Summary
Among the many inhabitants of planktonic communities, several lineages have biomineralized intricate skeletons. These have existed for millions of years and include the Radiolaria, a group of marine protists, many of which bear delicate mineral skeletons of different natures. Radiolaria are well known for their paleontological signatures, but little is known about the ecology of modern assemblages. They are found from polar to tropical regions, in the sunlit layers of the ocean down to the deep and cold bathypelagic. They are closely involved in the biogeochemical cycles of silica, carbon and strontium sulfate, carrying important amounts of such elements to the deep ocean. However, relatively little is known on the actual extent of genetic diversity or biogeographic patterns. The rapid emergence and acceptance of molecular approaches have nevertheless led to major advances in our understanding of diversity within and evolutionary relationships between major radiolarian groups. Here, we review the state of knowledge relating to the classification, diversity and ecology of extant radiolarian orders, highlighting the substantial gaps in our understanding of the extent of their contribution to marine biodiversity and their role in marine food webs.
Introduction
Planktonic realms have attracted attraction since the early ages of modern ocean exploration in the 19th century. Among the diverse publications from these early days, scientists like Christian Gottfried Ehrenberg (e.g. Ehrenberg, 1854), Johannes Müller (e.g. Müller, 1859) or later Ernst Haeckel depicted the beauty of plankton in their monographs. Haeckel's major taxonomic works on the Radiolaria were of particular impact. The first was his 1862 monograph ‘Die Radiolarian’; it earned him many accolades and is said to have astonished Charles Darwin (Richards, 2009). Die Radiolarian was followed by his renowned ‘The Voyage of H.M.S. Challenger’ report dedicated to Radiolaria (Haeckel, 1887). The massive work inspired not only several generations of researchers but also the general public via the presentation of hundreds of aesthetic drawings of radiolarians in 128 plates, which still inspire today a broad audience, from the new generation of scientists to modern designers (Williams et al., 2015).
But what exactly are Radiolaria? While the scientific definition has varied through time, and in particular during the last two decades, Radiolaria can be said to be a group of diverse unicellular eukaryotes (also known as protists) often bearing delicate and elegant mineral skeletons. The closest relative to Radiolaria are Foraminifera (Cavalier‐Smith et al., 2018), another group of skeleton‐bearing protist (calcium carbonate), also classified among the eukaryotic supergroup Rhizaria with the Radiolaria (Biard, in press). It is their skeletons that popularized Radiolaria to a broader scientific community. Indeed, upon the death of a radiolarian, only its skeleton remains and eventually sinks to the bottom of the ocean, where it will be slowly incorporated into sediments and rocks through diagenesis. The radiolarian skeleton, now fossilized, will record the imprint of its surrounding environment and save it for millennia, providing excellent tools to reconstruct past environments (De Wever et al., 2002). Along with Foraminifera, Radiolaria have the most extensive fossil record (starting as old as 521 Mya) of any other extant protistan lineage (Suzuki and Oba, 2015). At present though, knowledge of radiolarian fossil records is by far inversely proportional to the knowledge of extant radiolarians. We lack very basic knowledge concerning their ecology, this point being hampered by the lack of reliable estimates of their abundances across the various size classes (Biard et al., 2016). Knowledge on their contribution to contemporary biogeochemical cycles is more advanced. Radiolaria are active and important contributors to the carbon (Lampitt et al., 2009) and silicon cycles (Takahashi, 1987). Specifically, Acantharia exert a major control in the ocean's strontium budget (Bernstein et al., 1987). However, we still lack for Radiolaria full characterization of their contribution to global biogeochemical cycles, particularly with respect to their broad range of sizes. This imbalance is further exemplified by the current state of radiolarian classification, rapidly evolving for now more than 130 years. Haeckel's original radiolarian classification included four legions (Acantharia, Spumellaria, Nassellaria and Phaeodaria) subdivided into two subclasses (Porulosa and Osculosa) based on distribution patterns of openings (or pores) at the surface of their mineral skeleton. While this classification has influenced modern schemes through most of the 20th century, new classifications have separated Radiolaria into different groups, in particular, based on the mineral nature of their skeleton. Such recent developments, also based on molecular evidence, have separated Phaeodaria, long considered as radiolarians, from the core Radiolaria (Polet et al., 2004) but still classified among Rhizaria, within the phylum Cercozoa (Biard, in press). Therefore, Phaeodaria will not be considered in this review. For each core radiolarian order, taxonomic classifications are presented below, along with existing knowledge of their ecology and core information on environmental diversity signatures.
General morphology, ecology and classification
Morphology
According to the latest classification schemes (Sandin, 2019; Nakamura et al., 2021), Radiolaria are now divided into six orders: Acantharia, Taxopodida, Spumellaria, Nassellaria, Collodaria and Orodaria (Fig. 1). The last four orders are further grouped within the Class Polycystinea and hereafter refer as to polycystine radiolarians (Suzuki and Not, 2015). Two key morphological criteria help subdivide Radiolaria into the six orders: (i) the main skeletal chemical component, and (ii) the type/structure of skeleton. Acantharia (Fig. 2A and B) are unique with respect to their peculiar skeletons made out of strontium sulfate (SrSO4), which makes them the only known protist biomineralizing this element (Decelle and Not, 2015). All remaining radiolarians consistently biomineralize opaline silica (SiO2 nH2O) in a various range of skeleton types, mainly being shells. Taxopodida, however, possess oar‐like spines (referred to as axopodia) containing silica and are unique (Fig. 2C and D). Spumellaria (Fig. 2E and F) and Nassellaria (Fig. 2G and H) are likely the two best‐known radiolarian orders (due to their extensive fossil records), with their distinctive spherical and conical silicified skeletons respectively. The two remaining Polycystine orders, Orodaria and Collodaria, possess several unique morphological characters. Both often reach cell sizes of a thousand micrometres, while typical cell sizes for other radiolarians are a few hundred micrometres. Orodaria (Fig. 2I and J) possess the largest known silicified skeleton in any other extant radiolarian order (Nakamura et al., 2021). However, Radiolaria without silicified skeletons of the Collodaria order are able to form large colonies (up to 3 m for the largest reported collodarian; Swanberg and Anderson, 1981) composed of tens to thousands of single collodarian cells (Fig. 2K). The peculiar morphology of Collodaria is further exemplified by the existence of taxa bearing silicified spicules, resembling those of sponges, rather than the typical spherical skeleton found elsewhere in polycystines. The reduction of silicified structures within Collodaria is complete for some taxa lacking silicified structure in any forms (referred to as ‘naked’ collodarians). Finally, some Collodaria taxa can form large solitary cells consisting of one large (i.e. >1 mm length) collodarian cell surrounded by a large and dense network of pseudopods (Fig. 2L; Box 1).
Fig. 1.
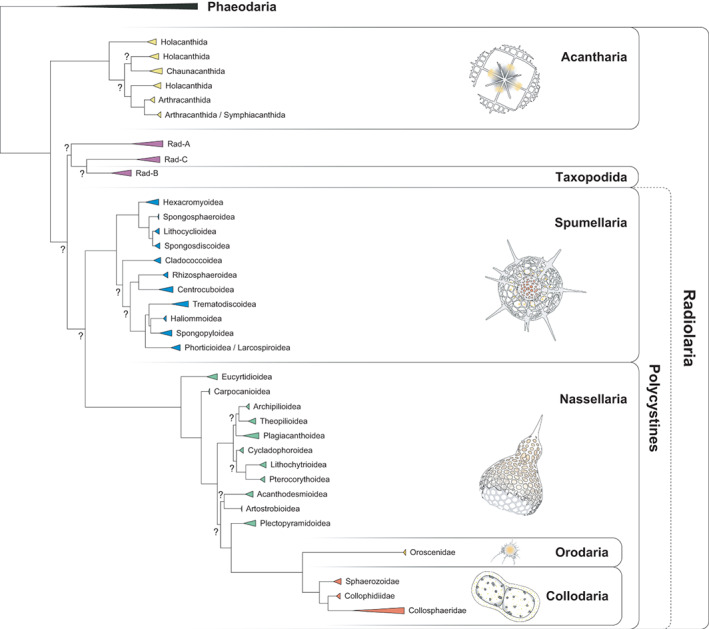
Schematic classification of Radiolaria based on ribosomal genes (18S and 28S rDNA) modified from Sandin (2019). Non‐supported nodes are reported by a surrounded question mark.
Fig. 2.
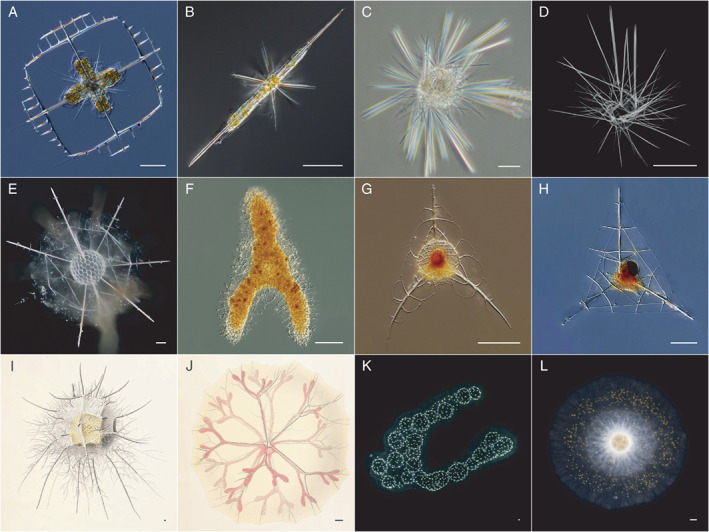
Diversity of the different extant Radiolaria orders.
A–B. Acantharia: Lithoptera fenestra and Amphilonche elongata.
C–D. Taxopodida: Sticholonche zanclea (C: live specimen; D: scanning electron microscopy image).
E–F. Polycystine ‐ Spumellaria: Diplosphaera spinosa and Euchitonia elegans.
G–H. Polycystine ‐ Nassellaria: Pteroscenium sp. and Plectaniscus sp.
I–J. Polycystine ‐ Orodaria: Oroscena regalis and Cytocladus tricladus.
K–L. Polycystine ‐ Collodaria: colonial (Collozoum sp.) and solitary (Thalassicolla nucleata) specimens. All scale bars are 50 μm. Images A–B and E–H courtesy of John Dolan (CNRS). Images C‐D courtesy of Karine Leblanc (CNRS). Monographs I–J from Haecker (1908). Many more images of Radiolaria and plankton can be found online at Aquaparadox and MIO Plankton images.
Box 1. Schematic classification of Radiolaria based on molecular and morphological criteria.
Phylogeny. Radiolaria are separated into three main phylogenetic entities: Acantharia, Taxopodida and Polycystines. Phaeodaria is no longer considered to belong to Radiolaria. Relationships between the three radiolarian main entities are still unclear (Sandin, 2019; Nakamura et al., 2021). See Fig. 1 for an illustration.
Morphology. Radiolaria can be separated into different orders based on two successive morphological criteria: (i) the composition of biomineralized structures and (2) the type of silicified structures. The first criterion separates Acantharia and their strontium sulfate skeletons, from Taxopodida and Polycystines, both having biomineralized structures made out of opaline silica. The second criterion pertains to silicified radiolarians, i.e. Taxopodida and Polycystines, the first having oar‐like silicified spines (Fig. 2C and D) while the second having classical silicified skeletons (Fig. 2E–J). Finally, among Polycystines, Collodaria (Fig. 2K and L) are unique due to the colonial nature of most species.
