Abstract
Introduction
Spirometry plays an important role in the assessment of possible respiratory failure in children with neuromuscular diseases (NMDs). However, obtaining reliable spirometry results is a major challenge. We studied the relation between oscillometry and spirometry results. Oscillometry is an easy, noninvasive method to measure respiratory resistance R and reactance X. We hypothesized an increased R and reduced X in patients with more reduced lung function.
Methods
In this prospective single‐center study, we included all children with NMDs able to perform spirometry. We consecutively measured R and X at 5, 11, and 19 Hz and (forced) vital capacity, peak expiratory flow. Spearman correlation coefficients and positive and negative predictive values were calculated. Regression curves were estimated.
Results
We included 148 patients, median age 13 years (interquartile range: 8–16). A negative correlation was found between R and spirometry outcomes (Spearman correlation coefficient [ρ]: −0.5 to −0.6, p < 0.001). A positive correlation was found between X (i.e., less negative outcomes) and spirometry outcomes (ρ: 0.4–0.6, p < 0.001). Highest correlation was found at lower frequencies. Regression analysis showed a nonlinear relation. Measurement of inspiratory and expiratory R and X did not provide added value. Positive predictive values of 80%–85% were found for z‐scores of R measured at 5 Hz versus (F)VC ≤ 60%.
Conclusion
We found a nonlinear relation between oscillometry and spirometry results with increased R and reduced X in patients with more restrictive lung function decline. Given the difficulties with performing spirometry, oscillometry may be a promising substitute.
Keywords: child, lung function, neuromuscular diseases
1. INTRODUCTION
Children with neuromuscular diseases (NMDs) may develop progressive respiratory failure due to respiratory muscle weakness. This leads to a decline in lung function, which is often further aggravated by a scoliosis and recurrent respiratory tract infections. Timing of respiratory failure varies in different NMDs. 1
Guidelines suggest to measure vital capacity (VC) in all patients with NMDs who are capable of performing spirometry as part of the respiratory assessment. 2
In children with NMDs spirometry results may steer clinical decision making with regard to respiratory care, support counseling about the timing of pending chronic respiratory failure, and may assist in the evaluation of the effects of NMD‐specific treatments (such as the recently introduced survival motor neuron protein augmenting therapies in spinal muscular atrophy [SMA]). 1 , 2 , 3 , 4 In healthy children reproducible spirometry is generally achievable from 6 years of age. In children of all ages who are weak, obtaining reliable spirometry results may be challenging, especially forced maneuvers. 2
Respiratory function in children with NMDs is often impaired by the age at which spirometry is feasible. 5 For this reason, there is an urgent need for alternative noninvasive lung function tests in children unable to perform spirometry. 1 Oscillometry may serve as a surrogate test in children unable to perform spirometry. Oscillometry is a noninvasive, versatile method to measure respiratory mechanics. Small‐amplitude pressure oscillations are superimposed on the normal breathing, thereby avoiding the need for any special breathing maneuver or any noticeable interference with respiration. 6 , 7 Oscillometry measures respiratory resistance R and respiratory reactance X. R describes the dissipative mechanical properties of the respiratory system, 5 and mainly reflects the frictional opposition offered by the conducting airways to the flow of air. 8 With progression of NMDs, it is expected that R values increase due to underinflation, secretions in the airways, and micro‐atelectasis. 5 X measures the relationship between pressure and volume (the elastic properties) at low oscillation frequencies and the relationship between pressure and volume acceleration (the inertive properties) which become progressively more important at increasing frequencies. 5 , 9 With progression of NMDs, X is expected to reduce due to less compliant chest wall and reduced lung volumes. 5 Different frequencies are used in oscillometry: lower frequency impulses travel deeper into the lung and reflect the mechanical behavior of smaller airways, while higher frequencies are more sensitive to upper airway pathology. 7
Previous studies have shown that oscillometry is feasible in children with SMA and Duchenne muscular dystrophy (DMD) as young as 3 years. 5 , 8 , 10 Here, we aimed to assess the relationship between results of oscillometry and spirometry in children with NMDs and hypothesized the presence of a correlation between these tests. We hypothesized an increased R and reduced X in patients with more restrictive lung function decline, due to reduced compliance of the chest wall and reduced lung volumes. 5
2. METHODS
In this prospective cross‐sectional study, all children with NMDs attending the outpatient department of the Center of Home Mechanical Ventilation of the University Medical Center Utrecht, and able to perform spirometry were included once between August 2019 and May 2021. Patients with tracheostomy were excluded.
