ABSTRACT
Objective
To develop a standardized sonographic evaluation and reporting system for Cesarean scar pregnancy (CSP) in the first trimester, for use by both general gynecology and expert clinics.
Methods
A modified Delphi procedure was carried out, in which 28 international experts in obstetric and gynecological ultrasonography were invited to participate. Extensive experience in the use of ultrasound to evaluate Cesarean section (CS) scars in early pregnancy and/or publications concerning CSP or niche evaluation was required to participate. Relevant items for the detection and evaluation of CSP were determined based on the results of a literature search. Consensus was predefined as a level of agreement of at least 70% for each item, and a minimum of three Delphi rounds were planned (two online questionnaires and one group meeting).
Results
Sixteen experts participated in the Delphi study and four Delphi rounds were performed. In total, 58 items were determined to be relevant. We differentiated between basic measurements to be performed in general practice and advanced measurements for expert centers or for research purposes. The panel also formulated advice on indications for referral to an expert clinic. Consensus was reached for all 58 items on the definition, terminology, relevant items for evaluation and reporting of CSP. It was recommended that the first CS scar evaluation to determine the location of the pregnancy should be performed at 6–7 weeks' gestation using transvaginal ultrasound. The use of magnetic resonance imaging was not considered to add value in the diagnosis of CSP. A CSP was defined as a pregnancy with implantation in, or in close contact with, the niche. The experts agreed that a CSP can occur only when a niche is present and not in relation to a healed CS scar. Relevant sonographic items to record included gestational sac (GS) size, vascularity, location in relation to the uterine vessels, thickness of the residual myometrium and location of the pregnancy in relation to the uterine cavity and serosa. According to its location, a CSP can be classified as: (1) CSP in which the largest part of the GS protrudes towards the uterine cavity; (2) CSP in which the largest part of the GS is embedded in the myometrium but does not cross the serosal contour; and (3) CSP in which the GS is partially located beyond the outer contour of the cervix or uterus. The type of CSP may change with advancing gestation. Future studies are needed to validate this reporting system and the value of the different CSP types.
Conclusion
Consensus was achieved among experts regarding the sonographic evaluation and reporting of CSP in the first trimester. © 2021 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: Cesarean scar pregnancy, cicatrix, classification, Delphi technique, pregnancy, ultrasonography
Short abstract
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
RESUMEN
Definición y sistema para informes ecográficos en el embarazo sobre cicatriz de cesárea en la gestación temprana: método Delphi modificado
Objetivo
Desarrollar un sistema estandarizado de evaluación e informe ecográfico para el embarazo sobre cicatriz de cesárea (CSP, por sus siglas en inglés) en el primer trimestre, para su uso tanto en ginecología general como en clínicas expertas.
Métodos
Se empleó un procedimiento Delphi modificado, en el que se invitó a participar a 28 personas expertas internacionales en ecografía obstétrica y ginecológica. Para participar se requería una amplia experiencia en el uso de la ecografía para evaluar las cicatrices de cesárea al comienzo del embarazo y/o publicaciones relativas a la evaluación del CSP o de la dehiscencia. Los elementos relevantes para la detección y evaluación del CSP se determinaron a partir de los resultados de una búsqueda bibliográfica El consenso se definió a priori como un nivel de acuerdo de al menos el 70% para cada elemento, y se planificó un mínimo de tres rondas Delphi (dos cuestionarios en línea y una reunión de grupo).
Resultados
Dieciséis personas expertas participaron en el estudio Delphi y se realizaron cuatro rondas Delphi. En total, se determinó que 58 elementos eran pertinentes. Se diferenció entre las mediciones básicas que se realizan en la práctica general y las mediciones avanzadas para centros expertos o con fines de investigación. El panel también formuló consejos sobre los indicios para la remisión a una clínica experta. Se llegó a un consenso para los 58 elementos relacionados con la definición, la terminología y los elementos pertinentes para la evaluación y los informes sobre el CSP. Se recomendó que la primera evaluación de la cicatriz de cesárea para determinar la localización del embarazo se realizara a las 6–7 semanas de gestación mediante ecografía transvaginal. Se consideró que el uso de imágenes por resonancia magnética no añadía valor al diagnóstico del CSP. Un CSP se definió como un embarazo implantado en la dehiscencia o en estrecho contacto con ella. Las personas expertas estuvieron de acuerdo en que un CSP sólo puede producirse cuando hay una dehiscencia y no en relación con una cicatriz de cesárea curada. Los elementos ecográficos relevantes que se deben registrar fueron el tamaño del saco gestacional (SG), la vascularidad, la ubicación en relación con los vasos uterinos, el grosor del miometrio residual y la ubicación del embarazo en relación con la cavidad uterina y la serosa. Según su ubicación, un CSP puede clasificarse como: (1) CSP en el que la mayor parte del SG sobresale hacia la cavidad uterina; (2) CSP en el que la mayor parte del SG está arraigado en el miometrio pero no cruza el contorno seroso; y (3) CSP en el que el SG está parcialmente situado más allá del contorno exterior del cuello uterino o del útero. El tipo de CSP puede cambiar con el avance de la gestación. Son necesarios estudios futuros para validar este sistema de informes y el valor de los diferentes tipos de CSP.
Conclusión
Se alcanzó un consenso entre personas expertas en cuanto a la evaluación ecográfica y los informes sobre CSP en el primer trimestre.
摘要
早期妊娠时剖宫产瘢痕妊娠的定义和超声报告系统:改良的德尔菲法
目的
为早期妊娠时的剖宫产瘢痕妊娠(CSP)开发一个标准化超声评估和报告系统,用于一般妇科医学以及专科门诊。
方法
展开了一次改良的德尔菲疗程,其中有28名产科及妇科超声波检查法的国际专家受邀参与。参与需有使用超声评估早期妊娠时剖宫产瘢痕的大量经验和/或关于CSP或微小裂孔(瘢痕缺损处)评估的发表文章。根据文献搜索结果确定了发现和评估CSP的相关项目。预先定义了当每项至少70%一致时达成共识,并计划了最少三个达尔菲循环(两个线上问卷调查和一个小组会议)。
结果
16名专家参与了德尔菲研究并执行了四个德尔菲循环。总共确定了58个具有相关性的项目。我们区分了在全科诊疗中执行的基本评估和在专科中心或为研究目的而执行的高级评估。专家组还明确建议了转介至专家门诊的相关适用症状。就所有58项的定义、术语、评估和报告CSP的相关项目达成一致意见。建议用于确定妊娠位置的首次CS瘢痕评估应在孕6‐7周使用经阴道超声进行。不认为使用磁共振成像为CSP的诊断带来附加价值。CSP定义为胚胎着床于或接近微小裂孔。专家们同意,CSP仅当有微小裂孔时才会发生并与痊愈的CS瘢痕无关。要记录的相关超声项目有:妊娠囊(GS)大小、血管分布情况、子宫血管相关位置、残留子宫肌层厚度,以及妊娠相对于子宫腔和绒毛膜的位置。根据其位置,CSP可分类为:(1)妊娠囊的最大部分向子宫腔突出的CSP;(2)妊娠囊的最大部分种植于子宫肌层但并未越过浆膜边界的CSP;和(3)妊娠囊部分位于子宫颈或子宫边界之外的CSP。CSP的类型可能随妊娠进入晚期而改变。需要将来的研究来验证本报告系统和不同CSP类型的价值。
结论
专家们就早期妊娠时的超声检查评估和报告CSP达成共识。
CONTRIBUTION —
What are the novel findings of this work?
Using a modified Delphi procedure, we have generated a standardized sonographic evaluation and reporting system for Cesarean scar pregnancy (CSP) in the first trimester, with specific recommendations for assessment in general practice and in expert clinics.
What are the clinical implications of this work?
These recommendations should guide gynecologists and ultrasound examiners in identifying CSP when performing ultrasound in early pregnancy, and provide a framework for experts to use during advanced evaluation of CSP. We hope that these recommendations will increase the awareness and recognition of CSP and, in so doing, help prevent life‐threatening complications.
INTRODUCTION
Rising rates of Cesarean delivery worldwide have resulted in increasing numbers of pregnant women with a Cesarean section (CS) scar 1 . Pregnancies occurring after Cesarean delivery are considered to be at high risk for Cesarean scar pregnancy (CSP), low‐implanted and invasive placenta (placenta accreta spectrum (PAS) 2 ), failure to progress during labor, and uterine dehiscence or rupture in the second or third trimester of pregnancy3, 4, 5, 6. A CSP occurs when the pregnancy implants on the uterine scar or in the niche after a previous CS 7 . Although a CSP is often considered for pregnancy termination, some cases have reportedly progressed towards an intrauterine pregnancy and resulted in viable births8, 9, 10.
Determination of the exact location of the gestational sac (GS) and invasion of the placenta is necessary to estimate the patient's risk and advise whether to terminate or continue the pregnancy. However, there is no uniform reporting system for CSP. Kaelin Agten et al. 6 distinguished between CSPs located on the ‘well‐healed’ Cesarean scar and those implanted in the dehiscent scar (or niche). Others used the level of invasion of the GS and the remaining myometrial thickness to classify CSPs11, 12, 13.
