Abstract
An anaerobic, H2-utilizing bacterium, strain RD-1, was isolated from the highest growth-positive dilution series of a root homogenate prepared from the sea grass Halodule wrightii. Cells of RD-1 were gram-positive, spore-forming, motile rods that were linked by connecting filaments. Acetate was produced in stoichiometries indicative of an acetyl coenzyme A (acetyl-CoA) pathway-dependent metabolism when RD-1 utilized H2-CO2, formate, lactate, or pyruvate. Growth on sugars or ethylene glycol yielded acetate and ethanol as end products. RD-1 grew at the expense of glucose in the presence of low initial concentrations (up to 6% [vol/vol]) of O2 in the headspace of static, horizontally incubated culture tubes; the concentration of O2 decreased during growth in such cultures. Peroxidase, NADH oxidase, and superoxide dismutase activities were detected in the cytoplasmic fraction of cells grown in the presence of O2. In comparison to cultures incubated under strictly anoxic conditions, acetate production decreased, higher amounts of ethanol were produced, and lactate and H2 became significant end products when RD-1 was grown on glucose in the presence of O2. Similarly, when RD-1 was grown on fructose in the presence of elevated salt concentrations, lower amounts of acetate and higher amounts of ethanol and H2 were produced. When the concentration of O2 in the headspace exceeded 1% (vol/vol), supplemental H2 was not utilized. The 16S rRNA gene of RD-1 had a 99.7% sequence similarity to that of Clostridium glycolicum DSM 1288T, an organism characterized as a fermentative anaerobe. Comparative experiments with C. glycolicum DSM 1288T demonstrated that it had negligible H2- and formate-utilizing capacities. However, carbon monoxide dehydrogenase was detected in both RD-1 and C. glycolicum DSM 1288T. A 91.4% DNA-DNA hybridization between the genomic DNA of RD-1 and that of C. glycolicum DSM 1288T confirmed that RD-1 was a strain of C. glycolicum. These results indicate that (i) RD-1 metabolizes certain substrates via the acetyl-CoA pathway, (ii) RD-1 can tolerate and consume limited amounts of O2, (iii) oxic conditions favor the production of ethanol, lactate, and H2 by RD-1, and (iv) the ability of RD-1 to cope with limited amounts of O2 might contribute to its survival in a habitat subject to daily gradients of photosynthesis-derived O2.
Sea grasses colonize shallow coastal marine environments (2, 26, 61) and thus are rooted in reduced, anoxic sediments where high rates of sulfate reduction can yield high concentrations of sulfide (5). Sediments colonized by the sea grass Halodule wrightii contain high numbers of readily culturable acetogens and acetate-utilizing sulfate reducers, and these numbers are significantly higher than in unvegetated sediments (38). Colonization of the sea grass rhizosphere by bacteria might give those organisms ready access to plant-derived substrates that could supply the energy needed for nitrogen fixation, thus yielding a beneficial plant-microbe interaction (11, 12). Although most rhizobacteria likely colonize root tips or outermost root cell layers (9), microautoradiographs of sea grass root thin sections hybridized with 33P-labeled probes revealed the presence of acetogens, clostridia, and sulfate-reducing bacteria in both the rhizoplane and deep cortex cells of sea grass roots (38).
The O2 produced by leaf photosynthesis is transported to the roots and generates transient O2 gradients around the roots (1, 33). Thus, anaerobic endorhizobacteria likely experience periods of elevated O2 tension, suggesting that such bacteria must cope with O2. Indeed, exposure of sea grass roots to O2 has minimal affect on root-surface-associated, sulfate-reducing activity (5). This observation is consistent with other findings demonstrating that some sulfate-reducing bacteria are aerotolerant (10, 19, 28).
Most acetogens have been isolated from habitats with stable anoxic conditions (e.g., sediments or sewage sludge) (21, 50). However, acetogens colonize the leaf litter and mineral soil of oxic forest soils (34, 37, 47), tolerate periods of oxygenation in soils (60), and are active in termite guts that have steep O2 gradients (7, 56). The objectives of this study were to (i) isolate an acetogen from sea grass roots, (ii) determine its survivability under oxic conditions, and (iii) investigate its physiological response and protective mechanisms to elevated O2 tensions.
MATERIALS AND METHODS
Collection of sea grass roots.
Sea grasses (H. wrightii) were sampled in clear, plastic cores from a depth of about 1 m below the surface of the overlying brackish water near Big Sabine Point in Santa Rosa Sound, located in northwestern Florida. The cores were released into a sterile glass dish, and the roots were carefully separated from the sediment. The pH of the sediment was 6.7, and the water temperature was 33°C. Healthy roots, white and free of lesions, were excised with a sterile razor blade and washed twice in sterile phosphate-buffered saline (120 mM sodium phosphate and 0.85% NaCl, pH 7.2).
Medium composition and growth conditions.
The anoxic, carbonate-buffered, undefined (U) medium contained yeast extract, vitamins, and trace metals but did not contain reducing agents (16). Usalt medium was U medium supplemented with NaCl (20 g liter−1) and MgCl2 (2 g liter−1). The defined medium was U medium without yeast extract. Media were dispensed under CO2 into 27-ml culture tubes (7 ml of medium per tube) or 1-liter infusion bottles (500 ml of medium per bottle, used for preparation of cell extracts), which were then sealed and autoclaved; the pH approximated 6.7. Tryptic soy broth (TSB) medium (28 g of Bacto TSB without glucose [Difco Laboratories, Detroit, Mich.] per liter) was made anoxic by boiling and cooling under 100% argon. The pH was adjusted with H3PO4 or NaOH to the indicated pH. Anoxic stock solutions of substrates (prepared under argon) were filter sterilized and were added by syringe injection using O2-free techniques. Unless otherwise indicated, culture tubes and bottles were incubated in a horizontal, static position, cultivation was in anoxic U medium, and the temperature of incubation was 30°C. Escherichia coli K12 (DSM 423) was cultivated in peptone-beef extract medium (5 g liter−1) (Difco Laboratories) at pH 7.0.
Alternative electron acceptors, reduction of C2H2, and toxic effects of oxygen.