Oscillometry (ResmonPro Restech®) and spirometry data (Geratherm Spirostik®) were measured consecutively at the department of pediatric pulmonology at the University Medical Center Utrecht. These lung function tests were performed by a small team of professionals experienced in conducting these tests in children.
To measure oscillometry, children were seated with the head in neutral position, connected to the oscillation device via a mouthpiece and a noseclip in place. Cheek and floor of mouth were supported by the lung function technician. Measurements were obtained according to the American Thoracic Society/European Respiratory Society guidelines. 11 Our oscillometry equipment measures at 5, 11, and 19 Hz to obtain more reliable measurements by not overlapping the impulses. We studied (Forced) vital capacity ((F)VC) and peak expiratory flow (PEF). Spirometry was measured and reported according to the European Respiratory Society guidelines. 12 All tests were performed in sitting position, without corsets or braces.
We studied the relation between R and X during inspiration (insp), expiration (exp), and total breath (tot) and (F)VC and PEF. We did a subgroup analysis in patients with SMA and DMD, the most common NMDs included in this study.
The study was approved by the medical ethical committee of the University Medical Center Utrecht (16‐563/c). Informed consent was obtained from all participants and/or their parents in case of minors.
2.1. Statistical analysis
We used descriptive statistics to describe baseline characteristics. Only measurements obtained during the first visit after inclusion were used for analyses. Z‐scores were calculated for oscillometry outcomes measured at 5 Hz. 13 We calculated the nonparametric two‐tailed spearman correlation coefficients (ρ) to describe the relation between oscillometry and spirometry results. Regression curve was estimated using IBM SPSS 26.0. To study the ability to predict and exclude moderate (i.e., ≤60% of predicted) lung function restriction based on abnormal or normal z‐scores of oscillometry results measured at 5 Hz, we calculated the positive and negative predictive values.
3. RESULTS
We included 148 patients with a median age of 13 years. One third of the patients were patients with SMA, one quarter of the patients were patients with DMD. About 15% of the patients were supported by home mechanical ventilation. Baseline characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Age in years, median (IQR) | 13 (8–16) |
| Male gender, n (%) | 92 (62) |
| Home mechanical ventilation, n (%) | 22 (15) |
| Body Mass Index, median (IQR) | 19 (16–22) |
| Underlying disease | |
| Spinal muscular atrophy | 46 (31) |
| Duchenne muscular dystrophy | 36 (24) |
| Other muscular dystrophy | |
| Limb girdle | 8 (5) |
| Ullrich | 5 (3) |
| Emery‐Dreifuss | 2 (1) |
| Becker | 2 (1) |
| Other | 5 (3) |
| Congenital myopathy | |
| Central core | 3 (2) |
| Nemaline | 2 (1) |
| Bethlem | 2 (1) |
| Other | 11 (7) |
| Myasthenic syndrome | 3 (2) |
| Hereditary sensory and motor neuropathy | 4 (3) |
| Mitochondrial myopathy | 8 (5) |
| Myotonic dystrophy | 11 (7) |
Abbreviations: IQR, interquartile range; n, number.
Oscillometry measurement was feasible in all children. Spearman correlation coefficients of the relation between oscillometry and spirometry results are shown in Table 2. All correlation coefficients were statistically significant with p < 0.001. A negative correlation was found between R and spirometry outcomes (Spearman correlation coefficient (ρ) between −0.5 and −0.6). A positive correlation was found between X (i.e., less negative outcomes) and spirometry outcomes (ρ between 0.4 and 0.6). Highest correlation was found at lower frequencies. This confirmed our hypothesis: an increased R and reduced X were observed in patients with lower (F)VC and PEF.
Table 2.
