Summary
Data on safety and success rates of ultrasound‐guided caudal blockade, performed on sedated children with an uninstrumented airway, are scarce. We performed a retrospective observational study of validated data from April 2014 to December 2020 in a paediatric cohort where the initial plan for anaesthetic management was sedation and caudal epidural without general anaesthesia or airway instrumentation. We examined success rates of this approach and rates of block failure and block‐related complications. In total, 2547 patients ≤ 15 years of chronological age met inclusion criteria. Among the 2547 cases, including 453 (17.8%) former preterm patients, caudal‐plus‐sedation success rate was 95.1%. The primary anaesthesia plan was abandoned for general anaesthesia in 124 cases. Pain‐related block failure in 83 (3.2%) was the most common cause for conversion. Complications included 39 respiratory events and 9 accidental spinal anaesthetics. Higher odds of pain‐related block failure were associated with higher body weight (adjusted OR 1.063, 95%CI 1.035–1.092, p < 0.001) as well as with mid‐abdominal surgery (e.g. umbilical hernia repair) (adjusted OR 15.11, 95%CI 7.69–29.7, p < 0.001), whereas extreme (< 28 weeks) former prematurity, regardless of chronological age, was associated with higher odds (adjusted OR 3.62, 95%CI 1.38–9.5, p = 0.009) for respiratory problems. Ultrasound‐guided caudal epidural, performed under sedation with an uninstrumented airway, is an effective technique in the daily clinical routine. Higher body weight and mid‐abdominal surgical procedures are risk factors for pain‐related block failure. Patients who, regardless of chronological age, had been born as extreme preterm babies are at the highest risk for respiratory events.
Keywords: anaesthesia, caudal, anaesthesia, epidural, infant, paediatrics
Introduction
Caudal epidural blockade is the most widely used technique of regional anaesthesia in children [1, 2]. For paediatric surgery below the umbilicus (e.g. orchidopexia or inguinal hernia repair), caudal blockade is predominantly combined with general anaesthesia [3]. To address the potential risk of postoperative apnoea and neurocognitive problems due to the substances involved, especially in neonates, efforts are made to avoid general anaesthesia and instead use awake spinal techniques in the management of neonatal hernia repair [4]. These considerations guided our team towards the concept of using caudal epidural blockade under sedation with preserved spontaneous breathing in infants and children. Starting in 2013, this concept was implemented as the standard approach in our clinical practice and has been enhanced by comprehensive utilisation of ultrasound guidance [5]. However, this strategy continues to be regarded with some scepticism in the specialist community. One subject of debate concerns the safety of regional anaesthetic techniques in sedated patients. Taenzer et al. reported that postoperative neurological symptoms occurred at a lower frequency in children under general anaesthesia than in sedated or awake patients [6]. Similarly, a survey of more than 100,000 regional blocks from the Paediatric Regional Anaesthesia Network reported that the risk of neurological complications is higher for blocks placed awake or sedated [7]. Conversely, regional anaesthesia, when used as an adjunct or alternative to general anaesthesia, can decrease volatile anaesthetic, sedative or opioid requirements, while providing effective intra‐ and postoperative analgesia [8]. Moreover, neuraxial regional techniques may avoid primary airway instrumentation and can reduce the incidence of postoperative apnoea and respiratory morbidity in high‐risk infants [8, 9]. We use ultrasound‐guided caudal epidural blocks for primary procedural analgesia with an uninstrumented airway in > 90% of eligible cases in paediatric surgery. This proportion contrasts with established processes in most centres and was the motivation to perform this study, alongside limited data in the current literature with regard to success rates and safety considerations.
We, therefore, retrospectively analysed our experience for the years 2014–2020 of this standardised approach. Validated data that had been prospectively collected from patients ≤ 15 y of age presenting for procedures amenable to caudal blockade with sedation were included. The primary outcome was the failure rate of the primary technique. Secondary outcomes included the incidence of respiratory and technique‐related complications. An additional objective was to evaluate factors associated with higher risk for pain‐related block failure and, on a separate basis, for respiratory side effects.
Methods
The institutional review board approved the current study. Collected data were retrospectively analysed and validated in accordance with the STROBE statement for observational studies [10]. The study was conducted at the Department of Anaesthesia, General Intensive Care Medicine and Pain Medicine (Division of Paediatric Anaesthesia) of the Medical University of Vienna, Austria, a major tertiary‐care centre with a catchment area of 3.5 million people. Considered for inclusion in this examination were all children aged ≤ 15 y admitted for paediatric surgery between April 2014 and December 2020, provided that caudal epidural blockade with an uninstrumented airway was the initial anaesthetic plan. Orthopaedic and trauma patients were not considered as the paediatric anaesthesia team at our centre is not by default in charge of managing these patients and to avoid further heterogeneity of the study population.
