Abstract
Our study evaluated whether exposure to naphthenic acid fraction compounds (NAFCs) extracted from oil sands process–affected waters (OSPW) has adverse effects on fish embryos that persist into later life. We exposed fathead minnow (Pimephales promelas) embryos to concentrations of NAFCs found in OSPW (2.5–54 mg/L) for 7 days (1 day postfertilization to hatch), then raised surviving larvae in outdoor mesocosms of uncontaminated lake water for 1 month. Embryos exposed to NAFCs were more likely to exhibit malformations (by up to 8‐fold) and had slower heart rates (by up to 24%) compared to controls. Fish raised in uncontaminated lake water following exposure to NAFCs as embryos, were 2.5‐fold less likely to survive during the larval stage than control fish. These fish also showed up to a 45% decrease in swim activity and a 36% increase in swim burst events during behavioral tests relative to controls. We conclude that exposure to NAFCs during the embryonic stage can have lasting effects on fish survival, physiology, and behavior that persist at least through the larval stage. These findings of delayed mortalities and persistent sublethal effects of embryonic NAFC exposure are relevant to informing the development of regulations on treated OSPW releases from mining operations. Environ Toxicol Chem 2022;41:1319–1332. © 2022 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
Keywords: Ecotoxicology, Aquatic toxicology, Exposure–response modeling, Industrial effluents, Behavioral toxicology
Embryonic exposure of fathead minnows (Pimephales promelas) to naphthenic acid fraction components derived from oil sands tailings in a seminatural setting had adverse effects on survival, development, and behavior that persisted into larval stages. NAFC = naphthenic acid fraction component.

INTRODUCTION
The Canadian oil sands industry generates approximately 3 million barrels of bitumen per day (Alberta Energy Regulator, 2021a). Extracting bitumen from oil sands is a water‐intensive process that produces large volumes of fluid tailings that are stored in temporary settling basins (hereafter, tailings ponds). The water portion of these tailings becomes separated from suspended solids after the solids settle out in tailings ponds and is called oil sands process‐affected water (OSPW). The OSPW from tailings ponds is used repeatedly for bitumen extraction, causing it to become concentrated with salts, metals, residual bitumen, and a complex suite of dissolved organic compounds derived from natural bitumen. It is well known that OSPW is toxic to invertebrates, fish, amphibians, birds, and mammals (Li et al., 2017). Currently, the oil sands industry stores over 1.4 billion m3 of OSPW in tailings ponds (Alberta Energy Regulator, 2021b). Holding large volumes of OSPW in tailings ponds poses environmental risks because of possible groundwater contamination and accidental breaches of impoundment structures (Commission for Environmental Cooperation, 2020; Frank et al., 2014; Hewitt et al., 2020). To mitigate these risks, plans are underway to treat OSPW and release it to the environment (Alberta Energy Regulator, 2017; Government of Alberta, 2015). A greater understanding of the effects of the toxic compounds in OSPW on aquatic biota under field conditions is needed to inform the regulations currently under development for the safe release of treated OSPW to the environment.
Naphthenic acid fraction compounds (NAFCs), an acid‐extractable organic fraction of OSPW, are important contributors to the toxicity of OSPW (Morandi et al., 2015). Classical naphthenic acids are characterized by the general formula C n H2n + Z O2, where n represents the carbon number and Z represents the number of hydrogen atoms lost to a ring structure or double bond (Richardson & Ternes, 2018). The term NAFC includes classical naphthenic acids as well as more complex structures that contain three or more oxygen atoms and heteroatoms of sulfur and nitrogen (Rowland et al., 2011). Concentrations of NAFCs range from 20 to 80 mg/L in OSPW tailings ponds (Mahaffey & Dube, 2017) and 5 to 40 mg/L in reclamation ponds (Anderson et al., 2012; Kavanagh et al., 2013) and are <1 mg/L in surface waters of the Athabasca River (Sun et al., 2017). Because NAFCs are highly persistent in the environment and can remain in OSPW in tailings ponds for decades (Marentette, Frank, Bartlett, et al., 2015), it is critical to understand both the immediate effects such as outright lethality and any sublethal, persistent effects of NAFC exposures that may affect aquatic species later in life.
The period of the typical fish life cycle most sensitive to environmental stressors is in the early stages, spanning immediate postfertilization through embryogenesis to the larval free‐feeding stage (Organisation for Economic Co‐operation and Development [OECD], 2013). Most published studies investigating the effects of NAFCs on fish focus on early‐life exposures, and several studies have investigated embryonic exposures specifically to OSPW‐derived NAFCs from active tailings ponds in the Canadian oil sands region (see Loughery et al., 2019; Madison et al., 2020; Marentette, Frank, Bartlett, et al., 2015; Marentette, Frank, Hewitt, et al., 2015; Peters et al., 2007). Fish embryos exposed to NAFCs tend to have lower survival, to hatch prematurely, and to be smaller when they hatch. These fish also exhibit reduced embryonic heart rates and heart malformations, such as pericardial edemas (Loughery et al., 2019; Madison et al., 2020; Marentette, Frank, Bartlett, et al., 2015; Marentette, Frank, Hewitt, et al., 2015; Peters et al., 2007). These impairments may lead to complications later in life that could affect the fitness of fish populations.
While these studies investigating fish embryonic and larval exposures to NAFCs provide important information about their immediate toxicity, few experiments ran longer than 3 weeks or investigated sublethal endpoints; and thus, there is limited information about any lasting sublethal effects of NAFC exposure across multiple life stages in fish. Identifying possible sublethal effects of environmentally relevant OSPW fractions including NAFCs is vital for the development of NAFC environmental quality guidelines, which have yet to be defined by the Canadian Council of Ministers of the Environment (2020) or the Government of Canada (2021). The overall aim of the present study was to examine whether embryonic exposure to NAFCs has chronic effects on the survival, development, and behavior of larval fish reared in lake water under seminatural field conditions. Specifically, we were interested in whether a short‐term exposure to NAFCs derived from OSPW during the embryonic stage can cause adverse, sublethal effects that manifest themselves during the larval stage. Investigating persistent effects of NAFC exposure on fish across multiple life stages is vital to fully understand the potential impacts of OSPW releases into the environment (be they intentional or accidental) and for making evidence‐informed decisions regarding OSPW management. Because recent end‐of‐pipe regulations and effects‐based monitoring frameworks for the oil sands sector will permit OSPW release into natural surface waters once it is deemed to be in a “ready‐to‐release state” (Alberta Energy Regulator, 2017; Government of Alberta, 2015), it is essential to define threshold NAFC concentrations to inform the specific criteria for “ready‐to‐release” OSPW. We chose to investigate early‐life effects on development following NAFC exposure because if fish physiology is impaired either structurally or functionally during this critical life‐history stage, impacts may persist into adulthood and manifest in essential characteristics like swimming performance (Incardona et al., 2015). We also chose to investigate behavioral endpoints because changes in behavior have immediate consequences for fish growth, reproduction, and survival (Smith & Blumstein, 2008). Thus, if NAFCs impair fish development and behavior, there could be ecologically important consequences that influence fish populations in the Canadian oil sands region.
In the present study, we exposed embryonic fathead minnow (Pimephales promelas), a robust indicator species that is tolerant to harsh environmental conditions and is native to the Canadian oil sands region (Ankley & Villeneuve, 2006; Wallace & McCart, 1984), to different environmentally relevant concentrations of NAFCs that are found in OSPW tailings ponds. We then raised the larvae in uncontaminated lake water for 1 month in outdoor mesocosms, to test the following hypotheses: embryonic exposure to NAFCs impairs development in fish in a exposure‐dependent manner, thereby reducing embryonic survival and hatching viability and increasing incidences of malformations (hypothesis 1 [H 1]); and impaired development attributable to embryonic NAFC exposure has sublethal effects that persist into later life stages, including decreased larval survival and growth and altered larval behavior (hypothesis 2 [H 2]).
MATERIALS AND METHODS
Study species
Fathead minnows occur naturally in the Athabasca River watershed, a major waterway running directly through the oil sands region of Alberta, Canada. Fathead minnows can be found in the main stem of the river, its tributaries, and surrounding lakes (Wallace & McCart, 1984). Therefore, fathead minnows in the oil sands region may be potentially exposed to NAFCs by controlled releases of treated OSPW into the Athabasca River, accidental breaches of tailings ponds into the watershed, or contamination of groundwater via leaching of OSPW‐derived chemicals from tailings ponds (Commission for Environmental Cooperation, 2020). Furthermore, fathead minnows are fairly tolerant to harsh environmental conditions and are a reliable model species in aquatic toxicology studies (Ankley & Villeneuve, 2006).
