Abstract
Tofacitinib is an oral small molecule JAK inhibitor for the treatment of ulcerative colitis. Relationships between plasma tofacitinib concentration and efficacy were characterized using exposure‐response (E‐R) models, with demographic and disease covariates evaluated as potential predictors of efficacy. Data were from phase II and III (OCTAVE Induction 1 and 2) induction studies, and a phase III maintenance study (OCTAVE Sustain). Induction studies included 1,355 patients (tofacitinib 0.5, 3, 10, or 15 mg b.i.d. or placebo). The maintenance study included 592 patients (tofacitinib 5 or 10 mg b.i.d. or placebo). E‐R models, including induction patients predicted placebo‐adjusted remission rates of 6.4% and 12.7% at week 8 for tofacitinib 5 and 10 mg b.i.d., respectively; corresponding rates in patients without prior tumor necrosis factor inhibitor (TNFi) failure were 12.8% and 20.4%. Estimates to achieve/maintain remission at week 52 of maintenance were 29% and 18% (tofacitinib 5 mg b.i.d.), and 41% and 26% (tofacitinib 10 mg b.i.d.), for patients in remission or not following induction, respectively. During maintenance, patients with prior TNFi failure had lower probability of remission on 5 mg b.i.d. (24.9%) than 10 mg b.i.d. (35.0%). Results indicated tofacitinib 10 mg b.i.d. was an appropriate induction dose but suggested efficacy with 5 mg b.i.d. in patients without prior TNFi failure. Tofacitinib 5 mg b.i.d. was efficacious for maintenance, although patients with prior TNFi failure might see additional benefit on 10 mg b.i.d. Per product labeling, recommended tofacitinib induction dose is 10 mg b.i.d., then maintenance at 5 mg b.i.d. For patients who lose response during maintenance, 10 mg b.i.d. may be considered, limited to the shortest duration.
Clinicaltrials.gov: NCT00787202; NCT01465763; NCT01458951; and NCT01458574.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Exposure‐response analyses can help predict which dosing regimens might be of most benefit to patients.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This study characterized the relationship between tofacitinib exposure and efficacy end points for both induction and maintenance therapy in patients with ulcerative colitis (UC), and identified patient‐specific factors that are important determinants of efficacy.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Results support the use of tofacitinib 10 mg b.i.d. for induction in patients with moderate to severe UC. However, they also suggest tofacitinib 5 mg b.i.d. might be efficacious for induction in patients without prior tumor necrosis factor inhibitor (TNFi) failure. Although 5 mg b.i.d. was efficacious for maintenance, patients with prior TNFi failure might see additional benefit on 10 mg b.i.d. Patients with lower baseline disease activity were more likely to achieve efficacy end points at the end of maintenance.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The findings highlight that prior TNFi status and baseline disease activity are important considerations that may impact efficacy of tofacitinib induction and maintenance therapy in patients with UC.
Ulcerative colitis (UC) is a chronic inflammatory disease localized to the colon, characterized by abdominal symptoms, including diarrhea, abdominal pain, rectal bleeding, and urgency. 1 , 2 Treatments available for patients with UC include 5‐aminosalicylates (5‐ASA), corticosteroids, thiopurines, methotrexate, tumor necrosis factor inhibitors (TNFi; e.g., adalimumab, infliximab, and golimumab), integrin inhibitors (e.g., vedolizumab), interleukin‐12/23 inhibitors (e.g., ustekinumab), and Janus kinase (JAK) inhibitors (e.g., tofacitinib and filgotinib). 3 , 4 , 5 , 6 The aim of treatment is to induce and maintain remission, with a goal of sustained steroid‐free remission. 3
Tofacitinib is an oral small molecule JAK inhibitor for the treatment of UC. The efficacy and safety of tofacitinib 10 mg twice daily (b.i.d.) in patients with moderate to severe UC has been demonstrated as induction therapy in 8‐week phase II (NCT00787202) and phase III (OCTAVE Induction 1 and 2; NCT01465763 and NCT01458951) studies. 7 , 8 Both tofacitinib 5 and 10 mg b.i.d. were investigated further in a 52‐week phase III maintenance study (OCTAVE Sustain; NCT01458574). 8 The approved tofacitinib dose for induction therapy is 10 mg b.i.d., followed by maintenance at 5 mg b.i.d. 9 , 10 For patients with loss of response during maintenance therapy, tofacitinib 10 mg b.i.d. may be considered and limited to the shortest duration.
Population pharmacokinetics (PKs) of tofacitinib have been characterized for patients with UC using nonlinear mixed‐effects modeling of pooled data from phase II/III studies. Tofacitinib PKs were linear, with a dose‐proportional increase in exposure. Oral clearance of tofacitinib did not change significantly over time, and dose adjustments for age, weight, sex, race, or baseline disease severity were not required. 11 In the phase II study, baseline disease activity was the most important predictor of efficacy. 12
The aim of this analysis was to characterize the relationship between tofacitinib exposure and efficacy end points for both induction and maintenance therapy in patients with UC, using data from phase II/III studies, and identify patient‐specific (demographic and disease) factors that are important determinants of efficacy.
METHODS
Study design and patients
These post hoc analyses included data from a phase II dose‐ranging induction study, two phase III induction studies (OCTAVE Induction 1 and 2), and a phase III maintenance study (OCTAVE Sustain). 7 , 8 These studies were approved by the institutional review boards or independent ethics committees for each center. 7 , 8
The phase II induction study was an 8‐week, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study involving patients with moderate to severe UC. Disease activity was assessed using the Mayo score (range 0–12 points; higher scores indicate higher disease activity; a full description of the Mayo score is provided in the Supplementary Information ). 13 Moderate to severe UC was defined as a total Mayo score ≥ 6. Patients were randomized to receive tofacitinib 0.5, 3, 10, or 15 mg b.i.d., or placebo. Local read of endoscopy was used for efficacy assessments.
Patients enrolled in OCTAVE Induction 1 or 2 had moderate to severe UC (defined as a total Mayo score ≥ 6, a rectal bleeding subscore ≥ 1, and an endoscopic subscore ≥ 2), and had failed or were intolerant to ≥ 1 prior UC treatments (oral or intravenous corticosteroids, azathioprine/mercaptopurine, or TNFi). Patients were randomized 4:1 to receive tofacitinib 10 mg b.i.d. or placebo. A total of 22 patients in OCTAVE Induction 1 and 2 received tofacitinib 15 mg b.i.d. 8 Stable doses of concomitant oral 5‐ASA and oral corticosteroids (≤ 25 mg/day prednisone equivalent) were permitted. Concomitant therapy with TNFi, azathioprine, methotrexate, or 6‐mercaptopurine was prohibited. The primary end point was remission at week 8 (defined as a total Mayo score of ≤ 2 points with no individual subscore > 1 point, and a rectal bleeding subscore of 0). The key secondary end point was endoscopic improvement at week 8 (a Mayo endoscopic subscore of 0 or 1; defined as mucosal healing in the original OCTAVE protocols). Local and central reads of endoscopy were performed; and registration end points were based on central reads.