For premedication, midazolam (Dormicum™; Roche, Vienna, Austria) was used rectally at 0.5 mg kg‐1 (not exceeding 15 mg) in 6‐ to 12‐month old patients, flavoured midazolam syrup in older children or midazolam at 0.1 mg kg‐1 if intravenous access was already in place. In cases without an intravenous line (predominantly patients aged ≤ 1 y), we induced mild sedation with inhalational sevoflurane via facemask to establish the intravenous line and stopped the sevoflurane immediately after placement. In older and compliant children, the intravenous line was placed without inhalational sedation. Cases with pre‐existing intravenous access and those after intravenous placement were sedated using propofol boluses (up to 2 mg kg‐1) to facilitate caudal injection, if necessary. Sedation with propofol was continued intra‐operatively (up to 5 mg kg‐1 h‐1). The infusion rate was modified as required, aiming for a sedation level where the patient was spontaneously breathing as required and arousable by significant physical stimulation. Continuous propofol sedation was not used for short surgical or diagnostic procedures (< 30 min) in infants aged ≤ 1 y (Fig. 1).
Figure 1.

Overview of caudal epidural anaesthesia with an uninstrumented airway; standard procedure at our centre. I.V., intravenous; LA, local anaesthetic. All photographs were taken with the consent of a parent or legal guardian.
After premedication and sedation, the patient was turned to a left lateral position with the hips and knees flexed. First, we performed a short ultrasound examination of the anatomical landmarks (SonoSite M‐Turbo; FUJIFILM Corporation, Bothell, WA, USA) with a high‐frequency linear ultrasound probe (38 or 50 mm active area) including transverse and longitudinal views to illustrate the sacrococcygeal ligament, the two sacral cornua and the dura mater with epidural space. After establishing sterile conditions (including the ultrasound probe), we use a combination of a landmark‐based and ultrasound‐guided puncture of the sacral hiatus. We use 30 mm 24G facette tip needles with an injection line for caudal blockade (Marhofer Set; Pajunk, Geisingen, Germany). For real‐time ultrasound visualisation of local anaesthetic spreading (see also online Supporting Information Video S1) into the epidural space, the sterile covered ultrasound probe was placed longitudinally in a position slightly paramedian to the lumbar spine cranial to the puncture site. This requires a three‐hand technique, with one anaesthetist performing the puncture and local anaesthetic injection, and another performing the real‐time ultrasound visualisation. Standardised dosages for caudal injection of 0.8 ml kg‐1 for sacral dermatomes, 1.0 ml kg‐1 for lumbar dermatomes and 1.2 ml kg‐1 for lower thoracic dermatomes of ropivacaine 3.8 mg ml‐1 were used. An overview of the clinical workflow, including photographs of signs to watch out for during the ultrasound examination, is given in Figs. 1 and 2.
Figure 2.
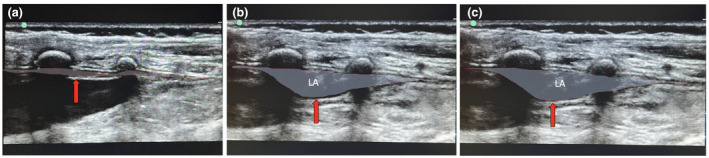
Ultrasound showing the spread of local anaesthetic (LA) during caudal blockade in a 14‐month‐old child. The chronological sequence of the pictures is from left to the right (a–c). The red arrow indicates the dura mater, which moves in an anterior direction during injection of the LA. The dotted red lines encircle the epidural space before (a) and after LA injection (b, c). The entire depth of the image is 27 mm; right side = caudal; upper side = dorsal. All photographs were taken with the consent of a parent or legal guardian.
Block failure, due to pain or any complications necessitating general anaesthesia with airway management, were followed by applying a defined departmental standard of either tracheal intubation (careful bag‐mask ventilation with < 10 mmHg of inspiratory pressure, followed by induction using propofol 2–4 mg kg‐1, fentanyl 3–5 μg kg‐1 and rocuronium 0.3–0.6 mg kg‐1) or placement of a supraglottic airway device (propofol 2–3 mg kg‐1 and fentanyl 2–3 μg kg‐1). Standard monitoring for every patient included ECG, non‐invasive arterial pressure and peripheral oxygen saturation (SpO2). The first hour of the procedure included an infusion of 10 ml kg‐1 h‐1 Elo‐Mel isotone or, in those aged < 1 y, Elo‐Paed balanced (Fresenius Kabi, Graz, Austria). Oxygen‐enriched air (FiO2 0.40) was administered through a facemask attached by adhesive tape in all sedated patients.
All relevant data were retrieved from two independent electronic systems by systematic interrogation. The first one is the electronic medical record (EMR) system of our institution, which is used in each of its operating theatres and intermediate or intensive care units. The second system, AKIM (which literally translates as ‘General Hospital Information Management’), is connected to a research, documentation and analysis database. This AKIM system, assisted by the database, was also used to systematically extract information on gestational age and birth weight.
Both the EMR and AKIM are separate patient documentation and information systems that operate in near real time with data quality assurance performed via exporting and validating data on a regular basis. The EMR holds a copy of each anaesthesia protocol since 2014 and collects data on (e.g.) vital signs, medications, regional anaesthesia or airway devices. It includes sections called ‘primary and secondary anaesthesia plan’ and ‘unexpected (critical) events’ for entry of potential complications (e.g. cardiac arrest, high spinal, laryngo/bronchospasm). Importantly, attending anaesthetists need to complete all required fields from within the operating theatre to close a case for ‘electronic transfer’ of the patient to the recovery room or intermediate/intensive care units.