Two‐dimensional (2D) B‐mode transvaginal ultrasound (TVS) alone or in conjunction with three‐dimensional (3D) ultrasound and color Doppler has been generally considered to be the gold standard for the diagnosis of CSP 14 . Some authors have also described the use of magnetic resonance imaging (MRI)15, 16, 17. However, there is no standardized guideline on how to locate the GS in relation to the CS scar in early pregnancy by using ultrasound. The ESHRE (European Society of Human Reproduction and Embryology) Working Group on Ectopic Pregnancy recently published recommendations on the terminology of normally sited and ectopic pregnancies, in which CSP is described briefly 18 .
The aim of this study was to develop a basic and advanced standardized sonographic evaluation and reporting system for CSP in early gestation.
METHODS
Design of a modified Delphi study
A modified Delphi procedure was conducted to achieve consensus (Figure 1). We performed a systematic literature search to discover available literature on the assessment of CSP, and to identify relevant items on the subject that could be used in the development of the first questionnaire. The modified Delphi procedure contained repeat rounds of questionnaires; after each round, answers were analyzed and results were presented to the experts, including their relevant feedback. Based on the outcomes, new questions were formulated concerning topics on which consensus had not been achieved. In this way, the experts participating in the study were able to reflect on the results of each previous questionnaire round in a structured manner. The experts participating in the Delphi study answered online questionnaires anonymously. We continued to the next Delphi round until all items reached consensus. It was predetermined that the process would include at least three rounds (two online questionnaire rounds and one face‐to‐face meeting). The data were collected between July 2018 and August 2020.
Figure 1.
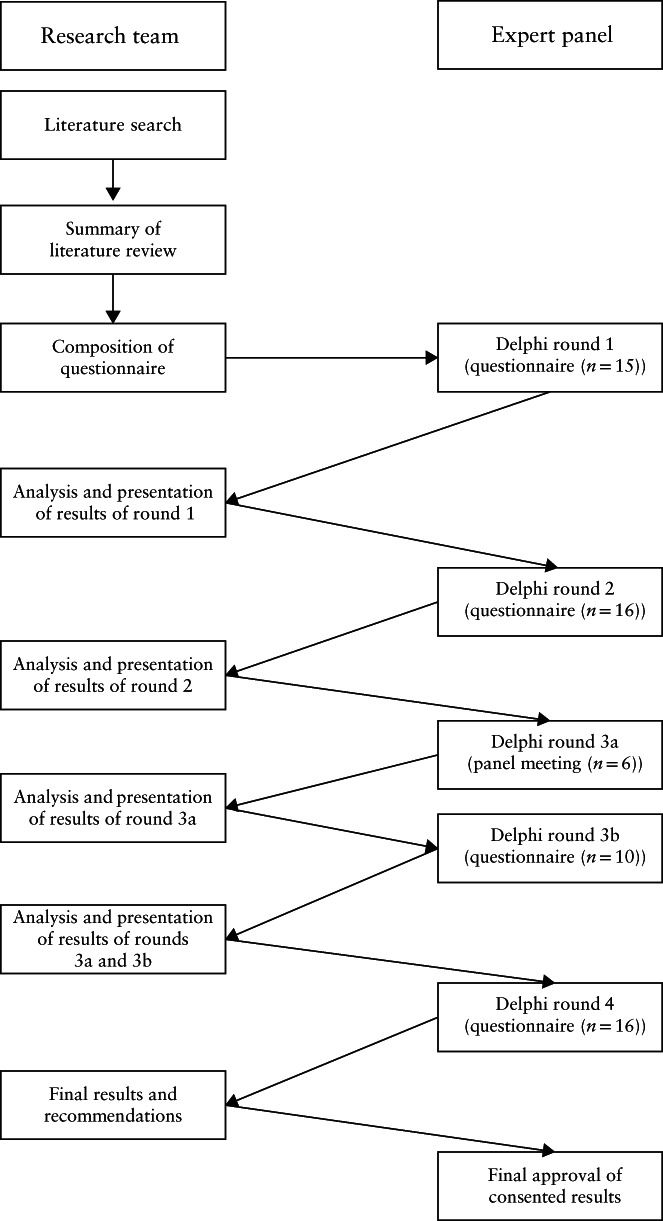
Study design: stepwise modified Delphi method used to reach consensus on the definition of Cesarean scar pregnancy and sonographic evaluation of the uterine scar in the first trimester of pregnancy.
Literature search and development of first Delphi questionnaire
The electronic databases PubMed and EMBASE were searched for articles published on the sonographic evaluation of CSP from inception to January 2018, with the assistance of a clinical librarian. The search strategy is provided in Appendix S1. Duplicate articles were excluded. All English or Dutch full‐text articles were included if they reported on the definition and evaluation of CSP using ultrasound and if they addressed one or more of the research questions predefined by J.A.F.H., R.A.d.L. and I.P.M.J. to use in the first questionnaire. These questions concerned: (1) sonographic criteria to define CSP; (2) classification based on CSP type; (3) method to locate a CSP using TVS; (4) optimal timing to check for the presence of CSP; (5) relevance of color Doppler ultrasound in the diagnosis of CSP; (6) relevance of pulsed Doppler ultrasound; (7) relevance of 3D (Doppler) ultrasound; (8) value of MRI in assessment and diagnosis of CSP. Relevant items were extracted from all the reviewed and included papers and were used in the first questionnaire. In addition, the relevant items were presented in a separate background‐information file that was provided for the experts in case it was needed to fill out the questionnaire.
Expert panel recruitment
Obstetrics and gynecology clinicians with expertise in advanced ultrasound evaluation of CS scars in early pregnancy and diagnosing CSP were invited to participate in this Delphi study. The experts were selected based on their membership of the International Niche Society, including the European Niche Taskforce, and the interest group of the International Society of Ultrasound in Obstetrics & Gynecology (ISUOG) or their authorship of publications concerning the use of ultrasound in the evaluation of CSP or CS scar/niche. All the invited experts were asked to recommend other experts who were also known to have extensive experience in the field. For the purpose of this Delphi procedure, ‘experts’ were defined as clinicians with substantial experience in advanced ultrasound evaluation of CS scars or CSP, or who had published at least one article on CSP or CS niche evaluation. Initially, 22 experts were invited; six further experts were invited subsequently after recommendation by their colleagues. Experts had to confirm their expertise to be included.
Delphi rounds and structural consensus method
All experts received an e‐mail containing a unique link to the online questionnaire after confirmation of their participation. After each round, the answers from all the experts were analyzed for each question. Consensus was predefined as a rate of agreement (RoA) of > 70%, where RoA = (agreement − disagreement)/(agreement + disagreement + indifferent) × 10019, 20, 21. Questions were transferred to the second round if no consensus was reached, and the results of the first round were fed back anonymously, including the reasoning of the respondents. Additional questions requiring clarification were added as appropriate. Furthermore, the experts were given the opportunity to add important relevant items, which were used in the next questionnaire. All the experts who agreed to participate in the Delphi procedure were invited to participate in each round, whether they had replied to the previous questionnaire or not. A draft set of recommendations was designed based on the results of the second round. These results were presented in a face‐to‐face meeting in October 2019, and the items without consensus were discussed. All comments and recommendations made in that meeting were recorded. The experts could reflect on their reasoning and, if necessary, reconsider their opinion. Experts who were unable to participate in the meeting could express their opinion in a third online questionnaire reflecting the results of the face‐to‐face meeting. All results of the agreed items during the three rounds were presented to the experts, then a need for more detailed clarification of a few items led to a fourth digital questionnaire. The results of the agreed items were sent for final approval to all the experts who participated in the Delphi procedure.
Some adjustments were made to the manuscript during the peer‐review process; these were submitted to and approved by all the experts.
RESULTS
Literature search
A systematic search for literature about CSP evaluation and niche evaluation in pregnancy resulted in 1735 articles after removal of duplicates (Figure S1). Of the 471 papers that were considered eligible after screening the title and abstract, 28 articles that reported on our predetermined research questions were finally included after full‐text review. The results of the search are presented in Table S1. Some of the papers6, 14, 22, 23, 24, 25, 26, 27 were used for multiple questions. In total, 15 articles6, 14, 22, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 described various criteria for the diagnosis of a CSP. Six6, 11, 12, 26, 27, 38 articles introduced a classification according to multiple CSP types or grades. Only Timor‐Tritsch et al. 22 described in detail how to locate a pregnancy using ultrasound in order to differentiate between an intrauterine pregnancy and a CSP. The use of Doppler ultrasound, pulsed Doppler, 3D (Doppler) ultrasound and MRI for the assessment and diagnosis of CSP were described in, respectively, three14, 23, 24, two14, 24, four14, 25, 39, 40 and eight14, 23, 25, 41, 42, 43, 44, 45 papers. None of the papers defined the optimal gestational age for assessing the presence of a CSP.
The results of the literature search were used in the development of the first questionnaire and included in the background‐information document provided to the experts. The results of a previous Delphi procedure on uterine niche measurement in non‐pregnant women 46 , and the ISUOG recommendations on the performance of ultrasound in the first trimester of pregnancy 47 , were also provided as background information.
