Abstract
Abstract
Aerobic exercise is an effective intervention in preventing obesity and is also an important factor associated with thermogenesis. There is an increasing interest in the factors and mechanisms induced by aerobic exercise that can influence the metabolism and thermogenic activity in an individual. Recent studies suggest that exercise induced circulating factors (known as ‘exerkines’), which are able to modulate activation of brown adipose tissue (BAT) and browning of white adipose tissue. However, the underlying molecular mechanisms associated with the effect of exercise‐induced peripheral factors on BAT activation remain poorly understood. Furthermore, the role of exercise training in BAT activation is still debatable. Hence, the purpose of our study is to assess whether exercise training affects the expression of uncoupled protein 1 (UCP1) in brown adipocytes via release of different blood factors. Four weeks of exercise training significantly decreased the body weight gain and fat mass gain. Furthermore, trained mice exhibit higher levels of energy expenditure and UCP1 expression than untrained mice. Surprisingly, treatment with serum from exercise‐trained mice increased the expression of UCP1 in differentiated brown adipocytes. To gain a better understanding of these mechanisms, we analysed the conditioned media obtained after treating the C2C12 myotubes with an AMP‐activated protein kinase (AMPK) activator (AICAR; 5‐aminoimidazole‐4‐carboxamide ribonucleotide), which leads to an increased expression of UCP1 when added to brown adipocytes. Our observations suggest the possibility of aerobic exercise‐induced BAT activation via activation of AMPK in skeletal muscles.
Key points
Exercise promotes thermogenesis by activating uncoupling protein 1 (UCP1), which leads to a decrease in the body weight gain and body fat content. However, little is known about the role of exerkines in modulating UCP1 expression and subsequent brown adipose tissue (BAT) activation.
Four weeks of voluntary wheel‐running exercise reduces body weight and fat content.
Exercise induces the increase in AMP‐activated protein kinase (AMPK) and slow‐type muscle fibre marker genes in skeletal muscles and promotes UCP1 expression in white and brown adipose tissues.
Incubation of brown adipocytes with serum isolated from exercise‐trained mice significantly increased their UCP1 gene and protein levels; moreover, conditioned media of AMPK‐activator‐treated C2C12 myotubes induces increased UCP1 expression in brown adipocytes.
These results show that aerobic exercise‐induced skeletal muscle AMPK has a significant effect on UCP1 expression in BAT.
Keywords: AMPK, BAT activation, brown adipocyte, exercise, exerkine, myokine, UCP1
Abstract figure legend Exercise induces the release of several factors called ‘exerkines’ that can help to modulate the metabolic as well as thermogenic activity of an individual. However, there is limited knowledge regarding the underlying molecular mechanisms that enable these factors to stimulate brown adipose tissue activity. Our study demonstrates that exercise induces activation of AMP‐activated protein kinase in skeletal muscles, which, in turn, presumably through the release of exerkines from muscles, can modulate the uncoupled protein 1 expression in brown adipocytes. We believe that this paper provides an understanding of the molecular basis of adipocyte browning during aerobic exercise, and this knowledge may be useful in developing novel strategies for preventing the onset of/overcoming obesity.
Introduction
The metabolic functions of brown adipose tissue (BAT) are well documented, and recent studies suggest that BAT can be regulated via certain factors secreted from skeletal muscle as well as non‐skeletal muscle tissues in response to exercise (Laurens et al., 2020; Safdar & Tarnopolsky, 2018; Sanchez‐Delgado et al., 2015). Over the past 20 years, several studies have defined myokines as the exercise‐induced circulating factors or cytokines released by skeletal muscles, which interact with adipose tissues and the liver (Hoffmann & Weigert, 2017; Magliulo et al., 2021; Severinsen & Pedersen, 2020). Mostly, myokines have been reported to play multiple roles in regulating the metabolic functions of an individual, depending on the type, intensity and duration of exercise. However, at present, humoral biomolecules, including hormones, cytokines and exosomes, that are released from any tissue during exercise, are defined as exerkines (Castano et al., 2019; Magliulo et al., 2021; Safdar & Tarnopolsky, 2018). Based on these observations, there has been an increased focus on determining the underlying mechanisms by which exerkines modulate white fat to beige fat conversion and BAT activation, ultimately leading to exercise‐induced metabolic improvement.
The available evidence suggests that exercise promotes thermogenesis through the expression of mitochondrial uncoupling protein 1 (UCP1), which uncouples respiration from ATP synthesis (De Matteis et al., 2013; Flouris et al., 2017; Shirkhani et al., 2018). High levels of UCP1 contribute to whole‐body energy expenditure, which, in turn, leads to a reduction in body fat and body weight in both rodents and humans (Feldmann et al., 2009; Okamatsu‐Ogura et al., 2020). Therefore, UCP1 is used as the most common and representative molecular marker of the browning of adipose tissue. However, the role of exercise‐induced circulating factors in modulating the UCP1‐induced regulation of BAT is poorly understood. Furthermore, there is still a debate about whether exercise induces BAT activation at all.
In this study, we first verified that 4 weeks of exercise training leads to an increased expression of peroxisome proliferator‐activated receptor‐gamma coactivator‐1 alpha (PGC‐1α) and UCP1, along with activation of BAT. This observation led us to hypothesize that the exercise‐induced activation of AMP‐activated protein kinase (AMPK) in the skeletal muscles may be, at least in part, responsible for BAT activation. After the 4 weeks of training, we collected blood from the hearts of the mice, isolated the serum, and incubated fully differentiated brown adipocytes in it to investigate whether the blood factors that are altered during exercise can directly modulate the expression of UCP1 in the brown adipocytes.
Additionally, we performed another experiment using a C2C12 skeletal muscle cell line to analyse the effect of muscle‐derived factors released during aerobic exercise on brown adipocytes. For this, we treated the conditioned medium (CM) of the C2C12 myotubes with an AMPK agonist (AICAR; 5‐aminoimidazole‐4‐carboxamide ribonucleotide), which is widely used to mimic the condition of aerobic exercise (Iglesias et al., 2002; Jakobsen et al., 2001; Sanchez et al., 2013), and incubated the brown adipocytes in it. Based on this in vitro system, we further investigated whether exercise‐induced AMPK activity in skeletal muscles can modulate the expression of browning markers in brown adipocytes.
Materials and methods
Ethical approval
All animal experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University, Seoul, Korea (Permit Number: SNU‐191014‐2) and performed according to the Guide for Animal Experiments (edited by the Korean Academy of Medical Sciences).
Animal studies
We obtained 8‐week‐old male C57BL/6N mice from DBL (Seoul, Korea). The mice were housed in specific pathogen‐free conditions and had ad libitum access to a normal chow diet (Ziegler Bros, NIH‐41) and tap water. All mice were maintained under a 12/12 h light/dark cycle, 60 ± 5% humidity, and 23 ± 5°C temperature. The C57BL/6 mice strain has been shown to be a suitable strain mimicking the human metabolism observed in obesity (Lang et al., 2019; Mori et al., 2010).
The mice were randomly divided into the control and exercise groups. Our previous study had demonstrated that voluntary wheel‐running was more effective in reducing body weight and fat content in mice, as compared to the treadmill exercise (Kim et al., 2020). Therefore, we chose to conduct voluntary wheel‐running as the mode of aerobic exercise. The exercise group performed this activity every day for 4 weeks. The daily running distance was recorded using an activity wheel‐running counter machine (STARR Life Science, PA, USA). Additionally, the body weights of all the mice were measured once a week, and the food intake was measured 4 days/week. After the 4 weeks of training, all mice were kept sedentary for 4 h before being killed, to abolish the last bout effect.
Body composition
Fat mass and lean mass of the mice were recorded using nuclear magnetic resonance methods with Minispec LF‐50 (Bruker, Germany).
Indirect calorimetry
For measurements of respiratory gas exchange and energy expenditure, sedentary and trained mice were housed individually in cages, with free access to food and water under a 12/12 h light/dark cycle. Mice were monitored using PhenoMaster 7.5.6 (TSE system, Germany) for 3 days.