Three consultant anaesthetists (PO, MO and FK) worked independently in validating the patient cohort returned by the initial database search. The process of validating each case included: baseline characteristics of patients; primary and secondary anaesthesia plan; documented technique of regional anaesthesia; use of an airway device and (if applicable) ventilation parameters; sequence of steps and events in the operating theatre; critical events; lowest heart rate; lowest SpO2 value; discharge records; anaesthesia consent form; and (if applicable and/or needed) digital intermediate/intensive care units records. Validation was aimed at detecting artefacts, verifying that complication(s) had actually occurred, finding out if anaesthetic procedures were actually performed as originally planned and retrieving any missing values (e.g. body weight from different sources).
This first round of each of the three examiners validating 928 cases resulted in ratings of: (A) obviously straightforward caudal epidural without airway instrumentation, no complication; (B) obviously converted to general anaesthesia following an initially planned caudal epidural without airway instrumentation; (C) any complication (e.g. cardiac arrest, high spinal, laryngo‐ or bronchospasm); (D) obviously primary general anaesthesia supplemented by caudal epidural anaesthesia; and (E) needs re‐assessment. Following this first round and exclusion of cases in category D (n = 229), a second round of validation was performed in categories B, C and E with each case assessed by a different examiner than in the first round. Finally, all cases in the categories C and E were handed over to the most experienced anaesthetist for a final assessment; they also acted as an adjudicator when there was disagreement on conversion to general anaesthesia due to pain or respiratory failure or other complications.
The primary outcome involved yes/no decisions on whether or not any of the initially planned caudal blocks under sedation with an uninstrumented airway had, in effect, been followed through or had been abandoned for general anaesthesia with intubation or a supraglottic airway device. Secondary outcomes were the particular aetiology for conversion of the primary anaesthesia plan to general anaesthesia and included residual pain, block‐related technical complications in the form of spinal injection or intoxication with local anaesthetic and respiratory events (e.g. laryngospasm, bronchospasm, apnoea, cardiac arrest to hypoxia or any less well‐specified respiratory events in this category requiring action by the anaesthetist). All these parameters for analysis were not established for this study but are implemented in our EMR system.
Given the clinical plausibility of distinguishing between risks for pain‐related ‘true’ block failures and risks for respiratory complications, multiple logistic regression analyses were performed separately for the dichotomous (yes/no) endpoints of pain‐related transitions to airway management and respiratory complications, regardless of whether these cases were transitioned to airway management. The results are reported as raw and confounder‐adjusted OR with 95%CI. Suspicions that specific variables may be significant confounders were expressed based on established relationships between explanatory and outcome variable, biological plausibility and inhomogeneous distribution at p < 0.1, and were considered as co‐variables in the multiple regression model. Subsequently, a stepwise logistic regression model was used to assess the independence of any association between the potential predictor and a primary or secondary outcome. Co‐variables were included after testing for interactions and collinearity with calculation of variance inflation factors (1/1 − Ri 2) [11]. The variance inflation factor estimates how much the variance of a regression coefficient is inflated due to multicollinearity in the model. Thereby, the numerical value reflects the percentage the variance inflated for each coefficient. Values > 5 show a high correlation.
All data were screened for completeness, consistency and outliers before analysis. Where values were missing, alternative data sources were explored before imputation was considered. Values were then replaced by appropriate sub‐group medians if ≤ 5% were missing. Continuous data were evaluated using non‐continuous Mann–Whitney U‐ or Kruskal–Wallis tests and cross‐tab calculations compared with Pearson's test. All tests were carried out two‐sided, considering differences to be significant at p < 0.05, and adjusting multiple hypotheses for α‐error accumulation by Bonferroni correction. For all operations in our analysis, SPSS® Statistics (version 24.0.0.0; IBM, Armonk, NY, USA) and R (R‐Foundation, Vienna, Austria) were used.
Results
A total of 2547 paediatric patients were analysed. Figure 3 illustrates the application of exclusion criteria to potential patients over the study period (April 2014 to December 2020). In all included cases, the primary plan for anaesthetic management was caudal epidural blockade under sedation with an uninstrumented airway. In all 2547 cases, ultrasound guidance was used to identify anatomical landmarks including the dura mater and epidural space. Relevant characteristics in patients in whom the primary decision for regional anaesthesia was followed through (2423 out of 2547, success rate: 95.1%) vs. patients in whom it was changed to general anaesthesia with airway management (124 out of 2547, failure rate: 4.9%) are listed in Table 1. In the 2547 cases, these secondary changes of plan were due to block‐related complications or respiratory events in 35 (1.4%), pain‐related block failures in 83 (3.3%) and anatomical impediments to puncture in 6 (0.2%). The overall rate of former preterm patients was as high as 453 (17.8%), including 142 (5.6%) extreme cases (< 28 weeks of gestation). The surgical procedures covered by all these primary epidurals vs. secondary general anaesthesia procedures are summarised in online Supporting Information Table S1. The conversion rates to general anaesthesia (stratified by age groups) are shown in Fig. 4.