Delphi procedure
The first questionnaire consisted of 43 relevant items comprising 93 questions. These items were categorized as: CSP definition and location; CS scar evaluation in the first trimester of pregnancy; differentiation between CSP and cervical pregnancy/miscarriage; evaluation in the transverse plane; gestational age and CSP; Doppler ultrasound and CSP; pulsed Doppler and CSP; 3D (Doppler) ultrasound and CSP; MRI and CSP; niche measurement in CSP; differentiation between basic measurements to be performed in general practice and advanced measurements to be performed in expert centers or research settings. In the second Delphi round, 10 further items were added based on the input given, and one additional category was included: referral to an expert clinic. Moreover, in the fourth Delphi round, one category (heterotopic pregnancy) and five items were added (Figure 2). An overview of the questions in all questionnaires and subjects discussed during the face‐to‐face meeting and their level of agreement are presented in Table S2.
Figure 2.
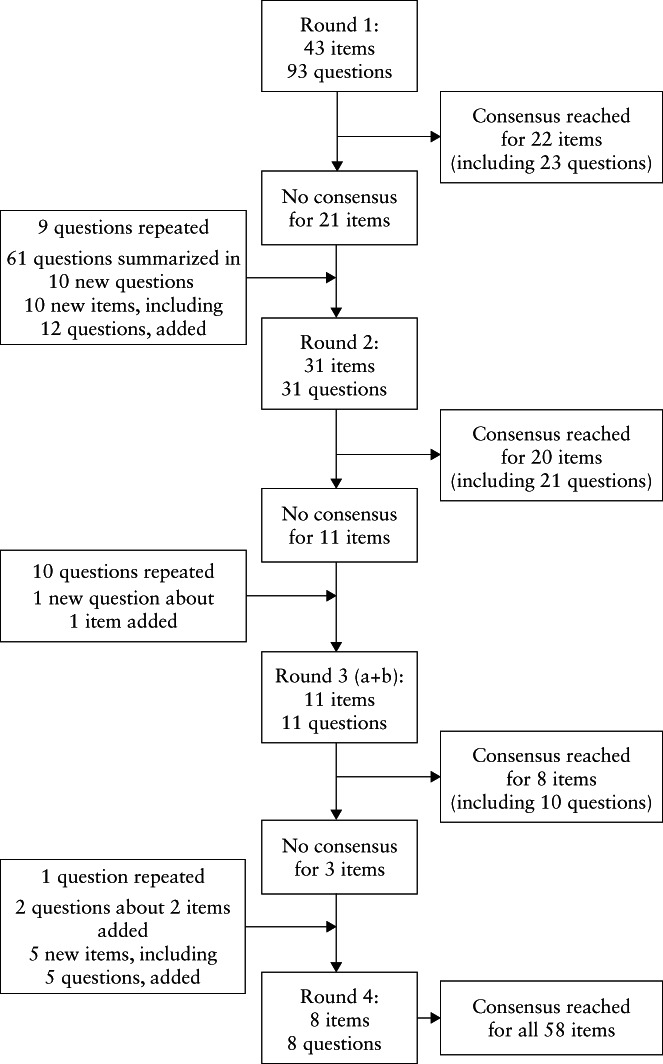
Flow diagram summarizing agreement with or rejection of items during Delphi procedure. Items were accepted if consensus agreement of at least 70% was reached.
Of the 28 experts contacted, one reported to have insufficient expertise. Of the remaining 27 experts, 16 agreed to participate in the Delphi study. Two junior researchers, who are also coauthors (I.P.M.J. and C.V.), facilitated the study; they did not complete the questionnaires and are not included in the table. All 16 participating experts completed the second, third and fourth Delphi rounds; 15 (94%) completed the first online questionnaire. Consensus was reached for all 58 items (Figure 2). The mean consensus achieved per item in each round of the Delphi procedure is presented in Table S3.
Agreed recommendations and statements
A complete overview of the agreed statements is presented in Table S4, and an overview of our primary research questions and recommendations is provided in Table S5.
Method of CS scar evaluation in first trimester of pregnancy
Localization of the GS and placenta depends on gestational age. For evaluation of a CSP, it was agreed that the optimal gestational age to carry out these examinations is 6–7 weeks (88–94% agreement). However, the recommendations apply for use during the entire first trimester of pregnancy (until 12 weeks). The gestational age should be based on the first day of the last menstrual period, if applicable; otherwise, it should be based on measurement of the GS or crown–rump length (81% agreement). CSP evaluation is recommended in women with a previous CS if ultrasound is performed because of symptoms, viability evaluation or other reasons such as a previous CSP.
Eighty percent of the experts agreed that the proposed standardized approach for imaging by TVS and reporting of the lower uterine segment in the first trimester of pregnancy as described by Kuleva et al. 48 can be used to evaluate the CS scar (see Figure S2).
CSP definition and location in first trimester of pregnancy
The first‐round questionnaire contained 34 questions about defining a CSP. We observed different use of the terms ‘CSP’ and ‘niche pregnancy’, resulting in inconsistent answers. Based on this observation, we proposed a uniform definition of CSP, which should be differentiated from a low‐implanted pregnancy and from an ongoing miscarriage or pregnancy remnant.
Most (94%) experts agreed that CSP can be used as a collective term that includes all pregnancies (GS and/or placenta) with implantation in, or in close contact with, the niche. The experts agreed that a CSP can occur only when a niche is present and not in relation to a healed CS scar. It should be noted that a diagnosis of CSP does not automatically mean that the pregnancy needs to be treated as discussed later. A pregnancy that is located near the CS scar should be called ‘low‐implanted pregnancy’ and not a CSP (94% agreement). A low‐implanted pregnancy is defined as any pregnancy implanted near the niche/CS scar without being in direct contact with it (Figure 3).
Figure 3.
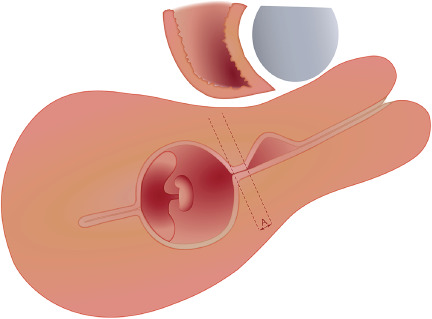
A pregnancy located near the Cesarean scar/niche without being in direct contact with it should be called ‘low‐implanted pregnancy’. ‘Distance A’ is the distance between the proximal border of the niche and the most distal border of the gestational sac.
There was consensus (94%) on describing a CSP depending on the GS crossing two imaginary lines: the ‘uterine cavity line’ (UCL) and/or the ‘serosal line’ (SL) (Figure 4). Specifically, it was agreed that a CSP can be described as follows: (1) CSP in which the largest part of the GS crosses the uterine cavity/cervical canal (the UCL) (Figure 5a,b); (2) CSP in which the largest part of the GS is embedded in the myometrium and does not cross the UCL, and the GS does not cross the SL (Figure 5c,d); and (3) CSP in which the GS crosses the SL; the pregnancy is covered by a thin layer of myometrium/visceral peritoneum and is herniating towards the vesicouterine pouch or into the broad ligament (Figure 5e,f). The definitions of a niche and related features were taken from a previous Delphi study concerning niche measurement in non‐pregnant women 46 .
Figure 4.
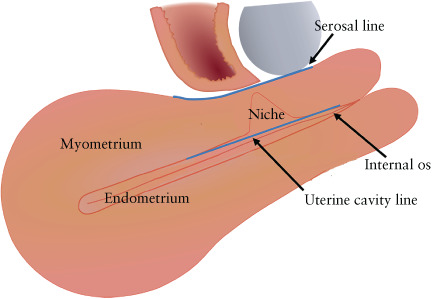
Differentiation of Cesarean scar pregnancy according to position of the gestational sac in relation to two imaginary lines: the ‘uterine cavity line’, i.e. the imaginary line at the transition of the endometrium and myometrium, and the ‘serosal line’, i.e. the imaginary line at the outer border of the myometrium.
Figure 5.
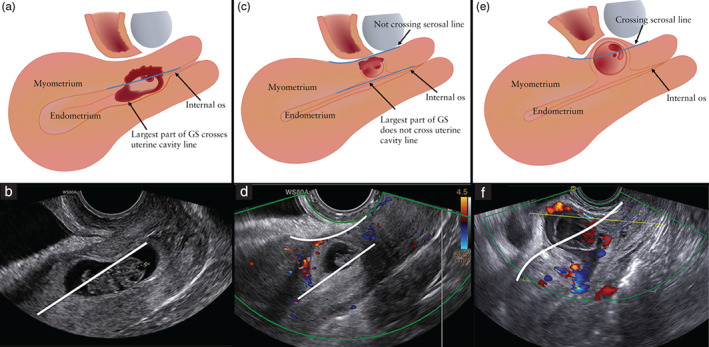
Schematic (a,c,e) and ultrasound (b,d,f) images, showing differentiation of Cesarean scar pregnancy (CSP) according to position of the gestational sac (GS) in relation to the uterine cavity line and the serosal line. (a,b) CSP with the largest part of the GS crossing the uterine cavity line. (c,d) CSP with the largest part of the GS embedded in the myometrium and not crossing the uterine cavity line, and the GS not crossing the serosal line. (e,f) CSP crossing the serosal line.