Diversity. While assessing the number of extant radiolarian species is a difficult task (Suzuki and Not, 2015), the three main radiolarian groups differ widely, not only from a morphological perspective but also from the putative number of known genera: from one genus for Taxopodida to several hundred for Polycystines (Adl et al., 2019).
Ecology
As many other protistan lineages, there are currently no cultures of any radiolarian species, despite several attempts made in the past (Anderson, 1978; Matsuoka, 1992), which failed to maintain radiolarian through successive generations. Therefore, knowledge of radiolarian ecology is limited by our ability to sample living organisms from various ecosystems or work with ‘omics’ data (e.g. transcriptomics, genomics, etc.; Caron et al., 2012). Despite the inherent complexity of collecting and studying them (see above), their relative wide occurrence in marine ecosystems has allowed a few studies that have provided precious information regarding radiolarian ecology. One of the most striking features of radiolarians biotic interactions, which was highlighted early on (e.g. Brandt, 1881), is their intricate relationships with photosymbionts (Anderson, 2012; Decelle et al., 2015). A large number of radiolarian species are associated with diverse types of photosymbionts, from prokaryotes to dinoflagellates, where the radiolarian host could acquire carbon‐rich products from the photosynthetic activity of the symbionts, and the symbiont could benefit from a protected micro‐environment. However, recurring evidence suggest that only the host could benefit from this association, the symbionts undergoing substantial morphological and physiological alterations (Uwizeye et al., 2021). While the complex nature of these associations has not been thoroughly investigated, it is believed that photosymbiosis could drive the latitudinal and vertical distribution of some radiolarian taxa (Suzuki and Not, 2015; Biard and Ohman, 2020).
Less is known about preys and predators of radiolarians and their influence on such biogeographical patterns. Most radiolarians are phagotrophs, feeding on a broad range (both in diversity and size) of prey without any obvious prey preferences: bacteria, small autotrophs (e.g. diatoms, dinoflagellates), protists (e.g. mostly ciliates and tintinnids) or even multicellular heterotrophs (e.g. copepods, large mollusc larvae; Anderson, 1977; Sugiyama and Anderson, 1998; Bernstein et al., 1999; Matsuoka, 2007; Sugiyama et al., 2008; Suzuki and Not, 2015; Mars Brisbin et al., 2020). A few reports, however, indicate that some radiolarians could be detritivores (Anderson, 1983).
Capture and digestion mechanisms are still elusive processes that often involve a few different types of pseudopodia (i.e. root‐like branching projections arising from the cytoplasm; Fig. 3). Capture of prey by Nassellaria involves the ‘axial projection’, the thickest and longest pseudopod emerging outward from the centre of the skeleton aperture (Fig. 3A). This AxP can be accompanied by several ‘terminal projections’, thick pseudopods emerging outwards too, forming altogether the ‘terminal cone’ (Sugiyama et al., 2008). The extent of the TC can be as long as two to three cell lengths, allowing the nassellarian cell to sweep large volumes for prey. Once captured, the prey is brought back within a few minutes toward the oral aperture of the skeleton, after which the prey will be digested within specific vacuoles. Prey capture among Spumellaria is unlike the TC found in Nassellaria. Rather than projecting their pseudopods toward one direction, Spumellaria possess a large number of pseudopods (often referred to as axopodia) radiating in all directions (Fig. 3B). Prey is captured by adhesion while swimming in the pseudopodial network. One detailed study revealed that Spumellaria pseudopodia follow a regular rhythmic cycle of extension and contraction, repeating successively every 10 min (Suzuki and Sugiyama, 2001). Prey capture among Collodaria is likely passive and can involve a large number of pseudopods radiating in all directions, similar to the feeding behaviour of Spumellaria (Fig. 3C). Prey (or sometime predators aiming to prey on Collodaria) swimming near the pseudopodial network get glued on it and will be slowly be digested in specific vacuoles. Prey capture by Acantharia is still an unresolved process, despite a recent study (Mars Brisbin et al., 2020) reporting that acantharians in the near‐surface layer consistently displayed long pseudopodial extensions terminating in drop‐shaped structures, which could serve as a fishing apparatus similar to those observed in polycystine radiolarians. Oroscenids feeding behaviour is primarily unknown. However, the absence of mouth‐like structures, or similar openings in their skeleton, would either suggest that Oroscenids are filter‐feeders like mesopelagic phaeodarians, or that feeding activities (including digestion) occur outside the skeleton (Nakamura et al., 2021).
Fig. 3.
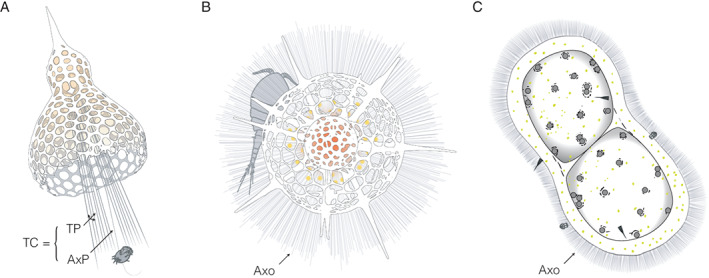
Schematic illustration of prey capture among different radiolarian orders.
A. Nassellaria: the ‘terminal cone (TC)’ comprises several ‘terminal projections’ (TP) and the ‘axial projection’ (AxP). Prey capture (here a ciliate) involves the different pseudopodia of the ‘terminal cone’.
B. Spumellaria: prey capture (here a copepod) involves many axopodia (Axo) radiating in all directions and forming a dense pseudopodial network.
C. Collodaria: prey capture (here some tintinnids) is similar to Spumellaria and involves a dense axopodial network surrounding the specimen.
To date, thanks mostly from gut content analyses, 12 taxonomic groups have been identified as potential predators of radiolarians (Table 1). These include a few crustacean species of amphipods, decapods, euphausids, isopods, ostracods and mysids, but the most diverse predators are undoubtedly copepods, with ca. 63 species in which radiolarian remains have been found in their gut contents. Other predators include molluscs (pteropods), annelids (polychaetes), gelatinous plankton like salps or doliolids, but also fishes (adults and larvae) whom seem to preferentially feed on larger radiolarians such as colonial Collodaria. Interestingly, some other taxa avoid consumption due to sterol compounds produced by collodarian's photosymbionts (Anderson, 1983; O'Rorke et al., 2012). While gut content analyses offer a good overview of potential predators of radiolarians (thanks to their skeletal remains being mostly preserved from digestion, which might not be the case for Acantharia), we cannot rule out that radiolarian remains in guts could be the result of a species feeding on detrital material (i.e. marine snow), leaving unknown the full extent of radiolarian predators. Despite the detailed nature of existing information, it should be noted that these observations are still restricted to a handful of specimens and studies. More than the lack of knowledge on specific predators, it is the lack of rate measurements that prevent the inclusion of radiolarians in marine food web models, precluding our understanding of their role there.
Table 1.
Summary of known radiolarian predators and occurrence of radiolarians in gut contents.
| Radiolaria group | Predator | Species | Frequency in gut contents |
|---|---|---|---|
| Acantharia (include Acanthometra, Dorataspis, Amphilonche) | Copepod | Canthocalanus pauper a , Pleuromamma robusta, Rhincalanus gigas | 0.04–0.15 |
| Fish | Myctophid b , c | n.a. | |
| Isopod | Acanthamunnopsis milleri c , Munneurycope murrayi c | n.a. | |
| Pteropod | Cuvierina columnella, Hyalaea trispinosa, Tiedemannia sp. | n.a. | |
| Collodaria | Fish | Bathylagus sp. a , Myctophid b , c | n.a. |
| Fish larvae | Anguilla anguilla a | n.a. | |
| Spiny lobster larvae | Panulirus cygnus a | n.a. | |
| Orodaria | Salp | Unidentified species | n.a. |
| Spumellaria (include Actinomma) | Copepod | Scopalatum vorax c | n.a. |
| Pteropod | Cuvierina columnella | n.a. | |
| Taxopodida | Copepod | Aetideopsis antarctica, Calanus propinquus, C. sinicus c , Centropages velificatus c , Euchaeta antarctica, Microcalanus pygmaeus, Paracalanus parvus c , P. quasimodo c , Parvocalanus crassirostris c , Temora stylifera c , T. turbinata c | 0.01–0.39 |
| Ostracod | Conchoecia belgicae | 0.07 | |
| Euphausiid | Euphausia sp. c | n.a. | |
| Radiolaria overall | Amphipod | Cyllopus lucasii, Epimeriella macronyx, Orchomene plebs | 0.03–0.06 |
| Copepod | Aetideopsis antarctica, A. rostrata, Calanus propinquus, C. similimus, C. tenuicornis, Centropages violaceus, Chiridius polaris d , Chiridius sp. d , Chirundina streetsii, Diaxis sp. d , Disseta palumboi, Euchaeta norvegica, E. sp., Euchirella rostromagna, Gaetanus simplex, G. tenuispinus, G. sp., Gaidius affinis, G. columbiae, Haloptilus ocellatus, Heterorhabdus sp. c , Heterostylites major, Lucicutia wolfendeni, Metridia discreta, M. gerlachei c , M. ornata, M. pacifica c , M. princeps, Nannocalanus minor, Neocalanus sp., Neoscolecithrix magna d , Oncaea mediterranea c , Pleuromamma robusta, P. scutullata c , P. xiphias, Pseudochirella polyspina, Rhincalanus gigas, Scaphocalanus subbrevicornis d , S. vervoorti, S. sp. d , Scopalatum sp. d , Scottocalanus securifrons, Spinocalanus abyssalis, S. antarcticus, S. magnus, S. spinosus, S. sp. d , Teneriforma sp. d , Undinella sp. d , Xanthocalanus sp. d , Zenkevitchiella sp. d | 0.01–1 | |
| Decapod | Gennadas valens, Hymenodora glacialis, Nematocarcinus lanceopes, Penaeus semisulcatus, Sergestes grandis, S. henseni, Systellaspis debilis | 0.02–0.33 | |
| Euphausiid | Euphausia crystallorophias, E. superba, Thysanoessa macrura | 0.03–0.46 | |
| Fish | Bathylagus antarctica, Trimmutom sp. | 0.31 | |
| Fish larvae | Anguilla anguilla a , A. japonica a , Clupea harengus pallasi, Etrumeus teres a , Paraliparis dipterus, Sardina pilchardus, Sardinops melanostictus a | n.a. | |
| Isopod | Acanthamunnopsis milleri c , Bathynomus doederleinii, Eurycope inermis, Ilyarachna hitrticeps, Munneurycope murrayi c | 0.02–0.1 | |
| Mysid | Antarctomysis ohlinii | 0.36 | |
| Polychaete | Poebius meseres | n.a. | |
| Pteropod | Tiedemannia sp. | n.a. | |
| Salp | Salpa thompsoni | 0.62 | |
| Tunicate | Dolioletta gegenbauri a | n.a. |
Additional information about location and depths of observation and a complete list of references are provided in Supplementary Information Table S1.
Observation from DNA sequences (clone or high‐throughput sequencing).
