, 93, 107, 121 | 68.9a ± 4.5 | 0.8d ± 0.0 | 75.9a ± 7.5 | 1.0c ± 0.0 | 108.0b ± 9.1 | 1.6d ± 0.1 | 474.8a ± 7.7 | 6.5c ± 0.1 | 1,2,3 |
| 4 | 964 | 976 | Sabinene | 41, 77, 91, 93 | 0.9c ± 0.0 | 1.0c ± 0.0 | 1.7 ± 0.0 | 1,2 | |||||
| 5 | 970 | 982 | β-Pinene | 69, 77, 93, 121 | 15.6a ± 1.1 | 0.4d ± 0.0 | 15.9a ± 0.7 | 0.4c ± 0.0 | 18.8b ± 1.3 | 0.6d ± 0.0 | 138.7a ± 10.5 | 2.7c ± 0.0 | 1,2,3 |
| 6 | 984 | 985 | 3-Octanone | 43, 57, 71, 99 | 0.1c ± 0.0 | 0.1c ± 0.0 | 1,2 | ||||||
| 7 | 979 | 989 | Myrcene | 41, 69, 79, 93 | 591.8b ± 3.6 | 7.4c ± 0.1 | 633.7a ± 18.5 | 7.5c ± 0.3 | 316.5b ± 3.9 | 2.6d ± 0.1 | 456.3a ± 16.2 | 4.5c ± 0.0 | 1,2,3 |
| 8 | 985 | 1002 | 3-Octanol | 41, 59, 83, 101 | 0.1c ± 0.0 | 0.1c ± 0.0 | 1,2 | ||||||
| 9 | 999 | 1010 | α-Phellandrene | 77, 93, 119, 136 | 33.2a ± 2.1 | 0.4c ± 0.0 | 22.4b ± 1.4 | 0.3d ± 0.0 | 16.6b ± 1.1 | 0.2d ± 0.0 | 25.2a ± 2.8 | 0.4c ± 0.0 | 1,2,3 |
| 10 | 1005 | 1012 | 3-Carene | 77, 79, 91, 93 | 0.1 ± 0.0 | 1,2 | |||||||
| 11 | 1008 | 1019 | α-Terpinene | 77, 93, 121, 136 | 408.2a ± 14.2 | 4.0c ± 0.0 | 149.6b ± 0.3 | 3.1d ± 0.1 | 107.7a ± 7.6 | 2.2c ± 0.1 | 20.4b ± 1.0 | 0.3d ± 0.0 | 1,2,3 |
| 12 | 1011 | 1027 | p-Cymene | 91, 117, 119, 134 | 156.7a ± 11.3 | 3.0c ± 0.0 | 119.2b ± 7.5 | 2.3d ± 0.1 | 1260.7a ± 13.0 | 29.2c ± 0.9 | 74.4b ± 4.9 | 1.3d ± 0.0 | 1,2,3 |
| 13 | 1020 | 1029 | Limonene | 68, 79, 93, 121 | 230.5a ± 15.0 | 2.7d ± 0.0 | 234.9a ± 5.4 | 3.0c ± 0.1 | 139.8a ± 12.6 | 1.9c ± 0.1 | 67.6b ± 6.9 | 0.8d ± 0.0 | 1,2,3 |
| 14 | 1024 | 1031 | Z-β-Ocimene | 41, 79, 93, 105 | 92.2a ± 4.7 | 0.7c ± 0.0 | 74.0b ± 5.4 | 0.5d ± 0.0 | 55.6b ± 5.0 | 0.4d ± 0.0 | 66.6a ± 2.3 | 0.8c ± 0.1 | 1,2,3 |
| 15 | 1023 | 1034 | 1,8-Cineole | 43, 81, 93, 108 | 12.5a ± 1.1 | 0.3c ± 0.0 | 12.8a ± 0.6 | 0.3c ± 0.0 | 130.5b ± 11.1 | 3.1d ± 0.0 | 1569.7a ± 9.9 | 36.9c ± 0.3 | 1,2,3 |
| 16 | 1036 | 1041 | E-β-Ocimene | 41, 79, 93, 105 | tr | tr | 0.1d ± 0.0 | 4.5c ± 0.0 | 1,2 | ||||
| 17 | 1053 | 1059 | γ-Terpinene | 77, 93, 121, 136 | 413.7a ± 21.4 | 7.7c ± 0.1 | 369.5b ± 13.4 | 6.1d ± 0.2 | 276.5a ± 13.3 | 4.8c ± 0.1 | 117.9b ± 7.7 | 1.8d ± 0.0 | 1,2,3 |
| 18 | 1070 | 1072 | E-Sabinene hydrate | 77, 93, 121, 136 | 164.2a ± 10.2 | 2.4d ± 0.0 | 186.1a ± 15.0 | 2.7c ± 0.0 | 0.1d ± 0.0 | 31.3 ± 1.8 | 0.4c ± 0.0 | 1,2,3 | |
| 19 | 1080 | 1088 | Terpinolene | 93, 105, 121, 136 | 1.4c ± 0.0 | 1.3d ± 0.0 | 0.4c ± 0.0 | 0.2d ± 0.0 | 1,2 | ||||
| 20 | 1089 | 1090 | Z-Lilalool oxide | 43, 59, 68, 111 | 0.2c ± 0.0 | 0.2c ± 0.0 | 0.1 ± 0.0 | 1,2 | |||||
| 21 | 1081 | 1105 | Linalool | 41, 69, 93, 121 | 1867.8b ± 33.9 | 41.3d ± 0.2 | 2064.7a ± 32.6 | 43.4c ± 0.4 | 630.7a ± 13.5 | 12.5c ± 0.1 | 495.9b ± 14.0 | 7.8d ± 0.1 | 1,2,3 |
| 22 | 1089 | 1110 | Hotrienol | 71, 82, 119, 134 | 0.7c ± 0.0 | 0.7c ± 0.0 | 1,2 | ||||||
| 23 | 1071 | 1110 | β-Terpinene | 77, 93, 121, 136 | 0.4c ± 0.0 | 0.5c ± 0.0 | 0.1d ± 0.0 | 0.2c ± 0.0 | 1,2 | ||||
| 24 | 1110 | 1132 | 6-Camphenol | 41, 77, 93, 108 | 0.5d ± 0.0 | 0.5c ± 0.0 | 1,2 | ||||||
| 25 | 1132 | 1152 | Hexyl isobutyrate | 43, 56, 71, 89 | 0.3c ± 0.0 | 0.3c ± 0.0 | 0.1d ± 0.0 | 0.2c ± 0.0 | 1,2 | ||||
| 26 | 1148 | 1157 | Camphor | 81, 95, 108, 152 | 18.3b ± 0.4 | 0.2d ± 0.0 | 26.5a ± 0.5 | 0.3c ± 0.0 | 31.3a ± 0.8 | 0.4d ± 0.0 | 803.0a ± 20.1 | 9.4c ± 0.1 | 1,2,3 |
| 27 | 1157 | 1179 | Lavandulol | 41, 69, 111, 123 | 0.1 ± 0.0 | 0.1d ± 0.0 | 0.6c ± 0.0 | 1,2 | |||||
| 28 | 1174 | 1181 | Borneol | 41, 95, 110, 121 | 115.3b ± 3.9 | 1.5d ± 0.0 | 137.6a ± 3.8 | 1.8c ± 0.1 | 157.5b ± 4.1 | 2.1d ± 0.1 | 419.3a ± 13.3 | 2.6c ± 0.1 | 1,2,3 |
| 29 | 1161 | 1187 | Terpinen-4-ol | 71, 93, 121, 136 | 391.4a ± 8.4 | 13.0c ± 0.1 | 389.9a ± 15.5 | 12.9c ± 0.3 | 155.9a ± 6.2 | 5.5c ± 0.1 | 37.0b ± 1.2 | 1.1d ± 0.0 | 1,2,3 |
| 30 | 1172 | 1197 | p-Cymene-8-ol | 65, 91, 117, 132 | 0.1c ± 0.0 | 0.1c ± 0.0 | 0.2 ± 0.0 | 1,2 | |||||