Preparation and characterization of the NAFC extract and treatment solutions
Approximately 2000 L of fresh OSPW was collected from an active tailings pond in 2011 (Industry A Fresh; Bartlett et al., 2017; Marentette, Frank, Bartlett, et al., 2015). Total NAFCs were extracted and purified from this sample as described by Frank et al. (2006). This extract has been extensively investigated for embryo‐larval toxicity in fathead minnow and walleye (Sander vitreus; Marentette, Frank, Bartlett, et al., 2015; Marentette, Frank, Hewitt, et al., 2015) and acute toxicity to three aquatic invertebrates and chemically compared to commercial naphthenic acid mixtures (Bartlett et al., 2017). Moreover, Marentette, Frank, Bartlett, et al. (2015) measured the presence of five alkylated polycyclic aromatic hydrocarbons (PAHs) and 15 US Environmental Protection Agency (USEPA) priority PAHs in their highest exposure solution prepared from this NAFC extract. These compounds were detected at a concentration of 0.005 µg/ml of alkylated PAHs (C1‐phenanthrene, C1‐chrysene, and C2‐chrysene) and 0.002 µg/ml of USEPA priority PAHs (e.g., phenanthrene and chrysene; see Marentette, Frank, Bartlett, et al., 2015).
For the present study, the NAFC concentration in the extract was measured by negative‐ion electrospray ionization high‐resolution mass spectrometry (ESI‐HRMS; Orbitrap) following methods in Headley et al. (2013). In brief, analysis was carried out using a dual‐pressure linear ion trap‐Orbitrap mass spectrometer (LTQ Orbitrap Elite; Thermo Fisher Scientific) equipped with an ESI interface operated in negative ion mode. Data were acquired in full‐scan mode from a mass to charge ratio (m/z) 100 to 600, and the average mass resolving power (m/Δm 50%) was 242,000 at m/z 250. N‐Butyl benzenesulfonamide was used as lock mass compound for scan‐to‐scan mass calibration correction. A previously characterized Athabasca OSPW large‐volume extract was used as a standard for the quantification of NAFCs in the samples (Rogers et al., 2002). The NAFC concentration of the extract as measured by ESI‐HRMS was 2636 mg/L. This concentration is lower than that reported for the same extract previously (i.e., 1998 mg/L of NAFCs by a liquid chromatography/quadrupole‐time of flight [LC‐QToF/MS] method). This discrepancy is likely due to the different analytical methods employed (ESI‐HRMS vs. LC‐QToF/MS and calibrations therein) because evaporation of the extracts would have been negligible under the storage conditions. The NAFC extract was stored in sealed amber glass bottles in darkness at 4 °C and preserved in 0.05 M NaOH.
The NAFC extract was nominally diluted with filtered lake water to working stock solutions of 2.5, 6.5, 10, 14.5, 21, 29.5, 40, and 54 mg/L, in 10‐L polyethylene containers. These NAFC concentrations are environmentally relevant and can be found in tailings ponds and reclamation ponds in Alberta (Anderson et al., 2012; Kavanagh et al., 2013; Mahaffey & Dube, 2017). The pH of the working stock solutions was adjusted to 8.3 ± 0.1 using 0.1 M hydrochloric acid, to match the pH of the lake water controls. Water samples were collected from treatment solutions throughout the exposure period (on days 0, 3, and 6) for further chemical analyses and stored in 250‐ml amber glass bottles at 4 °C. The class distribution, size of compounds (carbon number and double‐bond equivalents), and total NAFC concentrations present in the samples were determined by Orbitrap ESI‐HRMS (Headley et al., 2013). Class distributions were established with acquired accurate mass data and Composer, Ver 1.5.3 (Sierra Analytics), with mass accuracies of <2 ppm. Measured NAFC concentration values were approximately half of the NAFC values that were nominally calculated (Figure 1C). We suspect there was a reduction in sample recovery by ESI‐HRMS due to matrix‐related ion suppression effects caused by the lake water present in the samples; therefore, we retained the nominal concentration values of NAFCs for all subsequent analyses, following Marentette, Frank, Bartlett et al. (2015) and Bartlett et al. (2017). The concentration of NAFC compounds detected in the treatment solutions did not change (i.e., degrade) over the 7‐day exposure period (Figure 1D).
Figure 1.

Chemical analysis of naphthenic acid fraction compounds (NAFCs) measured in seminatural exposure vessels during the 7‐day embryonic exposure. Percentage abundance of compounds by size for a representative NAFC sample from the highest‐concentration NAFC solution (A, nominal 54 mg/L NAFCs) and the control (B, filtered lake water). The size of compounds is represented as the number of double‐bond equivalents (1.5–8.5) and the number of carbons present (7–20). Samples shown were collected on the first day of exposure, which did not change in abundance by more than ±0.3% in all samples by the end of exposure. Relationship between nominal and measured NAFC concentrations in exposure vessels throughout the exposure period (C); diagonal dashed line indicates 1:1 relationship compared to the line of best fit for measured versus nominal NAFC concentrations (y = 0.38x + 0.74). Temporal trends in NAFC concentrations (D), with vertical dashed lines indicating dates when NAFC solution was added to exposure vessels.
Experimental design
We conducted our experiment at the Queen's University Biological Station (44°33′55″N 76°19′35″W) from July 15 to August 21, 2019. Water for the experiment was sourced from Opinicon Lake adjacent to the field station (water chemistry described in Supporting Information, Table S1). Fathead minnow embryos (n = 1637, >24 h postfertilization [hpf]) from a minimum of 10 breeding pairs were obtained from Aquatox Testing and Consulting, to ensure that life stages of embryos were consistent for embryotoxicity assessment. The experiment consisted of two phases: a 7‐day static exposure phase, where embryos were continuously exposed to different concentrations of NAFCs from 24 hpf to hatch, and a 1‐month postexposure phase, where larvae were raised in lake water (filtered through a 500‐μm stainless steel sieve) to monitor for persistent or latent effects of their prior NAFC exposure. The seminatural study simulated fish exposure to NAFCs in an exposure–response design using environmentally relevant NAFC concentrations, investigating contaminant exposure over multiple life stages of fish, and assessing sensitive early–life stage toxicology endpoints.
The exposure phase took place outdoors in a tent that allowed embryos to experience natural variations in air temperature and sunlight (Supporting Information, Figure S1). On the first day of the experiment (hereafter, day 0), clumps of embryos were gently removed from the spawning tiles following a standard procedure for culturing fathead minnow embryos (USEPA, 2006) and randomly distributed among exposure vessels (1‐L glass jars, 18 cm height × 8.5 cm diameter, n = 27; Bernardin). Exposure vessels were filled with one of the eight NAFC concentrations (2.5, 6.5, 10, 14.5, 21, 29.5, 40, and 54 mg/L) or filtered lake water as the control. There were three exposure vessel replicates per treatment, each containing 49–65 embryos. Exposure of embryos to NAFCs lasted between 4 and 6 days, depending on time to hatch. Each exposure vessel was maintained above 7 mg/L dissolved oxygen via continuous aeration with an aquarium air pump (Hailea HAP60), and exposure vessels were held in a water bath (60‐L container). Treatment solutions were 90% renewed on day 3 of the exposure, except for the 40‐ and 54‐mg/L treatments, where we observed nearly 100% embryonic mortality by day 3; and thus, these treatments were thereafter terminated. Exposure concentrations did not change significantly over the course of the exposure phase (Figure 1); thus, exposure solutions were not renewed more frequently to minimize external disturbances to embryos. All animal procurement and experimental procedures were carried out in accordance with the Queen's University Animal Care Committee (protocol no. 2018‐1829).
After hatch, larvae were monitored for 3 days in glass jars (filled with uncontaminated lake water) and, after the onset of exogenous feeding, transferred to mesocosms for the 1‐month postexposure phase. Larvae (total n = 430) from the control, 2.5‐, 6.5‐, 10‐, 14.5‐, and 21‐mg/L treatments (three replicates/treatment) were transferred to outdoor mesocosms (92 cm height × 75 cm diameter, white opaque plastic, n = 18) filled with 300 L of filtered lake water (Supporting Information, Figure S2). The higher treatments (i.e., 29.5, 40, and 54 mg/L) were not included in the postexposure phase because very few embryos survived to day 7 at these concentrations (Figure 2A). Larvae were fed brine shrimp (Artemia franciscana) to satiation twice daily. Larvae were also observed consuming algae and insect larvae growing in the mesocosms. Given the volume of the mesocosms, water was not exchanged during the postexposure phase, to reduce disturbance to developing fish.
Figure 2.