The dissimilation of nitrate or sulfate was evaluated with TSB medium supplemented with 10 mM glucose and 5 mM NaNO3 or 5 mM Na2SO4, respectively. The reduction of Fe(III) was determined by assessing the growth-dependent production of white Fe(II) precipitates in medium formulated for Fe(III)-reducing bacteria containing U growth factors (8). Nitrogenase activity was determined with a modification of the C2H2 reduction method (30, 41, 58). Sterile O2 was added to the headspace of culture tubes to the concentrations (vol/vol) indicated.
Enrichment cultures.
Washed roots (5 g) were brought into an O2-free chamber (Mecaplex, Grenchen, Switzerland) (100% N2 gas phase; room temperature) and were homogenized with a grinder in 45-ml basal salt solution (16). The root suspension was serially diluted and transferred to culture tubes containing Usalt medium and H2-CO2 (80:20 [vol/vol]) in the headspace. Stable acetogenic enrichment cultures were obtained from the highest growth-positive dilution series and were subsequently streaked onto solidified Usalt medium. Isolated colonies were picked with a sterile needle and were transferred to liquid Usalt medium; cultures were subsequently restreaked onto solidified Usalt medium, and isolated colonies were again transferred to liquid Usalt medium. This procedure was repeated two additional times, and isolates were examined microscopically for purity.
Electron microscopy.
Cells were cultivated in Usalt medium or on solidified Usalt medium (1% Gelrite; Carl Roth GmbH, Karlsruhe, Germany); both media were supplemented with 10 mM glucose. For negative staining, cells were fixed for 30 min in liquid medium by adding glutaraldehyde to a final concentration of 2% (vol/vol). Cells were harvested by gentle centrifugation (1,000 × g; 15 min), adsorbed to carbon film (59), and stained with aqueous uranyl acetate solution (2% [wt/vol]). For preparation of thin sections, cells were fixed in glutaraldehyde-OsO4 (39, 57).
Preparation of cell extracts and enzyme assays.
Cell extracts were prepared under anoxic conditions (35, 42). Membranous and cytoplasmic fractions were prepared under oxic conditions (27). Oxidoreductase activities were assayed spectrophotometrically at 20°C in Tris-HCl (50 mM, pH 7.5) buffer containing 0.5 mM benzyl viologen (16). Assay tubes contained 20% H2 (vol/vol, with N2 in gas phase) for hydrogenase, 20% CO (vol/vol, with N2 in gas phase) for carbon monoxide dehydrogenase or 4 mM sodium formate for formate dehydrogenase (100% N2 in gas phase). These enzyme activities are expressed in micromoles of substrate oxidized minute−1.
Catalase, peroxidase, NADH oxidase, and superoxide dismutase activities were assayed according to standard protocols (3, 4, 52, 53). Catalase, peroxidase, NADH oxidase, and superoxide dismutase activities are expressed in the following units, respectively: 1 μmol of H2O2 consumed min−1, 1 mg of pyrogallol oxidized min−1, 1 μmol of NADH oxidized min−1, and 1 μmol of unreduced Nitro Blue Tetrazolium chloride min−1. RD-1 was grown in U medium with 10 mM glucose and 5% (vol/vol) O2 in the headspace when these enzymes were evaluated.
Redox difference spectra.
Membranous and cytoplasmic fractions were reduced with sodium dithionite, and redox difference spectra were obtained with a Uvikon 930 (Kontron Instruments, Milan, Italy) double-beam recording spectrophotometer (34).
G+C content.
Cells were treated with penicillin G (250 μg ml−1) 3 h before harvesting. DNA extraction included treatments with lysozyme/proteinase K and RNase/proteinase K (43). The G+C content was determined by high-performance liquid chromatography using nonmethylized lambda DNA for calibration (45, 54).
Phylogenetic analysis.
A total of 1,317 bases of the 16S rRNA gene of RD-1 were sequenced. DNA extraction, PCR-mediated amplification of the 16S rRNA gene, and purification of the PCR products were performed according to published protocols (20, 49). Cells were lysed by boiling at approximately 100°C. Purified PCR products were sequenced by using an ABI PRISM Ready Reaction Dye Terminator kit (Applied Biosystems, Foster City, Calif.). Sequence reaction mixtures were electrophoresed with an Applied Biosystems model 373A DNA sequencer. Alignment of the sequence data and sequence similarity calculations were performed using the tools of the ARB software package (http://www.mikro.biologie.tu-muenchen.de).
DNA-DNA hybridzation.
DNA-DNA hybridization was determined by spectrophotometric reassociation kinetics according to published protocols (13, 17, 24, 31).
Additional analytical methods.
Growth was measured as optical density at 660 nm; the optical-path width (inner diameter of culture tubes) was 1.6 cm. Uninoculated medium served as a reference. Protein in cell extracts was determined colorimetrically (6). Substrates and products were determined by high-performance liquid chromatography and gas chromatography (16, 36, 44). Nitrate was measured colorimetrically (14). Sulfate was analyzed by ion chromatography (36). Results are representative of replicate experiments.
Accession numbers.
RD-1 has been deposited at the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) under accession number DSM 13865. The 16S rRNA gene sequence of RD-1 has been deposited at the EMBL Nucleotide Sequence Database (Cambridge, United Kingdom) under accession number AJ291746.
RESULTS
Acetogenic enrichment cultures and isolates.
When root homogenates were serially diluted in Usalt medium containing H2-CO2, stable enrichment cultures that produced 1 mol of acetate per 5 mol of H2 consumed were obtained from the two highest growth-positive dilutions, 10−4 and 10−5. Two gram-positive, rod-shaped isolates, RD-1 (obtained from the 10−5 growth-positive dilution) and RD-3 (obtained from the 10−4 growth-positive dilution), converted H2-CO2 to acetate in stoichiometries indicative of an acetyl coenzyme A (acetyl-CoA) pathway-dependent metabolism: 4 H2 + 2 CO2 → CH3COOH + 2 H2O (21). Colonies of both isolates were shiny, convex, white to beige, and 2 to 3 mm in diameter. Initial screening of growth substrates indicated that RD-1 and RD-3 were the same organism, and RD-1 was selected for further characterization.
Morphology and ultrastructure of RD-1.
