Abbreviations
- BL
baseline
- CM
chronic migraine
- DBP
double‐blind period
- EM
episodic migraine
- MMDs
monthly average number of migraine days
- SE
standard error
INTRODUCTION
Fremanezumab, a humanized monoclonal antibody (IgG2Δa) that selectively targets calcitonin gene‐related peptide, is approved in Europe, the United States, and many other countries for the prevention of migraine attacks in adults. 1 , 2 In some countries, prior inadequate response to multiple preventive medications, which may include onabotulinumtoxinA, is required by health authorities prior to initiation of fremanezumab in individuals with chronic migraine (CM). The randomized, double‐blind, placebo‐controlled FOCUS study (ClinicalTrials.gov identifier: NCT03308968) demonstrated efficacy and tolerability for fremanezumab in individuals with CM or episodic migraine (EM) and documented inadequate response, as defined in the clinical trial protocol, to two to four prior migraine preventive treatment classes. 3 In the present post hoc analysis, we evaluated the efficacy of fremanezumab in the subgroup of participants with CM from the FOCUS study who had prior inadequate response to onabotulinumtoxinA and either topiramate or valproic acid. We hypothesized that efficacy would be comparable in this subgroup and the subgroup with CM who had not previously experienced inadequate response to onabotulinumtoxinA and topiramate or valproic acid.
METHODS
Details of the methods for the FOCUS study have been published previously. 3 The FOCUS study was performed in line with Good Clinical Practice Guidelines from the International Council for Harmonization (ICH) and with relevant national and local laws. All participants provided written informed consent before participating in the FOCUS study, and the study protocol and informed consent form were reviewed and approved by an independent ethics committee/institutional review board at all FOCUS study sites. 3
The FOCUS study included a 12‐week, double‐blind, randomized, placebo‐controlled period, and a subsequent 12‐week open‐label treatment period. This study enrolled adults (18–70 years of age; N = 838) with diagnosed CM or EM who had documented inadequate response to two to four prior migraine preventive medication classes (beta blockers, anticonvulsants, tricyclic antidepressants, calcium channel blockers, angiotensin II receptor antagonists, onabotulinumtoxinA, and valproic acid) over the past 10 years. Inadequate response to prior treatment was defined by no clinically meaningful improvement after greater than or equal to 3 months of stably dosed treatment, based on the treating physician’s judgment; discontinuation of treatment due to intolerability; or contraindication or unsuitability of the migraine preventive treatment for the patient. During an initial 12‐week, double‐blind, placebo‐controlled period, participants were randomized (1:1:1) to receive quarterly fremanezumab (months 1, 2, and 3: 675 mg/placebo/placebo), monthly fremanezumab (675 mg [CM]/225 mg [EM]/225 mg/225 mg), or matched monthly placebo. All patients completing treatment in the double‐blind period entered the subsequent open‐label period and received three monthly doses of fremanezumab 225 mg. Efficacy evaluations were based on patient‐reported daily electronic diary data. 3
For this post hoc subgroup analysis, efficacy was assessed based on change from baseline (BL) in the monthly average number of migraine days (MMDs) during 12 weeks of double‐blind treatment (primary end point of the FOCUS study) and proportions of participants with greater than or equal to 30% and greater than or equal to 50% reductions in MMDs during 12 weeks. In addition, a treatment‐by‐subgroup interaction analysis was performed between this subgroup of participants with prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid and the complementary subgroup without prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid to assess for a difference in efficacy between the two subgroups. Treatment‐by‐subgroup interactions were assessed using an analysis of covariance model for the efficacy outcomes. In this analysis, treatment, sex, region, and special group of inadequate response (prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid) were included as fixed effects.