Patients with clinical response (defined as a decrease from induction study baseline total Mayo score of ≥ 3 points and ≥ 30%, plus a decrease in rectal bleeding subscore of ≥ 1 point or an absolute rectal bleeding subscore of 0 or 1) at week 8 of OCTAVE Induction 1 or 2 were eligible to participate in the maintenance study, OCTAVE Sustain. Patients were re‐randomized 1:1:1 to receive tofacitinib 5 or 10 mg b.i.d. or placebo. Patients were required to remain on stable doses of their concomitant medications; per protocol, patients were required to taper off corticosteroids. The primary end point was remission at week 52. Key secondary end points were endoscopic improvement at week 52 and sustained steroid‐free remission (defined as being in remission, in addition to not requiring any treatment with corticosteroids for ≥ 4 weeks prior to the visit) among patients in remission at baseline (i.e., patients in remission following induction therapy). Local and central reads of endoscopy were performed; end points were based on central reads.
Pharmacokinetics
Individual PK parameters for exposure index were reported previously. 11 Average and trough concentrations (Cavg and Ctrough, respectively) were derived from the dose‐normalized, individual empirical Bayes estimates obtained from the population PK model. Although both Cavg and Ctrough were evaluated in exposure‐response (E‐R) analyses, only models using Cavg as predictor are presented.
Exposure‐response models
The E‐R analyses were performed using maximum likelihood methods. SAS (version 9.2; SAS Institute, Cary, NC) was used for data handling and data analysis. Parameters describing E‐R relationship (population typical value and residual variance) were estimated and potential covariate effects were investigated as predictors of variability. End points were modeled as a function of exposure.
The binary efficacy end points for induction studies, measured at the end of the study (week 8), were modeled using logistic regression models evaluating either linear or nonlinear (maximum effect (Emax) model) relationships with tofacitinib exposures, as reported previously. 12 Base E‐R models were developed first, using pooled central‐read data from phase III induction studies (OCTAVE Induction 1 and 2), followed by evaluation of the effect of covariates on model parameters using step‐wise covariate modeling with forward selection and backward elimination steps. Separately, E‐R analysis of pooled phase II/III induction studies was performed using locally read endoscopy end points. Only locally read endoscopies were performed in the phase II study, according to the regulatory guidance at that time.
Data from the phase II study were included as a wider range of tofacitinib doses were available (tofacitinib 0.5, 3, 10, and 15 mg b.i.d., as well as placebo), to better inform induction efficacy at tofacitinib doses lower than 10 mg b.i.d. (the only tofacitinib dose evaluated in phase III induction studies).
For the maintenance study, clinical end points (based on central reads) at weeks 24 and 52 were modeled using a Markov transition model and a longitudinal logistic regression model using linear or Emax link functions. The Markov model described the probabilities of transition from achiever to non‐achiever status, and vice versa, for each binary end point over time (Figure S1 ). Nonresponder imputation was used. E‐R models were first run without covariates using data from OCTAVE Sustain. E‐R models with covariates were used to identify significant predictors of efficacy (based on central reads).
For both induction and maintenance studies, covariates included in the full model development were: baseline Mayo score (based on central or local endoscopic reads), extent of baseline disease, prior TNFi failure, prior immunosuppressant use, concomitant oral corticosteroid use, concomitant 5‐ASA use, age, body weight, sex, and race (Asian vs. non‐Asian). Albumin and C‐reactive protein correlated with baseline Mayo score and were therefore not included in this analysis (albumin was also shown not to be independently correlated with efficacy in a previous analysis of these studies). 14 Covariates were evaluated on placebo (intercept) and drug (Emax) effect parameters (Table S1 ), and tested using a stepwise (forward and backward) method. Backward models were established using statistically significant (P < 0.05) covariates in the forward process. Final models were established using statistically significant (P < 0.01) covariates in the backward process. For nested models, significant covariates were determined using a variant of the traditional stepwise selection, where decisions on which potential covariate to add or drop at any step and when to terminate the selection, were based on a likelihood ratio test.
RESULTS
Patients
Baseline demographic and clinical characteristics for all patients have been reported previously. 7 , 8 These analyses included data from 1,355 patients treated with tofacitinib 0.5, 3, 10, or 15 mg b.i.d., or placebo, in the phase II/III induction studies, and 592 patients treated with tofacitinib 5 or 10 mg b.i.d., or placebo, in OCTAVE Sustain.
For all patients included in the E‐R analysis, demographics and clinical characteristics by phase and by treatment group are shown in Table 1 . Overall, at baseline of induction studies, 46.9% of patients had prior TNFi failure and 69.2% of patients had previously used immunosuppressants. Mean total Mayo score, determined by local read of endoscopy, was lower in the phase II induction study (8.2, SD 1.6) than in OCTAVE Induction 1 and 2 (9.0, SD 1.5). Demographics and clinical characteristics for patients in OCTAVE Sustain were similar to those in OCTAVE Induction 1 and 2, except for a lower mean total Mayo score and a greater proportion taking concomitant 5‐ASA. Demographics and clinical characteristics for patients without prior TNFi failure in induction studies were similar to those of the overall population (Table S2 ).
Table 1.