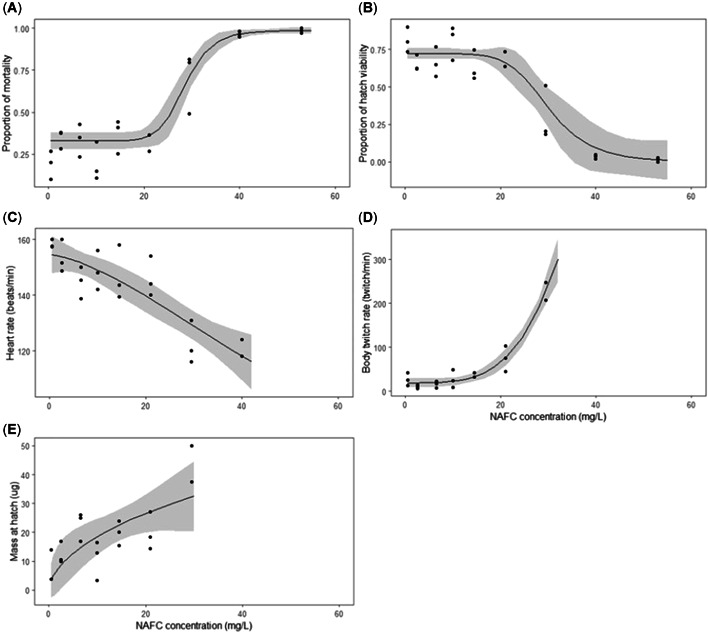
Embryonic mortality (A), hatch viability (B), heart rate at 96 h postfertilization (C), body twitching rate at hatch (D), and mass at hatch (E) in fathead minnows (Pimephales promelas) as functions of exposure to nominal concentrations of naphthenic acid fraction compounds (NAFCs). Solid lines represent the line of best fit, gray‐shaded areas are the ±95% confidence intervals, and each circle represents one replicate exposure vessel. Exposure to NAFCs significantly increased embryo mortality and decreased hatch viability (generalized linear mixed‐effects model [GLMM], Wald's Chi‐squared (1,18) = 84.7, p < 0.0001), decreased heart rate (linear mixed‐effects model [LME] F(1,18.1) = 32.9, p < 0.001), increased twitching rate (LME, F(1,17.3) = 17.5, p < 0.001), and a trend towards increased mass at hatch (GLM, F(1,24) = 3.13, P = 0.089).
Light intensity and water temperature were continuously monitored using HOBO data loggers (Onset) and placed inside the water bath housing exposure vessels as well as inside one exposure vessel and four mesocosms filled with filtered lake water (Supporting Information, Table S2). We measured pH and dissolved oxygen daily using a Hana meter (H198129) and conductivity and salinity using a Hach meter (H140d) on the first day of the experiment in exposure vessels and daily in mesocosms (Supporting Information, Table S3). All water parameters remained within the suggested ranges for fathead minnow early–life stage toxicity testing (Supporting Information, Tables S2 and S3; OECD, 2013).
Exposure‐phase endpoints
During the exposure phase, we assessed the following endpoints: embryonic mortality, embryonic heart rate, full‐body twitching rate (i.e., any rapid full‐body movement), dry mass, malformations, and viability at hatch.
Mortality and hatch viability were assessed daily in the morning. Embryos were counted as dead and removed if an opaque discoloration and an absence of movement were observed (OECD, 2013). Hatch viability was defined as the proportion of embryos that produced fully hatched, viable embryos; fish exhibiting more than two mild malformations or more than one moderate or one severe malformation were considered nonviable (after Marentette, Frank, Bartlett, et al., 2015).
Heart rate was assessed at 96 hpf (following Marentette, Frank, Bartlett, et al., 2015), where embryos (n = 9 per treatment, n = 3 per replicate exposure vessel) were randomly chosen and gently pipetted in 24‐well microplates (Thermo Scientific™ BioLite Microwell Plate; 1 ml volume/well) in their corresponding treatment solution. Because there was 100% mortality by day 3 in the highest NAFC treatment (54 mg/L; Figure 2A), heart rate analysis was not possible for that treatment. Embryos were videotaped using a custom‐built camera with a macro zoom lens for 30 s to estimate average heartbeat per minute, as described by Marentette, Frank, Bartlett, et al. (2015). Individual heart rates were counted manually from the videos by an observer unaware of NAFC treatment.
Dry mass at hatch was assessed by defrosting newly hatched sac fry (n = 279) that were previously euthanized and frozen. Vials were centrifuged briefly and then placed in a drying oven for 24 h at 70 °C (Quincy Lab; model A1‐2052). Vials were weighed using an analytical balance (±0.001 g, P‐403; Denver Instrument). Because there was close to 100% mortality in the 40‐ and 54‐mg/L NAFC treatments (Figure 2A), embryos from these treatments were excluded from further analyses.
Full‐body twitching rate was assessed by randomly choosing a subset of newly hatched sac fry (n = 9 per treatment) and gently pipetting them into six‐well microplates (Thermo Scientific BioLite Microwell Plate; 3 ml volume/well). A total of three fish per exposure vessel were placed inside one well, and only one well was used at a time per microplate. Groups of fish were acclimated for 3 min, before a 3‐min activity assessment began. Video cameras (Canon HF R800) were mounted overhead, and recordings were initiated prior to the acclimation period to minimize external disturbances to the fish. We recorded the total number of muscular twitches (any rapid full‐body movement) per fish to calculate the average twitching rate per minute. Following each trial, fish were euthanized in 0.2% buffered tricaine methanesulfonate (MS‐222) solution (sodium bicarbonate ratio 2:1, m/v; Acros Organics, Thermo Fisher Scientific), photographed, and stored in vials at −20 °C.
Malformations were assessed in a subset of newly hatched sac fry (n = 45–50 per treatment) that were euthanized in MS‐222 and photographed using the custom‐built camera with a macro zoom lens. Embryos that hatched prematurely (prior to 120 hpf) were underdeveloped and could not be included in the analysis. Photographs were later analyzed for cardiovascular (pericardial edema, hemorrhaging), craniofacial (anomaly in structure and size of head, eyes, and jaw), myoskeletal (spinal curvatures), and peritoneal (yolk sac edema) malformations using a modified scoring index from Villalobos et al. (2000; Supporting Information, Tables S4 and S5). Malformations were scored from 0 to 3, where 0 = no observable effect, 1 = mild effect, 2 = moderate effect, and 3 = severe effect, then summed to obtain the overall severity index score per fish. For total severity scores, 0 = no observable effect, 1–4 = mild effect, 5–8 = moderate effect, and 9–12 = severe effect. Analyses were performed independently by three researchers naive to treatment, and the overall severity index was the average of the three scores. Carcasses were grouped by replicate and stored at −20 °C.
Postexposure‐phase endpoints
Mortality, size, and behavior of larvae were assessed after 1 month of rearing in mesocosms supplied with filtered Opinicon Lake water. Larval mortality was determined by assessing the number of individuals alive in the mesocosms at the start of the postexposure phase and assessing the number of individuals alive in the mesocosms 1 month later. For all fish alive, fork length was measured from digital photographs (EOS 100D; Canon) taken at the beginning and end of the rearing period and analyzed using ImageJ2 software (Rueden et al., 2017). Wet mass of individuals was measured using an analytical balance (±0.001 g, P‐403; Denver Instrument) at the end of the rearing period. The condition factor was calculated as Fulton's index (Froese, 2006).
Larval behavior was assessed with and without a food stimulus. Behavioral test arenas consisted of plastic Petri dishes (6 × 6 × 1 cm) filled with filtered lake water. Five larvae were placed in each test arena and allowed to acclimate for 10 min. The larvae were then filmed with a video camera (Canon HF R800). After regular activity was recorded for 120 s without a food stimulus, 1 ml of brine shrimp was introduced to the center of the test arena via transfer pipette, and larvae were recorded for an additional 120 s. Using the Behavioral Observation Research Interactive Software (Ver 7.9.8; Friard & Gamba, 2016), an individual larva in each video was assessed for three behavioral metrics: time spent swimming, number of burst events (i.e., sudden increase in velocity), and time spent in the middle of the arena. All video tests had coded labels so that the individual analyzing behavior videos was naive to each treatment.
Statistical analyses
Statistical analyses were performed using R, Ver 3.6.2 (R Foundation for Statistical Computing, 2020). Model residuals were visually assessed to ensure that model assumptions for normality and homogeneity were met. If a data set did not meet assumptions (i.e., larval swimming activity), data were square root–transformed. To test the influence of NAFC concentration on fish, we used linear mixed‐effects models (LMEs) to assess the following endpoints: embryonic heart rate, dry mass, and malformations at hatch; larval growth (mass, length, and condition factor); and larval behavior (swimming activity, burst events, and space use). For all models, NAFC treatment was treated as a linear predictor, and replicate was treated as a random effect. To assess the influence of NAFC concentration on embryonic and larval mortality and hatch viability, we used generalized linear mixed‐effect models (GLMMs) with mortality or hatch viability as binary response variables, NAFC treatment as a linear predictor, and replicate as a random error term.