| 31 | 1192 | 1202 | α-Terpineol | 68, 93, 121, 136 | 126.3a ± 6.5 | 1.6c ± 0.0 | 137.9a ± 5.5 | 1.8c ± 0.1 | 147.8b ± 5.7 | 2.3c ± 0.0 | 174.4a ± 8.1 | 2.4c ± 0.1 | 1,2,3 |
| 32 | 1206 | 1210 | E-Dihydrocarvone | 67, 95, 109, 152 | 0.1d ± 0.0 | 0.2c ± 0.0 | 1,2 | ||||||
| 33 | 1204 | 1214 | Verbenone | 91, 107, 135, 150 | 12.3 ± 0.1 | 0.2c ± 0.0 | 0.1d ± 0.0 | 69.9 ± 2.1 | 0.8 ± 0.1 | 1,2,3 | |||
| 34 | 1206 | 1224 | Carveol | 91, 105, 119, 134 | 0.1c ± 0.0 | 0.1c ± 0.0 | 1,2 | ||||||
| 36 | 1215 | 1230 | Methyl ether of thymol | 91, 119, 149, 164 | 0.1c ± 0.0 | 0.1c ± 0.0 | 0.3 ± 0.0 | 1,2 | |||||
| 37 | 1208 | 1232 | Isobornyl formate | 93, 95, 121, 136 | 0.1d ± 0.0 | 0.2c ± 0.0 | 1,2 | ||||||
| 38 | 1231 | 1239 | Methyl ether of carvacrol | 77, 91, 117, 134 | 8.8a ± 0.3 | 0.1c ± 0.0 | 5.4b ± 0.5 | 0.1d ± 0.0 | 22.5 ± 0.3 | 0.3 ± 0.0 | 1,2,3 | ||
| 39 | 1236 | 1246 | Linalyl acetate | 41, 69, 93, 121 | 54.0a ± 1.8 | 0.9c ± 0.0 | 51.2a ± 1.2 | 0.9c ± 0.0 | 42.0a ± 0.7 | 0.7c ± 0.0 | 38.7b ± 1.1 | 0.6d ± 0.0 | 1,2,3 |
| 40 | 1232 | 1256 | Geraniol | 41, 69, 79, 93 | 8.1a ± 0.7 | 0.1c ± 0.0 | 9.3a ± 0.6 | 0.1c ± 0.0 | 6.0b ± 0.3 | 0.1d ± 0.0 | 15.3a ± 0.4 | 0.2c ± 0.0 | 1,2,3 |
| 41 | 1272 | 1283 | Lavandulyl acetate | 43, 69, 93, 121 | 0.1 ± 0.0 | 1,2 | |||||||
| 42 | 1285 | 1288 | Bornyl acetate | 43, 95, 121, 136 | 0.1d ± 0.0 | 8.5 ± 0.2 | 0.2c ± 0.0 | 8.8b ± 0.5 | 0.2d ± 0.0 | 28.7a ± 0.7 | 0.5c ± 0.0 | 1,2,3 | |
| 44 | 1266 | 1309 | Thymol | 91, 115, 135, 150 | 101.4a ± 1.5 | 1.0c ± 0.1 | 18.1b ± 0.5 | 0.1d ± 0.0 | 1210.6 ± 6.6 | 18.5 ± 0.9 | 1,2,3 | ||
| 45 | 1278 | 1315 | Carvacrol | 77, 91, 135, 150 | 9.5 ± 0.0 | 0.1 ± 0.0 | 75.1 ± 1.8 | 1.8 ± 0.1 | 1,2,3 | ||||
| 47 | 1334 | 1335 | δ-Elemene | 79, 93, 107, 121 | 0.1d ± 0.0 | 0.3c ± 0.0 | 1,2 | ||||||
| 48 | 1360 | 1379 | Geranyl Acetate | 41, 69, 93, 121 | 0.1d ± 0.0 | 0.2c ± 0.0 | 5.8b ± 0.3 | 0.1d ± 0.0 | 23.9a ± 0.6 | 0.8c ± 0.0 | 1,2,3 | ||
| 49 | 1421 | 1427 | E-β-Caryophyllene | 41, 79, 93, 133 | 25.5b ± 0.1 | 1.1c ± 0.0 | 26.8a ± 0.8 | 1.2c ± 0.1 | 25.6b ± 0.4 | 1.1d ± 0.0 | 49.4a ± 1.3 | 2.0c ± 0.1 | 1,2,3 |
| 50 | 1455 | 1447 | Aromadendrene | 91, 133, 161, 204 | 0.1 ± 0.0 | 1,2 | |||||||
| 51 | 1454 | 1467 | α-Humulene | 80, 93, 121, 147 | tr | 0.1 ± 0.0 | 0.1d ± 0.0 | 5.8 ± 0.3 | 0.2c ± 0.0 | 1,2,3 | |||
| 52 | 1475 | 1475 | α-Amorphene | 105, 119, 161, 204 | 0.3 ± 0.0 | 1,2 | |||||||
| 53 | 1480 | 1488 | Germacrene D | 91, 105, 119, 161 | tr | 0.1 ± 0.0 | 0.2 ± 0.0 | 1,2 | |||||
| 54 | 1496 | 1496 | Ledene | 107, 135, 171, 204 | 0.1d ± 0.0 | 1.2c ± 0.1 | 1,2 | ||||||
| 55 | 1514 | 1527 | δ-Cadinene | 134, 161, 189, 204 | 0.1d ± 0.0 | 0.6c ± 0.0 | 1,2 | ||||||
| 56 | 1567 | 1562 | Sesquisabinene hydrate | 69, 119, 161, 207 | 0.3 ± 0.0 | 1,2 | |||||||
| 57 | 1575 | 1594 | Caryophyllene oxide | 41, 79, 91, 105 | 9.9b ± 0.2 | 0.1c ± 0.0 | 11.5a ± 0.8 | 0.1c ± 0.0 | 15.9a ± 1.2 | 0.1c ± 0.0 | 13.5a ± 4.0 | 0.1d ± 0.0 | 1,2,3 |
| 58 | 1595 | 1623 | α-Cedrol | 91, 119, 161, 204 | 0.2 ± 0.0 | 1,2 | |||||||
| Oxygenated terpenes: | |||||||||||||
| Alcohol | 62.6 | 64.3 | 43.1 | 15.4 | |||||||||
| Ketone | 0.5 | 0.6 | 1.2 | 9.4 | |||||||||
| Aldehyde | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||
| Ester | 1.5 | 1.9 | 1.1 | 2.1 | |||||||||
| Ether | 0.8 | 0.8 | 3.9 | 37.0 | |||||||||
| Monoterpene hydrocarbons | 33.2 | 30.5 | 48.8 | 30.2 | |||||||||
| Oxygenated monoterpenes | 65.1 | 67.3 | 49.1 | 63.5 | |||||||||
| Sesquiterpene hydrocarbons | 1.2 | 1.6 | 1.7 | 5.7 | |||||||||
| Oxygenated sesquiterpenes | 0.1 | 0.1 | 0.1 | 0.4 | |||||||||
| Total terpene hydrocarbons | 34.4 | 32.1 | 50.5 | 35.9 | |||||||||
| Total oxygenated terpenes | 65.2 | 67.4 | 49.2 | 63.9 | |||||||||
| Non isoprenoid components | 0.4 | 0.5 | 0.3 | 0.2 | |||||||||
IM = Identification method: 1 = by LRI, 2 = by NIST 08 & Wiley 7, 3 = by comparison with pure compounds. tr = Traces (<0.1%).