Demographics and clinical characteristics at baseline in phase II and phase III induction studies and the phase III maintenance study, for patients included in these analyses
| Induction studies (by phase) | Pooled data from the phase II induction study and OCTAVE Induction 1 and 2 | OCTAVE Sustain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Treatment | |||||||||
|
Phase II (N = 194) |
Phase III (N = 1,161) |
Placebo (N = 282) |
0.5 mg b.i.d. (N = 31) |
3 mg b.i.d. (N = 33) |
10 mg b.i.d. (N = 938) |
15 mg b.i.d. (N = 71) |
Placebo (N = 198) |
5 mg b.i.d. (N = 198) |
10 mg b.i.d. (N = 196) |
|
| Female, n (%) | 88 (45.4) | 481 (41.4) | 127 (45.0) | 14 (45.2) | 14 (42.4) | 381 (40.6) | 33 (46.5) | 82 (41.4) | 95 (48.0) | 86 (43.9) |
| Age, years, mean (SD) | 42.5 (13.7) | 41.1 (13.9) | 41.4 (14.4) | 43.8 (13.4) | 42.5 (14.3) | 41.3 (13.8) | 40.3 (13.2) | 43.4 (14.0) | 41.9 (13.7) | 43.0 (14.4) |
| Total Mayo score, central, mean (SD) a | — | 9.0 (1.5) | 9.0 (1.4) b | — | — | 9.0 (1.5) b | 8.9 (1.5) b | 3.3 (1.8) | 3.3 (1.8) | 3.4 (1.8) |
| Total Mayo score, local, mean (SD) | 8.2 (1.6) | 9.0 (1.5) | 8.8 (1.5) | 8.6 (1.6) | 8.3 (1.5) | 9.0 (1.5) | 8.3 (1.5) | 3.0 (1.9) | 3.0 (1.8) | 3.2 (1.9) |
| C‐reactive protein, mean (SD), mg/L | 13.8 (20.9) | 11.6 (18.9) | 11.6 (18.6) | 18.8 (29.4) | 12.6 (13.2) | 11.3 (18.6) | 17.5 (24.4) | 3.31 (6.12) | 2.17 (3.84) | 3.96 (9.31) |
| Serum albumin, mean (SD), g/dL | 4.22 (0.38) | 4.16 (0.39) | 4.17 (0.37) | 4.12 (0.42) | 4.19 (0.38) | 4.17 (0.39) | 4.19 (0.46) | 4.49 (0.28) | 4.48 (0.32) | 4.51 (0.33) |
| Prior TNFi treatment, n (%) | 38 (19.6) | 630 (54.3) | 142 (50.4) | 3 (9.7) | 7 (21.2) | 494 (52.7) | 22 (31.0) | 92 (46.5) | 90 (45.5) | 100 (51.0) |
| Prior TNFi failure, n (%) | 36 (18.6) | 599 (51.6) | 136 (48.2) | 2 (6.5) | 6 (18.2) | 471 (50.2) | 20 (28.2) | 89 (44.9) | 83 (41.9) | 92 (46.9) |
| Prior immunosuppressant use, n (%) | 79 (40.7) | 859 (74.0) | 180 (63.8) | 13 (41.9) | 12 (36.4) | 699 (74.5) | 34 (47.9) | 134 (67.7) | 149 (75.3) | 144 (73.5) |
| Concomitant 5‐ASA use, n (%) | 133 (68.6) | 656 (56.5) | 170 (60.3) | 20 (64.5) | 22 (66.7) | 524 (55.9) | 53 (74.6) | 192 (97.0) | 194 (98.0) | 191 (97.4) |
| Concomitant oral corticosteroid use, n (%) | 56 (28.9) | 488 (42.0) | 113 (40.1) | 9 (29.0) | 8 (24.2) | 392 (41.8) | 22 (31.0) | 95 (48.0) | 94 (47.5) | 78 (39.8) |
Data are from the phase II (NCT00787202) and phase III (NCT01465763, NCT01458951) induction studies, and the phase III maintenance study (NCT01458574).
5‐ASA, 5‐aminosalicylates; N, number of patients in the treatment group; n, number of unique patients; TNFi, tumor necrosis factor inhibitor.
Central reads of endoscopy were not performed in the phase II Induction study.
The number of patients in each treatment group were: placebo, N = 233; tofacitinib 10 mg b.i.d., N = 903; tofacitinib 15 mg b.i.d., N = 22; All, N = 1,158.
Exposure‐response models for tofacitinib as induction therapy
For E‐R analyses using data from induction studies, an Emax logistic regression model was selected for all binary end points, as model fits were found to be acceptable for all end points. Cavg was used as the predictor, as this was previously shown to be the most relevant drug‐exposure measure of tofacitinib efficacy based on the drug’s mechanism of action, temporal response across diseases, and examination of results in rheumatoid arthritis. 15
Base model: Evidence of greater efficacy for tofacitinib 10 vs. 5 mg b.i.d. in induction studies
Patients enrolled in OCTAVE Induction 1 or 2 were randomized to receive either tofacitinib 10 mg b.i.d. or placebo, although 22 patients received tofacitinib 15 mg b.i.d. E‐R model predictions for the pooled OCTAVE Induction 1 and 2 studies were consistent with observed data and indicated that the probability of remission or endoscopic improvement increased with increasing Cavg (Figure 1 ). At week 8, Cavg values were similar in patients in remission to those not in remission (Figure S2 ). Probability estimates for achievement of remission or endoscopic improvement by the logistic Emax model were 6.5% and 14.8% for placebo, 12.8% and 24.8% for tofacitinib 5 mg b.i.d., and 19.1% and 32.6% for tofacitinib 10 mg b.i.d., respectively (Table 2 ). Predicted placebo‐adjusted remission and endoscopic improvement rates were 12.7% and 17.8% for tofacitinib 10 mg b.i.d., and 6.4% and 10.1% for tofacitinib 5 mg b.i.d., respectively (Table 2 ).
Figure 1.
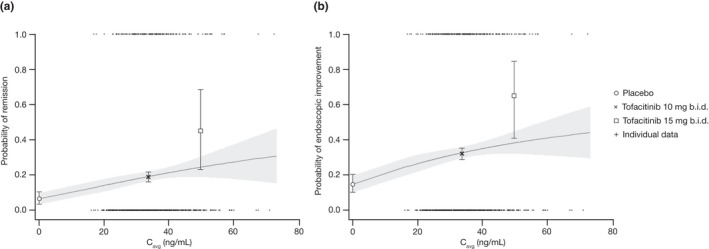
Probability of (a) remission and (b) endoscopic improvement at week 8 in OCTAVE Induction 1 and 2. The solid lines represent model‐predicted probability and the shaded areas represent the 95% CI. Observed probabilities by dose (placebo, tofacitinib 10 mg b.i.d., and tofacitinib 15 mg b.i.d.; black symbols) are plotted at the geometric mean of individual Cavg values for tofacitinib 10 mg b.i.d. (33.6 ng/mL) and 15 mg b.i.d. (50.4 ng/mL), error bars represent 95% CI. Cavg, average concentration during dosing interval; CI, confidence interval.
Table 2.
Probability estimates to achieve remission or endoscopic improvement at week 8 of OCTAVE Induction 1 or 2, by logistic Emax model a
| Treatment | Estimate | 95% CI | Δ(tofacitinib‐placebo) | |
|---|---|---|---|---|
| Estimate | 95% CI | |||
| Probability estimates to achieve remission | ||||
| Placebo | 0.065 | 0.032–0.097 | — | — |
| Tofacitinib 5 mg b.i.d. b | 0.128 | 0.092–0.164 | 0.064 | 0.023–0.104 |
| Tofacitinib 10 mg b.i.d. c | 0.191 | 0.163–0.219 | 0.127 | 0.083–0.170 |
| Probability estimates to achieve endoscopic improvement | ||||
| Placebo | 0.148 | 0.101–0.194 | — | — |
| Tofacitinib 5 mg b.i.d. b | 0.248 | 0.198–0.298 | 0.101 | 0.041–0.160 |
| Tofacitinib 10 mg b.i.d. c | 0.326 | 0.293–0.359 | 0.178 | 0.121–0.236 |
Δ, difference; Cavg, average concentration during dosing interval; CI, confidence interval; Emax, maximum effect.
Covariates were not explored in this analysis.
Probability of achieving end point for tofacitinib 5 mg b.i.d. was estimated by using the geometric mean Cavg at 5 mg b.i.d. (16.8 ng/mL).
