Abstract
Vibrio vulnificus serovar E (formerly biotype 2) is the etiologic agent that is responsible for the main infectious disease affecting farmed eels. Although the pathogen can theoretically use water as a vehicle for disease transmission, it has not been isolated from tank water during epizootics to date. In this work, the mode of transmission of the disease to healthy eels, the portals of entry of the pathogen into fish, and their putative reservoirs have been investigated by means of laboratory and field experiments. Results of the experiments of direct and indirect host-to-host transmission, patch contact challenges, and oral-anal intubations suggest that water is the prime vehicle for disease transmission and that gills are the main portals of entry into the eel body. The pathogen mixed with food can also come into the fish through the gastrointestinal tract and develop the disease. These conclusions were supported by field data obtained during a natural outbreak in which we were able to isolate this microorganism from tank water for the first time. The examination of some survivors from experimental infections by indirect immunofluorescence and scanning electron microscopy showed that V. vulnificus serovar E formed a biofilm-like structure on the eel skin surface. In vitro assays demonstrated that the ability of the pathogen to colonize both hydrophilic and hydrophobic surfaces was inhibited by glucose. The capacity to form biofilms on eel surface could constitute a strategy for surviving between epizootics or outbreaks, and coated survivors could act as reservoirs for the disease.
Vibrio vulnificus serovar E (formerly biotype 2) is a primary pathogen for eels and a secondary pathogen for humans (2, 35). As a human pathogen, this serovar probably behaves like the biotype 1 of the species, causing sporadic diseases and outbreaks in immunocompromised hosts (27, 36). As an eel pathogen, this serovar causes a primary septicemia, named vibriosis, that affects captured eels maintained in farms, occasionally resulting in economic losses (5, 7, 8, 13, 18). The incidence of the vibriosis in natural populations of wild eels is unknown. In farms, the disease can suddenly appear and cause high mortality rates (7, 8, 13, 18). After antibiotic treatment, the disease usually disappears and reappears as recurrent outbreaks that are often associated with stress factors such as changes in pH and nitrite levels (R. Barrera, personal communication). The onset of a new outbreak can be delayed by lowering water salinity, which partially inhibits the pathogen's ability to survive and spread (3, 21). However, the origin of the infection, the mode of transmission, and the reservoir between outbreaks or epizootics have yet to be determined.
It has been suggested that eel-virulent strains, like the avirulent ones (biotype 1), are natural inhabitants of aquatic ecosystems (3, 21). This hypothesis is mainly based on laboratory results that demonstrate the ability of eel-virulent isolates to survive in artificial seawater microcosms for years (21) and to use water as a vehicle for infection (3). However, field data do not support this hypothesis because attempts to isolate eel-virulent strains from sources other than moribund eels, including tank water sampled during epizootics, have been unsuccessful to date (4, 7, 18). In these studies, the isolation method followed was that developed for biotype 1 of the species: preenrichment in alkaline peptone water (APW) (1% NaCl [pH 8.6]), supplemented or not with antibiotics, followed by seeding on different selective and differential media (cellobiose-polymyxin B-colistin [CPC] agar or CC agar) (4, 7, 18). Collection strains of V. vulnificus serovar E grow well on both media, developing yellow colonies due to the fermentation of cellobiose (7, 18). The absence of natural water isolates of this pathogenic serovar could suggest that eel-virulent strains do not survive in natural waters and that the disease is mainly transmitted by direct host-to-host contact.
In this work, the mode of transmission of the disease to healthy eels and the putative reservoirs of the pathogen between outbreaks or epizootics have been investigated by means of laboratory and field experiments. Firstly, direct and indirect host-to-host transmission was evaluated by cohabitation experiments between donor (diseased) and recipient (healthy) eels. Second, the portals of entry into the eel body were studied by patch contact challenges and oral-anal intubations. Third, the role of survivors as disease carriers was investigated by bacteriological analysis and microscopic examination of infected eel tissues. Finally, the presence of this microorganism in tank water as well as the time course of the disease were monitored during one natural outbreak registered in an eel farm. To isolate this microorganism from the fish farm tank water, two selective media were employed after enrichment with APW: CPC agar (23) and VVM agar (11). The VVM agar has been designed recently and has not been used for the isolation of serovar E from fish farm water before.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strain CECT 4604 of V. vulnificus serovar E was used in this study. This strain was originally isolated as a pure culture of opaque (encapsulated) colonies from internal organs of a diseased eel in a Spanish fish farm (7). The strain was maintained as lyophilized stock at room temperature (25°C) and as frozen stock at −80°C in Marine Broth (Difco) plus 20% (vol/vol) glycerol. Cells were cultured in modified salt water-yeast extract (MSWYE) broth (28) with shaking or on MSWYE agar at 25°C for 24 h.
Experimental fish.
European elvers (Anguilla anguilla) (body weight ranging from 8 to 10 g) from a freshwater eel farm which had no history of V. vulnificus infections were used in this study. Fish were held at 25°C in 80-liter glass tanks of saline water (1% NaCl) with a system of filtration and recirculation. For the challenge experiments, fish were held in 30-liter plastic containers with similar filtration and aeration systems. Water was replaced daily in the case of patch contact and gastrointestinal challenges.
Challenge experiments.
The challenge procedures were performed according to Amaro et al. (3) and Kanno et al. (19). All experiments were performed in triplicate. Moribund fish were removed and bacteriologically analyzed before death occurred. Mortalities were recorded daily and were considered only if the pathogen was reisolated as a pure culture from livers or kidneys of moribund elvers. Portions of internal elver tissues and the surface of survivors were directly streaked onto plates with Tryptone soy agar supplemented with 0.5% (wt/vol) NaCl (TSA-1). Identification of the pathogen was carried out by agglutination of cells taken from the suspect colonies with rabbit antiserum raised against whole cells of strain CECT 4604 (22).
(i) Immersion challenge.
Bath challenges were performed according to Amaro et al. (3). Briefly, elvers were immersed for 1 h in saline water (1% NaCl) at 26 ± 2°C inoculated with stationary-phase cells of strain CECT 4604 at a final concentration of approximately 105 to 106 CFU ml−1. Mortalities were recorded for 14 days, and the 50% lethal dose (LD50) was calculated by the method of Reed and Münch (32). Experiments were repeated at that dose and, after 24 and 48 h, fish that showed external signs of vibriosis but were not moribund were transferred to new containers and used as donors (diseased fish) in cohabitation experiments. For contact experiments, moribund fish were used as donors.
