Abstract
The SARS-CoV-2 variant of concern, α, spread worldwide at the beginning of 2021. It was suggested that this variant was associated with a higher risk of mortality than other variants. We aimed to characterize the genetic diversity of SARS-CoV-2 variants isolated from patients with severe COVID-19 and unravel the relationships between specific viral mutations/mutational patterns and clinical outcomes. This is a prospective multicenter observational cohort study. Patients aged ≥18 years admitted to 11 intensive care units (ICUs) in hospitals in the Greater Paris area for SARS-CoV-2 infection and acute respiratory failure between 1 October 2020 and 30 May 2021 were included. The primary clinical endpoint was day-28 mortality. Full-length SARS-CoV-2 genomes were sequenced by means of next-generation sequencing (Illumina COVIDSeq). In total, 413 patients were included, 183 (44.3%) were infected with pre-existing variants, 197 (47.7%) were infected with variant α, and 33 (8.0%) were infected with other variants. The patients infected with pre-existing variants were significantly older (64.9 ± 11.9 vs. 60.5 ± 11.8 years; p = 0.0005) and had more frequent COPD (11.5% vs. 4.1%; p = 0.009) and higher SOFA scores (4 [3–8] vs. 3 [2–4]; 0.0002). The day-28 mortality was no different between the patients infected with pre-existing, α, or other variants (31.1% vs. 26.2% vs. 30.3%; p = 0.550). There was no association between day-28 mortality and specific variants or the presence of specific mutations. At ICU admission, the patients infected with pre-existing variants had a different clinical presentation from those infected with variant α, but mortality did not differ between these groups. There was no association between specific variants or SARS-CoV-2 genome mutational pattern and day-28 mortality.
Keywords: COVID-19, SARS-CoV-2, variant of concern, acute respiratory failure, intensive care unit
1. Introduction
As SARS-CoV-2 evolves and new variants continuously emerge worldwide, the sustained monitoring and rapid assessment of genetic changes are required to inform its public health response and health-care management. Since the fall of 2020, the emergence of so-called SARS-CoV-2 “variants of concern” (VOC) and of several “variants of interest” (VOI) appears to have resulted from the adaptation of the virus, combined with strong selective pressures on viral spread in the context of ongoing collective immunization [1]. Many of the substitutions that characterize emerging variants (VOC and VOI) are located in the Spike (S) protein, the protein involved in viral attachment and entry into cells and the target of neutralizing antibodies.
SARS-CoV-2 VOC bearing multiple signature (lineage-defining) deletions and amino-acid substitutions, such as N501Y and/or E484K (variants α (B.1.1.7), β (B.1.351) and γ (P.1)) spread worldwide, including in France, from early 2021 onwards. These changes have been suggested to result in increased infectivity, with 50–74% increased transmissibility for VOC α (B.1.1.7). More recently, variant δ (B.1.617.2) carrying L452R, T478K, and P681R substitutions, with enhanced transmissibility compared to other VOC, has spread and replaced most of the circulating variants in many parts of the world. Beyond transmissibility and immune evasion, the viral factors that explain the severity of infection and the corresponding case-fatality rate in some patients remain unclear. Mutations located outside of the Spike gene in the SARS-CoV-2 genome have been suggested to contribute to enhanced virulence, but these results have not been confirmed in other studies [2,3,4]. Similarly, the specific role of each VOC in the severity of the disease is debated. In a preclinical Syrian golden hamster model, the animals infected with variant α (B.1.1.7) produced significantly higher levels of proinflammatory cytokines than those infected with other variants, but there was no evidence for an altered phenotype [5]. In clinical settings, preliminary analyses of the prognosis in patients infected with variant α (B.1.1.7) have suggested that this variant could be associated with a higher risk of hospital and ICU admission [6,7] and mortality [8,9] compared with infection with other variants. By contrast, other studies found no association between mortality and infection with variant α (B.1.1.7) in patients admitted to hospital [10]. Very few clinical studies have evaluated whether infection with VOC and VOI would modify the prognosis in the subset of patients with severe disease admitted to intensive care units (ICUs) [6].
Most of the prognostic studies describing the role of VOC compared to pre-existing variants are based on PCR-based screening tests, such as S-gene molecular diagnostic assay failure (SGTF) for variant α (B.1.1.7) [6,8,11], which did not discriminate between different variants sharing the same pattern of mutations and could not evaluate the association of mutational hotspots with clinical outcomes. Genomic surveillance by means of full-length viral-genome sequencing has become critical to identifying circulating variants and the evolution of SARS-CoV-2 genomes over time. Different variants, including VOC and VOI, circulate at the same time, and their effects on disease severity and on the prognosis of severe forms remain unknown. At a mutational level, the presence of a single substitution (e.g., E484K) or a panel of mutations/deletions could specifically modulate immune response, with a potential impact on the clinical course of the disease [12].