Probability of achieving endpoint for tofacitinib 10 mg b.i.d. was estimated by using the geometric mean Cavg for tofacitinib 10 mg b.i.d. (33.6 ng/mL).
Estimated concentration at half‐maximum effect from base models for remission and endoscopic improvement were 56.5 and 49.0 ng/mL, respectively. Model‐predicted placebo‐adjusted remission and endoscopic improvement rates with tofacitinib 10 mg b.i.d. were 12.7% and 17.8%, respectively (Table 2 ). The geometric mean Cavg at 10 mg b.i.d. (33.6 ng/mL; derived from previously reported PK parameters) 11 corresponded to ED67 on the E‐R curve for remission.
Covariate effects on base exposure‐response model parameters for induction efficacy
The step‐wise covariate modeling approach was used to evaluate the effect of covariates. In the final model, only prior TNFi failure and baseline Mayo score were significant predictors of remission and endoscopic improvement (Table S3 ). Estimated parameters (95% confidence interval (CI)) for remission were: prior TNFi failure on intercept, 1.23 (0.39–2.08); prior TNFi failure on Emax, 1.61 (−0.26 to 3.48); and baseline Mayo score on intercept, 0.14 (0.080–0.20). For endoscopic improvement, estimated parameters (95% CI) were: prior TNFi failure on intercept, 0.61 (0.31–0.91); and baseline Mayo score on intercept, 0.30 (0.19–0.40).
Evidence of efficacy for tofacitinib 5 mg b.i.d. as induction therapy in patients without prior TNFi failure
E‐R modeling of phase III induction data identified prior TNFi failure status as a significant predictor of efficacy, therefore further E‐R analyses were performed to examine if tofacitinib 5 mg b.i.d. could be an effective induction dose in patients without prior TNFi failure. As OCTAVE Induction 1 and 2 only included patients who received tofacitinib 10 or 15 mg b.i.d., or placebo, data from the phase II induction study were included as these data included both lower (tofacitinib 0.5 and 3 mg b.i.d.) and higher (tofacitinib 10 and 15 mg b.i.d.) tofacitinib doses relative to the proposed 5 mg b.i.d. dose. Efficacy end points were based on local reads. Given the objective of this analysis, the dataset included only the pooled subpopulation of patients without prior TNFi failure in phase II/III induction studies. Approximately 80% of the phase II population had no prior TNFi failure, 12 therefore, robust dose‐ranging information was available for this subpopulation.
The subpopulation of patients without prior TNFi failure consisted of 687 TNFi‐naïve patients and 33 patients who previously received a TNFi without treatment failure. Of these 720 patients, 712 were included in the final E‐R analysis dataset; two patients were excluded due to missing baseline Mayo scores and six patients were excluded due to a baseline Mayo score < 6 (per enrollment criteria).
In patients without prior TNFi failure, model‐predicted E‐R relationships of remission in the phase II/III induction studies were shown to adequately describe the observed data (Figure 2 ). Model predictions (95% CI) for the proportion of patients without prior TNFi failure in remission, based on pooled phase II/III data, were 12.7% (7.5–17.8) for placebo, 25.4% (17.8–33.0) for tofacitinib 5 mg b.i.d., and 33.1% (29.0–37.2) for tofacitinib 10 mg b.i.d.; with placebo‐adjusted effects of 12.8% (3.1–22.4) and 20.4% (13.5–27.3) for the 5 and 10 mg b.i.d. groups, respectively.
Figure 2.
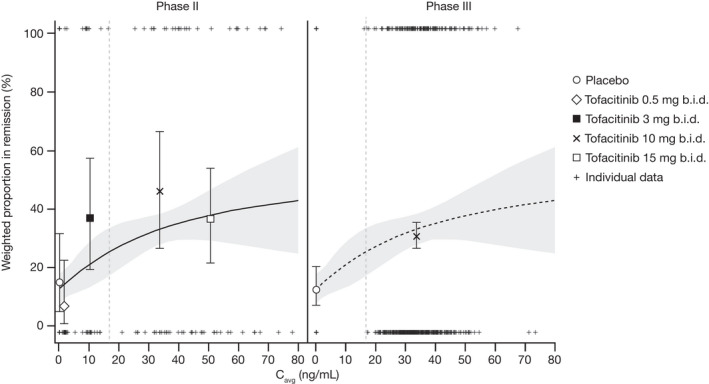
Observed (symbols) and model predicted (solid line and shaded area) remission rate based on E‐R analysis of pooled phase II and phase III induction data in the subpopulation of patients without prior TNFi failure. Pooled data are displayed separately for phase II and phase III. The solid lines represent model‐predicted probability and the shaded areas represent the 95% CI. The vertical dashed lines indicate median Cavg for tofacitinib 5 mg b.i.d. (16.8 ng/mL), derived from the dose‐normalized, individual empirical Bayes estimates obtained from the population PK model. Observed probabilities by dose are plotted at the geometric mean of individual Cavg values at tofacitinib 0.5 mg b.i.d. (1.68 ng/mL), 3 mg b.i.d. (10.08 ng/mL), 10 mg b.i.d. (33.6 ng/mL), and 15 mg b.i.d. (50.4 ng/mL), error bars represent 95% CI. Cavg, average concentration during dosing interval; CI, confidence interval; E‐R, exposure‐response; PK, pharmacokinetic; TNFi, tumor necrosis factor inhibitor.
Exposure‐response models for tofacitinib as maintenance therapy
A longitudinal E‐R analysis of binary efficacy end points at weeks 24 and 52 of OCTAVE Sustain was performed using a binomial transition model with Markov dependence. A logistic regression model was applied as an alternate model to evaluate the sensitivity of results to model assumptions. Consistent with the induction analyses, Cavg was used as the predictor for maintenance analyses.
Base model: Evidence of greater efficacy for tofacitinib maintenance therapy in patients in remission or with endoscopic improvement at baseline
E‐R model predictions using the Markov transition model (without covariates) were carried out at week 52 by baseline remission or endoscopic improvement (i.e., endoscopic subscore of 0 or 1) status using expected Cavg values at tofacitinib 5 and 10 mg b.i.d. doses. At weeks 24 and 52, Cavg values were similar in patients in remission to those not in remission (Figure S2 ). E‐R model predictions were consistent with observed data and indicated efficacy in maintenance increased with increasing tofacitinib exposure (Figure 3 ). Markov transition model predictions for all efficacy end points were also consistent with observed data at week 24, and from weeks 24–52 (Figure S3 ).
Figure 3.