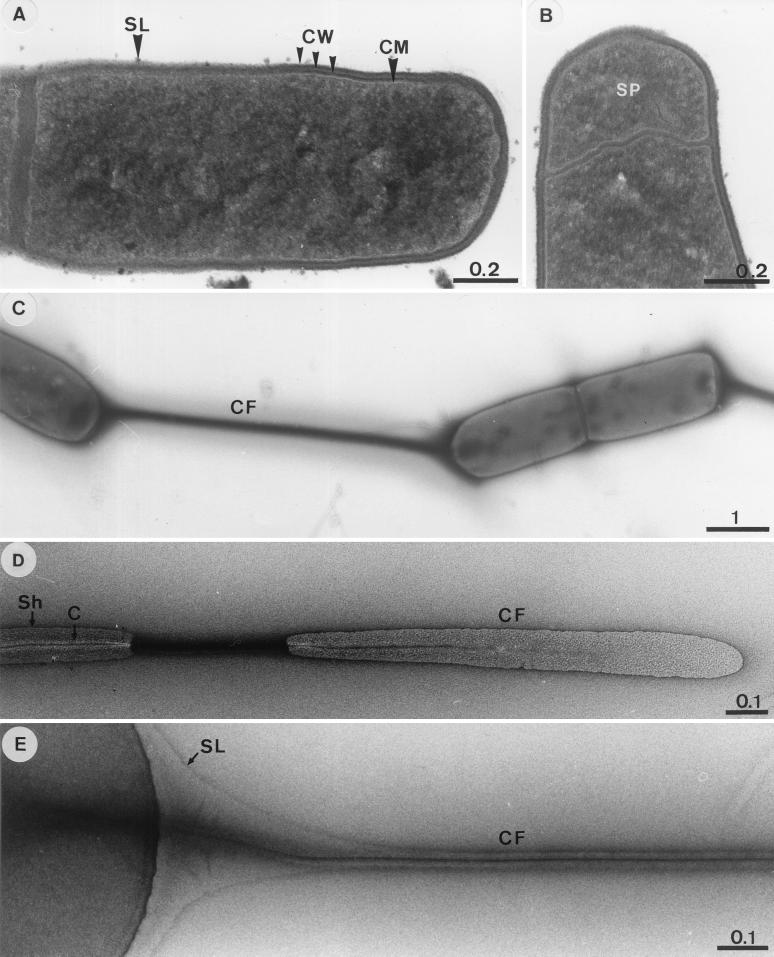
Cells were 4 by 0.8 to 6 by 0.8 μm (Fig. 1A) and were motile in wet mounts. Thin sections revealed a multilayered cell wall but no outer membrane (Fig. 1A). Cells formed terminal spores (Fig. 1B); free spores were rarely observed in cultures under the light microscope. Up to seven cells of RD-1 were often tethered to one another by connecting filaments (Fig. 1C). The connecting filament consisted of a core and a surrounding sheath (Fig. 1D). A morphological continuity was apparent between the surface of the cell and the sheath (Fig. 1E). However, the sheath was not always present, indicating that it could be dissociated from the core. The negatively stained core usually revealed a darkened center (Fig. 1D), which might have been due to a high affinity of the inner portion of the core for uranyl acetate.
FIG. 1.
Transmission electron micrographs of RD-1. (A) Thin section of a dividing cell. (B) Thin section showing formation of a terminal spore. (C to E) Negatively stained specimens revealing the ultrastructural features of connecting filaments. Abbreviations: SL, surface layer; CW, cell wall; CM, cytoplasmic membrane; SP, prespore; CF, connecting filament; Sh, outer sheath; C, core. Bars are in micrometers.
Growth properties of RD-1.
Growth was observed after cultures were heated for 15 min at 80°C, confirming the occurrence of spores. Growth could not be maintained in defined medium, indicating that RD-1 required some undefined factors for growth. In TSB medium, growth occurred at pH 6.1 to 8.2; growth did not occur at pH 5.6 and 8.5. The optimal pH was 7.0 to 7.5. RD-1 grew at 25 to 42°C in Usalt medium (pH 7.0) supplemented with fructose; no growth occurred at 20 and 45°C. At pH 7.0, growth rates and cell yields were optimal at 37 to 40°C; under these conditions, the doubling time was 2.9 h.
Substrate range and fermentation stoichiometries of RD-1.
The following substrates were utilized in Usalt medium: fructose, glucose, maltose, xylose, pyruvate, lactate, formate, and H2-CO2. RD-1 produced acetate from H2-CO2, formate, pyruvate, and lactate in stoichiometries indicative of acetogenesis (22) (Table 1 and data not shown). Fructose, glucose, maltose, and xylose were fermented to acetate and ethanol and small amounts of H2 (Table 1 and data not shown). The presence of elevated salt concentrations affected the ratio of acetate produced to fructose consumed (Table 2). Smaller amounts of acetate but greater amounts of ethanol and H2 were produced when RD-1 was grown on fructose in Usalt medium than when RD-1 was grown on fructose in U medium. Growth on yeast extract (i.e., U medium without supplemental substrates) yielded acetate and isovalerate. Ethylene glycol was fermented to acetate and ethanol, and propylene glycol was fermented to propionate and propanol and small amounts of acetate. Neither growth nor utilization of substrate was observed with galactose, sucrose, melitose, cellobiose, mannose, lactose, vanillate, syringate, ferulate, methanol, ehanol, propanol, 2,3-butandiol, citrate, succinate, fumarate, butyrate, oxalate, acetate, or CO. RD-1 did not dissimilate nitrate, sulfate, or Fe(III) and did not produce methane. In medium lacking inorganic nitrogen compounds, RD-1 did not reduce C2H2, indicating that RD-1 did not produce nitrogenase.
TABLE 1.
Product profiles of RD-1 cultivated in Usalt mediuma
| Substrate | Optical density at 660 nm | Substrate consumed (mM) | Acetate produced (mM) | Ethanol produced (mM) | H2 produced (mmol liter of culture fluid−1) | Acetate/substrate ratiob |
|---|---|---|---|---|---|---|
| H2 | 0.03 | 42.5 | 9.8 | NDc | 0 | 0.23 (0.25) |
| Formate | 0.01 | 15.4 | 4.7 | 0 | 0 | 0.31 (0.25) |
| Pyruvate | 0.05 | 3.7 | 5.7 | 0 | ND | 1.54 (1.25) |
| Glucose | 0.41 | 5.0 | 9.6 | 3.3 | Trace levels | 1.9 (3.0) |
| Xylose | 0.22 | 5.4 | 8.7 | 2.3 | Trace levels | 1.6 (2.5) |
Lactate was not detected in any culture. Values were corrected with control values obtained from cultures lacking additional substrates and are means of three replicates. The standard deviations were less than 10%.
