Abstract
Many animals have strict diel activity patterns, with unique adaptations for either diurnal or nocturnal activity. Diel activity is phylogenetically conserved, yet evolutionary shifts in diel activity occur and lead to important changes in an organism's morphology, physiology, and behavior. We use phylogenetic comparative methods to examine the evolutionary history of diel activity in skinks, one of the largest families of terrestrial vertebrates. We examine how diel patterns are associated with microhabitat, ambient temperatures, and morphology. We found support for a nondiurnal ancestral skink. Strict diurnality in crown group skinks only evolved during the Paleogene. Nocturnal habits are associated with fossorial activity, limb reduction and loss, and warm temperatures. Our results shed light on the evolution of diel activity patterns in a large radiation of terrestrial ectotherms and reveal how both intrinsic biotic and extrinsic abiotic factors can shape the evolution of animal activity patterns.
Keywords: Activity times, ancestral state reconstruction, macroevolution, MCMCglmm, phylogenetic ordinal regression, Scincidae
Almost all organisms have evolved in a rhythmically changing environment. Daily patterns in an animal's behavior and physiology result from an integration between its endogenous circadian rhythms, generated by an internal clock mechanism, and the influence of the environment (entrainment; Kuhlman et al. 2018). Circadian rhythms, which generate behavioral timing and activity patterns, allow the anticipation of external and environmental events (Daan and Aschoff 1982; Horton 2001). These events can be both abiotic (e.g., insolation, ambient temperatures, humidity, light intensity) and biotic (e.g., predation risk, timing of reproduction, food availability). The circadian clock evolves to adjust the animal's behavior both to its changing environment and to stable conditions in their habitat, allowing it to anticipate and react to predictive changes around it (DeCoursey 2004). Activity patterns can, thus, converge in distantly related taxa that occupy similar habitats. Alternatively, their fitness‐inducing role may lead activity patterns to be phylogenetically conserved despite divergence in other axes of niche spaces such as their spatial distributions, microhabitat use, or dietary preferences (Daan 1981; Roll et al. 2006).
A strong phylogenetic signal in activity times has been found across tetrapods as a whole (Anderson and Wiens 2017), and in tetrapod taxa, such as the Mammalia (Maor et al. 2017) and Rodentia (Roll et al. 2006), suggesting this trait is phylogenetically conserved. However, ecological temporal partitioning in the time niche axis sometimes manifests as divergence in activity times. Although such shifts are considered rare (Kronfeld‐Schor and Dayan 2003), frequent shifts in activity times have occurred in some lineages. For example, simiiform primates, squirrels, elephant shrews, and mongooses have all independently shifted to diurnality, perhaps following the K‐Pg mass extinction and the likely release of diurnal niche space (Maor et al. 2017). Anderson and Wiens (2017) found that, while tetrapods were probably ancestrally nocturnal, and many have remained nocturnal to this day, shifts to diurnality and back to nocturnality have occurred multiple times. These shifts can also be important for broader‐scale macroevolutionary dynamics. For example, diurnal lineages tend to diversify faster than nocturnal lineages (Santini et al. 2015; Anderson and Wiens 2017) and this may explain why some diurnal tetrapod clades are extremely species rich despite being relatively young (e.g., Aves; Anderson and Wiens 2017).
Activity times are strongly influenced by environmental conditions such as ambient temperature (Helm et al. 2017). Lower temperatures at night, and longer nights during the activity seasons, can lead to diurnality being more common in colder habitats (e.g., for rodents and geckos; Roll et al. 2006; Vidan et al. 2017). This can be particularly important for reptiles. As ectotherms, reptiles depend on ambient heat, which they use to regulate their body temperature behaviorally and achieve suitable body temperatures for biochemical processes (Pianka and Vitt 2003). Most reptiles are diurnal, although some large clades, such as geckos and snakes, are predominantly nocturnal (Pianka and Vitt 2003; Vitt et al. 2003; Vitt and Caldwell 2014; Meiri 2020). Vidan et al. (2017) have shown that nocturnal reptiles are absent from the coldest regions in Eurasia. The body temperatures of nocturnal and cathemeral reptiles are correlated with ambient temperatures, whereas those of diurnal species are not (Meiri et al. 2013), and nocturnal species generally have lower body temperatures than diurnal species (Meiri et al. 2013; Moreira et al. 2021). These observations suggest that low ambient temperatures may serve as a filter for the presence of nocturnal ectotherms. Thus, we can expect nocturnal species to be more common in warmer regions, and completely absent from areas where night‐time temperatures would be too low to enable activity.
Activity time can be related to body size through the mediating effect of thermoregulation. Thermoregulatory efficiency is determined, in part, by the size of the thermoregulating animal. Heat gain and loss is faster in smaller ectotherms (Carothers et al. 1997; Seebacher and Shine 2004; Zamora‐Camacho et al. 2014), due to differences in volume and surface area. If it manages to obtain operating body temperature, a larger ectotherm can thus be active for longer periods in cooler environments, since it can retain its heat longer before needing to bask again (Klingenböck et al. 2000). We could expect that nocturnal ectotherms would be larger, since larger size would allow them to be active for longer periods of the night while maintaining effective body temperatures gained during the day (Feldman and Meiri 2014). Large body size, however, may also be a detriment to nocturnal activity, since during the night there is no direct source of heat through basking. Therefore, too large a size may be detrimental for nocturnal ectotherms, since heat gain would be too slow (Carothers et al. 1997) without prolonged cryptic basking before sunset. We could then hypothesize there might be a “Goldilocks” zone of body size for nocturnal ectotherms—not so small as to lose heat too rapidly, but not so large as to be unable to heat up in the first place.
Nocturnal habits are often associated with unique adaptations. These can range from greater reliance on acoustic or olfactory sensory information and communication (Healy and Guilford 1990; Barton 2006; Chen and Wiens 2020), and improved locomotion at low temperatures (Autumn et al. 1999; Bars‐Closel et al. 2018), to visual adaptations for low‐light conditions such as large eyes, lack of foveae, or rod‐like receptors in the retina without oil droplets (Walls 1942; Underwood 1951; Underwood 1970; Pinto et al. 2019; Röll 2000a, 2000b). Fossorial habits offer low‐light conditions and, at least during hot days, reduced temperatures, much like nocturnal habits (Wu et al. 2009). Some fossorial reptiles exhibit similar metabolic adaptations to nocturnal reptiles (Withers 1981; Andrews and Pough 1985; Wu et al. 2009; Bars‐Closel et al. 2018), and fossoriality and nocturnality have often been thought to be strongly correlated in reptiles (Thomas and Thomas 1978; Vitt and Caldwell 2014), although this hypothesized correlation was never formally tested. Fossorial reptiles are often (Brandley et al. 2008; Skinner et al. 2008; Siler and Brown 2011; Camaiti et al. 2021), but not always (Wiens et al. 2006), limbless or limb reduced. Limbs are reduced or lost, and bodies become elongated and streamlined, to lower lateral drag while moving through substrate, thus improving locomotor efficiency (Gans 1962, 1975; Lee 1998; Camaiti et al. 2021, 2019). Since fossorial and nocturnal species are thought to experience similar selective pressures, we can expect nocturnal habits to be associated both with fossorial habits and the evolution of limb‐reduced and limbless body forms in squamates.
Skinks (Scincidae) comprise the largest family of lizards, with 1727 species currently described worldwide (Uetz et al. 2021). They occur in almost all terrestrial habitats and display remarkable ecological diversity (Chapple et al. 2021). Most species are diurnal, but many are nocturnal or cathemeral (Vitt and Caldwell 2014). With their almost worldwide distribution, and presence in a wide range of environments (Roll et al. 2017), skinks are one of the most evolutionarily successful radiations of tetrapods. Skinks have evolved dramatic morphological, physiological, and behavioral changes, such as total limb reduction and viviparity, many times independently in relatively short time spans (e.g., Skinner et al. 2008). This makes them an excellent case study to examine the evolution of activity times in a large radiation of vertebrates.
We examine the evolution of activity patterns in the family Scincidae. We test the following hypotheses regarding activity times and time shifts in skinks, and their evolutionary drivers:
The ancestral skinks were diurnal, as is thought to be the case for most squamates apart from geckos and snakes (Gamble et al. 2015; Anderson and Wiens 2017), and since skinks are predominantly diurnal today (see below).
Fossorial species are often nocturnal or cathemeral (Roll et al. 2006; Vitt and Caldwell 2014). Fossoriality is also associated with limb reduction and loss in reptiles in general as well as in skinks (Camaiti et al. 2021 and references therein). We thus expect limb reduction and loss to be associated with nocturnal or cathemeral habits.
Nocturnality will more commonly evolve in warmer habitats, whereas in colder habitats, low night temperatures will preclude nocturnal activity (Vidan et al. 2017). We, therefore, expect nocturnality to be associated with warm temperatures across the skink phylogeny.
Large body sizes improve heat retention (Klingenböck et al. 2000; Zamora‐Camacho et al. 2014), but negatively affect heat gain (Carothers et al. 1997). We thus expect the probability of diurnality to be a U‐shaped function of size (both the smallest and largest skinks are more likely to be diurnal).