Method of CSP evaluation in first trimester of pregnancy
2D ultrasound
It was agreed that the residual myometrial thickness (RMT) and adjacent myometrial thickness (AMT) in the sagittal plane should be measured and reported in cases of CSP, as illustrated in Figure 6. Measurements of the niche (length, depth and width) in cases of CSP were found irrelevant because of its change as the pregnancy progresses (100% agreement). Measurement of the position of the GS in relation to the external os (88% agreement) and in relation to the vesicovaginal fold (94% agreement) may be performed in the research setting and is not mandatory for basic evaluation.
Figure 6.
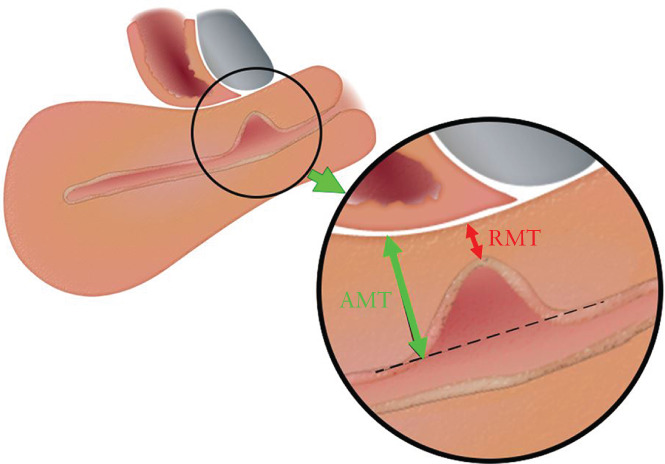
Measurement of residual myometrial thickness (RMT) and adjacent myometrial thickness (AMT) in the sagittal plane in cases of a niche in the non‐pregnant state. Adapted with permission from Jordans et al. 46 .
Color (flow) Doppler
According to the expert panel, color (flow) Doppler helps in the evaluation of trophoblast invasion, recognition of a CSP and differentiating a CSP from a low‐implanted pregnancy or miscarriage. It is therefore advisable to use color‐flow Doppler in case of a suspected CSP, but it is not mandatory in all pregnancies in women with a previous CS (88% agreement). For a suspected CSP, evaluation of the vascular pattern and its relation to the niche, cervix and adjacent uterine vascular anatomy using color (flow) Doppler is recommended (80% agreement). A proposal for evaluation of the CSP in the transverse plane, including its location in relation to the uterine arteries, reached consensus (80%) and was added to the reporting system for CSP (Figure 7).
Figure 7.
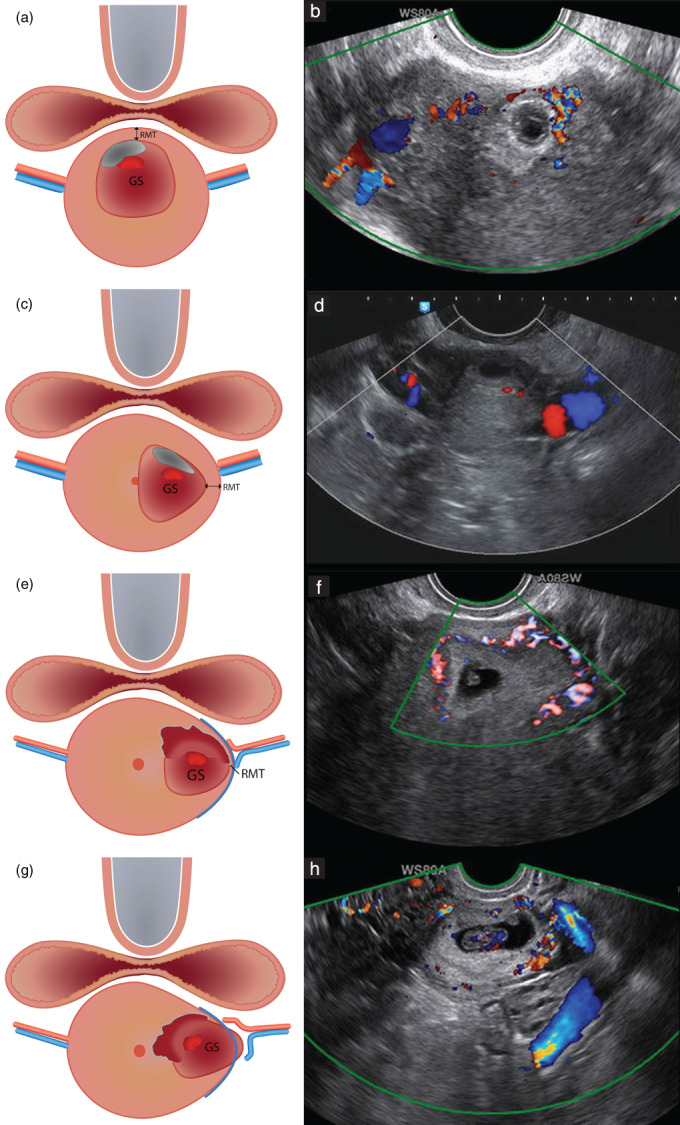
Schematic (a,c,e,g) and ultrasound (b,d,f,h) images showing assessment of location of Cesarean scar pregnancy (CSP) in relation to the uterine arteries in the transverse plane. (a,b) Median location of CSP. (c,d) Eccentric location of CSP; the gestational sac (GS) is connected with the cervical canal and is within the outer cervical contour. (e,f) Lateral location of CSP; the GS protrudes towards the broad ligament within the virtual outer cervical contour and the residual myometrium is visible (CSP with largest part of GS embedded in the myometrium and not crossing the serosal line). (g,h) Lateral location of CSP; the GS is bulging beyond the outer cervical contour and residual myometrium is absent (CSP crossing the serosal line). RMT, residual myometrial thickness.
Remnants of placental tissue within the uterine scar following partial spontaneous expulsion of a CSP can cause persistent bleeding with the risk of intermittent major hemorrhages 49 . These remnants can also often be seen following medical treatment of CSP and after incomplete surgical evacuation50, 51. Retained placental tissue can be difficult to differentiate from blood clots on ultrasound and it may resemble other uterine abnormalities such as fibroids. The experts agreed that color Doppler examination is essential for the differential diagnosis of remnants of placental tissue and to search for the signs of enhanced myometrial vascularity, which is associated with a high risk of bleeding with both conservative and surgical management of CSP (94% agreement).
Some of the experts stressed that the value of quantitative color (flow) Doppler parameters (i.e. vascular score, vessel diameter, flow velocity) should be evaluated in further research. Most respondents did not consider that these quantitative color features should be part of the basic or advanced evaluation. It is important to stress that proper Doppler settings are essential for flow detection, description of the vessel pattern and flow‐velocity measurement in order to obtain reproducible results. The optimal settings for the use of color Doppler in CSP should be ascertained in future research.
Pulsed Doppler, 3D (Doppler) ultrasound and MRI
Most experts agreed that pulsed Doppler (81% agreement) and 3D (Doppler) ultrasound (88% agreement) are not mandatory for routine evaluation of CSP, but may be relevant in a research setting. MRI does not add value to the diagnosis of a CSP according to 73% of the experts.
Differentiation between CSP and cervical pregnancy or miscarriage
A flowchart was introduced that presents different situations that can be encountered during sonographic evaluation of an early pregnancy in women with a previous CS (Figure 8). A pregnancy can be located high in the uterine cavity or low in the uterine cavity or in the cervical canal, the latter two being difficult to distinguish from a CSP. If located low in the uterus or in the cervix, it can be a low‐implanted pregnancy, a CSP or a miscarriage. The site of trophoblast invasion and vascularity are relevant for their discrimination. Note that the type of CSP may change over time as described earlier. Agreement (94%) was reached for the content of the flowchart and for the different steps to be used in clinical practice (Figure 8).
Figure 8.