N = Reference number for statistical PCA graphs.
LRI = Linear Retention Index from databases NIST 08 & Wiley 7.
LRI = Linear Retention Index calculated from the homologous series of n-alkanes (C7–C30).
Ions used for quantitation are in bold.
Oxygenated monoterpenes are predominant in five of the eight samples (Table 1). The other three samples, i.e., Tzt2, Tzt3 and Th1, have a similar percentage (around 50%) of oxygenated and hydrocarbonated monoterpenes. Alcohol (>40%) is the most abundant organic functional group in all cases except for Th2, where ether is the most abundant functional group (37%) and alcohol is the second most abundant functional group (15.4%).
3.1.2. Multivariate statistic PCA
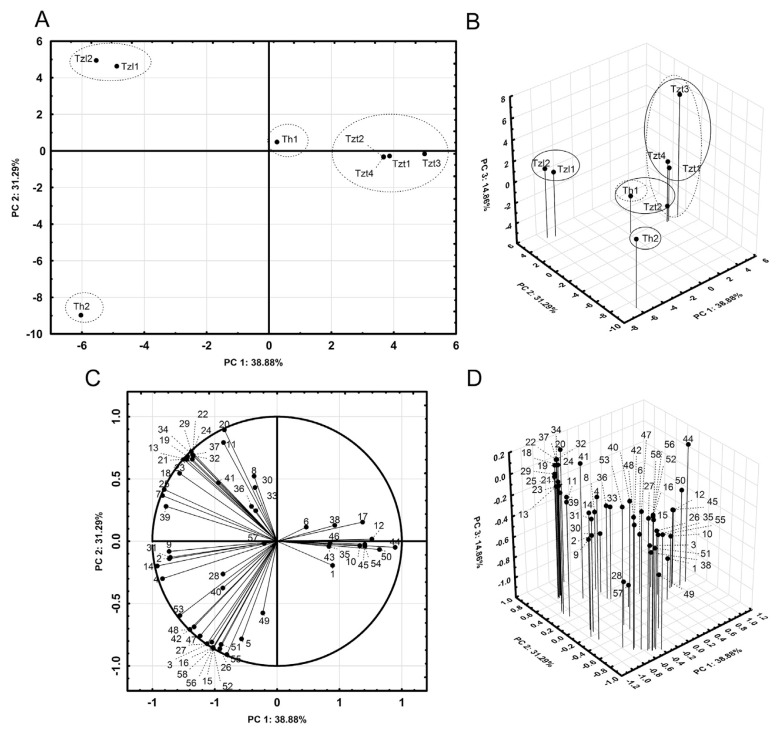
PCA is a multivariate statistic method based on the covariance matrix between linear combinations of the experimental variables (Table 1). PCA is useful for data reduction (Fig. 1), the detection of similarities between EOs (Fig. 1A and B) and identification of the characteristic compounds of EOs (Fig. 1C and D).
Fig. 1.
PCA score and loading plots. (A) Score plot of PC2 vs. PC1. (—) Tentative two-dimensional clustering. (B) 3D-score plot of PC3 vs. PC2 and PC1. (—) Tentative two-dimensional clustering. (−) Tentative three-dimensional clustering. (C) Loading plot of PC2 vs. PC1. (D) 3D-loading plot of PC3 vs. PC2 and PC1.
The total variance (Fig. 1B) consisted of three principal components, PC1 (38.88%), PC2 (31.29%) and PC3 (14.86%). Thus, the cumulative proportion of PC1 + PC2 + PC3 account for 85.03% of the total variance, which is a good starting point for data analysis.
The score plot of PC2 vs. PC1 (Fig. 1A) reveals possible clusters of EOs: (1) Tzt1, Tzt2, Tzt3 and Tzt4; (2) Th1; (3) Tzl1 and Tzl2; (4) Th2. The score plot with PC3 on the Z axis (Fig. 1B) might also lead to the above clusters, but could reveal other alternative clusters: (1) Tzt1, Tzt4 and Tzt3; (2) Tzt2 and Th1; (3) Tzl1 and Tzl2; (4) Th2. Therefore, the introduction of additional PCs identifies clusters other than those derived only from PC2 vs. PC1.
The loading plot of PC2 vs. PC1 (Fig. 1C), as well as the addition of PC3 on the Z axis (Fig. 1D), are useful tools for identifying “characteristic” compounds of each EO cluster. The loadings of compounds on a PC are standardized. Thus, a high load of a given compound could correspond to a high or low percentage of area in the EOs (Table 1). However, a high load of a compound indicates that its presence (high or low) in an EO is “characteristic” of it, and therefore useful for differentiating a particular EO from others (Fig. 1C and D, Table 1).
The biosynthetic pathway of thymol [1,19] [γ-terpinene (17) → p-cymene (12) → thymol (44)] explains the high proportion of thymol (44) and its precursors in Tzt1, Tzt4 and Tzt3, as well as their medium level proportions in Tzt2 and Th1, and low proportions in Tzl1 and Tzl2 and Th2 (Fig. 1C and D, Table 1).
Characteristic compounds of Tzl1 and Tzl2 are revealed by the high proportions of linalool (21) and terpinen-4-ol (29), as well as the medium level proportions of myrcene (7), α-terpinene (11), limonene (13) and terpinolene (19).
Characteristic compounds of Th2 are the high proportion of 1,8-cineole (15), the medium proportion of camphor (26) and camphene (3) and the low, but exclusive, proportion of αcedrol (58).
It was considered useful to confront the partial information (PCs) and qualitative similarities for the clusters from PCA (Fig. 1), with the overall information (Table 1) and quantitative similarities about clusters considered in the AHC (Section 3.1.3).
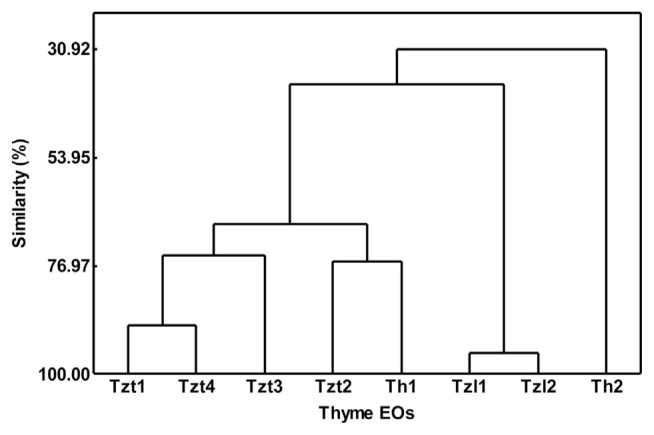
3.1.3. Multivariate statistic AHC
The statistical data and the corresponding dendrogram plot (Fig. 2) of AHC, based on Euclidean distance applied to the relative area of components, reveal four clusters for the studied EOs. Cluster 1: the pair Tzt1 and Tzt4 (89.7% similarity) coupled to Tzt3 (74.8% similarity). Cluster 2: Tzt2 and Th1, with a similarity of 76.1% despite being different species. However, these two samples shared growing location, which might have influenced the composition of the final EO. Cluster 3: Tzl1 and Tzl2 are the most similar samples (95.6% similarity). Cluster 4: Th2 is the most different sample, joining the group formed by the rest of the samples with 30.9% similarity. Thus, consideration of the whole EOs (Table 1) in AHC leads to a more accurate clustering (Fig. 2) than preliminary estimations with only two (Fig. 1A) or three (Fig. 1B) PCs from PCA analysis.