In this study, we aimed to characterize the genetic diversity of the SARS-CoV-2 variants involved in severe COVID-19 cases, in a population of patients hospitalized in ICUs for acute respiratory failure following severe SARS-CoV-2 infection between October 2020 and May 2021, i.e., before the emergence and spread of variant δ (B.617.2) in France. We further aimed to unravel the relationships between specific viral mutations/mutational patterns and the clinical outcomes of COVID-19 in these patients.
2. Materials and Methods
2.1. Study Design and Patients
This is a prospective multicenter observational cohort study. Patients admitted between 1 October 2020 (week 40/20) and 30 May 2021 (week 21/21) to one of the 11 participating ICUs from the hospitals in the Greater Paris area and included in the ANTICOV study (NCT04733105) were eligible for study inclusion. Inclusion criteria were as follows: age ≥18 years, SARS-CoV-2 infection confirmed by a positive reverse-transcriptase–polymerase-chain-reaction (RT–PCR), patient admitted to the ICU for acute respiratory failure (SpO2 ≤ 90% and need for supplemental oxygen or any kind of ventilator support), and patient or next of kin informed of study inclusion. Patients with SARS-CoV-2 infection but no acute respiratory failure or with a PCR cycle threshold (Ct) > 32 in nasopharyngeal samples were not included in the study. The study was approved by the Comité de Protection des Personnes Nord-Ouest IV (N° EudraCT/ID-RCB: 2020-A03009-30). Informed consent was obtained from all patients or their relatives.
Demographics and clinical and laboratory variables were recorded upon ICU admission and during ICU stay. Patients’ frailty was assessed using the Clinical Frailty Scale [13]. The severity of the disease upon ICU admission was assessed using the World Health Organization (WHO)’s 10-point ordinal scale [14], the sequential organ failure assessment (SOFA [15]) score, and the simplified acute physiology (SAPS) II score [16]. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition [17]. The primary clinical endpoint of the study was day-28 mortality.
SARS-CoV-2 Genome Sequence Analysis
The full-length SARS-CoV-2 genomes from all included patients were sequenced by means of next-generation sequencing. Briefly, viral RNA was extracted from nasopharyngeal swabs in viral transport medium using NucliSENS® easyMAG kit on EMAG device (bioMérieux, Marcy-l’Étoile, France). Sequencing was performed with the Illumina COVIDSeq Test (Illumina, San Diego, CA, USA), which uses 98-target multiplex amplifications along the full SARS-CoV-2 genome. The libraries were sequenced with NextSeq 500/550 High-Output Kit v2.5 (75 Cycles) on a NextSeq 500 device (Illumina). The sequences were demultiplexed and assembled as full-length genomes by means of the DRAGEN COVIDSeq Test Pipeline on a local DRAGEN server (Illumina). Lineages and clades were interpreted using Pangolin and NextClade, before being submitted to the GISAID database (https://www.gisaid.org; accessed on 1 June 2021; the GISAID IDs of the sequenced sampled are listed in the Supplementary Materials). Phylogeny was performed after full-length genome alignment with Muscle v3.8.31 (maximum-likelihood model GTR + I; 1000 bootstrap replicates), by means of IQ-Tree v1.3.11.1 and iTOL.
2.2. Statistical Analysis
Descriptive results are presented as means (±standard deviation [SD]) or medians (1st–3rd tertials) for continuous variables, and as numbers with percentages for categorical variables. Two-tailed p-values < 0.05 were considered statistically significant. Unadjusted comparisons between the main variants were performed using ANOVA or Kruskal–Wallis tests for global comparisons of continuous variables, and Chi-squared or Fisher’s exact tests for categorical variables, as appropriate. In case of global significance, post hoc pairwise comparisons were performed using t-tests or Mann–Whitney tests for continuous variables, and Chi-squared or Fisher’s exact tests for categorical variables, as appropriate, applying a Sidak correction to account for multiple testing. Adjusted analyses of the association between variants or mutations and 28- and 90-day mortality relied on logistic regression models, systematically adjusting for age, SOFA score at admission, gender and dexamethasone treatment, computing adjusted odds ratios (ORs) along with their 95% confidence intervals. Analyses were performed using Stata V16.0 statistical software (StataCorp, College Station, TX, USA), and R 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
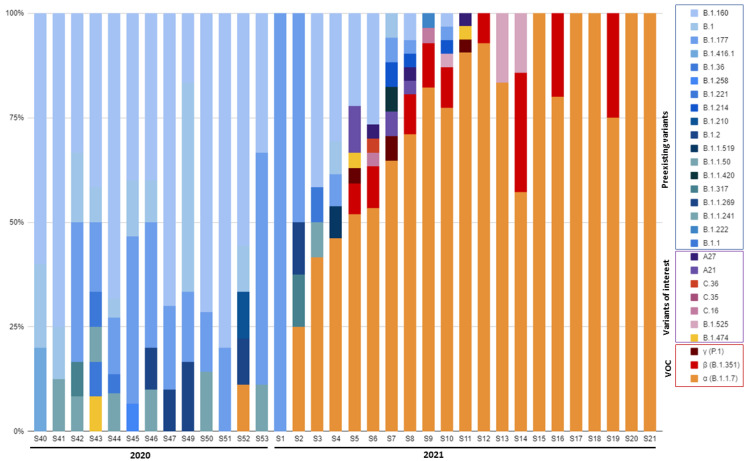
Between 1 October 2020 and 30 May 2021, 845 patients were admitted to 11 participating ICUs. Of these patients, 737 had at least one positive SARS-CoV-2 RNA RT–PCR, according to a nasopharyngeal swab sample in the hospital; 413 patients with a Ct ≤ 32 and a nasopharyngeal sample maintained at −80 °C that could be used for full-length viral genome sequence analysis were included in this study. Among these 413 patients, 183 (44.3%) were infected with so-called “pre-existing” variants, i.e., variants circulating before the emergence of variant α, 197 (47.7%) were infected with variant α (B.1.1.7), and 33 (8.0%) were infected with other variants, including β (B.1.351) (n = 19, 4.6%), γ (P.1) (n = 2, 0.5%), and other variants of interest (n = 12, 2.9%). Figure 1 illustrates the time course of the emerging SARS-CoV-2 variants during the study period (see Supplementary Table S1 for the detailed number of collected samples per week). Variant α (B.1.1.7) was first detected during the last week of 2020 in 11.1% of the patients, became predominant during the fourth week of January 2021 (51.9% of the patients) and remained so until the last week of May (100% of patients), which corresponded to the end of the inclusion period.