The C2C12 cell cultures
The C2C12 mouse myoblast cells were obtained from the American Type Culture Collection (ATCC, USA). These cells were seeded at a density of 2.5 × 105 cells/well in a 6‐well plate with Dulbecco's modified Eagle's medium (DMEM; Welgene, Korea) containing 10% fetal bovine serum (FBS; Gibco, USA) and 1% penicillin‐streptomycin (P/S; Welgene). When the cells reached 90% confluence, the maintenance medium was replaced with the differentiation medium (DMEM containing 2% horse serum (#H1270, Sigma, USA) and 1% P/S); subsequently, this medium was changed every 48 h for 4 days. After the cells were completely differentiated, the C2C12 myotubes were treated with 1 mm AICAR (#A9978, Sigma) for 24 h. Finally, the supernatant was harvested from the AICAR‐treated cells using CM to obtain the brown adipocytes.
Transfection of siRNA
Fully differentiated C2C12 myotubes were transfected with negative control siRNA or siRNA targeting AMPKα1 (30 nm) and AMPKα2 (30 nm) using the Lipofectamine RNAiMAX reagent (#13778075, Thermo Scientific, USA). The siRNA sequences are presented in Table 1.
Table 1.
Primer sequences for real‐time quantitative polymerase chain reaction (qPCR)
| Gene | Forward (5’→3’) | Reverse (5’→3’) |
|---|---|---|
| Myh2 | GCGACTTGAAGTTAGCCCAGGA | CTCGTCCTCAATCTTGCTCTGC |
| Myh7 | GCTGGAAGATGAGTGCTCAGAG | TCCAAACCAGCCATCTCCTCTG |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT |
| Ucp1 | ACTGCCACACCTCCAGTCATT | CTTTGGCTCACTCAGGATTGG |
| Pgc‐1α | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC |
|
Prkaa1 siRNA (AMPKα1) |
CUGAAAGAGUACCGUUCUU | AAGAACGGUACUCUUUCA |
|
Prkaa2 siRNA (AMPKα1) |
CUGUGAAUUCGCUGUAGAU | AUCUACAGCGAAUUCACAG |
Treatment of IL‐6 neutralizing antibody
Fully differentiated C2C12 myotubes were maintained in differentiation media with IL‐6 neutralizing antibody (1 μg/ml; MAB‐406, R&D systems, USA) for 24 h. After 24 h, the myotubes were treated with a combination of IL‐6 neutralizing antibody and AICAR (1 mM).
Immortalized brown pre‐adipocyte cell culture
Immortalized brown pre‐adipocytes (iBPA) (Ohno et al., 2013; Uldry et al., 2006) were obtained from Korea Research Institute of Bioscience and Biotechnology (KRIBB). These cells were seeded at a density of 5 × 104 cells/well in a 12‐well plate with DMEM (Gibco) supplemented with 10% FBS (Gibco). When the cells reached 90–95% confluence, the maintenance medium was replaced with the induction medium, which comprised DMEM with 10% FBS, 0.5 mm isobutylmethylxanthine (IBMX; #I7018, Sigma), 125 μm indomethacin, 0.5 μm dexamethasone (#R2408, Sigma), 1 nm T3, and 20 nm insulin (#sc‐360 248, Santa Cruz). After 2 days of induction, the medium was changed to the insulin medium, that is, DMEM with 10% FBS, 20 nm insulin and 1 nm T3, and this was subsequently changed every 48 h for 4 days. After the cells had completely differentiated to brown adipocytes, the 500 μl of CM from the C2C12 cell culture was added to each well.
Histology
Inguinal white adipose tissue (iWAT) and BAT were fixed with 4% paraformaldehyde (PFA; #HP2031, Biosesang, Korea), and 3 μm thin paraffin‐embedded sections were created. Haematoxylin and eosin (H&E) staining was performed following the standard procedures. For immunohistochemical analysis, the iWAT and BAT specimens were incubated with anti‐UCP1 (#ab10987, Abcam, UK; 1:400) overnight at 4°C. All slides were stained using a horseradish peroxidase/3,3′‐diaminobenzidine (HRP/DAB) detection immunohistochemistry (IHC) kit (#ab64261, Abcam). All slides were analysed using a Pannoramic Scanner (3DHISTECH, Hungary).
Fluorescence microscopy analysis
The cultured and treated brown adipocytes were fixed with 4% PFA for 10 min, permeabilized, and blocked with PBS containing 0.2% Triton X‐100 (0.2% PBST) and 1% bovine serum albumin for 15 min. The cells were incubated with anti‐UCP1 (#ab10987, Abcam; 1:400) overnight at 4°C. Subsequently, the cells were incubated with alexa‐488 conjugated goat anti‐rabbit immunoglobulin G (IgG) secondary antibody (Invitrogen) for 30 min at room temperature. Finally, the secondary antibody‐treated cells were stained with mounting media containing 4′,6‐diamidino‐2‐phenylinodole (DAPI; #D9542, Sigma) and analysed using a confocal microscope (Carl Zeiss, Germany).
Western blot
Protein concentrations were measured using a bicinchoninic acid (BCA) protein assay kit (#23227, Thermo Scientific, USA). Equal amounts of proteins were loaded, subjected to 10% SDS‐PAGE, and transferred to polyvinylidene fluoride membranes. Thereafter, the membranes were incubated overnight at 4°C with the following primary antibodies: anti‐UCP1 (#ab10987, Abcam; 1:2000), anti‐PGC‐1α (#ab54481, Abcam; 1:1000), anti‐α‐tubulin (#A01080, Abbkine; 1:5000), anti‐α‐actin (#A2066, Sigma; 1:5000), anti‐succinate dehydrogenase complex, subunit A (anti‐SDHA; #5839, Cell Signalling, USA; 1:2000), anti‐p‐AMPK (Thr172) (#5256, Cell Signalling; 1:1000) and anti‐AMPK (#2532, Cell Signalling; 1:1000). Finally, the membranes were probed with appropriate secondary antibodies (#PI‐1000‐1, Vector Laboratories, USA) for 2 h at room temperature. The target proteins were detected with enhanced chemiluminescence reagents (#170‐5061, Bio‐Rad, USA) and analysed with Chemi‐Doc XRS+ System (Bio‐Rad). The protein levels were normalized against α‐tubulin expression levels using Image J software (NIH, USA).
Quantitative real‐time polymerase chain reaction (qPCR)
The reverse transcribed cDNA was subjected to real‐time qPCR using the Sensi‐Fast SYBR Green Hi‐ROX Kit (#BIO‐92005, Meridian Bioscience, USA), according to the manufacturer's instructions. The mRNA expression levels of the target genes were normalized to the expression level of 36B4. The primer sequences used for this PCR analysis are presented in Table 1.
The 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide (MTT) assay
Cell viability was measured using the MTT assay (#M5655, Sigma). After treating the cells with serum or CM, the medium was removed and the cells were washed twice with Dulbecco's PBS. Thereafter, 200 μl of MTT solution (5 mg/ml) was added to each well and incubated for 3 h. The absorbance was measured at 595 nm using a microplate reader.
Cytokine assay
Serum from mice or CM from C2C12 adipokine and cytokine (#MADKMAG‐71K, Millipore, USA) levels were analysed using the MAGPIX assay kit (Luminex, USA). All experiments were performed according to the manufacturer's protocol.
Statistical analysis
Data are presented as means ± SD. Differences between the two groups were analysed using a two‐tailed t test. Prism 7.00 was used for all statistical analyses. Statistical significance was set at P < 0.05. *P < 0.05, **P < 0.01 and ***P < 0.001.
Results
Effects of 4 weeks of exercise training on fat mass and weight gain in C57BL/6 mice
The experimental design is shown in Fig. 1A . The daily running distance recorded over a period of 4 weeks is 8–10 km (Fig. 1B ). As shown in Fig. 1C , the daily cumulative food consumption is significantly higher in the exercise group of mice, as compared with that in the control group. Incidentally, despite the intake of a high amount of food for 4 weeks, the voluntary wheel‐running exercise markedly lowers the body weight of the exercise group (Fig. 1D ). Moreover, these exercise‐trained mice have a significantly lower fat mass, fat mass/body weight ratio (Fig. 1E ) and a higher lean mass/body weight ratio (Fig. 1F ), as compared with those of the control mice. Interestingly, the multiplex adipokine assay results are significantly correlated with the phenotype data. Particularly, the serum insulin and leptin levels are significantly lower in the serum of the exercise group of mice than those in the serum of the control mice (Fig. 1G ). These results demonstrate that 4 weeks of voluntary wheel‐running exercise training is effective enough for weight loss and modification of the serum insulin and leptin levels.
Figure 1. Voluntary wheel‐running exercise training improves metabolic phenotype.