Values in parentheses are the theoretical acetate-to-substrate ratios if substrates were converted to acetate via the acetyl-CoA pathway.
ND, not determined.
TABLE 2.
Effect of increasing salt concentrations on the fructose (5 mM)-dependent product profiles of RD-1a
| Additional concn of NaCl and MgCl2, respectively (g liter−1) | Products
|
Acetate:fructose ratio | Recovery of reducing equivalents (%) | ||
|---|---|---|---|---|---|
| Acetate (mM) | Ethanol (mM) | H2 (mmol liter of culture fluid−1) | |||
| 0, 0 | 11.7 | 2.3 | 0.09 | 2.34 | 101 |
| 5, 0.05 | 11.3 | 2.4 | 0.11 | 2.26 | 99 |
| 10, 0.1 | 11.1 | 2.9 | 0.15 | 2.22 | 103 |
| 15, 0.15 | 9.9 | 3.2 | 0.13 | 1.98 | 98 |
| 20, 0.2 | 9.6 | 3.0 | 0.18 | 1.92 | 93 |
Fructose was totally consumed in all cultures. Lactate was not detected in any culture. Values were corrected with control values obtained from cultures lacking fructose and are means of three replicates. The standard deviations were less than 10%.
Effect of O2 on growth of RD-1.
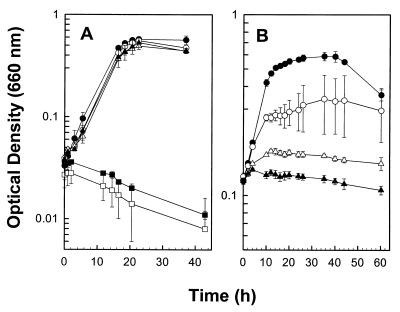
Glucose-dependent growth rates and cell yields were not significantly affected by up to 6% (vol/vol) O2 in the headspace of static, horizontally incubated tubes that were periodically shaken prior to measuring optical densities (Fig. 2A). When culture tubes were continuously shaken (60 rpm), RD-1 grew in the presence of up to 4% (vol/vol) O2 in the headspace (Fig. 2B).
FIG. 2.
Effect of O2 on the glucose-dependent growth of RD-1. Culture tubes were either incubated horizontally and shaken prior to each measurement (A) or continuously shaken (B). Data are the means of three replicates (± standard deviations). The initial concentrations of O2 (vol/vol) in the headspace were ●, 0%; ○, 4%; ▵, 5%; ▴, 6%; ▪, 7%; and □, 9% (A) or ●, 0%; ○, 2%; ▴, 3%; and ▵, 6% (B).
Effect of O2 on physiological capabilities of RD-1.
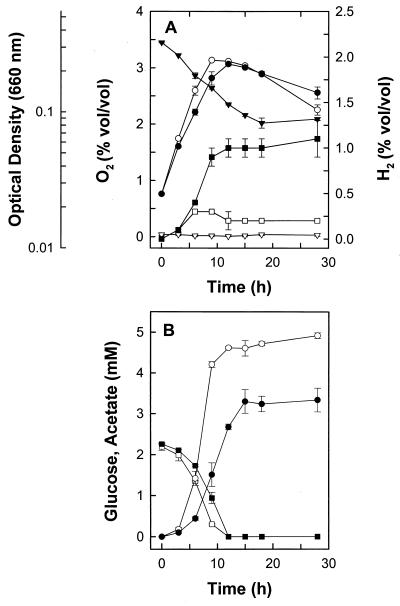
When RD-1 was cultivated on glucose with 0.3, 2.1, 2.8, 3.8, 4.9, or 5.8% (vol/vol) O2 in the headspace of static, horizontally incubated tubes, O2 decreased to 0, 1.5, 2.0, 3.1, 3.9, or 4.7% (vol/vol), respectively, by the end of incubation (2 days). O2 was not consumed in uninoculated controls. RD-1 consumed O2 concomitantly with exponential growth and the consumption of glucose (Fig. 3).
FIG. 3.
Effect of O2 on the glucose-derived product and growth profiles of RD-1. Data are the means of three replicates (± standard deviations). (A) ▾, O2 in cultures containing supplemental O2; ▿, O2 in cultures without supplemental O2; ●, optical density in cultures containing supplemental O2; ○, optical density in cultures without supplemental O2; ▪, H2 in cultures containing supplemental O2; □, H2 in cultures without supplemental O2. (B) ▪, glucose in cultures containing supplemental O2; □, glucose in cultures without supplemental O2; ●, acetate in cultures containing supplemental O2; ○, acetate in cultures without supplemental O2. Acetate values were corrected with control values from cultures lacking glucose. The small amounts of ethanol formed from 2 mM glucose could not be accurately quantitated.
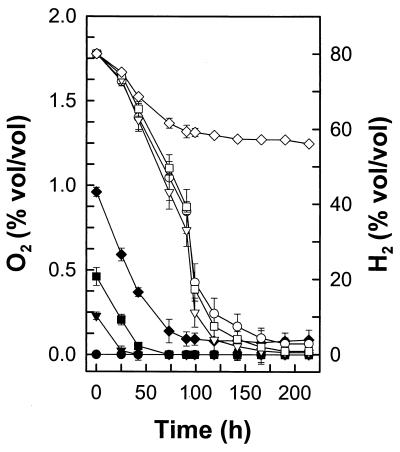
Acetate production decreased, higher amounts of ethanol were produced, and H2 (0.6 mmol l−1 culture fluid) and lactate (0.4 mM) became significant end products in the presence of O2 (Table 3). The recovery of reductant was low in cultures containing O2. Thus, unrecovered reductant derived from the oxidation of glucose appeared to be stored in unidentified products or biomass. The addition of up to 0.5% (vol/vol) O2 to the headspace of cultures did not affect the capacity of RD-1 to consume H2 (Fig. 4). H2 was not consumed when O2 in the headspace of cultures exceeded 1% (vol/vol).