We test these hypotheses using a large‐scale dataset on skink activity times, distribution, and morphology, using a phylogenetic framework. We use a time‐calibrated phylogeny of skinks, reconstruct ancestral character histories, and test for correlated evolution between activity time, morphology, and habitat preferences.
Methods
DATA COLLECTION
We used published data on maximum lizard mass, activity time, and microhabitat use (Slavenko et al. 2016; Meiri 2018) supplemented based on personal observations in the field and additional published sources. We classify skinks based on their degree of limb reduction based on morphological data in Meiri (2018), updated where necessary using a dataset supplied by Camaiti et al. (In Press). We collected activity time data for 1300 species, microhabitat data for 1452 species, and limb reduction data for all 1727 currently recognized skink species (as of May 23, 2021). Overall, we had activity time and microhabitat data for 1030 species. We refer to this as the “original dataset.”
We classified skinks as belonging to one of three discrete activity time categories: “diurnal,” “cathemeral” (including both cathemeral and crepuscular species), and “nocturnal.” Cathemeral species were any that showed a mixed diel activity pattern, ranging from fully cathemeral (active independent of daily cycle) to mostly, but not strictly, diurnal or nocturnal (e.g., usually diurnal but with observations of rare nocturnal activity on warm nights). Diurnal and nocturnal species were those that were reported with strictly diurnal or nocturnal activity, with no evidence of daily or seasonal shifts in diel activity patterns. Since our method to classify species as cathemeral is necessarily subjective, we assessed the sensitivity of our results to this subjectivity by rerunning the ancestral state reconstruction (see below) on a “strict dataset,” where we coded species as “cathemeral” only if they were explicitly stated to be cathemeral or crepuscular. Species that were described as predominantly diurnal or nocturnal, but with occasional or rare activity in the opposing time period, were coded according to their predominant activity time, and species that could not be confidently assigned under this classification were omitted. This resulted in 56 differently coded species compared to the original classification—45 as diurnal, 6 as nocturnal, and 5 omitted.
We further classified skinks as belonging to one of six discrete microhabitat categories based on substrate data. Substrate data were recorded from the literature and, in few cases, from our own observations in the field (Meiri 2018). Data refer to the substratum a reptile is active on (or in) when engaging in foraging, social, or active thermoregulatory behavior. We defined substrates as terrestrial (on the ground, including in leaf litter), fossorial (burrowing below ground), cryptic (species found almost always beneath objects such as rocks), arboreal (climbing vegetation), saxicolous (climbing rocks, boulders, nonhorizontal rocky structures), or (semi) aquatic (swimming in freshwater). In our final categorization, we generated the following microhabitat categorizations: “terrestrial,” “scansorial” (arboreal or saxicolous substrates, or both), “fossorial” (combination of fossorial and cryptic substrates), “semifossorial” (fossorial or cryptic and either terrestrial or saxicolous substrates), “semiaquatic,” and “variable” (mixed terrestrial and either arboreal, or saxicolous, or both). Finally, we classified skinks as belonging to one of three discrete limb reduction categories: “fully developed,” “reduced,” and “limbless.” Limb reduced skinks were those with either front limb length ≤ 15% of SVL (snout‐vent‐length) or hind limb length ≤20% of SVL (or both) following Camaiti et al. (In Press). Limbless skinks were those without limbs or remnants thereof (i.e., length of forelimb and hindlimb equals zero, without any spurs or vestigial limbs breaking the body wall).
We used a large‐scale, time‐calibrated phylogeny of squamates (Zheng and Wiens 2016) and pruned it to only include species for which we had data for all traits. We refer to this as the “final dataset,” comprising 595 skink species, with representatives from 131 out of 161 currently recognized genera. The missing 30 genera are predominantly species‐poor (≤nine species, 15 of the 30 are monotypic). The phylogenetically pruned “strict dataset” (different coding of cathemerality; see above) comprised of 594 species (missing only Amphiglossus reticulatus).
To explore patterns of diel activity in different skink clades, we follow the higher order classification of skinks of Shea (2021), which recognizes three subfamilies of skinks: Acontiinae, Scincinae, and Lygosominae, the latter of which is comprised of seven tribes: Lygosomini, Ateuchosaurini, Tiliquini, Eugongylini, Ristellini, Sphenomorphini, and Mabuyini. However, we note that whereas most published phylogenies place Eugongylus as the earliest branching lineage within Eugongylini (e.g., Pyron et al. 2013), the phylogeny we use (Zheng and Wiens 2016) has Eugongylus nested within Sphenomorphini, which also leads to Lygosomini being paraphyletic. Therefore, we treat Eugongylus as a member of Sphenomorphini for the purposes of this analysis following Zheng and Wiens (2016), although recognizing that the evidence for this placement is weak (Shea 2021). This placement is used for the sake of convenience and does not represent any taxonomic changes or decisions. All analyses were performed in R version 4.0.2 (R Core Team 2020).
ANCESTRAL STATE RECONSTRUCTION
We used the “fitDiscrete” function from the geiger package (Pennell et al. 2014) to fit several different models of discrete character evolution to select the most fitting transition rate matrix for each trait. We fitted three different models for all three traits: “ER” (equal transition rates between all states), “SYM” (symmetrical transition rates between states, i.e., q12 = q21 but q12 ≠ q23, where qij is the transition rate between state i to state j), and “ARD” (all transition rates differ). Furthermore, for activity time and limb reduction, we fitted two additional models: “MER‐SYM” (meristic model where transitions occur in a stepwise manner, i.e., 1 to 2, 2 to 3, but not 1 to 3, with symmetrical transition rates), and “MER‐D” (meristic model where transitions occur in a stepwise manner and all pairwise transition rates differ). We defined the stepwise progression for activity time as “diurnal” ↔ “cathemeral” ↔ “nocturnal,” and for limb reduction as “fully developed” ↔ “reduced” ↔ “limbless.” We then compared models via AICc scores to select the best supported model for each trait (Burnham and Anderson 2002).
We reconstructed ancestral states using Stochastic Character Mapping (SCM; Bollback 2006) with the “make.simmap” function in the phytools package (Revell 2012). We ran 100 iterations with the best‐fitting discrete character evolution model for each trait to obtain posterior probabilities (PP) for each character state at each internal node in the phylogeny. We considered a PP of 0.67 or higher as strong support for the ancestral state at the internal node, as it represents a best‐supported state that is at least twice as likely as the next best‐supported state (Maor et al. 2017). We also obtained posterior distributions for the number of shifts between each pair of character states during the evolutionary history of skinks. To ascertain the robustness of our results to competing methods of ancestral state reconstruction, we also performed maximum likelihood estimation of ancestral states using the “ace” function in the ape package (Paradis and Schliep 2019), and ancestral state estimation with the rerooting method (Yang et al. 1995) using the “rerootMethod” function in the phytools package (Revell 2012). We then compared the estimated probabilities for ancestral states derived using the three methods.
To assess whether our ancestral reconstruction is robust to the inclusion of particular taxa, we performed two supplementary analyses: in the first, we included representatives of the three other scincoid families that together are sister to the Scincidae (Pyron et al. 2013; Zheng and Wiens 2016)—the predominantly diurnal Cordylidae (42 species included) and Gerrhosauridae (28 species included), and the predominantly nondiurnal Xantusiidae (11 species included). We refer to this as the “extended dataset.” In the second, we excluded the skink subfamily Acontiinae from the analysis, due to them being the sister clade of all other skinks and their unusual combination of features fixed across the entire lineage (acontiines are all limbless, nondiurnal, and fossorial; see Results). We refer to this as the “reduced dataset.” We repeated the procedures described above, including selection of best model of trait evolution and ancestral state reconstruction, using both the “extended dataset” of skinks and closely related taxa (676 species), and the “reduced dataset” of skinks excluding Acontiinae (587 species).
Since our results are robust (see below) both to different coding of the cathemeral state (“strict dataset”), and to the inclusion of outgroups and exclusion of Acontiinae (“extended dataset” and “reduced dataset,” respectively), all results shown below refer to the “final dataset” unless stated otherwise.
EVOLUTIONARY DRIVERS OF ACTIVITY TIMES
We tested for correlations between the evolution of activity times and microhabitat, and between activity times and degrees of limb reduction, using posterior predictive tests (Huelsenbeck et al. 2003), as implemented with SCM (Bollback 2006). We used the “Dtest” function in the phytools package (Revell 2012), to measure the difference between the expected and observed association between each two pairs of character states from the different traits’ simulated character histories (Leschen and Buckley 2007). Negative values of the association test statistic, D, indicate that character states are associated less than expected by chance, whereas positive values indicate associations that are more common than expected by chance. Associations were assessed by calculating p values, with significance set at an α of 0.05.
Next, we examined correlated evolution between activity time and body size (measured as maximum mass; data from Slavenko et al. 2016 and subsequent additions) and mean temperature (BIO1 from CHELSA; Karger et al. 2017). Mean temperature was used as a proxy for the typical temperature regimes species are exposed to, although we recognize that in some cases, temperature minima or maxima may be more relevant to biological function. To calculate the mean temperature for each species, we overlapped polygons of species ranges from an updated version of Roll et al. (2017) over the BIO1 raster at a 30 arc‐second resolution in ArcGIS. We then averaged the values of BIO1 across each species range to arrive at one mean value per species. The dataset for this analysis included only 590 species, since five species lacked climatic data.