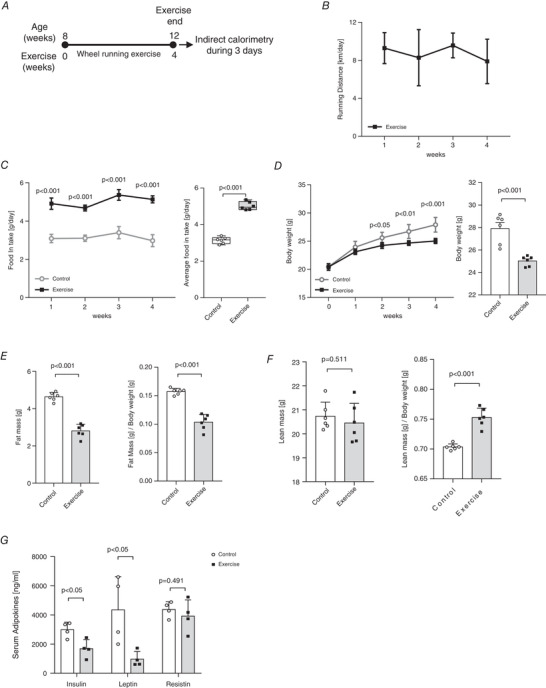
A, scheme of 4 weeks of voluntary wheel‐running exercise training. B, daily running distance (km) during exercise training, n = 6 for exercise group. C, daily food intake (g) during exercise training and average food intake (g/day) during experiment period, n = 6 for all groups. D, weekly body weight (0–4 weeks) and body weight after exercise, n = 6 for all groups. E, F, body composition analysis, n = 6 for all groups. E, fat mass (g) and fat mass (g)/body weight (g). F, lean mass (g) and lean mass (g)/body weight (g). G, serum adipokine levels (ng/ml) after exercise, n = 4 for all groups. Data are represented as means ± SD. Differences between two groups were analysed using a two‐tailed t test.
Exercise training increases the thermogenic capacity of white and brown adipose tissue
To investigate whether exercise induces weight loss through the activation of browning capacity of adipose tissues, we compared UCP1 expression and the browning marker gene levels in iWAT and BAT after exercise training. The H&E staining of adipose tissue sections demonstrate that the size of adipocytes in the iWAT and BAT is noticeably smaller in the exercise‐trained mice, as compared with that in sedentary mice (Fig. 2A ). Furthermore, exercise training not only activates BAT, but also stimulates iWAT to undergo a change to resemble the BAT‐like phenotype. We also performed immunohistochemical staining of iWAT and BAT with anti‐UCP1 antibody. As shown in Fig. 2B , both iWAT and BAT of the exercise‐trained mice exhibit a highly positive stain for UCP1. Similarly, western blot analysis also shows that the UCP1 protein expression level is significantly high (P < 0.05) in both iWAT and BAT of the exercise group of mice. Additionally, even though the PGC‐1α protein level is significantly increased in the BAT of the exercise group, there is no statistically significant difference in its levels between the iWATs of the exercise and control groups (P = 0.079) (Fig. 2C ). Based on the occurrence of this phenotype, we also investigated the Ucp1 gene expression levels in iWAT and BAT of the mice. Incidentally, the exercise‐trained mice show a significant increase (P < 0.05) in the level of Ucp1 mRNA in their iWAT as well as BAT (Fig. 2D ).
Figure 2. Voluntary wheel‐running exercise training induces browning of inguinal white adipose tissue (iWAT) and activation of brown adipose tissue (BAT).
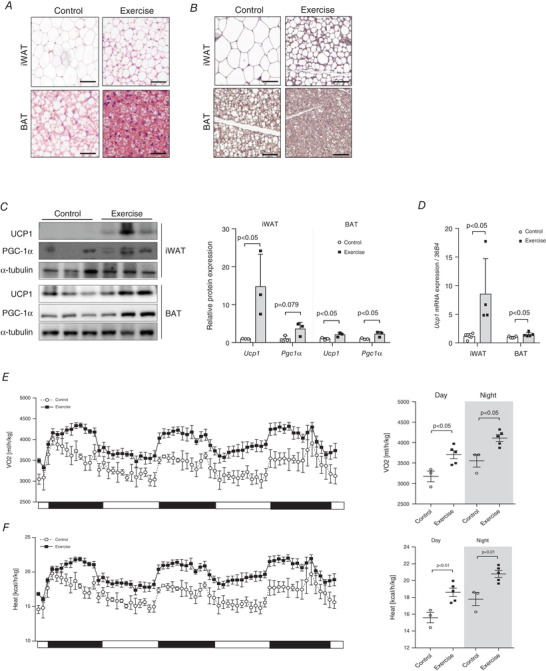
A, haematoxylin and eosin (H&E) staining of iWAT and BAT tissues, n = 3 for all groups. B, immunohistochemical staining of iWAT and BAT for detection of uncoupled protein‐1 (UCP1) levels, n = 3 for all groups (scale bar = 50 μm). C, UCP1 and peroxisome proliferator‐activated receptor‐gamma coactivator‐1 alpha (PGC‐1α) protein expressions in iWAT and BAT, n = 3 for all groups (scale bar = 100 μm); quantification of protein expression, n = 3 for all groups. D, Ucp1 gene expression in iWAT and BAT, control (CON) = 5, exercise (EX) = 4. The expression levels were normalized to the expression of 36B4 gene. E, whole‐body oxygen consumption rate (VO2) (ml/h/kg) were monitored in mice, CON = 3, EX = 5. F, whole‐body heat generation (kcal/h/kg) was monitored in mice, CON = 3, EX = 5. Data are represented as means ± SD. Differences between two groups were analysed using a two‐tailed t test. [Colour figure can be viewed at wileyonlinelibrary.com]
We then evaluated the physiological mechanism underlying the exercise‐induced thermogenic capacity using indirect calorimetry. VO2 (Day, P < 0.05; Night, P < 0.05) and heat generation (Day, P < 0.01; Night, P < 0.01) were significantly increased in the exercise‐trained mice compared with the untrained mice for 3 days from the end of the exercise (Fig. 2E and F ).
Activation of AMPK in skeletal muscles following voluntary wheel‐running exercise
In response to the wheel‐running exercise, there is a significant effect (P < 0.001) on AMPK phosphorylation in the soleus muscle of the mice (Fig. 3A ). Furthermore, the 4 weeks of exercise training led to the increased expression of slow fibre marker myosin heavy chain 7 (Myh7) and intermediate fibre marker Myh2 in the transverse abdominal (TA) (Myh7 and Myh2: P < 0.05), gastrocnemius (Myh7: P < 0.05 and Myh2: P < 0.01) and plantaris (Myh7: P = 0.452 and Myh2: P < 0.001) muscles. However, there is no statistically significant difference in the expression of these marker genes in the soleus muscle of the two groups (Myh7: P = 0.225, Myh2: P = 0.665) (Fig. 3B ).
Figure 3. Exercise‐induced activation of AMP‐activated protein kinase (AMPK) in skeletal muscle.
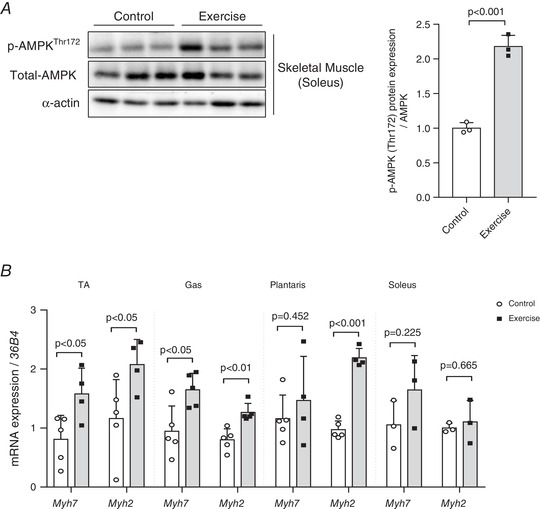
A, exercise increases AMPK phosphorylation in soleus muscle, n = 3 for all groups. B, four weeks of exercise training increases myosin heavy chain 7 (Myh7) and Myh2 gene expression in the hindlimb muscles (transverse abdominal muscle (TA): control = 5, exercise = 4; gastrocnemius (Gas): control = 5, exercise = 5; plantaris: control = 5, exercise = 4; soleus: control = 3, exercise = 3). The expression of target genes was normalized to the expression of 36B4 gene. Data are represented as means ± SD. Differences between two groups were analysed using a two‐tailed t test.