Fig. 2.
AHC dendrogram. Percentage of similarities between studied EOs and clusters.
Based on the data obtained from 3.1.2 to 3.1.3 it can be concluded that Tzt1 and Tzt4, both grown in the Lower Meso-Mediterranean zone, have developed the thymol biosynthetic pathway, with low concentrations of thymol precursors. Tzt3 has a high concentration of γ-terpinene and Tzt2 high concentrations of γ-terpinene and p-cymene (precursors of thymol) having grown in the Upper Meso-Mediterranean and Supra Mediterranean zones, respectively.
Similarly, Th2 shows a higher concentration of 1,8-cineole (final step of the pathway) having grown in the Upper Meso-Mediterranean zone, and Th1 shows a higher concentration of limonene (a precursor of 1,8-cineole), having grown in the Supra Mediterranean zone.
The Lower Meso-Mediterranean zone is the hottest and driest zone and the Supra Mediterranean zone is the coolest and wettest zone. Thus, hot temperatures and not excessive rainfall appears to be important for the development of the biosynthetic pathways towards the latest steps in the case of Thymus sp.
3.1.4. Comparison with other regions and countries
T. zygis chem. thymol EO is the most widely studied EO in the literature, and p-Cymene is found at a similar concentration in all studied samples [44] except in an EO from plants grown in Almeria (Spain) [8]. For their part, thymol and γ-terpinene were found among the main components in all reported EOs except one from Portugal [45] and another from Almeria [8]. Portuguese samples [15,28] were the only ones showing similar concentrations of myrcene and α-terpinene to studied here. The reports from Murcia, Córdoba (Spain) and Portugal describe similar concentrations of thymol [2,28,46]. The samples studied in this work show the highest concentrations of p-cymene and γ-terpinene mentioned in the revised literature.
In the case of T. hyemalis EOs, p-cymene, γ-terpinene, thymol and borneol are present in all EOs studied in the literature, whereas β-pinene, terpinen-4-ol, α-terpineol and geraniol only form part of the main components in the studied samples. Only EOs from plants grown in an experimental Spanish crop [30] and those studied here have linalool as a main component. The EO from Almeria [9] had similar concentration of camphene to the studied samples, those from experimental Spanish crops [4,30] showed similar concentrations of p-cymene and borneol and a Turkish EO [29] a similar concentration of thymol to Th1. The studied samples contained the highest concentrations of camphene, 1,8-cineole and camphor among those mentioned in the revised literature.
Myrcene, γ-terpinene, linalool and terpinen-4-ol were present in most of the samples of T. zygis chem. linalool EOs reported in the literature, confirming them as among the main components of the chemotype. The T. zygis EO from Murcia [2] had similar concentration of linalool, p-cymene and myrcene to those reported in this study. The samples studied, along with those reported by Rota et al. [30], have similar values of αterpinene, limonene and α-terpineol. The samples from Almeria [8] and central Portugal [15] showed the greatest differences from the studied samples, with higher values of p-cymene, borneol, terpinen-4-ol, thymol and E-β-caryophyllene and lower values of linalool and myrcene.
3.2. Comparison with the International Standard
The International Organization for Standardization (ISO) provides an International Standard for T. zygis EO (ISO 14715:2010). Our results are compared with the ISO norm in Table S2. As can be seen, some constituents exceed the maximum relative concentration allowed; for example, γterpinene in Tzt2, Tzt3 and Tzt4, p-cymene, linalool and E-βcaryophyllene in Tzt2 and thymol in Tzt4.
3.3. EsGC/MS study
The enantiomeric determinations of EO molecules from T. zygis and T. hyemalis are shown in Table 2. There were no adulterations with synthetic racemates of the main molecules, such as linalool or E-sabinene hydrate. The (+)-enantiomer predominates in the case of α-pinene, limonene, Esabinene hydrate, bornyl acetate, terpinen-4-ol and α-terpineol, while the (−)-enantiomer predominates in camphene, α-phellandrene, linalool, camphor, borneol, E-βcaryophyllene and caryophyllene oxide.
Table 2.
Enantiomeric ratios of Thymus sp. EO compounds.a
| t R | Compound (X) | Tzt1 | Tzt2 | Tzt3 | Tzt4 | Tzl1 | Tzl2 | Th1 | Th2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
||||||||||
| (+)–X (min) | (−)–X (min) | (+)–[X] (%) | (−)–[X] (%) | (+)–[X] (%) | (−)–[X] (%) | (+)–[X] (%) | (−)–[X] (%) | (+)–[X] (%) | (−)–[X] (%) | (+)–[X] (%) | (−)–[X] (%) | (+)–[X] (%) | (−)–[X] (%) | (+)–[X] (%) | (−)–[X] (%) | (+)–[X] (%) | (−)–[X] (%) | |
| 7.79 | 7.52 | α-Pinene | 95 | 5 | 46a | 54a | 95 | 5 | 73a | 27a | 90 | 10 | 85 | 15 | 90 | 10 | 38a | 62a |
| 8.47 | 8.24 | Camphene | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 |
| 8.89 | 9.16 | β-Pinene | 65 | 35 | 36a | 64a | 95a | 5a | 68 | 32 | 54 | 46 | 53 | 47 | 44 | 56 | 45 | 55 |
| 10.36 | 9.39 | α-Phellandrene | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 |
| 10.52 | 10.00 | Limonene | 95 | 5 | 95 | 5 | 95 | 5 | 85 | 15 | 95 | 5 | 90 | 10 | 95 | 5 | 88 | 12 |
| 14.28 | 14.51 | E-Sabinene hydrate | 95 | 5 | 95 | 5 | N/D | N/D | 88 | 12 | 95 | 5 | 95 | 5 | N/D | N/D | 95 | 5 |
| 15.73 | 15.57 | Linalool | 5 | 95 | 5 | 95 | 5 | 95 | 2 | 98 | 2 | 98 | 2 | 98 | 5 | 95 | 5 | 95 |
| 16.72 | 16.46 | Camphor | 5 | 95 | 5 | 95 | 5 | 95 | 38a | 62a | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 |
| 18.02 | 18.18 | Bornyl acetate | – | – | – | – | – | – | – | – | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 |
| 18.32 | 18.51 | Terpinen-4-ol | 63 | 37 | 64 | 36 | 82a | 18a | 61 | 39 | 73 | 27 | 72 | 28 | 71 | 29 | 49a | 51a |
| 20.10 | 19.76 | α-Terpineol | 95 | 5 | 95 | 5 | 95 | 5 | 40a | 60a | 90 | 10 | 90 | 10 | 39a | 61a | 66a | 34a |
| 20.15 | 19.58 | Borneol | 5 | 95 | 5 | 95 | – | – | 7 | 93 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 |
| 24.00 | 22.81 | E-β-Caryophyllene | 5 | 95 | 5 | 95 | 5 | 95 | 7 | 93 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 |
| – | 28.81 | Caryophyllene oxide | 5 | 95 | 5 | 95 | – | – | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 | 5 | 95 |
N/D = not detectable. SD lower than ±5%.