Figure 3.
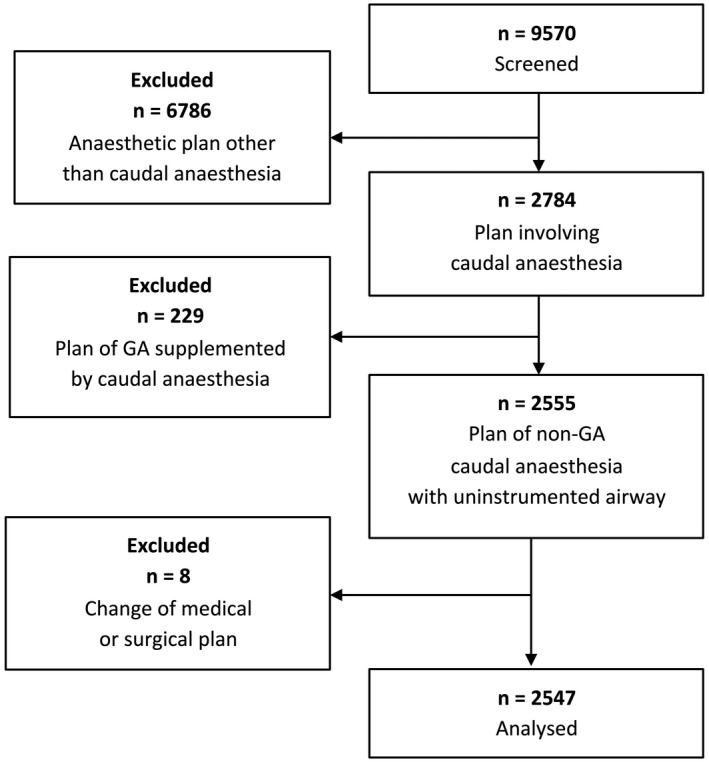
Flow chart illustrating the logic of database interrogation for this study. GA, general anaesthesia.
Table 1.
Baseline characteristics of patients and anaesthesia‐related data of paediatric cases with ultrasound‐guided caudal anaesthesia and uninstrumented airway or secondary conversion to general anaesthesia with airway. Values are median (IQR [range]) or number (proportion).
| Primary plan for caudal anaesthesia with uninstrumented airway | Secondary general anaesthesia with airway management* due to | p‐value | ||
|---|---|---|---|---|
| Successful (n = 2423) | Complications † (n = 41) | Pain (n = 41) | ||
| Chronological age, y | 1 (0–4 [0–14]) | 1 (0–5 [0–9]) | 3 (1–6 [0–11]) | < 0.001 |
| ASA physical status | 0.846 | |||
| 1 | 1823 (75.2%) | 32 (78%) | 68 (81.9%) | |
| 2 | 428 (17.7%) | 6 (14.6) | 12 (14.5%) | |
| 3 | 168 (6.9%) | 3 (7.3%) | 3 (3.6%) | |
| 4 | 4 (0.2%) | 0 | 0 | |
| Body weight, kg | < 0.001 | |||
| 0 to < 5 | 336 (13.9%) | 9 (22%) | 2 (2.4%) | |
| 5 to < 10 | 653 (27%) | 8 (19.5%) | 14 (16.9%) | |
| 10 to < 20 | 996 (41.1%) | 15 (36.6%) | 39 (47%) | |
| 20 to < 30 | 356 (14.7%) | 4 (9.8%) | 21 (25.3%) | |
| 30 to < 40 | 82 (3.4%) | 5 (12.2%) | 7 (8.4%) | |
| Sex, female | 460 (19%) | 5 (12.2%) | 22 (26.5%) | 0.12 |
| Gestational week at birth | 0.147 | |||
| < 28 (extreme preterm) | 132 (5.4%) | 6 (14.6%) | 4 (4.8%) | |
| 28 to < 37 (very‐late preterm) | 298 (12.3%) | 4 (9.8%) | 9 (10.8%) | |
| > 37 (term) | 1993 (82.3%) | 31 (75.6%) | 70 (84.3%) | |
| Surgery < 46 weeks after conception (yes/no) | 323 (13.3%) | 7 (17.1%) | 1 (1.2%) | 0.004 |
| Bronchopulmonary dysplasia (yes/no) | 30 (1.2%) | 3 (8.6%) | 2 (2.4%) | 0.002 |
| Puncture attempts, n | 1 (1–2 [1–5]) | 1 (1–2 [1–5]) | 1 (1–2 [1–4]) | 0.002 |
| Caudal block until skin incision, min | 12 (9–15 [2–69]) | 16 (10–20 [2–40]) | 18 (13–22 [2–30]) | < 0.001 |
| Blood aspiration during caudal block (yes/no) | 37 (1.5%) | 0 | 2 (2.4%) | 0.588 |
Supraglottic airway device or intubation.
Respiratory or technique‐related complications (n = 35) or site anatomy (n = 6).
Figure 4.