Effect of incubating differentiated brown adipocytes with serum extracted from exercise‐trained mice
The experimental design of incubating differentiated brown adipocytes with serum extracted from exercise‐trained mice is shown in Fig. 4A . As shown in Fig. 4B , post‐incubation with the mouse serum, the differentiated brown adipocytes did not exhibit any significant concentration‐dependent difference in cell viability. Subsequently, we examined the UCP1 protein level in these brown adipocytes to determine whether they undergo activation upon incubation with serum containing exercise‐induced factors. As expected, the UCP1 protein level is robustly increased in the cells treated with serum extracted from exercise‐trained mice (Fig. 4C ). Furthermore, western blot analysis showed that UCP1 (P < 0.05) protein levels were significantly elevated in the adipocytes treated with serum (1% and 5%, respectively) obtained from the exercise mice group, compared with that in the adipocytes treated with serum obtained from the control mice group (Fig. 4D ). However, SDHA protein levels were significantly elevated only in the cells treated with 5% serum (P < 0.05). Additionally, Ucp1 mRNA expression is significantly increased (P < 0.01) in the adipocytes treated with the serum of exercise‐trained mice in a concentration‐dependent manner (Fig. 4E ).
Figure 4. Exercise‐induced circulating factors in the serum of exercise‐trained mice can activate brown adipocytes by modulating uncoupled protein 1 (UCP1) expression.
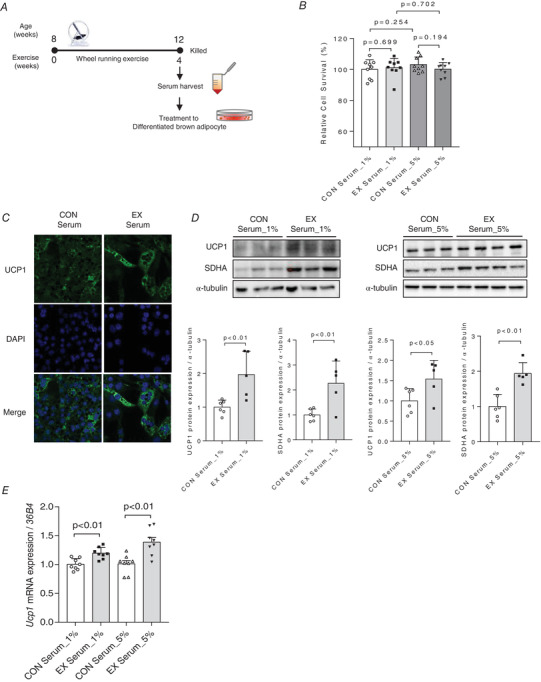
A, scheme of conditioned medium (CM) harvest and transfer experiment. B, relative cell survival (%) of differentiated brown adipocytes treated with 1% and 5% serum obtained from exercise‐trained mice assessed by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide (MTT) assays, n = 9 for all groups. C, immunofluorescence staining of differentiated brown adipocytes treated with 5% serum for UCP1 detection, n = 3 for all groups. D, UCP1 protein expression was elevated in cells treated with 1% and 5% serum obtained from exercise‐trained mice. Succinate dehydrogenase complex, subunit A (SDHA) protein expression was elevated in cells treated with 5% serum obtained from exercise‐trained mice; bands were quantified, control (CON) serum: n = 6; exercise (EX) serum: n = 5. E, Ucp1 mRNA expression in differentiated brown adipocytes treated with 1% and 5% serum obtained from exercise‐trained mice, n = 8 for all groups. The expression of target genes was normalized to the expression of 36B4 gene. Data are represented as means ± SD. Differences between two groups were analysed using a two‐tailed t test. Differences between the groups were analysed using one‐way ANOVA, post hoc Tukey's test. P < 0.05 was considered statistically significant. [Colour figure can be viewed at wileyonlinelibrary.com]
Effect of AICAR treatment on C2C12 myotubes
Fully differentiated C2C12 myotubes were treated with AICAR to induce effects similar to aerobic exercise, thereby leading to the presence of the exercise‐induced secretome in the CM. We determined whether the C2C12 myoblasts had completely differentiated into myotubes with skeletal muscle properties by checking the Myh7 (D0 vs. D2, D4 and D6; P < 0.001) and Myh2 (D0 vs. D2, D4 and D6; P < 0.001) mRNA levels (Fig. 5B and C ). To evaluate the effect of AICAR treatment on myotubes, we investigated the extent of AMPK phosphorylation (P < 0.01) and confirmed its significant increase post‐AICAR treatment (Fig. 5D ). Furthermore, AICAR treatment increases Myh7 (P < 0.05) and Myh2 (P < 0.05) mRNA expressions in the C2C12 myotubes (Fig. 5E ).
Figure 5. Administration of 5‐aminoimidazole‐4‐carboxamide ribonucleotide (AICAR) increases activation of AMP‐activated protein kinase (AMPK) in C2C12 myotubes.
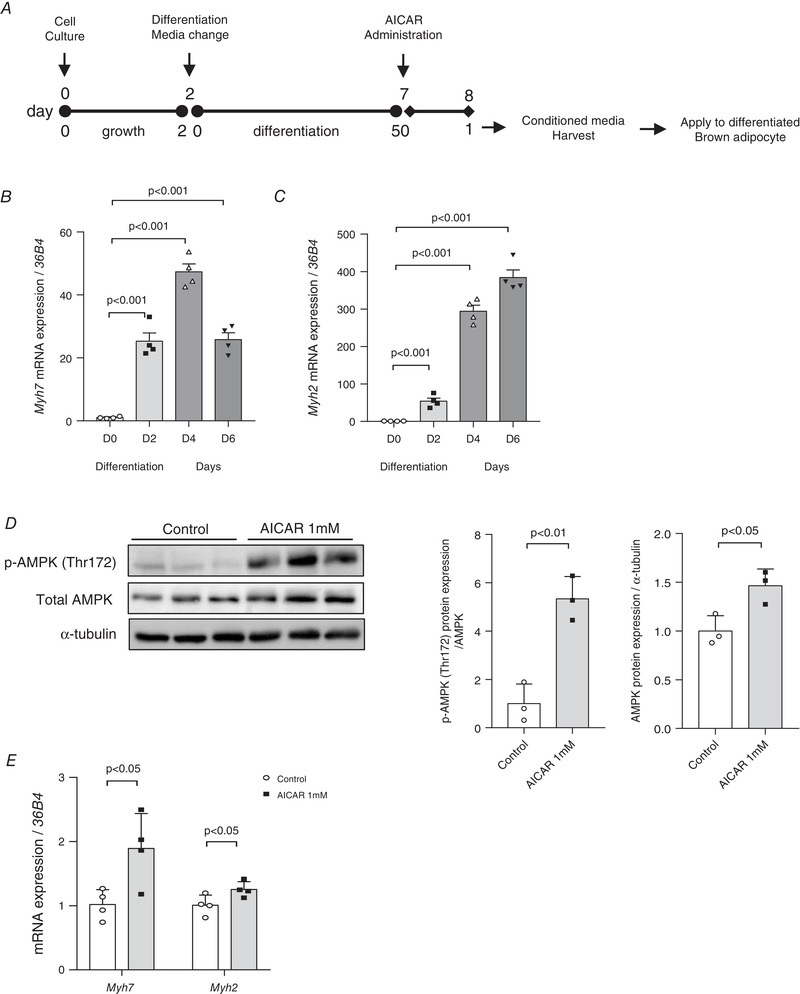
A, scheme of cell culture and conditioned medium (CM) transfer experiments. B, myosin heavy chain 7 (Myh7) mRNA expression levels during differentiation of C2C12 cells, n = 4 for all groups. C, Myh2 mRNA expression levels during differentiation of C2C12 cells, n = 4 for all groups. D, phosphorylated‐AMPK (p‐AMPK) (Thr172) and total AMPK protein levels in differentiated C2C12 myotubes treated with 1 mm AICAR. Protein expressions were quantified, n = 3 for all groups. E, Myh7 and Myh2 gene levels post‐AICAR treatment, n = 4 for all groups. Data are represented as means ± SD. Differences between the two groups were analysed using a two‐tailed t test. To compare more than two groups, one‐way ANOVA, Tukey's multiple comparisons test and Dunnett's test were used. P < 0.05 was considered statistically significant.