Observation from fish gut contents, where organisms could have been attached to plastic particles.
Observation from scanning electron microscopy.
Observation from transmission electron microscopy.
Evolutionary relationships
Hypotheses concerning the evolutionary relationships among the different radiolarian taxa have evolved constantly in the last century, but many clarifications of unresolved placements have been solved within the last two decades with the advent of integrative morpho‐molecular classifications. This is particularly true for Acantharia (Decelle et al., 2012a), Collodaria (Biard et al., 2015), Nassellaria (Sandin et al., 2019), Spumellaria (Sandin et al., 2021) and Orodaria (Nakamura et al., 2021). To date, only Taxopodida are lacking a detailed morpho‐molecular classification. However, clarification of the overall radiolarian classification scheme is still required as the most recent phylogenies including all known radiolarian orders have highlighted discrepancies (Sandin, 2019; Nakamura et al., 2021). These later studies suggested that Orodaria and Collodaria, rather than being monophyletic clades independent of other radiolarian orders, could be considered as sub‐clades merged within the order Nassellaria. Despite uncertainties related to long‐branch attraction artefact (known to be present within Nassellaria/Collodaria/Orodaria), this separation of polycystine Radiolaria into only two different orders (Spumellaria and Nassellaria, including Orodaria and Collodaria) would challenge any previous classification schemes, as Collodaria have never been classified as Nassellaria before (but were considered in the past to be part of Spumellaria due to the resemblance between collodarian and spumellarian skeletons; Anderson, 1983). Furthermore, this could result in deep modification of our understanding of radiolarian evolution and in particular the evolution of radiolarian skeleton, Collodaria and Orosphaeridae likely being the youngest radiolarian lineages (Suzuki and Oba, 2015) and possessing either giant silicified skeletons or lacking any traces of silicification.
Acantharia
Diversity and classification
Unlike most radiolarians with silicified skeletons, there is no fossil record of any Acantharia (Decelle et al., 2012b). This is because the high under‐saturation of strontium in seawater leads to rapid dissolution of their skeleton upon death (Beers and Stewart, 1970; Decelle and Not, 2015). Therefore, the establishment of classification frameworks has relied from early on, solely on morphological criteria of extant taxa. Such work has long history: it was initiated by Müller (1859), then his student Haeckel (1887)) and later stabilized by Schewiakoff (1926). This later work, emended in the four last decades, distinguishes 145 morphospecies, classified into 49–50 genera and four suborders: Hollacanthida, Chaunacanthida, Symphiacanthida and Arthracanthida (Suzuki and Not, 2015; Adl et al., 2019). Despite extensive taxonomic analyses that led to this classification framework, morphological identification of Acantharia actually still relies on a handful of criteria, difficult to diagnose because of the fragility and alterability of skeletal elements from dissolution (Decelle and Not, 2015). Therefore, the benefits of molecular approaches to study acantharian classification have been substantial and helped clarified the evolutionary relationships between acantharian suborders.
The latest phylogeny of acantharian ribosomal genes revealed the existence of six molecular clades (Clades A to F) (Decelle et al., 2012a). At the suborder level, only Chaunacanthida (Clade C) are monophyletic while the three remaining suborders are polyphyletic (Fig. 3A). Hollacanthida of Clade A represents the most basal and likely the most ancient lineage overall. Evolutionary relationships between this basal clade, and clades B and C (Chaunacanthida) are yet to be clarified. However, molecular clock analyses date the appearance of these two clades substantially ahead of Clades D, E and F, the latter two being Arthracanthida and Symphiacanthida respectively (Decelle et al., 2012a).
Compared with morphological classification, molecular phylogenies not only highlighted some major discrepancies between traditional taxonomical and molecular schemes but also provided substantial insights into the evolutionary history of acantharian skeleton and ecology (Decelle et al., 2012a). The three early clades (A–C) possess less complex skeleton, with 10–20 needle‐like spicules with no junction or loosed attached, while complexity increases (e.g. tight junction of spicules, presence of shell, etc.) for the recently diverged Clades D, E and F (Fig. 3B and C).
Photosymbiosis and biogeography
Acantharians are commonly found in surface waters from polar to equatorial regions, and from surface illuminated waters to the deep sea (Bernstein et al., 1999). They are, however, more commonly found in surface waters of tropical oligotrophic regions where they can represent a substantial fraction of protist abundances and overall primary production (Michaels, 1988; Michaels et al., 1995). The ecological success of such Acantharia is likely in part related to the fact that many species live in photosymbiosis with photosynthetic partners (Decelle et al., 2015). However, not all Acantharia have been found living such an intricate life (Fig. 4B). Indeed, only taxa of the Arthracanthida and Symphiacanthida (e.g. Clades E and F) have been repeatedly observed living together with tens to hundreds of haptophyte Phaeocystis cells (Khmeleva, 1967) and such symbioses likely date from between 100 and 200 Mya when inorganic nutrients were in short supply in the seas (Decelle et al., 2012b). Detailed investigation of intracellular processes related to this symbiosis revealed that Phaeocystis symbionts are heavily modified (both their morphology and metabolism) upon acquisition by their acantharian host, suggesting that this seemingly mutualism could be a ‘farming strategy’ (Decelle et al., 2019). These acantharians with symbionts often represent the majority of acantharian biomass in surface waters (Michaels, 1988; Stoecker et al., 1996). Despite Acantharia–Phaeocystis being the most widely distributed form of symbiosis among Acantharia, a few rarer, yet more complex, symbiosis cases have been observed among Chaunacanthida (i.e. Clade C). This symbiosis involves an acantharian cell with multiple partners (i.e. dinoflagellates and haptophyte) in a three‐way original symbiosis being a likely remnant of primitive symbiosis (Decelle et al., 2012c).
Fig. 4.
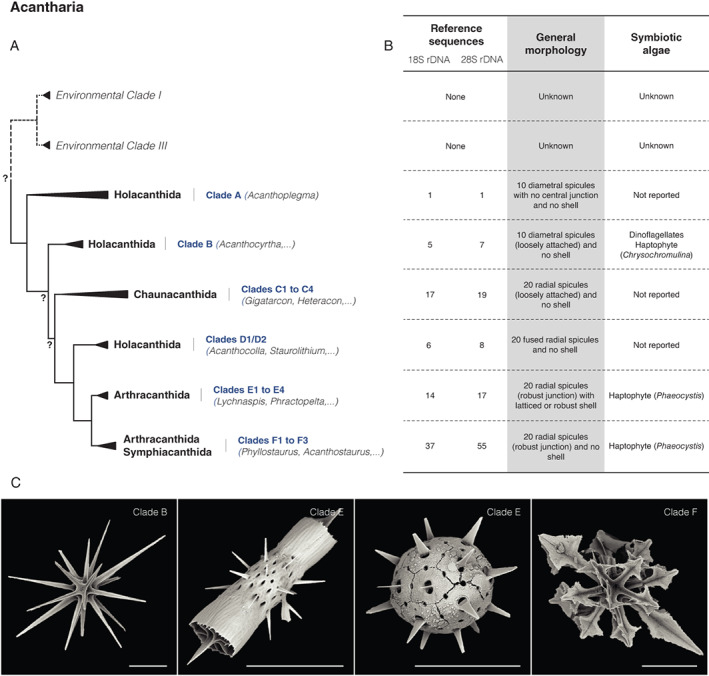
Schematic morpho‐molecular classification of Acantharia based on ribosomal genes (18S and 28S rDNA) from Decelle et al. (2012a).
A. Phylogeny showing molecular clades, taxonomic suborders and environmental clades. Non‐supported nodes are reported by a surrounded question mark.
B. Corresponding extent of the morpho‐molecular framework and main morphological features (including the presence and type of symbionts reported).
C. Scanning electron microscopy illustrations of typical strontium sulfate structures found in three acantharian clades. All scale bars are 50 μm. Images courtesy of Karine Leblanc (CNRS).
Environmental genetic diversity
Little is known about acantharian diversity in marine ecosystems and overall, only a handful of studies have looked specifically into the distribution patterns at regional or global scales. This is likely due to a combination of several factors: rapid dissolution of acantharians in fixed (e.g. formalin) samples (Beers and Stewart, 1970), the fact that acantharian skeletons are easily damaged upon collection, and commonly a lack of the taxonomic expertise required for identification (Decelle and Not, 2015). Consequently, investigations relying on the analysis of acantharian DNA from diverse environments have provided valuable, unique information. Of the six molecular clades described by Decelle et al. (2012a), only the most basal Clade A (Hollacanthida) was not retrieved in early molecular environmental surveys. The remaining clades are found in various ecosystems, including a wide range of trophic conditions, from the surface to deep‐sea ecosystems. Surprisingly, two molecular clades (described as Clade I and III; Decelle et al., 2012a) are composed exclusively of DNA sequences retrieved from environmental surveys and could not be assigned to any known acantharian morphospecies or suborder (Fig. 4A). Furthermore, these environmental clades were samples in extreme ecosystems such as deep‐water masses, hydrothermal vents or high‐latitude ecosystems. However, the study of such environmental sequences comes with inherent limitation. Most of these sequences come from the early age of molecular environmental surveys, using sequencing technics (e.g. clone library) nowadays obsolete. Such surveys only provide a relatively limited snapshot of diversity at a given location compared to the more recent DNA metabarcoding approaches.
DNA metabarcoding (i.e. high‐throughput sequencing of a constrained DNA region, often for marine protist communities the small‐subunit ribosomal RNA gene) has allowed much more extensive exploration of diversity patterns (Caron et al., 2004, 2012). Surveys of acantharian metabarcodes retrieved in the surface and deep‐sea (>200 m) zones suggested higher acantharian diversity (mainly dominated by Clades E and F) in surface waters (Decelle et al., 2013; Mars Brisbin et al., 2020). Similar to clone library surveys (see above), DNA metabarcoding systematically highlighted an overwhelming dominance of the environmental Clade I in deep waters, regardless of the sampling location (i.e. Indian, Atlantic, Antarctic or Pacific Oceans). Therefore, the existence of a deep‐sea and diverse acantharian population should be considered and deserves further attention. However, given the evolutionary history of acantharian skeleton (Decelle et al., 2012a; Decelle and Not, 2015), with basal clades represented by rudimentary skeletons (see above), we cannot rule out that this environmental clade I, never morphologically characterized, lack the typical acantharian strontium sulfate skeleton (Mars Brisbin et al., 2020). At broader geographical scales, despite a lack of detailed annotation and pattern analyses, DNA metabarcoding of surface waters suggested that Acantharia are one of the 11 most diverse eukaryotic lineages (de Vargas et al., 2015), with 1043 operational taxonomic units (can be considered a proxy for species richness to some extent; Caron and Hu, 2018). In the global bathypelagic realm, DNA metabarcoding suggested much more reduced acantharian diversity (i.e. 75 OTUs), despite any further detailed annotations (Pernice et al., 2016). Overall, environmental diversity of Acantharia is yet to be fully characterized but will undoubtedly reveal their substantial high diversity throughout a broad range of ecosystems.