Figure 1.
Time course of emerging SARS-CoV-2 variants during the study period. VOC, variants of concern.
3.1. SARS-CoV-2 Variant Association with Clinical Phenotypes at ICU Admission
The patients infected with pre-existing variants were older and had significantly more frequent chronic kidney disease and COPD than those infected with other variants (Table 1). Other comorbidities, including diabetes, peripheral vascular disease, solid cancer, and hypertension followed the same trend, although the differences were not statistically different.
Table 1.
Characteristics of patients with severe SARS-CoV-2 infection (n = 413) at the time of intensive-care-unit admission, according to SARS-CoV-2 variants.
| Pre-Existing Variants | Alpha (B.1.1.7) |
Other Variants |
p-Values | ||||
|---|---|---|---|---|---|---|---|
| N = 183 | N = 197 | N = 33 | Global Comparison | Pre-Existing vs. Alpha a | Pre-Existing vs. Others a | Alpha vs. Others a | |
| Demographics and comorbidities | |||||||
| Gender, male | 124 (67.8%) | 140 (71.1%) | 18 (54.5%) | 0.165 | - | - | - |
| Age, years | 65.0 (±11.9) | 60.5 (±11.8) | 62.2 (±10.7) | 0.001 | 0.001 | 0.531 | 0.823 |
| Diabetes | 67 (36.6%) | 59 (29.9%) | 7 (21.2%) | 0.141 | - | - | - |
| Hypertension | 108 (59.0%) | 98 (49.7%) | 16 (48.5%) | 0.159 | - | - | - |
| Peripheral vascular disease | 28 (15.3%) | 19 (9.6%) | 1 (3.0%) | 0.075 | - | - | - |
| Chronic heart failure | 19 (10.4%) | 20 (10.2%) | 2 (6.1%) | 0.851 | - | - | - |
| Chronic kidney disease | 34 (18.6%) | 23 (11.7%) | 1 (3.0%) | 0.025 | 0.178 | 0.063 | 0.517 |
| Cirrhosis | 3 (1.6%) | 4 (2.0%) | 0 (0.0%) | 1.000 | - | - | - |
| Cancer | 15 (8.2%) | 8 (4.1%) | 1 (3.0%) | 0.180 | - | - | - |
| HIV infection | 1 (0.5%) | 3 (1.5%) | 1 (3.0%) | 0.274 | - | - | - |
| Corticosteroids | 14 (7.7%) | 9 (4.6%) | 1 (3.0%) | 0.445 | - | - | - |
| COPD | 21 (11.5%) | 8 (4.1%) | 2 (6.1%) | 0.021 | 0.021 | 0.904 | 0.953 |
| Tobacco | 26 (14.2%) | 23 (11.7%) | 4 (12.1%) | 0.769 | - | - | - |
| BMI, kg/m2 | 30.2 (±6.8) | 29.8 (±6.6) | 30.2 (±6.0) | 0.857 | - | - | - |
| Clinical frailty scale | 3.0 (2.0;4.0) | 3.0 (2.0;4.0) | 3.0 (2.0;3.0) | 0.069 | - | - | - |
| Disease severity upon ICU admission and biological features | |||||||
| WHO 10-point scale | 6 (6;8) | 6 (6;8) | 6 (6;8) | 0.114 | - | - | - |
| SAPS II score | 37 (30;50) | 30 (24;40) | 32 (24;41) | 0.000 | 0.000 | 0.025 | 0.971 |
| SOFA score | 4 (3;8) | 3 (2;4) | 4 (2;4) | 0.000 | 0.000 | 0.156 | 0.935 |
| Blood neutrophils, G/L | 7.9 (5.3;11.3) | 6.3 (4.6;9.1) | 8.7 (5.8;12.2) | 0.006 | 0.017 | 0.737 | 0.061 |
| Blood lymphocytes, G/L | 0.6 (0.4;0.9) | 0.7 (0.4;0.8) | 0.8 (0.6;1.0) | 0.217 | - | - | - |
| Blood urea level, mM | 8.4 (6.0;12.5) | 6.5 (4.9;10.2) | 6.3 (5.5;8.7) | 0.000 | 0.000 | 0.038 | 0.999 |
| D-dimers, ng/mL | 1441 (771;2614) | 1310 (931;2183) | 1300 (799;2030) | 0.917 | - | - | - |
| Bacterial coinfection | 25 (13.7%) | 22 (11.2%) | 2 (6.1%) | 0.494 | - | - | - |
| Organ support and management during the first 24 h b | |||||||
| Oxygen | 5 (2.7%) | 6 (3.0%) | 4 (12.1%) | 0.045 | 1.000 | 0.095 | 0.114 |
| High-flow oxygen | 105 (57.4%) | 140 (71.1%) | 23 (69.7%) | 0.017 | 0.016 | 0.458 | 0.998 |