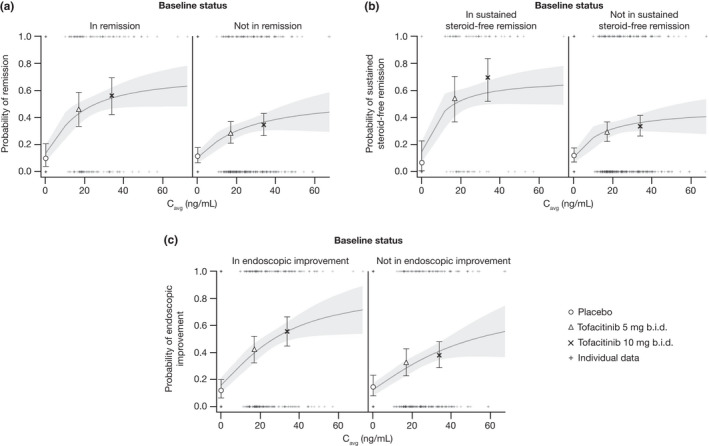
Probability of (a) remission, (b) sustained steroid‐free remission, and (c) endoscopic improvement at week 52 by baseline status in OCTAVE Sustain, using the basic Markov transition model. The solid lines represent model‐predicted probability, the shaded area represents 95% prediction interval and the error bars represent 95% CI. The symbols represent the observed ratio for tofacitinib 5 mg b.i.d., tofacitinib 10 mg b.i.d., and placebo. Typical Cavg values were 16.8 and 33.6 ng/mL for the tofacitinib 5 and 10 mg b.i.d. groups, respectively. Cavg, average concentration during dosing interval; CI, confidence interval.
Probability estimates to achieve or maintain remission, sustained steroid‐free remission, or endoscopic improvement at week 52 by Markov transition model are provided in Table 3 ; parameter estimates for the basic Markov transition model are provided in Table S4 . For both the tofacitinib 5 and 10 mg b.i.d. groups, estimated placebo‐adjusted probabilities of achieving/maintaining remission or sustained steroid‐free remission at week 52 were greater for patients in remission at baseline than for those not in remission (Table 3 ). For patients receiving tofacitinib 5 mg b.i.d., the predicted relative probabilities of achieving/maintaining remission and sustained steroid‐free remission at week 52 were 29% and 26%, respectively, for patients in remission at baseline, and 18% and 9%, respectively, for patients not in remission at baseline. The predicted relative probabilities of achieving/maintaining remission and sustained steroid‐free remission with tofacitinib 10 mg b.i.d. were 41% and 38%, respectively, for patients in remission at baseline, and 26% and 13%, respectively, for patients not in remission at baseline (Table 3 ). Similar findings were seen for the end point of endoscopic improvement (Table 3 ).
Table 3.
Probability estimates to achieve efficacy end points at week 52 of OCTAVE Sustain, predicted probability of response (difference from placebo), and relative efficacy of tofacitinib 5 and 10 mg b.i.d. by baseline status using the Markov transition model
| Treatment | Baseline status | Estimate | 95% CI | Δ(tofacitinib‐placebo) | Ratio (Δtofacitinib 10 mg b.i.d.: Δtofacitinib 5 mg b.i.d.) | ||
|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | ||||
| Probability estimates to achieve remission | |||||||
| Placebo | In remission | 0.135 | 0.073–0.196 | — | — | — | — |
| Not in remission | 0.104 | 0.062–0.146 | — | — | — | — | |
| 5 mg b.i.d. | In remission | 0.427 | 0.344–0.509 | 0.29 | 0.19–0.40 | — | — |
| Not in remission | 0.280 | 0.223–0.337 | 0.18 | 0.11–0.24 | — | — | |
| 10 mg b.i.d. | In remission | 0.543 | 0.464–0.621 | 0.41 | 0.31–0.51 | 1.27 | 1.02–1.53 |
| Not in remission | 0.365 | 0.302–0.428 | 0.26 | 0.18–0.34 | 1.30 | 1.00–1.61 | |
| Probability estimates to achieve sustained steroid‐free remission | |||||||
| Placebo | In remission | 0.132 | 0.072–0.191 | — | — | — | — |
| Not in remission | 0.105 | 0.062–0.147 | — | — | — | — | |
| 5 mg b.i.d. | In remission | 0.427 | 0.346–0.509 | 0.26 | 0.16–0.37 | — | — |
| Not in remission | 0.279 | 0.223–0.335 | 0.09 | 0.05–0.13 | — | — | |
| 10 mg b.i.d. | In remission | 0.537 | 0.458–0.617 | 0.38 | 0.27–0.49 | 1.34 | 1.00–1.67 |
| Not in remission | 0.356 | 0.294–0.417 | 0.13 | 0.08–0.19 | 1.33 | 0.97–1.69 | |
| Probability estimates to achieve endoscopic improvement | |||||||
| Placebo | In endoscopic improvement | 0.155 | 0.099–0.212 | — | — | — | — |
| Not in endoscopic improvement | 0.124 | 0.077–0.171 | — | — | — | — | |
| 5 mg b.i.d. | In endoscopic improvement | 0.392 | 0.319–0.465 | 0.24 | 0.14–0.33 | — | — |
| Not in endoscopic improvement | 0.282 | 0.224–0.340 | 0.16 | 0.09–0.23 | — | — | |
| 10 mg b.i.d. | In endoscopic improvement | 0.552 | 0.483–0.622 | 0.40 | 0.30–0.49 | 1.41 | 1.14–1.68 |
| Not in endoscopic improvement | 0.406 | 0.341–0.472 | 0.28 | 0.20–0.37 | 1.44 | 1.15–1.74 | |
Δ, difference; CI, confidence interval.
Efficacy in maintenance increased with increasing tofacitinib exposure, with predicted relative increase in remission and endoscopic improvement between tofacitinib 5 and 10 mg b.i.d. ranging from 27–44% (based on the ratio of tofacitinib 10 mg b.i.d.:tofacitinib 5 mg b.i.d. response ranging from 1.27–1.44) in the overall population, based on the geometric mean Cavg at these doses (Table 3 ). The relative increase in efficacy from tofacitinib 5 to 10 mg b.i.d. was 35–37% for sustained remission, 53–59% for sustained endoscopic improvement, and 33–34% for sustained steroid‐free remission.
Covariate effects on base exposure‐response model parameters for maintenance efficacy
Models were used to identify covariates that were predictive of response at week 52 of the maintenance study. In the final model, baseline Mayo score, oral corticosteroid use, and age were significant predictors of remission and endoscopic improvement (Table S5 ). Estimated parameters (95% CI) for remission were: effect of baseline Mayo score on intercept nonachievers, 0.1609 (0.07861–0.2432); effect of age on intercept for nonachievers, −0.00841 (−0.01428 to −0.00254); and effect of oral corticosteroid use at baseline on Emax for achievers, −0.3582 (−0.5804 to −0.1360). For endoscopic improvement, estimated parameters (95% CI) were: effect of baseline Mayo score on intercept for nonachievers, 0.1321 (0.03725–0.2270); effect of age on intercept nonachievers, −0.00977 (−0.01703 to −0.00252); and effect of oral corticosteroid use at baseline on intercept for achievers, 0.6382 (0.09051–1.1858). The effects of selected covariates (corticosteroid use at baseline; induction treatment (placebo or tofacitinib 15 mg b.i.d. (remission and sustained steroid‐free remission only)); baseline Mayo score (0, 2, or 10); and age (18 or 80 years)) are shown in Figure S4 . Covariates were not significantly associated with the achievement of remission, sustained steroid‐free remission, or endoscopic improvement at week 52, with the exception of a baseline Mayo score of 10, which was highly correlated with achievement of all efficacy end points, especially in baseline nonachievers.