Biomolecular marker of the EO chemotype.
Some compounds may be considered useful as biomolecular markers of the chemotype origin; for example, α-pinene for Tzt2, Tzt4 and Th2; β-pinene for Tzt2 and Tzt3; camphor for Tzt4; terpinen-4-ol for Tzt3 and Th2 and α-terpineol for Tzt4, Th1 and Th2. Based on the enantiomeric distribution of the compounds already mentioned for Tzt4, it is possible to differentiate the Tzt1 sample from the very similar Tzt4 sample.
According to the reported data from Israel [18], (1S,2R)-(−)-borneol has high purity in both Thymus sp. A substantial degree of variability in the α-pinene and limonene enantiomers present in Thymus sp. EOs has been observed in studies worldwide [19]. The (R)-(+)-α-pinene and (R)-(+)-limonene found in the samples studied here could be useful for assessing the origin and authenticity of the EOs in question.
To our knowledge, this is the first comprehensive chiral characterization of the EOs from T. zygis and T. hyemalis grown in Spain.
3.4. Antioxidant activity
3.4.1. ORAC
The ORAC antioxidant activity of the eight EO samples is expressed in TEAC units (μmol TE/μL EO) and was (Table 3) as follows:
Table 3.
Antioxidant capacity of Thymus sp. EOs and main individual compounds.a
| EO/compound | ORAC (μmol TE/μL X) | ABTS (μmol TE/mL X) | DPPH (μmol TE/mL X) | ChP (μmol EDTAE/mL X) | RdP (μmol AAE/L X) | TBARS (μmol BHTE/mL X) |
|---|---|---|---|---|---|---|
| Tzt1 | 1.7b ± 0.1 | 3117.7b ± 179.8 | 14.5b ± 0.9 | – | 205.0a ± 10.8 | 746.9a ± 98.7 |
| Tzt2 | 1.5c ± 0.1 | 1941.4d ± 91.5 | 15.4b ± 0.5 | – | 172.0b ± 7.4 | 566.1b ± 32.0 |
| Tzt3 | 1.4c ± 0.1 | 2560.5c ± 194.6 | 28.3a ± 2.8 | – | 101.1c ± 4.5 | 835.8a ± 34.9 |
| Tzt4 | 2.0a ± 0.1 | 3675.6a ± 267.1 | 14.1b ± 1.2 | – | 181.1b ± 9.1 | 749.0a ± 36.2 |
| Tzl1 | 1.3c ± 0.1 | 60.5f ± 4.5 | 0.3d ± 0.1 | 2.4c ± 0.3 | 15.3e ± 1.7 | 37.6d ± 2.8 |
| Tzl2 | 1.4c ± 0.1 | 14.9f ± 0.2 | 0.4c,d ± 0.0 | 3.8b ± 0.3 | 9.7e ± 0.6 | 14.2d ± 2.7 |
| Th1 | 1.4c ± 0.1 | 1161.9e ± 12.4 | 3.8c ± 0.3 | – | 61.9d ± 2.8 | 328.3c ± 18.8 |
| Th2 | 0.7d ± 0.0 | 3.0f ± 0.1 | 0.4c,d ± 0.0 | 6.5a ± 0.3 | 25e ± 1.1 | 8.8d ± 0.7 |
| α-Pinene | 0.2 ± 0.0 | N/D | 0.1 ± 0.0 | 104.8 ± 7.0 | N/D | 16.3 ± 0.5 |
| Camphene | N/D | 0.8 ± 0.0 | N/D | 9.9 ± 0.7 | 3238.9 ± 157.4 | N/D |
| β-Pinene | N/D | 0.7 ± 0.0 | 0.1 ± 0.0 | 11.7 ± 0.7 | N/D | 28.9 ± 1.9 |
| Myrcene | N/D | N/D | N/D | 14.4 ± 1.0 | N/D | N/D |
| α-Terpinene | 0.4 ± 0.0 | 24.6 ± 1.2 | 1.7 ± 0.1 | 385.6 ± 29.0 | 11,531.4 ± 637.1 | N/D |
| p-Cymene | N/D | 0.6 ± 0.0 | N/D | 124.7 ± 9.8 | N/D | N/D |
| Limonene | 0.8 ± 0.1 | 3.6 ± 0.2 | N/D | 36.7 ± 2.3 | N/D | N/D |
| 1,8-Cineole | N/D | 0.2 ± 0.0 | N/D | 6.7 ± 0.4 | N/D | N/D |
| γ-Terpinene | 1.1 ± 0.1 | 15.6 ± 0.7 | 2.1 ± 0.1 | 2.0 ± 0.1 | 1990.6 ± 160.2 | 273.2 ± 24.4 |
| Sabinene hydrate | 0.3 ± 0.0 | 3.2 ± 0.2 | N/D | 44.9 ± 3.7 | N/D | 97.2 ± 4.2 |
| Linalool | 1.9 ± 0.1 | 0.4 ± 0.0 | N/D | 541.6 ± 32.7 | N/D | N/D |
| Camphor | N/D | N/D | N/D | N/D | N/D | N/D |
| Borneol | N/D | N/D | N/D | N/D | N/D | N/D |
| Terpinen-4-ol | 2.2 ± 0.1 | 1.8 ± 0.1 | 0.3 ± 0.0 | 10.3 ± 0.7 | N/D | 50.2 ± 10.6 |
| α-Terpineol | 1.9 ± 0.1 | 1.1 ± 0.1 | N/D | 29.9 ± 1.9 | N/D | 15.6 ± 0.5 |
| Linalyl acetate | 1.0 ± 0.1 | 0.3 ± 0.0 | N/D | 125.7 ± 9.4 | N/D | 19.6 ± 0.6 |
| Bornyl acetate | N/D | N/D | N/D | 0.1 ± 0.0 | N/D | N/D |
| Thymol | 2.1 ± 0.1 | 6897.1 ± 225.0 | 34.9 ± 2.1 | N/D | 3353.9 ± 133.4 | 869.4 ± 255.2 |
| Carvacrol | 2.8 ± 0.1 | 7804.4 ± 268.1 | 34.9 ± 2.5 | N/D | 3239.6 ± 143.0 | 600.7 ± 102.2 |
| β-Caryophyllene | 1.7 ± 0.1 | N/D | N/D | 31.6 ± 2.2 | N/D | 76.5 ± 3.4 |
N/D = Activity lower than 0.05 units at a maximum assay concentration of 100 mM. X = EO or compound.
The antioxidant activity of each EO is related to its composition (Table 3), not only the intrinsic antioxidant activity of each of the compounds, but also the synergistic and antagonistic effects among them. Three oxygenated components are important for explaining the ORAC value of the EOs, namely thymol, carvacrol and terpinen-4-ol. The first is the most important molecule because of its high concentration and ORAC value (Table 3). Tzt3 had a high concentration of thymol, but lower concentrations of other antioxidant compounds, such as, linalool, terpinen-4-ol, carvacrol and β-caryophyllene. Similar results were reported in another study with Thymus vulgaris EOs from Barcelona (Spain) [47].