Association between oscillometry results (measured at 5, 11, and 19 Hz) and spirometry results using Spearman correlation coefficients
| VC | FVC | PEF | |
|---|---|---|---|
| R | |||
| 5 Hz | |||
| insp | −0.564 | −0.500 | −0.619 |
| exp | −0.559 | −0.502 | −0.637 |
| tot | −0.568 | −0.508 | −0.637 |
| 11 Hz | |||
| insp | −0.576 | −0.512 | −0.633 |
| exp | −0.564 | −0.518 | −0.638 |
| tot | −0.578 | −0.521 | −0.644 |
| 19 Hz | |||
| insp | −0.537 | −0.462 | −0.584 |
| exp | −0.558 | −0.501 | −0.605 |
| tot | −0.551 | −0.486 | −0.597 |
| X | |||
| 5 Hz | |||
| insp | 0.610 | 0.596 | 0.579 |
| exp | 0.512 | 0.524 | 0.539 |
| tot | 0.626 | 0.612 | 0.622 |
| 11 Hz | |||
| insp | 0.557 | 0.542 | 0.596 |
| exp | 0.511 | 0.508 | 0.574 |
| tot | 0.548 | 0.537 | 0.600 |
| 19 Hz | |||
| insp | 0.488 | 0.493 | 0.521 |
| exp | 0.435 | 0.434 | 0.522 |
| tot | 0.480 | 0.474 | 0.551 |
Abbreviations: exp, expiratory; FVC, forced vital capacity; Hz, Hertz; insp, inspiratory; PEF, peak expiratory flow; R, respiratory resistance; tot, total breath (inspiratory and expiratory); VC, vital capacity; X, respiratory reactance.
Measurement of inspiratory and expiratory R and X did not provide added value, as correlation coefficients were similar to total R and X. Regression curves showing the relation between both R and X measured at 5 Hz and spirometry results are shown in Figures 1 and 2, respectively.
Figure 1.
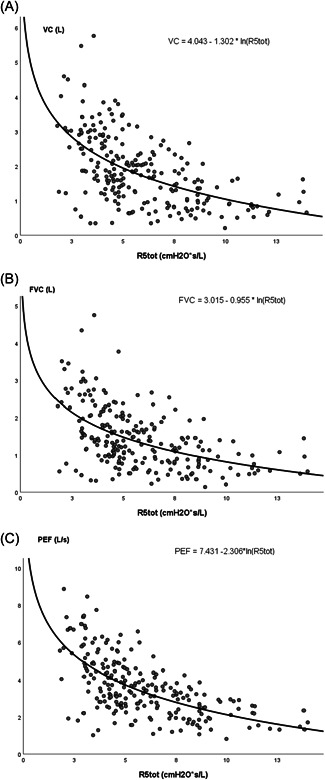
Regression curves showing the relation between respiratory resistance measured at 5 Hz (R5tot) and spirometry results. (A) Vital capacity (VC). (B) Forced vital capacity (FVC). (C) Peak expiratory flow (PEF). L, liter; R5tot, respiratory resistance measured at 5 Hz; s, second.
Figure 2.
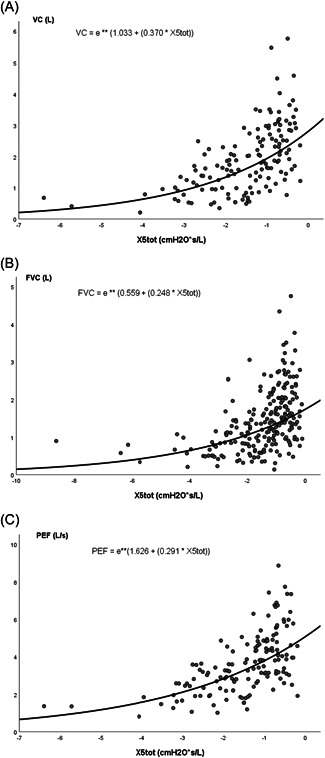
Regression curves showing the relation between respiratory reactance measured at 5 Hz (X5tot) and spirometry results. (A) Vital capacity (VC). (B) Forced vital capacity (FVC). (C) Peak expiratory flow (PEF). L, liter; s, second; X5tot, respiratory reactance measured at 5 Hz.