| NIV/C-PAP | 55 (30.1%) | 51 (25.9%) | 12 (36.4%) | 0.392 | - | - | - |
| Invasive MV | 88 (48.1%) | 78 (39.6%) | 12 (36.4%) | 0.178 | - | - | - |
| Prone position | 79 (43.2%) | 92 (46.7%) | 15 (45.5%) | 0.786 | - | - | - |
| ECMO | 24 (13.1%) | 9 (4.6%) | 3 (9.1%) | 0.010 | 0.010 | 0.989 | 0.770 |
| ARDS criteria | 147 (80.3%) | 136 (69.0%) | 21 (63.6%) | 0.018 | 0.035 | 0.098 | 0.901 |
| Vasopressors | 35 (19.1%) | 34 (17.3%) | 4 (12.1%) | 0.654 | - | - | - |
| Antibiotics | 123 (67.2%) | 115 (58.4%) | 19 (57.6%) | 0.175 | - | - | - |
a Pairwise comparisons applying a Sidak correction to account for multiple testing; b more than one type of respiratory support may have been used per patient during the first 24 h; thus the total may be more than 100%. COPD: chronic obstructive pulmonary disease; BMI: body mass index; SAPS: simplified acute physiology score; SOFA: sequential organ failure assessment; NIV: non-invasive ventilation; C-PAP: continuous positive airway pressure; MV: mechanical ventilation; ECMO: extracorporeal membrane oxygenation; ARDS: acute respiratory distress syndrome.
There were also marked differences between the variant groups regarding the severity of disease at ICU admission. Indeed, the patients infected with preexisting variants exhibited significantly higher severity-of-illness scores, with not only more severe respiratory disease, as assessed by the respiratory component of the SOFA score, but also more frequent extra-pulmonary organ failure compared with the patients infected with variant α (B.1.1.7) and other emerging variants (Table 1 and Figure 2). As a result, the patients infected with the pre-existing variants received less frequent high-flow oxygen therapy and more frequent extracorporeal membrane oxygenation (ECMO) support than the other patients within the first 24 h of ICU admission, while they met the ARDS-definition criteria more frequently.
Figure 2.
SOFA score and its organ system components in patients with pre-existing, α (B.1.1.7), or other variants. The p values are from ANOVA.
3.2. Relationship between SARS-CoV-2 Variants and Patient Outcomes
Although the patients infected with pre-existing variants were older, had more comorbidities, and had more severe disease at ICU admission, they did not show different outcomes from those infected with the other variants. Overall, the need for invasive mechanical ventilation or ECMO support did not significantly differ between the different variant groups, nor did the duration of these supports. There were also no significant differences between groups regarding extra-pulmonary organ support (i.e., vasopressors and renal replacement therapy) during ICU stay (Table 2). The patients with pre-existing variants received less dexamethasone and tocilizumab than the others, likely reflecting the changes in practice during the study period. As shown in Table 2, the day-28 and day-90 mortality rates were not statistically different between the groups.
Table 2.
Intensive-care management and outcomes of patients with severe SARS-CoV-2 infection (n = 413) during intensive-care-unit stay, according to SARS-CoV-2 variants.