Exposure–response relationships for embryonic mortality, hatch success, heart rate, and twitching rate, as well as larval mortality, were modeled using log‐logistic (logit) regression with the drc package (Ritz et al., 2015), and the bmd package (Jensen et al., 2020) was used to estimate the benchmark concentration (to the 10th percentile with 95% confidence interval [CI]) for NAFCs (Jenson et al., 2020; Ritz & Streibig, 2005). The four‐parameter log‐logistic function (LL.4) was used in exposure–response models for binary endpoints (embryonic and larval mortality, embryonic hatch viability), and the three‐parameter log‐logistic function (LL.3) was used in exposure–response models for all other endpoints (embryonic heart rate and twitching rate). Lethal and effective concentrations for the 50th percentile (LC50 and EC50) with 95% CIs were calculated from the logit regression models.
RESULTS
NAFCs in treatment solutions
Qualitative characterization of treatment solutions was consistent with those reported in prior studies that utilized the same NAFC extract (Bartlett et al., 2017; Marentette, Frank, Bartlett, et al., 2015; Marentette, Frank, Hewitt, et al., 2015). Treatment solutions were composed primarily of O2 species (i.e., monocarboxylic acids with the general structure C n H2n+Z O2; Table 1). The percentage of O2 species between treatment solutions increased from 77% (2.5 mg/L of NAFCs) to 96% (10 mg/L of NAFCs) of the total class distribution, compared to 6% in filtered lake water (Table 1). The O2 species with double‐bond equivalent of 3.5–4.5 were most prevalent in all treatment solutions and collectively comprised >60% of the total proportion of O2 species (Supporting Information, Table S6). The class distribution increased to include O3, O5–O8, N2O2, N2O3, N2O6, NO2S, and OS2 species as more lake water was present in the sample (Table 1). The proportion of O3–O8 species was much higher in lake water controls, which collectively comprised 75% of the class distribution. Furthermore, treatment solutions contained low–molecular weight compounds of fewer than 22 C atoms (Figure 1A). The dominant structures, comprising >50% of NAFCs detected in treatment solutions, were two‐ to four‐ringed compounds with 3.5–4.5 double‐bond equivalent, each containing 13–16 C atoms. In comparison, filtered lake water was comprised of 26% noncyclic compounds with 16 C atoms and a range of compounds (spanning 3.5–7.5 double‐bond equivalent and 9–15 C atoms), each present at <5% of the total composition (Figure 1B).
Table 1.
Chemical class distributions of eight naphthenic acid fraction compounds (NAFC) treatment solutions and one control treatment (filtered lake water)
| Class | Class distribution (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Treatments—nominal NAFC concentration (mg/L) | ||||||||
| 2.5 | 6.5 | 10 | 14.5 | 21 | 30 | 40 | 54 | ||
| N2 | 1.13 | 0.19 | 0.22 | 0.21 | 0.22 | 0.34 | 0.37 | 0.47 | 0.34 |
| O | 0.95 | 1.33 | 0.83 | 0.54 | 0.74 | 0.49 | 0.51 | 0.58 | 0.46 |
| O2 | 6.03 | 77.1 | 93.9 | 95.8 | 93.4 | 94.3 | 94.3 | 94.8 | 94.8 |
| O2S | 1.14 | 1.75 | 2.28 | 2.36 | 2.55 | 2.55 | 2.75 | 2.21 | 2.41 |
| O4 | 11.5 | 1.31 | 0.46 | 1.89 | 1.89 | 1.67 | 1.69 | 1.31 | 1.43 |
| S2 | 0.11 | – | – | 0.18 | 1.21 | 0.63 | 0.34 | 0.71 | 0.54 |
| O3 | 6.87 | 1.31 | 0.11 | – | – | – | – | – | – |
| O6 | 18.4 | 3.44 | 0.46 | – | – | – | – | – | – |
| N2O2 | 2.75 | 0.11 | – | – | – | – | – | – | – |
| N2O3 | 0.14 | 0.24 | – | – | – | – | – | – | – |
| N2O6 | 0.08 | 1.75 | – | – | – | – | – | – | – |
| NO2S | 0.06 | 0.08 | – | – | – | – | – | – | – |
| O5 | 15.9 | 4.33 | – | – | – | – | – | – | – |
| O7 | 13.65 | 4.95 | – | – | – | – | – | – | – |
| O8 | 8.18 | 3.49 | – | – | – | – | – | – | – |
| OS2 | 0.17 | 0.09 | – | – | – | – | – | – | – |
| N | 0.06 | – | – | – | – | – | – | – | – |
| N2O | 0.29 | – | – | – | – | – | – | – | – |
| N2O4 | 0.29 | – | – | – | – | – | – | – | – |
| N2O5 | 0.07 | – | – | – | – | – | – | – | – |
| N3OS | 0.13 | – | – | – | – | – | – | – | – |
| N3S | 0.18 | – | – | – | – | – | – | – | – |
| NO2 | 0.08 | – | – | – | – | – | – | – | – |
| NO3 | 0.50 | – | – | – | – | – | – | – | – |
| NO4 | 0.51 | – | – | – | – | – | – | – | – |
| NO5 | 0.58 | – | – | – | – | – | – | – | – |
| NO6 | 0.40 | – | – | – | – | – | – | – | – |
| NO7 | 0.20 | – | – | – | – | – | – | – | – |
| NO8 | 0.05 | – | – | – | – | – | – | – | – |
| O10 | 1.25 | – | – | – | – | – | – | – | – |
| O2S2 | 0.22 | – | – | – | – | – | – | – | – |
| O3S | 1.45 | – | – | – | – | – | – | – | – |
| O3S2 | 0.36 | – | – | – | – | – | – | – | – |
| O9 | 3.42 | – | – | – | – | – | – | – | – |
| OS | 0.38 | – | – | – | – | – | – | – | – |
| OS4 | 0.14 | – | – | – | – | – | – | – | – |
| S3 | 2.04 | – | – | – | – | – | – | – | – |
Data shown are from samples collected on the first day of the experiment. Abundance of chemical classes did not change by more than ±0.3% in all samples by the end of exposure.
Exposure‐phase endpoints
Embryonic mortality increased significantly with increasing NAFC concentrations ((GLMM, Wald's Chi‐squared (1,18) = 84.7, p < 0.0001) Figure 2A). Mortality of control embryos was 18% on average and increased >3.7‐fold when embryos were exposed to NAFC concentrations of ≥40 mg/L. Embryos exposed to the 54‐mg/L treatment died between days 1 and 2 of exposure (48–72 hpf). Embryonic LC50 was 28.5 mg/L of NAFCs (95% CI 26.8–30.3 mg/L), and the 10% benchmark concentration was 22.3 mg/L NAFCs (95% CI 19.4–26.4 mg/L).
Hatch viability of embryos significantly decreased with increasing NAFC concentrations (Figure 2B), with 85% of embryos from controls viable at the end of exposure and a 3‐fold reduction in viability following exposure to 29.5 mg/L of NAFCs. The EC50 for hatch viability was 30 mg/L NAFCs (95% CI 26.8–33.2 mg/L), and the 10% benchmark concentration was 21.7 mg/L NAFCs (95% CI 16.1–27.4 mg/L).
Embryonic heart rate was reduced as a function of exposure to increasing NAFC concentrations (LME, F(1,18.1) = 32.9, p < 0.0001; Figure 2C). At 96 hpf, the average heart rate of control embryos was 158 ± 7 beats/min, whereas the heart rate of embryos exposed to NAFCs was reduced by up to 24% in 40 mg/L NAFCs. The 10% benchmark concentration for heart rate was 18.6 mg/L NAFCs (95% CI 11.1–26.1 mg/L).
Twitching rate of newly hatched sac fry increased significantly with increasing NAFC concentrations (LME, F(1,17.3) = 17.5, p < 0.001; Figure 2D). The average twitching rate of control embryos ranged from 3 to 79 twitches/min, and this increased by up to 7.7‐fold in the 29.5‐mg/L treatment. In addition, twitches of fish from the 21‐mg/L and 29.5‐mg/L treatments were characterized by repetitive, circular movements that did not propel the individual forward but rather led to loss of equilibrium.
Average dry mass of newly hatched sac fry tended to increase with increasing NAFC concentrations, but this relationship was not significant (GLM, F(1,24) = 3.13, p = 0.089; Figure 2E). Hatched fish from controls (n = 40–48) weighed 8.9 μg on average, and hatched fish from the 29.5‐mg/L NAFC treatment (n = 10) were 5‐fold larger.
The prevalence and severity of malformations consistently increased as a function of increasing NAFC concentrations (GLM, F(1,16) = 13.9, p < 0.01; Figure 3A). At hatch, 77% of control fish had an severity index score of 0, indicating normal development. The proportion of malformations increased 4.8‐ to 8‐fold across indices following exposure to 21 mg/L of NAFCs, which was the highest treatment assessed for malformations because of high mortality rates in the 29.5‐, 40‐, and 54‐mg/L treatments. Almost half of the fish that hatched in the 21‐mg/L treatment had an severity index score in the moderate to high severity range, with severe pericardial edemas being the most prevalent malformation (47% of malformed fish; Figure 3B) and with spinal curvatures (kyphosis, lordosis, and bent) present at high severity in 20% of these fish (Figure 3C). Severely underdeveloped craniofacial structures including the head and jaw were observed in 28% of fish from the 21‐mg/L treatment, whereas another 15% exhibited moderate craniofacial malformations (Figure 3D), and fluid accumulations around the yolk sac (peritoneal edemas) were present at high severity in 15% of these fish (Figure 3E). Control fish that were malformed (19%) had severity index scores in the mild severity range, with relatively few fish (4%) exhibiting severe pericardial edemas.