Effect of AICAR treatment‐induced secretome derived from C2C12 myotubes on activation of differentiated brown adipocytes
We attempted to verify whether the differentiated brown adipocytes could be activated by the secretome of the C2C12 myotubes released upon induction by mimetics of aerobic exercise. The experimental design of the C2C12 cell CM transfer into the differentiated brown adipocyte culture plates is shown in Fig. 5A . After incubating the brown adipocytes with the CM of the AICAR‐treated C2C12 myotubes, we determined its effects on the activity of differentiated brown adipocytes. Firstly, there is no significant toxicity in cell viability of the CM‐transferred differentiated brown adipocytes (Fig. 6A ). Moreover, the Ucp1 mRNA level (P < 0.01) and the expressions of other thermogenic marker genes (Pgc‐1α, P < 0.01 and Cidea, P < 0.05) are significantly increased in the CM‐treated cells (Fig. 6B ). Western blot analysis shows that incubation of differentiated brown adipocytes for 24 h with AICAR‐treated CM significantly increases UCP1 (P < 0.01) and SDHA (P < 0.05) protein expressions, as compared with that in the control CM (Fig. 6C ).
Figure 6. Conditioned medium (CM) of the 5‐aminoimidazole‐4‐carboxamide ribonucleotide (AICAR)‐treated C2C12 cells activates brown adipocytes by modulating uncoupled protein 1 (UCP1) expression.
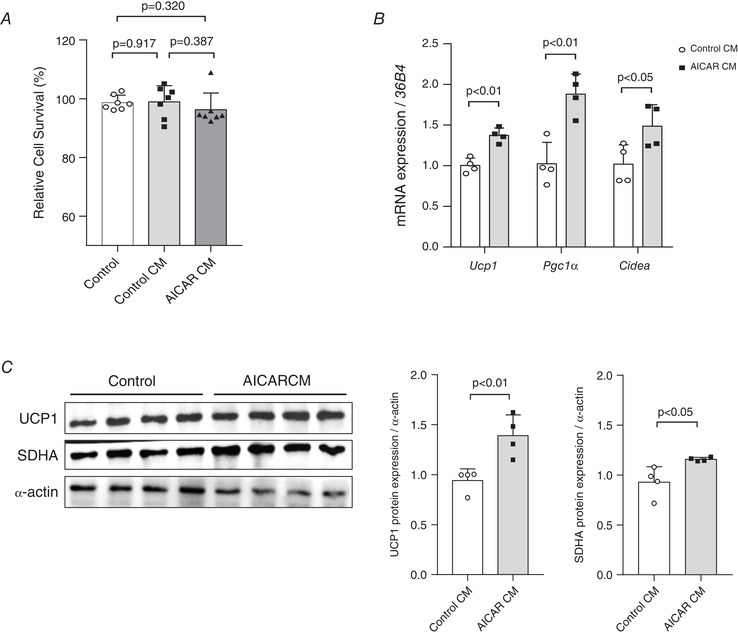
A, relative cell survival (%) of differentiated brown adipocytes incubated with CM of AICAR‐treated, differentiated C2C12 cells determined by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide (MTT) assays, n = 7 for all groups. B, brown adipose tissue (BAT) activation‐associated marker gene expression in differentiated brown adipocytes incubated with CM, n = 4 for all groups. The expression of target genes was normalized to the expression of the 36B4 gene. C, UCP1 and succinate dehydrogenase complex, subunit A (SDHA) protein levels in differentiated brown adipocytes incubated with CM of the AICAR‐treated differentiated C2C12 cells; protein expression levels were quantified, n = 4 for all groups. Data are represented as means ± SD. Differences between the two groups were analysed using a two‐tailed t test. For a comparison of more than two groups, the one‐way ANOVA and Tukey's multiple comparisons tests were used.
Effect of AICAR treatment‐induced AMPK activation on UCP1 expression in differentiated brown adipocytes
To investigate the AMPK‐dependent expression of UCP1 in brown adipocytes, AMPKα1 and AMPKα2 were knocked down in C2C12 myotubes and then treated with AICAR (Fig. 7A ). There was no significant toxicity, as observed from the cell viability of the AMPKα1 and AMPKα2 knock‐down C2C12 myotubes (Fig. 7B ). The expression of AMPK protein was suppressed in C2C12 myotubes transfected with target siRNA compared with that of non‐target siRNA (P < 0.01) (Fig. 7C ). Furthermore, phosphorylation and expression of AMPK levels were significantly abolished despite the AICAR (Fig. 7D ).
Figure 7. Conditioned medium (CM) of the AICAR‐treated AMPK knock‐down C2C12 cells failed to modulate UCP1 expression in brown adipocytes.
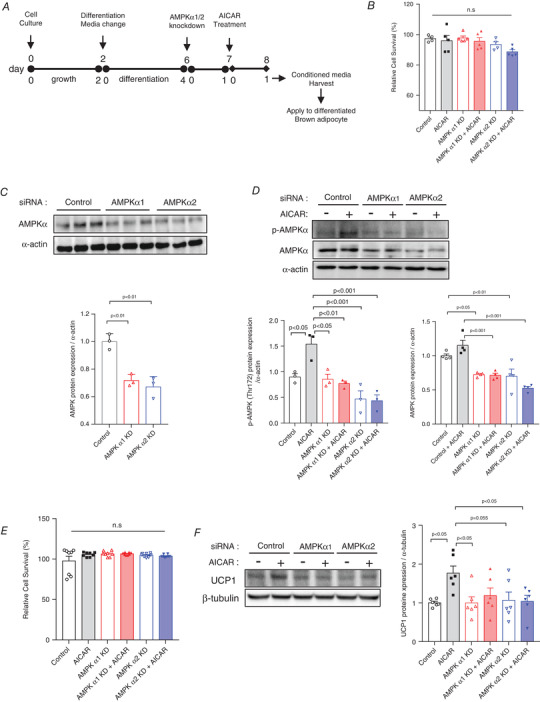
A, scheme of cell culture, siRNA treatment, AICAR treatment, and CM transfer experiments. B, relative cell survival (%) of differentiated C2C12 myotubes treated with 30 nm of AMPKα1 and AMPKα2 siRNA with or without AICAR, n = 4−5. C, AMPK protein levels in differentiated C2C12 myotubes treated with 30 nm of AMPKα1 and AMPKα2 siRNA. Protein expressions were quantified, n = 3 for all groups. D, AMPK phosphorylation and expression levels with and without AICAR treatment in differentiated C2C12 myotubes treated with 30 nm of AMPKα1 and AMPKα2 siRNA. Protein expressions were quantified, n = 3−4 for all groups. E, relative cell survival (%) of differentiated brown adipocytes incubated with CM of AMPK knock‐down with AICAR‐treated, differentiated C2C12 cells determined, n = 8 for all groups. F, UCP1 protein levels in differentiated brown adipocytes incubated with CM of the AICAR‐treated AMPK knock‐down C2C12 myotubes. Protein expression levels were quantified, n = 6 for all groups. Data are represented as means ± SD. Differences between the groups were analysed using one‐way ANOVA and post hoc Tukey's multiple comparisons test. P < 0.05 was considered statistically significant. [Colour figure can be viewed at wileyonlinelibrary.com]
After incubating the brown adipocytes with the CM of the AICAR‐treated AMPKα1 and α2 knock‐down C2C12 myotubes, we determined CM effects on the activity of differentiated brown adipocytes. Firstly, there was no significant toxicity, as observed from the cell viability of the CM‐transferred differentiated brown adipocytes (Fig. 7E ). Western blot analysis showed that the incubation of differentiated brown adipocytes for 24 h with AICAR‐treated CM significantly increased UCP1 protein expression (P < 0.05), compared with that in the control CM. However, the expression of UCP1 protein was significantly suppressed in AMPK α1 and α2 knock‐down brown adipocytes despite the AICAR treatment (Fig. 7F ).
AICAR treatment induces changes in cytokine levels released by C2C12 myotubes
AICAR‐treated C2C12 myotubes exhibit a significantly high release of interleukin 6 (IL‐6; P < 0.001) (Fig. 8A ), whereas the release of monocyte chemoattractant protein‐1 (MCP‐1) is significantly low (P < 0.01) (Fig. 8B ).