Nassellaria
Diversity and classification
Initial classification schemes for nassellarians have long relied on expert analyses of specific morphological features, mainly the architecture of the initial spicule (De Wever et al., 2002). Such schemes have been derived mainly from fossil specimens, and fewer attempts have been made to compare these classifications on living nassellarians. Despite the inherent complexity of discriminating such criteria, approximatively 140 genera and 430 species are presently recognized (Suzuki and Not, 2015). While divided at higher levels into seven super‐families until recently (Suzuki and Not, 2015), the latest classification suggests the existence of 16 super‐families, 14 corresponding to extant nassellarian super‐families (Suzuki et al., 2021). This revision of classification scheme at high taxonomic level was partially due to Sandin et al. (2019) latest morpho‐molecular classification.
In this classification, phylogenetic relationships among Nassellaria discriminate four ‘Molecular lineages’ that can be considered as the suborder level (Fig. 5A). Out of the 14 extant nassellarian families, 11 are covered by molecular data, thus allowing an extensive description of evolutionary relationships between super‐families. The first lineage (I) corresponds to the Eucyrtidioidea and represents the most basal and likely the most ancient lineage overall, according to molecular clock analyses (Sandin et al., 2019). Hereafter, Lineage II encompasses four super‐families (Acanthodesmioidea, Artostrobioidea, Carpocanioidea and Plectopyramidoidea) for which phylogenetic relationships are still unclear, particularly due to the weak amount (<20) of reference sequences available. The phylogenetic position is yet unclear for the two remaining lineages III (Archipilioidea, Theopilioidea and Plagiacanthoidea) and IV (Cycladophoroidea, Lithochytridoidea and Pterocorythoidea).
Fig. 5.
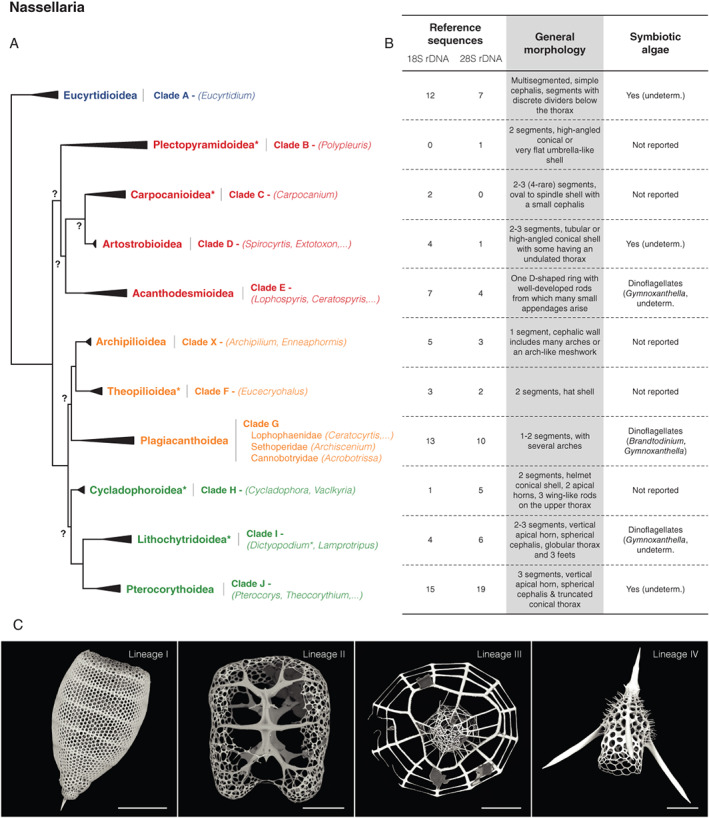
Schematic morpho‐molecular classification of Nassellaria based on ribosomal genes (18S and 28S rDNA) from Sandin et al. (2019).
A. Phylogeny showing molecular clades, taxonomic super‐families. Group with an asterisk denotes changes in taxonomic nomenclature from Sandin et al. (2019) following the latest classification scheme for Radiolaria (Suzuki et al., 2021). Non‐supported nodes are reported by a surrounded question mark. Colours correspond to molecular lineages (ML): blue = ML I, red = ML II, orange = ML III and green = ML IV.
B. Corresponding extent of the morpho‐molecular framework and main morphological features (including the presence and type of symbionts reported).
C. Scanning electron microscopy illustrations of typical silicified structures found in the four main nasellarian molecular lineages. All scale bars are 50 μm. Images courtesy of Miguel Sandin (Uppsala University).
When combined with morphological analyses of skeleton (Fig. 5B and C), the phylogeny revealed substantial discrepancies with the traditional classification (based in particular on the initial spicular system). Molecular phylogeny suggests that the overall morphology (i.e. number, sizes and shapes of the different segments, presence/absence of projections, etc.) of the specimen must be considered for accurate taxonomic identification at high taxonomical level.
Photosymbiosis and biogeography
There is a striking difference in our knowledge of the ecological relationships of fossil nassellarians, and polycystines overall, which have been used for paleoenvironmental reconstructions (i.e. the use of fossils to determine the climate for a given era in the past; Lazarus et al., 2021), and our knowledge of nassellarian ecology in modern ecosystems. Most of our knowledge of extant species is derived from sediment samples (e.g. Boltovskoy and Correa, 2016) from which ecological niche preferences of living nassellarians can be inferred. Temperature appears to be by far the most significant covarying factor structuring ecological assemblages of nassellarians. However, some species are representatives of particular trophic conditions: Acanthodesmia vinculata for surface oligotrophic waters, Eucyrtidium acuminatum for surface temperate areas (Suzuki and Not, 2015). While all Radiolaria are marine species inhabiting saline waters (salinities above 30 PSU; Boltovskoy et al., 2017), there is one nassellarian species, Lophophaena rioplatensis, who represents the only known radiolarian species thriving in brackish waters (Boltovskoy et al., 2003).
In addition to their phagotrophic behaviour (Fig. 3), numerous nassellarian species have been observed to be associated with photosynthetic partners (Fig. 5B). While most of these observations did not investigate the exact nature of the symbionts, often described as ‘golden dots scattered within the cell’, a few studies have narrowed their identities to two dinoflagellate species: Zooxanthella nutricula (often referred to as Brandtodinium nutricula; Probert et al., 2014) and Gymnoxanthella radiolariae (Yuasa et al., 2016). The numbers of symbionts (5–13 μm in size) per host vary considerably from a few cells up to more than 50 symbionts scattered within the host cytoplasm. While these symbiotic dinoflagellates have been identified in several nassellarian species (Fig. 5B), a few super‐families have never been observed associated with symbionts. However, given the lack of systematic analyses search for their presence, it is currently impossible to draw a conclusion regarding a possible co‐evolution between the symbionts and Nassellaria.
Environmental genetic diversity
Little is known about biogeographical patterns of nassellarian diversity in the global ocean. This observation is clearly contrasted with the extent of knowledge gathered from their microfossils (De Wever et al., 2002). Only a handful of study has investigated the extent of their distribution in modern oceans (Boltovskoy et al., 2010; Boltovskoy and Correa, 2016). A number of nassellarian species have been reported from the deep ocean (Boltovskoy and Correa, 2016), some of them being characteristic of deep‐water masses (e.g. Cycladophora davisiana for the mesopelagic ocean, 0–1000 m; Cornutella profunda for the bathypelagic, 1000–2000 m; Suzuki and Not, 2015). Yet, all these studies relied on challenging morphological identifications (see above) therefore hampering our understanding of diversity patterns.
By comparing environmental sequences from the previous expedition to the latest morpho‐molecular framework for Nassellaria, Sandin et al. (2019) provided the first assessment of nassellarian environmental diversity from molecular data. A large majority of the environmental sequences were assigned to the lineages II and III (in particular the Plagiacanthoidea), from various types of ecosystems (e.g. open ocean and nutrient‐poor, deep‐sea anoxic waters, etc.). However, clone data unlikely provide the most extensive overview of Nassellaria environmental diversity. Considering the two most recent and global oceanographic expeditions, high‐throughput sequencing for metabarcoding suggests a pool of approximatively 400 OTUs (i.e. proxy for species richness) for Nassellaria, equally spread across a size range from 0.8 to 2000 μm, and in the surface ocean (Tara Ocean Expedition; de Vargas et al., 2015). This number is similar to the number of identified species based on morphological analyses (Suzuki and Not, 2015), while they are likely not both covering the same size fraction (sizes <20 μm rarely being studied in microscopy for Nassellaria). From the Malaspina Expedition covering the global deep bathypelagic ocean, no sequences were assigned to Nassellaria (Pernice et al., 2016), suggesting a potential absence in deep oceans. However, since several deep‐sea nassellarians are well‐known (Suzuki and Not, 2015), the absence of deep‐sea sequences assigned to nassellarians could be due to the paucity of reference sequences available (Fig. 5B), or to the difficulties in DNA amplification for Nassellaria (Sandin et al., 2019).
Spumellaria
Diversity and classification
Traditionally (i.e. sensu Haeckel), radiolarians with spherical skeletons have been classified as Spumellaria (or Entactinaria, although recent molecular evidences questioned its existence as extant group; Nakamura et al., 2020; Suzuki et al., 2021). In recent years, several molecular studies have greatly clarified the phylogeny and taxonomy of Spumellaria (Yuasa et al., 2005; Kunitomo et al., 2006; Ishitani et al., 2012; Sandin et al., 2021). As for Nassellaria, most of the early taxonomic work on Spumellaria was based on fossil specimens, leaving the complex task of identifying living spumellarians. Nowadays, approximatively 110 genera and 380 species have been distinguished and divided into nine super‐families (Suzuki and Not, 2015). However, recently Spumellaria have been classified into 13 extant super‐families, thanks to the latest morpho‐molecular classification for this order (Sandin et al., 2021).
Phylogenetic relationships among Spumellaria (Fig. 6A) discriminate four ‘Molecular lineages’ encompassing distinct monophyletic super‐families. While the existence of these lineages is statistically supported, inter‐relations between spumellarian lineages are still unclear. Unlike other radiolarians orders, morphological classification and molecular phylogeny are in general agreement with the classification at the clade level. The overall skeleton symmetry and morphology (i.e. overall shape, central structure patterns, etc.; Fig. 6B and C) of the specimen must be considered for accurate taxonomic identification. Internal structures like the initial spicular system, believed to be critical taxonomic criteria for spumellarian identification (De Wever et al., 2002), therefore appear to be a secondary criterion enabling finer taxonomic assignation, while the overall shape and symmetry prove to be efficient and sufficient at higher taxonomic ranks.
Fig. 6.