To implement the best‐supported meristic model of activity time evolution (see below), we performed phylogenetic ordinal regression using the MCMCglmm package (Hadfield 2010). We used activity time as an ordinal response value (arbitrarily assigning “Diurnal” to 0, “Cathemeral” to 1, and “Nocturnal” to 2, to replicate the stepwise progression between the states), and maximum body mass (in g, log10‐transformed) and mean temperature (in°C) as fixed effects. We also examined quadratic effects for mass and temperature, and scaled them to have a mean of 0 and variance of 1 to allow easier comparison between their effect sizes. We also tested a model with an interaction term between the two predictors, but the interaction was found to be nonsignificant (results not shown). We used the “ordinal” family with a probit link function and assigned species as a random factor to account for phylogenetic relatedness. Following recommendations for models with the “ordinal” family (Hadfield 2010), we fixed the prior for residual variance at a value of 1 and set a weak prior for the random effect variance by using a low value of nu (0.002). We allowed for parameter expansion by setting the prior means (alpha.mu) to 0, and the prior covariance matrix (alpha.V) to 10000. We ran the MCMC algorithm for 5 × 108 generations, sampling every 2.5 × 105 generations, and discarded the first 10% as burn‐in. We ascertained that acceptance ratios were above 0.25, visually assessed trace plots (Supporting information Fig. S1), and calculated effective sample sizes (ESS) to ensure proper mixing and exploration of parameter space.
Results
Skinks are mostly diurnal. We classify 63.7% of species in the “final dataset” as diurnal, 31.3% as cathemeral, and 5.0% as nocturnal (379, 186, and 30 species, respectively). These proportions are similar to those in the original dataset (72.3% diurnal, 21.5% cathemeral, and 6.2% nocturnal species). The proportion of diurnal species varies greatly between skink clades (Fig. 1; Supporting information Table S1).
Figure 1.
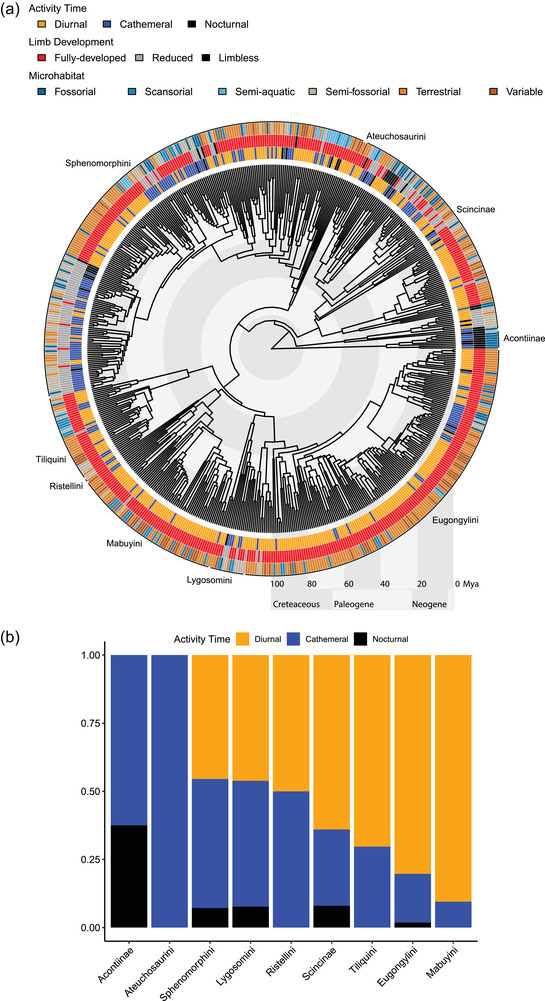
(a) Phylogeny of skinks, with tips color‐coded according to discrete traits. The inner ring encodes activity time, the middle ring encodes limb reduction, and the outer ring encodes microhabitat. (b) Bar plots designating the proportions of species of each activity time in the nine clades of skinks annotated on the phylogeny in panel A.
TRANSITIONS BETWEEN ACTIVITY TIMES AND HABITAT USE AND ANCESTRAL STATES
The MER‐D model (transitions occur in a stepwise manner and all differ) was best supported for activity times (“diurnal” ↔ “cathemeral” ↔ “nocturnal”; Fig. 2), and for limb reduction (“fully developed” ↔ “reduced” ↔ “limbless”), whereas the ARD model (transition rates between all character states differ) was the best supported for microhabitat use (Supporting information Table S2). The highest reconstructed rate was for shifts from nocturnality to cathemerality, almost twice as high as the rate for shifts from cathemerality to diurnality, itself twice as high as either shifts from diurnality to cathemerality, or from cathemerality to nocturnality (Fig. 2B). Across the 100 iterations, there were on average 238.7 transitions in activity times along the skink phylogeny in the simulated character histories (Supporting information Table S3; Fig. S2). The most common were shifts from cathemerality to diurnality, which occurred on average 104.8 times per simulated character history. The least common shifts were from nocturnality to cathemerality (31.8 times on average), despite the high reconstructed rate for these shifts—likely due to the rarity of the nocturnal state in the data.
Figure 2.
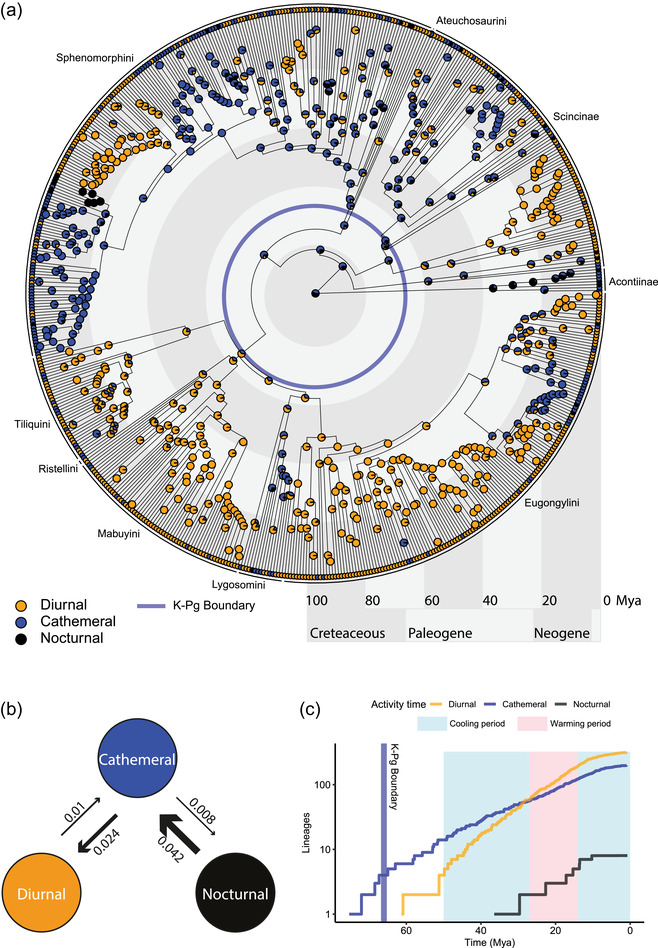
(a) Ancestral character state reconstruction of activity time for skinks, generated using stochastic character mapping based on 100 simulated character histories. The different activity times are coded to different colors: yellow for diurnal, blue for cathemeral, and black for nocturnal. The pie charts at the interior nodes denote the posterior probabilities of each character state in that node. The K‐Pg boundary is denoted by a pale blue circle. (b) Transition rates between the different activity times, calculated based on a meristic model of discrete trait evolution. The widths of the arrows are proportional to the transition rate between each pair of states. (c) Lineages‐through‐time plot showing the accumulation of lineages with strong support (posterior probability at node > 0.67) from each of the activity times. Periods of cooling and warming during the Paleogene are marked by light blue and pink rectangles, respectively.
Our ancestral state reconstruction suggests that the common ancestor of all skinks was most probably not strictly diurnal (PP = 0.06), with similar support for a cathemeral (PP = 0.43) and nocturnal (PP = 0.51) ancestral state (Table 1 and Fig. 2). This was likely also true for the common ancestors of the Scincinae (both clades) and of the massive radiation of sphenomorphin skinks. The first moderately supported shift to diurnality occurred roughly around the K‐Pg boundary (∼64.6 Mya; Table 1) in the common ancestor of Ristellini, Tiliquini, Mabuyini, and Lygosomini (Fig. 2A), the latter three of which are also the three clades with highest proportion of diurnal species and almost no strictly nocturnal species (Fig. 1B). Two major shifts to diurnality also occurred on the branch leading to the mostly North American genera Plestiodon and Mesoscincus (Scincinae), and on the branch leading to the Australian genus Ctenotus (Sphenomorphini). Most Tiliquini, Mabuyini, and Lygosomini retained the diurnal state despite several reversions to cathemerality, most prominently in a cathemeral subclade within the New Zealand endemic genus Oligosoma, and in the New Caledonian endemic genus Nannoscincus (all cathemeral). The reconstruction of the last common ancestor of skinks as nondiurnal is robust to a different coding of cathemerality using the “strict dataset” (Supporting information Fig. S3), to the inclusion of Cordylidae, Gerrhosauridae, and Xantusiidae as outgroups in the “extended dataset” (Supporting information Fig. S4A), to the exclusion of Acontiinae in the “reduced dataset” (Supporting information Fig. S4B), and to different methods of ancestral state reconstruction (Supporting information Fig. S5).