TABLE 3.
Effect of increasing O2 concentrations on the glucose (5 mM)-dependent product profiles of RD-1a
| Initial O2 concn (%) | O2 consumed (mmol liter of culture fluid−1) | Products (mM)
|
Acetate/glucose ratio | Recovery of reducing equivalents (%)b | |||
|---|---|---|---|---|---|---|---|
| Acetate | Ethanol | Lactate | H2 | ||||
| Anoxic | NAc | 12.1 | 0.8 | 0 | 0.02 | 2.42 | 89 |
| 0.3 | 0.29 | 11.6 | 0.8 | 0 | 0.02 | 2.32 | 85 (86) |
| 2.0 | 2.11 | 7.5 | 2.6 | 0.3 | 0.54 | 1.50 | 81 (88) |
| 2.8 | 2.22 | 6.4 | 2.9 | 0.3 | 0.52 | 1.28 | 75 (83) |
| 3.7 | 2.17 | 6.8 | 1.5 | 0.4 | 0.53 | 1.36 | 66 (73) |
Glucose was totally consumed in all cultures. Values were corrected with control values obtained from cultures lacking glucose and are means of three replicates. The standard deviations were less than 10%.
Values in parentheses take into account the amount of reductant that was theoretically required for the reduction of O2.
NA, not applicable.
FIG. 4.
Effect of O2 on the consumption of H2 by RD-1. Culture tubes contained an H2-CO2 gas phase (80:20%, vol/vol). Symbols: ♦, ▪, and ▾, O2 in cultures with supplemental O2; ●, O2 in cultures without supplemental O2; ⋄, □, and ▿, H2 in cultures containing 1, 0.5, and 0.25% (vol/vol) O2, respectively; and ○, H2 in cultures without supplemental O2.
Oxidative stress enzyme activities of RD-1.
NADH oxidase, superoxide dismutase, peroxidase, and catalase activities in the cytoplasmic fraction of RD-1 approximated 4.3, 0.4, 0.1, and 0 U mg−1 of protein, respectively. These enzyme activities were not detected in the membrane fraction. NADH oxidase, superoxide dismutase, peroxidase, and catalase activities in the cytoplasmic fraction of E. coli K12 approximated 3.3, 0.5, 0.5, and 230 U mg−1 of protein, respectively.
Oxidoreductase activities and redox difference spectra of membranes of RD-1.
Carbon monoxide dehydrogenase, hydrogenase, and formate dehydrogenase activites in cell extracts obtained from fructose-cultivated cells of RD-1 approximated 0.6, 0.9, and 0.2 U mg−1 of protein, respectively. No absorption maxima indicative of cytochromes were detected in the membranous or cytoplasmic fractions of RD-1 (data not shown).
Phylogenetic analysis, G+C content, DNA-DNA hybridization, and protein profiles.
The 16S rRNA gene sequence of RD-1 was most closely related to that of bacteria that group in cluster XI of the genus Clostridium (15). The highest sequence similarity value (99.7%) was to C. glycolicum DSM 1288T. The DNA base composition of RD-1 was 31.6 (±0.8) (n = 3) mol%. The G+C mol% of the DNA of C. glycolicum DSM 1288T is 29 (32). DNA-DNA hybridization experiments revealed a 91.4% genome sequence similarity between RD-1 and C. glycolicum DSM 1288T. Cells of RD-1 and of C. glycolicum DSM 1288T that had been cultivated on fructose yielded nearly identical protein profiles when cell extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (data not shown).
Metabolic capacities of C. glycolicum DSM 1288T
In U medium, C. glycolicum DSM 1288T fermented fructose (10 mM) to acetate (21 mM) and ethanol (2.2 mM). In Usalt medium, fructose (10 mM) was fermented to acetate (17.8 mM), ethanol (2.5 mM), and trace levels of H2. In contrast to RD-1, C. glycolicum DSM 1288T did not catalyze the H2- or formate-dependent formation of acetate. Nonetheless, carbon monoxide dehydrogenase and hydrogenase activities were detected in cell extracts of fructose-cultivated cells of C. glycolicum DSM 1288T (activities approximated 1.1 and 0.6 U mg−1 of protein, respectively). The temperature optimum of C. glycolicum DSM 1288T was 30°C.
DISCUSSION
RD-1 was isolated as an anaerobe with acetogenic capabilities yet was also able to grow in the presence of 4 and 6% (vol/vol) O2 in the headspace of continuously and periodically shaken culture tubes, respectively. The capacity of RD-1 to consume small amounts of O2 without an apparent lag phase suggests that this metabolic capacity was constitutive. Many so-called obligately anaerobic bacteria and archaea exhibit some degree of aerotolerance (48). NADH oxidase is used by the aerotolerant anaerobe Brachyspira hyodysenteriae for the consumption of O2 (51). Facultative bacteria and certain obligate anaerobes contain superoxide dismutase or NADH oxidase (48, 51). Superoxide dismutase, peroxidase, and NADH oxidase activities were detected in the cytoplasmic fraction of RD-1, indicating that RD-1 can disproportionate superoxide and reduce hydrogen peroxide or O2. The oxidative stress enzyme catalase, present in certain methanogens (40), was not detected in RD-1. Desulfoferrodoxin and rubrerythrin function together as an oxidative stress defense mechanism in the sulfate reducer Desulfovibrio vulgaris (42), and genes for similar proteins have been identified in the acetogen Moorella thermoacetica (A. X. Das and L. G. Ljungdahl, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. H-117, p. 374, 2000). In comparison to the ability of RD-1 to tolerate relatively high amounts of O2, the acetogens Acetobacterium woodii, M. thermoacetica, Clostridium magnum, and Sporomusa silvacetica can only tolerate 0.5, 1, 2, and 2% (vol/vol) O2, respectively, in the headspace of culture tubes when cultivated on glucose (A. Karnholz, K. Küsel, and H. L. Drake, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. I-91, p. 401, 2000; unpublished data). Methanogenic (40) and acetogenic (H. Boga and A. Brune, Abstr. Ann. Meet. Verein. Allgem. Angewand. Mikrobiol. BioSpectrum, abstr. 15.P.11.33, p. 143, 2000) termite gut isolates can grow and consume O2 in agar-O2-gradient tubes. Thus, certain acetogens, like other so-called obligate or strict anaerobes, have the capacity to cope with oxidative stress, and it should not be considered a paradox that acetogens occur in habitats subject to fluxes of O2.