Subgroup analysis in 46 patients with SMA and 36 patients with DMD showed that the correlation between oscillometry and spirometry was poor in patients with DMD compared to patients with SMA. Highest correlation was found in patients with SMA between oscillometry results and PEF (ρ > 0.5; Table 3).
Table 3.
Association between respiratory oscillometry results (measured at 5, 11, and 19 Hz) and spirometry results using Spearman correlation coefficients in patients with Duchenne muscular dystrophy and spinal muscular atrophy
| NMDs | n | VC | FVC | PEF | |
|---|---|---|---|---|---|
| R | |||||
| 5 Hz | Total group | 148 | −0.568 | −0.508 | −0.637 |
| SMA | 46 | −0.500 | −0.401 | −0.550 | |
| DMD | 36 | −0.262 | −0.203 | −0.355 | |
| 11 Hz | Total group | 148 | −0.578 | −0.521 | −0.644 |
| SMA | 46 | −0.508 | −0.447 | −0.588 | |
| DMD | 36 | −0.261 | −0.195 | −0.354 | |
| 19 Hz | Total group | 148 | −0.551 | −0.486 | −0.597 |
| SMA | 46 | −0.477 | −0.397 | −0.553 | |
| DMD | 36 | −0.261 | −0.195 | −0.354 | |
| X | |||||
| 5 Hz | Total group | 148 | 0.626 | 0.612 | 0.622 |
| SMA | 46 | 0.450 | 0.413 | 0.587 | |
| DMD | 36 | 0.483 | 0.455 | 0.304 | |
| 11 Hz | Total group | 148 | 0.548 | 0.537 | 0.600 |
| SMA | 46 | 0.462 | 0.362 | 0.547 | |
| DMD | 36 | 0.295 | 0.357 | 0.359 | |
| 19 Hz | Total group | 148 | 0.480 | 0.474 | 0.551 |
| SMA | 46 | 0.457 | 0.356 | 0.559 | |
| DMD | 36 | 0.187 | 0.255 | 0.324 |
Abbreviations: DMD, Duchenne muscular dystrophy; FVC, forced vital capacity; Hz, Hertz; n, number; PEF, peak expiratory flow; R, respiratory resistance; SMA, spinal muscular atrophy; VC, vital capacity; X, respiratory reactance.
Median z‐scores of R and X measured at 5 Hz were −0.16 (interquartile range [IQR]: −0.77 to 0.93) and 0.27 (IQR: −0.48 to 0.76) respectively. We found no significant correlation between calculated z‐scores of R and X measured at 5 Hz and standardized lung function test results.
Finally, we studied if (ab)normal z‐scores of R and X measured at 5 Hz could exclude or predict the presence of moderate lung function restriction by calculating negative and positive predictive values. Negative predictive values of 65%, 32%, and 55% were found for R and X versus VC, FVC, and PEF. Positive predictive values of 80%, 85%, and 62% were found for R versus VC, FVC, and PEF.
4. DISCUSSION
In this study, we have shown that oscillometry may be used as a substitute for spirometry in children with NMDs, especially oscillometry measurements at lower frequencies. An increased R and reduced X were observed in patients with lower (F)VC and PEF. Positive predictive values of 80%–85% were found for z‐scores of R measured at 5 Hz versus (F)VC ≤ 60%, suggesting that oscillometry may be used to predict moderate lung function restriction, that is, (F)VC ≤ 60% of predicted, in children with NMDs.
Although obtaining reliable spirometry results is challenging in young or severely affected patients with NMDs, spirometry results are important outcomes in patients with NMDs. For example, in these patients significantly lowered FVC values, that is, ≤60% of predicted, are associated with an increased risk of REM‐ and NREM‐related sleep‐disordered breathing, 2 , 14 and PEF has been shown to be a sensitive marker to monitor respiratory muscle strength in patients with DMD. 15 However, limited data are available on oscillometry in patients with NMDs. 5 , 8 , 10 , 16 Although oscillometry provides an objective measure, it does not measure the same aspects of respiratory function as other tests. 5 With progression of NMDs, it is expected that R values increase due to underinflation, secretions in the airways, and micro‐atelectasis. 5 In this study we confirmed increased R values with decreased spirometry results. In patients with NMDs, X is expected to be reduced due to reduced compliance of the chest wall and reduced lung volumes. 5 In this study we confirmed more reduced, that is, more negative, X values in patients with more reduced spirometry outcomes. We found the highest correlation at lower frequencies, probably explained by the involvement of smaller airways due to micro‐atelectasis and secretions.