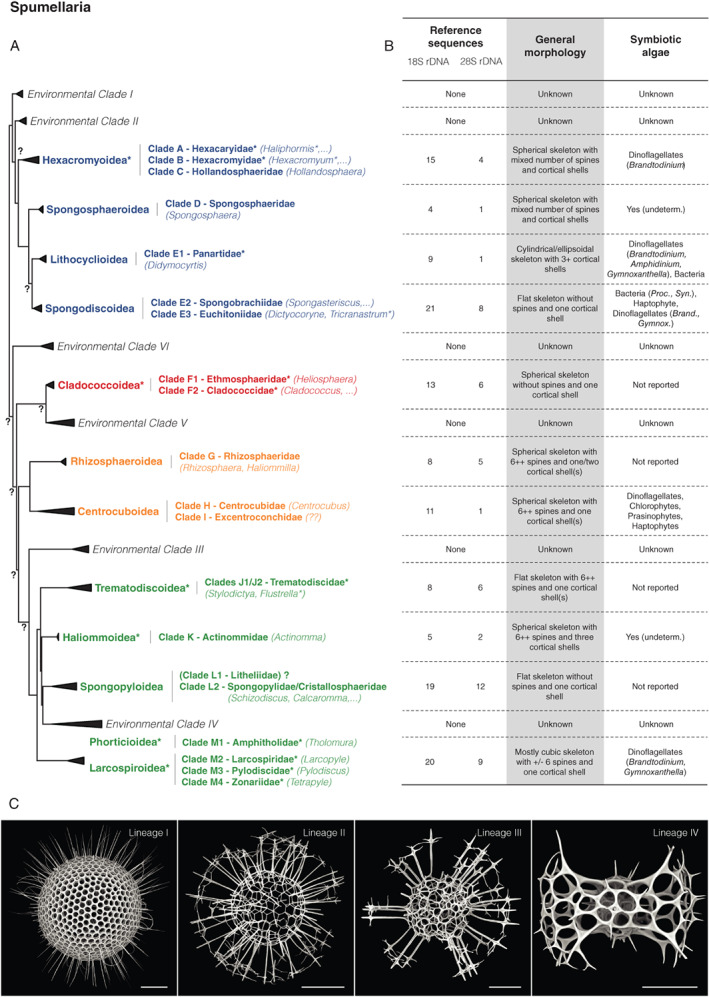
Schematic morpho‐molecular classification of Spumellaria based on ribosomal genes (18S and 28S rDNA) from Sandin et al. (2021).
A. Phylogeny showing molecular clades, taxonomic super‐families and environmental clades. Non‐supported nodes are reported by a surrounded question mark. Group with an asterisk denote changes in taxonomic nomenclature from Sandin et al. (2021) following the latest classification scheme for Radiolaria (Suzuki et al., 2021). Colours correspond to molecular lineages (ML): blue = ML I, red = ML II, orange = ML III and green = ML IV.
B. Corresponding extent of the morpho‐molecular framework and main morphological features (including the presence and type of symbionts reported).
C. Scanning electron microscopy illustrations of typical silicified structures found in the four main spumellarian molecular lineages. All scale bars are 50 μm. Images courtesy of Miguel Sandin (Uppsala University).
Photosymbiosis and biogeography
Our knowledge of spumellarian ecology has the same inherent limitations that apply to other extant radiolarians. Similar to Nassellaria, sea‐surface temperatures shape most spumellarian ecological assemblages (Boltovskoy and Correa, 2016). However, for most subtropical spumellarians, vertical distribution patterns are driven by their mixotrophic strategy, as they host one of the most diverse number of photosymbionts known among Radiolaria. In all four spumellarian molecular lineages, the presence of symbiotic partners has been reported, covering more than half of the super‐families (Fig. 6B). Photosymbionts include some of the algae groups identified among Nassellaria and Collodaria, such as Zooxanthella nutricula/Brandtodinium nutricula (Probert et al., 2014) and Gymnoxanthella radiolariae (Yuasa et al., 2016), but also other symbionts apparently exclusive to Spumellaria. These include unidentified species of Chlorophytes, Prasinophytes and Haptophytes (Gast and Caron, 2001; Anderson, 2012; Yuasa et al., 2019), as well the marine prokaryotes Prochlorococcus (Foster et al., 2006) and Synechococcus (Yuasa et al., 2012). The number of symbionts per host varies considerably between species of symbionts and hosts, from a few dinoflagellate cells up to hundreds of bacterial symbionts scattered within the host's cytoplasm. Unlike Acantharia, the lack of consistent search for spumellarian photosymbionts and their broad distribution among super‐families prevent drawing any conclusion regarding the origin of spumellarian symbiosis.
Environmental genetic diversity
As for Nassellaria, there is a major imbalance between the knowledge of spumellarian species distribution based on the presence of their skeletons in marine sediments (e.g. Boltovskoy et al., 2010) and diversity patterns in modern oceans. A few dedicated studies suggest that sets of few dominant species can characterize a latitudinal gradient in the North Pacific, ranging from subtropical areas to subarctic regions (Suzuki and Not, 2015). Similar patterns are observed between photic and deeper water layers with both patterns being partially explained by the presence of symbionts and/or the influence of temperature. A number of spumellarian species have been reported from deep waters (Reshetnyak, 1955) with a few being characteristic of deep‐water masses (e.g. Spongopyle osculosa for the mesopelagic ocean, Saturnalis circularis for the bathypelagic; Suzuki and Not, 2015).
A larger set of environmental sequences in shallow waters can be assigned to Spumellaria, compared to Acantharia or Nassellaria (Sandin et al., 2021), and a similar trend is observed in deep environments and particular ecosystems such as anoxic basins. Among the breadth of environmental sequences, several sequences cannot be clustered with reference sequences (i.e. one of the 13 molecular clades) and form six environmental clades (referred to as Env 1 to 6), of unknown morphologies (Fig. 6A). Among these environmental clades, Sandin et al. (2021) hypothesized that Env1 could contain a group of naked symbiotic spumellarians developing within skeleton‐bearing species. However, this hypothesis is currently not supported by any physical sample hampering any further investigation of the possible naked spumellarians.
At larger geographic scales, a metabarcoding survey in the surface ocean (i.e. Tara Ocean Expedition) highlighted a slightly more diverse spumellarian order (with approximatively 600 OTUs, twice more than the latest estimates from plankton samples; Suzuki and Not, 2015) compared to Nassellaria (de Vargas et al., 2015). However, considering metabarcode abundances (referred to as ‘read abundance’, a proxy for cell abundances), Spumellaria are among the nine most abundant eukaryotic lineages. Interestingly, 80% of these sequences/reads were extracted from the 0.8–5 μm size fraction, an unlikely size for typical spumellarians (generally larger than 60 μm). This imbalance in favour of the smaller size fraction could, however, strengthen the hypothesis of naked symbiotic spumellarians. Alternatively, the presence of spumellarian in such a small size fraction could be explained by cell breakage during sampling or the existence of spumellarian swarmers (i.e. flagellated cells of 2.5–10 μm corresponding to a sexual reproductive stage of radiolarians) reproducing in the deep ocean (Li and Endo, 2020), in a similar fashion to Foraminifera. Deeper in the oceans, Spumellaria appear less diverse (68 OTUs) in the Malaspina Expedition metabarcoding survey, but they comprise one species (Cladococcus viminalis) with the fourth largest read abundance overall (Pernice et al., 2016). This exploration of spumellarian environmental signatures altogether highlights that there are substantial gaps in the understanding of the likely underestimated spumellarian diversity (i.e. existence of clades without any known morphologies) and its distribution in modern oceans.
Collodaria
Diversity and classification
Compared to other radiolarian orders, only handful studies have investigated collodarian diversity, both in fossil and living organisms. Collodaria were described in the late 19th century (Haeckel, 1882) but were considered for decades to be part of the order Spumellaria (e.g. Anderson, 1983). Nowadays, Collodaria are formerly recognized as an independent radiolarian order, separate from Spumellaria (De Wever et al., 2002). However, recent molecular evidence have highlighted that Collodaria could be included as a new nassellarian family, rather than an independent molecular clade (Sandin, 2019; Nakamura et al., 2021). Nevertheless, until further morpho‐molecular analyses and for ease of understanding, Collodaria are here discussed as an independent entity separate from Nassellaria. Approximatively 80 collodarian species have been identified and regrouped in 20 genera (Suzuki and Not, 2015). Collodaria were originally separated into three main families, Thalassicollidae, Sphaerozoidae and Collosphaeridae, based on two key morphological criteria: as either colonial (Sphaerozoidae and Collosphaeridae) or solitary (Thalassicollidae) organisms and the presence or absence of cortical shells (Müller, 1859).
Recent morpho‐molecular classifications have expanded our knowledge of classification at the higher taxonomic levels among collodarians, nowadays classified into four families (Fig. 7A): the three former families in addition to the newly created Collophidiidae, another family of colonial collodarians (Biard et al., 2015). Interestingly, while coloniality was a key criterion, phylogenies suggested that Thalassicollidae do not form a monophyletic clade, but instead is spread in the three ‘colonial’ families (Fig. 7B). This major conflict between morphological taxonomy and molecular phylogeny has likely its origin in collodarian biology, where solitary forms are putatively reproductive stages of colonial organisms (Biard et al., 2015). Phylogenetic relationships further separate Sphaerozoidae from Collophidiidae and Collosphaeridae, the latter two being more closely related. Collosphaeridae are separated into six phylogenic clades where no clear patterns can associate the different clades with known genera. Historically gathering all skeleton‐bearing collodarian species, morpho‐molecular analyses highlighted the coexistence of naked specimens with skeleton‐bearing species among Collosphaeridae (Fig. 7C). Similar to the mix between colonial and solitary species, the coexistence of naked and skeleton‐bearing Collosphaeridae specimens is likely due to different ontogenic stages (Biard et al., 2015). In the newly formed family Collophidiidae, all three phylogenic clades are associated to the genus Collophidium, where all specimens lack silicified structures (Fig. 7B). Finally, the Sphaerozoidae are the most diverse family with 11 phylogenetic clades, yet not clearly separating the different genera or morphotypes. Indeed, spicule bearing species (identified as Sphaerozoum and Rhaphidozoum) are spread among naked specimens of the genus Collozoum, leaving unknown the evolutionary history of silicified structures among Collodaria, one of the most recent Radiolaria lineages (Suzuki and Not, 2015).
Fig. 7.
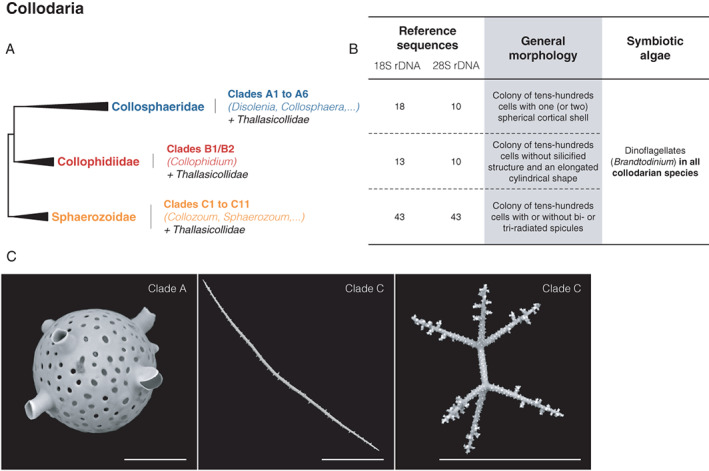
Schematic morpho‐molecular classification of Collodaria based on ribosomal genes (18S and 28S rDNA) from Biard et al. (2015).
A. Phylogeny showing molecular clades and taxonomic families.
B. Corresponding extent of the morpho‐molecular framework and main morphological features (including the presence and type of symbionts reported).
C. Scanning electron microscopy illustrations of typical silicified structures found in the three main collodarian clades. All scale bars are 50 μm.