Table 1.
Posterior probabilities of each of the three character states for activity time (diurnal, cathemeral, nocturnal), estimated using SCM, for the nodes denoting the common ancestors of several key clades in the phylogeny of skinks
| Clade | Diurnal | Cathemeral | Nocturnal | Crown Age |
|---|---|---|---|---|
| Scincidae | 0.06 | 0.43 | 0.51 | 97.3 |
| Acontiinae | 0.00 | 0.24 | 0.76 | 36.4 |
| Scincinae (Brachyemeles) | 0.19 | 0.57 | 0.24 | 58.5 |
| Scincinae (all else) | 0.13 | 0.66 | 0.21 | 69.8 |
| Ophiomorus + Plestiodon + Mesoscincus | 0.48 | 0.41 | 0.11 | 58.7 |
| Lygosominae | 0.16 | 0.70 | 0.14 | 75.3 |
| Ateuchosaurini + Sphenomorphini | 0.13 | 0.74 | 0.13 | 72.0 |
| Sphenomorphini | 0.16 | 0.76 | 0.08 | 65.0 |
| Australian Sphenomorphini | 0.00 | 1.00 | 0.00 | 33.6 |
| Ctenotus | 0.78 | 0.22 | 0.00 | 25.4 |
| Tiliquini + Ristellini + Mabuyini + Lygosomini + Eugongylini | 0.64 | 0.35 | 0.01 | 64.6 |
| Tiliquini | 0.77 | 0.22 | 0.01 | 51.2 |
| Ristellini | 0.67 | 0.32 | 0.01 | 49.8 |
| Mabuyini | 0.92 | 0.08 | 0.00 | 51.2 |
| Lygosomini + Eugongylini | 0.68 | 0.31 | 0.01 | 61.3 |
| Lygosomini | 0.61 | 0.36 | 0.03 | 56.6 |
| Eugongylini | 0.97 | 0.03 | 0.00 | 47.9 |
| Cathemeral Oligosoma | 0.04 | 0.96 | 0.00 | 11.9 |
| Nannoscincus | 0.03 | 0.97 | 0.00 | 20.4 |
For each clade, the crown age (in Mya) is also listed. The state with the highest posterior probability for each node is shaded in grey—if the state is also strongly supported (i.e., PP > 0.67), it is in bold.
All shifts to diurnality occurred, with high probability, after the K‐Pg boundary (Fig. 2 and Table 1; Supporting information Fig. S6). Shifts back to nocturnality predominantly occurred much later, with the median ages for strongly supported nocturnal nodes younger than the median age for either diurnal or cathemeral nodes (Fig. S6). Following the K‐Pg boundary there was also an increase in the rate of accumulation of diurnal lineages, overtaking the accumulation of cathemeral lineages toward the mid‐Paleogene (Fig. 2C).
EVOLUTIONARY DRIVERS OF ACTIVITY TIMES
Diurnal skinks almost always have fully developed limbs (Table 2; Fig. 1A) and are most often terrestrial or partially terrestrial (“variable”; Table 3; Fig. 1A). Both cathemeral and nocturnal habits are strongly associated with reduced limbs (Table 2; Fig. 1A) and with fossorial or semifossorial habits (Table 3; Fig. 1A), although some species do not fit this pattern. For example, the giant slender bluetongue (Cyclodomorphus maximus) is a nocturnal terrestrial skink with fully‐developed limbs, and several species of water skink (Tropidophorus spp.) are nocturnal and semiaquatic with fully‐developed limbs. Scansoriality is positively associated with diurnal habits, and semiaquatic species are often cathemeral—but neither of these associations are statistically significant (Table 3).
Table 2.
Summary of posterior predictive tests for activity time and limb reduction
| Fully‐developed | Limbless | Limb Reduced | |
|---|---|---|---|
| Cathemeral | −0.07 (1.00) | 0.01 (0.17) | 0.06 (0.00) |
| Diurnal | 0.09 (0.00) | −0.02 (0.96) | −0.07 (0.99) |
| Nocturnal | −0.02 (1.00) | 0.01 (0.02) | 0.01 (0.03) |
| Cathemeral | 105 species | 11 species | 70 species |
| Diurnal | 342 species | 9 species | 28 species |
| Nocturnal | 10 species | 5 species | 15 species |
For each pairwise combination of character states, D statistic is listed implying the strength of the association between the two, and the p value in parentheses. Significant associations are shaded grey. The bottom three rows show the numbers of species in each pair of categories.
Table 3.
Summary of posterior predictive tests for activity time and microhabitat
| Fossorial | Scansorial | Semiaquatic | Semifossorial | Terrestrial | Variables | |
|---|---|---|---|---|---|---|
| Cathemeral | 0.03 (0.02) | −0.01 (0.81) | 0.01 (0.11) | 0.05 (0.01) | −0.04 (0.98) | −0.03 (1.00) |
| Diurnal | −0.04 (0.98) | 0.01 (0.21) | −0.01 (0.89) | −0.06 (0.99) | 0.06 (0.00) | 0.04 (0.00) |
| Nocturnal | 0.01 (0.03) | 0.00 (0.62) | 0.00 (0.11) | 0.01 (0.03) | −0.02 (0.99) | −0.01 (1.00) |
| Cathemeral | 40 species | 7 species | 9 species | 67 species | 59 species | 4 species |
| Diurnal | 12 species | 41 species | 15 species | 34 species | 199 species | 78 species |
| Nocturnal | 10 species | 2 species | 1 species | 13 species | 3 species | 1 species |
For each pairwise combination of character states, D statistic is listed implying the strength of the association between the two, and the p value in parentheses. Significant associations are shaded grey. The bottom three rows show the numbers of species in each pair of categories.
Diel activity time is positively associated with mean temperature (p = 0.06), with a significant negative quadratic term (p = 0.02; Supporting information Fig. S7; Table S4). In our model, all activity times are common at high temperatures, but skinks inhabiting low temperatures are highly likely to be diurnal (Fig. 3), and the probability of being cathemeral or nocturnal increases with increasing temperature, but plateaus around ∼15°C. Diel activity is also positively associated with mass (p = 0.01; Fig. 3), but its quadratic term is not significant (p = 0.34; Supporting information Fig. S7; Table S4), such that the probability of being cathemeral or nocturnal generally increases with increasing body mass.
Figure 3.
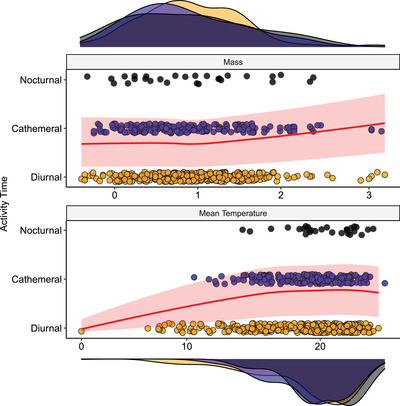
Phylogenetic ordinal regression, showing the probability of occurring in different activity times (diurnal in yellow, cathemeral in blue, and nocturnal in black). The model includes maximum mass (in g, log10‐transformed) and mean temperature (in°C) as fixed effects. The red line represents the predicted probability (and confidence interval) for mass (top) and mean temperature (bottom) when the other predictor is held constant at its mean value. The density plots on the top and bottom of the panels represent the distribution of mass and mean temperature (respectively) in the three activity time states.
Discussion
Shifts in activity times occurred often across skink evolution—at least 167 times—and were often associated with shifts in microhabitat preferences and the degree of limb development. In particular, low temperatures appear to be a strong driver of skink diurnality. Surprisingly, and although there is uncertainty around our ancestral reconstructions, we reconstructed the common ancestor of all skinks as not diurnal (Fig. 2). This finding contradicts our hypothesis (a), despite most extant skink species being diurnal (Fig. 1B; Vitt and Caldwell 2014), and squamate reptiles likely being ancestrally diurnal (Anderson and Wiens 2017).