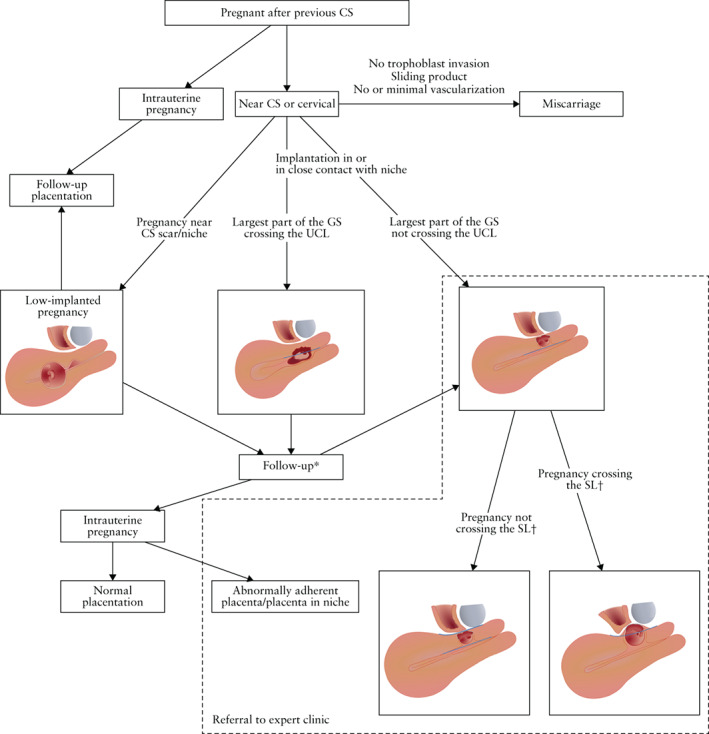
Flowchart showing evaluation of the Cesarean section (CS) scar in first trimester of pregnancy. Step 1: determination of location of the pregnancy: intrauterine pregnancy, low‐implanted pregnancy, Cesarean scar pregnancy (CSP) or miscarriage. Step 2: determination of type of CSP depending on whether the largest part of the gestational sac (GS) is crossing the uterine cavity line (UCL): (a) if the largest part of the GS is crossing the UCL, it should be determined whether the location of the largest part of the GS is in the uterine cavity or in the cervical canal; (b) if the largest part of the GS is not crossing the UCL, the existence of bulging should be determined: (i) if there is no bulging, i.e. the pregnancy is located completely within the level of the serosa/serosal line (SL), it is a CSP with the largest part of the GS in the myometrium and not crossing the SL; (ii) if there is bulging, i.e. the pregnancy is located partly beyond the contour of the outer cervix/SL, it is a CSP crossing the SL. Step 3: determination of location of the placenta: in the niche, near the niche or placenta previa. Step 4: evaluation of presence of signs of abnormally adherent placenta: yes or no? *Management regarding follow‐up or treatment will depend on patient characteristics and wishes. †To be evaluated in future cases and validated by peer‐reviewed articles.
Various sonographic features were agreed upon by the experts to differentiate between the three distinct clinical situations of CSP, cervical pregnancy and ongoing miscarriage. It needs, however, to be emphasized that the signs of a CSP may change over time with advancing gestation, and that the signs described in this paper are applicable in early pregnancy (up to 12 weeks' gestation). First, bulging of the GS towards the bladder is relevant for differentiating between a CSP and a cervical pregnancy (87% agreement). Second, if sliding tissue is visible at the level of the CS scar, it is more likely to be an ongoing or incomplete miscarriage than a CSP (100% agreement). Additionally, vascularization, the location of implantation and trophoblast invasion are useful features for discriminating between a CSP, a low‐implanted pregnancy and an ongoing miscarriage (all 88% agreement), for which the use of color (flow) Doppler is endorsed. According to 73% of the experts, the shape of the GS is not relevant for discriminating between a CSP and a cervical pregnancy. Evaluation of the presence of a GS, fetal pole or yolk sac with or without heart activity can be used to differentiate a CSP from another structure (artifact, nabothian cyst, miscarriage, inclusion cyst and a remnant after miscarriage of a CSP) according to 84% of the experts.
Approximately half of CSPs that are diagnosed contain a living embryo, while the remaining pregnancies are classified as failing49, 52. All the experts agreed that the criteria used for the differential diagnosis between normally developing pregnancies within the uterine cavity and miscarriages53, 54 can also be applied in cases of CSP to differentiate between failing CSP and those with potential to grow beyond the first trimester (Table S6).
Required sonographic items of CS scar evaluation in cases of CSP in first trimester of pregnancy
Basic assessment
An overview of agreed items that should be evaluated during routine ultrasound in cases of low‐implanted pregnancy or CSP is shown in Table 1. In cases of low‐implanted pregnancy, the location of the pregnancy/placenta in relation to the uterine scar was agreed to be more important than the precise location of the pregnancy (88% agreement). Evaluation of placental location, placental or trophoblast invasion into the myometrium and presence of a niche and CSP is recommended in early pregnancy (up to 12 weeks) in all women with a previous CS, if an ultrasound scan is carried out because of symptoms or to assess viability. Also, when there is an intracavitary pregnancy, an additional CSP should be excluded (73–75% agreement). The latter is also relevant to exclude the existence of a heterotopic pregnancy (one intracavitary and one CSP, as discussed later). RMT and AMT are required measurements in the sagittal plane (93–100% agreement). Furthermore, the exact amount of protrusion of the GS beyond the UCL, and the SL if applicable, should be estimated. The location of the GS in relation to the external os and in relation to the vesicovaginal fold is not mandatory for basic evaluation, as described earlier.
Table 1.
Overview of items that should be evaluated in the first trimester after previous Cesarean section in cases of low‐implanted pregnancy or Cesarean scar pregnancy, according to Delphi consensus
| Item | Consensus (%) |
|---|---|
| Basic evaluation | |
| Sagittal plane | |
| Location of GS | 100 |
| Presence of embryonic cardiac activity | 100 |
| Location of placenta in relation to uterine scar | 93 |
| Presence of placenta previa | 94 |
| Placenta or trophoblast invasion into myometrium (experts' advice: color Doppler) | 75 |
| Presence of niche | 87 |
| RMT or LUS thickness | 93 |
| AMT | 100 |
| Bulging of GS beyond serosa towards bladder or bowels | 87 |
| Bulging of placental vessels beyond serosa towards bladder or bowels | 73 |
| Exact amount of protrusion of GS beyond uterine cavity line and serosal line | 100 |
| Advanced evaluation/research setting * | |
| Sagittal plane | |
| Circular flow around GS (color Doppler) | 100 |
| Lining of endometrium covering niche may be relevant to detect an abnormally adherent placenta | 81 |
| Placental/trophoblast location (color Doppler) | 88 |
| Placental ingrowth and its relation to myometrium/serosa/bladder (color Doppler) | 80 |
| Distance between vessels of placenta and serosa (to give some indication concerning chance of presence of PAS) | 75 |
| Use of pulsed Doppler (research setting) | 81 |
| Use of 3D (Doppler) ultrasound (research setting) | 88 |
| Transverse plane | |
| Distance between GS and uterine arteries (color Doppler) | 73 |
| Level of protrusion in relation to outer serosal contour | 100 |
Additional items besides those of basic evaluation.
3D, three‐dimensional; AMT, adjacent myometrial thickness; GS, gestational sac; LUS, lower uterine segment; PAS, placenta accreta spectrum; RMT, residual myometrial thickness.
Advanced assessment
In expert centers, color Doppler ultrasound should be used to evaluate circular flow around the GS (100% agreement). This helps to determine the site of implantation and the degree of placental myometrial invasion. It also helps to determine the depth of placental invasion in relation to the arcuate and main uterine arteries. The location of the GS in relation to the uterine arteries was considered relevant when choosing different treatment options, in addition to the RMT in the sagittal plane (73% agreement). Also, the experts agreed that the level of CSP sac herniation should be assessed in both the sagittal and transverse planes if therapy is considered (100% agreement).
Heterotopic pregnancy
Although considered rare, CSP may coincide with a normally sited pregnancy within the uterine cavity or with ectopic pregnancies in other locations within or outside the uterus 55 . It was agreed that the possibility of a heterotopic pregnancy in the CS scar should be considered in all women with a previous CS (94% agreement). In cases of assisted reproductive techniques (ART), heterotopic pregnancies occur more frequently 56 . Therefore, it is advised that a CSP is excluded in all women with a previous CS and an apparent singleton pregnancy conceived following ART and in those with evidence of multiple ovulation on ultrasonography (94% agreement).
Referral to expert clinic
Expert clinics are considered to have extensive experience in CSP evaluation and management. In cases of CSP with the largest part of the GS located in the myometrium, whether or not it crosses the SL, it is recommended that the patient be referred to an expert clinic for ultrasound evaluation and further management (88% agreement). Doubt about the diagnosis and type of CSP and suspicion of an abnormally adherent placenta are also reasons to refer the patient to a specialized clinic (81% and 94% agreement, respectively). According to all the experts, a solitary finding of a thin residual myometrium in a patient with an intrauterine pregnancy, or a suspicion of placenta previa without abnormal invasion, is not necessarily an indication for referral. However, in cases in which the gynecologist/sonographer is not sure about the diagnosis or further management, referral is advised. Referral to an expert clinic is preferred over MRI in cases of a suspected CSP with the largest part of the GS located in the myometrium whether or not it crosses the SL (100% agreement) or in cases of diagnostic uncertainty. For a CSP with the largest part of the GS crossing the UCL, referral can also be considered in case of doubt about further management or lack of experience as to how to treat patients with PAS.
Advanced gestational age and follow‐up
As pregnancy progresses, evaluation of a CSP becomes more difficult because the GS and placenta are growing and vascularization increases. Furthermore, in case of a CSP, there is a high risk of PAS due to extensive trophoblast invasion49, 57. The type of CSP may also change with advancing gestation. For example, a CSP in which the largest part of the GS is located in the myometrium may progress into a CSP that crosses the imaginary SL but it can also progress into a CSP in which the largest part of the GS crosses the UCL or an intrauterine pregnancy with a placenta (partly) located in the niche or PAS (as illustrated in Figure S3) 57 . Also, in cases of a low‐implanted pregnancy, PAS may occur. The progress of a CSP or low‐implanted pregnancy depends on the size of the niche (RMT), degree of trophoblast invasion and gestational age.
