Abstract
Objective
The objective of this paper was to explore the utility of time to maximum concentration (Tmax)‐based target mismatch on computed tomography perfusion (CTP) in predicting radiological and clinical outcomes in patients with acute ischemic stroke (AIS) with anterior circulation large vessel occlusion (LVO) selected for endovascular treatment (EVT).
Methods
Patients with AIS underwent CTP within 24 hours from onset followed by EVT. Critically hypoperfused tissue and ischemic core volumes were automatically calculated using Tmax thresholds >9.5 seconds and >16 seconds, respectively. The difference between Tmax > 9.5 seconds and Tmax > 16 seconds volumes and the ratio between Tmax > 9.5 seconds and Tmax > 16 seconds volumes were considered ischemic penumbra and Tmax mismatch ratio, respectively. Final infarct volume (FIV) was measured on follow‐up non‐contrast computed tomography (CT) at 24 hours. Favorable clinical outcome was defined as 90‐day modified Rankin Scale 0 to 2. Predictors of FIV and outcome were assessed with multivariable logistic regression. Optimal Tmax volumes for identification of good outcome was defined using receiver operating curves.
Results
A total of 393 patients were included, of whom 298 (75.8%) achieved successful recanalization and 258 (65.5%) achieved good outcome. In multivariable analyses, all Tmax parameters were independent predictors of FIV and outcome. Tmax > 16 seconds volume had the strongest association with FIV (beta coefficient = 0.596 p <0.001) and good outcome (odds ratio [OR] = 0.96 per 1 ml increase, 95% confidence interval [CI] = 0.95–0.97, p < 0.001). Tmax > 16 seconds volume had the highest discriminative ability for good outcome (area under the curve [AUC] = 0.88, 95% CI = 0.842–0.909). A Tmax > 16 seconds volume of ≤67 ml best identified subjects with favorable outcome (sensitivity = 0.91 and specificity = 0.73).
Interpretation
Tmax target mismatch predicts radiological and clinical outcomes in patients with AIS with LVO receiving EVT within 24 hours from onset. ANN NEUROL 2022;91:878–888
A growing body of data suggest that computed tomography perfusion (CTP) is a useful tool for the selection of patients with acute ischemic stroke (AIS) with anterior circulation large vessel occlusion (LVO) for endovascular treatment (EVT). 1 A favorable CTP profile was successfully used to establish the eligibility for EVT of patients with AIS with LVO in 2 late time window (6–24 hours) randomized controlled trials (RCTs), namely DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) 2 and DAWN (Triage of Wake‐up and Late Presenting Strokes Undergoing Neurointervention With Trevo), 3 and in 2 early time window (0–6 hours) RCTs, namely SWIFT PRIME (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment) 4 and EXTEND‐IA (Extending the Time for Thrombolysis in Emergency Neurological Deficits‐Intra‐Arterial). 5 In all these four CTP‐guided RCTs, 2 , 3 , 4 , 5 patients with AIS were selected for therapy if meeting inclusion criteria consisting of a combination of parameters collectively called target mismatch. Although cutoff values of target mismatch varied across these studies, they were always automatically calculated with a dedicated software (RAPID; Rapid Processing of Perfusion and Diffusion; iSchemaView, Menlo Park, CA), based on the assumption that critically hypoperfused tissue is indicated by absolute time to the peak of the residual function (time to maximum concentration [Tmax]) threshold values more than 6 seconds (Tmax > 6 seconds) and ischemic core corresponds to relative cerebral blood flow (rCBF) threshold values less than 30% of normally perfused tissue (rCBF < 30%). In this model, the difference between Tmax > 6 seconds and rCBF < 30% volumes (Tmax‐CBF mismatch) represents ischemic penumbra, whereas the ratio between Tmax > 6 and rCBF < 30% volume is the mismatch ratio. Of note, CTP‐based and non CTP‐based RCTs shared the same limitation, a suboptimal patient selection as reflected by rates of functional dependency of around 50% despite successful recanalization. 2 , 3 , 6 Recent publications that use the GE CTP 4D show that a Tmax value >16 seconds optimally identified follow‐up infarct when non‐contrast CT (NCCT) to recanalization time was within 90 minutes versus a Tmax value >9.5 seconds that identified follow‐up infarct best when early reperfusion was not achieved. 7 Thus, in patients who achieved recanalization >90 minutes after NCCT Tmax > 16 seconds can be presumed to be the critically hypoperfused tissue. The difference between Tmax > 9.5 seconds and Tmax > 16 seconds volumes (Tmax–Tmax mismatch) can therefore be operationally considered as penumbra. Tmax is a relatively robust parameter to measure on CTP. The aim of the present study was to assess the utility of this Tmax based mismatch paradigm in predicting imaging and clinical outcomes. Additionally, this analysis sought to identify the optimal Tmax volume thresholds that would identify patients with favorable functional outcome on follow‐up.
Patients and Methods
This cohort study was approved by the local ethics boards of each participating site in which CT scans were performed and clinical information were recorded during routine clinical activity. Written informed consent was obtained from each patient or from their legally authorized representatives at admission or waived by the institutional review board.
Patient Selection
This was a retrospective analysis conducted on a prospectively collected cohort of consecutive patients with AIS with anterior circulation LVO treated with EVT and admitted from January 2013 to July 2017 at 2 academic Italian centers: S. Anna University Hospital of Ferrara and Careggi University Hospital of Florence. At both institutions, all patients presenting with suspected AIS with LVO, and no history of renal failure or contrast allergy routinely undergo NCCT, CT angiography (CTA) of the cervical and intracranial vessels and CTP at admission if they arrive at the hospital within 24 hours of symptom onset. If diagnosis of AIS is confirmed by neuroimaging findings, patients with AIS receive EVT according to the current recommended guidelines. 8 , 9 Patients were included if they presented to the emergency department with the following criteria: (1) NCCT Alberta Stroke Programme Early Computed Tomography Score (ASPECTS) ≥ 6; (2) diagnosis of AIS within 24 hours from witnessed symptom onset or time last seen well; (3) evidence of internal carotid artery (ICA) and/or middle cerebral artery (MCA) M1 or M2 segment occlusion on CTA; (4) CTP performed at admission; (5) selected for receiving EVT; and (6) follow‐up NCCT imaging performed at 24 hours. Exclusion criteria were: (1) NCCT ASPECTS < 6; (2) age < 18 years; (3) pregnancy; (4) severe pre‐stroke disability defined as modified Rankin Scale (mRS) ≥3; (5) detection of intracerebral hemorrhage on admission NCCT; (6) contraindications to iodinated contrast agent; (7) poor quality of CT acquisition due to motion artifacts; and (8) inability to complete multimodal CT protocol at baseline and/or 24‐hour follow‐up NCCT. NCCT ASPECTS (ASPECTS ≥ 6) was used for establishing patient eligibility for EVT before publication of the 2015 American Hospital Association / American Society of Anesthesiologists (AHA/ASA) guidelines, 9 based on the analysis by Puez and colleagues. 10 As suggested in the 2013 AHA/ASA guidelines, 8 CTP was used to provide additional information regarding diagnosis. No patient was excluded from the study due to a CTP unfavorable profile because CTP was not performed in patients with low ASPECTS (NCCT ASPECTS < 6). Figure S1 illustrates the cohort selection process.