| Preexisting Variants | Alpha (B.1.1.7) |
Other Variants |
p-Values | ||||
|---|---|---|---|---|---|---|---|
| N = 183 | N = 197 | N = 33 | Global Comparison | Preexisting vs. Alpha a | Preexisting vs. Others a | Alpha vs. Others a | |
| Invasive MV | 125 (68.3%) | 120 (60.9%) | 22 (66.7%) | 0.312 | - | - | - |
| MV duration, days | 16 (9;27) | 14 (8;23) | 16.5 (10;30) | 0.362 | - | - | - |
| ECMO support | 29 (15.8%) | 19 (9.6%) | 6 (18.2%) | 0.133 | - | - | - |
| Duration of ECMO, days | 17.0 (6.0;31.0) | 12.0 (4.0;17.0) | 34.5 (8.0;55.0) | 0.186 | - | - | - |
| Vasopressor support | 87 (47.5%) | 94 (47.7%) | 17 (51.5%) | 0.912 | - | - | - |
| Duration of vasopressors, days | 7 (3;15) | 9 (4;16) | 9 (3;14) | 0.862 | - | - | - |
| RRT | 47 (25.7%) | 53 (26.9%) | 7 (21.2%) | 0.784 | - | - | - |
| Pulmonary thrombosis | 10 (5.5%) | 11 (5.6%) | 1 (3.0%) | 1.000 | - | - | - |
| VAP | 88 (48.1%) | 85 (43.4%) | 15 (45.5%) | 0.654 | - | - | - |
| Dexamethasone | 143 (78.1%) | 168 (85.3%) | 31 (93.9%) | 0.038 | 0.199 | 0.101 | 0.444 |
| Tocilizumab | 6 (3.3%) | 25 (12.7%) | 4 (12.1%) | 0.002 | 0.003 | 0.139 | 1.000 |
| Day-28 mortality | 57 (31.1%) | 51 (26.2%) | 10 (30.3%) | 0.550 | - | - | - |
| Day-90 mortality | 74 (40.4%) | 61 (31.6%) | 13 (39.4%) | 0.189 | - | - | - |
a Pairwise comparisons applying a Sidak correction to account for multiple testing. MV: mechanical ventilation; ECMO: extracorporeal membrane oxygenation; RRT: renal replacement therapy; VAP: ventilator-acquired pneumonia.
3.3. Relationship between SARS-CoV-2 Spike Mutations and Mortality
Pre-existing and emerging variants (VOC or VOI) are characterized by multiple lineage-specific deletions and amino-acid substitutions. Spike mutations undergo evolutive convergence at several signature sites. The spike mutations at sites identified as undergoing convergent mutational evolution were selected a priori. There was no significant relationship between any of these mutations (n = 17) and day-28 mortality in univariate analysis (Figure 3). The selected mutations were also included in multivariate logistic regression models to determine their relationship with day-28 (Table 3) and day-90 (Supplementary Table S1) mortality, adjusting for age, gender, SOFA score at ICU admission, and dexamethasone treatment. None of them were significantly associated with mortality at either time point. The variant status was not associated with mortality either.
Figure 3.
Frequency of preselected SARS-CoV-2 mutations in spike according to day-28 mortality status. There was no statistical difference in any of the comparisons performed (gray bars indicate survivors, black bars indicate deceased).
Table 3.
Association between SARS-CoV-2 variants, relevant mutations ((substitutions and deletions (Del)) in spike selected a priori and day-28 mortality by multivariable logistic regression analysis.
| All Patients | Day-28 Survivors | Day-28 Non-Survivors |
aOR a (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|
| N = 411 | N = 293 | N = 118 | ||||
| Variants | Alpha | 195 (47.4%) | 144 (49.1%) | 51 (43.2%) | 1 (ref) | 0.347 |
| Pre-existing variant | 183 (44.5%) | 126 (43.0%) | 57 (48.3%) | 0.75 (0.45;1.25) | 0.263 | |
| Others | 33 (8.0%) | 23 (7.8%) | 10 (8.5%) | 1.31 (0.55;3.13) | 0.549 | |
| N501Y | 220 (53.5%) | 162 (55.3%) | 58 (49.2%) | 1.29 (0.80;2.11) | 0.299 | |
| N501Y x Alpha b | None | 191 (46.5%) | 131 (44.7%) | 60 (50.8%) | 1 (ref) | 0.565 |
| N501Y | 25 (6.1%) | 18 (6.1%) | 7 (5.9%) | 1.45 (0.53;3.97) | 0.473 | |
| N501Y and Alpha | 195 (47.4%) | 144 (49.1%) | 51 (43.2%) | 1.28 (0.77;2.11) | 0.342 | |
| Del 69–70 | 193 (47.0%) | 143 (48.8%) | 50 (42.4%) | 1.22 (0.75;1.98) | 0.426 | |
| Del 140–145 | 195 (47.4%) | 145 (49.5%) | 50 (42.4%) | 1.20 (0.74;1.95) | 0.468 | |
| Del 242–244 | 20 (4.9%) | 15 (5.1%) | 5 (4.2%) | 1.34 (0.44;4.09) | 0.607 | |
| L5F | 16 (3.9%) | 11 (3.8%) | 5 (4.2%) | 1.02 (0.32;3.27) | 0.978 | |
| L18F | 17 (4.1%) | 13 (4.4%) | 4 (3.4%) | 1.19 (0.34;4.10) | 0.788 | |
| D80A/G | 20 (4.9%) | 15 (5.1%) | 5 (4.2%) | 1.21 (0.40;3.66) | 0.733 | |
| S98F | 13 (3.2%) | 10 (3.4%) | 3 (2.5%) | 0.76 (0.18;3.17) | 0.703 | |
| K417N/T | 22 (5.4%) | 17 (5.8%) | 5 (4.2%) | 1.02 (0.34;3.02) | 0.976 | |
| L452R | 8 (1.9%) | 5 (1.7%) | 3 (2.5%) | 1.57 (0.33;7.41) | 0.569 | |
| S477N | 76 (18.5%) | 49 (16.7%) | 27 (22.9%) | 0.79 (0.43;1.45) | 0.447 | |
| E484K | 25 (6.1%) | 19 (6.5%) | 6 (5.1%) | 1.06 (0.38;2.94) | 0.908 | |
| A570D | 192 (46.7%) | 142 (48.5%) | 50 (42.4%) | 1.23 (0.76;2.00) | 0.406 | |
| D614G | 400 (97.3%) | 286 (97.6%) | 114 (96.6%) | 0.65 (0.16;2.68) | 0.550 | |
| H655Y | 6 (1.5%) | 4 (1.4%) | 2 (1.7%) | 1.07 (0.17;6.59) | 0.946 | |
| P681H | 190 (46.2%) | 140 (47.8%) | 50 (42.4%) | 1.29 (0.79;2.09) | 0.310 | |
| P681R | 9 (2.2%) | 5 (1.7%) | 4 (3.4%) | 2.72 (0.61;12.05) | 0.189 | |
a Multivariable analysis adjusted for age, SOFA score at admission, gender, and dexamethasone treatment; b This variable is from the interaction of the variable «alpha variant » and the variable « N501Y mutation»; aOR: adjusted odds ratio; 95% CI: 95% confidence interval.