Two previous studies by the same group have shown that the use of oscillometry as a surrogate outcome to measure lung function was feasible in children with SMA. Gauld et al. 5 showed a linear relationship between X measured at 8 Hz and FVC in four children with SMA. Kapur et al. 10 showed that children with SMA requiring noninvasive ventilation (NIV) (n = 10) had an abnormal R measured at 8 Hz compared to children not using NIV (n = 15). Although not linear, we confirmed the significant relation between spirometry and R and X in this larger number of patients. A study in patients with DMD showed a poor correlation between oscillometry outcomes and spirometric variables, all expressed as z‐scores, in healthy subjects as well as in patients with DMD. 8 These poor correlations were confirmed in our study.
Oscillometry has the potential of separate analysis of inspiratory and expiratory R and X, to determine disease‐specific changes in the mechanical behavior over the breathing cycle. Inspiratory R has been proven to be a very sensitive index of airway caliber. 7 Airway caliber may be reduced in smaller airways of children with NMDs due to reduced compliance, micro‐atelectasis, and retention of airway secretions. This study did not show the added value of measuring inspiratory and expiratory R and X separately.
Our study has several limitations. First, the lack of global reference equations for oscillometry is regrettable. 17 Generally, there is significant variability in the reported R and X values, depending on the characteristics of the examined populations and the equipment and technique used. 7 We calculated z‐scores based on a Polish study, which estimated regression equations based on oscillometry results in 626 healthy children aged 3–18 years. 13 Our study population had a heterogeneous ethnic background and we used different equipment. For this reason, calculated z‐scores in this study may not be fully accurate.
Also, the calculation of z‐scores was limited to measurements at 5 Hz, as we were unable to find reference equations for oscillometry measurements at 11 and 19 Hz in this age group.
Second, as this study was not aimed at studying the feasibility of oscillometry measurements in young or less cooperative children, we only included patients able to perform spirometry. It therefore remains unclear whether our results are generalizable to much younger children with NMDs, although we expect this to be the case as oscillometry has been shown to allow for evaluations of respiratory mechanics in neonates 18 and young children. 5 , 8 , 10 , 17 , 19
Although the number of included patients in this study was much higher than in previous studies in patients with NMDs, we did not study follow‐up data of these patients. Research is required to include repeated measures over time, to study the ability to predict lung function decline by using oscillometry.
5. CONCLUSION
We observed a nonlinear relation between oscillometry measurements and spirometry results in children with NMDs. We confirmed our hypothesis and found an increased R and reduced X in patients with more restrictive lung function decline. Oscillometry may be promising as a surrogate measure of lung function in these children.
AUTHOR CONTRIBUTIONS
Esther S. Veldhoen: Conceptualization (lead); data curation (equal); formal analysis (lead); methodology (lead); writing – original draft (lead). Johan H. Roos: Conceptualization (equal), data curation (equal), formal analysis (equal), methodology (equal), writing – review and editing (equal). Rolien Bekkema: data curation (equal), writing – review and editing (equal). Ludo W. van der Pol: Data curation (equal), writing – review and editing (equal). Marcel H. B. Tinnevelt: Data curation (equal), writing – review and editing (equal). Laura P. Verweij‐van den Oudenrijn: Data curation (equal); writing – review and editing (equal). Roelie M. Wosten‐van Asperen: Data curation (equal), writing – review and editing (equal). Erik H. J. Hulzebos: Data curation (equal), writing – review and editing (equal). Camiel A. Wijngaarde: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Cornelis K. van der Ent: Conceptualization (equal), data curation (equal), formal analysis (equal), methodology (equal), writing – review and editing (equal).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
The funding information is not available.