Figure 8. AICAR‐induced IL‐6 secreted conditioned medium (CM) increases the expression of UCP1 in brown adipocytes.
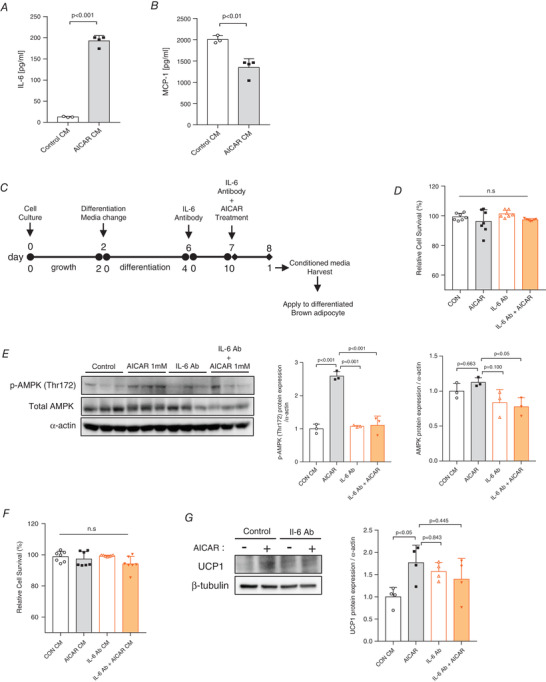
A, serum interleukin‐6 (IL‐6) concentration, n = 4 for all groups. B, serum monocyte chemoattractant protein 1 (MCP‐1) concentration, n = 4 for all groups. C, scheme of cell culture, IL‐6 neutralizing antibody (IL‐6 Ab) treatment, AICAR treatment and CM transfer experiments. D, relative cell survival (%) of differentiated C2C12 myotubes treated with IL‐6 Ab with or without AICAR, n = 7. E, phosphorylation and expression of AMPK protein levels in differentiated C2C12 myotubes treated with 1 μg/ml of IL‐6 Ab with or without AICAR. Protein expressions were quantified, n = 3 for all groups. F, relative cell survival (%) of differentiated brown adipocytes incubated with the CM of IL‐6 Ab with or without AICAR, n = 7 for all groups. G, UCP1 protein levels in differentiated brown adipocytes incubated with the CM of IL‐6 neutralized with or without AICAR from C2C12 myotubes. Protein expression levels were quantified, n = 4 for all groups. Data are expressed as means ± SD. Differences between the groups were analysed using one‐way ANOVA and post hoc Tukey's test. P < 0.05 was considered statistically significant. [Colour figure can be viewed at wileyonlinelibrary.com]
Next, we investigated the myotubes treated with the combination of IL‐6 neutralizing antibody and AICAR (Fig. 8C ). IL‐6 neutralizing antibody (1 μg) effectively blocked IL‐6 release in C2C12 myotubes without affecting cell viability (Fig. 8D ). Phosphorylation and expression of UCP1 were significantly abolished by IL‐6 Ab despite the treatment with AICAR (Fig. 8E ). IL‐6 Ab‐treated CM did not abolish AICAR‐induced UCP1 expression in differentiated brown adipocytes without affecting cell viability (Fig. 8F and G ).
Discussion
The current study examined the role of exercise‐induced secretory factors in stimulating the expression of UCP1 in brown adipocytes. We observed that the serum extracted from exercise‐trained mice could induce an increased expression of the UCP1 gene and protein in brown adipocytes. Furthermore, SDHA protein levels were also significantly increased. These results suggest that exercise‐induced serum factors are potent regulators that positively modulate mitochondrial function and UCP1 expression in BAT.
The UCP1 protein is generally considered as a potent regulator of whole‐body energy metabolism in both rodents and humans because it is abundantly present in the mitochondria, and it regulates heat generation (Feldmann et al., 2009; Flouris et al., 2017; Ikeda & Yamada, 2020; Okamatsu‐Ogura et al., 2020). Since the metabolic benefits of browning of adipose tissue are well‐established, there has been an increased focus on the various factors that stimulate UCP1 expression and the browning of the adipocytes. Although UCP1 levels are mainly controlled by cold exposure or β3‐adrenergic receptor activation during activation of BAT and browning of WAT (Chouchani et al., 2019; Feldmann et al., 2009; Ikeda & Yamada, 2020; Okamatsu‐Ogura et al., 2020; Winn et al., 2017), accumulating evidence has revealed that exercise is also a potent stimulator of UCP1 expression in adipose tissues.
Several studies have reported an increase in UCP1 during the exercise‐induced conversion of white to beige adipocytes, especially in iWAT (Knudsen et al., 2014; Lehnig & Stanford, 2018; Ringholm et al., 2013; Shirkhani et al., 2018; Stanford et al., 2015; Xiong et al., 2019). However, there are conflicting reports regarding the effectiveness of exercise in BAT activation. One study has reported that 6 weeks of swimming training increases BAT mass and the amount of UCP1 protein in BAT (Ohishi et al., 1996). Another study has demonstrated that 8 weeks of swimming exercise increases mitochondrial activity and iodothyronine deiodinase 2 (Dio2) enzyme activity in rats (Ignacio et al., 2012). In contrast, recent studies have indicated a decrease in BAT activity in response to exercise in both rodents and humans. For instance, 8 weeks of treadmill exercise decreased UCP1 expression in the BAT of rats (Wu et al., 2014). Furthermore, a human study showed that endurance exercise training decreases cold‐induced BAT activation and supraclavicular BAT mass in young male athletes (Vosselman et al., 2015). These contradictory results emphasise the necessity of studying the effects of exercise training on BAT metabolism. Therefore, our experimental design is an extremely important and unique method for investigating whether the exercise‐induced circulating factors closely affect the BAT activation. Although this study did not identify the specific, exercise‐induced, circulatory factors, our results demonstrate that 4 weeks of exercise training can increase activation of BAT; moreover, when the serum extracted from these exercise‐trained mice was added to fully differentiated brown adipocytes, there was an increase in the UCP1 expression. We believe that these observations will form an important clue in the discussions regarding the conflicting role of exercise in activating BAT.
Many studies have explored the nature of UCP1 expression during aerobic exercise due to the high rate of oxygen consumption and free fatty acid mobilization during such exercises (Gollisch et al., 2009; Joyner & Green, 2009). Aerobic exercise stimulates a multi‐step signal transduction that occurs through AMPK activation; in fact, AMPK has been identified as a sensor for intracellular energy stress in skeletal muscles, liver and adipose tissues (Jakobsen et al., 2001; Musi & Goodyear, 2003; van der Vaart et al., 2021). Therefore, we hypothesized that factors released upon AMPK activation in the skeletal muscles may induce the expression of UCP1 in brown adipocytes. To mimic the effects of aerobic exercise on skeletal muscle cells, we treated fully differentiated C2C12 myotubes with AICAR. Thereafter, the CM of the AICAR‐treated C2C12 myotubes was collected, and the brown adipocytes were incubated with it. Surprisingly, AICAR‐induced activated AMPK significantly increased the UCP1 expression. These results show that aerobic exercise‐induced AMPK has a significant effect on UCP1 expression in BAT.
Finally, we measured the cytokine levels in the CM of AICAR‐treated C2C12 myotubes to determine what led to these changes. Incidentally, the AICAR‐treated C2C12 myotubes released a significantly high amount of IL‐6 and a significantly low amount of MCP‐1. These results are consistent with those of previous studies, which report an increase in IL‐6 and a decrease in MCP‐1 during exercise (Diniz et al., 2019; Troseid et al., 2004). Moreover, previous studies have also reported that an increase in IL‐6 promotes UCP1 expression, and a decrease in MCP‐1 promotes browning of adipocytes (Laurens et al., 2020; Rajasekaran et al., 2019; Severinsen & Pedersen, 2020). Similarly, our observations suggest that activation of AMPK in skeletal muscle induces activation of brown adipocytes by promoting IL‐6 release and inhibiting MCP‐1 release. However, since several factors are associated with AICAR‐treated CM, reasoning that the increase in IL‐6 or the decrease in MCP1 had a significant effect on the expression of UCP1 would be far‐fetched and could indicate a limitation. In addition, the expression of UCP1 protein was not suppressed upon treatment with IL‐6‐neutralized CM. Presumably, other factors contributed to the expression of UCP1. However, the inability to identify the specific factors involved is a limitation of our study.