3.4.2. ABTS
The ABTS antioxidant activity is expressed in TEAC units (μmol TE/mL EO) and was (Table 3) as follows:
The overall activity is the result of the different activities of its components (Table 3). Thymol had a great impact in the ABTS method, the results obtained in this method are strongly related with the concentration of thymol. The contributions of the rest of compounds to the ABTS value were less significant.
3.4.3. DPPH
The DDPH antioxidant activity is expressed in TEAC units (μmol TE/mL EO) and was (Table 3) as follows:
The activity of each individual compound was also studied (Table 3). Thymol and carvacrol are the molecules with the highest antioxidant activity in this method, although γ-terpinene contributed significantly to the results.
3.4.4. ChP
The ChP activity is expressed in EDTAE units (μmol EDTAE/mL EO) (Table 3):
In this case, only three of the EOs showed great activity (Table 3).
The highest concentrations of 1,8-cineole and camphor were found in Th2. The higher concentration of linalool in Tzl2 was the main difference between Tzl1 and Tzl2. In the case of Th2, a high concentration of 1,8-cineole and the low concentrations of other highly active compounds like α-pinene, β-pinene, α-terpinene, p-cymene, linalool, terpinen-4-ol, α-terpineol, linalyl acetate and β-caryophyllene may have behaved synergistically, making Th2 the most active sample as regard ChP.
Phenolic compounds like thymol, with the electronic density of the oxygen atom delocalized by the action of the aromatic ring, do not show any ChP (Table 3). Nevertheless, aromatic and non-aromatic rings without oxygen substituents had a high chelating activity e.g., α-terpinene and p-cymene. Also, linalool and linalyl acetate had high ChP, due to the high electronic densities of the oxygen atoms of the carbonyl and alcohol groups.
3.4.5. RdP
The RdP antioxidant activity is expressed in AAE units (μmol AAE/L EO) (Table 3) as follows:
When the activity of each individual compound was studied (Table 3), thymol, carvacrol and camphene were seen to have great influence in this assay.
3.4.6. TBARS
The thiobarbituric acid reactive substances generated by oxidation were measured and expressed in BHTE units (μmol BHTE/mL EO) (Table 3) as follows:
When the activity of each individual compound was studied (Table 3), thymol was seen to have a strong influence since the order of the TBARS values reflected the decreasing concentration of thymol, except in Tzt3. The highest concentration of γ-terpinene was seen in Tzt3. According to our results and those mentioned in the literature, γ-terpinene has strong TBARS antioxidant activity [48].
3.5. Inhibitory activity on LOX
The results of the LOX inhibitory activity were obtained as described in Section 2.7, measuring the IC50 (μL(EO)/L):
The LOX inhibitory activity of the main commercially available compounds of thyme EO was tested, providing IC50 (μL (Compound)/L) values as follows: thymol (23 ± 2), limonene (58 ± 5), bornyl acetate (75 ± 3), p-cymene (79 ± 5), camphor (422 ± 13) and linalool (599 ± 8).NDGA was used as the reference compound (IC50 = 339 ± 9 μM), as in other similar studies [49].
The anti-LOX effect of each EO may not be the same as the sum of the anti-enzymatic activities of its compounds, because synergistic or antagonistic effects may occur. In our case, the inhibitory activities of the T. zygis and T. hyemalis EOs were clearly due to a combination of compounds with high inhibitory activity and high-to-moderate concentration, namely thymol, p-cymene, limonene and linalool.
3.6. Antimicrobial activity: determination of MIC and MBC or MFC
The results show that T. zygis chem. thymol EOs (Tzt1, Tzt2, Tzt3 and Tzt4) are more effective against S. aureus (Gram +) than E. coli (Gram −) and C. albicans (yeast) (Table 4). The MIC values for P. aeruginosa (Gram −) were higher than 10 μL/mL with all the EOs tested. These results are similar to other studies with EOs from Thymus saturejoides [50] and Etlingera fimbriobracteata [51]. The two samples of T. zygis chem. linalool EOs (Tzl1 and Tzl2) were effective against S. aureus due to the high amount and activity of linalool (Table 4). T. hyemalis EOs showed fewer differences between the tested microorganisms because of the lower amounts of thymol and linalool. For both S. aureus and C. albicans, sample Th1 was highly effective, probably due to the high amount of thymol and p-cymene. All the EOs tested showed bactericidal effects because the MBC/MIC was lower than 4, as mentioned for Annona senegalensis [52].
Table 4.
Antibacterial and antifungal capacities of Thymus sp. EOs and main individual compounds.
| EO/compound | Escherichia coli | Staphylococcus aureus | Candida albicans | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MFC (μL/mL) | |
| Tzt1 | 1.3 | 1.3 | 0.2 | 0.2 | 1.3 | 1.3 |
| Tzt2 | 1.3 | 1.3 | 0.3 | 0.3 | 1.3 | 2.5 |
| Tzt3 | 1.3 | 2.5 | 0.3 | 0.3 | 1.3 | 1.3 |
| Tzt4 | 1.3 | 1.3 | 0.3 | 0.3 | 1.3 | 1.3 |
| Tzl1 | 2.5 | 2.5 | 1.3 | 2.5 | 2.5 | 5.0 |
| Tzl2 | 2.5 | 2.5 | 1.3 | 2.5 | 2.5 | 5.0 |
| Th1 | 2.5 | 2.5 | 0.6 | 1.3 | 1.3 | 1.3 |
| Th2 | 5.0 | 5.0 | 5.0 | 5.0 | 2.5 | 5.0 |
| α-Pinene | 0.6 | 1.2 | 2.4 | >2.4 | 0.6 | 0.6 |
| Camphene | >2.4 | >2.4 | >2.4 | >2.4 | >2.4 | >2.4 |
| β-Pinene | >2.3 | >2.3 | >2.3 | >2.3 | >2.3 | >2.3 |
| Myrcene | >2.6 | >2.6 | >2.6 | >2.6 | >2.6 | >2.6 |
| α-Terpinene | >2.4 | >2.4 | 2.4 | >2.4 | >2.4 | >2.4 |
| p-Cymene | 1.2 | 2.4 | >2.4 | >2.4 | 0.6 | 0.6 |
| Limonene | 2.4 | 2.4 | 0.3 | 0.3 | 1.2 | 1.2 |
| 1,8-Cineole | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 |
| γ-Terpinene | >2.4 | >2.4 | >2.4 | >2.4 | >2.4 | >2.4 |
| Sabinene hydrate | >2.2 | >2.2 | 2.2 | 2.2 | >2.2 | >2.2 |
| Linalool | 1.3 | 2.7 | 0.7 | 1.3 | 2.7 | 2.7 |
| Camphor | >2.3 | >2.3 | >2.3 | >2.3 | >2.3 | >2.3 |
| Borneol | 1.1 | 1.1 | 0.3 | 0.3 | 0.6 | 0.6 |
| Terpinen-4-ol | 2.5 | 2.5 | 1.2 | 2.5 | >2.5 | >2.5 |
| α-Terpineol | 2.5 | 2.5 | 0.6 | 1.2 | >2.5 | >2.5 |
| Linalyl acetate | >3.3 | >3.3 | 3.3 | >3.3 | >3.3 | >3.3 |
| Bornyl acetate | >2.9 | >2.9 | 2.9 | >2.9 | >2.9 | >2.9 |
| Thymol | 0.6 | 0.6 | 0.1 | 0.1 | 0.6 | 1.2 |
| Carvacrol | 1.2 | 1.2 | 0.1 | 0.1 | 0.6 | 0.6 |
| β-Caryophyllene | >3.4 | >3.4 | >3.4 | >3.4 | >3.4 | >3.4 |
| Ledene | >3.3 | >3.3 | 1.7 | 1.7 | >3.3 | >3.3 |
4. Conclusions
T. zygis and T. hyemalis EOs contained seven common principal biomolecules: α-pinene, myrcene, α-terpinene, p-cymene, γ-terpinene, linalool, and terpinen-4-ol. Thymol was the main biomolecule for T. zygis while 1,8-cineole was present at a high concentration in T. hyemalis samples. Oxygenated monoterpenes accounted for more than 46% of the total compounds, alcohol being the most abundant functional group, except for Th2, in which ether was the most abundant functional group. Great variability between samples was found, even in the case of the same species and chemotype, mainly due to the different bioclimatic zones in which the plants were grown. The multivariate statistical analyses (PCA and AHC) revealed the similarities (%) within the EO samples of each cluster: (1) Tzt1, Tzt4 and Tzt3 with 74.8% similarity; (2) Tzt2 and Th1 with 76.1% similarity; (3) Tzl1 and Tzl2 with 95.6% similarity; (4) Th2 with 30.9% similarity respect to other clusters.