Clinical Assessment
Demographic and clinical variables were collected by investigators blinded to the outcomes of interest. In particular, we obtained data on age, sex, pre‐stroke functional status (mRS), known versus unknown stroke onset time, intravenous fibrinolysis with recombinant tissue plasminogen activator (r‐TPA), time from onset to baseline CT, and time from baseline CT to EVT conclusion. Baseline stroke severity was measured with the National Institute of Health Stroke Scale (NIHSS). Clinical outcome was measured using mRS at 3 months. The mRS ≤ 2 and >2 were defined as good and poor outcome, respectively.
Imaging Acquisition
All imaging was conducted on 64‐slice scanners (GE Healthcare, Waukesha, WI). NCCT helical scans were performed from the skull base to the vertex using the following imaging parameters: 120 kV, 340 mA, 4 × 5‐mm collimation, 1 second/rotation, and table speed of 15 mm/rotation. CTA of the cervical and intracranial vessels was performed as follows: 0.7 ml/kg contrast (maximum 90 ml), 5‐ to 10‐second delay from injection to scanning, 120 kV, 270 mA, 1 second/rotation, 1.25‐mm thick slices, and table speed 3.75 mm/rotation. CTA covered from the carotid bifurcation to vertex. CTP studies were obtained with a dynamic first‐pass bolus‐tracking methodology according to a 2‐phase imaging protocol, to avoid the truncation of time density curves, with axial shuttle mode. The 2‐phase acquisition consisted of a first phase every 2.8 seconds for 60 seconds and an additional second phase every 15 seconds for 90 seconds, which started 5 seconds after the automatic injection of 40 ml of non‐ionic contrast agent followed by a saline flush of 40 ml at the rate of 4 ml/s. Sections of 8 cm thickness were acquired at 5 mm slice thickness. The other acquisition parameters were 80 kV, 140 mAs, and 0.5 rotation time. All CTP source images were reconstructed with the standard filter and display field of view (DFOV) of 25 cm.
Imaging Processing and Analysis
The extent of early ischemic changes was evaluated on baseline NCCT using the ASPECTS methodology. 10 Each CTP study was processed by commercially available delay‐insensitive deconvolution software (CT Perfusion 4D; GE Healthcare), as described elsewhere. 7 In‐plane patient motion was corrected using an automated registration program included in the software, and the images with extreme motion at specific time points were manually removed, as needed, by visual inspection of the cine series and time density curve (TDC). For each study, the TDC for the arterial input function (AIF) and for venous output function (VOF) were measured from the basilar artery, ICA, or anterior cerebral artery and from the superior sagittal sinus, respectively using a 2 voxel × 2 voxel (in‐slice) regions of interest (ROIs). The VOF‐TDC was used to correct for partial volume averaging in the AIF. CBF, cerebral blood volume (CBV), and Tmax maps were generated for each patient by deconvolving the AIF from tissue TDCs. CBF, CBV, and Tmax values were expressed in ml·min−1·(100 g)−1, ml·(100 g)−1 and seconds, respectively. Average CTP maps were created by averaging the cine (dynamic) CTP source images over the duration of the first pass of contrast. These average CTP images were used to exclude cerebrospinal fluid (CSF) and skull from analysis using Hounsfield unit (HU) thresholds. Large blood vessels were automatically excluded from calculation by the software. Critically hypoperfused tissue and ischemic core volumes were defined as ischemic brain regions with Tmax values >9.5 seconds and >16 seconds, respectively, and were automatically segmented and calculated by the software (Figure 1). According to the Tmax target mismatch paradigm, 2 , 4 , 5 the difference between Tmax > 9.5 seconds and Tmax > 16 secondsvolumes (Tmax mismatch) was considered as ischemic penumbra, and the ratio between Tmax > 9.5 seconds and Tmax > 16 seconds volumes was defined as the Tmax mismatch ratio. Occlusion sites were identified on CTA and classified as cervical ICA, terminal ICA, middle cerebral artery (MCA) M1 segment, or MCA M2 segment occlusions. CTA collateral supply was graded on a 4‐point scale according to a previously published scoring system in which collaterals were categorized as absent (score 0), >0% but ≤50% (score 1), >50% but <100% (score 2) and 100% (score 3) of the occluded territory. 11 Recanalization was assessed on conventional digital subtraction angiography (DSA) at the end of endovascular therapy using the modified treatment in cerebral ischemia (mTICI) scale. 12 Patients with mTICI score of 2b or 3 were considered as successfully recanalized, whereas patients with mTICI score ranging from 0 to 2a were classified as not. Hemorrhagic transformation (HT) was classified on NCCT at 24 hours from symptom onset/last known well according to the European Cooperative Acute Stroke Study (ECASS)‐II criteria into four different categories: hemorrhagic infarction type 1 (HI1), HI type 2 (HI2), Parenchymal hemorrhage type 1 (PH1), and PH type 2 (PH2). 13 Symptomatic intracranial hemorrhage (sICH) was considered as any intracranial hemorrhage associated with a ≥4‐point increase in NIHSS. Final infarct volume (FIV) was measured on follow‐up NCCT at 24 hours after symptom onset/last known well with a multislice planimetric method by summation of the hypodense areas, manually traced on each slice in which they were detectable, multiplied by slice thickness. 14
FIGURE 1.