3.4. Relationship between SARS-CoV-2 Gene-Mutation Hotspots and Mortality
Overall, 1017 non-synonymous mutations (including 953 amino acid substitutions, 52 deletions, and 11 insertions) were detected in full-length viral genomes in at least one variant. There were no significant relationships between any of these mutations and day-28 mortality in the univariate analysis (Table 3). Although 11 mutations were found to be associated with mortality (p < 0.05), the number of patients harboring these mutations was considered too small (range: 2–6) to derive conclusions.
We then focused on the mutations previously reported to correlate with worse clinical outcomes [2,4,18]. We found that Q57H in Orf3a (but not other mutations, including P25L in Orf3a and S194L, R203K, or G204R in N) was highly prevalent in our cohort (31.8%). Q57H was more prevalent in patients who were dead at day 28 (35.6%) than in those who were still alive, but the difference did not reach significance (29.0%; p = 0.196). A phylogenetic tree (Supplementary Figure S1) shows that the Q57H mutation is mainly harbored by variants from the lineages B.1.160 and B.1.351.
4. Discussion
The full-length SARS-CoV-2 genomes of 413 critically ill COVID-19 patients from 11 ICUs, who were predominantly infected with pre-existing and α (B.1.1.7) variants, were sequenced, and the relationship between the viral sequences and their clinical presentation and outcomes was studied. The main results of this study are as follows. (i) Compared with the other patients, the patients infected with the α (B.1.1.7) variant showed a different clinical phenotype at ICU admission, characterized by younger age, fewer comorbidities, and less-severe clinical presentation. (ii) Despite these different initial clinical presentations, there were no significant differences in day-28 and day-90 mortality between the different SARS-CoV-2 variant groups. (iii) There was no statistically significant relationship between the presence of any of the 17 relevant spike substitutions and deletions selected a priori and mortality. (iv) A comprehensive full-length SARS-CoV-2 genome sequence analysis exploring all the mutations found failed to identify a relationship between the presence of any of them and day-28 mortality.
The patients infected with variants that emerged prior to variant α at the end of 2020 showed significant clinical differences, including older age and more comorbidities, when compared with the patients infected with other variants, particularly variant α (B.1.1.7), a result that was in keeping with those from previous cohorts [6,10,11]. Importantly, our results show differences in the clinical severity of the disease, resulting in different organ support requirements during the first 24 h of management in the ICU, in the patients infected with different SARS-CoV-2 variants. Indeed, the patients infected with pre-existing variants exhibited more severe pulmonary and non-pulmonary organ failure than those infected with variant α (B.1.1.7). Surprisingly, although the severity-of-illness scores on admission (i.e., SAPS II [16] and SOFA [15]) predicted higher mortality in the patients infected with the pre-existing variants, there was no significant difference in their mortality rates compared to the patients infected with variant α (B.1.1.7). This finding, together with the fact that the patients infected with variant α (B.1.1.7) had similar organ support requirements during their ICU stay (Table 2), indicates that the severity of the disease and the time course of organ failures were delayed in the patients infected with variant α compared to the patients infected with the pre-existing variants. Whether this different time course should be ascribed to host-related factors (e.g., age, comorbidities), a different pathogenesis/virulence of the variant, or differences in the type of patient management, is unclear. Previous large-scale data have suggested that patients infected with variant α (B.1.1.7), identified using the SGTF proxy had a higher risk of dying [6,8,11]. However, in a study by Frampton et al., which included 341 hospitalized patients, no association was found between the severity of the disease, death, and the viral lineage [10]. Interestingly, following SARS-CoV-2-infected patients with different disease severities through the pathway of disease management, Grint et al. reported a weakening association between variant-α (B.1.1.7) infection and mortality, which was highest in the primary-care population, lower in the hospitalized population, and non-significant in patients admitted in the ICU [11]. Indeed, other determinants of COVID-19 mortality, including male gender, age, and associated comorbidities, have been shown to have a major impact on outcomes [19]. Our results thus do not contradict those of previous studies showing an increased association of mortality with variant α (B.1.1.7) in the community [6,8]. The results are in keeping with data specific to ICU patient populations [6,11].