Photosymbiosis and biogeography
As for other radiolarians, Collodaria ecology has long remained elusive, but a number of studies in the last four decades have substantially expanded knowledge of this group. The most striking feature of Collodaria is their exclusive and tight association with one photosymbiont species, Zooxanthella nutricula/Brandtodinium nutricula (Probert et al., 2014). While B. nutricula has been observed sporadically in other radiolarian taxa, all known collodarian species have been described associated with a photosymbiont, later revealed to be B. nutricula. This dependence of Collodaria on a singular photosymbiont raises many questions about the specificity and functioning of this association, still unanswered to date. The number of B. nutricula symbionts per collodarian host varies considerably between colonial and solitary specimens, but can include a few hundred cells for solitary, up to several thousand scattered in the colonial host's cytoplasm. This high number of photosymbionts likely enables the host to obtain a considerable quantity of carbon‐rich products from its partner photosynthetic activity and likely thus enhance its survival rates (Anderson, 1983).
Despite what we know about prey and predators of Collodaria (Fig. 3; Table 1), the influence of biotic interactions on Collodaria distribution in modern oceans is unknown. A few studies have nevertheless shown the influence of abiotic factors on collodarian populations (Biard and Ohman, 2020). Mostly restricted to photic layers, likely to sustain the activity of their photosymbionts, Collodaria abundances in off the California coast appear to be influenced by temperature and the depth of the deep Chlorophyll a maximum. However, these factors only explain a minor fraction (25%) of the population's variability, suggesting that other factors (biotic or abiotic) influence Collodaria in these regions.
Environmental genetic diversity
The diversity of Collodaria in modern ecosystems has been assessed sporadically from microscopical analyses of surface sediment or plankton net samples, but without any detailed description of diversity patterns (e.g. Boltovskoy et al., 2010). A major breakthrough occurred in our understanding of collodarian diversity thanks to high‐throughput environmental sequencing. In early environmental surveys based on clone libraries, many sequences have been associated with Collodaria in various types of ecosystems, suggesting a potential high hidden pool of diversity for this order (Biard et al., 2015). A majority of these clone sequences, coming from deep environments for the most part, have been associated with the newly formed family Collophidiidae, suggesting that this family could be a major representative of Collodaria in the deep ocean (Biard et al., 2015).
In more recent environmental surveys, Collodaria have been in the spotlight as one of the most represented taxa in metabarcoding surveys. From the Tara Ocean Expedition global survey of the photic oceans, Collodaria were described as the fourth most hyperdiverse eukaryotic lineage (with nearly 6000 OTUs, a number exceeding by far the 80 morpho‐species described previously) and would be the most abundant (read abundances) protistan lineage, only exceeded in numbers by multicellular organisms (de Vargas et al., 2015). Similar trends were observed from the Malaspina metabarcoding survey: Collodaria would be the most abundant (read abundances) and fifth‐most diverse (197 OTUs) eukaryotic lineage in the bathypelagic ocean (Pernice et al., 2016). This study provided more precise identifications of these deep bathypelagic collodarian communities, systematically composed of Collophidiidae, a trend similar to clone libraries. However, if Collophidiidae represent a deep‐water collodarian family, their morphology in such an environment is yet to be determined, as no colonial or solitary collodarian specimens have ever been consistently observed in the deep ocean.
While Collodaria are now often recovered in metabarcoding analyses, one detailed study enabled a better understanding of their prominence in such surveys (Biard et al., 2017). Indeed, the number of DNA gene copies per collodarian cell is some of the highest in eukaryotic lineages. These high numbers are further amplified when considering collodarian colonies, with dozens of millions rDNA copies, being the highest rDNA copy number ever recorded in any marine protists. Such high numbers of copies are as high as the number of rDNA copies estimated for multi‐cellular crustacean copepods. Combined with the likely high DNA intracellular variability (observed in other radiolarian taxa; Decelle et al., 2014) and potential cell breakage during collection, these rDNA copy numbers have likely led to an over‐representation of Collodaria in metabarcoding surveys and suggest that diversity and relative abundance estimates have been likely over‐estimated.
Despite complex inclusion in metabarcoding surveys scaled to planktonic communities, direct comparisons between collodarian metabarcodes revealed marked patterns (Biard et al., 2017). Collodaria diversity shows an increase from the poles to equatorial regions and an increase from coastal regions to offshore areas. This second trend further revealed a clear distinction between collodarian communities along a gradient of distances to coasts, with Sphaerozoidae (comprising mostly naked collodarian, or with reduced silicified structures) dominating coastal areas, and Collosphaeridae (collodarian bearing silicified skeletons) dominating offshore.
Taxopodida and Orodaria
Diversity and classification
The last two remaining radiolarian orders, Taxopodida (Fig. 2C and D) and Orodaria (Fig. 2I and J) are two phylogenetic distant entities (Fig. 1) and they do not share any morphological similarities except their silicified skeletons. Nonetheless, both orders are currently the last remaining radiolarians without detailed morpho‐molecular frameworks, despite the recent placement of Orodaria in the radiolarian phylogeny (Nakamura et al., 2021). Orodaria are phylogenetically close to Collodaria and Nassellaria, but distant from Spumellaria and Phaeodaria, two families where Orodaria used to be classified based on morphological resemblances (Haeckel, 1887; Haecker, 1908; De Wever et al., 2002). Morpho‐molecular sequences currently cover only the family Oroscenidae, leaving unknown the phylogenetic placement of the second Orodaria family, Thalassothamnidae (Nakamura et al., 2021).
Taxopodida remains one of the most elusive radiolarian orders to date, due to the almost entire lack of knowledge about this group. It is currently composed of a single described species Sticholonche zanclea, for which a handful of molecular sequences have been produced. The phylogenetic placement of Taxopodida and its relation with other radiolarian orders is therefore complex and uncertain. Depending on the gene used, Taxopodida are alternatively a sister clade of Spumellaria or Acantharia, and less often a basal clade to Foraminifera and Radiolaria altogether (Sandin, 2019). Whilst no complete morpho‐molecular analyses have been carried out for Taxopodida, this order seemingly appeared highly diverse from environmental molecular surveys.
Photosymbiosis and biogeography
Orodaria are inhabitants of the deep ocean, dwelling below the photic zone (Nakamura et al., 2021). Consequently, unlike other radiolarians, Oroscenids have never been observed associated with photosymbionts or bacterial symbionts. However, the lack of observation of live Oroscenids prevents understanding of their ecology. Information about Taxopodida ecology is even more scarce and they remain the most elusive radiolarian order to date. Like Orodaria, no symbionts have been observed in any taxopodids. Because of such fragmentary information, the place of Orodaria and Taxopodida in marine food webs or biogeographical patterns is currently unknown, but given their peculiar natures, they should attract further attention in the future.
Environmental genetic diversity
Early on, during the environmental clone sequencing era, Taxopodida revealed a diverse signature, with many sequences from various ecosystems (surface, meso‐ and bathypelagic) clustering with the single S. zanclea reference sequence (Not et al., 2007). Following the rapid acceptance of environmental molecular surveys, more sequences were assigned to the Taxopodida cluster, hereafter described as the “Rad‐B" clade (Suzuki and Not, 2015). Two sister clades, Rad‐A and Rad‐C, were associated with the Rad‐B/Taxopodida cluster, without any evidence suggesting that these could altogether represent the order Taxopodida. However, given the breadth of sequences available (further exemplified by the ~250 and 85 OTUs in the Tara Expedition and Malaspina metarbarcoding datasets; De Vargas et al., 2015; Pernice et al., 2016) and the variety of ecosystems from which these sequences were produced, the Taxopodida hold a substantial pool of unexplored diversity waiting to be characterized.
Considering the extant of radiolarian diversity in environmental surveys, there is a striking difference with Orodaria. To date, no environmental sequences have been ever formally retrieved from clone sequencing or metabarcoding surveys. Due to the lack of reference sequences for Orodaria until recently (Nakamura et al., 2021), it cannot rule out that environmental sequences associated with Orodaria have been misidentified or unlabelled, leaving unknown the extent of orodarian environmental diversity.
Concluding remarks
The last few decades have seen a growing interest in modern Radiolaria, likely thanks to large‐scale studies that highlighted their apparently substantial contribution to marine biodiversity (de Vargas et al., 2015), carbon pool (Biard et al., 2016) or biogeochemical cycles (Lampitt et al., 2009; Biard et al., 2018). Coupled with the democratization and decreasing costs of molecular approaches, major breakthroughs in the understanding of radiolarian classification, at orders level, have set the baseline (i.e. extended morpho‐molecular reference sequences available on public platforms; Guillou et al., 2013; Clark et al., 2016) for further large‐scale observation of their diversity patterns.
Despite this new exciting dynamic in radiolarian research (Box 2), several elements need to be dealt with caution. For example, the overwhelming numbers of environmental sequences assigned to radiolarians might have a direct origin in the vast number of rDNA gene copies per radiolarians (Biard et al., 2017). The regularly high diversity estimates for Radiolaria could be biased by their high intracellular variability of gene, leading to artificial diversity even within the same cell (Decelle et al., 2014; unpublished data). A full characterization of such processes is therefore essential to fully understand their contribution to marine biodiversity.
Box 2. Summary of diversity, ecology and distribution for each radiolarian order.
Order (size range in μm); Number of extant genera and species; Fossil record; Reported numbers of Operational Taxonomic Units for the Tara Ocean Expedition and Malaspina Expedition; Trophic strategy; Symbiosis; Horizontal and vertical distribution.
Acantharia (50–1000); 49 genera and 145 species; Fossil record absent likely due to rapid dissolution; Tara = 1043 OTUs, Malaspina = 75 OTUs; Phagotroph; Mostly Haptophytes, Dinoflagellates less common, all restricted to three clades; Cosmopolite, with high abundances in coastal areas, and species distributed from the surface to the deep ocean.
Taxopodida also referred as to Rad‐B (200–800); one genus and one species; Fossil record absent; Tara = 250 OTUs, Malaspina = 85 OTUs; Trophic strategy unknown; No symbionts reported; Cosmopolite and species distributed from the surface to the deep ocean.
Spumellaria (50–2000); 110 genera and 380 species; Extensive fossil record; Tara = 600 OTUs, Malaspina = 68 OTUs; Phagotrophs; Diverse symbionts (e.g. Dinoflagellates, Haptophytes, Bacteria, etc.) found in all four molecular lineages but not in all families; Cosmopolite and species distributed from the surface to the deep ocean.
Nassellaria (50–300); 140 genera and 430 species; Extensive fossil record; Tara = 400 OTUs, Malaspina = absent; Phagotrophs; Dinoflagellates and unknown symbionts in half of the families; Cosmopolite and species distributed from the surface to the deep ocean.
Orodaria (1000–7000); At least four genera and an unknown number of species; Fossil record common; No OTUs reported; Likely phagotroph; No symbionts reported; Restricted to the deep ocean.
Collodaria (100–3 000 000); 20 genera and 80 species; Fossil record limited to skeleton‐bearing species (i.e. Collosphaeridae); Tara = 6000 OTUs, Malaspina = 197 OTUs; Phagotroph; Dinoflagellate Zooxanthella nutricula/Brandtodinium nutricula found in all collodarian species; Cosmopolite, with higher abundances in oligotrophic regions. Species are distributed mostly in the surface ocean, but many environmental sequences are found in the deep ocean.