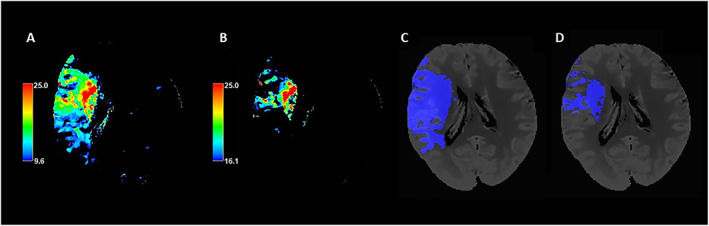
Automatic segmentation of critically hypoperfused tissue and infarct core volumes based on Tmax >9.5seconds and >16 seconds threshold values. Panel A: Color coded CTP Tmax map with scale set at 9.6 to 25 seconds. Panel B: Color coded CTP Tmax map with scale set at 16.1 to 25 seconds. Panel C: Tmax >9.5 seconds volume automatically segmented on CTP averaged images. Panel D: Tmax >16 seconds volume automatically segmented on CTP averaged images. CTP = computed tomography perfusion; Tmax = time to maximum concentration. [Color figure can be viewed at www.annalsofneurology.org]
Statistical Analysis
Continuous variables were summarized as median (interquartile range [IQR]) or mean (standard deviation [SD]) as appropriate based on their distribution assessed with the Shapiro–Wilk test. Mann–Whitney test and Student's t test were used to compare continuous variables with non‐normal and normal distributions, respectively. Categorical variables were summarized as count (percentage) and compared using the chi‐square test. Good functional prognosis (defined as mRS 0–2) at 90 days from stroke onset and FIV and were the main outcomes of interest. Variables associated with good functional outcome were assessed using multivariable logistic regression, adjusting for age, admission NIHSS, ASPECTS score, collateral score, reperfusion status, and any variable showing significance at p < 0.1 in univariable analysis. Variables associated with FIV were explored with multivariable linear regression after testing for normality of residuals and heteroskedasticity (with log‐transformation of FIV). Models were adjusted for age, admission NIHSS score, ASPECTS score, collateral score, reperfusion status (defined as mTICI score 2b/3) and variables with p < 0.1 in univariable analysis. Backward elimination was used in both models to reach to a final parsimonious model that avoids model overfitting. Interaction terms were used in regression models to test the interaction among Tmax parameters, reperfusion status, and time to reperfusion (defined as time from baseline NCCT to DSA end). The utility of different Tmax volumes and Tmax mismatch ratio for identification of patients with good functional outcome was analyzed using area under the curve (AUC) Receiver Operating Characteristic (ROC) curves and optimal sensitivity, specificity, positive predictive value, and negative predictive values identified using the Youden Index. Comparison of models with Tmax > 9.5 seconds, Tmax > 16 seconds, mismatch volume and mismatch ratios as independent variables and the mRS 0 to 2 at 90 days as dependent variable was performed using the DeLong test. 15 A secondary analysis was focused on subjects presenting after 6 hours from symptom onset or time last seen well, with the same outcomes of interest explored in the main analysis. In this subgroup of patients, the model building strategy was the same as in the main analyses. Finally, a sensitivity analysis explored the diagnostic performance of Tmax parameters in patients who achieved mTICI (2b/3) post EVT versus those achieving mTICI ≤ 2a. All analyses were performed with the statistical packages SPSS version 21.0 (www.spss.com) and MedCalc (www.medcalc.org). Statistical significance was set at two‐sided p < 0.05.
Results
We screened 477 potentially eligible patients with AIS with anterior circulation LVO, of whom 84 patients were excluded due to the presence of low ASPECTS (<6) on baseline NCCT, baseline intracerebral hemorrhage (n = 19), inability to complete multimodal CT protocol at baseline and/or 24‐hour follow‐up NCCT (n = 15), poor quality of CT acquisition due to motion artifacts (n = 12), contraindications to iodinated contrast agent (known contrast allergy, renal failure) (n = 8), pre‐stroke mRS >3 (n = 5), pregnancy (n = 3), and < 18 of age (n = 2). The study selection flowchart is illustrated in Figure S1.
Overall, 393 patients (median age = 73, IQR = 65–78, 54.7% men, median NIHSS = 14, IQR = 10–20) met study eligibility criteria. Of these, 314 were selected for EVT in the early (<6 hours) and 79 in the late (6–24 hours) time windows. Most of the included patients had a middle cerebral artery occlusion in the M1 segment and around one third of the study population received r‐TPA before EVT. Table 1 summarizes the patient characteristics and shows the comparison between patients with good and poor functional outcome at 3 months. In univariable analyses, patients with good prognosis had lower Tmax > 9.5 seconds volumes, Tmax > 16 seconds volumes, Tmax mismatch volumes, and higher Tmax mismatch ratio. As shown in Table 2, all these associations between Tmax parameters and clinical outcome remained significant in multivariable logistic regression analysis after adjustment for potential confounders. No statistically significant interaction was noted among Tmax > 9.5 seconds volume, Tmax > 16 seconds volume, and reperfusion time or reperfusion status in association with 90 day clinical outcome (all p values for interaction > 0.1). Conversely, the association between Tmax mismatch volume and outcome was significant only in patients with time from CT to reperfusion <165 minutes (p value for interaction < 0.001). Moreover, Tmax mismatch ratio predicted functional outcome only in patients achieving a mTICI score of 2b/3 (p for interaction = 0.017). The ROC curve analysis shown in Figure 2 demonstrated that Tmax > 16 seconds volume had the highest discriminative ability (AUC 0.878; 95% confidence interval [CI] = 0.842–0.909) for 90 day clinical outcome. When Tmax volumes and Tmax mismatch ratio were analyzed as dichotomous variables, the optimal cutoff points for predicting favorable 90‐day clinical outcome were ≤111.6 ml for Tmax > 9.5 seconds, ≤67.0 ml for Tmax > 16 seconds, ≤58.3 ml for Tmax mismatch volume, and >2.5 for Tmax mismatch ratio. Tmax > 16 seconds volume ≤67.0 ml had the highest sensitivity (0.91; 95% CI = 0.87–0.94) for identification of subjects with favorable 90‐day clinical outcome, whereas Tmax > 9.5 seconds volume ≤111.6 ml showed the highest specificity (0.82; 95% CI = 0.74–0.88). The test characteristics of different Tmax volumes are summarized in Table 3. In a secondary analysis stratified by mTICI status, results remained consistent. In particular, Tmax > 16 secondsvolume ≤67.0 ml remained the most sensitive (>0.90) parameter for the identification of patients with good prognosis, regardless of the degree of recanalization (Table S1).