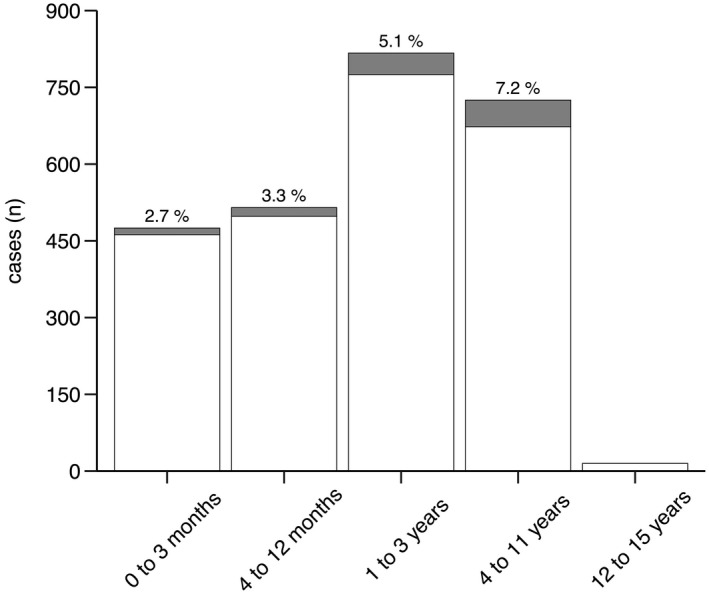
Rates of conversion from caudal anaesthesia with an uninstrumented airway to general anaesthesia with airway management in different age groups. White = caudal block with sedation; shaded = conversion to general anaesthesia.
In total, there were 48 cases with block‐related technical or respiratory complications, 13 of which could be managed within the primary non‐general anaesthesia plan, thus not requiring airway management. Respiratory incidents were the most frequent adverse events (39 or 1.5%). All other cases (9 or 0.4%) were due to accidental spinal injection of local anaesthetic with subsequent apnoea. All nine were transitioned to airway management, regained spontaneous breathing within 20–30 min of the initial event and took uneventful clinical courses after the airway device was removed near the end of surgery. The distribution and frequencies of respiratory events and complications after caudal blockade can be seen in the online Supporting Information (Figure S1).
Independent associations with higher risk for pain‐related transition to airway management from the fully adjusted regression model were found to include higher body weight (adjusted OR 1.063, 95%CI 1.035–1.092, p < 0.001); more puncture attempts (adjusted OR 1.48, 95%CI 1.13–1.95, p = 0.004); and mid‐abdominal (e.g. umbilical, para‐ and supraumbilical hernia) surgery (adjusted OR 15.11, 95%CI 7.69–29.7, p < 0.001). Longer block‐to‐incision intervals were identified as a confounder (OR 1.077, 95%CI 1.05–1.1, p < 0.001) (Table 2).
Table 2.
Results of logistic regression to assess potential factors associated with the probability of pain‐related block failure in paediatric cases with ultrasound‐guided caudal anaesthesia.
| Univariate analysis (crude) | Multivariate analysis (adjusted) | |||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Chronological age | 1.17 (1.098–1.25) | < 0.001 | Not included (collinearity)* | VIF = 6.54 |
| Weight | 1.06 (1.036–1.085) | < 0.001 | 1.063 (1.035–1.092) | < 0.001 |
| Sex, female | 0.64 (0.39–1.06) | 0.084 | 0.89 (0.5–1.56) | 0.68 |
| Prematurity | ||||
| < 28 weeks of gestation | 0.83 (0.3–2.3) | 0.735 | 1.79 (0.6–5.3) | 0.29 |
| 28 to < 37 weeks of gestation | 0.86 (0.42–1.74) | 0.679 | 1.35 (0.61–2.9) | 0.45 |
| Puncture attempts, n | 1.5 (1.2–1.98) | < 0.001 | 1.48 (1.13–1.95) | 0.004 |
| Blood aspiration (yes/no) | 1.62 (0.38–6.83) | 0.51 | Not included | |
| History of previous caudal blocks, n | 1.16 (0.83–1.63) | 0.369 | Not included | |
| Ropivacaine 0.38% dose, ml.kg‐1 | 1.068 (1.043–1.093) | < 0.001 | Not included (collinearity)* | VIF = 16.75 |
| Caudal block‐to‐skin incision time, min | 1.069 (1.044–1.095) | < 0.001 | 1.07 (1.05–1.1) | < 0.001 |
| Mid‐abdominal surgery (e.g. umbilical hernia repair) (yes/no) | 19.79 (10.6–36.87) | < 0.001 | 15.11 (7.69–29.7) | < 0.001 |
Collinear variables are body weight, age and amount of local anaesthetic (dosed at 1 ml.kg‐1). VIF, variance inflation factor.