Evidence of greater efficacy for tofacitinib 10 mg b.i.d., vs. 5 mg b.i.d., as maintenance therapy in patients with prior TNFi failure
In the covariate analyses, prior TNFi failure was not a significant predictor of response for the maintenance study, due to its correlation with baseline Mayo score and baseline remission status, which were included in the model. Therefore, the effect of this important covariate was characterized using a logistic Emax model.
The probability of predicting remission, sustained steroid‐free remission among patients in remission at baseline, or endoscopic improvement at week 52 of OCTAVE Sustain by prior TNFi failure status was assessed (Table 4 ). Among patients without prior TNFi failure in the maintenance study, 310 patients were TNFi‐naïve and 18 patients had previously received a TNFi without treatment failure.
Table 4.
Probability predicted by logistic Emax model for remission, sustained steroid‐free remission among patients who were in remission at baseline, or endoscopic improvement at week 52, and relative efficacy of tofacitinib 5 and 10 mg b.i.d.
| Treatment | Prior TNFi failure | Estimate | 95% CI | Ratio (tofacitinib 10 mg b.i.d.: tofacitinib 5 mg b.i.d.) | |
|---|---|---|---|---|---|
| Estimate | 95% CI | ||||
| Probability estimates to achieve remission | |||||
| Placebo | No | 0.109 | 0.059–0.159 | — | — |
| Yes | 0.110 | 0.057–0.164 | — | — | |
| Tofacitinib 5 mg b.i.d. | No | 0.394 | 0.308–0.480 | — | — |
| Yes | 0.249 | 0.179–0.319 | — | — | |
| Tofacitinib 10 mg b.i.d. | No | 0.468 | 0.383–0.553 | 1.19 | 0.87–1.51 |
| Yes | 0.350 | 0.285–0.416 | 1.41 | 1.08–1.73 | |
| Probability estimates to achieve sustained steroid‐free remission among patients who were in remission at baseline | |||||
| Placebo | No | 0.053 | −0.019 to 0.126 | — | — |
| Yes | 0.043 | −0.042 to 0.128 | — | — | |
| Tofacitinib 5 mg b.i.d. | No | 0.424 | 0.271–0.576 | — | — |
| Yes | 0.241 | 0.069–0.413 | — | — | |
| Tofacitinib 10 mg b.i.d. | No | 0.489 | 0.325–0.653 | 1.16 | 0.57–1.75 |
| Yes | 0.353 | 0.173–0.533 | 1.47 | 0.49–2.44 | |
| Probability estimates to achieve endoscopic improvement | |||||
| Placebo | No | 0.133 | 0.079–0.188 | — | — |
| Yes | 0.133 | 0.077–0.190 | — | — | |
| Tofacitinib 5 mg b.i.d. | No | 0.396 | 0.309–0.482 | — | — |
| Yes | 0.280 | 0.206–0.355 | — | — | |
| Tofacitinib 10 mg b.i.d. | No | 0.526 | 0.443–0.609 | 1.33 | 1.00–1.66 |
| Yes | 0.411 | 0.340–0.481 | 1.46 | 1.18–1.75 | |
CI, confidence interval; Emax, maximum effect; TNFi, tumor necrosis factor inhibitors.
For patients receiving placebo, the predicted probabilities of achieving each efficacy end point were similar between patients with vs. without prior TNFi failure (4.3–13.3% and 5.3–13.3%, respectively). In contrast, the probabilities of achieving these end points were lower in patients with prior TNFi failure (tofacitinib 5 mg b.i.d., 24.1–28.0%; tofacitinib 10 mg b.i.d., 35.0–41.1%) than in patients without prior TNFi failure (tofacitinib 5 mg b.i.d., 39.4–42.4%; tofacitinib 10 mg b.i.d., 46.8–52.6%; Table 4 ). The relative increase in efficacy between tofacitinib 5 and 10 mg b.i.d. was also assessed (Table 4 ). For each efficacy end point, patients with prior TNFi failure had a higher probability of response to tofacitinib 10 vs. 5 mg b.i.d. (ratios: 40.6–46.5%). Although tofacitinib 10 mg b.i.d. was also more efficacious than 5 mg b.i.d. in patients without prior TNFi failure, the relative increases in efficacy were smaller (ratios: 15.5–32.9%).
DISCUSSION
Previous E‐R analyses of the 8‐week, phase II induction study characterized the relationship between tofacitinib dose and plasma concentration, and provided the basis for selection of tofacitinib 10 mg b.i.d. in the phase III induction studies (OCTAVE Induction 1 and 2). 12 , 16 Induction efficacy estimates for remission in patients without prior TNFi failure indicated overall consistency between the phase II study and the phase III program, despite some differences between study populations. Results from our E‐R modeling analysis suggest clinically meaningful induction efficacy may be achieved with tofacitinib 5 mg b.i.d. in patients without prior TNFi failure. However, because phase III induction studies evaluated tofacitinib 10 mg b.i.d. only, confirmation of tofacitinib 5 mg b.i.d. induction efficacy in a phase III study may be required. Findings from the E‐R analysis support the use of tofacitinib 10 mg b.i.d. as induction therapy for patients with moderate to severe UC.