(ii) Cohabitation challenge.
Ten donors were transferred to each of two aquaria that contained 10 uninfected fish (recipients) (ratio of donors to recipients, 1:1). In one tank, recipients were in direct contact with donors, and in the other one, donors were placed in a water-permeable basket to avoid direct contact with recipients. Two control experiments were made using healthy elvers as donors. Mortalities were recorded over 22 days and coded as percent mortality. To confirm shedding of the pathogen from donors, samples from the upper layer of water, that is, at the air-water interface, were taken on days 14 and 21 and analyzed for the reisolation of V. vulnificus serovar E according to Amaro et al. (3).
(iii) Direct-contact challenge.
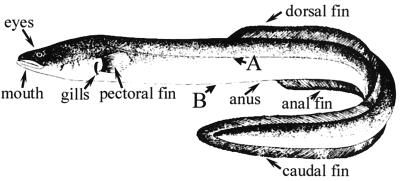
A group of 20 healthy elvers, anesthetized with benzocaine (200 mg/liter) (Guinama), were individually brought into physical contact with donors by gently rubbing their bodies either along its dorsal and ventral length or at specific points on the lateral or ventral zones (sites A and B, respectively, in Fig. 1). Afterwards, recipients were returned to a separate tank. Control groups were rubbed against uninfected fish in the same way. Mortalities were recorded for 40 days and coded as percent mortality.
FIG. 1.
Eel drawing indicating the specific rubbing sites (points A and B) between healthy and infected (by strain CECT 4604) eels in direct-contact challenges, and the sites where pieces of filter paper soaked in a cell suspension of this strain were placed in patch contact challenges.
(iv) Patch contact challenge.
Pieces (2.5 mm2) of sterilized filter paper were soaked in a cell suspension of strain CECT 4604 containing 109 CFU ml−1. Patches loaded with bacteria were applied for 1 min at different places on the surface of anesthetized healthy elvers: eyes, mouth (outside), gills (inserted between them), anus, pectoral fins, and anal, dorsal, and caudal zones of the long joined fin (Fig. 1). To determine whether sites other than the contacted point were contaminated with the pathogen, samples of elver surface were seeded onto TSA-1 plates. Fish were then returned to aquaria. Control groups were treated with patches soaked in phosphate-buffered saline solution supplemented with 1% (wt/vol) NaCl (PBS-1) in the same way. Mortalities were recorded for 40 days and coded as percent mortality.
(v) Gastrointestinal tract challenges.
A volume of 0.1 ml of a serial 10-fold dilution (from 109 to 105 CFU ml−1) of strain CECT 4604 was inoculated into groups of six elvers through the mouth to the stomach or through the anus to the intestine with a sterilized silicone tube (1 mm in diameter) attached to a plastic syringe. Control fish were given saline solution (0.85% NaCl, pH 7.0) in the same way. To determine the importance of transmission through food, intragastric intubations employing commercial feed contaminated with CECT 4604 were performed. The feed was autoclaved (121°C for 20 min) to avoid possible competition with any microorganism present in the sample. Then, it was aseptically homogenized with PBS-1 at a ratio of 1:4, and the mixture was incubated for 24 h at 28°C. Afterwards, this homogenate was diluted with an equal volume of bacterial suspension and maintained at room temperature for 30 min to allow the adsorption of the bacterial cells to the particulate material. The doses assayed and the procedure used were the same as in the oral inoculation of bacterial suspension alone. Control fish were challenged with sterile feed homogenate. Mortalities were recorded for 14 days and coded as percent mortality.
Survival in eel skin mucus.
Eel mucus was collected by placing healthy elvers in sterile flasks for approximately 5 min (12). After removing the fish, the mucous material within each flask was taken and filtered through 0.8- and 0.45-μm-pore-size filters (Millipore) and stored at −80°C until used. Survival of strain CECT 4604 in mucus solution was assayed in duplicate by inoculating bacterial stationary-phase cells resuspended in PBS-1 in samples of mucus at a level of around 105 CFU ml−1. The mixture was incubated at room temperature for 4 h, sampling every 60 min for plate counts on TSA-1.
Detection by immunofluorescence.
Tissue samples from moribund and survivor elvers positive for V. vulnificus serovar E were processed for examination by immunofluorescent antibody technique (IFAT) as described by Marco-Noales et al. (22). Briefly, tissue smears of gills, body surface, intestine, kidney, blood, and liver were placed within the circled areas of black slides, dried, fixed with 2% (vol/vol) formalin, and covered for 1 h with a primary antibody solution (diluted in PBS-1) at room temperature. After being washed, slides were incubated for 1 h with a solution of the secondary antibody at the same temperature. PBS-1 and tissue samples from healthy elvers were used as negative controls. Finally, slides were mounted with 50% (vol/vol) glycerol in PBS-1 with 25 mg of 1,4-diazabicyclo-(2,2,2)-octane (DABCO; Sigma) ml−1 and examined at a magnification of ×1,250 with a Zeiss epifluorescence microscope using a 450- to 490-nm band pass filter, FT510 beam splitter, and LP520 barrier filter.
Scanning electron microscopy.
Scanning electron microscopy was used to analyze the tissues of survivors that were positive for V. vulnificus serovar E isolation. Pieces of elver tissue were fixed with 2.5% (vol/vol) glutaraldehyde in 0.1 M phosphate buffer (0.48% [wt/vol] K2HPO4, 1.12% [wt/vol] Na2HPO4, pH 7.5) at room temperature for 2 h and then postfixed in 1% (wt/vol) osmium tetroxide for another 2 h. After being washed in distilled water (three times, 10 min each), samples were dehydrated in a graded alcohol series (30, 50, and 70% for 5 min each and then 100% for 20 min) and critical-point dried in CO2 in a Tousimis Autosamdri model 814 critical-point dryer. Finally, samples were coated with AuPd in a Bio-Rad model E5600 sputtering apparatus for examination with a Hitachi H-4100 scanning electron FE microscope at 5 to 10 kV of accelerating potential. Micrographs were made with Agfapan ISO100 film.
Biofilm formation assays.