Nowadays, among the six radiolarian orders described, two are lacking detailed morpho‐molecular characterization: Orodaria (despite a few sequences recently acquired; Nakamura et al., 2021) and Taxopodida. Until these two groups are fully characterized, it is impossible to understand the full evolutionary history of Radiolaria, which would be a key to understand how Radiolaria evolved since the Cambrian, from simple silicified spicules (i.e. Archeospicularia) to complex large colonial organisms, sometimes without any silicified structures and filled with photosymbiotic algae. Furthermore, characterizing the full morpho‐molecular framework of each radiolarian order by achieving broader sampling efforts would likely enable the description of environmental clades, such as those found among Acantharia and Spumellaria, but also finally naming the clades “Rad‐A" and “Rad‐C", who appear close to the only Taxopodida sequence available (Suzuki and Not, 2015).
In parallel with a full morpho‐molecular classification, remains the extensive problem of radiolarian ecology. There are many questions that remain unsolved and too many to be fully listed here: for example, who are the predators of radiolarians? Answering this simple question would have deep consequences in our understanding of radiolarian ecology and would finally allow the inclusion of Radiolaria in food web models. Given their substantial abundances in some location, it is clear that Radiolaria can represent a large amount of food for their putative predators, from copepods to fishes, both in the photic layers but also below in the deep ocean; What do radiolarians eat? As a number of radiolarians are associated with photosymbionts, what is the amount of ingested food they need to capture? Basic questions such as will likely remain partially answered because of our incapacity to culture radiolarians. Therefore, unless a major breakthrough occurs, we will rely on freshly collected radiolarian specimens, difficult to maintain through a complete reproductive cycle. Alternatively, the emergence of microfluidics technics (also known as Lab‐on‐a‐Chip; Girault et al., 2019) comes with a new existing alternative for radiolarian culture and a likely promise for major breakthroughs in our understanding of this wonderful group that is Radiolaria.
Supporting information
Appendix S1. Supplementary Information.
Acknowledgements
This work was supported by the ANR RhiCycle project, grant ANR‐19‐CE01‐0006 of the French National Research Agency. I am grateful to John Dolan (CNRS) for his valuable comments on the manuscript, and Miguel Sandin for his useful discussions on radiolarian classification. I would like to thank Karine Leblanc (CNRS) for sharing images of plankton from the Plankton MIO website (http://plankton.mio.osupytheas.fr), and Inigo Montoya (a.k.a. JRD) for sharing images of plankton from the Aquaparadox website (http://www.obs-vlfr.fr/LOV/aquaparadox/).
References
- Adl, S.M. , Bass, D. , Lane, C.E. , Lukeš, J. , Schoch, C.L. , Smirnov, A. , et al. (2019) Revisions to the classification, nomenclature, and diversity of eukaryotes. J Eukaryot Microbiol 66: 4–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, O.R. (1977) Cytoplasmic fine structure of nassellarian Radiolaria. Mar Micropaleontol 2: 251–264. [Google Scholar]
- Anderson, O.R. (1978) Light and electron microscopic observations of feeding behavior, nutrition, and reproduction in laboratory cultures of Thalassicolla nucleata. Tissue Cell 10: 401–412. [DOI] [PubMed] [Google Scholar]
- Anderson, O.R. (1983) Radiolaria: New York: Springer‐Verlag. [Google Scholar]
- Anderson, O.R. (2012) Living together in the plankton: a survey of marine protist symbioses. Acta Protozool 52: 1–10. [Google Scholar]
- Beers, J.R. , and Stewart, G.L. (1970) The preservation of Acantharians in fixed plankton samples. Limnol Oceanogr 15: 825–827. [Google Scholar]
- Bernstein, R.E. , Betzer, p.R. , Feely, R.A. , Byrne, R.H. , Lamb, M.F. , and Michaels, A.F. (1987) Acantharian fluxes and strontium to chlorinity ratios in the North Pacific Ocean. Science 237: 1490–1494. [DOI] [PubMed] [Google Scholar]
- Bernstein, R.E. , Kling, S.A. , and Boltovskoy, D. (1999) Acantharia. In South Atlantic Zooplankton, Boltovskoy, D. (ed). Leiden: Backhuys Publishers, pp. 77–147. [Google Scholar]
- Biard, T. , Bigeard, E. , Audic, S. , Poulain, J. , Gutierrez‐Rodriguez, A. , Pesant, S. , et al. (2017) Biogeography and diversity of Collodaria (Radiolaria) in the global ocean. ISME J 11: 1331–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biard, T. , Krause, J.W. , Stukel, M.R. , and Ohman, M.D. (2018) The significance of Giant Phaeodarians (Rhizaria) to biogenic silica export in the California current ecosystem. Global Biogeochem Cycles 32: 987–1004. [Google Scholar]
- Biard, T. , and Ohman, M.D. (2020) Vertical niche definition of test‐bearing protists (Rhizaria) into the twilight zone revealed by in situ imaging. Limnol Oceanogr 65: 2583–2602. [Google Scholar]
- Biard, T. , Pillet, L. , Decelle, J. , Poirier, C. , Suzuki, N. , and Not, F. (2015) Towards an integrative morpho‐molecular classification of the Collodaria (Polycystinea, Radiolaria). Protist 166: 374–388. [DOI] [PubMed] [Google Scholar]
- Biard, T. , Stemmann, L. , Picheral, M. , Mayot, N. , Vandromme, P. , Hauss, H. , et al. (2016) In situ imaging reveals the biomass of giant protists in the global ocean. Nature 532: 504–507. [DOI] [PubMed] [Google Scholar]
- Boltovskoy, D. , Anderson, O.R. , and Correa, N.M. (2017) Radiolaria and Phaeodaria. In Handbook of the Protists, Archibald, J.M. , Simpson, A.G.B. , and Slamovits, C.H. (eds). Cham: Springer International Publishing, pp. 731–763. [Google Scholar]
- Boltovskoy, D. , and Correa, N. (2016) Biogeography of Radiolaria Polycystina (Protista) in the World Ocean. Prog Oceanogr 149: 82–105. [Google Scholar]
- Boltovskoy, D. , Kling, S.A. , Takahashi, K. , and Bjørklund, K. (2010) World atlas of distribution of recent polycystina (Radiolaria). Palaeontol Electron 13: 230. [Google Scholar]
- Boltovskoy, D. , Kogan, M. , Alder, V.A. , and Mianzan, H. (2003) First record of a brackish radiolarian (Polycystina): Lophophaena rioplatensis n. sp. in the Río de la Plata estuary. J Plankton Res 25: 1551–1559. [Google Scholar]
- Brandt, K.A.H. (1881) Über das Zusammenleben von Thieren und Algen. Arch Anat Physiol: 2, 570–574. [Google Scholar]
- Caron, D.A. , Countway, p.D. , and Brown, M.V. (2004) The growing contributions of molecular biology and immunology to protistan ecology: molecular signatures as ecological Tools1. J Eukaryot Microbiol 51: 38–48. [DOI] [PubMed] [Google Scholar]
- Caron, D.A. , Countway, p.D. , Jones, A.C. , Kim, D.Y. , and Schnetzer, A. (2012) Marine protistan diversity. Ann Rev Mar Sci 4: 467–493. [DOI] [PubMed] [Google Scholar]
- Caron, D.A. , and Hu, S.K. (2018) Are we overestimating protistan diversity in nature? Trends Microbiol 27: P197–P205. [DOI] [PubMed] [Google Scholar]
- Cavalier‐Smith, T. , Chao, E.E. , and Lewis, R. (2018) Multigene phylogeny and cell evolution of chromist infrakingdom Rhizaria: contrasting cell organisation of sister phyla Cercozoa and Retaria. Protoplasma 255: 1517–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, K. , Karsch‐Mizrachi, I. , Lipman, D.J. , Ostell, J. , and Sayers, E.W. (2016) GenBank. Nucleic Acids Res 44: D67–D72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vargas, C. , Audic, S. , Henry, N. , Decelle, J. , Mahé, F. , Logares, R. , et al. (2015) Eukaryotic plankton diversity in the sunlit ocean. Science 348: 1261605. [DOI] [PubMed] [Google Scholar]
- De Wever, P. , Dumitrica, P. , Caulet, J.P. , Nigrini, C. , and Caridroit, M. (2002) Radiolarians in the Sedimentary Record. London: CRC Press. [Google Scholar]
- Decelle, J. , Colin, S. , and Foster, R.A. (2015) Photosymbiosis in marine planktonic protists. In Marine Protists, Ohtsuka, S. , Suzaki, T. , Horiguchi, T. , Suzuki, N. , and Not, F. (eds). Japan: Springer, pp. 465–500. [Google Scholar]
- Decelle, J. , Martin, P. , Paborstava, K. , Pond, D.W. , Tarling, G. , Mahé, F. , et al. (2013) Diversity, ecology and biogeochemistry of cyst‐forming Acantharia (Radiolaria) in the oceans. PLoS One 8: e53598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decelle, J. , and Not, F. (2015) Acantharia. In eLS, eLS (ed). Chichester: John Wiley & Sons Ltd, pp. 1–10. [Google Scholar]
- Decelle, J. , Probert, I. , Bittner, L. , Desdevises, Y. , Colin, S. , de Vargas, C. , et al. (2012a) An original mode of symbiosis in open ocean plankton. Proc Natl Acad Sci U S A 109: 18000–18005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decelle, J. , Romac, S. , Sasaki, E. , Not, F. , and Mahé, F. (2014) Intracellular diversity of the V4 and V9 regions of the 18S rRNA in marine protists (radiolarians) assessed by high‐throughput sequencing. PLoS One 9: e104297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decelle, J. , Stryhanyuk, H. , Gallet, B. , Veronesi, G. , Schmidt, M. , Balzano, S. , et al. (2019) Algal remodeling in a ubiquitous planktonic photosymbiosis. Curr Biol 29: 968–978.e4. [DOI] [PubMed] [Google Scholar]
- Decelle, J. , Siano, R. , Probert, I. , Poirier, C. , and Not, F. (2012b) Multiple microalgal partners in symbiosis with the acantharian Acanthochiasma sp. (Radiolaria). Symbiosis 58: 233–244. [Google Scholar]
- Decelle, J. , Suzuki, N. , Mahé, F. , de Vargas, C. , and Not, F. (2012c) Molecular phylogeny and morphological evolution of the Acantharia (Radiolaria). Protist 163: 435–450. [DOI] [PubMed] [Google Scholar]
- Ehrenberg, C.G. (1854) Mikrogeologie. Leipzig: Voss. [Google Scholar]
- Foster, R.A. , Carpenter, E.J. , and Bergman, B. (2006) Unicellular cyanobionts in open ocean dinoflagellates, radiolarians, and tintinnids: ultrastructural characterization and immuno‐localization of phycoerythrin and nitrogenase. J Phycol 42: 453–463. [Google Scholar]