The logistic regression analysis showed an independent significant association of extreme ex‐prematurity regardless of chronological age (born < 28 weeks, adjusted OR 3.62, 95%CI 1.38–9.5, p = 0.009) with higher risk for respiratory complications, regardless of whether these cases were transitioned to general anaesthesia. Chronological age on the day of surgery, while considered as a covariate in the fully adjusted model, was not a significant effect modifier (adjusted OR 0.99, 95%CI 0.87–1.12). Neither moderate to late former prematurity (28 to < 37 weeks) nor the diagnosis of bronchopulmonary dysplasia was significantly associated with increased odds of respiratory complications. Longer block‐to‐incision intervals, by contrast, did increase the odds of a respiratory complication (adjusted OR 1.05: 95%CI 1.02–1.08, p < 0.001) (see also online Supporting Information Table S2).
Discussion
This survey of data on caudal epidural anaesthesia with an uninstrumented airway, encompassing what is the largest single‐centre cohort to date, adds to the body of evidence indicating an appropriate ultrasound‐guided strategy. What is more, the results demonstrate an acceptable safety margin and a high success rate achieved not by highly experienced paediatric anaesthetists in an interventional study, but indeed by routine procedures in daily clinical practice.
During our observation period of April 2014 to December 2020, nine spinal injections of local anaesthetic resulting in apnoea and airway management translated to 3.5 per 1000 caudal blocks. Veyckemans et al. reported a considerably lower rate of 0.9 per 1000 caudal blocks performed under general anaesthesia [1]. We can only speculate about the reasons, although it seems plausible that accidental spinal injections may go unnoticed in patients anaesthetised and ventilated under general anaesthesia. Alternatively, movement during caudal blockade under sedation could lead to needle advancement through the dura and injection of local anaesthetic despite an initial aspiration ‘negative for liquor’. Another explanation could lie in our role as a training hospital, where registrar doctors less experienced in paediatric anaesthesia perform caudal blocks under supervision. Registrars were found to require at least 32 blocks for an 80% success rate [12]. Our experience has been that ultrasound guidance for caudal blocks reduces the learning curve by making it easier to locate landmarks and by enabling visualisation of the epidural and subarachnoid space or of anatomical variations [2, 5]. Nevertheless, our data cannot prove that spinal injection of local anaesthesia occurs more frequently in the hands of less experienced anaesthetists.
The logistic regression analysis showed a significant association of higher body weight (per kg) with higher odds of pain‐related block failure (see Table 2). This finding reaffirms our clinical experience. The underlying data imply that pain‐related block failures are rare in children weighing < 5 kg, whereas the risk of switching over to general anaesthesia due to residual pain is highest among those weighing 10 to < 30 kg (see Table 1). One might criticise our use of body weight rather than BMI in the logistic regression model, but BMI data as an inclusion criterion would have meant we could not evaluate numerous patients due to an unacceptable proportion of missing values (> 5%) on body height. In addition, the vast majority of our paediatric dosing regimens, decisions and device selection are based on body weight and patient age in clinical practice.
Another finding was that mid‐abdominal (e.g. umbilical hernia repair) surgery was associated with a higher risk of block failure by a factor of 15 compared with more distal surgical procedures. Reduced success rates and unpredictable prospects of success for mid‐abdominal operations have been reported previously [2]. Reasons for this poor performance are age‐dependent differences in sensory analgesia and unpredictable secondary spreading of local anaesthetic after caudal blockade [13, 14]. Consequently, we will change our approach for most of these cases in the future. Interventions of this type may be better managed by rectus sheath blockade and general anaesthesia [15] or lumbar/thoracic epidural anaesthesia for selected cases [2].
To identify the most suitable management plan for each patient, clinicians need to reflect on risks associated with a potential difficult airway and with neuraxial anaesthesia. In 2017, the study by Habre et al., a large multicentre study, was published on children aged 0–15 y who underwent anaesthesia for diagnostic or surgical procedures [16]. Out of the 30,874 cases recorded from 261 centres over a 10‐month period, 94% underwent general anaesthesia. The incidence of peri‐operative severe critical events was 5.2% (95%CI 5.0–5.5), the most common being respiratory (3.1%, 95%CI 2.9–3.3) [16]. In contrast, a multicentre study of 18,650 cases regarding safety issues of caudal blocks yielded a 1.9% (1.7–2.1) incidence of complications [3]. Given only 1.1% of caudal blocks were performed on awake or sedated patients, however, that study population cannot be compared with our series as most procedures were performed under general anaesthesia and the focus was on block‐related events with no mention of respiratory events [3]. Furthermore, a different definition of ‘block failure’ (unable to place, difficult to inject and subcutaneous injection) was used in this study [3].
The proportion of 453 out of 2547 (17.8%) of former preterm patients, regardless of chronological age, in our study reflects a global trend [17] and indicates a challenge to paediatric anaesthetists arising from immature physiology [18, 19]. Given that prematurity implies higher risks for respiratory complications [20, 21] and higher risks due to acquired airway disorders [22], anaesthesia with an uninstrumented airway may, in selected cases, be beneficial to the sole reason of preventing postoperative ventilator dependency. This strategy is supported by a general trend towards non‐invasive ventilation to minimise the risks and long‐term sequelae of intubation and invasive ventilation in preterm babies [21, 22].