Biofilm formation was evaluated according to O'Toole and Kolter (31) by the ability of cells of strain CECT 4604 to adhere to the wells of 96-well microtiter dishes made of polystyrene (hydrophobic surface) or to glass tubes (hydrophilic surface). Three growth media were employed: MSWYE broth, MSWYE broth plus 1% (wt/vol) glucose (MSWYE + G), and eel skin mucus. The medium (100 μl/well or tube) was inoculated with an appropriate dilution from an overnight Luria-Bertani (LB) culture. The assay was started with a relatively small number of cells (around 5 × 106 CFU ml−1) in the case of MSWYE and MSWYE + G, or with a high one (approximately 107 to 108 CFU ml−1) in the case of eel mucus. The plates or tubes were incubated at 28°C for 5 and 10 h. Biofilm formation was quantified by the addition of 200 μl of 95% (vol/vol) ethanol to crystal violet-stained samples, and the absorbance was determined with a plate reader at 540 nm (Multiskan Askcent; Labsystems).
Eel farm monitoring during a natural outbreak.
The isolation of V. vulnificus serovar E from water was attempted during a natural outbreak registered in an eel farm by using a two-step procedure with enrichment and selection (18, 29, 34) immediately after the first dead animal appeared. The outbreak affected animals maintained in fresh water at 27°C. The vibriosis was confirmed at day 2 after isolation and biochemical and serological identification from moribund eels (7). A drug susceptibility test was performed according to Biosca et al. (8). Mortalities in terms of kilograms of dead fish were recorded daily. To isolate serovar E from water, volumes of 100 ml of tank water were filtered at days 2 and 7. Filters were incubated for 12 h in APW at room temperature. An aliquot of 1 ml from the enrichment broth was tested by spreading on selective CPC agar (23) and VVM agar (11) plates. The yellow colonies were purified on TSA-1 and biochemically and serologically identified.
RESULTS
Bath challenges.
Under the assay conditions of temperature and salinity, the LD50 was around 105 CFU ml−1. V. vulnificus serovar E was isolated as a pure culture from internal organs and the surface of moribund elvers. Fish started to die at day 2 postchallenge, but external hemorrhages and ulcers were already apparent in some animals by day 1. Fish with similar external signs, taken at days 1 and 2, were used as donors for cohabitation experiments. V. vulnificus serovar E was not isolated from the internal organs of survivors, but it was recovered as a mixed culture from surface samples.
Cohabitation challenges.
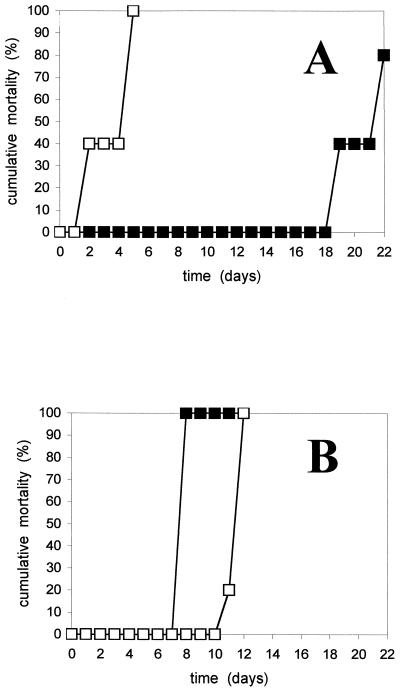
In all cohabitation experiments, mortalities were always higher than 80% in the recipient groups. The results of two representative experiments are shown in Fig. 2. Differences were found in the time of death between experiments with and without a physical barrier between donors and recipients. In the first case, it was clearly dependent on the infection stage of donors: deaths started much earlier if the elvers used as donors were taken at 48 h instead of 24 h post-water-borne infection (Fig. 2A). In the second case, the time of death was around 7 to 11 days after challenge, depending on the experiment, but was apparently independent of the donor's infection stage (Fig. 2B). Thus, interestingly, the presence of a physical barrier did not delay the time of death when donors were in an advanced stage of infection. In fact, mortality was higher and faster in the partitioned group than in the group cohabiting with 48-h-postinfection fish. It was confirmed that the donor fish shed cells of V. vulnificus serovar E into the water, since this microorganism was isolated on TSA-1 as a mixed culture from all water samples.
FIG. 2.
Cumulative mortality of recipient elvers challenged by cohabitation with donor elvers (ratio, 1:1) previously infected by immersion with strain CECT 4604. (A) No physical contact. (B) Physical contact. The experiments were performed with donor elvers infected for 24 h (▪) or 48 h (□) before the cohabitation challenge.
All moribund fish showed typical signs of vibriosis caused by V. vulnificus serovar E (7), and this microorganism was reisolated as a pure culture from internal organ and external surface. At 15 days after challenge, some survivors showed a thick mucous layer coating the body surface that was partly shed over time. V. vulnificus serovar E was recovered as a pure culture from this external layer. Control fish did not show signs of disease.
Direct-contact challenges.
Mortalities of 10% were reached when the tested group was rubbed against infected fish along the whole body. Direct contact at specific sites (in points of lateral and ventral zones [A and B in Fig. 1]) resulted in 100% survival of recipient fish at 40 days postchallenge. In the group rubbed against healthy fish, there was neither visible change nor mortality.
Patch contact challenge.
Immediately after the patch contact challenge, V. vulnificus serovar E was isolated from the specific contact site. However, because a mucous layer surrounds eels, this microorganism was occasionally isolated from other body surface areas near to the contact site. In general, this method of restrictive infection proved to be effective, since the results obtained in the different experiments were quite similar (Table 1). In all cases, the highest mortality was recorded when the gills were contacted with the vibrio-loaded filter paper. In this case, an average cumulative mortality of 62% was achieved before the first week. Lower cumulative mortalities were recorded when the eyes and the dorsal fin were contacted, and the lowest one when mouth or anal fin was challenged. Dead fish showed typical signs of vibriosis, with petechiae and general hemorrhages and exophthalmic and large ulcers, mainly at the contact site. In the control group, neither mortality nor external change was registered.
TABLE 1.