It is important to be aware of these changes with advancing gestational age, and the increased risk of PAS during follow‐up of a CSP or low‐implanted pregnancy. Furthermore, the importance of early detection of CSP was confirmed in a recent review in which CSP diagnosed at or before 9 weeks was associated with a significantly lower risk of composite adverse outcome (including massive hemorrhage and uterine rupture) than if diagnosed after 9 weeks (odds ratio, 0.14 (95% CI, 0.1–0.4); P < 0.001; I 2 = 1.6%) 58 .
DISCUSSION
Main findings
Our modified Delphi procedure resulted in consensus for all items concerning the ultrasound diagnosis, evaluation and reporting of CSP in early pregnancy (up to 12 weeks' gestation). Ultrasound evaluation of the CS scar/niche, to eliminate or confirm CSP, was recommended at 6–7 weeks using TVS in all women with a previous CS if an ultrasound scan is carried out because of symptoms or to assess viability. This is in line with previous literature 59 .
A CSP was defined as a pregnancy with implantation in, or in close contact with, the niche. The experts agreed that a CSP can occur only when a niche is present and not in relation to a healed CS scar. Relevant ultrasound features to record in cases of CSP included GS size, vascularity, location in relation to the uterine vessels, thickness of the residual myometrium and location of the pregnancy in relation to the uterine cavity and serosa. A CSP can be classified depending on the location of the largest part of the GS relative to the UCL, and on the existence of protrusion of the GS beyond the contour of the outer cervix/uterus. With advancing gestation, a CSP with the largest part of the GS in the myometrium and not crossing the SL may progress towards either of the other two CSP types or an intrauterine pregnancy (with or without PAS). In cases of a low‐implanted pregnancy, detailed follow‐up is required owing to the possibility of PAS. It should be stressed that identification of a CSP is not equivalent to an indication for treatment. CSP management depends on the gestational age at the time of the evaluation, the RMT, vascularity around the GS, the level of trophoblast invasion into the myometrium, location of the GS in relation to the UCL and SL, signs of PAS and upon the desire of the patient after evidence‐based counseling. However, evidence‐based counseling is possible only after the collection of evidence. Our reporting system for CSP should facilitate the collection of such information, but this should be confirmed by future studies.
Comparison with other studies
In the last decade, an increasing number of CSP cases and studies on CSP evaluation have been published. However, a standardized guideline on uterine‐scar evaluation in (early) pregnancy, including CSP, was lacking and different definitions of CSP are in use. Du et al. 60 classified CSPs according to the size of the CS diverticula. Kaelin Agten et al. 6 classified a CSP as ‘on the scar’ (partially or fully on top of a well‐healed scar) or ‘in the niche’ (within a deficient or dehiscent scar) depending on the level of invasion of the placenta into the CS scar. Others have proposed a more detailed classification system including different grades of CSP based on the level of protrusion into the uterine wall towards the bladder, some including vascularity at the site of the CS scar11, 12. However, some of these classifications may prove more difficult for clinicians who are not experts in early pregnancy ultrasound. Cali et al. 61 suggested a classification that is partly in line with our reporting system, including location of the GS with respect to the ‘endometrial line’, corresponding to our UCL. However, this classification is less detailed and the GS crossing the SL was not part of it. Balci and Ercan 38 described Type 1 and Type 2 CSPs as a GS that implants on the CS scar with progression in the cervicoisthmus and uterine cavity (Type 1) or with progression towards the myometrium (Type 2), without defining the depth. The experts in our study elected to use the latter proposal as a base and approved the use of some additional items from other classification systems to refine the type and reporting of CSPs. To improve the reproducibility of the reporting system, the experts defined a CSP based on the location of the GS in relation to its protrusion into the cervix or uterine cavity and the extent of myometrial involvement. It should be stated that the classification of a CSP is not fixed and that it may change with advancing gestation. Interpretation of the type of CSP becomes very difficult with advancing gestational age, which may have different consequences for treatment.
The ESHRE Working Group categorized CSPs as ‘partial CSP’, which corresponds to our suggested classification of CSP with the largest part of the GS crossing the UCL, and ‘complete CSP’, which corresponds to our classification of a CSP in which the largest part of the GS is located in the myometrium (crossing or not crossing the SL) 18 . We present a more detailed definition and description of CSP and provide an item list for reporting a CSP.
Strengths and limitations
A strength of our study lies in the use of a modified Delphi method, in which the participants' anonymity was ensured during the questionnaire rounds, preventing domination by any individual, and allowing participants to revise their opinion during successive rounds. Furthermore, all relevant literature available at that time was put at their disposal, by including it in the background information and questionnaires. Another strength is that we did not aim to provide a complete overview of CSP therapies. A third strength is the high response rate during all the rounds, emphasizing the agreement of the experts with the study content.
A limitation of the study is that not all the experts (10/16) participated in the face‐to‐face round. Therefore, we added two more questionnaires after which consensus was achieved for all items. Although we invited a number of international experts with extensive experience in the sonographic evaluation of the uterine CS scar in early pregnancy, we recognize that not all experts in the field were included in this study. Furthermore, our recommendations focus on early pregnancy, so the reporting system may be less suitable at advanced gestation. Validity of the construction and accuracy of the item list of sonographic CS scar features in pregnancy and the value of its use when developing treatment policies should be determined in future studies.
Future perspectives
These recommendations on the evaluation and reporting of a CSP are intended to guide gynecologists and ultrasound examiners when performing ultrasonography in early pregnancy, and provide a framework for experts to use during advanced evaluation. Several cases have been described in which a CSP was misdiagnosed as a cervical ectopic pregnancy, a miscarriage in progress or even as a malignant tumor, resulting in massive blood loss and/or emergency hysterectomy62, 63, 64. On the other hand, it is critical that we prevent the termination of potentially viable pregnancies that appear to be CSPs but that may progress towards intracavitary pregnancies with low‐located placentae; this is expected to occur in 75% of CSPs in which the largest part of the GS crosses the UCL (also called Type 1 in the literature) 49 . We hope that our recommendations will increase awareness and recognition of CSPs, and we aim to develop a free e‐learning program to ease implementation.
Although evaluation of CSP is advised at 6–7 weeks' gestation, our recommendations can be used during the whole first trimester (until 12 weeks). After 12 weeks, it becomes more difficult to evaluate the level of protrusion. In the case of a CSP that protrudes toward the uterine cavity in the late first trimester or early second trimester, the most relevant items to evaluate are its vascularity and its relation with the myometrial/uterine vascular architecture and bladder. These items determine future treatment policy.
The relevance of the different CSP types described in this paper needs to be evaluated in future research. This will be achievable only if future studies record the same features and use the same terminology as those recommended in this paper. In addition to the terminology, it is important to record the precise extent of protrusion beyond the UCL and SL to allow future (meta‐)analyses. To further research in this important area, we propose that all expert clinics submit their cases to the international Cesarean Scar Pregnancy registry, which can be found online (www.csp‐registry.com).
Surgical and medical management of CSPs and niche measurement (during pregnancy) of intracavitary pregnancies were beyond the scope of this Delphi study. International use of this reporting system should enable consistent data collection regarding treatment outcomes of CSP, allowing the development of evidence‐based guidelines in the future.
Conclusions
We have described recommendations for the evaluation and reporting of a CSP in early gestation that can be used by all sonographers in order to facilitate future studies and the development of guidelines. Consensus was achieved for all 58 items concerning the sonographic evaluation of CSP, using a modified Delphi procedure among experts in advanced ultrasound evaluation of CS scars or CSP. Treatment of different CSP types and cut‐off values of niche measurements in pregnancy have yet to be determined.
Supporting information
Appendix S1 Search strategy
Figure S1 Flowchart showing studies identified through literature search.
Figure S2 Principal setting of transvaginal ultrasound during evaluation of uterine scar in first trimester of pregnancy. Modified from Kuleva et al. 48 .
Figure S3 Example of a Cesarean scar pregnancy with the largest part of the gestational sac located in the myometrium, which progressed to intrauterine pregnancy with advancing gestation (1 week difference between ultrasound images).
Table S1 Results of literature search that identified 28 papers reporting on predefined items regarding Cesarean scar pregnancy definition, diagnosis and evaluation
Table S2 Overview of questions of all Delphi rounds, including answers and consensus rate
Table S3 Summary of consensus for definition, diagnosis and evaluation of Cesarean scar pregnancy (CSP) in the first trimester, presented per item
Table S4 Overview of agreed statements after four Delphi rounds, on the definition of Cesarean scar pregnancy (CSP) and sonographic evaluation of the Cesarean section (CS) scar in the first trimester of pregnancy
Table S5 Overview of recommendations on primary research questions
Table S6 Criteria for transvaginal ultrasonographic diagnosis of pregnancy failure, which can also be used to diagnose failing Cesarean scar pregnancy (CSP). Adapted from table 2 in Doubilet et al. 54
ACKNOWLEDGMENTS
We thank Hans Ket, librarian (Amsterdam UMC, location VUmc, Amsterdam, The Netherlands) for his assistance in our literature search. We thank Nazar Amso (Cardiff University, Cardiff, UK), Justin Clark (Birmingham Women's Hospital, Birmingham, UK), Tayfun Cok (Baskent University Hospital, Ankara, Turkey), Jon Einarsson (Brigham and Women's Hospital, Boston, MA, USA), Motti Goldenberg (Sheba Medical Center – Tel HaShomer, Tel Aviv, Israel) and Mary Connor (Sheffield Teaching Hospitals, Sheffield, UK), all members of the ESGE niche taskforce, for being a sounding board during the composition of the recommendations.