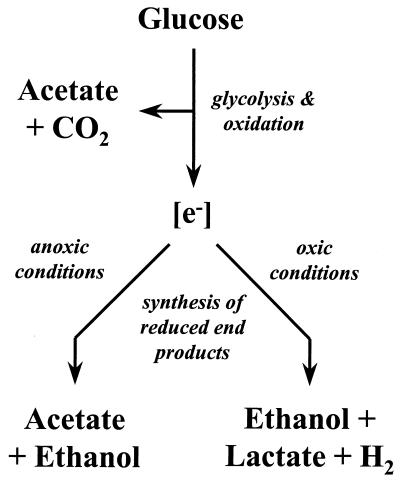
Under anoxic conditions, RD-1 utilized both ethanol fermentation and the acetyl-CoA pathway as terminal, electron-accepting processes when grown on glucose (Fig. 5). In contrast, growth on pyruvate did not yield ethanol as a product. Thus, the simultaneous engagement of acetogenesis and fermentation was substrate dependent. When RD-1 was grown on glucose in the presence of O2, ethanol and, to a lesser extent, lactate and H2 became the main reduced end products, indicating that reductant flow was diverted away from the acetyl-CoA pathway when redox conditions became more oxic (Fig. 5). Ethanol and lactate fermentation are also important energy-conserving processes for the acetogen Ruminococcus productus U1, and the simultaneous engagement of multiple catabolic processes might contribute to the in situ competitiveness of acetogens (22).
FIG. 5.
Hypothetical scheme illustrating how oxic conditions cause a shift in the flow of reductant ([e−]) by RD-1. The acetate formed under oxic conditions is derived from the oxidation of glucose (upper portion of scheme) rather than reductive synthesis from CO2 via the acetyl-CoA pathway (depicted as the acetate that is produced as a reduced end product under anoxic conditions (lower left branch of figure).
Many of the enzymes of the acetyl-CoA pathway are very sensitive to O2 (21), and H2-dependent acetogenesis by RD-1 was more sensitive to O2 than was fermentation. O2 is likewise inhibitory to the H2-dependent acetogenic capabilities of certain termite gut isolates (Boga et al., Abstr. Ann. Meet. Verein. Allgem. Angewand. Mikrobiol. BioSpectrum, abstr. 15.P.1133, p. 143, 2000). The ionic strength of the medium also caused a shift in the metabolism of RD-1 towards the synthesis of ethanol. However, lactate was only formed from sugars when RD-1 was subjected to oxidative stress.
Molecular characterization of RD-1 indicated that it was a strain of C. glycolicum. Strains of C. glycolicum have been isolated from soil, mud, bovine intestine, human feces, and human wounds (23, 25). C. glycolicum DSM 1288T is described as a saccharolytic fermenter that produces acetate, ethanol, CO2, and H2 (29). In contrast to RD-1, C. glycolicum DSM 1288T had negligible H2- and formate-utilizing capacities. RD-1 and C. glycolicum DSM 1288T also had dissimilar temperature optima. Although acetogenic capacities were not readily apparent with C. glycolicum DSM 1288T, the organism nonetheless contained carbon monoxide dehydrogenase. Carbon monoxide dehydrogenase activity is also present in the nonacetogenic butyrate fermenter Clostridium pasteurianum (18).
A phylogenetically uncharacterized H2-utilizing acetogen, Clostridium sp. strain 22 (ATCC 29797), might be a strain of C. glycolicum (46; information from the American Type Culture Collection [Manassas, Va.] Bacteriology Program). Strain 22 was isolated on H2-CO2 from sewage sludge, is a gram-positive rod that forms subterminal spores, and has a pH optimum of 8.0 (46). Since RD-1 formed terminal spores and had a pH optimum of 7.0 to 7.5, RD-1 and strain 22 appear to be dissimilar. C. glycolicum DSM 1288T might have lost its ability to reductively synthesize acetate from H2-CO2. Most acetogens grow poorly on H2-CO2, in part because of the thermodynamic constraints of the carbonyl branch of the acetyl-CoA pathway (21), and certain acetogens lose their ability to efficiently utilize H2 after prolonged cultivation in the laboratory on H2-CO2 (39).
Phylogenetically, acetogens are not tightly clustered but are widely dispersed throughout the low-G+C-content, gram-positive bacteria (55). To date, no general 16S rRNA gene probe is available to detect acetogens in natural samples. The 16S rRNA gene probes used to evaluate the occurrence of acetogens in sea grass roots (38) are specific for acetogenic species of Acetobacterium (probe AW), the acetogen Eubacterium limosum (probe AW), and Clostridium species that group in cluster I of their genus (probe Clost I), respectively (15). About 60% of the epidermal cells, 24% of the outermost cortex cell layers, and 2% of the deeper cortex cell layers of H. wrightii roots were colonized with acetogens that hybridized with probe AW (38). However, based on 16S rRNA gene sequence similarities, RD-1 grouped in cluster XI of the genus Clostridium (15) and would not be detected by the 16S rRNA gene probes AW and Clost I. Further studies will be required to elucidate the number and types of acetogens associated with sea grass rhizospheres and to understand how these microorganisms affect the viability of sea grasses.
ACKNOWLEDGMENTS
We are grateful to Anita Gößner for technical assistance and to Rita Grotjahn for preparation of the electron micrographs.
Support for this study was provided by the German Ministry of Education, Science, Research, and Technology (PT BEO 51–0339476C).