TABLE 1.
Study Population Characteristics
| All | mRS 0–2 | mRS 3–6 | ||
|---|---|---|---|---|
| n = 393 | n = 258 | n = 135 | p | |
| Age, median (IQR), yr | 73 (65–78) | 72 (64–78) | 75 (68–80) | <0.001 |
| Sex, males, n (%) | 215 (54.7) | 136 (52.7) | 79 (58.5) | 0.272 |
| Pre‐stroke mRS | ||||
| 0, n (%) | 324 (82.4) | 220 (85.3) | 104 (77.0) | 0.047 |
| 1, n (%) | 56 (14.2) | 33 (12.8) | 23 (17.0) | |
| 2, n (%) | 13 (3.3) | 5 (1.9) | 8 (5.9) | |
| Admission NIHSS, median (IQR) | 14 (10–20) | 12 (9–15) | 21 (17–23) | <0.001 |
| ASPECTS score, median (IQR) | 9 (8–10) | 9 (8–10) | 8 (7–10) | <0.001 |
| Occlusion site | ||||
| Cervical ICA, n (%) | 86 (21.9) | 44 (17.1) | 42 (31.1) | <0.001 |
| Terminal ICA, n (%) | 14 (3.6) | 4 (1.6) | 10 (7.4) | |
| MCA M1, n (%) | 266 (67.7) | 188 (72.9) | 78 (57.8) | |
| MCA M2, n (%) | 27 (6.9) | 22 (8.5) | 5 (3.7) | |
| r‐TPA before EVT, n (%) | 126 (32.1) | 88 (34.1) | 38 (28.1) | 0.229 |
| Time from onset to NCCT, median (IQR) minutes | 279 (118–333) | 281 (118–327) | 257 (116–354) | 0.961 |
| Time from NCCT to reperfusion, median (IQR), minutes | 124 (100–163) | 112 (96–138) | 151 (121–213) | <0.001 |
| Collateral score | ||||
| 0, n (%) | 14 (3.6) | 0 (0.0) | 14 (10.4) | <0.001 |
| 1, n (%) | 106 (27.0) | 17 (6.6) | 89 (65.9) | |
| 2, n (%) | 155 (39.4) | 134 (51.9) | 21 (15.6) | |
| 3, n (%) | 118 (30.0) | 107 (41.5) | 11 (8.1) | |
| mTICI score | ||||
| 0, n (%) | 34 (8.7) | 8 (3.1) | 26 (19.3) | <0.001 |
| 1, n (%) | 24 (6.1) | 1 (0.4) | 21 (17.0) | |
| 2a, n (%) | 37 (9.4) | 4 (1.6) | 33 (24.4) | |
| 2b, n (%) | 63 (16.0) | 40 (15.5) | 23 (17.0) | |
| 3, n (%) | 235 (59.8) | 205 (79.5) | 30 (22.2) | |
| Tmax >9.5 seconds volume, median (IQR), ml | 102 (55–160) | 69 (41–112) | 175 (121–218) | <0.001 |
| Tmax >16 seconds volume, median (IQR), ml | 30 (11–77) | 20 (6–35) | 93 (50–110) | <0.001 |
| Tmax mismatch volume, median (IQR), ml | 56 (35–91) | 48 (29–73) | 81 (51–117) | <0.001 |
| Tmax mismatch ratio, median | 2.7 (2.0–5.2) | 3.7 (2.4–6.7) | 2.0 (1.6–2.5) | <0.001 |
| Final infarct volume, median (IQR), ml | 29 (10–92) | 16 (5–29) | 103 (87–135) | <0.001 |
| Hemorrhagic transformation ECASS II | ||||
| None, n (%) | 252 (64.1) | 195 (75.6) | 57 (42.2) | <0.001 |
| HT1, n (%) | 63 (16.0) | 41 (15.9) | 22 (16.3) | |
| HT2, n (%) | 32 (8.1) | 11 (4.3) | 21 (15.6) | |
| PH1, n (%) | 20 (5.1) | 7 (2.7) | 13 (9.6) | |
| PH2, n (%) | 26 (6.6) | 4 (1.6) | 22 (16.3) | |
| sICH, n (%) | 39 (9.9) | 7 (2.7) | 32 (23.7) | <0.001 |
| Any hemorrhagic transformation, n (%) | 141 (35.8) | 78 (57.8) | 63 (24.4) | <0.001 |
ASPECTS = Alberta Stroke Program Early Computed Tomography Score; DSA = digital subtraction angiography; ECASS = European Cooperative Acute Stroke Study; EVT = endovascular treatment; HI1 = hemorrhagic infarction type 1; HI2 = hemorrhagic infarction type 2; ICA = internal carotid artery; IQR = interquartile range; MCA = middle cerebral artery; mRS = modified Rankin Scale; mTICI = modified treatment in cerebral infarction score; NCCT = non‐contrast computed tomography; NIHSS = National Institute of Health Stroke Scale; PH1 = parenchymal hemorrhage type 1; PH2 = parenchymal hemorrhage type 2; r‐TPA = recombinant tissue plasminogen activator; sICH = symptomatic intracerebral hemorrhage; Tmax = time to maximum concentration.
TABLE 2.