Insight on the ancestral activity pattern of skinks could be gained from examining adaptations they may have for different diel patterns. For instance, squamates have long been considered ancestrally diurnal (Vitt et al. 2003) even before explicit examination in a phylogenetic framework (Anderson and Wiens 2017). This was supported in part by their purely cone‐based visual systems (Underwood 1951, 1970). Similarly, geckos have been considered ancestrally nocturnal thanks to numerous elements of their visual systems being adapted to low‐light conditions such as large relative eye size, lack of foveae, and rod‐like photoreceptors (Walls 1942; Underwood 1951, 1970; Röll 2000a, 2000b; Gamble et al. 2015; Pinto et al. 2019). These adaptations suggest the entire clade is ancestrally adapted for nocturnal activity. Few studies, however, have examined the visual systems of skinks in depth. Some skinks possess classic diurnal adaptations, such as shallow foveae (Röll 2001c) and many contain oil droplets in the cones (New et al. 2012). However, not all skink retinas possess foveae (New et al. 2012; Zhao et al. 2019), and while many studies have focused on strictly diurnal surface‐dwelling species (such as Tiliqua rugosa; New et al. 2012), there is evidence that eyes of some fossorial species are adapted for low‐light conditions (Zhao et al. 2019). Furthermore, even in the ancestrally nocturnal geckos, species that have shifted to diurnality have secondarily evolved ocular adaptations for photopic environments such as foveae, smaller eyes, and cone‐like photoreceptors (Röll 2001a, 2001b). Since shifts in diel activity can lead to secondary evolution of ocular adaptations, we caution against deriving the ancestral diel activity of skinks based on the limited data currently available on the visual systems of extant species.
While our analyses show that the ancestral skink was probably not diurnal, we could not determine whether it was cathemeral or nocturnal (Fig. 2; Table 1). A cathemeral ancestral skink is plausible, at least under the liberal definition of cathemerality we use here (although we note this result still holds under a stricter definition of cathemerality in our “strict dataset”; Supporting information Fig. S3). Some of the skinks we classified as cathemeral lean more strongly toward either diurnality or nocturnality but can shift their activity patterns due to seasonal changes in temperature, precipitation, and food availability. For instance, multiple species in the genus Chalcides are more likely to be active nocturnally during summer, when nights are warm, and diurnally during winter (Schleich et al. 1996; Szczerbak 2003; pers. obs.). Thus, it is not unlikely that the ancestral skink, rather than being a diurnal or nocturnal specialist, was able to shift its activity patterns seasonally as do many extant skinks, particularly among scincid groups such as Scincinae.
What kind of animal was the ancestral skink? The well‐supported, earliest phylogenetic split in the skink tree is between Acontiinae and all other skinks (Wiens et al. 2012; Pyron et al. 2013; Lambert et al. 2015; Tonini et al. 2016; Zheng and Wiens 2016). Acontiines are all limbless, elongated, fossorial, and either nocturnal or cathemeral. However, they are unlikely to represent the ancestral skink condition and instead clearly possess a highly derived phenotype. Total limb loss is widely considered to be irreversible (Skinner et al. 2008; Camaiti et al. 2021), despite some evidence that limb reduction, but not loss, might be reversible (Bergmann et al. 2020). If so, while a limbless ancestral skink is unlikely, a limb‐reduced ancestral skink, at least through the measure of limb length as a proportion of body length, could give rise to secondarily fully‐limbed descendants. The ancestral skink might then have either had fully developed, relatively long limbs, or relatively short, reduced limbs.
We have uncovered limb reduction to be strongly associated with nocturnal and cathemeral habits, as per our hypothesis (b) (Fig. 1; Table 3), and the ancestral skink to have been nondiurnal (Fig. 2). This might suggest a scenario of an at least partially fossorial, cathemeral or nocturnal ancestral skink with relatively short limbs (e.g., “a somewhat clumsily built, rather long‐bodied and short‐legged creature”; Smith 1937). However, the earliest known stem scincoid fossils are fully limbed (Tałanda 2018), and the correlation between limb reduction and nondiurnality, while it exists (Table 3), is not absolute. Long‐limbed cathemeral or nocturnal skink species exist including many species in the New Zealandian genus Oligosoma and multiple nocturnal Sphenomorphus species. Therefore, while we can claim with confidence that it was likely not a strictly diurnal species, the exact condition of the ancestral skink in terms of activity time, microhabitat use, and limb development remains ambiguous at least until we have better representation in the fossil record of stem scincids.
As per our hypothesis (c), lower temperatures are associated with a greater probability of being diurnal. While in warm areas skinks show all activity patterns, nocturnal skinks are absent from regions with mean annual temperatures lower than 15°C, and cathemeral species are absent from regions colder than 10°C (Fig. 3). Our results therefore support previous findings in Palearctic lizards, few of which were skinks (Vidan et al. 2017), that suggest that night‐time temperatures are a strong limiting factor on the distribution of terrestrial nocturnal ectotherms. Simply put, if temperatures at night are too low, nocturnally active reptiles will have difficulty maintaining high enough body temperatures for prolonged activity.
We found conflicting support for our hypothesis (d) that intermediate body masses would be associated with increasing nocturnality. Our model predicts that the probabilities of being cathemeral or nocturnal increase with increasing mass (Fig. 3), but the quadratic term was nonsignificant (Supporting information Table S4). Many of the largest skinks, such as the Australian land mullet (Bellatorias major), are diurnal, yet there are a few exceptionally large cathemeral species (Fig. 3). These include the centralian bluetongue skink (Tiliqua multifasciata), of which at least some populations shift their activity patterns seasonally (Christian 1977), the crepuscular Solomon Island skink (Corucia zebrata; Mann and Meek 2004), and the extinct, cathemeral, Cape Verde giant skink (Chioninia coctei; Greer 1976). This result is not particularly surprising considering the mechanisms through which body size affects thermoregulation. Since rates of both heat gain and loss would be slower in large skinks, large cathemeral species would be able to absorb heat during the day and retain it long enough for activity after sunset—whereas large, strictly nocturnal species would be constrained by slow heat gain from even achieving optimal temperatures for activity unless they bask cryptically before sunset. Additionally, for fossorial species, nocturnal surface activity may actively reduce rates of heat loss via conduction, as the rapidly cooling ground would only be in contact with the abdomen, rather than the entire body if the animal is below the surface.
Our results suggest that lineages began to acquire the diurnal state more rapidly toward the end of the Paleogene, and the accumulation of diurnal lineages outpaced that of cathemeral lineages toward the end of the Paleogene (∼23 Mya; Fig. 2C; Table 1). In particular, the massive radiation of the almost entirely diurnal clade that is comprised of Tiliquini, Ristellini, Mabuyini, Lygosomini, and Eugongylini (Fig. 1B) only arose after the K‐Pg (Fig. 2). The early Paleogene was a period of warming. Temperatures reached their peak during the Early Eocene Climatic optimum (∼50 Mya; Zachos et al. 2001), followed by a period of relatively prolonged cooling until ∼27 MA. The onset of cooling (although in times when temperatures were still very warm), coincided with the large increase in the number of diurnal skink lineages (Fig. 2C). Conversely, strictly nocturnal skinks (crown acontiines and a subclade of Lerista) evolved during the Oligocene and early Miocene, a short period of relatively warm and stable climate following the Oligocene glaciation and lasting until the mid‐Miocene Climatic Optimum (∼14 Mya; Zachos et al. 2001) when cooling recommenced. However, the warmest temperatures during the Paleogene occurred during the Eocene, and so we must ask why no strictly nocturnal lineages evolved during this long period of warm climate. A possible explanation is that such lineages did evolve, but failed to persist to current times, perhaps due to rapid temperature drops during the Oligocene glaciation. However, this hypothesis will need to be rigorously tested by examining the fossil record and temporal dynamics in skink diversification. We further note that the interpretation of the diversification of lineages with different activity times relies on the assumptions that the reconstructed ages of branching events in the phylogeny are accurate, and that inferred lineage‐through‐time plots reflect the true evolutionary dynamics in diversification in this clade. However, uncertainty exists around dates calibrated using fossil taxa representing minimal ages of splits, and recent evidence suggests that diversification rates can be extremely difficult to accurately infer from reconstructed phylogenies (Louca and Pennell 2020). Despite this caveat, the general pattern of proliferation of diurnal species during the Paleogene, followed by a later evolution of nocturnal skinks, is likely to hold true even if the exact dates are uncertain, and might be tested by employing state‐dependent speciation and extinction models that can detect changes in diversification rates associated with shifts in discrete character states (Maddison et al. 2007; FitzJohn et al. 2009; Beaulieu and O'Meara 2016).
The end‐Cretaceous mass extinction event has previously been linked to the evolution of diurnality in mammals (Maor et al. 2017). One of the leading explanations for this pattern is the “nocturnal bottleneck” hypothesis, which posits that mammals were limited to nocturnal habits by antagonistic interactions with the ecologically dominant diurnal dinosaurs until their extinction at the K‐Pg (Walls 1942; Crompton et al. 1978; Gerkema et al. 2013). This “nocturnal bottleneck” hypothesis also originally posited that endothermy evolved in mammals in order to “escape” into the nocturnal niche due to competition and predation by diurnal ectothermic dinosaurs (Crompton et al. 1978). This concept has come under scrutiny because of conflicting evidence about the supposed ectothermy and diurnality of dinosaurs (Hut et al. 2012). Nevertheless, empirical evidence seems to support an acceleration of diurnal species diversification following the K‐Pg for mammals (Maor et al. 2017). The end‐Cretaceous mass extinction event likely also severely impacted squamates, with recovery lasting well into the Paleogene (Longrich et al. 2012; but see Rage 2013). Thus, the surviving skinks after the K‐Pg may have been ancestrally cathemeral and evolved strictly diurnal temporal niches during the Paleogene recovery of squamate diversity as cooling temperatures following the early eocene climatic optimum favored diurnal habits. This hypothesis needs to be carefully evaluated.