Our data show that patients who, regardless of their chronological age at the time of surgery, had been born as extreme preterm (< 28 weeks) were more frequently affected by respiratory events. A large meta‐analysis of 147,000 European children showed a positive association of preterm birth, irrespective of birth weight, with preschool wheezing and school‐age asthma [23]. A long‐term follow‐up study of adults born preterm, too, has revealed persistent abnormalities of respiratory and cardiovascular/cardiopulmonary function, possibly as a result of discordant growth and development, as young adults born preterm were found to have smaller airways limiting the expiratory airflow, abnormal respiratory mechanics, smaller cardiac chambers in the presence of normal total cardiac size and sometimes pulmonary hypertension suggestive of reduced pulmonary vascular capacity [24]. While these findings might explain the higher incidence of respiratory events in our study, its retrospective findings cannot demonstrate a causal relationship. It will take prospective studies to investigate the risk of anaesthesia‐related critical respiratory events in formerly premature children.
Since our departmental standard for subumbilical surgery is, indeed, caudal blockade under sedation with an uninstrumented airway, our data cannot be readily compared with previous cohorts, which predominantly reflect either combinations with general anaesthesia [1, 3] or awake caudal blocks [4]. It appears that our series, largely consisting of caudal blocks performed under sedation, represents rather a singularity, contrary to what is common practice in most centres [3, 25]. This also implies, however, that given no representative cohort of subumbilical surgery managed by general anaesthesia with supplementary caudal blockade at our centre, we are unable to demonstrate that our management strategy reduces the risk for anaesthesia‐related critical events. Our extensive validation process should have left no room for oversights of severe complications but naturally cannot eliminate the risk of non‐reporting and detection bias or differences between anaesthesia teams in judging or approaching clinical situations. On a similar note, the presented data are a reflection of caudal blocks in children of various ages and routinely performed by anaesthetists with different levels of training. These data may be encumbered by accuracy and quality concerns inherent in real‐time documentation and variable levels of experience, but we feel that the value of real‐life routine management being more truly reflected may outweigh these concerns.
Ultrasound‐guided caudal epidurals, performed under sedation with an uninstrumented airway, are an effective technique. Higher body weight and mid‐abdominal surgical procedures are factors associated with higher odds of pain‐related block failure. Patients who, regardless of chronological age at the time of surgery, had been born prematurely are at the highest risk for respiratory complications. The latter result emerged despite our uninstrumented airway approach and is expected to guide future decisions towards optimising and personalising our anaesthetic strategy for this specific sub‐group.
Supporting information
Figure S1 . Distribution and frequencies of respiratory events and complications after caudal blockade, expressed in absolute numbers.
Table S1 . Surgical procedures primarily planned as caudal anaesthesia with uninstrumented airway and conversion to general anaesthesia with airway management.
Table S2. Logistic regression evaluating potential factors associated with the probability for respiratory side‐effects during anaesthesia management (regardless of transition to airway management).
Video S1 . Spread of local anaesthetic during caudal blockade, using a Sono Site M‐Turbo (Fujifilm Corporation) ultrasound system with a high‐frequency linear probe (active area: 50 mm) in a 14‐month‐old child. The video was made with the consent of a parent or legal guardian.
Acknowledgements
The study was approved by the institutional review board (Ethics Commission at Medical University of Vienna) and registered on the German Clinical Trial Register. PO and FK contributed equally to this work. The authors thank W. Preinfalk for substantive language editing of the manuscript. They are indebted to I. Shulym, Centre for Medical Statistics, Informatics and Intelligent Systems, Medical University of Vienna, for assistance with data retrieval and providing detail data from the AKH Information Management system. Furthermore, they gratefully acknowledge assistance and critical review of their statistical analysis plan by H. Villamizar, Senior Data Scientist. No external funding or competing interests declared.