Spike mutations have dominated SARS-CoV-2 variant research due to concerns regarding the enhancement of viral replication and transmission via ACE-2 receptor binding and/or lower sensitivity to the action of naturally or vaccine-induced neutralizing antibodies. By contrast, little attention has been paid to variant-specific mutations in other viral proteins. Nevertheless, such mutations could be associated with different clinical outcomes. Previous large-scale studies based on the GISAID database and in vitro experiments suggested that amino-acid substitutions in the N and Orf3a regions could be associated with more severe disease. To study the potential link between SARS-CoV-2 genetic diversity outside of the spike protein and severe COVID-19-case outcomes, we analyzed all non-synonymous mutations in the full-length SARS-CoV-2 genomes and not only assessed the relationship between variant status and mortality, as already performed by others [6,8,10,11], but also performed a mutational analysis of all the viral genes. None of the relevant pre-selected spike substitutions/deletions were found to be associated with mortality, either in univariate or in multivariate analyses. When extending the analysis to all the viral genes, we found 11 mutations that were harbored by a very small number of patients (n ≤ 6) with a statistically significant difference, but without clinically relevant meaning. Together, our results indicate that in the patients with the most severe forms of COVID-19 who required ICU admission, there was no mutational pattern associated with mortality.
Limitations and Strengths of the Study
We acknowledge that our study has several limitations. The number of patients included was limited and, thus, the statistical power may have been too weak to show some between-group differences. However, there was no clear trend regarding associations between variant groups/mutations and mortality, suggesting that increasing the number of patients in the cohort would not have changed the results. Changes in management practices occurred during the study period, with more patients infected with variant α (B.1.1.7) and other variant groups receiving dexamethasone, with a possible impact on mortality [20]. This potential bias was accounted for in the multivariate analysis. We did not use a population-based database, precluding the study of relationships between the effects of the COVID-19 epidemic and risk of ICU death according to variant type. Additionally, the period of inclusion of the present study began on October 1, 2020, which corresponded in France to the beginning of the second COVID-19 wave, a period when ICU admission policies, ICU patient load and demand, and COVID-19 management strategies were more homogeneous among centers than during the first COVID-19 wave. We also did not record SARS-CoV-2 vaccination status as there was no anti-SARS-CoV-2 vaccine available at the time the study started. However, on May 1 2021, when the inclusion period ended, less than 10% of the French population had been fully vaccinated (https://covidtracker.fr/vaccintracker/; accessed on 1 June 2022), implying that the proportion of vaccinated patients in this cohort of critically ill patients was very low.
Our study also had strengths, including the constitution of a prospective multicenter cohort of well-phenotyped critically ill patients, and the fact that we performed full-length SARS-CoV-2 genome sequencing by means of up-to-date technology.
5. Conclusions
The patients with the pre-existing variants had a different clinical presentation from the patients infected with variant α (B.1.1.7) and other variants at ICU admission, characterized by older age, more comorbidities, and a more severe clinical presentation. However, there were no day-28 or day-90 mortality differences between the different groups. We found no association between the variant status or any mutational pattern in SARS-CoV-2 viral genes and mortality.
Acknowledgments
The authors wish to thank the patients involved in the study and all the nurses and physicians involved in patient care in the intensive care units.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14071529/s1. Table S1: Time course of emerging SARS-CoV-2 variants during the study period. Figure S1: Phylogenetic tree performed after full-length genome alignment.
Author Contributions
Concept and design: S.F., J.-M.P., and N.d.P.; acquisition, analysis, or interpretation of data: all authors; drafting of the manuscript: S.F. and N.d.P.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: E.A. (Etienne Audureau); acquisition of funding: N.d.P.; administrative, technical, and material support: S.F., C.R., and E.A. (Etienne Audureau), N.d.P.; supervision: S.F. and N.d.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Comité de Protection des Personnes Nord-Ouest IV (N° EudraCT/ID-RCB: 2020-A03009-30). Informed consent was obtained from all patients or their relatives.
Informed Consent Statement
Informed consent was obtained from all patients or their relatives.
Data Availability Statement
Original data are available on request from the corresponding author.
Conflicts of Interest
C.-E.L. has served as a consultant for Bayer Healthcare, Carmat, and Thermo Fisher Brahms, and received lecture fees from MSD, Aerogen, Advanzpharma, and BioMérieux, outside the submitted work. Other authors have no conflict of interest to disclose.