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. National Collaborating Centre for Women's and Children's Health . Caesarean section (NICE clinical guideliine 132). Royal College of Obstetricians and Gynaecologists (RCOG) Press: London, UK, 2011; 180–195. [Google Scholar]
- 2. Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence‐based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol 2018; 218: 75–87. [DOI] [PubMed] [Google Scholar]
- 3. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol 2013; 20: 562–572. [DOI] [PubMed] [Google Scholar]
- 4. Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol 2011; 117: 525–532. [DOI] [PubMed] [Google Scholar]
- 5. Timor‐Tritsch IE, Monteagudo A, Calì G, D'Antonio F, Agten AK. Cesarean Scar Pregnancy: Patient Counseling and Management. Obstet Gynecol Clin North Am 2019; 46: 813–828. [DOI] [PubMed] [Google Scholar]
- 6. Kaelin Agten A, Cali G, Monteagudo A, Oviedo J, Ramos J, Timor‐Tritsch I. The clinical outcome of cesarean scar pregnancies implanted “on the scar” versus “in the niche”. Am J Obstet Gynecol 2017; 216: 510.e1–6. [DOI] [PubMed] [Google Scholar]
- 7. Timor‐Tritsch IE, Monteagudo A, Cali G, D'Antonio F, Kaelin Agten A. Cesarean Scar Pregnancy: Diagnosis and Pathogenesis. Obstet Gynecol Clin North Am 2019; 46: 797–811. [DOI] [PubMed] [Google Scholar]
- 8. Nukaga S, Aoki S, Kurasawa K, Takahashi T, Hirahara F. A case of misdiagnosed cesarean scar pregnancy with a viable birth at 28 weeks. Case Rep Obstet Gynecol 2014; 2014: 375685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zosmer N, Fuller J, Shaikh H, Johns J, Ross JA. Natural history of early first‐trimester pregnancies implanted in Cesarean scars. Ultrasound Obstet Gynecol 2015; 46: 367–375. [DOI] [PubMed] [Google Scholar]
- 10. Ahmadi F, Moinian D, Pooransari P, Rashidi Z, Haghighi H. Ectopic pregnancy within a cesarean scar resulting in live birth: a case report. Arch Iran Med 2013; 16: 679–682. [PubMed] [Google Scholar]
- 11. Lin SY, Hsieh CJ, Tu YA, Li YP, Lee CN, Hsu WW, Shih JC. New ultrasound grading system for cesarean scar pregnancy and its implications for management strategies: An observational cohort study. PLoS One 2018; 13: e0202020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang H, Huang J, Wu X, Fan H, Li H, Gao T. Clinical classification and treatment of cesarean scar pregnancy. J Obstet Gynaecol Res 2017; 43: 653–661. [DOI] [PubMed] [Google Scholar]
- 13. RCOG . Diagnosis and Management of Ectopic Pregnancy: Green‐top Guideline No. 21. BJOG 2016; 123: e15–e55. [DOI] [PubMed] [Google Scholar]
- 14. Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG 2007; 114: 253–263. [DOI] [PubMed] [Google Scholar]
- 15. Wu R, Klein MA, Mahboob S, Gupta M, Katz DS. Magnetic resonance imaging as an adjunct to ultrasound in evaluating cesarean scar ectopic pregnancy. J Clin Imaging Sci 2013; 29: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riaz RM, Williams TR, Craig BM, Myers DT. Cesarean scar ectopic pregnancy: imaging features, current treatment options, and clinical outcomes. Abdom Imaging 2015; 40: 2589–2599. [DOI] [PubMed] [Google Scholar]
- 17. Osborn DA, Williams TR, Craig BM. Cesarean scar pregnancy: sonographic and magnetic resonance imaging findings, complications, and treatment. J Ultrasound Med 2012; 31: 1449–1456. [DOI] [PubMed] [Google Scholar]
- 18. ESHRE working group on Ectopic Pregnancy ; Kirk E, Ankum P, Jakab A, Le Clef N, Ludwin A, Small R, Tellum T, Töyli M, Van den Bosch T, Jurkovic D. Terminology for describing normally sited and ectopic pregnancies on ultrasound: ESHRE recommendations for good practice. Hum Reprod Open 2020; 2020: hoaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–1015. [PubMed] [Google Scholar]
- 20. Hsu C‐C, Sandford BA. The Delphi technique: making sense of consensus. PARA 2007; 12: 1–8. [Google Scholar]
- 21. Janssen PF, Brolmann HA, Huirne JA. Recommendations to prevent urinary tract injuries during laparoscopic hysterectomy: a systematic Delphi procedure among experts. J Minim Invasive Gynecol 2011; 18: 314–321. [DOI] [PubMed] [Google Scholar]
- 22. Timor‐Tritsch IE, Monteagudo A, Cali G, El Refaey H, Kaelin Agten A, Arslan AA. Easy sonographic differential diagnosis between intrauterine pregnancy and cesarean delivery scar pregnancy in the early first trimester. Am J Obstet Gynecol 2016; 215: 225.e1–7. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez N, Tulandi T. Cesarean Scar Pregnancy: A Systematic Review. J Minim Invasive Gynecol 2017; 24: 731–738. [DOI] [PubMed] [Google Scholar]
- 24. Honemeyer U, Alkatout I, Kupesic‐Plavsic S, Pour‐Mirza A, Kurjak A. The value of color and power doppler in the diagnosis of ectopic pregnancy. Donald School J Ultrasound Obstet Gynecol 2013; 7: 429–439. [Google Scholar]
- 25. Köroǧlu M, Kayhan A, Soylu FN, Erol B, Schmid‐Tannwald C, Gürses C, Karademir I, Ernst R, Yousuf A, Oto A. MR imaging of ectopic pregnancy with an emphasis on unusual implantation sites. Jpn J Radiol 2013; 31: 75–80. [DOI] [PubMed] [Google Scholar]
- 26. Li Y, Wang W, Yang T, Wei X, Yang X. Incorporating uterine artery embolization in the treatment of cesarean scar pregnancy following diagnostic ultrasonography. Int J Gynaecol Obstet 2016; 134: 202–207. [DOI] [PubMed] [Google Scholar]
- 27. Vial Y, Petignat P, Hohlfeld P. Pregnancy in a Cesarean scar. Ultrasound Obstet Gynecol 2000; 16: 592–593. [DOI] [PubMed] [Google Scholar]
- 28. Timor‐Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow‐up of cesarean scar pregnancy. Am J Obstet Gynecol 2012; 207: 44.e1–13. [DOI] [PubMed] [Google Scholar]
- 29. Cheng LY, Wang CB, Chu LC, Tseng CW, Kung FT. Outcomes of primary surgical evacuation during the first trimester in different types of implantation in women with cesarean scar pregnancy. Fertil Steril 2014; 102: 1085–1090.e2. [DOI] [PubMed] [Google Scholar]
- 30. Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril 1997; 67: 398–400. [DOI] [PubMed] [Google Scholar]
- 31. Jungkman O, Anderson J. Importance of early detection of cesarean scar ectopic pregnancy. J Diagnost Med Sonography 2015; 31: 318–321. [Google Scholar]
- 32. Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First‐trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 2003; 21: 220–227. [DOI] [PubMed] [Google Scholar]
- 33. Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol 2004; 23: 247–253. [DOI] [PubMed] [Google Scholar]
- 34. Seow K‐M, Hwang J‐L, Tsai Y‐L. Ultrasound diagnosis of a pregnancy in a Cesarean section scar. Ultrasound Obstet Gynecol 2001; 18: 547–549. [DOI] [PubMed] [Google Scholar]
- 35. Takahashi H, Matsubara S. Intrauterine hypoechoic mass cephalad to cesarean scar pregnancy. Acta Obstet Gynecol Scand 2015; 94: 670–671. [DOI] [PubMed] [Google Scholar]
- 36. Weilin C, Li J. Successful treatment of endogenous cesarean scar pregnancies with transabdominal ultrasound‐guided suction curettage alone. Eur J Obstet Gynecol Reprod Biol 2014; 183: 20–22. [DOI] [PubMed] [Google Scholar]
- 37. Yin XH, Yang SZ, Wang ZQ, Jia HY, Shi M. Injection of MTX for the treatment of cesarean scar pregnancy: comparison between different methods. Int J Clin Exp Med 2014; 7: 1867–1872. [PMC free article] [PubMed] [Google Scholar]
- 38. Balci O, Ercan F. Foley Balloon Catheter Application to Prevent Bleeding During Treatment for Cesarean Scar Pregnancy. Turkiye Klin J Gynecol Obstet 2017; 27: 64–70. [Google Scholar]
- 39. Pascual MA, Hereter L, Graupera B, Tresserra F, Fernandez‐Cid M, Simon M. Three‐dimensional power Doppler ultrasound diagnosis and conservative treatment of ectopic pregnancy in a cesarean section scar. Fertil Steril 2007; 88: 706.e5–7. [DOI] [PubMed] [Google Scholar]