Multivariable Predictors of Good Functional Outcome
| OR (95% CI) | p | |
|---|---|---|
| MODEL 1 | ||
| Age, yr | 0.96 (0.92–1.01) | 0.085 |
| Admission NIHSS | 0.84 (0.78–0.91) | <0.001 |
| Collateral score | 9.74 (4.90–19.34) | <0.001 |
| mTICI score | 5.03 (3.17–7.98) | <0.001 |
| Tmax >9.5 seconds volume, ml | 0.98 (0.97–0.99) | <0.001 |
| MODEL 2 | ||
| Admission NIHSS | 0.83 (0.76–0.90) | <0.001 |
| Collateral score | 7.29 (3.76–14.13) | <0.001 |
| mTICI score | 4.76 (3.06–7.42) | <0.001 |
| Tmax >16 seconds volume, ml | 0.96 (0.95–0.97) | <0.001 |
| MODEL 3 | ||
| Admission NIHSS | 0.82 (0.76.0.88) | <0.001 |
| Collateral score | 11.28 (5.91–21.54) | <0.001 |
| mTICI score | 4.53 (2.976.92) | <0.001 |
| Tmax mismatch volume, ml | 0.98 (0.97–0.99) | <0.001 |
| MODEL 4 | ||
| Admission NIHSS | 0.80 (0.74–0.87) | <0.001 |
| Collateral score | 8.58 (4.69–15.72) | <0.001 |
| mTICI score | 4.18 (2.83–6.18) | <0.001 |
| Tmax mismatch ratio | 1.17 (1.02–1.35) | 0.026 |
Logistic regression with backward elimination at p < 0.1. Variables entered into the model: age, pre‐stroke mRS, and NIHSS, ASPECTS, time from CT to recanalization, mTICI, collateral score, occlusion site; Tmax parameters entered separately into different models. ASPECTS = Alberta Stroke Programme Early Computed Tomography Score; CI = confidence interval; CT = computed tomography; mRS = modified Rankin Scale; mTICI = modified treatment in cerebral infarction score; NIHSS = National Institute of Health Stroke Scale; OR = odds ratio; Tmax = time to maximum concentration.
FIGURE 2.
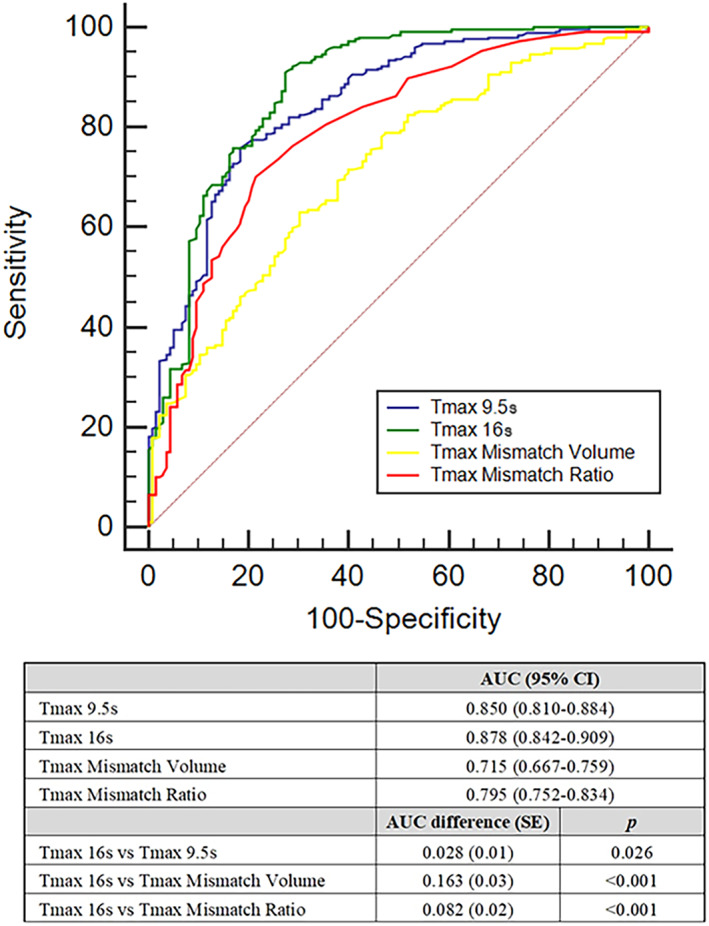
Tmax volumes and Tmax mismatch ratio optimal values for recognizing patients with AIS with good clinical outcome as calculated using ROC curves. AIS = acute ischemic stroke; AUC = area under the curve; CI = confidence interval; ROC = Receiver Operating Characteristic; SE = standard error; Tmax = time to maximum concentration; Tmax 9.5 seconds, Tmax >9.5 seconds volume; Tmax 16 seconds, Tmax >16 seconds volume. AUC comparison was performed with DeLong test. Outcome of interest: modified Rankin Scale 0 to 2 at 90 days. [Color figure can be viewed at www.annalsofneurology.org]
TABLE 3.
Test Characteristics of Tmax Volumes and Tmax Mismatch Ratio
| Sensitivity(95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|
| Tmax > 9.5 seconds volume ≤ 111.6 ml | 0.76 (0.70–0.81) | 0.82 (0.74–0.88) | 0.89 (0.84–0.92) | 0.64 (0.59–0.69) |
| Tmax > 16 seconds volume ≤ 67.0 ml | 0.91 (0.87–0.94) | 0.73 (0.64–0.80) | 0.86 (0.83–0.89) | 0.81 (0.74–0.87) |
| Tmax mismatch volume ≤ 58.3 ml | 0.63 (0.57–0.69) | 0.70 (0.61–0.77) | 0.80 (0–75‐0.84) | 0.50 (0.45–0.55) |
| Tmax mismatch ratio > 2.5 | 0.70 (0.64–0.76) | 0.79 (0.71–0.85) | 0.86 (0.82–0.90) | 0.58 (0.53–0.63) |
CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value; Tmax = time to maximum concentration. Outcome of interest: modified Rankin Scale 0 to 2 at 90 days.
As illustrated in Table 4, Tmax > 9.5 seconds volume, Tmax > 16 seconds volume, Tmax mismatch volume and Tmax mismatch ratio were all associated with the extent of FIV in unadjusted analysis. All Tmax variables remained independently associated with FIV (p < 0.001) in multivariable linear regression (Table 5). When the analysis was restricted to 79 patients presenting 6 hours from stroke onset, of whom 47 (59.5%) had favorable functional outcome, the discriminative ability of Tmax volumes and Tmax mismatch volume remained good (Tmax > 9.5 seconds volume, AUC 0.86, p < 0.001; Tmax > 16 seconds volume AUC 0.88, p < 0.001; Tmax mismatch volume AUC 0.74, p < 0.001; Tmax mismatch ratio AUC 0.77, p < 0.001). In this subgroup of patients, the optimal value for predicting good outcome was similar to that obtained in overall analyses with Tmax > 9.5 seconds volume (≤114.4 ml, sensitivity 0.72, specificity 0.84), Tmax > 16 secondsvolume (≤67.4 ml, sensitivity 0.89, and specificity 0.75) and Tmax mismatch ratio (>2.8, sensitivity 0.62 and specificity 0.84), whereas Tmax mismatch volume was lower than overall analysis (≤47.1 ml, sensitivity 0.53, and specificity 0.84). Tmax > 9.5 seconds volume ≤ 114.4, ml and Tmax > 16 seconds volume ≤ 67.4 ml were the most reliable parameters for identification of patients with favorable clinical outcome, with highest sensitivity (89%) and specificity (84%), respectively. Logistic and linear regression models showed that all Tmax parameters remained independently associated with functional outcome and FIV in these late presenters, except for the lack of association between Tmax mismatch ratio and outcome at 90 days (odds ratio [OR] = 1.11; 95% CI = 0.86–1.43; p = 0.431). Two illustrative cases showing the application of Tmax volumes based on the established thresholds of >9.5 seconds and >16 secondsare depicted in Figure 3.