Cumulative mortality of elvers (average weight, 10 g) challenged by patch contact at specific body sites with papers soaked in a cell suspension of strain CECT 4604
| Patch contact site | Cumulative % mortality ± SD on days:
|
|||
|---|---|---|---|---|
| 0–5 | 5–10 | 10–20 | 20–35 | |
| Anus | 0 | 0 | 0 | 54.7 ± 8.6 |
| Anal fin | 0 | 0 | 0 | 16 ± 2.1 |
| Caudal fin | 3.3 ± 3.3 | 40 | 40 | 57.3 ± 2.7 |
| Dorsal fin | 6.7 ± 4.2 | 32 ± 8 | 32 ± 8 | 46.7 ± 2.5 |
| Eyes | 0 | 8 ± 4.9 | 8 ± 4.9 | 10.7 ± 4.3 |
| Gills | 62.5 ± 16.5 | 100 | 100 | 100 |
| Mouth | 0 | 0 | 0 | 4 ± 2.1 |
| Pectoral fins | 0 | 8 ± 8 | 44 ± 2.7 | 61.3 ± 1.3 |
Gastrointestinal tract challenges.
Mortalities of 100% occurred at a dose of <2.9 × 103 CFU g−1 in the group challenged through the anus (Table 2). In fish challenged through the mouth with bacterial cells alone, no mortality was recorded at a dose of 2.5 × 107 CFU g−1 (Table 2). However, the intragastric inoculations with V. vulnificus serovar E-laden feed at a similar bacterial dose resulted in the death of 33% of the fish after 3 days (Table 2). Among control groups receiving only saline solution or only sterile feed homogenate, neither mortality nor visible change was observed. Moribund fish showed external hemorrhages, mainly in the ventral part of the body, and a hemorrhagic intestine.
TABLE 2.
Mortality of elvers (average weight, 10 g) after gastrointestinal challenge with strain CECT 4604
| Challenge zone | Dose (CFU/g of fish) | Inoculated material | % Mortality |
|---|---|---|---|
| Stomach (via mouth) | ≥2.5 × 107 | Bacterial cells | 0 |
| 1.5 × 107 | Bacterial cells + feed | 33 | |
| Intestine (via anus) | <2.9 × 103 | Bacterial cells | 100 |
Survival in skin mucus.
Cells of V. vulnificus serovar E survived and successfully multiplied in skin mucus, reaching a fourfold increase in number of viable cells at the end of the incubation period (data not shown).
Microscopic observations.
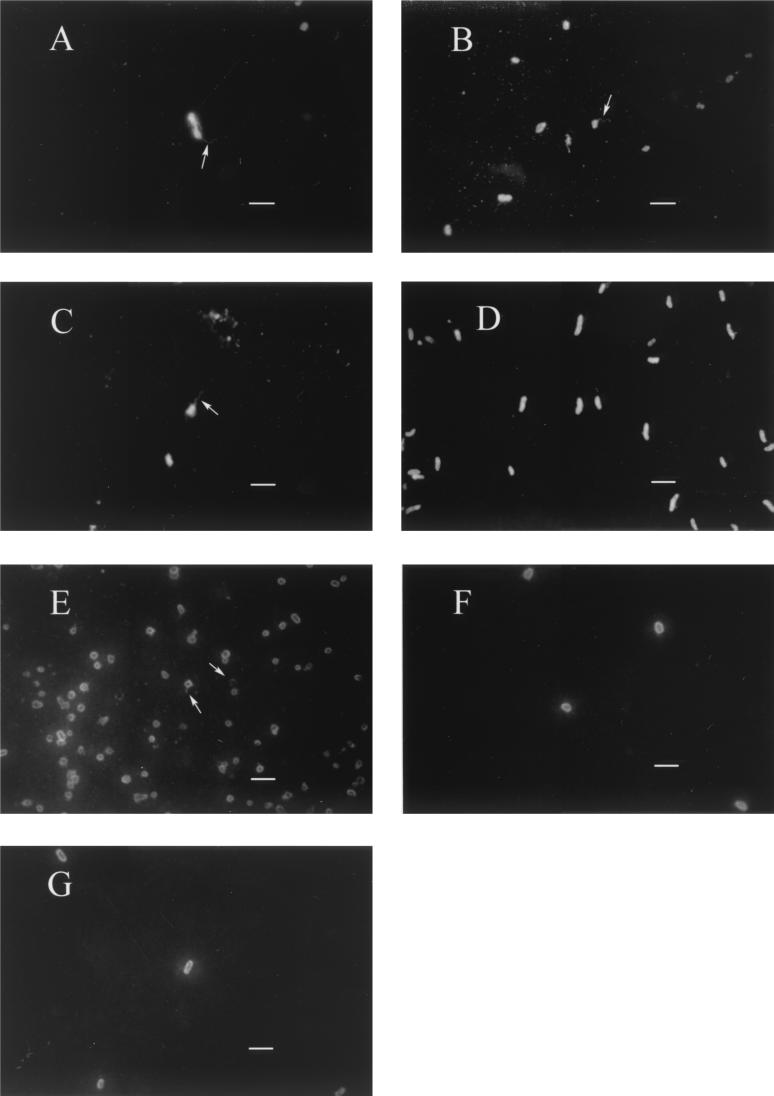
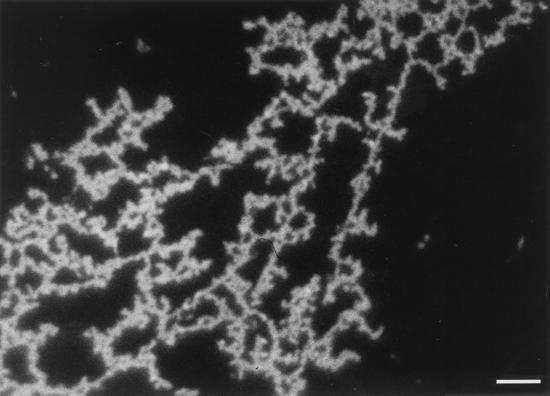
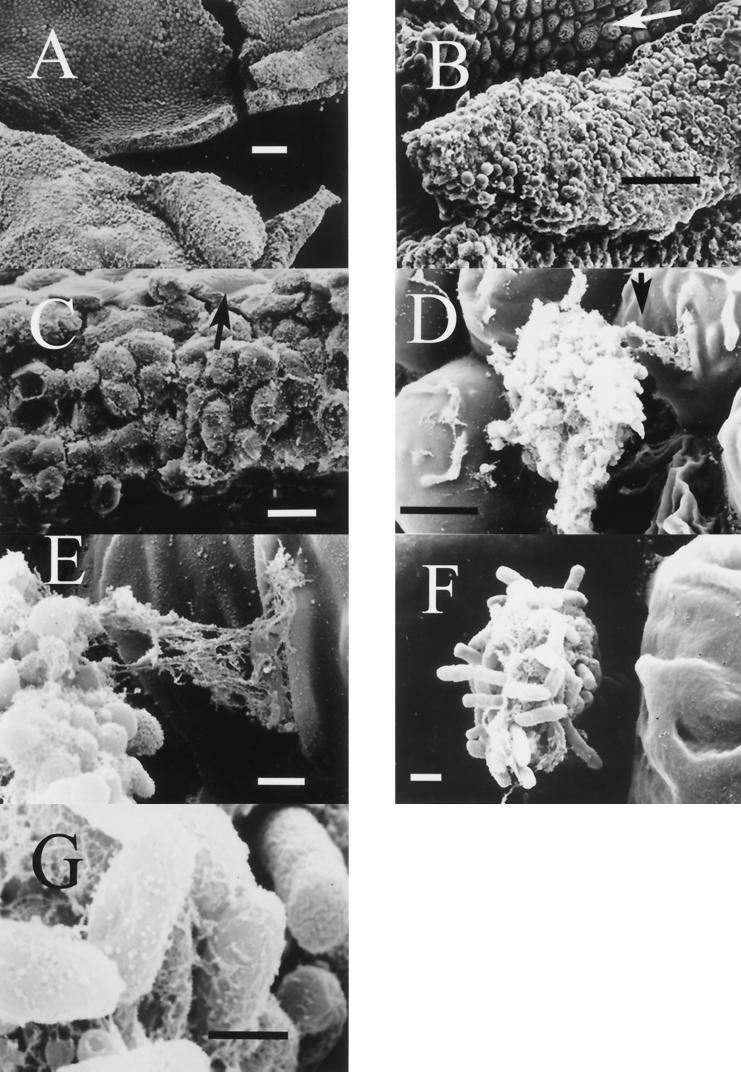
Tissue samples from external and internal organs of moribund eels that were positive for V. vulnificus serovar E isolation were processed by IFAT. Cells of the pathogen appeared clearly stained in fluorescent green, and some of them showed a polar flagellum (Fig. 3). In samples from fish challenged by patch contact, cells were detected in the specific contact site (Fig. 3G). IFAT was also used to analyze the mucous layer coating the body of survivors, which was positive for V. vulnificus serovar E isolation. Green cells forming a kind of network, kept together by an extracellular immunoreactive substance, were clearly observed (Fig. 4). Scanning electron micrographs of the same samples appear in Fig. 5. In these samples, the epidermis, the external layer made up of wrinkled cells (indicated by arrows in Fig. 5B and C), and the dermis, a thicker, internal layer placed under the first one (Fig. 5B and C), were clearly distinguishable. Among epidermal cells, that is, on the outer part of eel skin, microcolonies of bacteria adhering to the interstices were observed (Fig. 5D to F). Bacterial cells showed a great amount of fibrillar extracellular material (Fig. 5D to G) that formed bridges among bacteria and eel epidermal cells (Fig. 5D and E). Flagella were observed in some bacteria (Fig. 5F).