Figure 3.

Percent malformations at hatch in fathead minnows (Pimephales promelas) following a 7‐day exposure to nominal concentrations of naphthenic acid fraction compounds (NAFCs). Total (A); cardiovascular, including pericardial edema and hemorrhaging (B); myoskeletal, specifically spinal curvatures (C); craniofacial, including altered head, eye, and jaw development (D); and peritoneal, specifically yolk sac edema (E) malformations. Malformations for each index are further categorized as mild, moderate, or severe, based on the severity of the malformation. Images show representations of each malformation, scored from 0 (no malformation) to 3 (severe malformations). Data and images from high treatments (>21 mg/L NAFCs) were excluded because of high rates of mortality. The prevalence and severity of malformations increased significantly as a function of increasing NAFC concentrations (generalized linear model, GLM, F(1,16) = 13.9, p < 0.01).
Postexposure‐phase endpoints
Larval fish raised in uncontaminated lake water were less likely to survive the 1‐month rearing period if they were exposed to NAFCs as embryos (GLMM, Wald's Chi‐squared (1,12) = 9.06, p < 0.01; Figure 4A). As expected, mortality rate of larval fish increased as a function of NAFC concentration. Mortality in control mesocosms was 8.4 ± 5.1% but in fish exposed to NAFCs was up to 2.5‐fold higher. The 10% benchmark concentration was 7.1 mg/L NAFCs (95% CI 0–16 mg/L).
Figure 4.

Postembryonic exposure–phase larval mortality in fathead minnows (Pimephales promelas; A; gray areas represent ±95% confidence intervals, each point represents one replicate exposure vessel) and behavioral responses to a food stimulus, including burst events (B), swim duration (C), and duration in the middle of the arena (D), as functions of nominal concentrations of naphthenic acid fraction components (NAFCs). Points and error bars (B–D) are means ± standard deviation. Embryonic exposure to NAFCs significantly increased larval mortality GLMM, Wald's Chi‐squared(1,12) = 9.06, p < 0.01 and frequency of burst swims LME, F(1,16) = 6.47, p = 0.022 Swimming duration LME, F(1,16) = 2.66, p = 0.12 and duration in the middle of the arena LME, F(1,16) = 2.7, P = 0.12 did not vary significantly between treatment groups.
Larval behaviors when a food stimulus was added to the test arena were altered by embryonic NAFC exposure, as evidenced by a trend towards decreases in swimming time (Figure 4C; LME, F(1,16) = 2.67, p = 0.12) and a significant increase in frequency of burst swims (Figure 4B; LME, F(1,16) = 6.47, p = 0.022). Fish in the 21‐mg/L treatment demonstrated an average 8% decrease in swimming time compared to controls, but in one test we observed a decrease of 45% compared to controls. In addition, fish in the 21‐mg/L treatment demonstrated a 36% increase in burst frequency relative to controls. Space use did not vary significantly between treatment groups (Figure 4D; LME, F(1,16) = 2.7, p = 0.12).
Embryonic NAFC exposure did not significantly affect larval length LME, F(1, 16.4) = 2.37, p = 0.14 mass LME, F(1,16.1) = 4.11, p = 0.059 or condition factor LME, F(1,16.3) = 1.78, p = 0.2. Rather, fish density significantly influenced larval growth models, resulting in significant increases in larval length (Supporting Information, Table S8; ANOVA, χ 2[2] = 10.79, p < 0.01), mass (Supporting Information, Table S8; ANOVA, χ 2[3] = 102.41, p < 0.001), and condition factor (Supporting Information, Table S8; ANOVA, χ 2[3] = 78.18, p < 0.001).
DISCUSSION
Embryonic exposure to NAFCs resulted in decreased embryonic survival and hatch viability. It also resulted in increased malformations, locomotory impairments, and mass at hatch in exposed fathead minnows, supporting H 1. Furthermore, embryonic exposure to NAFCs had persistent adverse effects on larval fish survival and behavior after 1 month of rearing in filtered Opinicon Lake water, supporting H 2. These findings highlight the relevance of studies extending beyond embryonic exposures because we show that short embryonic exposure to NAFCs elicits chronic toxic effects on fish that persist into the larval stage. Understanding the effects of NAFCs across multiple life stages of fish is vital to understanding the implications of releasing OSPW into natural environments.
Embryonic NAFC exposure increases embryonic and larval mortality
Under seminatural field conditions, both embryonic and larval mortality of fathead minnows increased with increasing NAFC exposure concentrations. These findings differ from previous studies that reported that embryonic exposure to NAFCs or OSPW did not affect larval mortality at 16 (Marentette, Frank, Hewitt, et al., 2015; same extract as present study) or 28 (Siwik et al., 2000) days posthatch for fathead minnows. Though the immediate toxicity of NAFCs on fish is proposed to be a result of acute narcosis (Frank et al., 2009), the mechanism for NAFC toxicity persisting into the larval stage and causing increased larval mortality is not clear. In contrast to Marentette, Frank, Bartlett, et al. (2015) and Siwik et al. (2000), NAFC‐exposed fathead minnows in the present study were transferred to mesocosms filled with filtered lake water at hatch. It is possible that the differing exposure conditions that included lake water and natural light were sufficient to elicit the differences observed. Furthermore, the introduction to a novel environment posthatch may have posed an additional challenge to the fish exposed to NAFCs in the present study.
In the present study, the prevalence and severity of malformations at hatch increased as a function of NAFC concentration, with pericardial edemas being the most frequent malformation observed. These findings are consistent with previous studies that found similar cardiac malformations in fish exposed to NAFCs (Madison et al., 2020; Marentette, Frank, Bartlett, et al., 2015; Marentette, Frank, Hewitt, et al., 2015 [same extract as present study]; Peters et al., 2007) and polycyclic aromatic compounds (PACs) derived from crude oil (Incardona et al., 2009) during early‐life development. There are several possible mechanisms by which NAFC exposure causes malformations in embryos. First, restricted blood flow to tissues as a result of cardiac dysfunction could cause malformations at hatch. Fish exposed to commercial naphthenic acids show heart development problems and poor blood circulation with malformations at hatch (Peters et al., 2007), suggesting that any morphological defects are secondary to impaired blood circulation (Incardona et al., 2015). Second, the metabolism of NAFCs could be damaging cells and endogenous molecules within the embryo, thereby impairing normal development. Marentette et al. (2017) reported altered expression levels of genes involved in oxidative stress via the aryl‐hydrocarbon receptor (AhR) pathway in walleye embryos exposed to the same extract as used in the present study. The metabolism of aromatic hydrocarbons via the AhR pathway can cause oxidative stress by producing reactive oxygen species; these chemical species damage DNA, cause lipid and protein degradation and cell death, and are associated with a suite of malformations and deformations at hatch. Third, NAFCs could be altering genetic expression in embryos during organogenesis. Transcriptome profiling of fathead minnow embryos exposed to the same NAFC extract used in the present study displayed altered expression of gene networks involved in skeletal development, bone formation, and arteriogenesis, among other pathways (Loughery et al., 2019).
The impaired development we observed at the embryonic stage may have had subsequent and persistent effects on larval survival and driven the observed changes in behavior. Several studies have demonstrated that impaired heart development can have lasting effects on fish into adulthood. Hicken et al. (2011) showed that embryonic exposure to crude oil caused sublethal cardiotoxicity, altering heart morphology and reducing swim performance in fish after 1 year of being reared in clean water. Similarly, Incardona et al. (2015) found that embryonic exposure to crude oil causes both structural and functional changes in fish hearts that impair cardiorespiratory performance in adulthood. Berube et al. (2022) predicted that cardiovascular deformities in embryos exposed to conventional oil could explain the latent fish mortality observed in a depuration period following oil exposure. In the present study, embryos exposed to NAFCs showed decreases in average heart rates, consistent with other early–life stage exposure experiments using the same extract (see Marentette, Frank, Bartlett, et al., 2015). This reduction in heart contractility may be due to inhibited cardiac development and function because embryonic exposure to OSPW and NAFC extracts has been shown to alter the expression of genes involved in cardiomyocyte differentiation and calcium ion homeostasis in the heart (Loughery et al., 2019; Philibert et al., 2019). Calcium ions play a critical role in regulating heart contractility, and altered heart contractility during embryonic development can lead to defective cardiac morphogenesis or cardiac failure in more severe cases (Andrés‐Delgado & Mercader, 2016). Should the impaired heart development that we observed in fish embryos persist into later life stages, cardiovascular function essential for fish survival and swimming performance could be hindered. Though the exact mechanisms are still unclear, it is apparent that exposure to NAFCs impairs embryonic development in fish and may have lasting effects on fish survival.