In conclusion, we uncovered a surprisingly elaborate and complicated history of diel activity evolution in skinks. Despite common conceptions of them as generally diurnal animals, they are likely to have been ancestrally nondiurnal, with strict diurnality and nocturnality having only evolved more recently, possibly due to expansion of temporal niche space following shifting temperatures during the recovery phase after the K‐Pg mass extinction. We encourage more study on diel activity times of various species of skinks, and particularly on physiological and anatomical adaptations to different activity times. Such studies could help shed further light on the evolution of diel activity in one of the most ecologically diverse radiations of terrestrial vertebrates.
AUTHOR CONTRIBUTIONS
The study was designed by AS, LD, and SM. AS led the analyses and writing. All authors contributed significantly to data collection and writing.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
DATA ARCHIVING
All data associated with the study are available on Figshare https://doi.org/10.6084/m9.figshare.16578881. R code to run the analyses is available on Figshare https://doi.org/10.6084/m9.figshare.16578905.
Associate Editor: M. Zelditch
Handling Editor: T. Chapman
Supporting information
Table S1. Numbers and percentages (in parentheses) of species from the different skink clades in each activity time category in the original and final datasets.
Table S2. Model selection summary table for discrete character evolution models.
Table S3. Mean number of shifts between activity times, and proportion of mean total time spent in each state, across 100 simulated character histories.
Table S4. Summary of the MCMCglmm results.
Figure S1. Diagnostics of the MCMCglmm model fixed effects.
Figure S2. Posterior densities of shifts between different activity times across 100 simulated character histories.
Figure S3. Scatterplot comparing the posterior probabilities of ancestral states estimated using the “final dataset” (solid lines) and the “strict dataset” (dashed lines).
Figure S4. Ancestral character state reconstruction of activity time for (A) the “extended dataset” of Scincoidea, including 595 species in Scincidae, 70 species in Cordylidae, and 11 species in Xantusiidae, and (B) the “reduced dataset” of 587 species in Scincidae excluding 8 species of Acontiinae, both generated using stochastic character mapping based on 100 simulated character histories using a meristic model of trait evolution, where transitions occur in a stepwise manner (“diurnal” <‐> “cathemeral” <‐> “nocturnal”) and all rates differ.
Figure S5. Matrix comparing ancestral state reconstruction using three methods: Stochastic Character Mapping (SCM) with the scm function in the package “phytools”, joint maximum likelihood reconstruction with the ace function in the package “ape”, and marginal maximum likelihood reconstruction with the rerootingMethod function in the package “phytools”.
Figure S6. Density plot of internal nodes with posterior probabilities of 0.67 or higher for either diurnal (yellow), cathemeral (blue) or nocturnal (black) activity time, showing density of such nodes at different crown ages (Mya).
Figure S7. Posterior distributions of fixed effects from phylogenetic ordinal regression using MCMCglmm.
ACKNOWLEDGMENTS
We would like to thank Andrew Amey, Aaron M. Bauer, Rafe M. Brown, Harold Cogger, Werner Conradie, Patrick Couper, Indraniel Das, Paul Doughty, Ryan Ellis, Frank Glaw, L. Lee Grismer, Conrad Hoskins, Simon Jamison, Chris Jolly, Fred Kraus, Mike McCoy, Stewart McDonald, Paul Oliver, Magnus Peterson, Ross Sadlier, Brendan Schembri, Dane Trembath, Yuezhao Wang, Steve Wilson, and Stephen Zozaya for contributing their personal observations for classifying the diel activity of many skink species. The project was supported by funding from the Australian Friends of Tel Aviv‐Monash University Research Collaboration Award (to SM and DGC), a grant from the Australian Research Council (to DGC; FT200100108), and grants from the Holsworth Wildlife Research Endowment – Equity Trustees Charitable Foundation & the Ecological Society of Australia, and the Monash‐Museums Victoria Robert Blackwood scholarship (to MC). AS is supported by a Royal Society Grant No. RGF∖EA∖181082.
LITERATURE CITED
- Anderson, S.R. & Wiens, J.J. (2017) Out of the dark: 350 million years of conservatism and evolution in diel activity patterns in vertebrates. Evolution; Internation Journal of Organic Evolution,, 71, 1944‐1959. [DOI] [PubMed] [Google Scholar]
- Andrews, R.M. & Pough, F.H. (1985) Metabolism of squamate reptiles: allometric and ecological relationships. Physiological Zoology, 58, 214‐231. [Google Scholar]
- Autumn, K. , Jindrich, D. , DeNardo, D. & Mueller, R. (1999) Locomotor performance at low temperature and the evolution of nocturnality in geckos. Evolution; Internation Journal of Organic Evolution, 53, 580‐599. [DOI] [PubMed] [Google Scholar]
- Bars‐Closel, M. , Camacho, A. & Kohlsdorf, T. (2018) Shifts in space and time: ecological transitions affect the evolution of resting metabolic rates in microteiid lizards. Journal of Experimental Biology, 221, jeb175661. [DOI] [PubMed] [Google Scholar]
- Barton, R.A. (2006) Olfactory evolution and behavioral ecology in primates. American Journal of Primatology, 68, 545‐558. [DOI] [PubMed] [Google Scholar]
- Beaulieu, J.M. & O'Meara, B.C. (2016) Detecting hidden diversification shifts in models of trait‐dependent speciation and extinction. Syst. Biol, 65, 583‐601. [DOI] [PubMed] [Google Scholar]
- Bergmann, P.J. , Morinaga, G. , Freitas, E.S. , Irschick, D.J. , Wagner, G.P. & Siler, C.D. (2020) Locomotion and palaeoclimate explain the re‐evolution of quadrupedal body form in Brachymeles lizards. P. Roy. Soc. B., 287, 20201994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollback, J.P. (2006) SIMMAP: stochastic character mapping of discrete traits on phylogenies. Bmc Bioinformatics [Electronic Resource], 7, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandley, M.C. , Huelsenbeck, J.P. & Wiens, J.J. (2008) Rates and patterns in the evolution of snake‐like body form in squamate reptiles: evidence for repeated re‐evolution of lost digits and long‐term persistence of intermediate body forms. Evolution; Internation Journal of Organic Evolution, 62, 2042‐2064. [DOI] [PubMed] [Google Scholar]
- Burnham, K.P. & Anderson, D.R. (2002) Model selection and multimodel inference: a practical information‐theoretic approach. New York, NY: Springer Science & Business Media. [Google Scholar]
- Camaiti, M. , Evans, A.R. , Hipsley, C.A. & Chapple, D.G. (2021) A farewell to arms and legs: a review of limb reduction in squamates. Biol. Rev., 96, 1035‐1050. [DOI] [PubMed] [Google Scholar]
- Camaiti, M. , Villa, A. , Wencker, L.C. , Bauer, A.M. , Stanley, E.L. & Delfino, M. (2019) Descriptive osteology and patterns of limb loss of the European limbless skink Ophiomorus punctatissimus (Squamata, Scincidae). Journal of Anatomy, 235, 313‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaiti, M. , Evans, A.R. , Hipsley, C.A. , Meiri, S. , Hutchinson, M.N. , Anderson, R.O. , Slavenko, A. & Chapple, D.G. (In Press) A database of the morphology, ecology and literature of the world’s limb‐reduced skinks. Journal of Biogeography. 10.1111/jbi.14392 [DOI] [Google Scholar]
- Carothers, J.H. , Fox, S.F. , Marquet, P.A. & Jaksic, F.M. (1997) Thermal characteristics of ten Andean lizards of the genus Liolaemus in central Chile. Rev. Chil. Hist. Nat., 70, 297‐309. [Google Scholar]
- Chapple, D.G. , Roll, U. , Böhm, M. , Aguilar, R. , Amey, A.P. , Austin, C.C. et al. (2021) Conservation status of the world's skinks (Scincidae): taxonomic and geographic patterns in extinction risk. Biological Conservation, 257, 109101. [Google Scholar]
- Chen, Z. & Wiens, J.J. (2020) The origins of acoustic communication in vertebrates. Nat. Comm., 11, 1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian, T. (1977) Notes on Centralian Bluetongues (Tiliqua multifasciata). Newsl. Victorian Herpetol. Soc., 1, 8‐9. [Google Scholar]
- Crompton, A. , Taylor, C.R. & Jagger, J.A. (1978) Evolution of homeothermy in mammals. Nature, 272, 333‐336. [DOI] [PubMed] [Google Scholar]