FIG. 3.
Epifluorescence micrographs of tissue samples from elvers infected with V. vulnificus serovar E, stained by IFAT using an anti-whole-cell serum. Samples corresponding to blood (A), intestine (B), kidney (C), body surface (D), gills (E and G), and liver (F) are representative of different experiments: bath challenge (A and B), cohabitation challenge with contact between donor and recipient fish (C and E), cohabitation challenge without contact (D), and patch contact challenge in gills (F and G). Arrows indicate the polar flagellum. Bars, 2 μm.
FIG. 4.
Epifluorescence micrograph of the mucous layer coating the surface of one survivor elver from experimental cohabitation challenge. The sample was stained by IFAT with an anti-whole-cell serum against V. vulnificus serovar E. Bar, 5 μm.
FIG. 5.
Scanning electron micrographs of the eel body surface of one survivor from experimental cohabitation challenge. (A, B, and C) It can be observed that the layer is actually eel skin, distinguishing the epidermis and the dermis below. Among epidermal cells there were microcolonies of bacteria (D to G) adhering to eel epidermis by means of an extracellular mesh-like substance (D and E) which also covered the bacterial cells (E to G). Arrows indicate wrinkled cells of eel epidermis (B and C), a fragment of the photo amplified in picture E (D), and a bacterial flagellum (F). Bars: 100 μm (A), 50 μm (B), 10 μm (C), 5 μm (D), 1 μm (E and F), and 0.5 μm (G).
Biofilm formation assays.
To confirm that V. vulnificus serovar E was able to form a biofilm, we performed an in vitro assay previously described by O'Toole and Kolter (31). Strain CECT 4604 colonized both hydrophobic and hydrophilic surfaces when MSWYE and eel mucus were used as growth media (Table 3). The strain formed a biofilm at the interface between the air and the liquid medium and at the bottom of the wells or tubes. Biofilm production was inhibited by the addition of glucose to MSWYE (Table 3). In the presence of glucose, the growth kinetics were distinct: cells achieved the stationary phase earlier (at 5 h; data not shown) and the final cell rate was lower (around 108 CFU ml−1) (Table 3).
TABLE 3.
Biofilm formation on hydrophobic and hydrophilic surfaces of strain CECT 4604 after 10 h of incubation in different growth media, measured as absorbance at 540 nm after extraction with ethanol of crystal violet-stained cells
| Growth medium | Mean A540 ± SDa for growth on:
|
|
|---|---|---|
| Hydrophobic surface | Hydrophilic surface | |
| MSWYE | 0.55 ± 0.05 (1.2 × 109) | 0.65 ± 0.35 (5 × 109) |
| MSWYE + G | 0 ± 0.01 (2 × 108) | 0.05 ± 0.10 (4 × 108) |
| Eel mucus | 0.36 ± 0.03 (1 × 1010) | 0.33 ± 0.06 (1.5 × 109) |
The corresponding value in CFU per milliliter on TSA-1 is shown in parentheses.