RESULTS
In this subgroup (n = 138), mean age was comparable across treatment groups (44.7–47.7 years), and most participants (quarterly fremanezumab, 85% [44/52]; monthly fremanezumab, 80% [37/46]; and placebo, 88% [35/40]) were women. In the quarterly fremanezumab, monthly fremanezumab, and placebo groups, respectively, 96% (50/52), 96% (44/46), and 83% (33/40) of participants had prior inadequate response to topiramate and 21% (11/52), 35% (16/46), and 28% (11/40) had prior inadequate response to valproic acid. Clinical characteristics were generally comparable to the overall FOCUS study, 3 although this subgroup population had more severe disease, with a longer time since diagnosis (25.0–28.3 years vs. FOCUS overall, 24.0–24.3 years), a higher proportion with inadequate response to four prior migraine preventive medication classes (quarterly fremanezumab, 40% [21/52]; monthly fremanezumab, 50% [23/46]; placebo, 60% [24/40] vs. FOCUS overall, quarterly fremanezumab, 18% [49/276]; monthly fremanezumab, 18% [50/283]; placebo, 19% [54/279]), and a higher number of mean MMDs (17.8–19.4 vs. FOCUS overall, 14.1–14.3). Both this subgroup and the overall FOCUS population had more severe disease than other studies with fremanezumab, 4 , 5 which excluded individuals with greater than or equal to 2 clusters of inadequate response to prior preventive treatments.
The changes from BL in MMDs during the 12‐week treatment period were significantly greater with fremanezumab (quarterly fremanezumab, −3.8; monthly fremanezumab, −3.4) versus placebo (−0.9; quarterly fremanezumab, p = 0.003; monthly fremanezumab, p = 0.012; Figure 1A). Significant reductions from BL in MMDs were also observed with both fremanezumab dosing regimens at month 1 and month 3 and at month 2 with quarterly fremanezumab (Figure 1A). The proportions of participants with a greater than or equal to 30% reduction in MMDs from BL during the 12‐week treatment period were also significantly higher in the fremanezumab groups (quarterly fremanezumab, 44% [23/52]; monthly fremanezumab, 37% [17/46]) versus placebo (13% [5/40]; quarterly fremanezumab, p = 0.002; monthly fremanezumab, p = 0.017), as was the proportion with greater than or equal to 50% reduction from BL during the 12‐week treatment period with quarterly fremanezumab (25% [13/52] vs. placebo, 5% [2/40]; p = 0.024; Figure 2A). Proportions of participants with a greater than or equal to 30% reduction in MMDs from BL were also significantly higher at months 1, 2, and 3 with both fremanezumab dosing regimens compared with placebo (Figure 2A). The proportion of participants with a greater than or equal to 50% reduction in MMDs from BL was significantly higher with quarterly fremanezumab compared with placebo at months 1 and 2 (Figure 2A).