TABLE 4.
Univariable Predictors of Final Infarct Volume
| B (SE) | p | |
|---|---|---|
| Age, yr | 0.151 (0.003) | 0.003 |
| Sex, male | −0.021 (0.066) | 0.675 |
| Admission NIHSS | 0.581 (0.004) | <0.001 |
| ASPECTS score | −0.239 (0.021) | <0.001 |
| Carotid occlusion | 0.209 (0.073) | <0.001 |
| Unknown onset | −0.082 (0.092) | 0.106 |
| r‐TPA before EVT | 0.022 (0.071) | 0.664 |
| Time from onset to NCCT, minutes | 0.035 (0.000) | 0.492 |
| Time from NCCT to DSA end, minutes | 0.331 (0.000) | <0.001 |
| Collateral score | −0.482 (0.034) | <0.001 |
| mTICI 2b/3 | −0.428 (0.069) | <0.001 |
| Tmax > 9.5 volume, ml | 0.747 (0.000) | <0.001 |
| Tmax > 16 volume, ml | 0.773 (0.000) | <0.001 |
| Tmax mismatch volume, ml | 0.523 (0.001) | <0.001 |
| Tmax mismatch ratio | −0.460 (0.002) | <0.001 |
ASPECTS = Alberta Stroke Program Early Computed Tomography Score; B = beta coefficient; DSA = digital subtraction angiography; EVT = endovascular treatment; mTICI = modified treatment in cerebral infarction score; NCCT = non‐contrast computed tomography; NIHSS = National Institute of Health Stroke Scale; r‐TPA = recombinant tissue plasminogen activator; SE = standard error.
TABLE 5.
Multivariable Predictors of Final Infarct Volume
| B (SE) | p | |
|---|---|---|
| MODEL 1 | ||
| Admission NIHSS | 0.189 (0.004) | <0.001 |
| Time from NCCT to DSA end, minutes | 0.077 (0.000) | 0.017 |
| Collateral score | −0.090 (0.027) | 0.012 |
| mTICI 2b/3 | −0.169 (0.049) | <0.001 |
| Tmax > 9.5 seconds volume, ml | 0.544 (0.000) | <0.001 |
| MODEL 2 | ||
| Admission NIHSS | 0.203 (0.081) | <0.001 |
| Time from NCCT to DSA end, minutes | 0.075 (0.000) | 0.017 |
| Collateral score | −0.146 (0.048) | <0.001 |
| mTICI 2b/3 | 0.596 (0.001) | <0.001 |
| Tmax > 16 seconds volume, ml | 0.596 (0.001) | <0.001 |
| MODEL 3 | ||
| Admission NIHSS | 0.282 (0.004) | <0.001 |
| Time from NCCT to DSA end, minutes | 0.099 (0.000) | 0.007 |
| Collateral score | −0.199 (0.030) | <0.001 |
| mTICI 2b/3 | −0.203 (0.056) | <0.001 |
| Tmax mismatch volume, ml | 0.333 (0.001) | <0.001 |
| MODEL 4 | ||
| Admission NIHSS | 0.320 (0.004) | <0.001 |
| Time from NCCT to DSA end, minutes | 0.110 (0.000) | 0.003 |
| Collateral score | −0.195 (0.031) | <0.001 |
| mTICI 2b/3 | −0.205 (0.057) | <0.001 |
| Tmax mismatch ratio | −0.307 (0.002) | <0.001 |
Logistic regression with backward elimination at p < 0.1. Variables entered into the model: age, NIHSS, ASPECTS, carotid occlusion, time from CT to recanalization, collateral score, mTICI 2b/3, Tmax, Tmax parameters entered separately into different models.
B = beta coefficient; CT = computed tomography; DSA = digital subtraction angiography; EVT = endovascular treatment; mTICI = modified treatment in cerebral infarction score; NCCT = non‐contrast computed tomography; NIHSS = National Institute of Health Stroke Scale; r‐TPA = recombinant tissue plasminogen activator; Tmax = time to maximum concentration; SE = standard error.
FIGURE 3.