Because the molecular experiments required to understand obesity and metabolic syndrome to the degree required for discover cannot be easily performed in human subjects, translation from animals to humans is necessary. Although basic metabolic and physiological mechanisms are well conserved between mice and humans, there are still barriers to translating animal studies to humans. According to previous studies, increasing the housing temperature of mice from room temperature to 27−30°C (thermoneutrality) is amenable for translation between humans and mice (Keijer et al., 2019; Raun et al., 2020; Seeley & MacDougald, 2021). However, a recent study has suggested that studying mice at thermoneutrality is not conducive, because the thermal biology of mice is fundamentally different from that of humans and 30°C conditions could be activating the immune system (Skop et al., 2020). In addition, several groups have recently reported that the voluntary wheel‐running distance of mice is significantly reduced in thermoneutral conditions compared with room temperature (Raun et al., 2020; Takahashi et al., 2019). These findings signify limitations as to whether the exercise effect can be adequately explained when the mouse exercised in specific conditions. This is also a limitation of this study.
Fortunately, however, there have been well‐documented studies that have reported similarity in BAT functions between humans and mice. The studies have directly quantified UCP1 function in human BAT (Porter et al., 2016) and suggested that human BAT has a respiratory capacity greater than that of WAT and UCP1 function is similar in human and rodent BAT. Therefore, our findings could be expected to yield similar results when applied to humans.
In conclusion, the present data suggest that exercise can cause UCP1 activation in brown adipocytes, and this might help to resolve the previous conflicting reports. Although further studies are required to determine the details of the underlying molecular signalling pathways that contribute to the activation of BAT, the results of our study may provide a better understanding of exercise‐induced UCP1 expression in differentiated brown adipocytes.
Additional information
Competing interests
The authors have no conflicting interests.
Author contributions
H.J.K. and Y.J.K. designed the research, performed the experiments, analysed the data and wrote the manuscript under the supervision of J.K.S. All authors contributed to the discussion and revision of the manuscript.
Funding
This study was supported by the Korea Mouse Phenotyping Project (NRF‐2013M3A9D5072550) of the Ministry of Science and ICT, through the National Research Foundation. The work was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF‐2019R1I1A1A01061357).
Supporting information
Statistical Summary Document
Peer Review History
Biographies
Hye Jin Kim is a Research Professor at the Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul, working with Professor Dr Je Kyung Seong. She received her PhD and MS from Ewha Womans University, where she investigated the functional mechanisms of exercise, skeletal muscle and glucose homeostasis. Her area of research is obesity and metabolism with a specific focus on exercise physiology.

Youn Ju Kim is a PhD student at the Laboratory of Developmental Biology and Genomics, College of Veterinary Medicine, Seoul National University, Seoul, working with Professor Dr Je Kyung Seong. Her current work is focused on understanding the mechanisms underlying genes related to obesity and metabolism with a specific focus on the browning of adipose tissue in a mouse model.

Edited by: Michael Hogan & Bettina Mittendorfer
Linked articles: This article is highlighted in a Perspective article by Schönke & Gabriel. To read this article, visit https://doi.org/10.1113/JP283087.
The peer review history is available in the Supporting information section of this article (https://doi.org/10.1113/JP282999#support‐information‐section).
H. J. Kim and Y. J. Kim contributed equally to this work.
Data availability statement
All data supporting presented results are available from the corresponding author upon reasonable request.
References
- Castano, C. , Mirasierra, M. , Vallejo, M. , Brugnara, L. , Murillo, S. , Novials, A. , & Parrizas, M. (2019). Exosomes as mediators of the systemic adaptations to high intensity interval training. Diabetologia, 62, S110‐S110. [Google Scholar]
- Chouchani, E. T. , Kazak, L. , & Spiegelman, B. M. (2019). New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metabolism, 29, 27–37. [DOI] [PubMed] [Google Scholar]
- De Matteis, R. , Lucertini, F. , Guescini, M. , Polidori, E. , Zeppa, S. , Stocchi, V. , Cinti, S. , & Cuppini, R. (2013). Exercise as a new physiological stimulus for brown adipose tissue activity. Nutrition, Metabolism, and Cardiovascular, 23, 582–590. [DOI] [PubMed] [Google Scholar]
- Diniz, T. A. , Aquino, Jr J. C. J. , Mosele, F. C. , Cabral‐Santos, C. , Lima, Jr E. A. , Teixeira, A. A. S. , Lira, F. S. , & Rosa Neto, J. C. (2019). Exercise‐induced AMPK activation and IL‐6 muscle production are disturbed in adiponectin knockout mice. Cytokine, 119, 71–80. [DOI] [PubMed] [Google Scholar]
- Feldmann, H. M. , Golozoubova, V. , Cannon, B. , & Nedergaard, J. (2009). UCP1 ablation induces obesity and abolishes diet‐induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metabolism, 9, 203–209. [DOI] [PubMed] [Google Scholar]
- Flouris, A. D. , Dinas, P. C. , Valente, A. , Andrade, C. M. B. , Kawashita, N. H. , & Sakellariou, P. (2017). Exercise‐induced effects on UCP1 expression in classical brown adipose tissue: A systematic review. Hormone Molecular Biology and Clinical Investigation, 31 [DOI] [PubMed] [Google Scholar]
- Gollisch, K. S. C. , Brandauer, J. , Jessen, N. , Toyoda, T. , Nayer, A. , Hirshman, M. F. , & Goodyear, L. J. (2009). Effects of exercise training on subcutaneous and visceral adipose tissue in normal‐ and high‐fat diet‐fed rats. American Journal of Physiology – Endocrinology and Metabolism, 297, E495–E504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, C. , & Weigert, C. (2017). Skeletal muscle as an endocrine organ: The role of myokines in exercise adaptations. Cold Spring Harbor Perspectives in Medicine, 7, a029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias, M. A. , Ye, J. M. , Frangioudakis, G. , Saha, A. K. , Tomas, E. , Ruderman, N. B. , Cooney, G. J. , & Kraegen, E. W. (2002). AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin‐resistant high‐fat‐fed rats. Diabetes, 51, 2886–2894. [DOI] [PubMed] [Google Scholar]
- Ignacio, D. L. , Fortunato, R. S. , Neto, R. A. L. , Silvestre, D. H. D. , Nigro, M. , Frankenfeld, T. G. P. , Werneck‐de‐Castro, J. P. S. , & Carvalho, D. P. (2012). Blunted response of pituitary type 1 and brown adipose tissue type 2 deiodinases to swimming training in ovariectomized rats. Hormone and Metabolic Research, 44, 797–803. [DOI] [PubMed] [Google Scholar]
- Ikeda, K. , & Yamada, T. (2020). UCP1 dependent and independent thermogenesis in brown and beige adipocytes. Frontiers in Endocrinology, 11, 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen, S. N. , Hardie, D. G. , Morrice, N. , & Tornqvist, H. E. (2001). 5'‐AMP‐activated protein kinase phosphorylates IRS‐1 on Ser‐789 in mouse C2C12 myotubes in response to 5‐aminoimidazole‐4‐carboxamide riboside. Journal of Biological Chemistry, 276, 46912–46916. [DOI] [PubMed] [Google Scholar]