Veldhoen ES, Roos JH, Bekkema R, et al. Oscillometry: a substitute of spirometry in children with neuromuscular diseases? Pediatric Pulmonology. 2022;57:1618‐1624. 10.1002/ppul.25923
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Fauroux B, Khirani S. Neuromuscular disease and respiratory physiology in children: putting lung function into perspective. Respirology. 2014;19(6):782‐791. [DOI] [PubMed] [Google Scholar]
- 2. Hull J, Aniapravan R, Chan E, et al. Guideline for respiratory management of children with neuromuscular disease. Thorax. 2012;67:1‐40. [DOI] [PubMed] [Google Scholar]
- 3. Buu MC. Respiratory complications, management and treatments for neuromuscular disease in children. Curr Opin Pediatr. 2017;29:326‐333. [DOI] [PubMed] [Google Scholar]
- 4. Gower WA, Birnkrant DJ, Black JB, Noah TL. Pediatric Pulmonology Year in Review 2018: Rare lung disease, neuromuscular disease, and diagnostic testing. Pediatr Pulmonol. 2019;54:1655‐1662. [DOI] [PubMed] [Google Scholar]
- 5. Gauld LM, Keeling LA, Shackleton CE, Sly PD. Forced oscillation technique in spinal muscular atrophy. Chest. 2014;146(3):795‐803. [DOI] [PubMed] [Google Scholar]
- 6. King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55:1900753. [DOI] [PubMed] [Google Scholar]
- 7. Skylogianni E, Douros K, Anthracopoulos MB, Fouzas S. The forced oscillation technique in paediatric respiratory practice. Paediatr Respir Rev. 2016;18:46‐51. [DOI] [PubMed] [Google Scholar]
- 8. Gochicoa‐Rangel L, Vargas MH, Alonso‐Gómez JL, et al. Respiratory impedance in patients with Duchenne muscular dystrophy. Pediatr Pulmonol. 2016;51:1072‐1079. [DOI] [PubMed] [Google Scholar]
- 9. Oostveen E, Macleod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments on behalf of the ERS Task Force on Respiratory Impedance Measurements. Eur Respir J. 2003;22(6):1026‐1041. [DOI] [PubMed] [Google Scholar]
- 10. Kapur N, Deegan S, Parakh A, Gauld L. Relationship between respiratory function and need for NIV in childhood SMA. Pediatr Pulmonol. 2019;54(11):1174‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beydon N, Davis SD, Lombardi E, et al. An Official American Thoracic Society/European Respiratory Society Statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175(12):1304‐1345. [DOI] [PubMed] [Google Scholar]
- 12. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 13. Nowowiejska B, Tomalak W, Radliński J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3‐18 years. Pediatr Pulmonol. 2008;43:1193‐1197. [DOI] [PubMed] [Google Scholar]
- 14. Finkel RS, Mercuri E, Meyer OH, et al. Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28(3):197‐207. [DOI] [PubMed] [Google Scholar]
- 15. Buyse GM, Goemans N, Van Den Hauwe M, Meier T. Effects of glucocorticoids and idebenone on respiratory function in patients with Duchenne muscular dystrophy. Pediatr Pulmonol. 2013;48(9):912‐920. [DOI] [PubMed] [Google Scholar]
- 16. Wesseling G, Quaedvlieg FCM, Wouters EFM. Oscillatory mechanics of the respiratory system in neuromuscular disease. Chest. 1992;102(6):1752‐1757. [DOI] [PubMed] [Google Scholar]
- 17. Björn L, Erik M, Per T, Mikael N, Jenny H. Agreement between spirometry and impulse oscillometry for lung function assessment in 6‐year‐old children born extremely preterm and at term. Pediatr Pulmonol. 2020;55:2745‐2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klinger AP, Travers CP, Martin A, et al. Non‐invasive forced oscillometry to quantify respiratory mechanics in term neonates. Pediatr Res. 2020;88(2):293‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakarya A, Uyan ZS, Baydemir C, et al. Evaluation of children with cystic fibrosis by impulse oscillometry when stable and at exacerbation. Pediatr Pulmonol. 2016;51:1151‐1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