FIGURE 1.
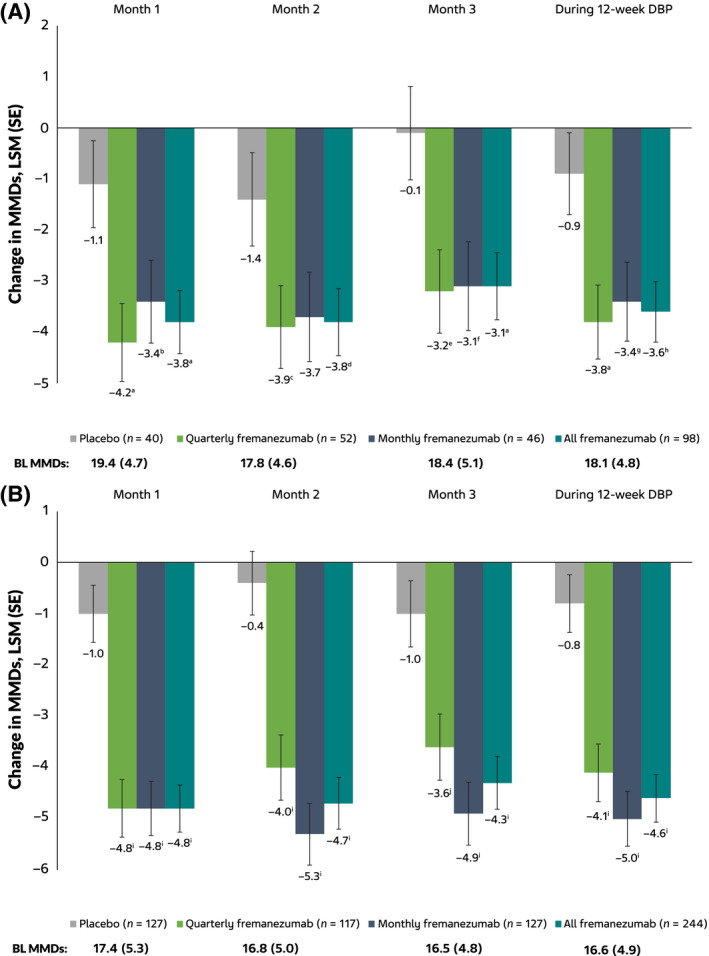
Change from BL in MMDs in the (A) subgroup with CM and prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid (B) subgroup with CM without prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid. BL, baseline; DBP, double‐blind period; LSM, least‐squares mean; MMDs, monthly average number of migraine days; SE, standard error. a p = 0.003 vs. placebo. b p = 0.032 vs. placebo. c p = 0.026 vs. placebo. d p = 0.017 vs. placebo. e p = 0.007 vs. placebo. f p = 0.011 vs. placebo. g p = 0.012 vs. placebo. h p = 0.002 vs. placebo. i p < 0.0001 vs. placebo. j p < 0.001 vs. placebo [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
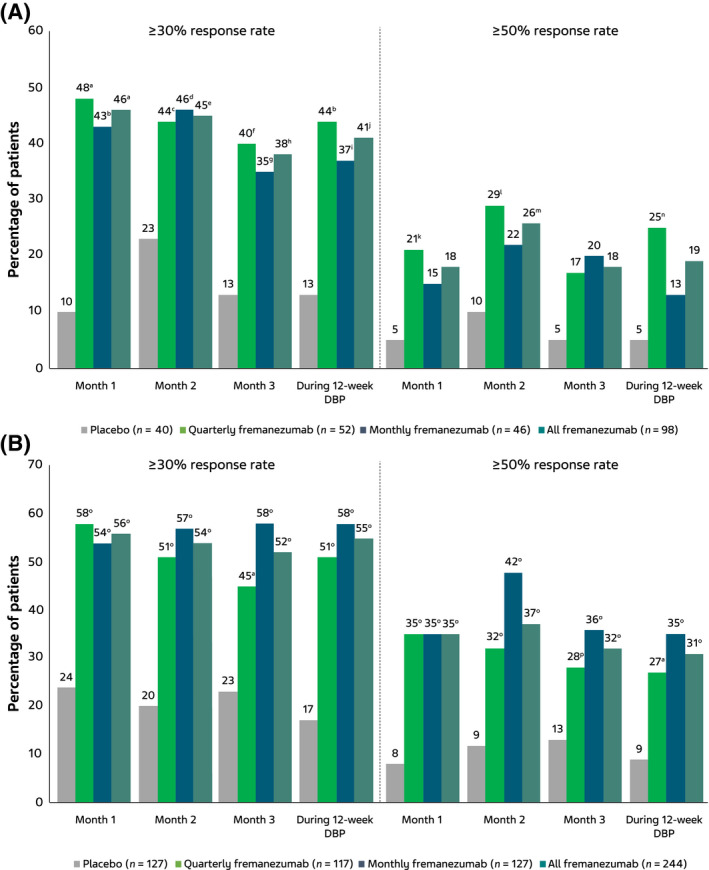
Proportion of participants with ≥30% and ≥50% reduction in MMDs in the (A) subgroup with CM and prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid and (B) subgroup with CM without prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid. CM, chronic migraine; DBP, double‐blind period; MMDs, monthly average number of migraine days. a p < 0.001 vs. placebo. b p = 0.002 vs. placebo. c p = 0.041 vs. placebo. d p = 0.039 vs. placebo. e p = 0.023 vs. placebo. f p = 0.006 vs. placebo. g p = 0.026 vs. placebo. h p = 0.007 vs. placebo. i p = 0.017 vs. placebo. j p = 0.003 vs. placebo. k p = 0.047 vs. placebo. l p = 0.033 vs. placebo. m p = 0.048 vs. placebo. n p = 0.024 vs placebo. o p < 0.0001 vs. placebo. p p = 0.004 vs. placebo [Color figure can be viewed at wileyonlinelibrary.com]
Results for the subgroup without prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid were generally comparable to the subgroup with prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid (Figures 1B and 2B). In a treatment‐by‐subgroup interaction analysis, no significant difference was shown in efficacy between participants in the current subgroup with prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid and the subgroup without prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid (estimate [standard error]: quarterly fremanezumab, 0.32 [1.13], p = 0.775; monthly fremanezumab, 1.58 [1.15], p = 0.173). These results indicate no statistically significant difference in efficacy between the subgroups.