Embryonic NAFC exposure did not affect larval growth
Though embryonic mass tended to increase as a function of NAFC exposure, NAFC exposure did not affect larval growth. Rather, variation in rearing densities between treatments likely influenced differences in larval growth. Similar to Smith et al. (1978), lower fish densities could have contributed to the increased growth that we observed in treatments with higher NAFC concentrations. The current literature on the effect of NAFC exposure on subsequent fish size is equivocal. While some studies reported increased size at hatch following embryonic OSPW exposure (Siwik et al., 2000), others found reductions in size (He et al., 2012; Peters et al., 2007), while yet others have reported no effect on size (Bauer et al., 2019; Marentette, Frank, Bartlett, et al., 2015). Studies involving fish in later life stages also show equivocal growth pattern results. Kavanagh et al. (2013) found that fathead minnows in OSPW ponds either did not change in size relative to reference ponds or were larger depending on the season. Similarly, Siwik et al. (2000) found no difference in fish growth at 28 or 56 days after OSPW exposure, although their OSPW‐exposed larvae were larger after 7 days. Fish growth is an integrative process that accounts for changes in a multitude of biotic and abiotic factors including population density, food abundance, and temperature (Siwik et al., 2000), complicating the interpretation of fish growth as a metric of contaminant exposure. Furthermore, the inconsistencies of the source material used across studies (e.g., tailings pond used, age of pond, extraction method) may preclude direct comparisons of results between studies. In the present study, variations in stocking density between rearing mesocosms appear to have had more influence on larval fish growth than embryonic NAFC exposure levels.
Embryonic NAFC exposure alters embryonic and larval activity
Both newly hatched embryos and 1‐month‐old larvae showed degrees of behavioral alterations increasing with NAFC exposure concentrations. This finding indicates that exposure to different NAFC concentrations affecting embryonic activity can also induce persistent effects on fish activity during the larval stage and possibly beyond. These behavioral changes are of importance because larvae displayed changes in behavior 1 month after removal from NAFC exposure, indicating that embryonic exposure to NAFCs has lasting effects on fathead minnow development that affect fish behavior in later life stages. To our knowledge, this is the first study to show that embryonic NAFC exposure can alter larval fish behavior and thus serves as an important consideration for evaluating the effects of NAFCs in aquatic ecosystems.
The observed changes in posthatch twitching activity are likely attributed to disrupted neurophysiological function from acute NAFC‐induced narcosis (Sharma, 2019). Compounds that disrupt cell membrane integrity can interfere with nerve functioning (van Wezel & Opperhuizen, 1995). It has been found that NAFCs disrupt cell membranes via narcosis (Frank et al., 2009); thus, they may have contributed to the changes in embryonic activity through this mechanism. One study showed that PACs, another family of compounds that can disrupt cell membranes, disrupted dopaminergic and serotonergic systems in the brain, causing increased neurotransmitter turnover and altered stress responses in fish (Gesto et al., 2009). These behavioral changes are important and ecologically relevant because impairments to nerve function could render exposed fish unable to swim away from a potential exposure of OSPW within natural ecosystems.
Chemical contaminants can alter fish behavior in multiple different ways, including changes in sensory or motor functions. The disrupted cardiovascular development we observed in fish embryos could have hindered cardiovascular performance as larvae, thus altering larval activity patterns. Furthermore, the observed changes in swimming and increase in burst events are indicative of freezing and darting behaviors, which are common anxiety‐ or fear‐related behaviors in many fish species (Cachat et al., 2010). Our findings are similar to those of Philibert et al. (2019), who found that embryonic exposure to OSPW altered larval activity levels and anxiety behaviors in adult fish and, moreover, that these altered behaviors were transgenerational. The observed changes in fish activity in the present study may have great ecological importance because changes in small‐scale activity can alter encounter rates with food, resources, mates, and predators (Saaristo et al., 2018). If fish embryos are exposed to stressors such as OSPW‐derived NAFCs during development, they may experience lasting effects on fish survival and behavior beyond the changes in larvae that we observed.
Our findings highlight that exposure to NAFCs impairs embryonic survival and development and that, subsequently, these embryonic exposures impair larval fish survival and behavior. Changes in fish development and behavior may have important implications if fish are exposed to NAFCs in the Canadian oil sands region. We showed that exposure to 28–30 mg/L NAFCs causes a 50% reduction in survival and hatch viability in embryonic fish. As well, we found that exposure to 21 mg/L NAFCs has persistent adverse effects on larval survival and behaviors that could influence fish survival. Even a small variation in mortality can have major impacts on fish recruitment (Scott & Sloman, 2004). Thus, if fish are exposed to similar mixtures of fresh, untreated NAFCs following the accidental release of OSPW in natural ecosystems, these exposures have the potential to impair fish recruitment and populations in the Canadian oil sands region.
CONCLUSION
In Canada, new end‐of‐pipe regulations and an effects‐based monitoring framework for the oil sands sector will ultimately permit OSPW release into natural surface waters once it is deemed to be in a ready‐to‐release state (Alberta Energy Regulator, 2017; Government of Alberta, 2015). The specific criteria for “ready‐to‐release” OSPW should be informed by threshold NAFC concentrations, but these safe release limits have yet to be defined by the Canadian Council of Ministers of the Environment (2020), though naphthenic acids are currently under development to be listed by the Government of Canada (2021). Evidence‐informed decisions regarding tailings pond management require more research on the effects of NAFCs on aquatic wildlife including fishes, particularly studies on sublethal effects of lower concentrations that could persist throughout life histories. In our study, two main findings emerged that are important for understanding the persistent effects of NAFCs on fish. First, we found that embryonic exposure to NAFCs impairs fish embryonic development and later was associated with decreased larval survival. Second, our study is the first to demonstrate that sublethal embryonic NAFC exposure alters larval fish behavior, a novel finding with important implications for the survival and performance of fish exposed to sublethal NAFC concentrations. Our findings point toward the potential for early‐life exposures to sublethal NAFC concentrations to induce persistent effects on the fitness of fish populations through altered survival and behavioral patterns later in life. However, more research is required to understand these long‐term implications. Findings from our study are pertinent for developing end‐of‐pipe regulations for intended releases of OSPW.
Supporting Information
The Supporting Information is available on the Wiley Online Library at https://doi.org/10.1002/etc.5314.
Author Contributions Statement
Jessie S. Reynolds: Conceptualization; Methodology; Investigation; Formal analysis; Writing – original draft; Writing – review and editing; Visualization. Brianna L. Jackson: Conceptualization; Methodology; Investigation; Formal analysis; Writing – original draft; Writing – review and editing; Visualization. Barry N. Madison: Conceptualization; Methodology; Investigation; Writing – review and editing; Supervision. Chris K. Elvidge: Formal analysis; Writing – original draft; Writing – review and editing. Caleb T. Hasler: Conceptualization; Writing – review and editing. Sarah B. Yakimowski: Formal analysis; Writing – review and editing. Richard A. Frank: Investigation; Writing – review and editing. John V. Headley: Investigation; Writing – review and editing. L. Mark Hewitt: Investigation; Writing – review and editing. Kerry M. Peru: Investigation; Writing – review and editing. Diane M. Orihel: Conceptualization; Methodology; Writing – original draft; Writing – review and editing; Supervision; Project administration; Funding acquisition.
This article has earned an Open Data badge for making publicly available the digitally shareable data necessary to reproduce the reported results. The data are available at https://github.com/JessieReynolds/NAFCEmbryoLarvalStudy. Learn more about the Open Practices badges from the Center for Open Science: https://osf.io/tvyxz/wiki.
Supporting information
This article includes online‐only Supporting Information.
Supporting information.
Acknowledgment
We thank E. Lopez for her hard work in the field; B. Pauli, Y. Wang, and P. Blanchfield for their expert advice on this project; and two anonymous reviewers for providing helpful comments that improved the manuscript. Financial support for this research was provided by a grant and contribution (GCXE19S019) from Environment and Climate Change Canada (to D. M. Orihel) and the Queen's Research Opportunities Funds (to D. M. Orihel).
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (diane.orihel@queensu.ca).