Field studies.
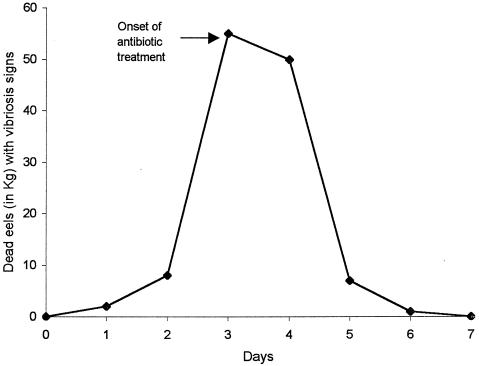
During a natural outbreak in an eel farm, affected fish showed external hemorrhages and ulcers mainly located near the head and in the ventral part of the body. Internally, the main signs were a pale liver and a hemorrhagic intestine. Pure cultures on TSA-1 were obtained from all internal organs sampled of moribund fish. The isolated strains presented API 20E profile 5006005 and agglutinated with the specific antiserum against serovar E. From these results, they were identified as V. vulnificus serovar E. The most effective antibiotic in the drug sensitivity test, tetracycline, was used to control the vibriosis (25 ppm as a bath followed by 80 mg/kg of fish per day for 12 days), and the antibiotic treatment started on day 3. After 24 h, the number of dying eels decreased, and the outbreak was finally controlled at day 7. The time course of the outbreak is shown in Fig. 6.
FIG. 6.
Time course of an outbreak in a Spanish eel farm, which affected fish maintained in fresh water at 27°C. The data show kilograms of dead eels with signs of vibriosis over time.
Samples of tank water were analyzed to check whether the pathogen was present in a viable form. After enrichment in APW and growth on selective media, mixed cultures were obtained, and yellow colonies suspected of being the eel pathogen were purified and identified. V. vulnificus serovar E was detected only from samples taken on day 2 and seeded on VVM. The water isolates showed the same biochemical profile in the API 20E system as the eel isolates and also agglutinated with the specific antiserum. Other yellow colonies from CPC agar and VVM agar were identified as V. vulnificus biotype 1 because they gave a code profile of 1146005 (99.9%) and were negative for agglutination with the specific antiserum against serovar E.
DISCUSSION
Transmission of the disease.
Results of the cohabitation experiments and direct-contact challenges suggest that the disease produced by V. vulnificus serovar E in eels is mainly transmitted by water. First, the disease was transmitted from donors to recipients who were separated by a physical barrier. Second, physical contact between donors and recipients did not enhance the effectiveness of transmission; mortality was always higher than 80% regardless of contact among fish. Third, the bacterium was isolated from the upper surface of the water, with which this species seems to be preferentially associated (3). These cells, probably originating from diseased and moribund fish, indicated that the pathogen was effectively released into water. Finally, the direct-contact challenges resulted in a low mortality (10%) when whole surfaces were contacted and in no mortality when contact took place at specific sites. In cohabitation experiments, the time of death in recipient eels was influenced by the infection stage of donor fish, especially when they were physically separated. In this case, the sole mode of disease transmission was through water, so that the more advanced the stage of the disease, the more bacteria were released and the more animals were infected in a short time. The influence of the infection stage of donors was less apparent when there was contact between donor and recipient fish, probably because the pathogen was also transmitted from infected to healthy individuals by the direct contact of their bodies. Kanno et al. (19) reached the same conclusion after a similar set of experiments performed with another fish pathogen, Vibrio anguillarum, in ayu (Plecoglossus altivelis).
The field results obtained during a natural outbreak affecting one eel farm support the hypothesis that water is the main vehicle for disease transmission. The shape of the outbreak curve, with a sharp rise to a peak, is more compatible with a common-source outbreak than with a host-to-host one, which is characterized by a relatively slow progressive rise (10). A common-source outbreak arises as the result of a large number of animals being infected by common source, such as water. In fact, we successfully isolated V. vulnificus serovar E from tank water during the outbreak, probably for two reasons. First, we used a new selective medium which has recently been described as the most efficient one for V. vulnificus isolation (11). This medium contains MgCl2 · 6H2O and KCl, which act as stimulation growth factors for pathogenic vibrios (15). Second, we were able to sample water from tanks before antibiotic treatment had started. Currently, fish farmers start chemotherapy when the first dead eels appear, before the diagnosis is complete. Unfortunately, this practice is generally extended because fish farmers fear the devastating effects of this vibriosis. The use of drugs seemed effective, at least short term, since the bacterium was not recovered from water after the antibiotic treatment, and the outbreak was apparently controlled in 1 week. From the results obtained in the present study, we can also conclude that the medium VVM was more effective than CPC agar in the recovery of V. vulnificus serovar E from water. The use of this medium in the isolation of this bacterium from aquatic ecosystems could improve our knowledge of the ecology of this pathogen.
Portals of entry.
Several authors have suggested that the potential routes for penetration into fish of pathogenic bacteria are the gills, the skin, and the digestive tract (6, 19, 26). Nevertheless, a pathogen can use more than one portal of entry to colonize the same fish (19, 26). The patch contact challenge experiments demonstrated that the main portals of entry used by V. vulnificus serovar E were the gills, followed by the pectoral and caudal fins and the anus. Cells were able to resist the bactericidal effect of the surface mucus (1) and even proliferate, which correlates with the fact that mucosal damage is not necessary to promote the disease caused by V. vulnificus serovar E (3). Gills and anus are also important portals of entry of V. anguillarum into rainbow trout (6) and ayu (19), respectively.
Results of gastrointestinal challenges revealed that V. vulnificus serovar E was rapidly destroyed in the stomach (probably due to pH and digestive enzymes), but it could cause disease if it arrived at the intestine. First, at low doses (103 CFU g−1), the pathogen provoked 100% mortality when administered by the anal route. Second, at high doses (107 CFU g−1), it was avirulent by the oral route. Finally, when the pathogen was administered associated with food material, some cells could arrive at the intestine, proliferate, and begin the septicemic process. In all cases, moribund eels showed external hemorrhages and a hemorrhagic intestine as the main signs. Previous studies had demonstrated that V. anguillarum induced vibriosis in ayu and eel by anal intubation but not by the oral route (19). In these studies the pathogen was not administered with the feed. Although many cells of V. vulnificus mixed with feed are necessary to develop vibriosis, we cannot discard the possibility that the pathogen can use the oral route to enter the fish body. In fact, one of the characteristic signs when the pathogen enters by the oral-anal route, the hemorrhagic intestine, was detected in naturally infected eels during the natural outbreak studied mentioned above.