Funding Statement
This research received funding from the Agence Nationale de la Recherche (ANR-21-COVR-0022).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martin D.P., Weaver S., Tegally H., San J.E., Shank S.D., Wilkinson E., Lucaci A.G., Giandhari J., Naidoo S., Pillay Y., et al. The Emergence and Ongoing Convergent Evolution of the SARS-CoV-2 N501Y Lineages. Cell. 2021;184:5189–5200.e7. doi: 10.1016/j.cell.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majumdar P., Niyogi S. ORF3a Mutation Associated with Higher Mortality Rate in SARS-CoV-2 Infection. Epidemiol. Infect. 2020;148:e262. doi: 10.1017/S0950268820002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young B.E., Fong S.-W., Chan Y.-H., Mak T.-M., Ang L.W., Anderson D.E., Lee C.Y.-P., Amrun S.N., Lee B., Goh Y.S., et al. Effects of a Major Deletion in the SARS-CoV-2 Genome on the Severity of Infection and the Inflammatory Response: An Observational Cohort Study. Lancet. 2020;396:603–611. doi: 10.1016/S0140-6736(20)31757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagy Á., Pongor S., Győrffy B. Different Mutations in SARS-CoV-2 Associate with Severe and Mild Outcome. Int. J. Antimicrob. Agents. 2021;57:106272. doi: 10.1016/j.ijantimicag.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelnabi R., Boudewijns R., Foo C.S., Seldeslachts L., Sanchez-Felipe L., Zhang X., Delang L., Maes P., Kaptein S.J.F., Weynand B., et al. Comparing Infectivity and Virulence of Emerging SARS-CoV-2 Variants in Syrian Hamsters. eBioMedicine. 2021;68:103403. doi: 10.1016/j.ebiom.2021.103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patone M., Thomas K., Hatch R., Tan P.S., Coupland C., Liao W., Mouncey P., Harrison D., Rowan K., Horby P., et al. Mortality and Critical Care Unit Admission Associated with the SARS-CoV-2 Lineage B.1.1.7 in England: An Observational Cohort Study. Lancet Infect. Dis. 2021;21:1518–1528. doi: 10.1016/S1473-3099(21)00318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funk T., Pharris A., Spiteri G., Bundle N., Melidou A., Carr M., Gonzalez G., Garcia-Leon A., Crispie F., O’Connor L., et al. Characteristics of SARS-CoV-2 Variants of Concern B.1.1.7, B.1.351 or P.1: Data from Seven EU/EEA Countries, Weeks 38/2020 to 10/2021. Eurosurveillance. 2021;26:2100348. doi: 10.2807/1560-7917.ES.2021.26.16.2100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies N.G., Jarvis C.I., CMMID COVID-19 Working Group. Edmunds W.J., Jewell N.P., Diaz-Ordaz K., Keogh R.H. Increased Mortality in Community-Tested Cases of SARS-CoV-2 Lineage B.1.1.7. Nature. 2021;593:270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giles B., Meredith P., Robson S., Smith G., Chauhan A., PACIFIC-19 and COG-UK Research Groups The SARS-CoV-2 B.1.1.7 Variant and Increased Clinical Severity-the Jury Is Out. Lancet Infect. Dis. 2021;21:1213–1214. doi: 10.1016/S1473-3099(21)00356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frampton D., Rampling T., Cross A., Bailey H., Heaney J., Byott M., Scott R., Sconza R., Price J., Margaritis M., et al. Genomic Characteristics and Clinical Effect of the Emergent SARS-CoV-2 B.1.1.7 Lineage in London, UK: A Whole-Genome Sequencing and Hospital-Based Cohort Study. Lancet Infect. Dis. 2021;21:1246–1256. doi: 10.1016/S1473-3099(21)00170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grint D.J., Wing K., Houlihan C., Gibbs H.P., Evans S.J.W., Williamson E., McDonald H.I., Bhaskaran K., Evans D., Walker A.J., et al. Severity of SARS-CoV-2 Alpha Variant (B.1.1.7) in England. Clin. Infect. Dis. 2021:ciab754. doi: 10.1093/cid/ciab754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H.K., Knabl L., Knabl L., Wieser M., Mur A., Zabernigg A., Schumacher J., Kaiser N., Furth P.A., Hennighausen L. Immune Transcriptomes from Hospitalized Patients Infected with the SARS-CoV-2 Variants B.1.1.7 and B.1.1.7 Carrying the E484K Escape Mutation. medRxiv. 2021 doi: 10.1101/2021.05.27.21257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockwood K., Song X., MacKnight C., Bergman H., Hogan D.B., McDowell I., Mitnitski A. A Global Clinical Measure of Fitness and Frailty in Elderly People. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H., Reinhart C.K., Suter P.M., Thijs L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. On Behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Le Gall J.R., Lemeshow S., Saulnier F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 17.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 18.Pang X., Li P., Zhang L., Que L., Dong M., Xie B., Wang Q., Wei Y., Xie X., Li L., et al. Emerging Severe Acute Respiratory Syndrome Coronavirus 2 Mutation Hotspots Associated with Clinical Outcomes and Transmission. Front. Microbiol. 2021;12:753823. doi: 10.3389/fmicb.2021.753823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data are available on request from the corresponding author.