REFERENCES
- 1.Armstrong W, Justin S H F W, Beckett P M, Lythe S. Root adaptation to soil water logging. Aquat Bot. 1991;39:57–73. [Google Scholar]
- 2.Barko J W, Gunnison D, Carpenter S R. Sediment interactions with submersed macrophyte growth and community dynamics. Aquat Bot. 1991;41:41–65. [Google Scholar]
- 3.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Ann Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 4.Beers R F, Jr, Sizers I W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133. [PubMed] [Google Scholar]
- 5.Blaabjerg V, Finster K. Sulphate reduction associated with roots and rhizomes of the marine macrophyte Zostera marina. Aquat Microb Ecol. 1998;15:311–314. [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Brune A, Emerson D, Breznak J A. The termite gut microflora as an oxygen sink: microelectrode determination of oxygen and pH gradients in guts of lower and higher termites. Appl Environ Microbiol. 1995;61:2681–2687. doi: 10.1128/aem.61.7.2681-2687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caccavo F, Jr, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducens sp. nov., a hydogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell R, Greaves M P. Anatomy and community structure of the rhizosphere. In: Lynch J M, editor. The rhizosphere. Chichester, United Kingdom: John Wiley & Sons; 1990. pp. 11–34. [Google Scholar]
- 10.Canfield D E, DesMarais D J. Aerobic sulphate reduction in microbial mats. Science. 1991;251:1471–1473. doi: 10.1126/science.11538266. [DOI] [PubMed] [Google Scholar]
- 11.Capone D C, Penhale P A, Oremland R S, Taylor B F. Relationship between productivity and N2 (C2H2) fixation in a Thalassia testudinum community. Limnol Oceanogr. 1979;24:117–125. [Google Scholar]
- 12.Capone D G, Taylor B F. N2 fixation in the rhizosphere of Thalassia testudinum. Can J Microbiol. 1980;26:998–1005. doi: 10.1139/m80-169. [DOI] [PubMed] [Google Scholar]
- 13.Cashion P, Holder-Franklin M A, McCully J, Franklin M. A rapid method for the base ratio determination of bacterial DNA. Anal Biochem. 1977;81:461–466. doi: 10.1016/0003-2697(77)90720-5. [DOI] [PubMed] [Google Scholar]
- 14.Cataldo D A, Haroon M, Schrader L E, Young V L. Rapid colorimetric determination of nitrate in plant tissue by titration of salicylic acid. Commun Soil Sci Plant Anal. 1975;6:81–90. [Google Scholar]
- 15.Collins M D, Lawson P A, Willems A, Cordoba J J, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow J A E. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 16.Daniel S L, Hsu T, Dean S I, Drake H L. Characterization of the H2- and CO-dependent chemolithotrophic potentials of the acetogens Clostridium thermoaceticum and Acetogenium kivui. J Bacteriol. 1990;172:4464–4471. doi: 10.1128/jb.172.8.4464-4471.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Ley J, Cattoir H, Reynaerts A. The quantitative measurement of DNA hybridization from renaturing rates. Eur J Biochem. 1970;12:133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 18.Diekert G B, Graf E G, Thauer R K. Nickel requirement for carbon monoxide dehydrogenase formation in Clostridium pasteurianum. Arch Microbiol. 1979;122:117–120. [Google Scholar]
- 19.Diling W, Cypionka H. Aerobic respiration in sulphate-reducing bacteria. FEMS Microbiol Lett. 1990;71:123–128. [Google Scholar]
- 20.Dorsch M, Stackebrandt E. Some modifications in the procedure of direct sequencing of PCR amplified 16S rDNA. J Microbiol Methods. 1992;16:271–279. [Google Scholar]
- 21.Drake H L. Acetogenesis, acetogenic bacteria, and the acetyl-CoA Wood/Ljungdahl pathway: past and current perspectives. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall, Inc.; 1994. pp. 3–60. [Google Scholar]
- 22.Drake H L, Daniel S L, Küsel K, Matthies C, Kuhner C, Braus-Stromeyer S. Acetogenic bacteria: what are the in situ consequences of their diverse metabolic versatilities? BioFactors. 1997;6:13–24. doi: 10.1002/biof.5520060103. [DOI] [PubMed] [Google Scholar]
- 23.Drasar B S, Goddard P, Heaton S, Peach S, West B. Clostridia isolated from faeces. J Med Microbiol. 1976;9:63–71. doi: 10.1099/00222615-9-1-63. [DOI] [PubMed] [Google Scholar]
- 24.Escara J F, Hutton J R. Thermal stability and renaturation of DNA in dimethyl sulfoxide solutions: acceleration of the renaturation rate. Biopolymers. 1980;19:1315–1327. doi: 10.1002/bip.1980.360190708. [DOI] [PubMed] [Google Scholar]
- 25.Finegold S M, Sutter V L, Mathisen G E. Normal indigenous intestinal flora. In: Hentges D J, editor. Human intestinal microflora in health and disease. New York, N.Y: Academic Press; 1983. pp. 99–108. [Google Scholar]
- 26.Fonseca M S, Meyer D L, Hall M O. Development of planted seagrass beds in Tampa Bay, Florida, USA. II. Faunal components. Mar Ecol Prog Ser. 1996;132:141–156. [Google Scholar]
- 27.Fröstl J M, Seifritz C, Drake H L. Effect of nitrate on the autotrophic metabolism of the acetogens Clostridium thermoautotrophicum and Clostridium thermoaceticum. J Bacteriol. 1996;178:4597–4603. doi: 10.1128/jb.178.15.4597-4603.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fründ C, Cohen Y. Diurnal cycles of sulfate reduction under oxic conditions in cyanobacterial mats. Appl Environ Microbiol. 1992;58:70–77. doi: 10.1128/aem.58.1.70-77.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaston L W, Stadtman E R. Fermentation of ethylene glycol by Clostridium glycolicum sp. n. J Bacteriol. 1963;85:356–362. doi: 10.1128/jb.85.2.356-362.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy R W F, Burns R C, Holsten R D. Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol Biochem. 1972;5:47–81. [Google Scholar]
- 31.Huss V A R, Festl H, Schleifer K H. Studies on the spectrometric determination of DNA hybridization from renaturation rates. J Syst Appl Microbiol. 1983;4:184–192. doi: 10.1016/S0723-2020(83)80048-4. [DOI] [PubMed] [Google Scholar]
- 32.Johnson J L, Francis B S. Taxonomy of the clostridia: ribosomal ribonucleic acid homologies among the species. J Gen Microbiol. 1975;88:229–244. doi: 10.1099/00221287-88-2-229. [DOI] [PubMed] [Google Scholar]