Our findings show that tofacitinib 5 mg b.i.d. was efficacious as maintenance therapy, with additional clinical benefit with 10 mg b.i.d. in the overall population. Patients with lower baseline disease activity were more likely to achieve efficacy endpoints after 52‐week maintenance treatment. This is consistent with previous E‐R analyses of the phase II induction study, which also showed that patients with lower disease activity were more likely to achieve remission after 8 weeks of treatment. 12 E‐R analyses of maintenance data also indicate that for patients with prior TNFi failure, additional benefit of tofacitinib 10 mg b.i.d., relative to 5 mg b.i.d., is possible. These findings are consistent with a post hoc analysis which found that for patients in OCTAVE Sustain, treatment effects were generally higher with tofacitinib 10 vs. 5 mg b.i.d., regardless of prior TNFi failure. 17 , 18 These results are also consistent with a recent analysis of filgotinib (JAK 1 inhibitor) in patients with UC who were stratified by line of therapy, which reported that filgotinib 200 mg was effective in the induction and maintenance of clinical remission in patients who were naïve to biologic therapies (bio‐naïve), as well as those who had previously received biologic therapy (bio‐experienced). The proportion of patients achieving clinical remission with filgotinib vs. placebo was numerically higher in the bio‐naïve group compared with the bio‐experienced group, however, no direct comparisons were carried out between groups. 19
The importance of baseline disease activity and prior treatment experience have also been demonstrated with other therapies for UC. An E‐R analysis of vedolizumab (integrin inhibitor) in patients with inflammatory bowel disease (including UC) reported that patients who had lower baseline disease activity (as measured by fecal calprotectin concentration) and no prior TNFi experience had a higher probability of achieving clinical remission, regardless of treatment (vedolizumab or placebo), and prior TNFi experience was identified as the covariate with the greatest impact on clinical outcome rates. 20 In addition, an E‐R analysis of ustekinumab (interleukin‐12/23 inhibitor) in patients with UC reported that the probability of achieving remission or endoscopic improvement after 8 weeks of induction therapy was higher in patients with lower vs. higher baseline Mayo scores, and in those without vs. with prior biologic treatment failure. 21 The previous findings with other UC therapies demonstrate the importance of baseline disease activity and prior treatment as covariates of efficacy. Along with the results of the current analysis, this suggests that patients who have previously received and/or failed TNFi or other biologic therapies, and induction nonresponders, may respond differently to treatment, and therefore may need an alternative therapeutic strategy with respect to treatment options and/or dosing.
Cavg was used as the predictor of efficacy in E‐R models, based in part on the observation that Cavg was a better predictor than Ctrough in E‐R models for the induction studies. Additionally, the half‐life of tofacitinib is short (~ 2.5 hours) 22 compared with the half‐lives of biologics (days to weeks) 23 , 24 , 25 ; therefore, variability associated with Ctrough is higher compared with Cavg. Use of Cavg as the predictor is consistent with E‐R model predictions for tofacitinib in rheumatoid arthritis. 15 Plasma concentrations of tofacitinib have been shown to be stable long‐term in patients with UC, providing there is no change in dose. 11 In contrast, biologic therapies can be susceptible to clearance mechanisms that result in low drug exposure and subsequent loss of response in some patients. 12 Trough plasma concentrations have been shown to be useful as predictors of efficacy for TNFi in patients with inflammatory bowel disease. 26 , 27 , 28 , 29 Therapeutic drug monitoring of trough plasma concentrations can inform on changes to treatment dose that may be required to compensate for loss of response due to drug clearance. 30 However, in contrast to biologic therapies, 30 , 31 the PK profile of tofacitinib in patients with UC indicates that plasma concentrations of tofacitinib are unaffected by colonic inflammation, and patient characteristics (e.g., age, body weight, sex, race, or baseline disease severity) do not impact tofacitinib exposure, 11 suggesting that therapeutic drug monitoring is unlikely to be of clinical value in patients receiving tofacitinib. 30
A limitation of this analysis was the small sample size in the phase II study, and a lack of clinical data at different doses in phase III studies. For example, in OCTAVE Sustain, data were only available for tofacitinib 5 and 10 mg b.i.d. This may have limited the ability to make predictions for tofacitinib 5 mg b.i.d. as maintenance therapy; however, this may have been mitigated by the wide range of exposures reported in patients who received tofacitinib 5 or 10 mg b.i.d. in OCTAVE Sustain, and the fact that E‐R models predict there would be suboptimal/clinically inadequate efficacy for maintenance doses < 5 mg b.i.d.
E‐R characterization of efficacy end points during induction indicated that tofacitinib 10 mg b.i.d. was an appropriate induction dose, although clinically meaningful induction efficacy may be achieved with tofacitinib 5 mg b.i.d. in patients without prior TNFi failure. Tofacitinib 5 mg b.i.d. was efficacious as maintenance therapy; additional clinical benefit at 10 mg b.i.d. was seen in the overall population but was most evident in patients with prior TNFi failure. It is important to note that the recommended tofacitinib dose for induction therapy is 10 mg b.i.d., followed by maintenance at 5 mg b.i.d. For patients with loss of response during maintenance therapy, tofacitinib 10 mg b.i.d. may be considered and limited to the shortest duration possible.
FUNDING
All aspects of this study were funded by Pfizer Inc. Authors employed by Pfizer were involved in the study design and in the collection, analysis, and interpretation of data.
CONFLICT OF INTEREST
A.M., S.T., T.N., J.A.C., I.M., and C.S. are employees and stockholders of Pfizer Inc. G.R.D’H. has served as an advisor for AbbVie, Ablynx, Amakem, AM Pharma, Avaxia, Biogen, Bristol‐Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Cosmo, Covidien, Dr. Falk Pharma, Engene, Ferring, Galapagos, Gilead Sciences, GSK, Hospira, Johnson and Johnson, Medimetrics, Millennium/Takeda, Mitsubishi Pharma, MSD, Mundipharma, Novo Nordisk, Pfizer Inc, Prometheus Laboratories/Nestle, Receptos, Robarts Clinical Trials, Salix, Sandoz, Setpoint, Shire, Teva, TiGenix, Tillotts, Topivert, Versant, and Vifor; and has received speaker fees from AbbVie, Ferring, Johnson and Johnson, Millennium/Takeda, MSD, Mundipharma, Norgine, Pfizer Inc, Shire, Tillotts, and Vifor. W.J.S. reports grants, personal fees, and non‐financial support from Pfizer Inc during the conduct of the study; research grants from AbbVie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Gilead Sciences, GSK, Janssen, Lilly, Pfizer Inc, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, and Theravance Biopharma; consulting fees from AbbVie, Abivax, AdMIRx, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health (Salix), BeiGene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol‐Meyers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pandion Therapeutics, Pfizer Inc, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonists Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vedanta Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, and Zealand Pharma; stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences, Prometheus Laboratories Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, and Vivreon Biosciences; and employment at Shoreline Biosciences. W.J.S.’s spouse reports consulting fees from Iveric Bio, Oppilan Pharma, and Prometheus Laboratories; stock options from Iveric Bio, Oppilan Pharma, Prometheus Biosciences, Prometheus Laboratories, Ventyx Biosciences, and Vimalan Biosciences; and stock from Progenity, Prometheus Biosciences, Ventyx Biosciences, and Vimalan Biosciences.
AUTHOR CONTRIBUTIONS
A.M., S.T., T.N., I.M., C.S., J.A.C., W.J.S., and G.R.D’H. wrote the manuscript. A.M., S.T., C.S., T.N., J.A.C., W.J.S., and G.R.D’H. designed the research. W.J.S. and G.R.D’H. performed the research. A.M., S.T., I.M., C.S., T.N., J.A.C., W.J.S., and G.R.D’H. analyzed the data.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material
Table S1
Table S2
Table S3
Table S4
Table S5
ACKNOWLEDGMENTS
The authors would like to thank the patients, investigators, and study teams who were involved in the phase II induction study, OCTAVE Induction 1 and 2, and OCTAVE Sustain. Medical writing support, under the guidance of the authors, was provided by Molly MacFadyen, MSc, CMC Connect, McCann Health Medical Communications, and Chris Guise, PhD, formerly of CMC Connect, McCann Health Medical Communications, and was funded by Pfizer Inc, New York, New York, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann. Intern. Med. 163, 461–464 (2015)).