Biofilm production and microscopic observations.
Cells of V. vulnificus serovar E were detected by IFAT in all tissue samples analyzed, as vibriosis is a septicemic process and the pathogen spreads to different eel organs (7). The bacterium was also detected on the surface of the eels. Many cells showed a polar flagellum, which points out that motility can be an important virulence factor in vivo for development of vibriosis caused by this pathogen, as it is for V. anguillarum (30).
Some survivor eels presented a mucous layer coating the body, which contained a network of cells of V. vulnificus serovar E linked by an extracellular and immunoreactive matrix. This pathogen produces a mucous layer of exopolysaccharidic nature (9), essential to water-borne infection (3), which could partially correspond to the immunoreactive matrix observed by IFAT. Scanning microcopy showed that putative V. vulnificus serovar E cells formed microcolonies adhering to epidermic eel tissue by the extracellular material. This material built up bridges among bacterial cells, bringing them together in aggregates placed in the midst of eel cells.
In vitro experiments confirmed that the strain was able to colonize both hydrophilic and hydrophobic surfaces, forming a biofilm on the walls of the vessels even when eel mucus was used as the growth medium. This ability has also been described for other pathogenic vibrios that form a biofilm microscopically similar to that observed by us (20, 37). Biofilm formation in V. vulnificus serovar E was inhibited by glucose. The addition of glucose to the growth medium also seemed to prevent capsule production, measured as colony opacity (J. D. Oliver, unpublished data). It has been reported previously that this compound inhibits the adhesion to siliconized glass of other marine vibrios (14). These results are in contrast to those found in Escherichia coli, Salmonella spp., and Pseudomonas fluorescens, in which glucose promotes biofilm formation (31) or the secretion of molecules involved in intercellular communication through quorum-sensing systems (24, 33). More studies are needed to demonstrate the role of glucose in biofilm formation and the relationship between biofilm formation and capsule production.
The exogenous matrix of the biofilms has been reported to contribute, in natural ecosystems, to (i) the attachment of bacteria to marine organisms, such as plankton and fishes (25, 37), (ii) protection against a variety of environmental stresses, such as pH shifts and osmotic shock (16), and (iii) preventing access of several antimicrobial agents (17). This fact may explain how V. vulnificus survives between outbreaks and resists the adverse physicochemical conditions imposed by dissolved antibiotics and low water salinity.
In summary, the primary mode of transmission to healthy eels of V. vulnificus serovar E is through water, and the main portal of entry is via the gills. Bacteria can be released into water, adhere to the eel surface, and multiply, forming a kind of biofilm, which could constitute a strategy to survive between outbreaks. The state in which fish carry a V. vulnificus serovar E biofilm on the body surface could be considered a carrier state. The carriers could act as reservoirs and develop the disease under stress conditions. Because V. vulnificus serovar E can also be an opportunistic human pathogen, it seems clear that it would be advisable to set up proper management procedures at fish farms, including the reduction of overcrowding (as direct contact between individual fish might accelerate the spread of disease in crowded ponds), the prompt removal of moribund fish, and the adoption of preventive measures by fish farmers to avoid risks inherent in manipulating eels.
ACKNOWLEDGMENTS
This work was partially supported by two projects from the Comisión Interministerial de Ciencia y Tecnología (PB98-1423 and IFD97-0800). E. Marco-Noales thanks Consellería de Cultura, Educación y Ciencia de la Generalitat Valenciana (Plan Valenciano de Ciencia y Tecnología) for a predoctoral fellowship.
We thank the Servicio de Microscopía Electrónica (Universidad de Valencia) for expert technical assistance; Rafael Ruano and José Tornero for supplying eels from the eel farm Poliñá, and F. Barraglough and D. Donnellan for helping with the English.
REFERENCES
- 1.Alexander J B, Ingram G A. Noncellular nonspecific defence mechanisms of fish. Annu Rev Fish Dis. 1992;2:249–279. [Google Scholar]
- 2.Amaro C, Biosca E G. Vibrio vulnificus biotype 2:pathogenic for eels, is also an opportunistic pathogen for humans. Appl Environ Microbiol. 1996;62:1454–1457. doi: 10.1128/aem.62.4.1454-1457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaro C, Biosca E G, Fouz B, Alcaide E, Esteve C. Evidence that water transmits Vibrio vulnificus biotype 2 infections to eels. Appl Environ Microbiol. 1995;61:1133–1137. doi: 10.1128/aem.61.3.1133-1137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arias C R. Ph.D. thesis. Valencia, Spain: Universidad de Valencia; 1998. [Google Scholar]
- 5.Austin B, Austin D A. Vibrionaceae representatives. In: Laird L M, editor. Bacterial fish pathogens: disease in farmed and wild fish. Chichester, England: Ellis Horwood Limited; 1993. pp. 265–294. [Google Scholar]
- 6.Baudin-Laurencin F, Germon E. Experimental infection of rainbow trout, Salmo gairdneri R., by dipping in suspensions of Vibrio anguillarum: ways of bacterial penetration; influence of temperature and salinity. Aquaculture. 1987;67:203–205. [Google Scholar]
- 7.Biosca E G. Ph.D. thesis. Valencia, Spain: Universidad de Valencia; 1994. [Google Scholar]
- 8.Biosca E G, Amaro C, Esteve C, Alcaide E, Garay E. First record of Vibrio vulnificus biotype 2 from diseased European eel Anguilla anguilla L. J Fish Dis. 1991;14:103–109. [Google Scholar]