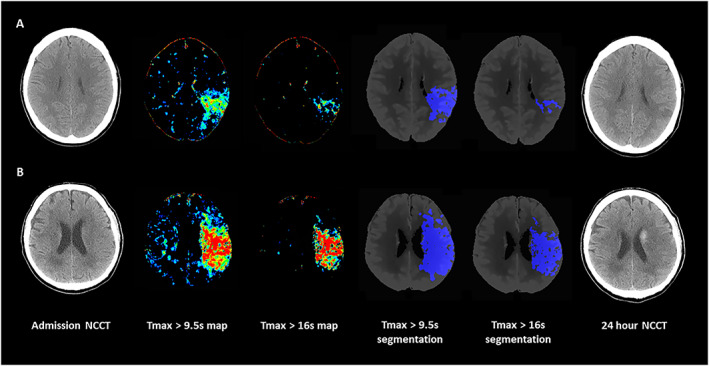
Tmax volumes in 2 patients with AIS and anterior circulation large vessel occlusion. Panel A: NCCT at admission, color coded CTP Tmax map with scale set at 9.6 to 25 seconds, Color coded CTP Tmax map with scale set at 16.1 to 25 seconds, Tmax > 9.5 seconds volume automatically segmented on CTP averaged images, Tmax > 16 seconds volume automatically segmented on CTP averaged images and NCCT at 24 hours in a 48 year‐old patient with AIS and ischemic lesion in left MCA territory who presented the following Tmax parameters: critically hypoperfused tissue = 39.5 ml; core volume = 8.7 ml; penumbra volume = 30.8 ml; and mismatch ratio = 4.5. FIV was 7.2 ml and mRS at 3 months was 0. Panel B: NCCT at admission, color coded CTP Tmax map with scale set at 9.6 to 25 seconds, Color coded CTP Tmax map with scale set at 16.1 to 25 seconds, Tmax >9.5 seconds volume automatically segmented on CTP averaged images, Tmax >16 seconds volume automatically segmented on CTP averaged images and NCCT at 24 hours with hemorrhagic transformation in a 67 year old patient with AIS and ischemic lesion in left MCA territory who presented the following Tmax parameters: critically hypoperfused tissue = 158.0 ml; core volume = 87.4 ml; penumbra volume = 70.6 ml; mismatch ratio = 1.8. FIV was 131.2 ml and mRS at 3 months was 4. AIS = acute ischemic stroke; CTP = computed tomography perfusion; FIV = final infarct volume; MCA = middle cerebral artery; mRS = modified Rankin Scale; NCCT = non‐contrast computed tomography; Tmax = time to maximum concentration. [Color figure can be viewed at www.annalsofneurology.org]
Discussion
The main finding of this study is that multiple CTP Tmax parameters are independently associated with functional outcome and with final infarct extent in patients with AIS undergoing EVT for LVO. In the context of RCTs showing the effectiveness of EVT in patients with AIS with LVO, CTP provided additional value in the early time window 1 , 4 and was mandatory in the late time window 2 , 3 for patients’ selection. Inclusion criteria in these RCTs were based on the assumption that Tmax > 6 seconds and rCBF < 30% threshold values represented critically hypoperfused tissue and ischemic core, respectively, and could be used to select patients for EVT. However, the identification of patients likely to benefit from EVT remains suboptimal as 50% of patients with AIS receiving successful reperfusion do not achieve a good functional outcome. 2 , 3 , 6 A recent study suggested that critically hypoperfused tissue and ischemic core could be better defined by Tmax > 9.5 seconds and Tmax > 16 seconds threshold values (GE CTP4D), respectively. 7 Our findings expand this previous observation demonstrating the ability of these parameters to predict radiological and clinical outcomes.
Our results are in line with previous investigations showing that in patients with AIS who underwent EVT < 6 hours 16 and 6–16 hours 17 after symptom onset/last known well time, ischemic core and hypoperfusion volumes were associated with FIV. In addition, as in a prior work on patients receiving EVT < 6 hours from onset, 18 we also confirm that the 2 variables indicating penumbra size, namely, Tmax mismatch volumes and Tmax mismatch ratio, predict FIV. The inclusion in our analysis of both patients who achieved reperfusion, in whom penumbral tissue is presumed to be saved, and patients who did not achieve reperfusion, in whom penumbra evolves into infarct, could in part explain our results. More interesting was the demonstration that Tmax > 9.5 seconds volumes, Tmax > 16 seconds volumes, Tmax mismatch volumes, and Tmax mismatch ratio were associated 90‐day good outcome. This association was in line with data from RCTs 1 , 2 , 3 , 4 and other studies 18 , 19 , 20 suggesting that patients with AIS with favorable target mismatch profile achieved a good response to EVT. In this setting, the high predictive value of Tmax > 9.5 seconds and the poor performance of Tmax mismatch ratio for favorable outcome are not unexpected. Whereas Tmax > 9.5 seconds volume represents critically hypoperfused tissue, including both ischemic core and ischemic penumbra, which strongly correlates with good prognosis, prior evidence suggests that mismatch ratios cannot be considered as robust markers of favorable outcome. 21 It is important to highlight that Tmax volumes and mismatch ratio were associated with FIV and 90 day clinical outcomes independently from NIHSS, recanalization, and collateral score, which are considered strong predictors of radiological and clinical outcomes. In particular, Tmax parameters and collaterals predicted outcome independently from each other, suggesting that Tmax is not an epiphenomenon of collateral extent, but a complementary marker of delay in vessel filling of ischemic tissue. 22
The optimal cutoffs for Tmax parameters that predict good clinical outcome are similar to those in previous studies: a hypoperfusion volume ≤ 111.6 ml was comparable to the optimal value selected in DEFUSE 2 (<100 ml), 23 whereas an infarct volume ≤ 67.0 ml was similar to a cutoff point used in EXTEND‐IA 5 and DEFUSE 3 2 (<70 ml). A penumbra volume ≤ 58.3 ml was very different from the cut‐off values used in EXTEND‐IA 5 (>10 ml), and in DEFUSE 32 and SWIFT PRIME 4 (>15 ml), but in agreement with the concept that a RAPID Tmax > 8 seconds volume > 85 mL 24 indicates a malignant profile reflecting severely hypoperfused tissue destined to progress into infarction. A Tmax mismatch ratio >2.5 in this analysis was higher than the thresholds used in SWIFT PRIME (>1.2), 4 and in EXTEND‐IA 5 and DEFUSE 3 2 (>1.8), but equivalent to the value proposed by other investigators (>2.6) 21 who recommended a larger mismatch ratio for better detecting the benefit of reperfusion therapies. Optimal thresholds to discriminate good outcome did not substantially change when the subset of patients presenting in the late time window were examined. Taken together, these data suggest that the possibility of achieving a favorable outcome increases in the presence of a small ischemic core volume, a significant mismatch between the extent of core and hypoperfusion, and a large, but not oversized, ischemic penumbra volume. Moreover, this study proposes a new CTP target mismatch based on specific threshold values of Tmax alone for identifying critically hypoperfused tissue (Tmax > 9.5 seconds) and ischemic core (Tmax > 16 seconds).