- 40. Wang CJ, Yuen LT, Yen CF, Lee CL, Soong YK. Three‐dimensional power Doppler ultrasound diagnosis and laparoscopic management of a pregnancy in a previous cesarean scar. J Laparoendosc Adv Surg Tech A 2004; 14: 399–402. [DOI] [PubMed] [Google Scholar]
- 41. Dibble EH, Lourenco AP. Imaging unusual pregnancy implantations: Rare ectopic pregnancies and more. AJR Am J Roentgenol 2016; 207: 1380–1392. [DOI] [PubMed] [Google Scholar]
- 42. Huang Q, Zhang M, Zhai RY. Comparison of gadolinium‐enhanced magnetic resonance imaging with ultrasound in evaluation of cesarean scar pregnancy. J Obstet Gynaecol Res 2014; 40: 1890–1893. [DOI] [PubMed] [Google Scholar]
- 43. Kao L, Dym R, Chernyak V, Scheinfeld M, Oh S, Rozenblit A. Beyond ultrasound: CT and MRI of ectopic pregnancy and its mimics. AJR Am J Roentgenol 2012; 198: 5. [DOI] [PubMed] [Google Scholar]
- 44. Peng KW, Lei Z, Xiao TH, Jia FG, Zhong WX, Gao Y, Shen BX, Xie JW. First trimester caesarean scar ectopic pregnancy evaluation using MRI. Clin Radiol 2014; 69: 123–129. [DOI] [PubMed] [Google Scholar]
- 45. Rosen T. Placenta accreta and cesarean scar pregnancy: overlooked costs of the rising cesarean section rate. Clin Perinatol 2008; 35: 519–529, x. [DOI] [PubMed] [Google Scholar]
- 46. Jordans IPM, de Leeuw RA, Stegwee SI, Amso NN, Barri‐Soldevila PN, van den Bosch T, Bourne T, Brolmann HAM, Donnez O, Dueholm M, Hehenkamp WJK, Jastrow N, Jurkovic D, Mashiach R, Naji O, Streuli I, Timmerman D, van der Voet LF, Huirne JAF. Sonographic examination of uterine niche in non‐pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol 2019; 53: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Salomon LJ, Alfirevic Z, Bilardo CM, Chalouhi GE, Ghi T, Kagan KO, Lau TK, Papageorghiou AT, Raine‐Fenning NJ, Stirnemann J, Suresh S, Tabor A, Timor‐Tritsch IE, Toi A, Yeo G. ISUOG practice guidelines: performance of first‐trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2013; 41: 102–113. [DOI] [PubMed] [Google Scholar]
- 48. Kuleva M, Castaing O, Fries N, Bernard JP, Bussieres L, Fontanges M, Moeglin D, Salomon LJ. A standardized approach for the assessment of the lower uterine segment at first trimester by transvaginal ultrasound: a flash study. J Matern Fetal Neonatal Med 2016; 29: 1376–1381. [DOI] [PubMed] [Google Scholar]
- 49. Cali G, Timor‐Tritsch IE, Palacios‐Jaraquemada J, Monteaugudo A, Buca D, Forlani F, Familiari A, Scambia G, Acharya G, D'Antonio F. Outcome of Cesarean scar pregnancy managed expectantly: systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2018; 51: 169–175. [DOI] [PubMed] [Google Scholar]
- 50. Dubuisson JB, Ben Ali N, Bouquet de Jolinière J, Haggenjos M, Feki A. Laparoscopic treatment of placenta percreta retention in a cesarean scar: a case report. Front Surg 2014; 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for caesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol 2011; 156: 209–211. [DOI] [PubMed] [Google Scholar]
- 52. Jurkovic D, Knez J, Appiah A, Farahani L, Mavrelos D, Ross JA. Surgical treatment of Cesarean scar ectopic pregnancy: efficacy and safety of ultrasound‐guided suction curettage. Ultrasound Obstet Gynecol 2016; 47: 511–517. [DOI] [PubMed] [Google Scholar]
- 53. Abdallah Y, Daemen A, Kirk E, Pexsters A, Naji O, Stalder C, Gould D, Ahmed S, Guha S, Syed S, Bottomley C, Timmerman D, Bourne T. Limitations of current definitions of miscarriage using mean gestational sac diameter and crown–rump length measurements: a multicenter observational study. Ultrasound Obstet Gynecol 2011; 38: 497–502. [DOI] [PubMed] [Google Scholar]
- 54. Doubilet PM, Benson CB, Bourne T, Blaivas M. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med 2013; 369: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 55. Laing‐Aiken Z, Robson D, Wu J. Surgical management of first‐trimester bleeding in a heterotopic caesarean scar pregnancy: A case report and review of literature. Case Rep Womens Health 2020; 27: e00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ouyang Y, Li X, Yi Y, Gong F, Lin G, Lu G. First‐trimester diagnosis and management of Cesarean scar pregnancies after in vitro fertilization–embryo transfer: a retrospective clinical analysis of 12 cases. Reprod Biol Endocrinol 2015; 13: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Timor‐Tritsch IE, Monteagudo A, Cali G, Vintzileos A, Viscarello R, Al‐Khan A, Zamudio S, Mayberry P, Cordoba MM, Dar P. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol 2014; 44: 346–353. [DOI] [PubMed] [Google Scholar]
- 58. Timor‐Tritsch I, Buca D, Di Mascio D, Cali G, D'Amico A, Monteagudo A, Tinari S, Morlando M, Nappi L, Greco P, Rizzo G, Liberati M, Jose Palacios J, D'Antonio F. Outcome of cesarean scar pregnancy according to gestational age at diagnosis: A systematic review and meta‐analysis. Eur J Obstet Gynecol Reprod Biol 2021; 258: 53–59. [DOI] [PubMed] [Google Scholar]
- 59. Timor‐Tritsch IE, D'Antonio F, Cali G, Palacios‐Jaraquemada J, Meyer J, Monteagudo A. Early first‐trimester transvaginal ultrasound is indicated in pregnancy after previous Cesarean delivery: should it be mandatory? Ultrasound Obstet Gynecol 2019; 54: 156–163. [DOI] [PubMed] [Google Scholar]
- 60. Du Q, Liu G, Zhao W. A novel method for typing of cesarean scar pregnancy based on size of cesarean scar diverticulum and its significance in clinical decision‐making. J Obstet Gynaecol Res 2020; 46: 707–714. [DOI] [PubMed] [Google Scholar]
- 61. Cali G, Forlani F, Timor‐Tritsch IE, Palacios‐Jaraquemada J, Minneci G, D'Antonio F. Natural history of Cesarean scar pregnancy on prenatal ultrasound: the crossover sign. Ultrasound Obstet Gynecol 2017; 50: 100–104. [DOI] [PubMed] [Google Scholar]
- 62. Soydinc H, Evsen M, Sak M, Gul T. Cesarean scar pregnancy mimicking malignant tumor: a case report. J Reprod Med 2011; 56: 518–520. [PubMed] [Google Scholar]
- 63. Einenkel J, Stumpp P, Kosling S, Horn LC, Hockel M. A misdiagnosed case of caesarean scar pregnancy. Arch Gynecol Obstet 2005; 271: 178–181. [DOI] [PubMed] [Google Scholar]
- 64. Collins K, Kothari A. Catastrophic consequences of a caesarean scar pregnancy missed on ultrasound. Australas J Ultrasound Med 2015; 18: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy
Figure S1 Flowchart showing studies identified through literature search.
Figure S2 Principal setting of transvaginal ultrasound during evaluation of uterine scar in first trimester of pregnancy. Modified from Kuleva et al. 48 .
Figure S3 Example of a Cesarean scar pregnancy with the largest part of the gestational sac located in the myometrium, which progressed to intrauterine pregnancy with advancing gestation (1 week difference between ultrasound images).
Table S1 Results of literature search that identified 28 papers reporting on predefined items regarding Cesarean scar pregnancy definition, diagnosis and evaluation
Table S2 Overview of questions of all Delphi rounds, including answers and consensus rate
Table S3 Summary of consensus for definition, diagnosis and evaluation of Cesarean scar pregnancy (CSP) in the first trimester, presented per item
Table S4 Overview of agreed statements after four Delphi rounds, on the definition of Cesarean scar pregnancy (CSP) and sonographic evaluation of the Cesarean section (CS) scar in the first trimester of pregnancy
Table S5 Overview of recommendations on primary research questions
Table S6 Criteria for transvaginal ultrasonographic diagnosis of pregnancy failure, which can also be used to diagnose failing Cesarean scar pregnancy (CSP). Adapted from table 2 in Doubilet et al. 54
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