DATA AVAILABILITY STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.
- 1. Fakhoury, M. , Negrulj, R. , Mooranian, A. & Al‐Salami, H. Inflammatory bowel disease: clinical aspects and treatments. J. Inflamm. Res. 7, 113–120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ungaro, R. , Mehandru, S. , Allen, P.B. , Peyrin‐Biroulet, L. & Colombel, J.‐F. Ulcerative colitis. Lancet 389, 1756–1770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubin, D.T. , Ananthakrishnan, A.N. , Siegel, C.A. , Sauer, B.G. & Long, M.D. ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. 114, 384–413 (2019). [DOI] [PubMed] [Google Scholar]
- 4. Harbord, M. et al. Third European evidence‐based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J. Crohns Colitis 11, 769–784 (2017). [DOI] [PubMed] [Google Scholar]
- 5. Ochsenkühn, T. , Tillack, C. , Szokodi, D. , Janelidze, S. & Schnitzler, F. Clinical outcomes with ustekinumab as rescue treatment in therapy‐refractory or therapy‐intolerant ulcerative colitis. United European Gastroenterol. J. 8, 91–98 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feagan, B.G. et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double‐blind, randomised, placebo‐controlled trial. Lancet 397, 2372–2384 (2021). [DOI] [PubMed] [Google Scholar]
- 7. Sandborn, W.J. et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 367, 616–624 (2012). [DOI] [PubMed] [Google Scholar]
- 8. Sandborn, W.J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736 (2017). [DOI] [PubMed] [Google Scholar]
- 9. European Medicines Agency Xeljanz® (tofacitinib): summary of product characteristics <https://www.ema.europa.eu/en/documents/product‐information/xeljanz‐epar‐product‐information_en.pdf> (2020). Accessed September 1, 2021.
- 10. Pfizer Inc Xeljanz® (tofacitinib): s of prescribing information <http://labeling.pfizer.com/ShowLabeling.aspx?id=959> (2020). Accessed October 14, 2021.
- 11. Vong, C. et al. Population pharmacokinetics of tofacitinib in patients with moderate to severe ulcerative colitis. Clin. Pharmacol. Drug Dev. 10, 229–240 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukherjee, A. et al. Exposure‐response characterization of tofacitinib efficacy in moderate to severe ulcerative colitis: results from a dose‐ranging phase 2 trial. Br. J. Clin. Pharmacol. 84, 1136–1145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schroeder, K.W. , Tremaine, W.J. & Ilstrup, D.M. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 317, 1625–1629 (1987). [DOI] [PubMed] [Google Scholar]
- 14. Lichtenstein, G.R. et al. Baseline albumin level is not a significant predictor of tofacitinib efficacy in patients with ulcerative colitis: results of multivariate exposure‐response analysis. Am. J. Gastroenterol. 113, S354–S355 (2018). [Google Scholar]
- 15. Lamba, M. et al. Model‐informed development and registration of a once‐daily regimen of extended‐release tofacitinib. Clin. Pharmacol. Ther. 101, 745–753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mukherjee, A. et al. Pharmacokinetics and exposure‐response of tofacitinib in a Phase 3 maintenance study in ulcerative colitis patients. J. Crohns Colitis 11(Suppl 1), S69–S70 (2017). [Google Scholar]
- 17. Dubinsky, M.C. et al. Efficacy of tofacitinib in patients with ulcerative colitis by prior tumor necrosis factor inhibitor treatment status: results from OCTAVE induction and maintenance studies. Am. J. Gastroenterol. 112, S354 (2017). [Google Scholar]
- 18. Sandborn, W.J. et al. Efficacy and safety of tofacitinib in ulcerative colitis based on prior tumor necrosis factor inhibitor failure status. Clin. Gastroenterol. Hepatol. 20, 591–601 (2022). [DOI] [PubMed] [Google Scholar]
- 19. Peyrin‐Biroulet, L. et al. Efficacy of filgotinib in patients with ulcerative colitis by line of therapy in the phase 2b/3 SELECTION trial. J. Crohns Colitis 15(Suppl 1), S024–S026 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosario, M. et al. Exposure‐efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn's disease. J. Crohns Colitis 11, 921–929 (2017). [DOI] [PubMed] [Google Scholar]
- 21. Xu, Y. et al. Population pharmacokinetics and exposure‐response modeling analyses of ustekinumab in adults with moderately to severely active ulcerative colitis. J. Clin. Pharmacol. 60, 889–902 (2020). [DOI] [PubMed] [Google Scholar]
- 22. Lawendy, N. et al. Effect of CP‐690,550, an orally active Janus kinase inhibitor, on renal function in healthy adult volunteers. J. Clin. Pharmacol. 49, 423–429 (2009). [DOI] [PubMed] [Google Scholar]
- 23. Hemperly, A. & Vande Casteele, N. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin. Pharmacokinet. 57, 929–942 (2018). [DOI] [PubMed] [Google Scholar]
- 24. Weisman, M.H. et al. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti‐tumor necrosis factor‐alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin. Ther. 25, 1700–1721 (2003). [DOI] [PubMed] [Google Scholar]
- 25. Zhuang, Y. et al. Golimumab pharmacokinetics after repeated subcutaneous and intravenous administrations in patients with rheumatoid arthritis and the effect of concomitant methotrexate: an open‐label, randomized study. Clin. Ther. 34, 77–90 (2012). [DOI] [PubMed] [Google Scholar]
- 26. Rutgeerts, P. , Vermeire, S. & Van Assche, G. Predicting the response to infliximab from trough serum levels. Gut 59, 7–8 (2010). [DOI] [PubMed] [Google Scholar]
- 27. Bortlik, M. et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J. Crohns Colitis 7, 736–743 (2013). [DOI] [PubMed] [Google Scholar]
- 28. Roblin, X. et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 12, 80–84 (2014). [DOI] [PubMed] [Google Scholar]
- 29. Adedokun, O.J. et al. Pharmacokinetics and exposure‐response relationship of golimumab in patients with moderately‐to‐severely active ulcerative colitis: results from phase 2/3 PURSUIT induction and maintenance studies. J. Crohns Colitis 11, 35–46 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee, S.D. et al. Therapeutic drug monitoring for current and investigational inflammatory bowel disease treatments. J. Clin. Gastroenterol. 55, 195–206 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hindryckx, P. et al. Review article: dose optimisation of infliximab for acute severe ulcerative colitis. Aliment. Pharmacol. Ther. 45, 617–630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material
Table S1
Table S2
Table S3
Table S4
Table S5
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.