COMMENT
These analyses were subject to certain limitations, including that this was a post hoc analysis in a subgroup of the overall FOCUS study population and may thus be subject to bias. The number of participants in this subgroup was relatively small, particularly compared to the overall FOCUS population, which may have contributed to a reduced ability to detect treatment effects. Nevertheless, results were generally comparable to those observed for the overall FOCUS population. Fremanezumab afforded significantly greater reductions in MMDs and higher responder rates compared with placebo in individuals with CM and prior inadequate response to onabotulinumtoxinA and topiramate or valproic acid, supporting the use of fremanezumab in such patients. This subgroup generally had more severe and longer‐term migraine than the overall FOCUS population; nevertheless, these participants experienced comparable treatment benefits to the general FOCUS population. These results may be particularly relevant for countries in which health authorities require prior inadequate response to multiple preventive medications, such as onabotulinumtoxinA, before initiating fremanezumab preventive treatment for CM.
CONFLICT OF INTEREST
M.D. Ferrari was an investigator on the FOCUS study, which was sponsored by Teva Pharmaceuticals. K.W.M. Zuurbier is an employee of Teva Netherlands B.V. S. Barash and X. Ning are employees of Teva Pharmaceuticals. J.M. Cohen is a former employee of Teva Pharmaceuticals.
AUTHOR CONTRIBUTIONS
Study concept and design: Karin W. M. Zuurbier. Acquisition of data: Steve Barash. Analysis and interpretation of data: Michel D. Ferrari, Karin W. M. Zuurbier, Steve Barash, Xiaoping Ning, Joshua M. Cohen. Drafting of the manuscript: Michel D. Ferrari, Karin W. M. Zuurbier, Steve Barash, Xiaoping Ning, Joshua M. Cohen. Revising it for intellectual content: Michel D. Ferrari, Karin W. M. Zuurbier, Steve Barash, Xiaoping Ning, Joshua M. Cohen. Final approval of the completed manuscript: Michel D. Ferrari, Karin W. M. Zuurbier, Steve Barash, Xiaoping Ning, Joshua M. Cohen.
ACKNOWLEDGEMENTS
Editorial assistance was provided by Megan Knagge, of MedErgy/Cello Health Communications, which was in accordance with Good Publication Practice (GPP3) guidelines and funded by Teva Netherlands B.V.
REFERENCES
- 1. Bigal ME, Walter S, Rapoport AM. Fremanezumab as a preventive treatment for episodic and chronic migraine. Expert Rev Neurother. 2019;19:719‐728. [DOI] [PubMed] [Google Scholar]
- 2. Hoy SM. Fremanezumab: first global approval. Drugs. 2018;78:1829‐1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double‐blind, placebo‐controlled, phase 3b trial. Lancet. 2019;394:1030‐1040. [DOI] [PubMed] [Google Scholar]
- 4. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113‐2122. [DOI] [PubMed] [Google Scholar]
- 5. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319:1999‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