- 9.Biosca E G, Llorens H, Garay E, Amaro C. Presence of a capsule in Vibrio vulnificus biotype 2 and its relationship to virulence for eels. Infect Immun. 1993;61:1611–1618. doi: 10.1128/iai.61.5.1611-1618.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brock T D, Madigan M T, Martinko J M, Parker J. Epidemiology and public health microbiology. In: Brock T D, editor. Biology of microorganisms. 7th ed. Englewood Cliffs, N.J: Prentice-Hall International, Inc.; 1994. pp. 506–523. [Google Scholar]
- 11.Cerdà-Cuéllar M, Jofre J, Blanch A R. A selective medium and a specific probe for detection of Vibrio vulnificus. Appl Environ Microbiol. 2000;66:855–859. doi: 10.1128/aem.66.2.855-859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collado R, Fouz B, Sanjuán E, Amaro C. Effectiveness of different vaccine formulations against vibriosis caused by Vibrio vulnificus serovar E (biotype 2) in European eels Anguilla anguilla. Dis Aquat Org. 2000;43:91–101. doi: 10.3354/dao043091. [DOI] [PubMed] [Google Scholar]
- 13.Dalsgaard I, Høi L, Siebeling R J, Dalsgaard A. Indole-positive Vibrio vulnificus isolated from disease outbreaks on a Danish eel-farm. Dis Aquat Org. 1999;35:187–194. doi: 10.3354/dao035187. [DOI] [PubMed] [Google Scholar]
- 14.Dawson M P, Humphrey B A, Marshall K C. Adhesion: a tactic in the survival strategy of a marine Vibrio during starvation. Curr Microbiol. 1981;6:195–199. [Google Scholar]
- 15.Donovan T J, Van Netten P. Culture media for the isolation and enumeration of pathogenic Vibrio species in foods and environmental samples. Int J Food Microbiol. 1995;26:77–91. doi: 10.1016/0168-1605(95)00015-c. [DOI] [PubMed] [Google Scholar]
- 16.Flemming H-C. Biofilms and environmental protection. Water Sci Technol. 1993;27:1–10. [Google Scholar]
- 17.Gilbert P, Das J, Foley I. Biofilms susceptibility to antimicrobials. Adv Dent Res. 1997;11:160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 18.Høi L. Ph.D. thesis. Copenhagen, Denmark: The Royal Veterinary and Agricultural University; 1998. [Google Scholar]
- 19.Kanno T, Nakai T, Muroga K. Mode of transmission of vibriosis among ayu Plecoglossus altivelis. J Aquat Anim Health. 1989;1:2–6. [Google Scholar]
- 20.Kanno T, Nakai T, Muroga K. Scanning electron microscopy on the skin surface of ayu Plecoglossus altivelis infected with Vibrio anguillarum. Dis Aquat Org. 1990;8:73–75. [Google Scholar]
- 21.Marco-Noales E, Biosca E G, Amaro C. Effects of salinity and temperature on long-term survival of the eel pathogen Vibrio vulnificus biotype 2 (serovar E) Appl Environ Microbiol. 1999;65:1117–1126. doi: 10.1128/aem.65.3.1117-1126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marco-Noales E, Biosca E G, Milán M, Amaro C. An indirect immunofluorescent antibody technique for detection and enumeration of Vibrio vulnificus serovar E (biotype 2): development and applications. J Appl Microbiol. 2000;89:599–607. doi: 10.1046/j.1365-2672.2000.01156.x. [DOI] [PubMed] [Google Scholar]
- 23.Massad G, Oliver J D. New selective and differential medium for Vibrio cholerae and Vibrio vulnificus. Appl Environ Microbiol. 1987;53:2262–2264. doi: 10.1128/aem.53.9.2262-2264.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClean R J C, Whiteley M, Stickler D J, Fuqua W C. Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiol Lett. 1997;154:259–263. doi: 10.1111/j.1574-6968.1997.tb12653.x. [DOI] [PubMed] [Google Scholar]
- 25.Morris J G, Jr, Sztein M B, Rice E W, Nataro J P, Losonsky G A, Panigrahi P, Tacket C O, Johnson J A. Vibrio cholerae O1 can assume a chlorine-resistant rugose survival form that is virulent for humans. J Infect Dis. 1996;174:1364–1368. doi: 10.1093/infdis/174.6.1364. [DOI] [PubMed] [Google Scholar]
- 26.Muroga K, De La Cruz M C. Fate and location of Vibrio anguillarum in tissues of artificially infected ayu (Plecoglossus altivelis) Fish Pathol. 1987;22:99–103. [Google Scholar]
- 27.Oliver J D. Vibrio vulnificus, In: Doyle M P, editor. Foodborne Bacterial Pathogens. New York, N.Y: Marcel Dekker, Inc; 1989. pp. 569–600. [Google Scholar]
- 28.Oliver J D, Colwell R R. Extractable lipids of gram-negative marine bacteria: phospholipid composition. J Bacteriol. 1973;114:897–908. doi: 10.1128/jb.114.3.897-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver J D, Guthrie K, Preyer J, Wright A C, Simpson L M, Siebeling R, Morris J G., Jr Use of colistin-polymixin B-cellobiose agar for isolation of Vibrio vulnificus from the environment. Appl Environ Microbiol. 1992;58:737–739. doi: 10.1128/aem.58.2.737-739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ormonde P, Hörstedt P, O'Toole R, Milton D L. Role of motility in adherence to and invasion of a fish cell line by Vibrio anguillarum. J Bacteriol. 2000;182:2326–2328. doi: 10.1128/jb.182.8.2326-2328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 32.Reed M J, Münch M. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 33.Surette M G, Bassler B L. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:7046–7050. doi: 10.1073/pnas.95.12.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamplin M L, Martin A L, Ruple A D, Cook D W, Kaspar C W. Enzyme immunoassay for identification of Vibrio vulnificus in seawater, sediment, and oysters. Appl Environ Microbiol. 1991;57:1235–1240. doi: 10.1128/aem.57.4.1235-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tison D L, Nishibuchi M, Greenwood J D, Seidler R J. Vibrio vulnificus biogroup 2: new biogroup pathogenic for eels. Appl Environ Microbiol. 1982;44:640–646. doi: 10.1128/aem.44.3.640-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veenstra J, Rietra J P G M, Coster J M, Stoutenbeek C P, Ter Laak E A, Haenen O L M, De Hier H H W, Dirsks-Go S. Human Vibrio vulnificus infections and environmental isolates in the Netherlands. Aquacult Fish Manag. 1993;24:119–122. [Google Scholar]
- 37.Wai S N, Mizunoe Y, Takade A, Kawabata S-I, Yoshida S-I. Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl Environ Microbiol. 1998;64:3648–3655. doi: 10.1128/aem.64.10.3648-3655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]