REFERENCES
- Alberta Energy Regulator . (2017). Directive 085: Fluid tailings management for oil sands mining projects. https://www.aer.ca/regulating-development/rules-and-directives/directives/directive-085
- Alberta Energy Regulator . (2021a). Alberta energy outlook: Executive summary. https://static.aer.ca/prd/documents/sts/ST98/2021/st98-2021-executivesummary.pdf
- Alberta Energy Regulator . (2021b). State of Fluid Tailings Management for Mineable Oil Sands. https://static.aer.ca/prd/documents/reports/2020-State-Fluid-Tailings-Management-Mineable-OilSands.pdf
- Anderson, J. , Wiseman, S. B. , Moustafa, A. , Gamal El‐Din, M. , Liber, K. , & Giesy, J. P. (2012). Effects of exposure to oil sands process–affected water from experimental reclamation ponds on Chironomus dilutus . Water Research, 46(6), 1662–1672. [DOI] [PubMed] [Google Scholar]
- Andrés‐Delgado, L. , & Mercader, N. (2016). Interplay between cardiac function and heart development. Biochimica et Biophysica Acta, 1863(7), 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley, G. T. , & Villeneuve, D. L. (2006). The fathead minnow in aquatic toxicology: Past, present and future. Aquatic Toxicology, 78, 1, 91–102. [DOI] [PubMed] [Google Scholar]
- Bartlett, A. J. , Frank, R. A. , Gillis, P. L. , Parrott, J. L. , Marentette, J. R. , Brown, L. R. , Hooey, T. , Vanderveen, R. , McInnis, R. , Brunswick, P. , Shang, D. , Headley, J. V. , Peru, K. M. , & Hewitt, L. M. (2017). Toxicity of naphthenic acids to invertebrates: Extracts from oil sands process‐affected water versus commercial mixtures. Environmental Pollution, 227, 271–279. [DOI] [PubMed] [Google Scholar]
- Bauer, A. E. , Hewitt, L. M. , Parrott, J. L. , Bartlett, A. J. , Gillis, P. L. , Deeth, L. E. , Rudy, M. D. , Vanderveen, R. , Brown, L. , Campbell, S.D. , Rodrigues, M. R. , Farwell, A. J. , Dixon, D. G. , & Frank, R. A. (2019). The toxicity of organic fractions from aged oil sands process‐affected water to aquatic species. Science of the Total Environment, 669, 702–710. [DOI] [PubMed] [Google Scholar]
- Berube, R. , Lefbvre‐Raine, M. , Gauthier, C. , Bourdin, T. , Bellot, P. , Triffault‐Bouchet, G. , Langlois, V. S. , & Couture, P. (2022). Comparative toxicity of conventional and unconventional oils during rainbow trout (Oncorhynchus mykiss) embryonic development: From molecular to health consequences. Chemosphere, 288(2), Article 13251. [DOI] [PubMed] [Google Scholar]
- Cachat, J. , Stewart, A. , Grossman, L. , Gaikwad, S. , Kadri, F. , Chung, K. M. , Wu, N. , Wong, K. , Roy, S. , Suciu, C. , Goodspeed, J. , Elegante, M. , Bartels, B. , Elkhayat, S. , Tien, D. , Tan, J. , Denmark, A. , Gilder, T. , Kyzar, E. , … Kalueff, A. V. (2010). Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nature Protocols, 5, 1786–1799. [DOI] [PubMed] [Google Scholar]
- Canadian Council of Ministers of the Environment . (2020). Canadian environmental quality guidelines. https://ccme.ca/en/current-activities/canadian-environmental-quality-guidelines
- Commission for Environmental Cooperation . (2020). Alberta tailings ponds II. Factual record regarding submission SEM‐17‐001. http://www.cec.org/wp-content/uploads/wpallimport/files/17-1-ffr_en.pdf
- Frank, R. A. , Fischer, K. , Kavanagh, R. , Burnison, B. K. , Arsenault, G. , Headley, J. V. , Peru, K. M. , Van Der Kraak, G. , & Solomon, K. R. (2009). Effect of carboxylic acid content on the acute toxicity of oil sands naphthenic acids. Environmental Science & Technology, 43(2), 266–271. [DOI] [PubMed] [Google Scholar]
- Frank, R. A. , Kavanagh, R. , Burnison, B. K. , Headley, J. V. , Peru, K. M. , Van Der Kraak, G. , & Solomon, K. R. (2006). Diethylaminoethyl‐cellulose clean‐up of a large volume naphthenic acid extract. Chemosphere, 64, 1346–1345. [DOI] [PubMed] [Google Scholar]
- Frank, R. A. , Roy, J. W. , Bickerton, G. , Rowland, S. J. , Headley, J. V. , Scarlett, A. G. , West, C. E. , Peru, K. M. , Parrott, J. L. , Conly, F. M. , & Hewitt, L. M. (2014). Profiling oil sands mixtures from industrial developments and natural groundwaters for source identification. Environmental Science & Technology, 48(5), 2660–2670. [DOI] [PubMed] [Google Scholar]
- Friard, O. , & Gamba, M. (2016). BORIS: A free, versatile open‐source event‐logging software for video/audio coding and live observations. Methods in Ecology and Evolution, 7, 1325–1330. [Google Scholar]
- Froese, R. (2006). Cube law, condition factor and weight–length relationships: History, meta‐analysis and recommendations. Journal of Applied Ichthyology, 22(4), 241–253. [Google Scholar]
- Gesto, M. , Tintos, A. , Alvarez, R. , Soengas, J. L. , & Miguez, J. M. (2009). Alterations in the brain monoaminergic neurotransmitters of rainbow trout related to naphthalene exposure at the beginning of vitellogenesis. Fish Physiology and Biochemistry, 35, 453–465. [DOI] [PubMed] [Google Scholar]
- Government of Alberta . (2015). Lower Athabasca region: Tailings management framework for the mineable Athabasca oil sands. https://open.alberta.ca/dataset/962bc8f4-3924-46ce-baf8-d6b7a26467ae/resource/7c49eb63-751b-49fd-b746-87d5edee3131/download/2015-larp-tailingsmgtathabascaoilsands.pdf
- Government of Canada . (2021). Federal environmental quality guidelines. Chemical substances fact sheets and frequently asked questions. https://www.canada.ca/en/health-canada/services/chemical-substances/fact-sheets/federal-environmental-quality-guidelines.html#shr-pg0
- He, Y. , Patterson, S. , Wang, N. , Hecker, M. , Martin, J. W. , El‐Din, M. G. , Giesy, J. P. , & Wiseman, S. B. (2012). Toxicity of untreated and ozone‐treated oil sands process–affected water (OSPW) to early life stages of the fathead minnow (Pimephales promelas). Water Research, 46, 6359–6368. [DOI] [PubMed] [Google Scholar]
- Headley, J. V. , Peru, K. M. , Mohamed, M. H. , Frank, R. A. , Martin, J. W. , Hazewinkel, R. R. , Humphries, D. , Gurprasad, N. P. , Hewitt, L. M. , Muir, D.C. , Lindeman, D. , Strub, R. , Young, R. F. , Grewer, D. M. , Whittal, R. M. , Fedorak, P. M. , Birkholz, D. A. , Hindle, R. , Reisdorph, R. , … Wrona, F. J. (2013). Chemical fingerprinting of naphthenic acids and oil sands process waters—A review of analytical methods for environmental samples. Journal of Environmental Science and Health Part A, 48(10), 1145–1163. [DOI] [PubMed] [Google Scholar]
- Hewitt, L. M. , Roy, J. W. , Rowland, S. J. , Bickerton, G. , DeSilva, A. , Headley, J. V. , Milestone, C. B. , Scarlett, A. G. , Brown, S. , Spencer, C. , West, C. E. , Peru, K. M. , Grapentine, L. , Ahad, J. , Pakdel, H. , & Frank, R. A. (2020). Advances in distinguishing groundwater influenced by oil sands process–affected water (OSPW) from natural bitumen‐influenced groundwaters. Environmental Science & Technology, 54(3), 1522–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicken, C. E. , Linbo, T. L. , Baldwin, D. H. , Willis, M. L. , Myers, M. S. , Holland, L. , Larsen, M. , Stekoll, M. S. , Rice, S. D. , Collier, T. K. , Scholz, N. L. , & Incardona, J. P. (2011). Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proceedings of the National Academy of Sciences of the United States of America, 108(17), 7086–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona, J. P. , Carls, M. G. , Day, H. L. , Sloan, C. A. , Bolton, J. L. , Collier, T. K. , & Scholz, N. L. (2009). Cardiac arrhythmia is the primary response of embryonic pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environmental Science & Technology, 43, 201–207. [DOI] [PubMed] [Google Scholar]
- Incardona, J. P. , Carls, M. G. , Holland, L. , Linbo, T. L. , Baldwin, D. H. , Myers, M. S. , Peck, K. A. , Tagal, M. , Rice, S. D. , & Scholz, N. L. (2015). Very low embryonic crude oil exposures cause lasting cardiac defects in salmon and herring. Scientific Reports, 5, Article 13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson, S. M. , Kluxen, F. M. , Streibig, J. C. , Cedergreen, N. , & Ritz, C. (2020). BMD: An R package for benchmark dose estimation. PeerJ, 8, Article e10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh, R. J. , Frank, R. A. , Solomon, K. R. , & Van Der Kraak, G. (2013). Reproductive and health assessment of fathead minnows (Pimephales promelas) inhabiting a pond containing oil sands process‐affected water. Aquatic Toxicology, 130–131, 201–209. [DOI] [PubMed] [Google Scholar]