- 33.Kraemer G P, Alberte R S. Impact of daily photosynthetic period on protein synthesis and carbohydrate stores in Zostera marina L. (eelgrass) roots: implications for survival in light-limited environments. J Exp Mar Biol Ecol. 1995;185:191–202. [Google Scholar]
- 34.Kuhner C H, Frank C, Grießhammer A, Schmittroth M, Acker G, Gößner A, Drake H L. Sporomusa silvacetica sp. nov., an acetogenic bacterium isolated from aggregated forest soil. Int J Syst Bacteriol. 1997;47:352–358. doi: 10.1099/00207713-47-2-352. [DOI] [PubMed] [Google Scholar]
- 35.Kuhner C H, Matthies C, Acker G, Schmittroth M, Gößner A S, Drake H L. Clostridium akagii sp. nov. and Clostridium acidisoli sp. nov.: acid-tolerant, N2-fixing clostridia isolated from acidic forest soil and litter. Int J Syst Evol Microbiol. 2000;50:873–881. doi: 10.1099/00207713-50-2-873. [DOI] [PubMed] [Google Scholar]
- 36.Küsel K, Drake H L. Effects of environmental parameters on the formation and turnover of acetate by forest soils. Appl Environ Microbiol. 1995;61:3667–3675. doi: 10.1128/aem.61.10.3667-3675.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Küsel K, Wagner C, Drake H L. Enumeration and metabolic product profiles of the anaerobic microflora in the mineral soil and litter of a beech forest. FEMS Microbiol Ecol. 1999;29:91–103. [Google Scholar]
- 38.Küsel K, Pinkart H C, Drake H L, Devereux R. Acetogenic and sulfate-reducing bacteria inhabiting the rhizoplane and deep cortex cells of the sea grass Halodule wrightii. Appl Environ Microbiol. 1999;65:5117–5123. doi: 10.1128/aem.65.11.5117-5123.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Küsel K, Dorsch T, Acker G, Stackebrandt E, Drake H L. Clostridium scatologenes strain SL1 isolated as an acetogenic bacterium from acidic sediments. Int J Syst Evol Microbiol. 2000;50:537–546. doi: 10.1099/00207713-50-2-537. [DOI] [PubMed] [Google Scholar]
- 40.Leadbetter J R, Breznak J A. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl Environ Microbiol. 1996;62:3620–3631. doi: 10.1128/aem.62.10.3620-3631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Limmer C, Drake H L. Non-symbiotic N2-fixation in acidic and pH-neutral forest soils: aerobic and anaerobic differentials. Soil Biol Biochem. 1996;28:177–183. [Google Scholar]
- 42.Lumppio H L, Shenvi N V, Summers A O, Voordrouw G, Kurtz D M., Jr Rubrerythrin and rubredoxin oxidoreductase in Desulfovibrio vulgaris: a novel oxidative stress protection system. J Bacteriol. 2001;183:101–108. doi: 10.1128/JB.183.1.101-108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marmur J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 44.Matthies C, Freiberger A, Drake H L. Fumarate dissimilation and differential reductant flow by Clostridium formicoaceticum and Clostridium aceticum. Arch Microbiol. 1993;160:273–278. [Google Scholar]
- 45.Mesbah M, Premachandran U, Whitman W B. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol. 1989;39:159–167. [Google Scholar]
- 46.Ohwaki K, Hungate R E. Hydrogen utilization by clostridia in sewage sludge. Appl Environ Microbiol. 1977;33:1270–1274. doi: 10.1128/aem.33.6.1270-1274.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters V, Conrad R. Methanogenic and other strictly anaerobic bacteria in desert soil and other oxic soils. Appl Environ Microbiol. 1995;61:1673–1676. doi: 10.1128/aem.61.4.1673-1676.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolfe R D, Hentges D J, Benedict J C, Barrett J T. Factors related to the oxygen tolerance of anaerobic bacteria. Appl Environ Microbiol. 1978;36:306–313. doi: 10.1128/aem.36.2.306-313.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Schink B. Diversity, ecology, and isolation of acetogenic bacteria. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall, Inc.; 1994. pp. 197–235. [Google Scholar]
- 51.Stanton T B, Lebo D F. Treponema hyodysenteriae growth under various culture conditions. Vet Microbiol. 1988;18:177–190. doi: 10.1016/0378-1135(88)90063-6. [DOI] [PubMed] [Google Scholar]
- 52.Stanton T B, Jensen N S. Purification and characterization of NADH oxidase from Serpulina (Treponema) hyodysenteriae. J Bacteriol. 1993;175:2980–2987. doi: 10.1128/jb.175.10.2980-2987.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stellmach B, Gottschick W, Battermann F, Zabel K. Bestimmungsmethoden Enzyme. Darmstadt, Germany: Steinkopff Verlag; 1988. pp. 222–223. [Google Scholar]
- 54.Tamaoka J, Komagata K. Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol Lett. 1984;25:125–128. [Google Scholar]
- 55.Tanner R S, Woese C R. A phylogenetic assessment of the acetogens. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall, Inc.; 1994. pp. 254–272. [Google Scholar]
- 56.Tholen A, Brune A. Localization and in situ activities of homoacetogenic bacteria in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.) Appl Environ Microbiol. 1999;65:4497–4505. doi: 10.1128/aem.65.10.4497-4505.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Traub W H, Acker G, Kleber I. Ultrastructural surface alterations of Serratia marcescens after exposure to polymyxin B and/or fresh human serum. Chemotherapy (Basel) 1976;22:104–113. doi: 10.1159/000221919. [DOI] [PubMed] [Google Scholar]
- 58.Turner G L, Gibson A H. Measurement of nitrogen fixation by indirect means. In: Bergersen F J, editor. Methods for evaluating biological nitrogen fixation. New York, N.Y: John Wiley & Sons; 1980. pp. 111–138. [Google Scholar]
- 59.Valentine R C, Shapiro B M, Stadtman E R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968;7:143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- 60.Wagner C, Grießhammer A, Drake H L. Acetogenic capacities and the anaerobic turnover of carbon in a Kansas prairie soil. Appl Environ Microbiol. 1996;62:494–500. doi: 10.1128/aem.62.2.494-500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waisel Y, Agami M. Ecophysiology of roots of submerged aquatic plants. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1996. pp. 895–909. [Google Scholar]