- Daan, S. (1981) Adaptive daily strategies in behavior. In: J., Aschoff (Ed.) Biological rhythms. Boston, MA: Springer, pp. 275–298. [Google Scholar]
- Daan, S. & Aschoff, J. (1982) Circadian contributions to survival. In: J., Aschoff , S., Daan & Groos G. A. (Eds.) Vertebrate circadian systems. Berlin, Germany: Springer‐Verlag, pp. 305–321. [Google Scholar]
- DeCoursey, P.J. (2004) The behavioral ecology and evolution of biological timing systems. In: J., Loros , J. C., Dunlap and DeCoursey P. J. (Eds). Chronobiology: biological timekeeping. Sunderland, MA: Sinauer Associates, pp. 27–65. [Google Scholar]
- Feldman, A. & Meiri, S. (2014) Australian snakes do not follow Bergmann's rule. Evol. Biol., 41, 327‐335. [Google Scholar]
- FitzJohn, R.G. , Maddison, W.P. & Otto, S.P. (2009) Estimating trait‐dependent speciation and extinction rates from incompletely resolved phylogenies. Systematic Biology, 58, 595‐611. [DOI] [PubMed] [Google Scholar]
- Gamble, T. , Greenbaum, E. , Jackman, T.R. & Bauer, A.M. (2015) Into the light: diurnality has evolved multiple times in geckos. Biological Journal of the Linnean Society, 115, 896‐910. [Google Scholar]
- Gans, C. (1962) Terrestrial locomotion without limbs. American Zoologist, 2, 167‐182. [Google Scholar]
- Gans, C. (1975) Tetrapod limblessness: evolution and functional corollaries. American Zoologist, 15, 455‐467. [Google Scholar]
- Gerkema, M.P. , Davies, W.I. , Foster, R.G. , Menaker, M. & Hut, R.A. (2013) The nocturnal bottleneck and the evolution of activity patterns in mammals. P. Roy. Soc. B., 280, 20130508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer, A.E. (1976) On the evolution of the giant Cape Verde scincid lizard Macroscincus coctei . Journal of Natural History, 10, 691‐712. [Google Scholar]
- Hadfield, J.D. (2010) MCMC methods for multi‐response generalized linear mixed models: The MCMCglmm R package. J. Stat. Softw., 33, 1‐22.20808728 [Google Scholar]
- Healy, S. & Guilford, T. (1990) Olfactory‐bulb size and nocturnality in birds. Evolution; Internation Journal of Organic Evolution, 44, 339‐346. [DOI] [PubMed] [Google Scholar]
- Helm, B. , Visser, M.E. , Schwartz, W. , Kronfeld‐Schor, N. , Gerkema, M. , Piersma, T. et al. (2017) Two sides of a coin: Ecological and chronobiological perspectives of timing in the wild. Philos. T. Roy. Soc. B., 372, 20160246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, T.H. (2001) Conceptual issues in the ecology and evolution of circadian rhythms. In: A., Kramer and Merrow M. (Eds.) Circadian clocks. Boston, MA: Springer, pp. 45–57. [Google Scholar]
- Huelsenbeck, J.P. , Nielsen, R. & Bollback, J.P. (2003) Stochastic mapping of morphological characters. Systematic Biology, 52, 131‐158. [DOI] [PubMed] [Google Scholar]
- Hut, R.A. , Kronfeld‐Schor, N. , van der Vinne, V. & De la Iglesia, H. (2012) In search of a temporal niche: environmental factors. In: A., Kalsbeek , M., Merrow , T., Roennberg and Foster R. G. (Eds.) The neurobiology of circadian timing. Amsterdam, the Netherlands: Elsevier, pp. 281–304. [DOI] [PubMed] [Google Scholar]
- Karger, D.N. , Conrad, O. , Böhner, J. , Kawohl, T. , Kreft, H. , Soria‐Auza, R.W. et al. (2017) Climatologies at high resolution for the earth's land surface areas. Sci. Data., 4, 170122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenböck, A. , Osterwalder, K. & Shine, R. (2000) Habitat use and thermal biology of the “Land Mullet” Egernia major, a large Scincid lizard from remnant rain forest in southeastern Australia. Copeia, 2000, 931‐939. [Google Scholar]
- Kronfeld‐Schor, N. & Dayan, T. (2003) Partitioning of time as an ecological resource. Annual Review of Ecology, Evolution, and Systematics, 34, 153‐181. [Google Scholar]
- Kuhlman, S.J. , Craig, L.M. & Duffy, J.F. (2018) Introduction to chronobiology. CSH. Perspect. Biol., 10, a033613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, S.M. , Reeder, T.W. & Wiens, J.J. (2015) When do species‐tree and concatenated estimates disagree? An empirical analysis with higher‐level scincid lizard phylogeny. Molecular Phylogenetics and Evolution, 82, 146‐155. [DOI] [PubMed] [Google Scholar]
- Lee, M.S. (1998) Convergent evolution and character correlation in burrowing reptiles: towards a resolution of squamate relationships. Biological Journal of the Linnean Society, 65, 369‐453. [Google Scholar]
- Leschen, R.A.B. & Buckley, T.R. (2007) Multistate characters and diet shifts: evolution of Erotylidae (Coleoptera). Systematic Biology, 56, 97‐112. [DOI] [PubMed] [Google Scholar]
- Longrich, N.R. , Bhullar, B.‐A.S. & Gauthier, J.A. (2012) Mass extinction of lizards and snakes at the Cretaceous–Paleogene boundary. P. Natl. Acad. Sci. USA., 109, 21396‐21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca, S. & Pennell, M.W. (2020) Extant timetrees are consistent with a myriad of diversification histories. Nature, 580, 502‐505. [DOI] [PubMed] [Google Scholar]
- Maddison, W.P. , Midford, P.E. , Otto, S.P. & Oakley, T. (2007) Estimating a binary character's effect on speciation and extinction. Systematic Biology, 56, 701‐710. [DOI] [PubMed] [Google Scholar]
- Mann, S.L. & Meek, R. (2004) Understanding the relationship between body temperature and activity patterns in the giant Solomon Island skink, Corucia zebrata, as a contribution to the effectiveness of captive breeding programmes. Appl. Herpetol., 1, 287‐298. [Google Scholar]
- Maor, R. , Dayan, T. , Ferguson‐Gow, H. & Jones, K.E. (2017) Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nature Ecology & Evolution, 1, 1889‐1895. [DOI] [PubMed] [Google Scholar]
- Meiri, S. (2018) Traits of the lizards of the world: variation around a successful evolutionary design. Glob. Ecol. Biogeogr., 27, 1168‐1172. [Google Scholar]
- Meiri, S. (2020) Gekkota Mundi–the world of geckos. Israel J. Ecol. Evol., 66, 113‐116. [Google Scholar]
- Meiri, S. , Bauer, A.M. , Chirio, L. , Colli, G.R. , Das, I. , Doan, T.M. , Feldman, A. , Herrera, F.‐C. , Novosolov, M. , Pafilis, P. , et al. (2013) Are lizards feeling the heat? A tale of ecology and evolution under two temperatures. Glob. Ecol. Biogeogr., 22, 834‐845. [Google Scholar]
- Moreira, M.O. , Qu, Y.‐F. & Wiens, J.J. (2021) Large‐scale evolution of body temperatures in land vertebrates. Evol. Lett., 5, 484‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New, S.T. , Hemmi, J.M. , Kerr, G.D. & Bull, C.M. (2012) Ocular anatomy and retinal photoreceptors in a skink, the sleepy lizard (Tiliqua rugosa). Anatomical Record, 295, 1727‐1735. [DOI] [PubMed] [Google Scholar]
- Paradis, E. & Schliep, K. (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics, 35, 526‐528. [DOI] [PubMed] [Google Scholar]
- Pennell, M.W. , Eastman, J.M. , Slater, G.J. , Brown, J.W. , Uyeda, J.C. , FitzJohn, R.G. et al. (2014) geiger v2. 0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics, 30, 2216‐2218. [DOI] [PubMed] [Google Scholar]
- Pianka, E.R. & Vitt, L.J. (2003) Lizards: Windows to the evolution of diversity. Berkeley, CA: University California Press. [Google Scholar]
- Pinto, B.J. , Nielsen, S.V. & Gamble, T. (2019) Transcriptomic data support a nocturnal bottleneck in the ancestor of gecko lizards. Molecular Phylogenetics and Evolution, 141, 106639. [DOI] [PubMed] [Google Scholar]
- Pyron, R.A. , Burbrink, F.T. & Wiens, J.J. (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. Bmc Evolutionary Biology, 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2020) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rage, J.‐C. (2013) Mesozoic and Cenozoic squamates of Europe. Palaeobio. Palaeoenv., 93, 517‐534. [Google Scholar]
- Revell, L.J. (2012) phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217‐223. [Google Scholar]
- Röll, B. (2000a) Characterization of retinal oil droplets in diurnal geckos (Reptilia, Gekkonidae). Journal of Experimental Zoology, 287, 467‐476. [DOI] [PubMed] [Google Scholar]
- Röll, B. (2000b) Gecko vision—visual cells, evolution, and ecological constraints. Journal of Neurocytology, 29, 471‐484. [DOI] [PubMed] [Google Scholar]
- Röll, B. (2001a) Gecko vision—retinal organization, foveae and implications for binocular vision. Vision Research, 41, 2043‐2056. [DOI] [PubMed] [Google Scholar]
- Röll, B. (2001b) Multiple origin of diurnality in geckos: evidence from eye lens crystallins. Die Naturwissenschaften, 88, 293‐296. [DOI] [PubMed] [Google Scholar]