- Li, C. , Fu, L. , Stafford, J. , Belosevic, M. , & El‐Din, M. G. (2017). The toxicity of oil sands process–affected water (OSPW): A critical review. Science of the Total Environment, 601–602, 1785–1802. [DOI] [PubMed] [Google Scholar]
- Loughery, J. R. , Marentette, J. R. , Frank, R. A. , Hewitt, L. M. , Parrott, J. L. , & Martyniuk, C. J. (2019). Transcriptome profiling in larval fathead minnow exposed to commercial naphthenic acids and extracts from fresh and aged oil sands process–affected water. Environmental Science & Technology, 53, 10435–10444. [DOI] [PubMed] [Google Scholar]
- Madison, B. N. , Reynolds, J. , Halliwell, L. , Leshuk, T. , Gu, F. , Peru, K. M. , Headley, J. V. , & Orihel, D. M. (2020). Can the toxicity of naphthenic acids in oil sands process–affected water be mitigated by a green photocatalytic method? FACETS, 5(1), 474–487. [Google Scholar]
- Mahaffey, A. , & Dube, M. (2017). Review of the composition and toxicity of oil sands process–affected water. Environmental Reviews, 25(1), 97–114. [Google Scholar]
- Marentette, J. R. , Frank, R. A. , Bartlett, A. J. , Gillis, P. L. , Hewitt, L. M. , Peru, K. M. , Headley, J. V. , Brunswick, P. , Shang, D. , & Parrott, J. L. (2015). Toxicity of naphthenic acid fraction components extracted from fresh and aged oil sands process–affected waters, and commercial naphthenic acid mixtures, to fathead minnow (Pimephales promelas) embryos. Aquatic Toxicology, 164, 108–117. [DOI] [PubMed] [Google Scholar]
- Marentette, J. R. , Frank, R. A. , Hewitt, L. M. , Gillis, P. L. , Bartlett, A. J. , Brunswick, P. , Shang, D. , & Parrott, J. L. (2015). Sensitivity of walleye (Sander vitreus) and fathead minnow (Pimephales promelas) early‐life stages to naphthenic acid fraction components extracted from fresh oil sands process–affected waters. Environmental Pollution, 207, 59–67. [DOI] [PubMed] [Google Scholar]
- Marentette, J. R. , Sarty, K. , Cowie, A. M. , Frank, R. A. , Hewitt, L. M. , Parrott, J. L. , & Martyniuk, C. J. (2017). Molecular responses of walleye (Sander vitreus) embryos to naphthenic acid fraction components extracted from fresh oil sands process‐affected water. Aquatic Toxicology, 182, 11–19. [DOI] [PubMed] [Google Scholar]
- Morandi, G. D. , Wiseman, S. B. , Pereira, A. , Mankidy, R. , Gault, I. G. M. , Martin, J. W. , & Giesy, J. P. (2015). Effects‐directed analysis of dissolved organic compounds in oil sands process–affected water. Environmental Science & Technology, 49(20), 12395–12404. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co‐operation and Development . (2013). Test No. 210: Fish, early‐life stage toxicity test. OECD Guidelines for the Testing of Chemicals.
- Peters, L. E. , MacKinnon, M. , Van Meer, T. , van den Heuvel, M. R. , & Dixon, D. G. (2007). Effects of oil sands process–affected water and naphthenic acids on yellow perch (Perca flavescens) and Japanese medaka (Orizias latipes) embryonic development. Chemosphere, 67, 2177–2183. [DOI] [PubMed] [Google Scholar]
- Philibert, D. A. , Lyons, D. D. , Qin, R. , Huang, R. , El‐Din, M. G. , & Tierney, K. B. (2019). Persistent and transgenerational effects of raw and ozonated oil sands process–affected water exposure on a model vertebrate, the zebrafish. Science of the Total Environment, 693, Article 133611. [DOI] [PubMed] [Google Scholar]
- R Foundation for Statistical Computing . (2020). R: A language and environment for statistical computing. https://www.R-project.org/
- Richardson, S. D. , & Ternes, T. A. (2018). Water analysis: Emerging contaminants and current issues. Analytical Chemistry, 90(1), 398–428. [DOI] [PubMed] [Google Scholar]
- Ritz, C. , Baty, F. , Streibig, J. C. , & Gerhard, D. (2015). Dose–response analysis using R. PLoS One, 10(12), Article e0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz, C. , & Streibig, J. C. (2005). Bioassay analysis using R. Journal of Statistical Software, 12, 1–22. [Google Scholar]
- Rogers, V. V. , Liber, K. , & MacKinnon, M. D. (2002). Isolation and characterization of naphthenic acids from Athabasca oil sands tailings pond water. Chemosphere, 48(5), 519–527. [DOI] [PubMed] [Google Scholar]
- Rowland, S. J. , Scarlett, A. G. , Jones, D. , West, C. E. , & Frank, R. A. (2011). Diamonds in the rough: Identification of individual naphthenic acids in oil sands process water. Environmental Science & Technology, 45(7), 3154–3159. [DOI] [PubMed] [Google Scholar]
- Rueden, C. T. , Schindelin, J. , Hiner, M. C. , DeZonia, B. E. , Walkter, A. E. , Arena, E. T. , & Eliceiri, K. W. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics, 18(1), 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaristo, M. , Brodin, T. , Balshine, S. , Bertram, M. G. , Brooks, B. W. , Ehlman, S. M. , McCallum, E. S. , Sih, A. , Sundin, J. , Wong, B. , & Arnold, K. E. (2018). Direct and indirect effects of chemical contaminants on the behavior, ecology and evolution of wildlife. Proceedings of the Royal Society B, 285, Article 20181297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, G. R. , & Sloman, K. A. (2004). The effects of environmental pollutants on complex fish behavior: Integrating behavioral and physiological indicators of toxicity. Aquatic Toxicology, 68(4), 369–392. [DOI] [PubMed] [Google Scholar]
- Sharma, M. (2019). Behavioral responses in effect to chemical stress in fish: A review. International Journal of Fisheries and Aquatic Studies, 7(1), 1–5. [Google Scholar]
- Siwik, P. L. , Van Meer, T. , MacKinnon, M. D. , & Paszkowski, C. A. (2000). Growth of fathead minnows in oilsand‐processed wastewater in laboratory and field. Environmental Toxicology and Chemistry, 19, 1837–1845. [Google Scholar]
- Smith, B. R. , & Blumstein, D. T. (2008). Fitness consequences of personality: A meta‐analysis. Behavioral Ecology, 19(2), 448–455. [Google Scholar]
- Smith, H. T. , Schreck, C. B. , & Maughan, O. E. (1978). Effect of population density and feeding rate on the fathead minnow (Pimephales promelas). Journal of Fish Biology, 12(5), 449–455. [Google Scholar]
- Sun, C. , Shotyk, W. , Cuss, C. W. , Donner, M. W. , Fennell, J. , Javed, M. , Noernberg, T. , Poesch, M. , Pelletier, R. , Sinnatamby, N. , Siddique, T. , & Martin, J. W. (2017). Characterization of naphthenic acids and other dissolved organics in natural water from the Athabasca oil sands region, Canada. Environmental Science & Technology, 51, 9524–9532. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency . (2006). Culturing of fathead minnows (Pimephales promelas). https://www.epa.gov/sites/default/files/2016-02/documents/culturing_of_fathead_minnows_supplememt_to_training_video.pdf
- Van Wezel, A. P. , & Opperhuizen, A. (1995). Narcosis due to environmental pollutants in aquatic organisms: Residue‐based toxicity, mechanisms, and membrane burdens. Critical Reviews in Toxicology, 25, 255–279. [DOI] [PubMed] [Google Scholar]
- Villalobos, S. A. , Papoulias, D. M. , Meadows, J. , Blankenship, A. L. , Pastva, S. D. , Kannan, K. , Hinton, D. E. , Tillitt, D. E. , & Giesy, J. P. (2000). Toxic responses of medaka, d‐rR strain, to polychlorinated naphthalene mixtures after embryonic exposure by in ovo nanoinjection: A partial life‐cycle assessment. Environmental Toxicology and Chemistry, 19, 432–440. [Google Scholar]
- Wallace, R. R. , & McCart, P. J. (1984). The fish and fisheries of the Athabasca River basin: Status and environmental requirements. Oil Sands Research and Information Network (OSRIN), Government of Alberta Reports. Alberta Environment and Sustainable Resource Development. https://era.library.ualberta.ca/items/376ef8a1-0283-4978-846b-86a46468a86b
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supporting Information.
Supporting information.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (diane.orihel@queensu.ca).