- Joyner, M. J. , & Green, D. J. (2009). Exercise protects the cardiovascular system: Effects beyond traditional risk factors. The Journal of Physiology, 587, 5551–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijer, J. , Li, M. , & Speakman, J. R. (2019). What is the best housing temperature to translate mouse experiments to humans? Molecular Metabolism, 25, 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. J. , Kim, H. J. , Lee, W. J. , & Seong, J. K. (2020). A comparison of the metabolic effects of treadmill and wheel running exercise in mouse model. Laboratory Animal Research, 36, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen, J. G. , Murholm, M. , Carey, A. L. , Bienso, R. S. , Basse, A. L. , Allen, T. L. , Hidalgo, J. , Kingwell, B. A. , Febbraio, M. A. , Hansen, J. B. , & Pilegaard, H. (2014). Role of IL‐6 in exercise training‐ and cold‐induced UCP1 expression in subcutaneous white adipose tissue. Plos One, 9, e84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, P. , Hasselwander, S. , Li, H. G. , & Xia, N. (2019). Effects of different diets used in diet‐induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Scientific Reports, 9, 19556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens, C. , Bergouignan, A. , & Moro, C. (2020). Exercise‐released myokines in the control of energy metabolism. Frontiers in Physiology, 11, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnig, A. C. , & Stanford, K. I. (2018). Exercise‐induced adaptations to white and brown adipose tissue. Journal of Experimental Biology, 221, jeb161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliulo, L. , Bondi, D. , Pini, N. , Marramiero, L. , & Di Filippo, E. S. (2021). The wonder exerkines‐novel insights: a critical state‐of‐the‐art review. Molecular and Cellular Biochemistry, 477, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, M. A. , Liu, M. , Bezy, O. , Almind, K. , Shapiro, H. , Kasif, S. , & Kahn, C. R. (2010). A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes, 59, 2960–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi, N. , & Goodyear, L. J. (2003). AMP‐activated protein kinase and muscle glucose uptake. Acta Physiologica Scandinavica, 178, 337–345. [DOI] [PubMed] [Google Scholar]
- Ohishi, S. , Kizaki, T. , Toshinai, K. , Haga, S. , Fukuda, K. , Nagata, N. , & Ohno, H. (1996). Swimming training improves brown‐adipose‐tissue activity in young and old mice. Mechanisms of Ageing and Development, 89, 67–78. [DOI] [PubMed] [Google Scholar]
- Ohno, H. , Shinoda, K. , Ohyama, K. , Sharp, L. Z. , & Kajimura, S. (2013). EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature, 504, 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamatsu‐Ogura, Y. , Kuroda, M. , Tsutsumi, R. , Tsubota, A. , Saito, M. , Kimura, K. , & Sakaue, H. (2020). UCP1‐dependent and UCP1‐independent metabolic changes induced by acute cold exposure in brown adipose tissue of mice. Metabolism, 113, 154396. [DOI] [PubMed] [Google Scholar]
- Porter, C. , Herndon, D. N. , Chondronikola, M. , Chao, T. , Annamalai, P. , Bhattarai, N. , Saraf, M. K. , Capek, K. D. , Reidy, P. T. , Daquinag, A. C. , Kolonin, M. G. , Rasmussen, B. B. , Borsheim, E. , Toliver‐Kinsky, T. , & Sidossis, L. S. (2016). Human and mouse brown adipose tissue mitochondria have comparable UCP1 function. Cell Metabolism, 24, 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran, M. , Sul, O. J. , Choi, E. K. , Kim, J. E. , Suh, J. H. , & Choi, H. S. (2019). MCP‐1 deficiency enhances browning of adipose tissue via increased M2 polarization. Journal of Endocrinology, 242, 91–101. [DOI] [PubMed] [Google Scholar]
- Raun, S. H. , Henriquez‐Olguin, C. , Karavaeva, I. , Ali, M. , Moller, L. L. V. , Kot, W. , Castro‐Mejia, J. L. , Nielsen, D. S. , Gerhart‐Hines, Z. , Richter, E. A. , & Sylow, L. (2020). Housing temperature influences exercise training adaptations in mice. Nature Communication, 11, 1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringholm, S. , Grunnet Knudsen, J. , Leick, L. , Lundgaard, A. , Munk Nielsen, M. , & Pilegaard, H. (2013). PGC‐1alpha is required for exercise‐ and exercise training‐induced UCP1 up‐regulation in mouse white adipose tissue. Plos One, 8, e64123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar, A. , & Tarnopolsky, M. A. (2018). Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harbor Perspectives in Medicine, 8, a029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Delgado, G. , Martinez‐Tellez, B. , Olza, J. , Aguilera, C. M. , Gli, A. , & Ruiz, J. R. (2015). Role of exercise in the activation of brown adipose tissue. Annals of Nutrition & Metabolism, 67, 21–32. [DOI] [PubMed] [Google Scholar]
- Sanchez, J. , Nozhenko, Y. , Palou, A. , & Rodriguez, A. M. (2013). Free fatty acid effects on myokine production in combination with exercise mimetics. Molecular Nutrition & Food Research, 57, 1456–1467. [DOI] [PubMed] [Google Scholar]
- Seeley, R. J. , & MacDougald, O. A. (2021). Mice as experimental models for human physiology: When several degrees in housing temperature matter. Nature Metabolism, 3, 443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinsen, M. C. K. , & Pedersen, B. K. (2020). Muscle‐organ crosstalk: The emerging roles of myokines. Endocrine Reviews, 41, 594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirkhani, S. , Marandi, S. M. , Kazeminasab, F. , Esmaeili, M. , Ghaedi, K. , Esfarjani, F. , Shiralian‐Esfahani, H. , & Nasr‐Esfahani, M. H. (2018). Comparative studies on the effects of high‐fat diet, endurance training and obesity on Ucp1 expression in male C57BL/6 mice. Gene, 676, 16–21. [DOI] [PubMed] [Google Scholar]
- Skop, V. , Guo, J. , Liu, N. , Xiao, C. , Hall, K. D. , Gavrilova, O. , & Reitman, M. L. (2020). Mouse thermoregulation: introducing the concept of the thermoneutral point. Cell Reports, 31, 107501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford, K. I. , Middelbeek, R. J. W. , Townsend, K. L. , Lee, M. Y. , Takahashi, H. , So, K. , Hitchcox, K. M. , Markan, K. R. , Hellbach, K. , Hirshman, M. F. , Tseng, Y. H. , & Goodyear, L. J. (2015). A novel role for subcutaneous adipose tissue in exercise‐induced improvements in glucose homeostasis. Diabetes, 64, 2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H. , Alves, C. R. R. , Stanford, K. I. , Middelbeek, R. J. W. , Nigro, P. , Ryan, R. E. , Xue, R. D. , Sakaguchi, M. , Lynes, M. D. , So, K. W. , Mul, J. D. , Lee, M. Y. , Balan, E. , Pan, H. , Dreyfuss, J. M. , Hirshman, M. F. , Azhar, M. , Hannukainen, J. C. , Nuutila, P. , … Goodyear, L. J. (2019). TGF‐beta 2 is an exercise‐induced adipokine that regulates glucose and fatty acid metabolism. Nature Metabolism, 1, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troseid, M. , Lappegard, K. T. , Claudi, T. , Damas, J. K. , Morkrid, L. , Brendberg, R. , & Mollnes, T. E. (2004). Exercise reduces plasma levels of the chemokines MCP‐1 and IL‐8 in subjects with the metabolic syndrome. European Heart Journal, 25, 349–355. [DOI] [PubMed] [Google Scholar]
- Uldry, M. , Yang, W. L. , St‐Pierre, J. , Lin, J. D. , Seale, P. , & Spiegelman, B. M. (2006). Complementary action of the PGC‐1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metabolism, 3, 333–341. [DOI] [PubMed] [Google Scholar]
- van der Vaart, J. I. , Boon, M. R. , & Houtkooper, R. H. (2021). The role of AMPK signaling in brown adipose tissue activation. Cells‐Basel, 10, 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosselman, M. J. , Hoeks, J. , Brans, B. , Pallubinsky, H. , Nascimento, E. B. , van der Lans, A. A. , Broeders, E. P. , Mottaghy, F. M. , Schrauwen, P. , & van Marken Lichtenbelt, W. D. (2015). Low brown adipose tissue activity in endurance‐trained compared with lean sedentary men. International Journal of Obesity (London), 39, 1696–1702. [DOI] [PubMed] [Google Scholar]
- Winn, N. C. , Vieira‐Potter, V. J. , Gastecki, M. L. , Welly, R. J. , Scroggins, R. J. , Zidon, T. M. , Gaines, T. L. , Woodford, M. L. , Karasseva, N. G. , Kanaley, J. A. , Sacks, H. S. , & Padilla, J. (2017). Loss of UCP1 exacerbates Western diet‐induced glycemic dysregulation independent of changes in body weight in female mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 312, R74–R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M. V. , Bikopoulos, G. , Hung, S. , & Ceddia, R. B. (2014). Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats impact on whole‐body energy expenditure. Journal of Biological Chemistry, 289, 34129–34140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y. , Wu, Z. H. , Zhang, B. , Wang, C. , Mao, F. Y. , Liu, X. , Hu, K. P. , Sun, X. B. , Jin, W. , & Kuang, S. H. (2019). Fndc5 loss‐of‐function attenuates exercise‐induced browning of white adipose tissue in mice. Faseb Journal, 33, 5876–5886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document
Peer Review History
Data Availability Statement
All data supporting presented results are available from the corresponding author upon reasonable request.