- Röll, B. (2001c) Retina of Bouton's skink (Reptilia, Scincidae): visual cells, fovea, and ecological constraints. Journal of Comparative Neurology, 436, 487‐496. [DOI] [PubMed] [Google Scholar]
- Roll, U. , Dayan, T. & Kronfeld‐Schor, N. (2006) On the role of phylogeny in determining activity patterns of rodents. Evol. Ecol., 20, 479‐490. [Google Scholar]
- Roll, U. , Feldman, A. , Novosolov, M. , Allison, A. , Bauer, A. , Bernard, R. et al. (2017) The global distribution of tetrapods reveals a need for targeted reptile conservation. Nature Ecology & Evolution, 1, 1677‐1682. [DOI] [PubMed] [Google Scholar]
- Santini, L. , Rojas, D. & Donati, G. (2015) Evolving through day and night: origin and diversification of activity pattern in modern primates. Behav. Ecol., 26, 789‐796. [Google Scholar]
- Schleich, H.H. , Kästle, W. & Kabisch, K. (1996) Amphibians and reptiles of North Africa. Koenigstein, Germany: Koeltz Scientific Publishers. [Google Scholar]
- Seebacher, F. & Shine, R. (2004) Evaluating thermoregulation in reptiles: the fallacy of the inappropriately applied method. Physiological and Biochemical Zoology, 77, 688‐695. [DOI] [PubMed] [Google Scholar]
- Shea, G.M. (2021) Nomenclature of supra‐generic units within the Family Scincidae (Squamata). Zootaxa, 5067, 301‐351. [DOI] [PubMed] [Google Scholar]
- Siler, C.D. & Brown, R.M. (2011) Evidence for repeated acquisition and loss of complex body‐form characters in an insular clade of Southeast Asian semi‐fossorial skinks. Evolution; Internation Journal of Organic Evolution, 65, 2641‐2663. [DOI] [PubMed] [Google Scholar]
- Skinner, A. , Lee, M.S.Y. & Hutchinson, M.N. (2008) Rapid and repeated limb loss in a clade of scincid lizards. Bmc Evolutionary Biology, 8, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavenko, A. , Tallowin, O.J.S. , Itescu, Y. , Raia, P. & Meiri, S. (2016) Late quaternary reptile extinctions: Size matters, insularity dominates. Glob. Ecol. Biogeogr., 25, 1308‐1320. [Google Scholar]
- Smith, M.A. (1937) A review of the genus Lygosoma (Scincidae: Reptilia) and its allies. Rec. Indian. Mus., 39, 213‐234. [Google Scholar]
- Szczerbak, N.N. (2003) Guide to the reptiles of the eastern Palearctic. Malabar, FL: Krieger Publishing Company. [Google Scholar]
- Tałanda, M. (2018) An exceptionally preserved Jurassic skink suggests lizard diversification preceded fragmentation of Pangea. Palaeontology, 61, 659‐677. [Google Scholar]
- Thomas, K.R. & Thomas, R. (1978) Locomotor activity responses to photoperiod in four West Indian fossorial squamates of the genera Amphisbaena and Typhlops (Reptilia, Lacertilia). J. Herpetol., 12, 35‐41. [Google Scholar]
- Tonini, J.F.R. , Beard, K.H. , Ferreira, R.B. , Jetz, W. & Pyron, R.A. (2016) Fully‐sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biol. Cons., 204, 23‐31. [Google Scholar]
- Uetz, P. , Freed, P. , Aguilar, R. & Hošek, J. (2021) The Reptile Database. Available at: http://www.reptile‐database.org. Accessed 23 May 2021.
- Underwood, G. (1951) Reptilian retinas. Nature, 167, 183‐185. [DOI] [PubMed] [Google Scholar]
- Underwood, G. (1970) The eye. In: C., Gans and Parsons T. S. (Eds.) Biology of the reptilia, Volume 2, morphology B. London, UK: Academic Press, pp. 1–97. [Google Scholar]
- Vidan, E. , Roll, U. , Bauer, A. , Grismer, L. , Guo, P. , Maza, E. et al. (2017) The Eurasian hot nightlife: environmental forces associated with nocturnality in lizards. Glob. Ecol. Biogeogr, 26, 1316‐1325. [Google Scholar]
- Vitt, L.J. & Caldwell, J.P. (2014) Herpetology: an introductory biology of amphibians and reptiles, fourth edition. London, UK: Elsevier Inc., Academic Press. [Google Scholar]
- Vitt, L.J. , Pianka, E.R. , Cooper, J. , William, E. & Schwenk, K. (2003) History and the global ecology of squamate reptiles. American Naturalist, 162, 44‐60. [DOI] [PubMed] [Google Scholar]
- Walls, G.L. (1942) The vertebrate eye and its adaptive radiation. Bloomfield Hills, MI: Cranbrook Institute of Science. [Google Scholar]
- Wiens, J.J. , Brandley, M.W. & Reeder, T.W. (2006) Why does a trait evolve multiple times within a clade? Repeated evolution of snakeline body form in squamate reptiles. Evolution; Internation Journal of Organic Evolution, 60, 123‐141. [PubMed] [Google Scholar]
- Wiens, J.J. , Hutter, C.R. , Mulcahy, D.G. , Noonan, B.P. , Townsend, T.M. , Sites, J.W. Jr et al. (2012) Resolving the phylogeny of lizards and snakes (Squamata) with extensive sampling of genes and species. Biol. Letters., 8, 1043‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers, P.C. (1981) Physiological correlates of limblessness and fossoriality in scincid lizards. Copeia, 1981, 197‐204. [Google Scholar]
- Wu, Q. , Parker, S.L. & Thompson, M.B. (2009) Selected body temperature, metabolic rate and activity pattern of the Australian fossorial skink, Saiphos equalis . Herpetol. J., 19, 127‐133. [Google Scholar]
- Yang, Z. , Kumar, S. & Nei, M. (1995) A new method of inference of ancestral nucleotide and amino acid sequences. Genetics, 141, 1641‐1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos, J. , Pagani, M. , Sloan, L. , Thomas, E. & Billups, K. (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292, 686‐693. [DOI] [PubMed] [Google Scholar]
- Zamora‐Camacho, F.J. , Reguera, S. & Moreno‐Rueda, G. (2014) Bergmann's Rule rules body size in an ectotherm: heat conservation in a lizard along a 2200‐metre elevational gradient. Journal of Evolutionary Biology, 27, 2820‐2828. [DOI] [PubMed] [Google Scholar]
- Zhao, Z. , Goedhals, J. , Verdú‐Ricoy, J. , Jordaan, A. & Heideman, N. (2019) Comparative analysis of the eye anatomy in fossorial and surface‐living skink species (Reptilia: Scincidae), with special reference to the structure of the retina. Acta Zool., 101, 311‐323. [Google Scholar]
- Zheng, Y. & Wiens, J.J. (2016) Combining phylogenomic and supermatrix approaches, and a time‐calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Molecular Phylogenetics and Evolution, 94, 537‐547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Numbers and percentages (in parentheses) of species from the different skink clades in each activity time category in the original and final datasets.
Table S2. Model selection summary table for discrete character evolution models.
Table S3. Mean number of shifts between activity times, and proportion of mean total time spent in each state, across 100 simulated character histories.
Table S4. Summary of the MCMCglmm results.
Figure S1. Diagnostics of the MCMCglmm model fixed effects.
Figure S2. Posterior densities of shifts between different activity times across 100 simulated character histories.
Figure S3. Scatterplot comparing the posterior probabilities of ancestral states estimated using the “final dataset” (solid lines) and the “strict dataset” (dashed lines).
Figure S4. Ancestral character state reconstruction of activity time for (A) the “extended dataset” of Scincoidea, including 595 species in Scincidae, 70 species in Cordylidae, and 11 species in Xantusiidae, and (B) the “reduced dataset” of 587 species in Scincidae excluding 8 species of Acontiinae, both generated using stochastic character mapping based on 100 simulated character histories using a meristic model of trait evolution, where transitions occur in a stepwise manner (“diurnal” <‐> “cathemeral” <‐> “nocturnal”) and all rates differ.
Figure S5. Matrix comparing ancestral state reconstruction using three methods: Stochastic Character Mapping (SCM) with the scm function in the package “phytools”, joint maximum likelihood reconstruction with the ace function in the package “ape”, and marginal maximum likelihood reconstruction with the rerootingMethod function in the package “phytools”.
Figure S6. Density plot of internal nodes with posterior probabilities of 0.67 or higher for either diurnal (yellow), cathemeral (blue) or nocturnal (black) activity time, showing density of such nodes at different crown ages (Mya).
Figure S7. Posterior distributions of fixed effects from phylogenetic ordinal regression using MCMCglmm.
Data Availability Statement
All data associated with the study are available on Figshare https://doi.org/10.6084/m9.figshare.16578881. R code to run the analyses is available on Figshare https://doi.org/10.6084/m9.figshare.16578905.