This study is not without limitations. First, as this study was based on retrospective analysis, our findings require prospective validation. Second, this was a non‐randomized study consisting of a selected population in which eligibility for EVT was decided by local stroke team and data from patients not receiving EVT were lacking. Therefore, we cannot exclude the influence of unmeasured confounders and of selection bias in our analysis. Third, early presenters were more represented than late presenters, making the 2 groups unbalanced. Fourth, these results are conditional on the use of the GE CTP 4D algorithm; corresponding values for Tmax may need to be derived for other CTP algorithms. 25 , 26 Fifth, a comparison with RAPID thresholds as part of the DEFUSE 3 selection criteria was not performed, and therefore we are unable to comment whether Tmax target mismatch is different to DEFUSE 3 parameters based on Tmax‐CBF mismatch for differentiating good and poor radiological and clinical outcomes. Sixth, although consistent with some previous trials and meta‐analyses, 2 , 27 the use of a dichotomized mRS may have reduced statistical power and neglected possible outcome shifts in the mRS range 3 to 6. 28 Seventh, the different devices used in our study period and the evolution of EVT technologies therefore during this period 29 may have influenced the results of this analysis. Finally, the exclusion of patients with low ASPECTS scores did not allow us to verify the rates of patients with unfavorable NCCT and favorable CTP profiles who achieved a good functional outcome that was reported to be about 60% in a recent study. 19
In conclusion, we demonstrate the potential of a simple Tmax only target mismatch paradigm in predicting radiological and clinical outcomes in patients with AIS undergoing EVT both in the early and late time windows. Further prospective studies are warranted to clarify the actual applicability of Tmax target mismatch in selecting patients with AIS with LVO who can benefit from EVT.
Author Contributions
Conception and design of study: A.M. and E.F. Acquisition and analysis of data: all authors. Drafting manuscript and figures: all authors.
Potential Conflict of Interest
T.‐Y.L. has a licensing agreement with GE Healthcare for the CT Perfusion software. Dr. Goyal participated to ESCAPE, REVASCAT, and SWIFT PRIME trials. Dr. Demchuk contributed to ESCAPE and REVASCAT trials and Dr. Menon was involved in the ESCAPE trial.
Supporting information
Figure S1 Cohort selection flowchart. AIS = acute ischemic stroke; ASPECTS = Alberta Stroke Programme Early Computed Tomography Score; NCCT = non‐contrast computed tomography; mRS = modified Rankin Scale.
Table S1 Tmax performance for good outcome prediction stratified by mTICI status.
Acknowledgment
Open Access Funding provided by Universita degli Studi di Firenze within the CRUI‐CARE Agreement.
References
- 1. Campbell BCV. Optimal imaging at the primary stroke center. Stroke 2020;51:1932–1940. [DOI] [PubMed] [Google Scholar]
- 2. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 4. Saver JL, Goyal M, Bonafe A, et al. Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. N Engl J Med 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 5. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 6. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 7. d'Esterre CD, Boesen ME, Ahn SH, et al. Time‐dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke 2015;46:3390–3397. [DOI] [PubMed] [Google Scholar]
- 8. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 9. Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:3020–3035. [DOI] [PubMed] [Google Scholar]
- 10. Puetz V, Dzialowski I, Hill MD, et al. The Alberta stroke program early CT score in clinical practice: what have we learned? Int J Stroke 2009;4:354–364. [DOI] [PubMed] [Google Scholar]
- 11. Tan IYL, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 2009;30:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaidat OO, Yoo AJ, Khatri P, et al. Cerebral angiographic revascularization grading (CARG) collaborators; STIR revascularization working group; STIR thrombolysis in cerebral infarction (TICI) task force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hacke W, Kaste M, Fieschi C, et al. Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian acute stroke study investigators. Lancet 1998;352:1245–1251. [DOI] [PubMed] [Google Scholar]
- 14. Brott T, Marler JR, Olinger CP, et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke 1989;20:871–875. [DOI] [PubMed] [Google Scholar]
- 15. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics [online serial] 1988;44:837–845. [PubMed] [Google Scholar]
- 16. Albers GW, Goyal M, Jahan R, et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol 2016;79:76–89. [DOI] [PubMed] [Google Scholar]
- 17. Rao V, Christensen S, Yennu A, et al. Ischemic core and hypoperfusion volumes correlate with infarct size 24 hours after randomization in DEFUSE 3. Stroke 2019;50:626–631. [DOI] [PubMed] [Google Scholar]
- 18. Albers GW, Goyal M, Jahan R, et al. Relationships between imaging assessments and outcomes in solitaire with the intention for Thrombectomy as primary endovascular treatment for acute ischemic stroke. Stroke 2015;46:2786–2794. [DOI] [PubMed] [Google Scholar]
- 19. Sarraj A, Hassan AE, Grotta J, et al. Optimizing patient selection for endovascular treatment in acute ischemic stroke (SELECT): a prospective, multicenter cohort study of imaging selection. Ann Neurol 2020;87:419–433. [DOI] [PubMed] [Google Scholar]
- 20. Olivot JM, Albucher JF, Guenego A, et al. Mismatch profile influences outcome after mechanical thrombectomy. Stroke 2021;52:232–240. [DOI] [PubMed] [Google Scholar]
- 21. Kakuda W, Lansberg MG, Thijs VN, et al. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab 2008;28:887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. d'Esterre CD, Trivedi A, Pordeli P, et al. Regional comparison of multiphase computed tomographic angiography and computed tomographic perfusion for prediction of tissue fate in ischemic stroke. Stroke 2017;48:939–945. [DOI] [PubMed] [Google Scholar]
- 23. Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012;11:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mlynash M, Lansberg MG, De Silva DA, et al. Refining the definition of the malignant profile: insights from the DEFUSE‐EPITHET pooled data set. Stroke 2011;42:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kudo K, Sasaki M, Yamada K, et al. Differences in CT perfusion maps generated by different commercial software: quantitative analysis by using identical source data of acute stroke patients. Radiology 2010;254:200–209. [DOI] [PubMed] [Google Scholar]
- 26. Rava RA, Snyder KV, Mokin M, et al. Assessment of computed tomography perfusion software in predicting spatial location and volume of infarct in acute ischemic stroke patients: a comparison of sphere, Vitrea, and RAPID. J Neurointerv Surg 2021;13:130–135. [DOI] [PubMed] [Google Scholar]
- 27. Campbell BCV, Majoie CBLM, Albers GW, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta‐analysis of individual patient‐level data. Lancet Neurol 2019;18:46–55. [DOI] [PubMed] [Google Scholar]
- 28. Saver JL. Optimal end points for acute stroke therapy trials: best ways to measure treatment effects of drugs and devices. Stroke 2011;42:2356–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shafie M, Yu W. Recanalization therapy for acute ischemic stroke with large vessel occlusion: where we are and what comes next? Transl Stroke Res 2021;12:369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Cohort selection flowchart. AIS = acute ischemic stroke; ASPECTS = Alberta Stroke Programme Early Computed Tomography Score; NCCT = non‐contrast computed tomography; mRS = modified Rankin Scale.
Table S1 Tmax performance for good outcome prediction stratified by mTICI status.