References
- 1. Veyckemans F, Van Obbergh LJ, Gouverneur JM. Lessons from 1100 pediatric caudal blocks in a teaching hospital. Regional Anesthesia 1992; 17: 119–25. [PubMed] [Google Scholar]
- 2. Wiegele M, Marhofer P, Lönnqvist PA. Caudal epidural blocks in paediatric patients: a review and practical considerations. British Journal of Anaesthesia 2019; 122: 509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suresh S, Long J, Birmingham PK, De Oliveira GS Jr. Are caudal blocks for pain control safe in children? An analysis of 18,650 caudal blocks from the pediatric regional anesthesia network (PRAN) database. Anesthesia and Analgesia 2015; 120: 151–6. [DOI] [PubMed] [Google Scholar]
- 4. Frawley G, Bell G, Disma N, et al. Predictors of failure of awake regional anesthesia for neonatal hernia repair: data from the general anesthesia compared to spinal anesthesia study – comparing apnea and neurodevelopmental outcomes. Anesthesiology 2015; 123: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marhofer P, Lönnqvist PA. The use of ultrasound‐guided regional anaesthetic techniques in neonates and young infants. Acta Anaesthesiologica Scandinavica 2014; 58: 1049–60. [DOI] [PubMed] [Google Scholar]
- 6. Taenzer AH, Walker BJ, Bosenberg AT, et al. Asleep versus awake: does it matter? Pediatric regional block complications by patient state: a report from the pediatric regional anesthesia network. Regional Anesthesia and Pain Medicine 2014; 39: 279–83. [DOI] [PubMed] [Google Scholar]
- 7. Walker BJ, Long JB, Sathyamoorthy M, et al. Complications in pediatric regional anesthesia: an analysis of more than 100,000 blocks from the pediatric regional anesthesia network. Anesthesiology 2018; 129: 721–32. [DOI] [PubMed] [Google Scholar]
- 8. Heydinger G, Tobias J, Veneziano G. Fundamentals and innovations in regional anaesthesia for infants and children. Anaesthesia 2021; 76: 74–88. [DOI] [PubMed] [Google Scholar]
- 9. Davidson AJ, Morton NS, Arnup SJ, et al. Apnea after awake regional and general anesthesia in infants: the general anesthesia compared to spinal anesthesia study – comparing apnea and neurodevelopmental outcomes, a randomized controlled trial. Anesthesiology 2015; 123: 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–7. [DOI] [PubMed] [Google Scholar]
- 11. Everitt BS, Skrondal A. The Cambridge Dictionary of Statistics. Cambridge: Cambridge University Press, 2010. [Google Scholar]
- 12. Schuepfer G, Konrad C, Schmeck J, Poortmans G, Staffelbach B, Jöhr M. Generating a learning curve for pediatric caudal epidural blocks: an empirical evaluation of technical skills in novice and experienced anesthetists. Regional Anesthesia and Pain Medicine 2000; 25: 385–8. [DOI] [PubMed] [Google Scholar]
- 13. Lundblad M, Lönnqvist PA, Eksborg S, Marhofer P. Segmental distribution of high‐volume caudal anesthesia in neonates, infants, and toddlers as assessed by ultrasonography. Pediatric Anesthesia 2011; 21: 121–7. [DOI] [PubMed] [Google Scholar]
- 14. Lundblad M, Eksborg S, Lönnqvist PA. Secondary spread of caudal block as assessed by ultrasonography. British Journal of Anaesthesia 2012; 108: 675–81. [DOI] [PubMed] [Google Scholar]
- 15. Willschke H, Bösenberg A, Marhofer P, et al. Ultrasonography‐guided rectus sheath block in paediatric anaesthesia – a new approach to an old technique. British Journal of Anaesthesia 2006; 97: 244–9. [DOI] [PubMed] [Google Scholar]
- 16. Habre W, Disma N, Virag K, et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respiratory Medicine 2017; 5: 412–25. [DOI] [PubMed] [Google Scholar]
- 17. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012; 379: 2162–72. [DOI] [PubMed] [Google Scholar]
- 18. Somaini M, Ingelmo P. Anesthesia and analgesia in preterm infants is still something in between art and science. Minerva Anestesiologica 2020; 86: 699–700. [DOI] [PubMed] [Google Scholar]
- 19. Neumann RP, von Ungern‐Sternberg BS. The neonatal lung – physiology and ventilation. Pediatric Anesthesia 2014; 24: 10–21. [DOI] [PubMed] [Google Scholar]
- 20. Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Practice and Research. Clinical Obstetrics and Gynaecology 2018; 52: 3–12. [DOI] [PubMed] [Google Scholar]
- 21. Owen LS, Manley BJ, Davis PG, Doyle LW. The evolution of modern respiratory care for preterm infants. Lancet 2017; 389: 1649–59. [DOI] [PubMed] [Google Scholar]
- 22. Zhang H, Zhang J, Zhao S. Airway damage of prematurity: the impact of prolonged intubation, ventilation, and chronic lung disease. Seminars in Fetal and Neonatal Medicine 2016; 21: 246–53. [DOI] [PubMed] [Google Scholar]
- 23. Sonnenschein‐van der Voort AM, Arends LR, de Jongste JC, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta‐analysis of 147,000 European children. Journal of Allergy and Clinical Immunology 2014; 133: 1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duke JW, Lewandowski AJ, Abman SH, Lovering AT. Physiological aspects of cardiopulmonary dysanapsis on exercise in adults born preterm. Journal of Physiology 2022; 600: 463–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ecoffey C, Lacroix F, Giaufre E, et al. Epidemiology and morbidity of regional anesthesia in children: a follow‐up one‐year prospective survey of the French‐language Society of Paediatric Anaesthesiologists (ADARPEF). Pediatric Anesthesia 2010; 20: 1061–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 . Distribution and frequencies of respiratory events and complications after caudal blockade, expressed in absolute numbers.
Table S1 . Surgical procedures primarily planned as caudal anaesthesia with uninstrumented airway and conversion to general anaesthesia with airway management.
Table S2. Logistic regression evaluating potential factors associated with the probability for respiratory side‐effects during anaesthesia management (regardless of transition to airway management).
Video S1 . Spread of local anaesthetic during caudal blockade, using a Sono Site M‐Turbo (Fujifilm Corporation) ultrasound system with a high‐frequency linear probe (active area: 50 mm) in a 14‐month‐old child. The video was made with the consent of a parent or legal guardian.