- Gast, R.J. , and Caron, D.A. (2001) Photosymbiotic associations in planktonic foraminifera and radiolaria. Hydrobiologia 461: 1–7. [Google Scholar]
- Girault, M. , Beneyton, T. , del Amo, Y. , and Baret, J.‐C. (2019) Microfluidic technology for plankton research. Curr Opin Biotechnol 55: 134–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou, L. , Bachar, D. , Audic, S. , Bass, D. , Berney, C. , Bittner, L. , et al. (2013) The protist ribosomal reference database (PR2): a catalog of unicellular eukaryote small sub‐unit rRNA sequences with curated taxonomy. Nucleic Acids Res 41: D597–D604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeckel, E.H.p.A. (1882) Entwurf eines Radiolarien‐Systems auf Grund von Studien der Challenger‐Radiolarien. Jenaische Z Für Naturwissenschaft 15: 418–472. [Google Scholar]
- Haeckel, E.H.p.A. (1887) Report on the Radiolaria collected by H. M.S. Challenger during the years 1873–1876. Fortschr Zool 18: 1–1803. [Google Scholar]
- Haecker, V. (1908) Tiefsee‐Radiolarien. Spezieller Teil. Tripyleen, Collodarien und Mikroradiolarien der Tiefsee. Wiss Ergeb Dtsch Tiefsee‐Exped Auf Dem Dampfer Valdivia 1898‐1899 14: 1–476. [Google Scholar]
- Ishitani, Y. , Ujiié, Y. , de Vargas, C. , Not, F. , and Takahashi, K. (2012) Two distinct lineages in the radiolarian order Spumellaria having different ecological preferences. Deep Sea Res Part II Top Stud Oceanogr 61–64: 172–178. [Google Scholar]
- Khmeleva, N. (1967) Role of radiolarians in the estimation of the primary production in the Red Sea and the Gulf of Aden. Dokl Akad Nauk SSSR 172: 1430–1433. [Google Scholar]
- Kunitomo, Y. , Sarashina, I. , Iijima, M. , Endo, K. , and Sashida, K. (2006) Molecular phylogeny of acantharian and polycystine radiolarians based on ribosomal DNA sequences, and some comparisons with data from the fossil record. Eur J Protistol 42: 143–153. [DOI] [PubMed] [Google Scholar]
- Lampitt, R.S. , Salter, I. , and Johns, D. (2009) Radiolaria: major exporters of organic carbon to the deep ocean. Global Biogeochem Cycles 23: GB1010. [Google Scholar]
- Lazarus, D. , Suzuki, N. , Ishitani, Y. , and Takahashi, K. (2021) Paleobiology of the Polycystine Radiolaria, 1st ed: Hoboken, NJ: Wiley‐Blackwell. [Google Scholar]
- Li, L. , and Endo, K. (2020) Phylogenetic positions of “pico‐sized” radiolarians from middle layer waters of the tropical Pacific. Prog Earth Planet Sci 7: 70. [Google Scholar]
- Mars Brisbin, M. , Brunner, O.D. , Grossmann, M.M. , and Mitarai, S. (2020) Paired high‐throughput, in situ imaging and high‐throughput sequencing illuminate acantharian abundance and vertical distribution. Limnol Oceanogr 65: 2953–2965. [Google Scholar]
- Matsuoka, A. (1992) Skeletal growth of a spongiose radiolarian Dictyocoryne truncatum in laboratory culture. Mar Micropaleontol 19: 287–297. [Google Scholar]
- Matsuoka, A. (2007) Living radiolarian feeding mechanisms: new light on past marine ecosystems. Swiss J Geosci 100: 273–279. [Google Scholar]
- Michaels, A.F. (1988) Vertical distribution and abundance of Acantharia and their symbionts. Mar Biol 97: 559–569. [Google Scholar]
- Michaels, A.F. , Caron, D.A. , Swanberg, N.R. , Howse, F.A. , and Michaels, C.M. (1995) Planktonic sarcodines (Acantharia, Radiolaria, Foraminifera) in surface waters near Bermuda: abundance, biomass and vertical flux. J Plankton Res 17: 131–163. [Google Scholar]
- Müller, J. (1859) Über die Thalassicollen, Polycystinen und Acanthometren des Mittelmeeres. Abh K Akad Wiss Zu Berl: 1–62. [Google Scholar]
- Nakamura, Y. , Sandin, M.M. , Suzuki, N. , Tuji, A. , and Not, F. (2020) Phylogenetic revision of the order Entactinaria—Paleozoic relict Radiolaria (Rhizaria, SAR). Protist 171: 125712. [DOI] [PubMed] [Google Scholar]
- Nakamura, Y. , Tuji, A. , Kimoto, K. , Yamaguchi, A. , Hori, R.S. , and Suzuki, N. (2021) Ecology, morphology, phylogeny and taxonomic revision of giant radiolarians, Orodaria ord. nov. (Radiolaria; Rhizaria; SAR). Protist 172: 125808. [DOI] [PubMed] [Google Scholar]
- Not, F. , Gausling, R. , Azam, F. , Heidelberg, J.F. , and Worden, A.Z. (2007) Vertical distribution of picoeukaryotic diversity in the Sargasso Sea. Environ Microbiol 9: 1233–1252. [DOI] [PubMed] [Google Scholar]
- O'Rorke, R. , Lavery, S. , Chow, S. , Takeyama, H. , Tsai, P. , Beckley, L.E. , et al. (2012) Determining the diet of larvae of Western rock lobster (Panulirus cygnus) using high‐throughput DNA sequencing techniques. PLoS One 7: e42757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernice, M.C. , Giner, C.R. , Logares, R. , Perera‐Bel, J. , Acinas, S.G. , Duarte, C.M. , et al. (2016) Large variability of bathypelagic microbial eukaryotic communities across the world's oceans. ISME J 10: 945–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polet, S. , Berney, C. , Fahrni, J. , and Pawlowski, J. (2004) Small‐subunit ribosomal RNA gene sequences of Phaeodarea challenge the monophyly of Haeckel's Radiolaria. Protist 155: 53–63. [DOI] [PubMed] [Google Scholar]
- Probert, I. , Siano, R. , Poirier, C. , Decelle, J. , Biard, T. , Tuji, A. , et al. (2014) Brandtodinium gen. nov. and B. nutricula comb. nov. (Dinophyceae), a dinoflagellate commonly found in symbiosis with polycystine radiolarians. J Phycol 50: 388–399. [DOI] [PubMed] [Google Scholar]
- Reshetnyak, V. (1955) Vertical distribution of radiolarians in the Kuril‐Kamchatka trench. Tr Zool Inta Akad Nauk SSSR 21: 94–101. [Google Scholar]
- Richards, R.J. (2009) The Tragic Sense of Life: Ernst Haeckel and the Struggle over Evolutionary Thought. Chicago, IL: University of Chicago Press. [Google Scholar]
- Sandin, M.M. (2019) Diversity and Evolution of Nassellaria and Spumellaria (Radiolaria). Paris, France: Sorbonne Université. [Google Scholar]
- Sandin, M.M. , Biard, T. , Romac, S. , O'Dogherty, L. , Suzuki, N. , and Not, F. (2021) A morpho‐molecular perspective on the diversity and evolution of Spumellaria (Radiolaria). Protist 172: 125806. [DOI] [PubMed] [Google Scholar]
- Sandin, M.M. , Pillet, L. , Biard, T. , Poirier, C. , Bigeard, E. , Romac, S. , et al. (2019) Time calibrated morpho‐molecular classification of Nassellaria (Radiolaria). Protist 170: 187–208. [DOI] [PubMed] [Google Scholar]
- Schewiakoff, W.T. (1926) The Acantharia. Fauna E Flora Golfo Napoli 37: 1–755. [Google Scholar]
- Stoecker, D. , Gustafson, D. , and Verity, P. (1996) Micro‐ and mesoprotozooplankton at 140*W in the equatorial Pacific: heterotrophs and mixotrophs. Aquat Microb Ecol 10: 273–282. [Google Scholar]
- Sugiyama, K. , and Anderson, O.R. (1998) The fine structures of some living Spyrida (Nassellaria, Radiolaria) and their implications for nassellarian classification. Paleontol Res 2: 75–88. [Google Scholar]
- Sugiyama, K. , Hori, R.S. , Kusunoki, Y. , and Matsuoka, A. (2008) Pseudopodial features and feeding behavior of living nassellarians Eucyrtidium hexagonatum Haeckel, Pterocorys zancleus (Müler) and Dictyocodon prometheus Haeckel. Paleontol Res 12: 209–222. [Google Scholar]
- Suzuki, N. , and Not, F. (2015) Biology and ecology of Radiolaria. In Marine Protists, Ohtsuka, S. , Suzaki, T. , Horiguchi, T. , Suzuki, N. , and Not, F. (eds). Japan: Springer, pp. 179–222. [Google Scholar]
- Suzuki, N. , O'Dogherty, L. , Caulet, J.‐P. , and Dumitrica, P. (2021) A new integrated morpho‐ and molecular systematic classification of Cenozoic radiolarians (class Polycystinea) – suprageneric taxonomy and logical nomenclatorial acts. Geodiversitas 43(15): 405–573. [Google Scholar]
- Suzuki, N. , and Oba, M. (2015) Oldest fossil records of marine protists and the geologic history toward the establishment of the modern‐type marine protist world. In Marine Protists, Ohtsuka, S. , Suzaki, T. , Horiguchi, T. , Suzuki, N. , and Not, F. (eds). Japan: Springer, pp. 359–394. [Google Scholar]
- Suzuki, N. , and Sugiyama, K. (2001) Regular axopodial activity of Diplosphaera hexagonalis Haeckel (spheroidal spumellarian, Radiolaria). Paleontol Res 5: 131–140. [Google Scholar]
- Swanberg, N.R. , and Anderson, O.R. (1981) Collozoum caudatum sp. nov.: a giant colonial radiolarian from equatorial and Gulf Stream waters. Deep Sea Res Part Oceanogr Res Pap 28: 1033–1047. [Google Scholar]
- Takahashi, K. (1987) Radiolarian flux and seasonality: climatic and El Nino response in the subarctic Pacific, 1982–1984. Global Biogeochem Cycles 1: 213–231. [Google Scholar]
- Uwizeye, C. , Mars Brisbin, M. , Gallet, B. , Chevalier, F. , LeKieffre, C. , Schieber, N.L. , et al. (2021) Cytoklepty in the plankton: a host strategy to optimize the bioenergetic machinery of endosymbiotic algae. Proc Natl Acad Sci U S A 118: e2025252118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, p.J.L.B. , Evans, D.W. , Roberts, D.J. , and Thomas, D.N. (2015) Ernst Haeckel: Art Forms from the Abyss: Images from the HMS Challenger Expedition: Prestel. [Google Scholar]
- Yuasa, T. , Horiguchi, T. , Mayama, S. , Matsuoka, A. , and Takahashi, O. (2012) Ultrastructural and molecular characterization of cyanobacterial symbionts in Dictyocoryne profunda (polycystine radiolaria). Symbiosis 57: 51–55. [Google Scholar]
- Yuasa, T. , Horiguchi, T. , Mayama, S. , and Takahashi, O. (2016) Gymnoxanthella radiolariae gen. et sp. nov. (Dinophyceae), a dinoflagellate symbiont from solitary polycystine radiolarians. J Phycol 52: 89–104. [DOI] [PubMed] [Google Scholar]
- Yuasa, T. , Kawachi, M. , Horiguchi, T. , and Takahashi, O. (2019) Chrysochromulina andersonii sp. nov. (Prymnesiophyceae), a new flagellate haptophyte symbiotic with radiolarians. Phycologia 58: 1–14. [Google Scholar]
- Yuasa, T. , Takahashi, O. , Honda, D. , and Mayama, S. (2005) Phylogenetic analyses of the polycystine Radiolaria based on the 18s rDNA sequences of the Spumellarida and the Nassellarida. Eur J Protistol 41: 287–298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Information.