Some concentrations of characteristic biomolecules, like γterpinene, linalool and thymol, were found to exceed ISO limits in some samples, underlining the EOs as good sources of these biomolecules.
The enantiomeric profile showed that (R)-(+)-α-pinene, (R)-(+)-limonene, (R)-(−)-linalool, (1S, 2R)-(−)-borneol, (1R, 9S)-(−)-E-β-caryophyllene and (1R, 4R, 6R, 10S)-(−)-caryophyllene oxide were the predominant enantiomers in Spanish Thymus sp. This data could be useful for assessing the origin and the authenticity of the EOs.
T. zygis and T. hyemalis EOs showed good antioxidant capacities compared with reference antioxidants due to the complex mixture of compounds they contained. Nevertheless, the main contributors to the overall bioactivity can be identified as thymol and linalool.
As regard their potential use as anti-inflammatory agents, both T. zygis and T. hyemalis EOs were able to inhibit LOX activity, due to some of their individual compounds. These included thymol, p-cymene, linalool and limonene due to their high concentrations in the EOs.
Thymus EOs are effective antimicrobial and antifungal agents, which is mainly attributable to the high concentration of thymol, linalool and p-cymene, and the high activity shown by thymol and carvacrol.
Generally, T. zygis thymol chemotype was the most effective as regard all antioxidant, anti-enzymatic and antimicrobial bioactivities, mainly due to its higher concentration of thymol. The only exception was the Chelating Power, in which case the 1,8-cineole chemotype of T. hyemalis was more active.
The biochemical compositions and bioactivities of T. zygis and T. hyemalis EOs described in this study include absolute concentrations and chiral characterizations, which have not usually been reported in previous studies, but which are crucial for ascertaining the pharmacological and industrial potential applications of the studied EOs from Murcia (S.E. Spain).
Acknowledgments
This work was partially supported by Spanish organizations: Projects 19545/PI/14 (Fundacion Seneca, CARM, Murcia, Spain), UMU-15452 and UMU-17766 (Universidad de Murcia, Murcia, Spain). A.B.C. has a pre-doctoral contract from Spanish Ministry of Education, Culture and Sport [FPU13/04013].
Some results have been reported in the PhD Thesis of one of the coauthors (A. C.) of this manuscript.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2017.05.004.
REFERENCES
- 1.Morales R. The history, botany and taxonomy of the genus Thymus. Thyme: the genus. In: Stahl-Biskup E, Sáez F, editors. Thymus. Vol. 17. London: CRC Press; 2002. pp. 1–43. [Google Scholar]
- 2. Ballester-Costa C, Sendra E, Fernandez-Lopez J, Perez-Alvarez JA, Viuda-Martos M. Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind Crops Prod. 2013;50:304–11. [Google Scholar]
- 3. Jordan MJ, Martinez RM, Martinez C, Monino I, Sotomayor JA. Polyphenolic extract and essential oil quality of Thymus zygis ssp gracilis shrubs cultivated under different watering levels. Ind Crops Prod. 2009;29(1):145–53. [Google Scholar]
- 4. Jordan MJ, Martinez RM, Goodner KL, Baldwin EA, Sotomayor JA. Seasonal variation of Thymus hyemalis Lange and Spanish Thymus vulgaris L. essential oils composition. Ind Crops Prod. 2006;24(3):253–63. [Google Scholar]
- 5. Goodner KL, Mahattanatawee K, Plotto A, Sotomayor JA, Jordan MJ. Aromatic profiles of Thymus hyemalis and Spanish T. vulgaris essential oils by GC–MS/GC–O. Ind Crops Prod. 2006;24(3):264–8. [Google Scholar]
- 6. Martinez RM, Jordan MJ, Quilez M, Sotomayor JA. Effects of edaphoclimatic conditions on Thymus hyemalis L. essential oil yield and composition. J Essent Oil Res. 2005;17(6):614–8. [Google Scholar]
- 7. Perez-Sanchez R, Galvez C, Ubera JL. Bioclimatic influence on essential oil composition in south Iberian peninsular populations of Thymus zygis. J Essent Oil Res. 2012;24(1):71–81. [Google Scholar]
- 8. Saez F. Essential oil variability of Thymus zygis growing wild in southeastern Spain. Phytochemistry. 1995;40(3):819–25. [Google Scholar]
- 9. Saez F. Essential oil variability of Thymus hyemalis growing wild in southeastern Spain. Biochem Syst Ecol. 1995;23(4):431–8. doi: 10.1016/s0305-1978(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 10. Blanco Salas J, Ruiz Tellez T, Vazquez Pardo FM, Cases Capdevila MA, Perez-Alonso MJ, Gervasini Rodriguez C. Influence of phenological stage on the antioxidant activity of Thymus zygis. Span J Agric Res. 2012;10(2):461–5. [Google Scholar]
- 11. Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crops Prod. 2014;62(0):250–64. [Google Scholar]
- 12. Hazzit M, Baaliouamer A, Verissimo AR, Faleiro ML, Miguel MG. Chemical composition and biological activities of Algerian Thymus oils. Food Chem. 2009;116(3):714–21. [Google Scholar]
- 13. Yu YM, Chao TY, Chang WC, Chang MJ, Lee MF. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J Food Drug Anal. 2016;24(3):556–63. doi: 10.1016/j.jfda.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ehivet FE, Min B, Park M-K, Oh J-H. Characterization and antimicrobial activity of sweet potato starch-based edible film containing origanum (Thymus capitatus) oil. J Food Sci. 2011;76(1):C178–84. doi: 10.1111/j.1750-3841.2010.01961.x. [DOI] [PubMed] [Google Scholar]
- 15. Gonçalves MJ, Cruz MT, Cavaleiro C, Lopes MC, Salgueiro L. Chemical, antifungal and cytotoxic evaluation of the essential oil of Thymus zygis subsp sylvestris. Ind Crops Prod. 2010;32(1):70–5. [Google Scholar]
- 16. Simas DLR, de Amorim SHBM, Goulart FRV, Alviano CS, Alviano DS, da Silva AJR. Citrus species essential oils and their components can inhibit or stimulate fungal growth in fruit. Ind Crops Prod. 2017;98:108–15. [Google Scholar]
- 17. del Castillo MLR, Blanch GP, Herraiz M. Natural variability of the enantiomeric composition of bioactive chiral terpenes in Mentha piperita. J Chromatogr A. 2004;1054(1–2):87–93. [PubMed] [Google Scholar]
- 18. Ravid U, Putievsky E, Katzir I. Stereochemical analysis of borneol in essential oils using permethylated β-cyclodextrin as a chiral stationary phase. Flavour Fragr J. 1996;11(3):191–5. [Google Scholar]
- 19.Stahl-Biskup E, Sáez F. Thymus. CRC Press; 2002. Thyme: the genus. [Google Scholar]
- 20. Yuan GF, Chen XE, Li D. Chitosan films and coatings containing essential oils: the antioxidant and antimicrobial activity, and application in food systems. Food Res Int. 2016;89:117–28. doi: 10.1016/j.foodres.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 21. Butterfield DA, Sultana R. Redox proteomics: understanding oxidative stress in the progression of age-related neurodegenerative disorders. Expert Rev Proteomics. 2008;5(2):157–60. doi: 10.1586/14789450.5.2.157. [DOI] [PubMed] [Google Scholar]
- 22. Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic Biol Med. 2009;47(12):1673–706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chu J, Pratico D. The 5-Lipoxygenase as modulator of Alzheimer’s gamma-secretase and therapeutic target. Brain Res Bull. 2016;126:207–12. doi: 10.1016/j.brainresbull.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2015;1851(4):331–9. doi: 10.1016/j.bbalip.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 25. Perera H, Samarasekera J, Handunnetti SM, Weerasena O. In vitro anti-inflammatory and anti-oxidant activities of Sri Lankan medicinal plants. Ind Crops Prod. 2016;94:610–20. [Google Scholar]
- 26. Lin PC, Lee JJ, Chang IJ. Essential oils from Taiwan: chemical composition and antibacterial activity against Escherichia coli. J Food Drug Anal. 2016;24(3):464–70. doi: 10.1016/j.jfda.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dandlen SA, Sofia Lima A, Mendes MD, Graca Miguel M, Leonor Faleiro M, Joao Sousa M, et al. Antimicrobial activity, cytotoxicity and intracellular growth inhibition of Portuguese Thymus essential oils. Rev Bras Farmacogn. 2011;21(6):1012–24. [Google Scholar]
- 28. Pina-Vaz C, Rodrigues AG, Pinto E, Costa-de-Oliveira S, Tavares C, Satgueiro L, et al. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004;18(1):73–8. doi: 10.1111/j.1468-3083.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 29. Tepe B, Sarikurkcu C, Berk S, Alim A, Akpulat HA. Chemical composition, radical scavenging and antimicrobial activity of the essential oils of Thymus boveii and Thymus hyemalis. Rec Nat Prod. 2011;5(3):208–20. [Google Scholar]
- 30. Rota MC, Herrera A, Martinez RM, Sotomayor JA, Jordan MJ. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control. 2008;19(7):681–7. [Google Scholar]
- 31. Semeniuc CA, Pop CR, Rotar AM. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J Food Drug Anal. 2017;25(2):403–8. doi: 10.1016/j.jfda.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivas-Martínez S. Nociones sobre fitosociología, biogeografía y bioclimatología en la vegetación de España [Notions about phytosociology, biogeography and bioclimatology in the plants of Spain] In: Peinado M, Rivas-Martinez S, editors. La vegetación de España. Madrid, Spain: University of Alcalá de Henares; 1987. pp. 17–45. [Google Scholar]
- 33. Ou BX, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49(10):4619–26. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 34. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 35. Brandwilliams W, Cuvelier ME, Berset C. Use of a free-radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. [Google Scholar]
- 36. Miguel MG, Cruz C, Faleiro L, Simoes MTF, Figueiredo AC, Barroso JG, et al. Foeniculum vulgare essential oils: chemical composition, antioxidant and antimicrobial activities. Nat Prod Commun. 2010;5(2):319–28. [PubMed] [Google Scholar]
- 37. Oyaizu M. Studies on products of browning reaction – antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 38. Damien Dorman HJ, Deans SG, Noble RC, Surai P. Evaluation in vitro of plant essential oils as natural antioxidants. J Essent Oil Res. 1995;7(6):645–51. [Google Scholar]
- 39. Christopher J, Pistorius E, Axelrod B. Isolation of an isozyme of soybean lipoxygenase. Biochim Biophys Acta. 1970;198(1):12–9. doi: 10.1016/0005-2744(70)90028-8. [DOI] [PubMed] [Google Scholar]
- 40. Whent M, Ping T, Kenworthy W, Yu L. High-throughput assay for detection of soybean lipoxygenase-1. J Agric Food Chem. 2010;58(24):12602–7. doi: 10.1021/jf1028784. [DOI] [PubMed] [Google Scholar]
- 41.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—tenth edition Document M07-A10. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 42.CLSI. Reference method for broth dilution antifungal susceptibility testing of yeast; approved standard—third edition. Document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 43.Hahs-Vaughn DL. Applied multivariate statistical concepts. New York: Routledge; 2017. [Google Scholar]
- 44. Moldão-Martins M, Bernardo-Gil GM, da Costa LM. Sensory and chemical evaluation of Thymus zygis L. essential oil and compressed CO2 extracts. Eur Food Res Technol. 2002;214(3):207–11. [Google Scholar]
- 45. Dandlen SA, Lima AS, Mendes MD, Miguel MG, Faleiro ML, Sousa MJ, et al. Antioxidant activity of six Portuguese thyme species essential oils. Flavour Fragr J. 2010;25(3):150–5. [Google Scholar]
- 46. Penalver P, Huerta B, Borge C, Astorga R, Romero R, Perea A. Antimicrobial activity of five essential oils against origin strains of the Enterobacteriaceae family. Apmis. 2005;113(1):1–6. doi: 10.1111/j.1600-0463.2005.apm1130101.x. [DOI] [PubMed] [Google Scholar]
- 47. Bentayeb K, Vera P, Rubio C, Nerin C. The additive properties of oxygen radical absorbance capacity (ORAC) assay: the case of essential oils. Food Chem. 2014;148:204–8. doi: 10.1016/j.foodchem.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 48. Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69(2):167–74. [Google Scholar]
- 49. Kurihara H, Kagawa Y, Konno R, Kim SM, Takahashi K. Lipoxygenase inhibitors derived from marine macroalgae. Bioorg Med Chem Lett. 2014;24(5):1383–5. doi: 10.1016/j.bmcl.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 50. Kasrati A, Alaoui Jamali C, Fadli M, Bekkouche K, Hassani L, Wohlmuth H, et al. Antioxidative activity and synergistic effect of Thymus saturejoides Coss. essential oils with cefixime against selected food-borne bacteria. Ind Crops Prod. 2014;61:338–44. [Google Scholar]
- 51. Ud-Daula AFMS, Demirci F, Abu Salim K, Demirci B, Lim LBL, Baser KHC, et al. Chemical composition, antioxidant and antimicrobial activities of essential oils from leaves, aerial stems, basal stems, and rhizomes of Etlingera fimbriobracteata (K.Schum.) R.M.Sm. Ind Crops Prod. 2016;84:189–98. [Google Scholar]
- 52. Traoré Y, Ouattara K, Yéo D, Doumbia I, Coulibaly A. Recherche des activités antifongique et antibactérienne des feuilles d’Annona senegalensis Pers. (Annonaceae) J Appl Biosci. 2012;58:4234–42. [Google Scholar]